Abstract
Background and aims
Adenoma detection rate (ADR) in colon cancer screening is most important for cancer prophylaxis. This work is the first three‐armed randomised controlled clinical trial aimed at comparing a head‐to‐head setting standard colonoscopy (SC) with Endocuff‐assisted colonoscopy (EC) and cap‐assisted colonoscopy (CAC) for improvement of ADR.
Methods
Patients from Poland and Germany with independent indication for colonoscopy were randomised into three arms of this trial: EC, CAC and SC. Exclusion criteria were age <18 years, active Crohn's disease or ulcerative colitis, known stenosis and post‐colonic resection status.
Results
A total of 585 patients (195 SC, 189 EC and 186 CAC) were enrolled in this study. Indications were not different between the groups (colorectal cancer screening 51%, diagnostic colonoscopy in 31% and post‐polypectomy follow‐up in 18%; p = 0.94). Withdrawal time was a mean of 7 min in all groups (p = 0.658), and bowel preparation did not differ between the groups. The time to reach the caecum was significantly reduced when using the cap (a mean of 6 min for CAC vs. 7 min for SC; p = 0.0001). There was no significant difference in the primary outcome of the ADR between the groups (EC 32%, CAC 30%, SC 30%; p = 0.815). EC proved to be superior (EC vs. SC) in the sigmoid colon and transverse colon for polyp detection.
Conclusion
The use of EC increased the total number of polyps seen during colonoscopy. In contrast to recent studies, no significant improvement of the ADR was detected.
Keywords: adenoma detection rate, colon cancer, colonoscopy, Endocuff, screening
INTRODUCTION
Colon cancer remains a deadly disease. 1 Screening programmes have been shown to be efficient in preventing colon cancer death. 2 Among the screening tools, colonoscopy plays a pivotal role. 3 The quality of colonoscopy, which is measured by parameters such as adenoma detection rate (ADR), withdrawal time, examiner expertise and bowel preparation, is important for a reduced incidence of interval colon cancer. 4 Therefore, improving the ADR is important for successful colon cancer prevention. Among these, the Endocuff device has shown the ability to improve the ADR. 5 , 6 Cap‐assisted colonoscopy has shown benefit for the visualisation of polyps in the right colon flexure and the ascending colon. 7 However, results concerning the ADR remain inconclusive. 8 The aim of this study was a head‐to‐head comparison of Endocuff‐assisted colonoscopy (EC) versus cap‐assisted colonoscopy (CAC) compared to standard colonoscopy (SC). To the best of our knowledge, no head‐to‐head comparison of ADR‐improving devices has been published so far.
METHODS
Trial design
This study was designed as a prospective, international, multi‐centre, randomised clinical trial (RCT) with three arms. Parts of our data were published in an abstract for our oral presentation at DDW 2018. 9 A copy of our trial protocol and statistical analysis plan as well as a complete CONSORT trial checklist will be provided as supplement to this paper. A CONSORT flow diagram is provided in Figure 1. The study was approved by the ethical review board of the University of Goettingen, Germany (No. 26/7/14, 27 November 2014). Written informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
FIGURE 1.
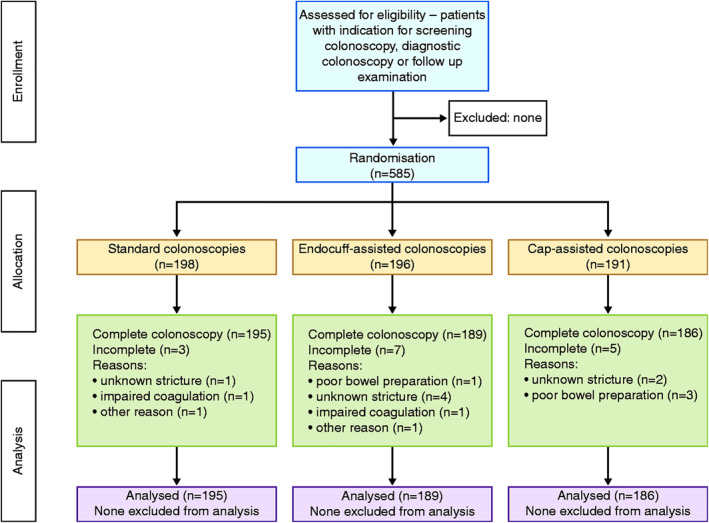
CONSORT chart of the study design
Indications for colonoscopy were screening, diagnostic colonoscopy and follow‐up examination. Exclusion criteria were age <18 years, pregnant or breastfeeding women, missing informed consent, impaired coagulation, active Crohn's disease or ulcerative colitis, known stenosis, poor bowel preparation, and history of colon resection.
Data security was guaranteed according to the European law. The present trial was registered before the first patient was included (Clinical Trials number NCT02331836; http://ClinicalTrials.gov).
The time measurement during colonoscopy was performed as described earlier by our work group. 10
Briefly, the procedure time started with the insertion of the colonoscope and ended with the removal of the colonoscope and included intervention time. Withdrawal time was the beginning of the withdrawal of the endoscope from the caecal pole without time spent for interventions. Removal of the colonoscope was the end point for withdrawal time. A microchronometer was used to measure the time. 10
Participants
Participating institutions were university hospitals as well as community hospitals: HELIOS AlbertSchweizer Hospital Northeim, HELIOS Klinikum Siegburg, HELIOS Hospital Helmstedt, University Hospital Muenster and University Medical Centre Goettingen, all of which are in Germany, and additionally University Hospital at Wroclaw Medical University, Poland. The study was also approved by the local ethics committees.
Interventions
A total of 585 patients from Poland and Germany were enrolled. Of these, 189 completed examination as EC, 186 CAC and 195 SC (Figure 1). The Endocuff was provided by Arc Medical Design Ltd. The cap was provided by Olympus Europa SE & Co. KG (Figure 2). The study was performed between 1 January 2015 and 31 December 2017. No patient refused to participate.
FIGURE 2.
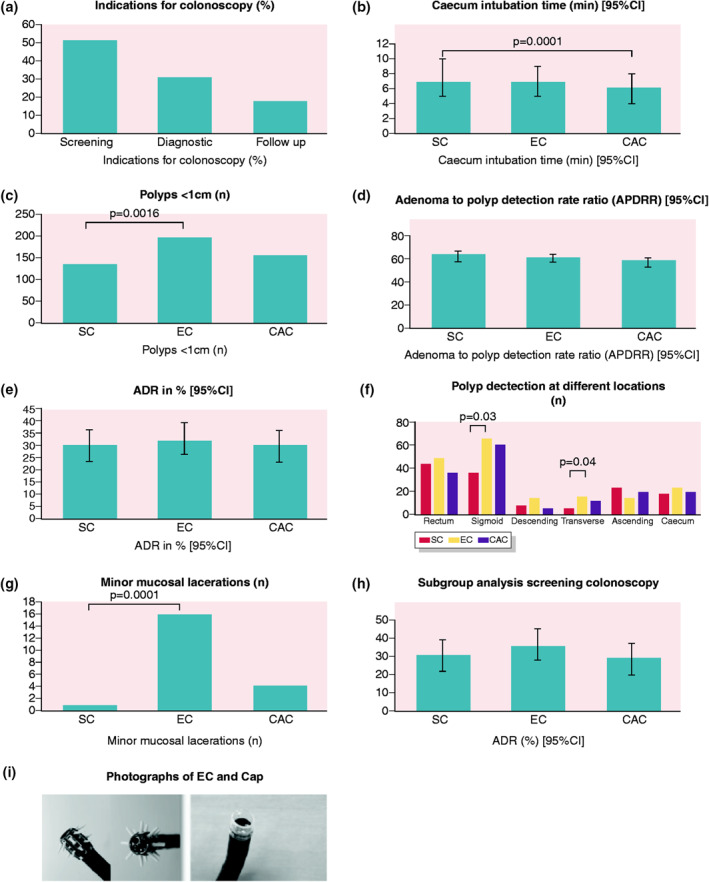
Results and devices. (a) Indications for colonoscopy. (b) Caecum intubation time. (c) Detection of polyps <1 cm. (d) Adenoma‐to‐polyp detection rate ratios. (e) Adenoma detection rate. (f) Polyp detection at different locations. (g) Adverse events: minor mucosal lacerations. (h) Subgroup analysis for screening colonoscopies: adenoma detection rate. (i) Photographs of the Endocuff (left) and cap (right) used for colonoscopy
Outcomes
The primary focus of this study was the proportion of examinations within a given arm in which adenomas were detected; here referred to as the ADR. Additionally, the following parameters were also considered for the study: polyp detection rate (PDR), age, sex, indication, previous abdominal surgery, first‐time colonoscopy, diabetes, device used, bowel preparation grade, ileum intubation, time to reach the caecum, procedure time, withdrawal time, occurrence of complications, total colonoscopy, partial colonoscopy, number of polyps, polyp location, polyp size, polyp type, histological results, and adverse effects. Bowel preparation grade was also measured, as described elsewhere. 11
Sample size calculation
The sample size was calculated on the basis of an average ADR of 25% in SC. From our earlier studies, 10 , 12 we assumed an increase of the ADR with Endocuff or CAC to 35%. A priori analysis was performed with a chi‐square test in order to estimate the sample size. We found a minimum of 179 patients per group to be necessary in order to achieve at least an 80% power to detect a 15% difference in the ADR. A type I error rate of 5% using two‐sided tests was used. Statistical power analysis was performed using G*Power v3.17. 10
Randomisation sequence generation
Block randomisation lists (block size 60) were created by a computed algorithm and sent to the different participating institutions. Block randomisation of the three groups (SC vs. EC vs. CAC) was performed for each participating centre. The unequal distribution of sample size between SC, EC and CAC is due to the individual randomisation lists for each centre.
Allocation concealment mechanism
The allocation assignment of each participating patient was performed by an independent medical doctor who was not involved as an examiner. The examiner had no ability to change the order of allocated exams in each arm.
Statistical methods
The study was designed in cooperation with a biostatistician from Muenster University. Statistical analysis was performed using SPSS v17.0 (SPSS, Inc.). Descriptive analysis was used to document the demographic and clinical data. Results are expressed as means ± standard deviation or medians (interquartile range). For detection of statistical significance, a two‐sided t‐test and Mann–Whitney U‐test were used when appropriate. A p‐value of <0.05 was considered statistically significant. 10
Implementation
Indications for colonoscopy were colorectal cancer screening (51% of cases), diagnostic colonoscopy due to anaemia or abdominal pain (31% of cases), and surveillance after polyectomy (18% of cases).
Twelve‐hour fasting before intervention and a standardised bowel preparation protocol using Moviprep (Norgine) were performed. Sedation was performed using propofol in repeated doses between 40 and 60 mg. After this, colonoscopy was performed. All endoscopy units used high‐end colonoscopes (EC‐590 WM4 and EC‐590 WL4; Fujifilm Europe; and CF H180 AI; Olympus). All patients signed the informed consent. The examiners had at least an experience level of 3000 colonoscopies as professional gastroenterologists (board certified).
The polyp specimens were evaluated by different pathology institutes belonging to the participating hospitals. All lesions were classified as hyperplastic polyps, tubular, tubulovillous, villous adenomas or carcinoma. As a grading system, the Vienna classification (discriminating high‐grade [HGIN] versus low‐grade adenomas [LGIN] and invasive carcinomas) was used. The pathologists who examined all specimens received from the study cohorts were blinded to the type of colonoscopy performed.
Blinding
This RCT was not blinded, since the devices used were observable by the examiner during the procedure.
RESULTS
Of the 585 patients enrolled between 2014 and 2018 in this study, 198 were enrolled to SC, and 195 completed the examination. A total of 196 were included in the EC group, of which 189 completed the examination. A total of 191 patients were recruited for the CAC group (see Figure 1 for details and drop‐outs), and 186 completed the examination. Recruiting ended after the enrolment of the necessary number of patients for data analysis according to the study protocol. No significant differences between the groups in baseline characteristics (age, sex, history of diabetes, first‐time colonoscopy or history of abdominal surgery) were seen in the composition of the study population (colorectal cancer screening in 51%, diagnostic colonoscopy due to anaemia or abdominal pain in 31% and post‐polypectomy follow‐up in 18%; no significant deviation between the groups, p = 0.94; Table 1).
TABLE 1.
Demographics and baseline data
| Variable | SC | EC | CAC | p‐Value |
|---|---|---|---|---|
| Patients, n | 195 | 189 | 186 | |
| Age (years), median (IQR) | 63 (52–73) | 62 (52–72) | 62 (53–72) | n.s. |
| Sex (male/female), n | 89/106 | 94/95 | 108/78 | n.s. |
| First‐time colonoscopy, n (%) | 61 (31) | 66 (35) | 58 (31) | n.s. |
| Diabetes, n (%) | 7 (3) | 10 (5) | 8 (4) | n.s. |
| Prior abdominal surgery, n (%) | 27 (14) | 36 (19) | 29 (15) | n.s. |
| Indication: screening colonoscopy | 101 | 91 | 99 | n.s. |
| Indication: follow‐up (former polypectomy) | 31 | 33 | 40 | n.s. |
| Indication: diagnostic | 63 | 63 | 45 | n.s. |
Abbreviations: CAC, cap‐assisted colonoscopy; EC, Endocuff‐assisted colonoscopy; IQR, interquartile range; n.s., not significant; SC, standard colonoscopy.
The SC ADR did not differ significantly between the institutions and examiners. The bowel preparation was good in all groups. No differences were seen for ileum intubation rate or rate of total colonoscopies (Table 2).
TABLE 2.
Colonoscopy performance data
| Variable | SC | EC | CAC | p‐Value |
|---|---|---|---|---|
| Caecum intubation, n (%) | 189 (97) | 185 (98) | 180 (97) | n.s. |
| Ileum intubation, n (%) | 125 (64) | 118 (62) | 133 (72) | n.s. |
| Caecum intubation time (min) | 7 (5–10) | 7 (5–9) | 6 (4–8) | 0.0001 |
| Procedure time (min), median (IQR) | 16 (13–22) | 16 (13–20) | 15 (12–20) | SC versus Cap: 0.020 |
| Withdrawal time (min), median (IQR) | 7 (6–8) | 7 (6–9) | 7 (6–8) | n.s. |
| Cleanliness score, median (IQR) | 1 (1–1) | 1 (1–2) | 1 (1–1.25) | n.s. |
| 1 = good, n (%) | 146 (75) | 125 (66) | 140 (76) | n.s. |
| 2 = fair, n (%) | 36 (19) | 48 (26) | 38 (21) | n.s. |
Abbreviations: CAC, cap‐assisted colonoscopy; EC, Endocuff‐assisted colonoscopy; IQR, interquartile range; n.s., not significant; SC, standard colonoscopy.
Withdrawal time (a mean of 7 min in all groups; p = 0.658) and bowel preparation (p = 0.15) did not differ between the groups. The time to reach the caecum was significantly reduced when using the cap (a mean of 6 min for CAC vs. 7 min for SC; p = 0.0001), while no differences were seen between EC and SC groups. Neoplastic PDR did not differ between the different arms (p = 0.95). There was no significant difference in the primary outcome of the ADR between the groups (EC 32%, CAC 30%, SC 30%; p = 0.815). The total number of polyps <1 cm was significantly higher in the EC group compared to the SC group or CAC group (198 EC vs. 137 SC vs. 158 CAC; p = 0.016). The adenoma‐to‐PDR ratio was 64% for the SC group, 62% for the EC group and 59% for the CAC group (Table 3).
TABLE 3.
Polyp detection rates and adenoma detection rates
| Variable | SC | EC | CAC | p‐Value |
|---|---|---|---|---|
| Polyp detection rate (95% CI) | 47% (40–54) | 52% (45–60) | 51% (43–58) | n.s. |
| Adenoma detection rate (95% CI) | 30% (23–36) | 32% (26–39) | 30% (23–36) | n.s. |
| Number of adenomas (LGIN) | 87 | 99 | 99 | n.s. |
| Number of adenomas (HGIN) | 3 | 0 | 4 | n.s. |
| Number of carcinomas | 2 | 2 | 7 | n.s. |
| Mean adenomas per procedure a (95% CI) | 1.59 (1.23–1.94) | 1.66 (1.39–1.92) | 2.0 (1.56–2.54) | n.s. |
| Polyp size >1 cm | 36 | 35 | 26 | n.s. |
| Polyp size <1 cm | 137 | 198 | 158 | 0.016 |
| Adenoma‐to‐polyp detection rate ratio (95% CI) | 64% (58–67) | 62% (58–65) | 59% (53–62) |
Abbreviations: CAC, cap‐assisted colonoscopy; CI, confidence interval; EC, Endocuff‐assisted colonoscopy; HGIN, high‐grade adenomas; LGIN, low‐grade adenomas; SC, standard colonoscopy.
Only procedures considered in which at least one adenoma was detected.
Looking at the total number of polyps sorted by colonic distribution, the maximum numbers of polyps were seen using the EC in the rectum, sigmoid colon, descending colon, left flexure, transverse colon, right flexure and caecum. Of these locations, EC proved superior (EC vs. SC) in the sigmoid colon and transverse colon (Table 4).
TABLE 4.
Polyp detection <1 cm at different colon sites
| Location | SC | EC | CAC | SC versus EC p‐value |
|---|---|---|---|---|
| Rectum, n | 44 | 49 | 37 | n.s. |
| Sigmoid, n | 37 | 67 | 62 | 0.03 |
| Descending, n | 8 | 15 | 5 | n.s. |
| Left flexure, n | 1 | 5 | 1 | n.s |
| Transverse, n | 5 | 16 | 11 | 0.04 |
| Right flexure, n | 1 | 7 | 4 | n.s. |
| Ascending, n | 23 | 15 | 19 | n.s. |
| Caecum, n | 18 | 24 | 19 | n.s. |
Abbreviations: CAC, cap‐assisted colonoscopy; EC, Endocuff‐assisted colonoscopy; SC, standard colonoscopy.
Detection of LGIN or HIGN did not differ between the three groups. The detection of polyps <1 cm increased significantly in the EC group (p = 0.016). Looking at adverse events, we found that EC was significantly associated with minor mucosal lacerations compared to SC or CAC. In all three arms, no major bleeding, perforation, tip device loss or oxygen desaturation occurred (Table 5).
TABLE 5.
Adverse events
| Adverse event | SC | EC | CAC | p‐Value |
|---|---|---|---|---|
| Minor mucosal laceration, n | 1 | 16 | 4 | 0.0001 |
| Major bleeding, n | 0 | 0 | 0 | |
| Perforation, n | 0 | 0 | 0 | |
| Loss of cuff/cap, n | n/a | 0 | 0 | |
| SpO2 decline (<90%), n | 0 | 0 | 0 |
Abbreviations: CAC, cap‐assisted colonoscopy; EC, Endocuff‐assisted colonoscopy; SC, standard colonoscopy.
For better comparison, we also performed a subgroup analysis including all colonoscopies which were indicated as colon cancer screening. We found a tendency for a better ADR with EC. However, this was not statistically significant. The ADR was 31% in SC, 36% in EC and 29% in CAC (p = 0.557; Table 6).
TABLE 6.
Subgroup analysis (screening colonoscopies)
| Variable | SC | EC | CAP | p‐Value |
|---|---|---|---|---|
| Adenoma detection rate % (95% CI) | 31 (22‐40) | 36 (22‐46) | 29 (20‐38) | n.s. |
| Polyp detection rate % (95% CI) | 52 (43‐62) | 54 (43‐64) | 49 (39‐60) | n.s. |
| Adenoma‐to‐polyp detection rate ratio % (95% CI) | 58.5 (51‐65) | 67.3 (51‐72) | 59 (51‐63) | n.s. |
Abbreviations: CAC, cap‐assisted colonoscopy; CI, confidence interval; EC, Endocuff‐assisted colonoscopy; n.s., non‐significant; SC, standard colonoscopy.
DISCUSSION
Screening colonoscopy plays a key role in reducing colon cancer death. The most important is the performance of a high‐quality colonoscopy. Withdrawal times are crucial for the ADR. The European Society of Gastrointestinal Endoscopy and the American Society of Gastrointestinal Endoscopy recommend a minimum of 6 min of withdrawal time. 11 In our study, withdrawal time was around 7 min, which exceeds the recommended minimum time. Longer withdrawal times do correlate with a higher ADR. 13 Furthermore, a minimum ADR of 25% is recommended for screening colonoscopies. 11 Our ADR in SC was 30%, which indicates that the quality of colonoscopy was sufficient in all centres. The adenoma‐to‐PDR ratio was 64% for the SC group, 62% for the EC group and 59% for the CAC group. No significant difference between the arms was seen. The ratios found in this study are similar to recently published data. 14
We found a relatively low ADR in the SC group when compared to the average European population. Our study was limited by the inclusion of 43 (7.3%) patients with a withdrawal time of less than 6 min (17 in the EC group, 14 in the SC and 12 in the CAC group). Furthermore, only 51% of the screening colonoscopies were included. Among the other 49%, patients were included who had colonoscopies earlier in their life and might have undergone previous screening. This group might have a lower risk of adenomatous polyps compared to the usual screening population, and this might have contributed to our results.
However, the ADR was not significantly improved by the use of EC or CAC. There was still a non‐significant positive tendency measured (ADR SC 30% vs. EC 32%). This result was surprising, since our group has published data from two different RCTs where an impact on the ADR was clearly seen. 10 , 12 However, a working group from the Netherlands found EC had no significant impact on the ADR. 15 Looking in detail, the prolonged withdrawal time of up to 13 min as well as an extraordinary ADR in SC might have contributed to the results found by group from The Netherlands. In our study, no prolonged withdrawal times were detected.
We have used the older Endocuff generation. During the course of the study, a newer generation of the Endocuff—the Endocuff Vision® (EV)—was introduced. 16 The effect of the new design on the ADR compared to the older model was not found to be significant according to Triantafyllou et al. 17 However, in 2019, according to the latest data from Ngu et al., 18 a significantly improved ADR with EV‐assisted colonoscopy was observed. A head‐to‐head comparison of both models was never performed, and meta‐analyses including both devices showed an overall benefit for the ADR. 17 Our own previous data from two RCTs showed a clear benefit with the older model. It remains uncertain whether the use of the former Endocuff design affected our results. Another limitation might be that no training protocol for Endocuff users was provided in our study, although most of the examiners had used it before, and the majority of the hospitals took part in our previous EC studies.
Taking into account our ex ante study design and power analysis, as well as experience from our earlier studies, we looked for differences from our control group using an estimated PDR in SC of around 30%. In fact, the PDR was 47% in our control group. Thus, the overall high PDR in our control group might have influenced our results, and this study might be underpowered. One could speculate that higher patient numbers might have revealed an improved ADR with EC usage.
However, compared to our previous data, the study population was significantly older. Furthermore, the population was more diversified due to the inclusion of university centres from Germany and Poland. Thus, a selection bias might have contributed to these conflicting results.
EC was useful for the detection of polyps <1 cm, which is consistent with earlier published data. 5 In more difficult‐to‐reach parts of the colon, EC proved superior to SC and CAC in relation to the total number of polyps. However, detected polyps were mainly <1 cm in diameter and hyperplastic.
The use of the cap had no impact on the ADR and PDR compared to SC. Earlier data have shown a benefit for CAC regarding polyp detection in the right colonic flexure. 8 However, our data did not demonstrate any advantage over SC in any special localisation. What we found was a significantly shorter time needed to reach the caecum. An average time benefit of one minute was seen. Although the sex distribution was not significantly different between the groups, a larger proportion of men were included in the CAC group compared to the SC group. We cannot exclude that sex effects might have influenced our findings.
No serious adverse events were detected. An increase of minor mucosal lacerations by using the Endocuff was detected, which was consistent with previously published data.
In conclusion, high‐quality colonoscopy performed by experienced hands for colon cancer screening remains the most important step in reducing colon cancer mortality. The use of the Endocuff might help to reach a better PDR and possibly ADR and therefore a good screening result, especially in more difficult‐to‐reach sections of the colon.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
ETHICS APPROVAL
The study was approved by the ethical review board of the University of Goettingen, Germany (No. 26/7/14, date: 27 November 2014).
INFORMED CONSENT
Written informed consent was obtained from each patient included in the study.
ACKNOWLEDGEMENT
We would like to thank Karly Conrads, MSc, for her review of our paper as a native English speaker.
The authors received no financial support for the research, authorship and/or publication of this article.
[Correction added on February 19, 2021 after first online publication: Katarzyna Neubauer's affiliation has been updated.]
DATA AVAILABILITY STATEMENT
The data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics. CA Cancer J Clin. 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
- 2. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N Engl J Med. 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaminski MF, Thomas‐Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49:378–97. [DOI] [PubMed] [Google Scholar]
- 4. Floer M, Meister T. Endoscopic improvement of the adenoma detection rate during colonoscopy ‐ where do we stand in 2015? Digestion. 2016;93:202–13. [DOI] [PubMed] [Google Scholar]
- 5. Facciorusso A, Triantafyllou K, Murad MH, et al. Compared abilities of endoscopic techniques to increase colon adenoma detection rates: a network meta‐analysis. Clin Gastroenterol Hepatol. 2019;17:2439–54. e25. [DOI] [PubMed] [Google Scholar]
- 6. Patel HK, Chandrasekar VT, Srinivasan S, et al. Second‐ generation distal attachment cuff improves adenoma detection rate: meta‐analysis of randomized controlled trials. Gastrointest Endosc. 2020. 10.1016/j.gie.2020.09.045. Epub ahead of print 5 October. [DOI] [PubMed] [Google Scholar]
- 7. Kim DJ, Kim HW, Park SB, et al. Efficacy of cap‐ assisted colonoscopy according to lesion location and endoscopist training level. World J Gastroenterol. 2015;21:6261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Adenoma detection with cap‐assisted colonoscopy versus regular colonoscopy: a randomised controlled trial. Gut. 2012;61:1426–34. [DOI] [PubMed] [Google Scholar]
- 9. Floer M, Tschaikowski L, Krueger H, et al. 396 Standard vs. Endocuff vs. cap assisted colonoscopy for polyp detection: a randomized controlled trial. Gastrointest Endosc. 2018;87:AB74. 10.1016/j.gie.2018.04.064. [DOI] [Google Scholar]
- 10. Biecker E, Floer M, Heinecke A, et al. Novel Endocuff‐ assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413–8. [DOI] [PubMed] [Google Scholar]
- 11. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. [DOI] [PubMed] [Google Scholar]
- 12. Floer M, Biecker E, Fitzlaff R, et al. Higher adenoma detection rates with Endocuff‐assisted colonoscopy ‐ a randomized controlled multicenter trial. PLoS One. 2014;9:e114267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajasekhar PT, Rees CJ, Bramble MG, et al. A multicenter pragmatic study of an evidence‐based intervention to improve adenoma detection: the Quality Improvement in Colonoscopy (QIC) study. Endoscopy. 2015;47:217–24. [DOI] [PubMed] [Google Scholar]
- 14. Schramm C, Scheller I, Franklin J, et al. Predicting ADR from PDR and individual adenoma‐to‐polyp‐detection‐ rate ratio for screening and surveillance colonoscopies: a new approach to quality assessment. United Eur Gastroenterol J. 2017;5:742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Doorn SC, Van Der Vlugt M, Depla A, et al. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2017;66:438–45. [DOI] [PubMed] [Google Scholar]
- 16. Rameshshanker R, Tsiamoulos Z, Wilson A, et al. Endoscopic cuff‐assisted colonoscopy versus cap‐ assisted colonoscopy in adenoma detection: randomized tandem study ‐ DEtection in Tandem Endocuff Cap Trial (DETECT). Gastrointest Endosc. 2020;91:894–904. e1. [DOI] [PubMed] [Google Scholar]
- 17. Triantafyllou K, Gkolfakis P, Tziatzios G, et al. Effect of Endocuff use on colonoscopy outcomes: a systematic review and meta‐analysis. World J Gastroenterol. 2019;25:1158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ngu WS, Bevan R, Tsiamoulos ZP, et al. Improved adenoma detection with Endocuff Vision: the ADENOMA randomised controlled trial. Gut. 2019;68:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon reasonable request to the corresponding author.


