Abstract
Background
Functional dyspepsia (FD) is one of the most common conditions in clinical practice. In spite of its prevalence, FD is associated with major uncertainties in terms of its definition, underlying pathophysiology, diagnosis, treatment, and prognosis.
Methods
A Delphi consensus was initiated with 41 experts from 22 European countries who conducted a literature summary and voting process on 87 statements. Quality of evidence was evaluated using the grading of recommendations, assessment, development, and evaluation (GRADE) criteria. Consensus (defined as >80% agreement) was reached for 36 statements.
Results
The panel agreed with the definition in terms of its cardinal symptoms (early satiation, postprandial fullness, epigastric pain, and epigastric burning), its subdivision into epigastric pain syndrome and postprandial distress syndrome, and the presence of accessory symptoms (upper abdominal bloating, nausea, belching), and overlapping conditions. Also, well accepted are the female predominance of FD, its impact on quality of life and health costs, and acute gastrointestinal infections, and anxiety as risk factors. In terms of pathophysiological mechanisms, the consensus supports a role for impaired gastric accommodation, delayed gastric emptying, hypersensitivity to gastric distention, Helicobacter pylori infection, and altered central processing of signals from the gastroduodenal region. There is consensus that endoscopy is mandatory for establishing a firm diagnosis of FD, but that in primary care, patients without alarm symptoms or risk factors can be managed without endoscopy. There is consensus that H. pylori status should be determined in every patient with dyspeptic symptoms and H. pylori positive patients should receive eradication therapy. Also, proton pump inhibitor therapy is considered an effective therapy for FD, but no other treatment approach reached a consensus. The long‐term prognosis and life expectancy are favorable.
Conclusions and Inferences
A multinational group of European experts summarized the current state of consensus on the definition, diagnosis and management of FD.
Keywords: consensus, endoscopy, evidence‐based medicine, functional dyspepsia, proton pump inhibitors
Key summary
Current knowledge
Functional dyspepsia is one of the most common conditions encountered in clinical practice.
There is a lack of guidance for clinicians in guiding diagnosis and treatment of this prevalent condition.
No treatments are currently approved for the treatment of functional dyspepsia in Europe.
What is new here
A Delphi panel consisting of 41 experts from 22 European countries established the level of consensus on 87 statements regarding functional dyspepsia.
The statements reaching consensus serve to guide clinicians in recognizing, diagnosing and treating FD in clinical practice.
Endoscopy is mandatory for establishing a firm diagnosis of functional dyspepsia D, but in primary care patients without alarm symptoms or risk factors can be managed without endoscopy.
Helicobacter pylori status should be determined in every patient with dyspeptic symptoms and H. Pylori positive patients should receive eradication therapy.
Proton pump inhibitor‐therapy is considered an effective therapy for FD, but no other treatment approach reached consensus support.
INTRODUCTION
Functional dyspepsia (FD), defined by the presence of recurrent or chronic epigastric symptoms in the absence of organic disease likely to explain them, is one of the most common conditions seen in clinical practice. 1 , 2 In spite of its prevalence, FD is associated with major uncertainties, as definitions and the symptom spectrum of FD have evolved over time, 3 the differential diagnosis is very broad, 1 the optimal diagnostic work‐up has not been defined, 4 , 5 and there is a lack of available treatments with established efficacy. 6 , 7
The aim of this project was to develop a European consensus on the definition, pathophysiological concepts, diagnosis, management, and prognosis of FD. The results of this consensus can offer the clinician guidance in diagnosing and managing FD patients to optimize clinical outcomes.
METHODS
The European Society for Neurogastroenterology and Motility (ESNM) initiated a Delphi process, funded by United European Gastroenterology, to develop consensus statements on different aspects of FD in collaboration with other European societies. The Delphi approach, which combines the principles of evidence‐based medicine, supported by systematic literature reviews and a voting process, aims at determining consensus for complex problems in medicine for which evidence from controlled trials is lacking. 8
The principal steps in the process were (1) selection of a working group of seven ESNM members with expertise in FD and/or Delphi consensus processes; (2) selection of a European Consensus Group consisting of experts in FD from different European countries, recruited through the ESNM board and through UEG Sister Societies; (3) drafting of statements allowing to evaluate the current knowledge on FD; (4) systematic literature reviews to identify evidence to support each statement; (5) two rounds of repeated voting of the statements and voting discussion until a stable level of consensus voting was reached; and (6) grading of the strength of evidence using accepted criteria.
For the Consensus Group, ESNM board members nominated experts from their respective national societies for participation, and the UEG Sister Societies (EAGEN, EHSMG, and ESPCG) nominated additional experts. A total of 41 experts from 22 European countries agreed to participate. The members had a background of expertise in gastroenterology, general practice, Helicobacter pylori infection or gastrointestinal motility. All members submitted a conflict of interest statement by December 2018.
The seven‐member Core Group drafted and finalized a list of 75 statements covering several aspects of FD. The finalized list was evaluated in the first voting round by all members in the second quarter of 2019, where each member indicated the degree of agreement for the statement using a 6‐point Likert scale (Table 1). Participants were blinded to the votes of other participants and also gave feedback on the clarity of the statement and made suggestions for adapting or splitting the statements into two or more questions, or for adding additional statements on a given topic. The Core Group adjusted the statement list, generating a total of 87 statements, and subdivided the Guideline Group members into 12 working groups with 3–4 members each. Each working group was allocated statements for which they needed to conduct a systematic literature search using several relevant keywords and provide narrative substantiation of the statements. The literature review and references were made available on a share‐point server, accessible to all members. This was finalized by the Summer of 2019, followed by a voting round in which each statement was presented with the evidence summary. The available members of the Guideline Group met in September 2019 at the occasion of the ESNM meeting in Lisbon and in October 2019 at the UEG week in Barcelona to discuss statements and voting outcomes. A final voting round was conducted between both meetings, focusing on statements that were adapted based on the evaluation at the ESNM meeting. Throughout the process, all votes were mutually anonymous and blinded.
TABLE 1.
Six‐point Likert scale
| Point | Description |
|---|---|
| A+ | Agree strongly |
| A | Agree with minor reservation |
| A− | Agree with major reservation |
| D− | Disagree with minor reservation |
| D | Disagree with major reservation |
| D+ | Disagree strongly |
When 80% of the Consensus Group agreed (A+ or A) with a statement, this was defined as consensus. The strength of evidence for each statement was scored using the GRADE system (Table 2). 9 After the final voting round (summarized in Table 3), the manuscript was drafted and circulated for the final approval by the participants. The references cited in this chapter are only a selection of the articles reviewed in each area, chosen to clarify the discussion. A final meeting planned in October 2020 was canceled because of the COVID−19 pandemic.
TABLE 2.
Grading of recommendations assessment, development and evaluation system 9
| Code | Quality of evidence | Definition |
|---|---|---|
| A | High | Further research is very unlikely to change our confidence in the estimate of effect |
| ||
| ||
| B | Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| ||
| ||
| C | Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| ||
| D | Very low | Any estimate of effect is very uncertain. |
| ||
| ||
|
TABLE 3.
All statements with endorsement and references
| Statement | Endorsement | Grade of Evidence | References |
|---|---|---|---|
| 1.1. Dyspepsia refers to a symptom or set of symptoms that is (are) considered to originate from the gastroduodenal region. | Yes | B | 1, 2, 3, 4 |
| 1.2. Early satiation, postprandial fullness, epigastric pain, and epigastric burning are the cardinal dyspeptic symptoms as defined by Rome IV. | Yes | B | 1, 2, 3, 4 |
| 1.3. Functional dyspepsia (FD) is a condition characterized by chronic dyspeptic symptoms in the absence of organic, systemic or metabolic condition(s) that is (are) likely to explain symptoms. | Yes | A | 1, 2, 3, 4 |
| 1.4. The vast majority of patients with dyspeptic symptoms and no alarm symptoms in the general population is identified as FD after investigation (if this would be done). | Yes | A | 1, 4 |
| 1.5. Two main subtypes of FD are distinguished which may overlap: postprandial distress syndrome (PDS) characterized by meal‐induced symptoms (early satiation, postprandial fullness) and epigastric pain syndrome (EPS), with epigastric pain and/or epigastric burning not necessarily associated with a meal. | Yes | B | 1, 3, 10, 11 |
| 1.6. Dyspeptic symptoms often co‐exist with other symptoms such as bloating in the upper abdomen, nausea and belching. | Yes | A | 1, 7 |
| 1.7. Bloating or visible distention in the upper abdomen is a dyspeptic symptom. | No | B | 1, 10, 11 |
| 1.8. The use of pictograms helps to characterize the presence and nature of dyspeptic symptoms. | No | C | 1, 10, 11 |
| 1.9. Typical reflux symptoms (heartburn, regurgitation) often co‐exist with dyspeptic symptoms in the general population. | Yes | A | 12, 13, 14, 15, 16 |
| 1.10. Gastro‐esophageal reflux disease may be distinguished from FD using dedicated questionnaires or good history taking. | No | C | 12, 13, 14, 15, 16 |
| 1.11. Irritable bowel syndrome often coexists with FD. | Yes | A | 12, 13, 14, 15, 16 |
| 2.1. (Functional) dyspepsia occurs at all ages but the highest incidence is in the middle age. | No | B | 12, 13, 14, 15, 16,19, 20, 21, 22 |
| 2.2.(Functional) dyspepsia is more prevalent in women than men | Yes | A | 12, 13, 14, 15, 16,19, 20, 21, 22 |
| 2.3. Acute GI infection is a risk factor for development of FD. | Yes | A | 23, 24, 25, 26, 27 |
| 2.4. NSAID intake is a risk factor for development of FD. | No | C | 28, 29, 30, 31 |
| 2.5. Antibiotic therapy is a risk factor for development of FD. | No | C | 28, 29, 30, 31 |
| 2.6. Anxiety is a risk factor for development of FD | Yes | A | 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 |
| 2.7. Depression is a risk factor for development of FD. | No | B | 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 |
| 2.8. Smoking is a risk factor for development of FD. | No | C | 49, 50, 51, 52, 53, 54 |
| 3.1. FD is a major source of healthcare costs. | Yes | A | 55, 56, 57, 58 |
| 3.2. FD is a major source of self‐costs to patients. | Yes | B | 55, 56, 57, 58 |
| 3.3. FD is an important source of loss of work productivity. | Yes | B | 56, 57, 59 |
| 3.4. FD is associated with a significant decrease in quality of life. | Yes | A | 60, 61, 62 |
| 3.5. FD is associated with psychosocial co‐morbidities such as anxiety and depression | Yes | A | 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 48 |
| 3.6. Weight loss can be consequence of FD | Yes | B | 49, 63, 64, 65, 66, 67 |
| 3.7. In case of weight loss, eating disorders must be ruled out. | No | C | 49, 63, 64, 65, 66, 67 |
| 3.8. Healthcare consulting behavior in FD is driven by symptom severity and impact. | Yes | B | 57, 68, 69, 70 |
| 3.9. Healthcare consulting behavior in FD is driven by psychosocial comorbidity. | Yes | B | 36, 71 |
| 3.10. Healthcare consulting behavior in FD is driven by access to the healthcare system. | No | B | 68, 70, 71 |
| 4.1. Dietary factors underlie symptom generation in FD. | No | C | 57, 73, 74, 75, 76, 77, 78, 79 |
| 4.2. H. pylori is a cause of symptoms in a subgroup of patients with dyspepsia and normal endoscopy. | Yes | B | 80, 81, 82 |
| 4.3. Impaired gastric accommodation is a pathophysiological mechanism in FD. | Yes | B | 63, 67, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93 |
| 4.4. Delayed gastric emptying is a pathophysiological mechanism in FD. | Yes | B | 91, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102 |
| 4.5. Rapid gastric emptying is a pathophysiological mechanism in FD. | No | C | 99, 101, 102 |
| 4.6. Hypersensitivity to gastric distention is a pathophysiological mechanism in FD. | Yes | B | 64, 91, 100, 101, 102, 103, 104, 105, 106, 107, 108 |
| 4.7. Duodenal mucosal alterations are a pathophysiological mechanism in FD. | No | B | 109, 110, 111, 112, 113, 114, 115, 116, 117 |
| 4.8. Altered gastric acid secretion is a pathophysiological mechanism in FD. | No | C | 118, 119, 120 |
| 4.9. Altered release of peptide hormones is a pathophysiological mechanism in FD. | No | C | 121, 122, 123, 124 |
| 4.10. Increased sensitivity to duodenal luminal content is a pathophysiological mechanism in FD. | No | C | 127, 128, 129, 130 |
| 4.11. Altered duodenal microbiota composition is a pathophysiological mechanism in FD. | No | C | 131, 132 |
| 4.12. Impaired vagus nerve function is a pathophysiological mechanism in FD. | No | C | 133, 134, 135, 136, 137, 138 |
| 4.13. Anxiety and stress are pathophysiological mechanisms in FD. | No | B | 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 |
| 4.14. Depression is a pathophysiological mechanism in FD. | No | B | 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 48 |
| 4.15. Disordered central processing of incoming signals from the gastroduodenal region is a pathophysiological mechanism in FD. | Yes | C | 139, 140, 141, 142, 143, 144 |
| 4.16. Genetic factors determine the susceptibility to FD. | No | C | 145, 146, 147, 148 |
| 5.1. Upper GI endoscopy is mandatory for establishing a diagnosis of FD. | Yes | A | 1, 10, 149, 150, 151 |
| 5.2. In primary care, uninvestigated dyspepsia can be managed without endoscopy if there are no alarm of risk factors. | Yes | A | 149, 150, 151 |
| 5.3. Upper GI endoscopy is mandatory if there are alarm symptoms or risk factors. | Yes | A | 1, 10, 149, 150, 151 |
| 5.4. Screening blood test are useful when considering a diagnosis of FD. | No | B | 149, 150, 151 |
| 5.5. Every patient with dyspeptic symptoms should be tested for H. pylori (non‐invasively or at gastroscopy). | Yes | A | 1, 10, 81, 149, 150, 153, 154 |
| 5.6. Patients with dyspepsia and H. pylori positive gastritis should be considered to have FD just if symptoms persist 6 to 12 months after H. pylori eradication. | Yes | B | 1, 81, 155 |
| 5.7. Patients with dyspepsia and HP negative gastritis should be considered to have FD. | Yes | B | 1, 81, 155 |
| 5.8. FD should be subdivided into EPS and PDS for further diagnostic and therapeutic approach. | Yes | B | 3, 11, 156, 157, 158, 159, 160, 161, 162 |
| 5.9. Upper abdominal ultrasound is useful when considering a diagnosis of FD. | No | B | 1, 150, 165, 166 |
| 5.10. A gastric emptying test is useful when considering a diagnosis of FD. | No | B | 1, 91, 150, 167, 168 |
| 5.11. Esophageal pH monitoring is useful in FD to rule out GERD. | No | B | 13, 169, 170, 171 |
| 5.12. Increased duodenal eosinophil count is a marker of FD. | No | C | 13, 169, 170, 171 |
| 5.13. Impaired nutrient volume tolerance is a marker of FD. | No | B | 63, 67, 86, 173, 174, 175, 176 |
| 6.1. Dietary adjustment improves symptoms in FD. | No | C | 57, 73, 74, 75, 76, 77, 78, 79 |
| 6.2. H. pylori positive FD patients should receive eradication therapy. | Yes | A | 1, 81, 150, 160, 177 |
| 6.3. PPI therapy is the most appropriate initial therapy for FD. | No | B | 150, 178, 179, 180, 181, 182, 183, 184, 185, 186 |
| 6.4. PPI therapy is an effective therapy for FD. | Yes | A | 150, 178, 179, 180, 181, 182, 183, 184, 185, 186 |
| 6.5. PPI therapy is most effective for EPS. | No | C | 150, 177, 186 |
| 6.6. Prokinetic therapy is the most appropriate initial therapy for FD. | No | C | 150, 187, 188, 189 |
| 6.7. Prokinetic therapy is an effective therapy for FD. | No | B | 150, 187, 188, 189 |
| 6.8. Prokinetic therapy is most effective for PDS. | No | B | 150, 187, 188, 189 |
| 6.9. Efficacy of prokinetics is not related to their enhancement of gastric emptying rate. | No | B | 150, 187, 188, 189 |
| 6.10. Itopride is effective for FD patients. | No | C | 190, 191, 192, 193 |
| 6.11. Tricyclic antidepressants are effective for epigastric pain syndrome (EPS). | No | B | 194, 195, 196, 197 |
| 6.12. Tricyclic antidepressants are effective for post‐prandial distress syndrome (PDS). | No | B | 194, 195, 196, 197 |
| 6.13. Tricyclic antidepressants are not effective for post‐prandial distress syndrome (PDS). | No | B | 194, 195, 196, 197,194, 195, 196, 197 |
| 6.14. Serotonin reuptake inhibitors are effective for FD. | No | B | 195, 198 |
| 6.15. Serotonin reuptake inhibitors are not effective for FD. | No | B | 195, 198 |
| 6.16. Serotonin noradrenaline reuptake inhibitors are effective for FD. | No | C | 195, 198 |
| 6.17. Serotonin noradrenaline reuptake inhibitors are not effective for FD. | No | C | 195, 198 |
| 6.18. Mirtazapine is effective for post‐prandial distress syndrome patients with weight loss. | No | B | 200, 201 |
| 6.19. 5‐HT1A agonists (tandospirone, buspirone, ….) are effective for PDS. | No | B | 202, 203, 204, 205, 206 |
| 6.20. Herbal therapies are effective for FD patients. | No | B | 209, 210 |
| 6.21. Iberogast (STW‐5) is effective for FD patients. | No | B | 192, 206, 207 |
| 6.22. Rifaximin is effective for FD patients. | No | C | 192, 206, 207 |
| 6.23. Hypnotherapy is effective for FD patients. | No | B | 192, 206, 207 |
| 6.24. Cognitive–behavioral therapy (CBT) is effective for FD patients. | No | B | 213, 214 |
| 6.25. Acupuncture is effective for FD patients. | No | B | 215, 216, 217, 218 |
| 6.26. Mindfulness is effective for FD patients. | No | B | 215, 216, 217, 218 |
| 6.27. In case of severe weight loss in FD, nutritional support may be needed. | Yes | B | 215, 216, 217, 218 |
| 7.1. The long‐term prognosis is favorable in the majority of patients with FD. | Yes | B | 49, 221, 222 |
| 7.2. Life expectancy in FD is similar to the general population. | Yes | A | 224, 225 |
RESULTS
-
1.
Definitions and symptom descriptors
-
1.1
Dyspepsia refers to a symptom or set of symptoms that is (are) considered to originate from the gastroduodenal region.
STATEMENT ENDORSED, overall agreement 98%: A+ 78%, A 20%, A− 0%, D− 2%, D 0%, D+ 0%. GRADE B
-
1.2
Early satiation, postprandial fullness, epigastric pain, and epigastric burning are the cardinal dyspeptic symptoms as defined by Rome IV.
STATEMENT ENDORSED, overall agreement 98%: A+ 83%, A 15%, A− 0%, D− 2%, D 0%, D+ 0%. GRADE B
-
1.3
Functional dyspepsia is a condition characterized by chronic dyspeptic symptoms in the absence of organic, systemic, or metabolic condition(s) that is (are) likely to explain symptoms
STATEMENT ENDORSED, overall agreement 93%: A+ 68%, A 25%, A− 7%, D− 0%, D 0%, D+ 0%: GRADE A
-
1.4
The vast majority of patients with dyspeptic symptoms and no alarm symptoms in the general population are identified as functional dyspepsia after investigation (if this would be done).
STATEMENT ENDORSED, overall agreement 93%: A+ 66%, A 27%, A− 2%, D− 0%, D 5%, D+ 0%: GRADE A
-
1.5
Two main subtypes of functional dyspepsia are distinguished which may overlap: postprandial distress syndrome (PDS) characterized by meal‐induced symptoms (early satiation, postprandial fullness) and epigastric pain syndrome (EPS), with epigastric pain and/or epigastric burning not necessarily associated with a meal.
STATEMENT ENDORSED, overall agreement 98%: A+ 78%, A 20%, A− 2%, D− 0%, D 0%, D+ 0%: GRADE B
-
1.6
Dyspeptic symptoms often coexist with other symptoms such as bloating in the upper abdomen, nausea, and belching.
STATEMENT ENDORSED, overall agreement 98%: A+ 73%, A 25%, A− 0%, D− 2%, D 0%, D+ 0%: GRADE A
-
1.7
Bloating or visible distention in the upper abdomen is a dyspeptic symptom.
STATEMENT NOT ENDORSED, overall agreement 61%: A+ 27%, A 34%, A− 29%, D− 0%, D 5%, D+ 5%: GRADE B
-
1.8
The use of pictograms helps to characterize the presence and nature of dyspeptic symptoms.
STATEMENT NOT ENDORSED, overall agreement 76%: A+ 27%, A 49%, A− 24%, D− 0%, D 0%, D+ 0%: GRADE C
-
1.9
Typical reflux symptoms (heartburn, regurgitation) often coexist with dyspeptic symptoms in the general population:
STATEMENT ENDORSED, overall agreement 98%: A+ 56%, A 42%, A− 2%, D− 0%, D 0%, D+ 0%: GRADE A
-
1.10
Gastroesophageal reflux disease may be distinguished from functional dyspepsia using dedicated questionnaires or good history taking.
STATEMENT NOT ENDORSED, overall agreement 46%: A+ 14%, A 32%, A− 24%, D− 10%, D 17%, D+ 2%. GRADE C
-
1.11
Irritable bowel syndrome often coexists with functional dyspepsia.
STATEMENT ENDORSED, overall agreement 95%: A+ 73%, A 22%, A− 5%, D− 0%, D 0%, D+ 0%: GRADE A
-
1.1
The definition of dyspepsia and FD has seen major evolutions over time. While early definitions included esophageal symptoms such as heartburn, as well as nausea, vomiting, and belching within the dyspeptic symptom complex, the Rome III and Rome IV consensus have significantly narrowed the symptom profile. 1 , 3 , 10 The recent Rome IV consensus, which defined dyspepsia as the presence of chronic symptoms thought to originate from the gastroduodenal region, is well accepted. 1 According to this consensus, the four cardinal dyspeptic symptoms are troublesome postprandial fullness, early satiation, epigastric pain, and non‐radiating epigastric burning. 1
FD is defined as the presence of chronic dyspeptic symptoms in the absence of organic disease that readily explains the symptoms. 1 Symptoms do not reliably distinguish between functional and organic dyspepsia. 1 , 12 Consequently, in clinical practice, upper endoscopy is regularly performed to rule out organic causes. The prevalence of clinically significant endoscopic findings in subjects with uninvestigated dyspepsia is low, but the high number of affected patients is relevant. Less than 10% of patients have a peptic ulcer, and less than 1% have gastroesophageal cancer. 4 Thus, based on the endoscopic findings, a systematic review and meta‐analysis found that more than 70% of subjects with dyspeptic symptoms qualify for a diagnosis of FD. 4
Besides the four cardinal symptoms, nausea, belching, and upper abdominal bloating are often found in FD patients and are considered adjunctive features of the FD spectrum. 1 Their presence may reflect common pathophysiological mechanisms such as altered motility or hypersensitivity. 1 , 7
The Rome IV criteria stress that heartburn is not a dyspeptic symptom but may often coexist with FD and that the presence of heartburn should not lead to the exclusion of FD as diagnosis. 1 , 3 Overall, one‐third of FD patients also experience typical symptoms of gastroesophageal reflux disease (GERD). 13 It seems that, in cases of overlap between FD and GERD, FD is often underestimated, favoring the diagnosis of GERD. 14 The substantial overlap of core symptoms of GERD and FD persists, regardless of the use of objective tools or evidence from upper endoscopies or esophageal pH studies. 12 , 14 Thus, separating out GERD and FD based on questionnaires and history taking alone can be quite difficult, if not impossible. 13 , 15 In addition, non‐acid‐related conditions such as functional heartburn frequently overlap with FD. 13 , 16 Irritable bowel syndrome (IBS) is another symptomatic condition which frequently overlaps with FD. 17 The high rate of overlap between FD and conditions like IBS or GERD may be explained by common etiological risk factors (e.g., acute infectious gastroenteritis, psychological disturbances) and pathophysiological mechanisms (e.g., visceral hypersensitivity, altered motility, etc.). 13 , 15 , 16 , 17 Severity and impact of symptoms are higher in those with overlapping conditions. 40
Over time, several subdivisions of the dyspeptic symptom pattern have also been proposed. 1 , 3 , 10 According to the Rome III consensus, and refined in Rome IV, two subgroups are identified within FD: the PDS and the EPS. 1 , 10 The PDS subgroup is characterized by symptom triggered or aggravated by a meal and includes postprandial fullness, early satiation, and other postprandial symptoms. The EPS subtype is defined by meal‐unrelated symptoms, such as epigastric pain and epigastric burning. 1 The existence of these subtypes in the general population is supported by epidemiological data. 3 With the Rome III definitions, a major overlap between EPS and PDS was found, but this is substantially decreased with the Rome IV update. 1 , 3 , 10 , 11 , 40
Assessing the presence of cardinal and accessory symptoms requires an accurate understanding of symptom descriptors by the patient. It may be difficult for patients to distinguish upper gastrointestinal symptoms based on verbal descriptors alone, but adding pictograms to verbal descriptors significantly improves the accuracy of symptom reporting by FD patients. 18 However, as this was done in a specific context (tertiary care, Belgium), confirmatory studies in a different medical and cultural–linguistic setting are needed.
-
2.
Epidemiology and risk factors
-
2.1
(Functional) Dyspepsia occurs at all ages but the highest incidence is in the middle age.
STATEMENT NOT ENDORSED, overall agreement 73%: A+ 24%, A 49%, A− 24%, D− 2%, D 0%, D+ 0%. GRADE B
-
2.2
(Functional) Dyspepsia is more prevalent in women than me.
STATEMENT ENDORSED, overall agreement 83%: A+ 51%, A 32%, A− 15%, D− 0%, D 3%, D+ 0%. GRADE A
-
2.3
Acute gastrointestinal infection is a risk factor for development of functional dyspepsia.
STATEMENT ENDORSED, overall agreement 90%: A+ 54%, A 36%, A− 5%, D− 0%, D 5%, D+ 0%. GRADE A
-
2.4
NSAID intake is a risk factor for development of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 61%: A+ 20%, A 41%, A− 20%, D− 0%, D 17%, D+ 2%. GRADE C
-
2.5
Antibiotic therapy is a risk factor for development of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 37%: A+ 5%, A 32%, A− 32%, D− 7%, D 24%, D+ 0%. GRADE C
-
2.6
Anxiety is a risk factor for development of functional dyspepsia.
STATEMENT ENDORSED, overall agreement 93%: A+ 34%, A 59%, A− 7%, D− 0%, D 0%, D+ 0%. GRADE A
-
2.7
Depression is a risk factor for development of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 76%: A+ 27%, A 49%, A− 20%, D− 2%, D 2%, D+ 0%. GRADE B
-
2.8
Smoking is a risk factor for development of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 37%: A+ 7%, A 30%, A− 46%, D− 2%, D 15%, D+ 0%. GRADE C
-
2.1
Approximately 10% of the adult population fulfills symptom‐based Rome IV criteria for (uninvestigated) FD, and its prevalence appears to disappear with increasing age. 2 , 19 In several studies, the peak incidence of FD seems to occur in the forties or fifties age segment. 19 , 20 , 21 , 22 A recent metA−analysis including 55 studies revealed a slightly higher pooled prevalence of dyspepsia in women compared with men. 19 The Rome Global Epidemiology Study, which used the most uniform criteria and approach, showed a significantly higher prevalence of (uninvestigated) Rome IV FD in women compared to men. 2
Acute gastroenteritis is associated with an increased risk of FD, with an estimated mean prevalence of 9.6% in adults. 23 Among pathogens suggested to be associated with post‐infectious FD (PI‐FD) are Norovirus, Giardia lamblia, Salmonella spp., Escherichia coli O157, and Campylobacter spp. 24 , 25 , 26 H. pylori does not seem to be a cause of PI‐FD. 23 , 27
Nonsteroidal anti‐inflammatory drug (NSAID) use has been identified as a risk factor for dyspepsia in two population‐based studies. 28 , 29 It has been suggested that the development of dyspeptic symptoms during treatment with NSAIDs could be linked to alterations in gastric mechanosensory function. 30 However, NSAID intake appears to be most relevant to uninvestigated dyspepsia. 31 Data supporting the role of drugs other than NSAIDs in the pathogenesis of dyspepsia in the general population are lacking. A nested case–control study in Olmsted County suggested that treatment with antibiotics for a non‐gastrointestinal infection was associated with the development of functional gastrointestinal disorders (FGIDs), but this needs confirmation in other cohorts. 32
Several cross‐sectional and population‐based studies have observed that anxiety is frequently associated with FD. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Some longitudinal studies have shown that mood disorders may precede dyspeptic symptoms and thus predispose to FD. 39 , 41 , 42 , 43 Similarly, cross‐sectional and population‐based studies have observed that depression is frequently encountered in patients with FD. 33 , 35 , 36 , 37 , 38 , 39 , 40 , 43 , 44 , 45 , 47 , 48 However, the Kalixanda study, an important cross‐sectional study carried out in a large sample of patients and its longitudinal 10‐year follow‐up investigation, failed to find a clear association between depression and the risk for development of FD. 41 , 42
Population‐ and endoscopy‐based studies suggested an association between smoking and FD. 20 , 49 , 50 , 51 In contrast, other population‐based studies failed to find an association after adjustment for confounders such as age, gender, and drugs. 52 , 53 , 54
-
3.
Impact of functional dyspepsia
-
3.1Functional dyspepsia is a major source of healthcare costs.STATEMENT ENDORSED, overall agreement 98%: A+ 78%, A 20%, A− 2%, D− 0%, D 0%, D+ 0%. GRADE A
-
3.2Functional dyspepsia is a major source of self‐costs to patients.STATEMENT ENDORSED, overall agreement 93%: A+ 64%, A 29%, A− 7%, D− 0%, D 0%, D+ 0%. GRADE B
-
3.3Functional dyspepsia is an important source of loss of work productivity.STATEMENT ENDORSED, overall agreement 88%: A+ 46%, A 42%, A− 10%, D− 2%, D 0%, D+ 0%. GRADE B
-
3.4Functional dyspepsia is associated with a significant decrease in quality of life.STATEMENT ENDORSED, overall agreement 100%: A+ 80%, A 20%, A− 0%, D− 0%, D 0%, D+ 0%. GRADE A
-
3.5Functional dyspepsia is associated with psychosocial co‐morbidities such as anxiety and depression.STATEMENT ENDORSED, overall agreement 100%: A+ 61%, A 39%, A− 0%, D− 0%, D 0%, D+ 0%. GRADE A
-
3.6Weight loss can be consequence of FD.STATEMENT ENDORSED, overall agreement 90%: A+ 43%, A 47%, A− 8%, D− 0%, D 2%, D+ 0%. GRADE B
-
3.7In case of weight loss, eating disorders must be ruled out.STATEMENT NOT ENDORSED, overall agreement 73%: A+ 36%, A 37%, A− 19%, D− 0%, D 7%, D+ 0%. GRADE C
-
3.8Healthcare consulting behavior in functional dyspepsia is driven by symptom severity and impact.STATEMENT ENDORSED, overall agreement 93%: A+ 42%, A 51%, A− 7%, D− 0%, D 0%, D+ 0%. GRADE B
-
3.9Healthcare consulting behavior in functional dyspepsia is driven by psychosocial comorbidity.STATEMENT ENDORSED, overall agreement 80%: A+ 29%, A 51%, A− 20%, D− 0%, D 0%, D+ 0%. GRADE B
-
3.10Healthcare consulting behavior in functional dyspepsia is driven by access to the healthcare system.STATEMENT NOT ENDORSED, overall agreement 71%: A+ 17%, A 54%, A− 19%, D− 0%, D 7%, D+ 0%. GRADE B
-
3.1
Studies conducted in several parts of the world have shown that FD is associated with significantly elevated health expenses related to medical consultations, diagnostic tests, and therapeutic measures. 55 , 56 , 57 , 58 In addition, FD patients incur both direct and indirect costs driven by over‐the‐counter medications, alternative therapies, medical consultations, and co‐financed treatments, as well as the cost of dietary modifications. 55 Studies conducted in several parts of the world report that FD patients have increased absenteeism, and reduced productivity at work compared to healthy subjects. 56 , 57 , 59
Multiple studies have shown that FD is associated with a reduction in quality of life. Factors found to be related to a greater reduction in quality of life are anxiety and depression, advanced age, female sex, severity of symptoms, and low or intermediate cultural level. 60 , 61 , 62
Several cross‐sectional studies have shown that FD frequently coexists with anxiety and depression and that the severity of symptoms correlates with scores on psychopathology questionnaires. 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 Furthermore, psychosocial factors, such as depression, history of childhood abuse, and somatization, have shown to contribute to symptom severity more than the degree of gastric sensorimotor dysfunction. 38
Weight loss occurs in a large subset of subjects with dyspeptic symptoms and is closely associated with symptoms of early satiation as well as epigastric pain, both at the population level and in tertiary care patients. 63 , 64 , 65 , 66 Important determinants of weight loss in FD are impaired accommodation and decreased nutrient volume tolerance. 63 , 67
Although dyspepsia affects approximately 10% of the general population, only half of these ever consult a doctor for their symptoms. Dyspeptic subjects who seek healthcare attention have more severe, frequent, and persistent dyspepsia symptoms. 57 , 68 , 69 , 70 In addition, dyspeptic patients with high scores on anxiety and depression questionnaires report higher consultation rates than those with lower scores, indicating that psychosocial factors also influence healthcare‐seeking behavior. 36 , 71 A longitudinal study also found that anxiety or depression precedes dyspepsia among consulters in a larger proportion than in non‐consulters, confirming that basal psychosocial comorbidity is associated with healthcare seeking. 71 Consultation rates for dyspepsia vary widely between countries and regions, suggesting that access to the healthcare system may influence healthcare‐seeking behavior. 70 However, differences in healthcare‐consulting behavior are also modulated by socioeconomic status. Several studies have shown that low socioeconomic status is associated with higher consultation rates for dyspepsia. 68 , 72
-
4.
Pathophysiology of functional dyspepsia
-
4.1
Dietary factors underlie symptom generation in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 51%: A+ 24%, A 27%, A− 34%, D− 2%, D 10%, D+ 2%. GRADE C
-
4.2
H. pylori is a cause of symptoms in a subgroup of patients with dyspepsia and normal endoscopy.
STATEMENT ENDORSED, overall agreement 81%: A+ 37%, A 44%, A− 17%, D− 2%, D 0%, D+ 0%. GRADE B
-
4.3
Impaired gastric accommodation is a pathophysiological mechanism in functional dyspepsia.
STATEMENT ENDORSED, overall agreement 93%: A+ 51%, A 42%, A− 7%, D− 0%, D 0%, D+ 0%. GRADE B
-
4.4
Delayed gastric emptying is a pathophysiological mechanism in functional dyspepsia.
STATEMENT ENDORSED, overall agreement 85%: A+ 39%, A 46%, A− 12%, D− 0%, D 2%, D+ 0%. GRADE B
-
4.5
Rapid gastric emptying is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 32%: A+ 2%, A 30%, A− 46%, D− 5%, D 15%, D+ 2%. GRADE C
-
4.6
Hypersensitivity to gastric distention is a pathophysiological mechanism in functional dyspepsia.
STATEMENT ENDORSED, overall agreement 93%: A+ 63%, A 30%, A− 7%, D− 0%, D 0%, D+ 0%. GRADE B
-
4.7
Duodenal mucosal alterations are a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 76%: A+ 17%, A 59%, A− 20%, D− 2%, D 0%, D+ 2%. GRADE B
-
4.8
Altered gastric acid secretion is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 29%: A+ 7%, A 22%, A− 32%, D− 7%, D 32%, D+ 0%. GRADE C
-
4.9
Altered release of peptide hormones is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 24%: A+ 7%, A 17%, A− 49, D− 12%, D 12%, D+ 2%. GRADE C
-
4.10
Increased sensitivity to duodenal luminal content is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 68%: A+ 17%, A 51%, A− 29%, D− 0%, D 0%, D+ 2%. GRADE C
-
4.11
Altered duodenal microbiota composition is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 34%: A+ 10%, A 24%, A− 39%, D− 12%, D 15%, D+ 0%. GRADE C
-
4.12
Impaired vagus nerve function is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 46%: A+ 15%, A 31%, A− 37%, D− 5%, D 10%, D+ 2%. GRADE C
-
4.13
Anxiety and stress are pathophysiological mechanisms in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 66%: A+ 20%, A 46%, A− 24%, D− 2%, D 7%, D+ 0%. GRADE B
-
4.14
Depression is a pathophysiological mechanism in functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 15%, A 39%, A− 24%, D− 7%, D 12%, D+ 2%. GRADE B
-
4.15
Disordered central processing of incoming signals from the gastroduodenal region is a pathophysiological mechanism in functional dyspepsia.
STATEMENT ENDORSED, overall agreement 85%: A+ 39%, A 46%, A− 12%, D− 0%, D 2%, D+ 0%. GRADE C
-
4.16
Genetic factors determine the susceptibility to functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 37%: A+ 22%, A 15%, A− 42%, D− 5%, D 15%, D+ 2%. GRADE C
-
4.1
Several studies, both in community‐based and in‐patient cohorts, have shown that food is a major trigger for FD symptoms. 57 , 73 , 74 Whether alterations in content or timing of meals in FD contributes to this triggering effect has been evaluated in only a few studies. A number of studies reported intake of a lower number of meals in FD patients compared to controls, in some cases with a tendency for more snacks between meals. 75 , 76 , 77 In terms of macronutrient intake, reduced fat intake has been reported in FD, 77 but also reduced carbohydrate has been reported in a mixed FD/IBS population. 78 A recent systematic review of 16 studies failed to show a consistent link between symptoms and dietary intake. 79 Taken together, there are not enough data to confirm that dietary habits induce symptoms in FD patients, but patients are likely to have adapted their food intake patterns in an attempt to decrease symptom occurrence and severity.
In FD patients with otherwise normal macroscopic findings at endoscopy, microscopic H. pylori infection has been considered a factor potentially involved in symptom generation. A cause–effect relationship between H. pylori infection and FD is supported by evidence that H. pylori eradication may lead to sustained symptom improvement in a subset of patients. 80 The Kyoto consensus proposed that H. pylori infection is associated with dyspepsia in a subset of patients, with a strong grade of recommendation and high evidence level, and referred to this entity as H. pylori‐associated dyspepsia. 81 The Rome IV consensus has adopted this view. 1 However, there is a possibility of intermittent peptic ulcer disease, missed at endoscopy for dyspeptic symptoms. 81 , 82 Furthermore, symptomatic improvement after eradication therapy may also be due to an effect of antibiotics on microbiota other than H. pylori infection since H. pylori eradication therapy has never been attempted in H. pylori‐negative FD patients.
Several studies have reported impaired gastric accommodation in 15%–50% of FD patients, 63 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 without differences according to Rome III subgroups. 91 The impairment of gastric accommodation was associated with reduced drinking capacity and symptoms such as early satiation, fullness, and weight loss. 63 , 67 , 92
Several studies reported delayed gastric emptying in FD for solids as well as liquids 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 without differences in the emptying times between Rome III subgroups. 91 A meta‐analysis described gastric emptying in patients with FD to be 1.46 times slower than in controls. 96 Delayed gastric emptying was associated with female sex, postprandial fullness, nausea, vomiting, bloating, and early satiety. 97 , 98 , 99 , 100 In spite of the presence of delayed emptying in a subset of patients, the association with symptoms is weak. The severity of gastric emptying delay is not a good determinant of symptom severity or pattern. 94 , 97 , 99 , 100 Few studies also reported an acceleration of gastric emptying in FD, 101 , 102 but this was not confirmed in others. 99
Hypersensitivity to gastric distention has been reported in 34%–65% of FD patients by several studies, 64 , 100 , 101 , 102 , 103 , 104 , 105 , 106 without difference between Rome III subgroups, 91 and postprandial sensitivity to gastric distention was even greater than fasting sensitivity. 107 Hypersensitivity to gastric distention was associated with a higher prevalence of postprandial pain, belching, and weight loss, 64 and an increase in gastrointestinal symptom severity was observed with increasing visceral sensitivity. 108
Several studies from around the world have reported increased numbers of activated eosinophils and mast cells in the duodenal mucosa of FD patients. 109 , 110 , 111 , 112 , 113 , 114 , 115 In addition, Ussing chamber experiments demonstrated that this is correlated with impaired duodenal integrity and changes in the expression of cell‐to‐cell adhesion proteins, as well as functional and structural submucosal neuronal changes. 110 , 112 Although the cause–consequence relationship of the barrier defect and immune activation is still unknown, persisting changes in duodenal mucosal immune cells in PI‐FD and systemic immune activation in acute compared to unspecified‐onset FD suggest the inability of the immune system to handle a triggering (infectious) insult in FD. 116 , 117
Although gastric acid secretion is reported as normal, 118 FD patients displayed increased spontaneous duodenal acid exposure during the daytime and the late postprandial phase with higher symptom severity in patients with high duodenal acid exposure. 119 However, the correlation between acid exposure and symptom severity was weak, and the increased duodenal acid exposure could be, at least in part, attributable to delayed duodenal acid clearance as FD patients display decreased duodenal motor activity in response to acid perfusion. 119 , 120
A number of studies suggest the implication of gut hormones in the pathophysiology of FD, but the studies are small and findings are heterogeneous. 121 Early studies focused on cholecystokinin (CCK), as a subset of FD patients had elevated plasma levels, intravenous administration of CCK worsened dyspeptic symptoms, and the selective CCKA antagonist dexloxiglumide reduced symptoms during gastric distention and duodenal lipid infusion. 122 , 123 , 124 In PDS, ghrelin plasma levels were reported to be reduced. 125 , 126 Further studies are needed to elucidate the potential implication of other gut hormones including gastrin, somatostatin, glucagon‐like peptide‐1, and peptide YY in FD symptom generation. 121
Duodenal hypersensitivity to luminal acid and lipids has been reported in FD, 127 , 128 with induction of nausea and decreased duodenal motor responses in response to acid infusion. 129 These alterations were found to be chemospecific as they did not occur during saline or dextrose infusion. 130 However, the number of studies and the sample size are generally low in the available studies.
Data on the duodenal mucosa‐associated microbiome in FD are limited to one pilot study involving nine patients, with an increase in Streptococcus and a decrease in the anaerobic genera Prevotella, Veillonella, and Actinomyces compared to healthy controls. 131 Interestingly, the total mucosal bacterial load correlated with meal‐related symptom severity and quality of life, indicating the potential of targeting the duodenal microbiome in FD. 131 Studies on the gastric microbiome in FD have shown a significant inverse correlation between the abundance of Prevotella in the gastric fluid and the severity of PDS. 132
Mucosal vagal sensory nerve endings are involved in the initiation of satiety, nausea, and vomiting by chemical and osmotic stimuli. The vagus nerve is also a major contributor to control upper gastrointestinal motility. 133 An early study in seven FD patients, using an insulin hypoglycemia test and plasma levels of pancreatic polypeptide, suggested a disturbed efferent vagal function. 134 The gastric response to sham feeding, a marker for vagal activity, was lower in PDS compared to controls. 135 Conversely, sham feeding was reported to improve the suppressed response to a liquid nutrient meal in FD. 136 Slow deep breathing, which is thought to activate the vagus nerve, was associated with improvement of nutrient volume tolerance and quality of life in FD. 137 Using spectral analysis of cardiac R‐R intervals to evaluate vagal tone, Guo et al. showed a decreased vagal tone that was associated with delayed gastric emptying. 138 Taken together, a number of observations suggest decreased vagal activity in FD, but the studies all occurred in laboratory settings in small groups of patients.
As mentioned above, there is an association of anxiety with FD, and anxiety may precede FD. Moreover, FD is associated with altered brain processing of gastrointestinal (GI) stimuli, altered central nervous system connectivity, and structure and altered expression of neurotransmitter pathways. 139 , 140 , 141 , 142 , 143 However, a causal relation between anxiety and FD has not been established. As mentioned above, depression is also associated with FD, but also in this case, there is a lack of evidence for a causal relation.
Numerous studies have shown that FD patients report more symptoms or earlier symptoms following gastroduodenal stimulation with either balloon, liquid volume, or food compared to healthy controls. A systematic review evaluated studies on central processing of signals from the gastroduodenal region in FD patients and controls, mostly by balloon distention of the stomach, using PET or fMRI technology. 144 The results show that FD is associated with functional abnormalities in sensory and pain modulation, emotion, saliency, and homeostatic processing regions, suggesting that disordered central processing of incoming signals from the gastroduodenal region is indeed a relevant pathophysiological mechanism at least in a subgroup of FD patients. However, this does not exclude an involvement of peripheral mechanisms as well.
Family studies support a genetic component in FD susceptibility. 145 A mea‐analysis of eight studies indicated that the GNβ3 C825T polymorphism is significantly associated with FD, and susceptible to racial variation. 146 However, a meta‐analysis based on 12 studies has failed to confirm a significant association. 147 Several additional gene polymorphisms including serotonin transporter promoter, interleukin‐17F, migration inhibitory factor, cholecystokinin‐1 intron 1, cyclooxygenase‐1, catechol‐o‐methyltransferase, transient receptor potential vanilloid 1 receptor, regulated upon activation normal T cell expressed and secreted, p22PHOX, Toll‐like receptor 2, SCN10A, CD14, adrenoreceptors, and others have been investigated in relation to FD; however, the results are contradictory. 148
-
5.
Diagnosis
-
5.1
Upper gastrointestinal endoscopy is mandatory for establishing a diagnosis of functional dyspepsia.
STATEMENT ENDORSED, overall agreement 80%: A+ 51%, A 29%, A− 15%, D− 0%, D 2%, D+ 2%. GRADE A
-
5.2
In primary care, uninvestigated dyspepsia can be managed without endoscopy if there are no alarm symptoms or risk factors.
STATEMENT ENDORSED, overall agreement 93%: A+ 32%, A 61%, A− 5%, D− 0%, D 2%, D+ 0%. GRADE A
-
5.3
Upper gastrointestinal endoscopy is mandatory if there are alarm symptoms or risk factors.
STATEMENT ENDORSED, overall agreement 93%: A+ 73%, A 20%, A− 5%, D− 0%, D 2%, D+ 0%. GRADE A
-
5.4
Screening blood tests are useful when considering a diagnosis of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 46%: A+ 10%, A 36%, A− 24%, D− 2%, D 24%, D+ 2%. GRADE B
-
5.5
Every patient with dyspeptic symptoms should be tested for Helicobacter pylori (non‐invasively or at gastroscopy).
STATEMENT ENDORSED, overall agreement 81%: A+ 39%, A 42%, A− 12%, D− 0%, D 7%, D+ 0%. GRADE A
-
5.6
Patients with dyspepsia and H. pylori‐positive gastritis should be considered to have functional dyspepsia just if symptoms persist 6 to 12 months after H. pylori eradication.
STATEMENT ENDORSED, overall agreement 83%: A+ 34%, A 49%, A− 12%, D− 0%, D 2%, D+ 2%. GRADE B
-
5.7
Patients with dyspepsia and H. pylori‐negative gastritis should be considered to have functional dyspepsia.
STATEMENT ENDORSED, overall agreement 85%: A+ 34%, A 51%, A− 10%, D− 5%, D 0%, D+ 0%. GRADE B
-
5.8
Functional dyspepsia should be subdivided into EPS and PDS for further diagnostic and therapeutic approach.
STATEMENT ENDORSED, overall agreement 83%: A+ 34%, A 49%, A− 15%, D− 0%, D 2%, D+ 0%. GRADE B
-
5.9
Upper abdominal ultrasound is useful when considering a diagnosis of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 27%: A+ 12%, A 15%, A− 24%, D− 10%, D 32%, D+ 7%. GRADE B
-
5.10
A gastric emptying test is useful when considering a diagnosis of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 34%: A+ 2%, A 32%, A− 27%, D− 20%, D 15%, D+ 5%. GRADE B
-
5.11
Esophageal pH monitoring is useful in functional dyspepsia to rule out GERD.
STATEMENT NOT ENDORSED, overall agreement 37%: A+ 7%, A 30%, A− 29%, D− 7%, D 24%, D+ 2%. GRADE B
-
5.12
Increased duodenal eosinophil count is a marker of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 20%: A+ 3%, A 17%, A− 37%, D− 15%, D 27%, D+ 2%. GRADE C
-
5.13
Impaired nutrient volume tolerance is a marker of functional dyspepsia.
STATEMENT NOT ENDORSED, overall agreement 63%: A+ 14%, A 49%, A− 22%, D− 5%, D 7%, D+ 2%. GRADE B
-
5.1
A distinction should be made between the management of uninvestigated dyspeptic symptoms and the diagnosis of FD. The Rome IV definition of FD implies that potential underlying organic disorders have been ruled out by endoscopy. 1 In a patient presenting with dyspeptic symptoms, all guidelines recommend a prompt upper GI endoscopy in patients aged over 45–60 years to rule out neoplasia, and to take biopsies to establish H. pylori status. 1 , 10 , 149 , 150 Endoscopy is also mandatory in younger patients presenting with alarm features, 1 , 10 although alarm symptoms have a limited value in predicting an organic disease. 5 , 151 In younger patients without alarming symptoms, guidelines agree that there is no need to perform a gastroscopy to detect malignancy, which is rare. Empiric therapy, either with proton pump inhibitors (PPIs), prokinetics or H. pylori eradication (“test and treat strategy”), is valuable for the management of uninvestigated dyspepsia. When considering the actual diagnosis of FD, endoscopy is mandatory to rule out not only malignancies, but also benign organic disorders which may explain the symptoms such as peptic ulcer (prevalence 8%), esophagitis (20%), or H. pylori‐associated gastritis. 6 The cutoff between young and old is now considered to be 60 years in the West, adjusted to additional risk factors and local incidence age of gastric cancer. 150
Overall, there is a lack of data on cost–benefit utility of laboratory testing in patients presenting with dyspeptic symptoms. A study from India found that apart from warning signs, blood tests for hemoglobin and albumin could discriminate functional from organic disease when placed in a risk model, 152 but the results have not been replicated in Western populations.
Most Western guidelines advocate testing for H. pylori in dyspeptic subjects and eradication in case of a positive test. In younger patients (<60), the most cost‐effective way to manage dyspeptic patients is to reduce or stop NSAID medication and/or to have a non‐invasive test for H. pylori. 153 , 154 Especially in high prevalence areas, the positive subjects should be considered for follow‐up surveillance of early gastric cancer. 81 If an endoscopy is performed, biopsies should be obtained to test for H. pylori status. 1 , 10 , 81 , 149 , 150
H. pylori eradication may improve symptoms in a subset of patients with investigated dyspepsia, that is, with normal macroscopic upper GI endoscopy, but studies suggest that the symptomatic benefit is only reached after 6–12 months. 1 , 81 This subgroup of patients, referred to as H. Pylori‐associated dyspepsia, is relatively small, since meta‐analysis of randomized controlled trials has shown a 10% relative risk reduction of persisting symptoms in the H. Pylori eradication group compared to placebo, with a number needed to treat (NNT) of 12.5 to cure one case of dyspepsia. 150
Although chronic superficial gastritis might affect a variety of gastric functions, there is no evidence to consider that the presence of gastric mucosal inflammation (with or without atrophy) may cause symptoms. 1 , 81 , 155 Therefore, if no H. Pylori infection can be demonstrated, a patient with dyspeptic symptoms and normal endoscopy should be considered to have FD even if gastritis is present, whatever its severity. 1
In population‐based symptom analyses, dyspeptic symptoms were shown to group around clusters, representing EPS and PDS. 3 , 156 The literature is divided on the usefulness of distinguishing PDS and EPS for patient management. When the Rome III subdivision is used, a major overlap is found between both, which is largely corrected with the Rome IV subdivision as a good separation between both subtypes is now found both in epidemiological studies and in clinical practice. 3 , 11 , 40 , 156 , 157 , 158 While some studies report different treatment responses, 159 , 160 others do not. 161 , 162 To date, no fully published study has evaluated differential pathophysiological mechanisms or treatment outcomes according to the Rome IV subdivision. 163 , 164
Kraag et al. performed a meta‐analysis of 21 controlled studies on the association between gallstones and dyspeptic symptoms and found no reasonable association between gallstones and “classical” dyspeptic symptoms other than upper abdominal pain. 165 In a systematic review of 24 publications, biliary colic was the only single symptom associated with gallstones. 166 Hence, guidelines do not recommend upper abdominal ultrasound for exclusion of biliary pathology in the diagnosis of FD. 1 , 150
The prevalence of delayed gastric emptying in FD ranges between 20% and 50%, but its association with symptoms and response to therapy has shown inconsistent results. 1 , 91 , 167 , 168 The American College of Gastroenterology and Canadian Association of Gastroenterology, as well as the Rome IV consensus did not recommend the use of gastric emptying testing in the diagnosis or management of FD. 1 , 150
Abnormal esophageal acid exposure on pH monitoring can be found in 20%–30% of patients presenting with dyspeptic symptoms without heartburn as a predominant symptom, and in up to 50% in the subgroup of patients with epigastric burning. 13 , 169 , 170 , 171 Randomized placebo‐controlled studies have shown a small but significant benefit of acid‐suppressive therapies. 1 , 150 However, there is no evidence that esophageal pH monitoring would help to identify patients who may respond to acid‐suppressive therapy. In addition, there is no evidence that pH monitoring would be useful in patients with dyspeptic symptoms refractory to acid‐suppressive therapy.
In a systematic review and meta‐analysis of 37 studies, both mast cell and eosinophil counts in the duodenum were increased in FD compared to controls, both in PDS and EPS. 172 Nevertheless, as there is overlap with health and as other conditions may also be associated with increased eosinophil numbers, this cannot be used as a diagnostic marker.
Several studies have reported decreased volume tolerance in FD compared to health, using liquid nutrients but also water intake. 63 , 67 , 86 , 173 , 174 , 175 A large drinking test study in secondary care FD patients confirmed decreased nutrient volume tolerance, and this is reproducible and correlates with symptom pattern and severity. 176 However, the use of different substances (water vs. nutrients) and different rates of ingestion and levels of blinding to the ingested nutrient volume have hampered the development of a standardized protocol that can be useful in the clinical setting.
-
6.
Treatment
-
6.1
Dietary adjustment improves symptoms in FD.
STATEMENT NOT ENDORSED, overall agreement 73%: A+ 22%, A 51%, A− 24%, D− 2%, D 0%, D+ 0%. GRADE C
-
6.2
H. pylori positive FD patients should receive eradication therapy.
STATEMENT ENDORSED, overall agreement 95%: A+ 59%, A 36%, A− 5%, D− 0%, D 0%, D+ 0%. GRADE A
-
6.3
PPI therapy is the most appropriate initial therapy for FD.
STATEMENT NOT ENDORSED, overall agreement 73%: A+ 27%, A 46%, A− 12%, D− 2%, D 12%, D+ 0%. GRADE B
-
6.4
PPI therapy is an effective therapy for FD.
STATEMENT ENDORSED, overall agreement 83%: A+ 25%, A 58%, A− 15%, D− 2%, D 0%, D+ 0%. GRADE A
-
6.5
PPI therapy is the most effective therapy for EPS.
STATEMENT NOT ENDORSED, overall agreement 59%: A+ 20%, A 39%, A− 22%, D− 2%, D 17%, D+ 0%. GRADE C
-
6.6
Prokinetic therapy is the most appropriate initial therapy for FD.
STATEMENT NOT ENDORSED, overall agreement 30%: A+ 18%, A 12%, A− 15%, D− 12%, D 38%, D+ 5%. GRADE C
-
6.7
Prokinetic therapy is an effective therapy for FD.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 17%, A 37%, A− 34%, D− 5%, D 7%, D+ 0%. GRADE B
-
6.8
Prokinetic therapy is the most effective therapy for PDS.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 22%, A 32%, A− 22%, D− 5%, D 17%, D+ 2%. GRADE B
-
6.9
Efficacy of prokinetics is not related to their enhancement of gastric emptying rate.
STATEMENT NOT ENDORSED, overall agreement 56%: A+ 7%, A 49%, A− 24%, D− 7%, D 10%, D+ 2%. GRADE B
-
6.10
Itopride is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 56%: A+ 12%, A 44%, A− 30%, D− 7%, D 7%, D+ 0%. GRADE C
-
6.11
Tricyclic antidepressants (TCAs) are effective for EPS.
STATEMENT NOT ENDORSED, overall agreement 78%: A+ 20%, A 58%, A− 15%, D− 0%, D 5%, D+ 2%. GRADE B
-
6.12
TCAs are effective for PDS.
STATEMENT NOT ENDORSED, overall agreement 32%: A+ 5%, A 27%, A− 24%, D− 12%, D 27%, D+ 5%. GRADE B
-
6.13
TCAs are not effective for PDS.
STATEMENT NOT ENDORSED, overall agreement 39%: A+ 10%, A 29%, A− 25%, D− 17%, D 17%, D+ 2%. GRADE B
-
6.14
Serotonin reuptake inhibitors are effective for FD.
STATEMENT NOT ENDORSED, overall agreement 20%: A+ 5%, A 15%, A− 17%, D− 12%, D 44%, D+ 7%. GRADE B
-
6.15
Serotonin reuptake inhibitors are not effective for FD.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 7%, A 47%, A− 29%, D− 7%, D 10%, D+ 0%. GRADE B
-
6.16
Serotonin noradrenaline reuptake inhibitors are effective for FD.
STATEMENT NOT ENDORSED, overall agreement 17%: A+ 0%, A 17%, A− 27%, D− 12%, D 39%, D+ 5%. GRADE C
-
6.17
Serotonin noradrenaline reuptake inhibitors are not effective for FD.
STATEMENT NOT ENDORSED, overall agreement 49%: A+ 5%, A 44%, A− 24%, D− 5%, D 17%, D+ 5%. GRADE C
-
6.18
Mirtazapine is effective for post‐prandial distress syndrome patients with weight loss.
STATEMENT NOT ENDORSED, overall agreement 68%: A+ 12%, A 56%, A− 27%, D− 0%, D 5%, D+ 0%. GRADE B
-
6.19
5‐HT1A agonists (tandospirone, buspirone, ….) are effective for PDS.
STATEMENT NOT ENDORSED, overall agreement 56%: A+ 5%, A 51%, A− 37%, D− 2%, D 5%, D+ 0%. GRADE B
-
6.20
Herbal therapies are effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 37%: A+ 12%, A 25%, A− 34%, D− 10%, D 17%, D+ 2%: . GRADE B
-
6.21
Iberogast (STW‐5) is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 12%, A 42%, A− 34%, D− 0%, D 12%, D+ 0%. GRADE B
-
6.22
Rifaximin is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 19%: A+ 2%, A 17%, A− 27%, D− 10%, D 37%, D+ 7%. GRADE C
-
6.23
Hypnotherapy is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 29%: A+ 5%, A 24%, A− 22%, D− 17%, D 32%, D+ 0%. GRADE B
-
6.24
Cognitive–behavioral therapy (CBT) is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 42%: A+ 10%, A 32%, A− 39%, D− 5%, D 14%, D+ 0%. GRADE B
-
6.25
Acupuncture is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 27%: A+ 0%, A 27%, A− 27%, D− 15%, D 22%, D+ 10%. GRADE B
-
6.26
Mindfulness is effective for FD patients.
STATEMENT NOT ENDORSED, overall agreement 27%: A+ 3%, A 24%, A− 12%, D− 10%, D 49%, D+ 2%. GRADE B
-
6.27
In case of severe weight loss in FD, nutritional support may be needed.
STATEMENT ENDORSED, overall agreement 90%: A+ 36%, A 54%, A− 7%, D− 0%, D 2%, D+ 0%. GRADE B
-
6.1
Although the majority of FD patients indicate that their symptoms are triggered by nutrient ingestion, there is a lack of controlled dietary intervention studies. It is reasonable to advise frequent small‐size meals and avoiding high‐fat food items, but the supporting evidence for this strategy is rather limited. Large‐scale randomized studies are required to evaluate the impact of dietary factors on symptoms of FD and the role of diet as a therapeutic strategy.
A review of 22 trials involving 4896 H. pylori‐positive FD patients showed that H. pylori eradication determined a small but statistically significant improvement of symptoms when compared with placebo (RR dyspepsia remaining = 0.91; 95% CI = 0.88–0.94; p < 0.00001). The observation period in most of the trials included in the review was 12 months, and the NNT was 12.5. 150 Although the overall therapeutic effect is modest, additional benefits of eradication therapy are the elimination of a putative pathogenic factor with prevention of peptic ulcer and possibly gastric cancer. 1 , 81 Pre‐Rome IV evidence suggests that eradication of H. pylori infection could be beneficial in both predominant epigastric pain and predominant dysmotility‐type symptoms, although a more recent study showed the main benefit in EPS. 160 , 177
Several guidelines recommend standard, once‐daily PPI therapy during 4–8 weeks as the first‐line treatment for patients with FD who remain symptomatic after eradication of H. pylori or who are negative for H. pylori. 150 , 178 , 179 , 180 A recent Cochrane meta‐analysis, including 6172 patients from 18 randomized controlled trials, confirmed that PPIs are more effective than placebo in the reduction of global symptoms of FD (RR of remaining dyspeptic 0.88; 95% CI 0.82–0.94; NNT 11). 181 There were no differences between low‐ and high‐dose PPI, type of PPI, and H. pylori status. 150 , 181 , 182 In a meta‐analysis of two studies including 740 FD patients, directly comparing PPI and histamine‐2 receptor antagonists, there was no difference between both treatments. 181 However, both studies are older, possibly including GERD patients, and one is only available in abstract form. 183 , 184 In areas of low (<20%) prevalence of H. pylori, a course of PPI has been suggested as the preferred first‐line option before a test‐and‐treat approach. 185 The Rome IV consensus stated that PPIs are ineffective in relieving PDS symptoms, based on older data. 1 Two Japanese studies that investigated the effect of PPI in the Rome III/IV subgroups found no significant difference between subgroups. 177 , 186 The ACG/CAG guidelines, based on an updated meta‐analysis, propose PPI as first‐line therapy, irrespective of the Rome IV subgroups. 150
A recent meta‐analysis of 29 studies involving 10,044 patients with FD demonstrated a significant effect of prokinetics in reducing dyspeptic symptoms (RR of ongoing dyspeptic symptoms 0.81; 95% CI [0.74–.89]) with an NNT of 7. 187 However, the studies showed significant heterogeneity and the funnel plot was asymmetrical, suggesting publication bias. Moreover, 12 studies involved cisapride, which has been withdrawn from the market because of cardiac adverse events. 188 When cisapride was removed from the meta‐analysis, the overall effect was still significant, but the NNT increased to 12. 187
The rationale for prokinetic therapy in FD is the presence of motor abnormalities such as delayed gastric emptying, especially in PDS, but in a large study, similar prevalence of gastric motor abnormalities was found in PDS, EPS, and the overlap group. 91 In the 2019 meta‐analysis, prokinetics demonstrated similar efficacy in PDS and EPS subgroups, although only two studies included a total of 124 EPS patients, 187 one of which is a study on cisapride from 1989. 189 The evidence supporting the use of prokinetics in FD is rather poor and no target subgroup can be defined based on the available evidence. Moreover, many prokinetics such as domperidone and acotiamide, are not widely available. Adverse effects, such as extrapyramidal syndrome for many dopamine‐2 (D2) receptor antagonists and QTc prolongation with domperidone, are limiting chronic use in FD. This has led the ACG/CAG guideline to recommend treatment with a TCA in patients refractory to PPI treatment before prokinetics. 150
A systematic analysis of 34 studies failed to demonstrate a correlation between the acceleration of gastric emptying and symptom improvement. 167 One possible explanation is the heterogeneous pathophysiology of FD, involving not only delayed gastric emptying but also impaired gastric accommodation and hypersensitivity to gastric distention which are often not taken into account. However, in a recent meta‐analysis by Vijayvargiya et al., the authors found that when optimal test methods were used, a selection of promotility agents significantly accelerated gastric emptying and produced significant symptom improvement in gastroparesis patients. 168 Nevertheless, it has not been established that the effect of prokinetics in FD depends on baseline emptying rate or improvement of emptying rate with therapy.
Itopride is a combined D2 antagonist and acetylcholinesterase inhibitor and is available in Asia and several countries in Eastern Europe. A phase IIb placebo‐controlled trial found significantly more responders to itopride, based on a global efficacy measure. 190 However, no significant improvement over placebo in reduction of FD symptoms was observed in two subsequent Phase III trials. 191 These trials suffered from issues with patients and endpoint selection, 192 but their negative outcome stopped further development of itopride in the West. In a recent controlled trial in Belgium, itopride seemed more effective in Rome IV PDS compared to Rome III PDS. 193
Psychotropic drugs appear to be an effective treatment for FD, as demonstrated by a systematic review and meta‐analysis, with an NNT of 6 when data from all studies were pooled (1241 patients, 673 assigned to psychoactive drugs, and 568 to placebo). However, this beneficial effect appeared to be limited to TCAs and antipsychotics. 194 In a randomized placebo‐controlled trial including 292 FD patients assigned to either placebo, 50 mg amitriptyline or 10 mg escitalopram for 12 weeks, subjects with “ulcer‐like” FD (likely equivalent to EPS) receiving amitriptyline reported more adequate relief of symptoms than those receiving placebo or escitalopram (p = 0.06). 195 There were adverse events in 30% (n = 29) individuals in the amitriptyline arm, leading to discontinuation of treatment in two of them. Those with delayed gastric emptying were less likely to report adequate relief on amitriptyline compared with FD patients with normal emptying, but this was not related to an amitriptyline‐induced delay in gastric emptying. 194 Amitriptyline appeared to derive its benefit predominantly through improving abdominal pain, since no change in psychological distress measures nor gastric emptying rates was found. 195 , 196 In the subset of patients with PDS, little evidence exists so far supporting the use of TCA's. A double‐blind, randomized controlled trial including 107 patients with refractory FD, treated with either imipramine or placebo for 12 weeks, showed efficacy in symptom relief (p = 0.0051), but 18% of the patients on imipramine discontinued the study due to adverse effects. In this study, no conclusion was made regarding efficacy in FD subtypes. 197 The ACG and CAG clinical guidelines on dyspepsia considered that FD patients failing to respond to PPI and H. pylori eradication treatment, should be offered TCA before prokinetics based on the superior evidence for TCA in this indication. No consideration was done based on FD subtypes EPS and PDS. 150
The systematic review and meta‐analysis on the efficacy of psychotropics in FD included two studies of selective serotonin reuptake inhibitors (sertraline 50 mg o.d. and escitalopram 10 mg o.d.), containing almost 400 patients, which were negative. 194 , 195 , 198 Thus, it seems reasonable to assume that these drugs are of no benefit in FD.
A double‐blind clinical trial randomly assigned 160 FD patients to 8 weeks of treatment with venlafaxine or placebo. 199 At none of the measurement times there was a statistically significant difference in symptom severity, quality of life or anxiety, and depression scores between venlafaxine and placebo. 199 The dropout rate among venlafaxine‐treated patients was high due to side effects. While this single study with venlafaxine in FD was negative, it remains to be elucidated whether certain groups of patients might benefit from treatment with serotonin/noradrenaline reuptake inhibitors with a more potent analgesic effect at lower doses, for example, duloxetine.
In FD, weight loss is normally considered an alarming symptom, but may be present in up to 40% of tertiary care FD patients. 10 , 65 , 66 A controlled trial to assess mirtazapine's efficacy in FD and weight loss randomly assigned 34 patients to placebo or mirtazapine 15 mg daily for 8 weeks. 200 Mirtazapine significantly improved early satiation scores and nutrient tolerance compared to placebo. A trend was found for overall dyspepsia symptom score at week 4 in the mirtazapine group, but not at week 8. Nevertheless, this was associated with significant recovery of weight loss, improvement of quality of life, and visceral specific anxiety score. Another trial treated 60 FD patients with depression and weight loss with either mirtazapine 30 mg daily, paroxetine 20 mg daily or conventional therapy, and showed that mirtazapine did not only alleviate symptoms associated with dyspepsia and depression linked to FD with weight loss, but also significantly increased body weight. 201 In summary, two limited size trials showed the efficacy of mirtazapine in FD, one in patients without and one in patients with coexisting depression.
Tandospirone citrate, a serotonin 1A receptor (5‐HT1A) agonist, was shown to improve abdominal symptom scores in FD patients. 200 However, R‐137696, another 5‐HT1A agonist, failed to improve symptoms or visceral hypersensitivity in FD patients. 203 In a small cross‐over controlled trial, buspirone significantly reduced the overall severity of symptoms of dyspepsia and individual symptoms of postprandial fullness, early satiation, and upper abdominal bloating, whereas placebo had no significant effect. 204 The presumed mechanism of action was an enhancement of gastric accommodation. 205 A meta‐analysis of the three available studies with 5‐HT1A agonists in FD showed no overall beneficial effect. 194
Meta‐analyses pooling data from different small clinical trials that fall under the FD category indicate that the European herbal combination drug STW‐5 and peppermint oil are superior compared to placebo in the treatment of FD symptoms. 192 , 206 , 207 A meta‐analysis of five controlled trials found superiority over placebo of an encapsulated peppermint/caraway oil preparation in FD for overall symptoms and epigastric pain and discomfort. 208
A meta‐analysis including 24 clinical trials evaluated the efficacy of the Japanese herbal KAMPO preparation Rikkunshito in FD. 209 No significant benefit was found when evaluating upper gastrointestinal symptoms based on the Gastrointestinal Symptom Rating Scale, but Rikkunshito was superior to placebo in improving symptoms based on a five‐point scale and in improving appetite, although the authors identified risk of bias for the majority of available studies.
Meta‐analyses of numerous controlled trials of low quality and small numbers of participants show that different Chinese Herbal Medicines, alone or in combination with variable prokinetic medications, may be effective and superior to prokinetic medication alone when evaluated with variable FD symptom scores. Effects of Chinese Herbal Medicines on individual FD symptoms are largely unreported. 210
A single double‐blind, placebo‐controlled randomized study examined the efficacy of rifaximin in subjects with Rome III criteria defined FD who were H. pylori negative. 211 The authors found that rifaximin was superior to placebo for the relief of global dyspeptic symptoms, postprandial fullness/bloating, and belching. Additional future trials are needed to examine the efficacy of rifaximin in FD and to elucidate the underlying mechanism of action.
In a 2017 review, a total of 12 controlled trials of psychological therapies involving 1563 FD patients were identified. 150 All trials reported a statistically significant benefit of psychological therapies over control, which was most commonly usual management. Information on individual types of psychological therapies is variable. For hypnotherapy in FD, only one small randomized controlled study reported benefit. 212 For CBT, two studies showed positive short‐term effects on FD symptoms. 213 , 214
Meta‐analyses of numerous low‐quality randomized, controlled studies suggest manual and electric acupuncture being effective in the treatment of FD, as shown by improved symptom scores and health‐related quality‐of‐life scores. 215 , 216 , 217 Effects are most pronounced in sham‐controlled trials and less pronounced in trials comparing to prokinetic medication or traditional Chinese medicine. A recent sham‐controlled trial adds that the effect of acupuncture, following 20 treatment sessions in a 4‐week episode, is sustained for 24 weeks. 218 Besides the overall small number of patients included and the different acupuncture protocols followed, selection bias, performing bias, reporting bias, attrition bias, and blinding difficulties remain the major concerns when interpreting findings in the meta‐analyses.
In a meta‐analysis, the quality and effectiveness of mindfulness‐based therapy in FGIDs was evaluated. 219 However, studies evaluating the effectiveness of mindfulness specifically in FD have not been found in the literature.
While severe weight loss may occur in FD patients, especially in those with PDS and food avoidance, few studies have addressed its management. The antidepressant mirtazapine seems to help with weight gain in these patients 200 , 201 and to improve FD symptoms, so the conclusion that other clinical management strategies for weight‐gain support may also be effective, is plausible but has not been tested. This holds true specifically for enteral and parenteral feeding. In one preliminary report of 19 FD patients with delayed gastric emptying and severe weight loss (15.8 ± 2.8 kg), enteral feeding through a percutaneous endoscopic gastrojejunostomy (PEG/J) generated weight gain in 63% of subjects, but there were many non‐life‐threatening complications. The PEG/J was electively removed after 19.9 ± 5.6 months and a mean weight gain of 6.1 ± 0.8 kg 220
-
7.
Prognosis of FD
-
7.1
The long‐term prognosis is favorable in the majority of patients with FD.
STATEMENT ENDORSED, overall agreement 85%: A+ 29%, A 56%, A− 12%, D− 0%, D 2%, D+ 0%. GRADE B
-
7.2
Life expectancy in FD is similar to the general population.
STATEMENT ENDORSED, overall agreement 100%: A+ 56%, A 44%, A− 0%, D− 0%, D 0%, D+ 0%. GRADE A
-
7.1
It is well established that symptoms in FD vary over time. Based on a cohort study, 1 year after initial diagnosis, 24% of patients reported their symptoms as unimproved, and these patients were younger and had higher symptom severity at the first diagnosis. 219 In a tertiary care cohort, half of the FD patients improved or became asymptomatic after a mean follow‐up of 5 years, with anxiety and weight loss at presentation as unfavorable predictors. 48 Population‐based reports of longer observation periods performed in Olmsted County, Minnesota, United States (12 years) and Iceland (10 years) report a favorable outcome with symptom resolution in 63%–67%, stable or persistent symptoms falling under the FD category in 16%–20%, and fluctuating or additional symptoms in 32%–35% confirming high symptom turnover among FGID patients. 222 , 223
In a population‐based cohort study, 5262 randomly selected subjects were screened. From 3933 eligible subjects included in the analysis, dyspepsia was diagnosed in 2% of subjects. No association with overall survival was detected for dyspepsia (HR = 1.08 (95% CI: 0.58–2.02)). 224 A 10‐year long‐term follow‐up study included 8323 people, of whom dyspepsia was diagnosed in 3169 patients (38.1%). After multivariate analysis, there was no significant difference in the likelihood of death at 10 years in those with dyspepsia (HR: 0.94; 99% CI: 0.58–1.54) compared to those who did not meet the criteria for FD. 225 Thus, available data show that life expectancy in FD is similar to life expectancy in the general population.
RECOMMENDATIONS
Based on the statements that achieved consensus (Table 3), a number of recommendations for understanding and managing FD can be made, which are summarized in Table 4. The Delphi process also identified several areas of uncertainty, which require additional evidence or further research. Figure 1 schematically summarizes the findings.
TABLE 4.
Summary of the ESNM consensus on FD
| Recommendations | Based on statement(s) |
|---|---|
| Dyspepsia refers to a symptom or set of symptoms that are considered to originate from the gastroduodenal region. Early satiation, postprandial fullness, epigastric pain and epigastric burning are the cardinal dyspeptic symptoms. | 1.1, 1.2 |
| FD is a condition characterized by chronic dyspeptic symptoms in the absence of organic, systemic or metabolic condition(s) that is (are) likely to explain symptoms. The vast majority of patients with dyspeptic symptoms and no alarm symptoms in the general population would be identified as FD after investigation (if performed). | 1.3, 1.4 |
| Two main subtypes of FD are distinguished which may overlap: postprandial distress syndrome (PDS) characterized by meal‐induced symptoms (early satiation, postprandial fullness) and epigastric pain syndrome (EPS), with epigastric pain and/or epigastric burning. | 1.5 |
| Dyspeptic symptoms often co‐exist with other symptoms such as bloating in the upper abdomen, nausea and belching. Typical reflux symptoms and irritable bowel syndrome often coexists with FD. | 1.6, 1.9, 1.11 |
| (Functional) dyspepsia is more prevalent in women than men. | 2.2 |
| Acute GI infection and anxiety are risk factors for development of FD. | 2.3; 2.6 |
| FD is a major source of healthcare costs, self‐costs to patients and loss of work productivity. | 3.1, 3.2, 3.3 |
| FD is associated with a significant decrease in quality of life and with psychosocial co‐morbidities. | 3.4, 3.5 |
| Weight loss can be consequence of FD. | 3.6 |
| Healthcare consulting behavior in FD is driven by symptom severity and impact, and by psychosocial co‐morbidities. | 3.8, 3.9 |
| H. pylori is a cause of symptoms in a subgroup of patients with dyspepsia and normal endoscopy. | 4.2 |
| Impaired gastric accommodation, delayed gastric emptying, hypersensitivity to gastric distention and disordered central processing of incoming signals from the gastroduodenal region are pathophysiological mechanisms in FD | 4.3, 4.4, 4.6, 4.15 |
| Upper GI endoscopy is mandatory for establishing a diagnosis of FD, but in primary care, dyspepsia can be managed without endoscopy if there are no alarm of risk factors. The endoscopy is mandatory if there are alarm symptoms or risk factors | 5.1, 5.2, 5.3 |
| Every patient with dyspeptic symptoms should be tested for H. pylori (Hp) (non‐invasively or at gastroscopy). H. pylori positive FD patients should receive eradication therapy. Patients with dyspepsia and H. pylori positive gastritis should be considered to have FD if symptoms persist 6 to 12 months after H. pylori eradication. Patients with dyspepsia and H. pylori negative gastritis should be considered to have FD. | 5.5, 5.6, 5.7, 6.2 |
| FD should be subdivided into EPS and PDS for further diagnostic and therapeutic approach | 5.8 |
| PPI‐therapy is an effective therapy for FD. | 6.4 |
| In case of severe weight loss in FD, nutritional support may be needed. | 6.27 |
| The long‐term prognosis is favorable in the majority of patients with FD, whose life expectancy is similar to that of the general population. | 7.1, 7.2 |
FIGURE 1.
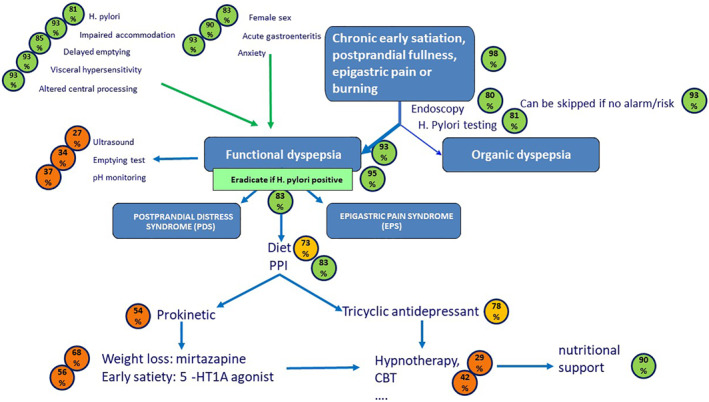
Schematic representation, in an algorithm‐like fashion, of the outcome of the consensus on functional dyspepsia management. The blue arrows depict the diagnostic and therapeutic flow of the patient. Green arrows refer to risk or pathophysiological factors. The circles depict the % of agreement, using a green color for ≥80% consensus, light orange for consensus between 70% and 80%, and dark orange for lower levels of consensus
The Rome IV definition with four cardinal symptoms (early satiation, postprandial fullness, epigastric pain, and epigastric burning), its subdivision into EPS and PDS, and accessory symptoms (upper abdominal bloating, nausea, belching) are well‐accepted. There is also consensus that the majority of uninvestigated dyspepsia in the general population represents FD, if an investigation would be done. Overlap with GERD and IBS is acknowledged. Also well accepted are the female predominance of FD, and acute gastrointestinal infections and anxiety as risk factors for developing FD. There is a clear consensus on the impact of FD on the personal level (quality of life, self‐cost), on healthcare costs, on the ability to work, and on psychosocial well‐being.
In terms of pathophysiological mechanisms that are relevant to FD, consensus supports a role for impaired gastric accommodation, delayed gastric emptying, hypersensitivity to gastric distention, H. pylori infection, and altered central processing of incoming signals from the gastroduodenal region. There is no consensus on duodenal mucosal alterations, sensitivity to luminal contents, peptide release, or microbiota. Anxiety is a risk factor for the development of functional dyspepsia; however, anxiety, depression, or stress is not considered a pathophysiological mechanism that underlies FD symptom generation.
There is consensus that endoscopy is mandatory for establishing a firm diagnosis of FD, but that patients in primary care with dyspeptic symptoms and no alarm symptoms or risk factors can be managed without endoscopy. There is consensus that endoscopy is mandatory in case of alarm symptoms or risk factors, and that H. pylori status should be determined at endoscopy or non‐invasively in every patient. There is no consensus on the benefit of additional examinations including laboratory testing, abdominal ultrasound, gastric emptying testing, or esophageal pH monitoring.
The biggest area of lack of consensus is the section on treatment approaches for FD. There is an agreement to use to subdivision in PDS and EPS to guide management, but the vast majority of treatment options are not supported for a specific subgroup. There is consensus to eradicate every H. pylori positive FD patient, and PPI therapy is considered an effective therapy for FD, although there is no consensus that it is the preferred initial therapy. There is no consensus on the indication and efficacy of prokinetics or antidepressants, but an almost‐agreement (78%) on the use of TCA in EPS. There is also no consensus on the use of other neuromodulators, herbal therapies, acupuncture, or psychological therapies in FD. There is agreement on the use of nutritional support in case of severe weight loss. Finally, there is consensus that the long‐term prognosis of FD is favorable and that life expectancy is not shortened in FD.
The areas of uncertainty revealed by this consensus are multiple. Further unraveling of the FD symptom pattern is useful, and especially the concept of using pictograms deserves additional, preferably multilingual and multicultural studies. These may aid further diagnostic refinement, where the currently only supported tools are endoscopy and H. pylori status assessments, with their limited sensitivity and impact on management and outcome. While there is acceptance of a role for gastric sensorimotor dysfunction in triggering FD symptoms, the role of duodenal luminal or mucosal alterations is not established, but the 76% amount of agreement is still considerable. The putative entity of H. pylori‐associated dyspepsia is poorly described and needs further characterizing studies, especially in the West. The voting on the therapy statements clearly establishes the need for more therapeutic trials, preferably in a multi‐center setting using validated endpoints. In the absence of a well‐established path to regulatory approval, scientific or professional organizations such as the ESNM or the Rome Foundation may consider taking the lead here.
CONCLUSION
FD is a highly prevalent and impactful clinical condition. This Delphi process used a multinational and multidisciplinary group of European experts to summarize the current state of consensus on definition, symptom characteristics, pathophysiology, diagnosis, treatment, and prognosis of this condition. The Consensus Group voted on several statements that may guide clinicians in recognizing, diagnosing, and treating FD in clinical practice, whereas the statements without consensus identify areas in need of future research.
AUTHOR CONTRIBUTIONS
Drafted consensus questions, drafted literature review sections, set up the voting and summaries access, participated in voting, summarized voting outcomes, reviewed and corrected the manuscript: L. Wauters. Drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript: R. Dickman. Drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript: V. Drug. Drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript: A. Mulak. Drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript: J. Serra. Co‐initiated the process, obtained funding , drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected manuscript content: P. Enck. Co‐initiated the process, drafted consensus questions, drafted literature review sections, wrote manuscript sections, participated in voting, wrote manuscript, reviewed and corrected content: J. Tack. Drafted consensus questions, wrote manuscript sections, participated in voting, reviewed and corrected content: All members of the ESNM FD consensus group (A. Accarino, G. Barbara, S. Bor, B. Coffin, M. Corsetti, H. De Schepper, D. Dumitrascu, A. Farmer, G. Gourcerol, G. Hauser, T. Hausken, G. Karamanolis, D. Kestzhelyi, C. Malagelada, T. Milosavljevic, J. Muris, C. O'Morain, A. Papathanasopoulos, D. Pohl, D. Rumyantseva, G. Sarnelli, E. Savarino, J. Schol, A. Sheptulin, A. Smet, A. Stengel, O. Storonova, M. Storr, H. Törnblom, T. Vanuytsel, M. Velosa, M. Waluga, N. Zarate, F. Zerbib).
ACKNOWLEDGEMENT
This consensus was supported by a grant from United European Gastroenterology.
REFERENCES
- 1. Stanghellini V, Talley N, Chan F, Hasler B, Malagelada J, Suzuki H, et al. Functional gastroduodenal disorders. Gastroenterology. 2016;150(6):1380‐92. [DOI] [PubMed] [Google Scholar]
- 2. Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. 2020;S0016‐5085:30487. 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Tack J, Talley NJ. Functional dyspepsia−symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10:134‐41. [DOI] [PubMed] [Google Scholar]
- 4. Ford AC, Marwaha A, Lim A, Moayyedi P. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2010;8(10):830‐7. [DOI] [PubMed] [Google Scholar]
- 5. Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta‐analysis. Gastroenterology. 2006;131(2):390‐401. [DOI] [PubMed] [Google Scholar]
- 6. Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol. 2018;43:111‐7. [DOI] [PubMed] [Google Scholar]
- 7. Van den Houte K, Carbone F, Tack J. Postprandial distress syndrome: stratification and management. Expert Rev Gastroenterol Hepatol. 2019;3(4):337‐43. [DOI] [PubMed] [Google Scholar]
- 8. Mullen PM. Delphi: myths and reality. J Health Organ Manag. 2003;17:37‐52. [DOI] [PubMed] [Google Scholar]
- 9. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
- 10. Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466‐79. [DOI] [PubMed] [Google Scholar]
- 11. Van den Houte K, Carbone F, Goelen N, Schol J, Masuy I, Arts J, et al. Effects of Rome IV definitions of functional dyspepsia subgroups in secondary care. Clin Gastroenterol Hepatol. 2020;620:S1542‐3565. 10.1016/j.cgh.2020.06.043. Online ahead of print.30906‐X [DOI] [PubMed] [Google Scholar]
- 12. Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle‐Bisset C, Holtmann G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081. [DOI] [PubMed] [Google Scholar]
- 13. Geeraerts A, Van Houtte B, Clevers E, Geysen H, Vanuytsel T, Tack J, et al. Gastroesophageal reflux disease‐functional dyspepsia overlap: do birds of a feather flock together? Am J Gastroenterol. 2020;115(8):1167‐82. [DOI] [PubMed] [Google Scholar]
- 14. Pleyer C, Bittner H, Locke GR, 3rd , Choung RS, Zinsmeister AR, Schleck CD, et al. Overdiagnosis of gastro‐esophageal reflux disease and underdiagnosis of functional dyspepsia in a USA community. Neuro Gastroenterol Motil. 2014;26(8):1163‐71. [DOI] [PubMed] [Google Scholar]
- 15. Quigley EM, Lacy BE. Overlap of functional dyspepsia and GERD−‐diagnostic and treatment implications. Nat Rev Gastroenterol Hepatol. 2013;10(3):175‐86. [DOI] [PubMed] [Google Scholar]
- 16. de Bortoli N, Tolone S, Frazzoni M, Martinucci I, Sgherri G, Albano E, et al. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: common overlapping gastrointestinal disorders. Ann Gastroenterol. 2018;31(6):639‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and metA−analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010;8(5):401‐9. [DOI] [PubMed] [Google Scholar]
- 18. Tack J, Carbone F, Holvoet L, Vanheel H, Vanuytsel T, Vandenberghe A. The use of pictograms improves symptom evaluation by patients with functional dyspepsia. Aliment Pharmacol Ther. 2014;40(5):523‐30. [DOI] [PubMed] [Google Scholar]
- 19. Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta‐analysis. Gut. 2015;64(7):1049‐57. [DOI] [PubMed] [Google Scholar]
- 20. Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A. Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. Domestic/International Gastroenterology Surveillance Study. Am J Gastroenterol. 1999;94(10):2845‐54. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Nie Y, Sha W, Su H. The link between psychosocial factors and functional dyspepsia: an epidemiological study. Chin Med J (Engl). 2002;115(7):1082‐4. [PubMed] [Google Scholar]
- 22. Hirakawa K, Adachi K, Amano K, Katsube T, Ishihara S, Fukuda R, et al. Prevalence of non‐ulcer dyspepsia in the Japanese population. J Gastroenterol Hepatol. 1999;14(11):1083‐7. [DOI] [PubMed] [Google Scholar]
- 23. Futagami S, Itoh T, Sakamoto C. Systematic review with metA−analysis: post‐infectious functional dyspepsia. Aliment Pharmacol Ther. 2015;41(2):177‐88. [DOI] [PubMed] [Google Scholar]
- 24. Porter CK, Faix DJ, Shiau D, Espiritu J, Espinosa BJ, Riddle MS. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis. 2012;55(7):915‐22. [DOI] [PubMed] [Google Scholar]
- 25. Dizdar V, Gilja OH, Hausken T. Increased visceral sensitivity in GiardiA−induced postinfectious irritable bowel syndrome and functional dyspepsia. Effect of the 5HT3‐antagonist ondansetron. Neuro Gastroenterol Motil. 2007;19(12):977‐82. [DOI] [PubMed] [Google Scholar]
- 26. Ford AC, Thabane M, Collins SM, Moayyedi P, Garg AX, Clark WF, et al. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: a cohort study. Gastroenterology. 2010;138(5):1727‐36. [DOI] [PubMed] [Google Scholar]
- 27. Tack J, Demedts I, Dehondt G, Caenepeel P, Fischler B, Zandecki M, et al. Clinical and pathophysiological characteristics of acute‐onset functional dyspepsia. Gastroenterology. 2002;122(7):1738‐47. [DOI] [PubMed] [Google Scholar]
- 28. Shaib Y, El‐Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99(11):2210‐6. [DOI] [PubMed] [Google Scholar]
- 29. Moayyedi P, Forman D, Braunholtz D, Feltbower R, Crocombe W, Liptrott M, et al. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti‐inflammatory drugs. Leeds HELP Study Group. Am J Gastroenterol. 2000;95(6):1448‐55. [DOI] [PubMed] [Google Scholar]
- 30. Holtmann G, Gschossmann J, Buenger L, Gerken G, Talley NJ. Do changes in visceral sensory function determine the development of dyspepsia during treatment with aspirin? Gastroenterology. 2002;123(5):1451‐8. [DOI] [PubMed] [Google Scholar]
- 31. Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12(17):2661‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paula H, Grover M, Halder SL, Locke GR, 3rd , Schleck CD, Zinsmeister AR, et al. Non‐enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neuro Gastroenterol Motil. 2015;27(11):1580‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lacy BE, Everhart K, Crowell MD. Functional dyspepsia is associated with sleep disorders. Clin Gastroenterol Hepatol. 2011;9(5):410‐4. [DOI] [PubMed] [Google Scholar]
- 34. Quadri A, Vakil N. Health‐related anxiety and the effect of open‐access endoscopy in US patients with dyspepsia. Aliment Pharmacol Ther. 2003;17(6):835‐40. [DOI] [PubMed] [Google Scholar]
- 35. Mak AD, Wu JC, Chan Y, Chan FK, Sung JJ, Lee S. Dyspepsia is strongly associated with major depression and generalised anxiety disorder ‐ a community study. Aliment Pharmacol Ther. 2012;36(8):800‐10. [DOI] [PubMed] [Google Scholar]
- 36. Hu WH, Wong WM, Lam CL, Lam KF, Hui WM, Lai KC, et al. Anxiety but not depression determines health care‐seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population‐based study. Aliment Pharmacol Ther. 2002;16(12):2081‐8. [DOI] [PubMed] [Google Scholar]
- 37. Mujakovic S, de Wit NJ, van Marrewijk CJ, Fransen GA, Laheij RJ, Muris JW, et al. Psychopathology is associated with dyspeptic symptom severity in primary care patients with a new episode of dyspepsia. Aliment Pharmacol Ther. 2009;29(5):580‐8. [DOI] [PubMed] [Google Scholar]
- 38. Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Fischler B, et al. Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation? Gut. 2008;57(12):1666‐73. [DOI] [PubMed] [Google Scholar]
- 39. Koloski NA, Jones M, Talley NJ. Evidence that independent gut‐to‐brain and brain‐to‐gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1‐year population‐based prospective study. Aliment Pharmacol Ther. 2016;44(6):592‐600. [DOI] [PubMed] [Google Scholar]
- 40. Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simrén M. Epidemiology, clinical characteristics, and associations for symptom‐based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross‐sectional population‐based study. Lancet Gastroenterol Hepatol. 2018;3(4):252‐62. [DOI] [PubMed] [Google Scholar]
- 41. Aro P, Talley NJ, Ronkainen J, Storskrubb T, Vieth M, Johansson SE, et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population‐based study. Gastroenterology. 2009;137(1):94‐100. [DOI] [PubMed] [Google Scholar]
- 42. Aro P, Talley NJ, Johansson SE, Agréus L, Ronkainen J. Anxiety is linked to new‐onset dyspepsia in the Swedish population: a 10‐year follow‐up study. Gastroenterology. 2015;148(5):928‐37. [DOI] [PubMed] [Google Scholar]
- 43. Jones MP, Oudenhove LV, Koloski N, Tack J, Talley NJ. Early life factors initiate a ‘vicious circle’ of affective and gastrointestinal symptoms: a longitudinal study. United European Gastroenterol J. 2013;1(5):394‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De la RocA−Chiapas JM, Solís‐Ortiz S, Fajardo‐Araujo M, Sosa M, CórdovA−Fraga T, RosA−Zarate A. Stress profile, coping style, anxiety, depression, and gastric emptying as predictors of functional dyspepsia: a case‐control study. J Psychosom Res. 2010;68(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 45. Filipović BF, Randjelovic T, Ille T, Markovic O, Milovanović B, Kovacevic N, et al. Anxiety, personality traits and quality of life in functional dyspepsiA−suffering patients. Eur J Intern Med. 2013;24(1):83‐6. [DOI] [PubMed] [Google Scholar]
- 46. Ly HG, Weltens N, Tack J, Van Oudenhove L. Acute anxiety and anxiety disorders are associated with impaired gastric accommodation in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2015;13(9):1584‐91.e3. [DOI] [PubMed] [Google Scholar]
- 47. Pinto‐Sanchez MI, Ford AC, Avila CA, Verdu EF, Collins SM, Morgan D, et al. Anxiety and depression increase in a stepwise manner in parallel with multiple FGIDs and symptom severity and frequency. Am J Gastroenterol. 2015;110(7):1038‐48. [DOI] [PubMed] [Google Scholar]
- 48. Kindt S, Van Oudenhove L, Mispelon L, Caenepeel P, Arts J, Tack J. Longitudinal and cross‐sectional factors associated with long‐term clinical course in functional dyspepsia: a 5‐year follow‐up study. Am J Gastroenterol. 2011;106(2):340‐8. [DOI] [PubMed] [Google Scholar]
- 49. Bernersen B, Johnsen R, Straume B. Non‐ulcer dyspepsia and peptic ulcer: the distribution in a population and their relation to risk factors. Gut. 1996;38(6):822‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zagari RM, Law GR, Fuccio L, Cennamo V, Gilthorpe MS, Forman D, et al. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010;138(4):1302‐11. [DOI] [PubMed] [Google Scholar]
- 51. Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Thjodleifsson B. Natural history of functional dyspepsia: a 10‐year population‐based study. Digestion. 2010;81(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 52. Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ, 3rd . Smoking, alcohol, and analgesics in dyspepsia and among dyspepsia subgroups: lack of an association in a community. Gut. 1994;35(5):619‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haque M, Wyeth JW, Stace NH, Talley NJ, Green R. Prevalence, severity and associated features of gastro‐oesophageal reflux and dyspepsia: a population‐based study. N Z Med J. 2000;113(1110):178‐81. [PubMed] [Google Scholar]
- 54. Chang FY, Chen PH, Wu TC, Pan WH, Chang HY, Wu SJ, et al. Prevalence of functional gastrointestinal disorders in Taiwan: questionnaire‐based survey for adults based on the Rome III criteria. Asia Pac J Clin Nutr. 2012;21(4):594‐600. [PubMed] [Google Scholar]
- 55. Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38(2):170‐7. [DOI] [PubMed] [Google Scholar]
- 56. Brook RA, Kleinman NL, Choung RS, Melkonian AK, Smeeding JE, Talley NJ. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8(6):498‐503. [DOI] [PubMed] [Google Scholar]
- 57. Piessevaux H, de Winter B, Louis E, Muls V, de Looze D, Pelckmans P, et al. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neuro Gastroenterol Motil. 2009;21(4):378‐88. [DOI] [PubMed] [Google Scholar]
- 58. Mahadeva S, Ford AC. Clinical and epidemiological differences in functional dyspepsia between the East and the West. Neuro Gastroenterol Motil. 2016;28(2):167‐74. [DOI] [PubMed] [Google Scholar]
- 59. Sander GB, Mazzoleni LE, Francesconi CF, Balbinotto G, Mazzoleni F, Wortmann AC, et al. Helicobacter eradication relief of dyspetic symptoms trial investigators. Influence of organic and functional dyspepsia on work productivity: the HEROES‐DIP study. Value Health. 2011;14(5 Suppl 1):S126‐9. [DOI] [PubMed] [Google Scholar]
- 60. Hantoro IF, Syam AF, Mudjaddid E, Setiati S, Abdullah M. Factors associated with health‐related quality of life in patients with functional dyspepsia. Health Qual Life Outcomes. 2018;16(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aro P, Talley NJ, Agréus L, Johansson SE, Bolling‐Sternevald E, Storskrubb T, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33(11):1215‐24. [DOI] [PubMed] [Google Scholar]
- 62. Van Oudenhove L, Vandenberghe J, Vos R, Holvoet L, Demyttenaere K, Tack J. Risk factors for impaired health‐related quality of life in functional dyspepsia. Aliment Pharmacol Ther. 2011;33(2):261‐74. [DOI] [PubMed] [Google Scholar]
- 63. Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346‐52. [DOI] [PubMed] [Google Scholar]
- 64. Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms accosiated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526‐35. [DOI] [PubMed] [Google Scholar]
- 65. Jones MP, Talley NJ, Eslick GD, Dubois D, Tack J. Community subgroups in dyspepsia and their association with weight loss. Am J Gastroenterol. 2008;103(8):2051‐60. [DOI] [PubMed] [Google Scholar]
- 66. Tack J, Jones MP, Karamanolis G, Coulie B, Dubois D. Symptom pattern and pathophysiological correlates of weight loss in tertiary‐referred functional dyspepsia. Neuro Gastroenterol Motil. 2010;22(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 67. Tack J, Caenepeel P, Piessevaux H, Cuomo R, Janssens J. Assessment of meal‐induced gastric accommodation by a satiety drinking test in health and in functional dyspepsia. Gut. 2003;52:1271‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ford AC, Forman D, Bailey AG, Cook MB, Axon AT, Moayyedi P. Who consults with dyspepsia? Results from a longitudinal 10‐yr follow‐up study. Am J Gastroenterol. 2007;102(5):957‐65. [DOI] [PubMed] [Google Scholar]
- 69. Ahlawat SK, Richard Locke G, Weaver AL, Farmer SA, Yawn BP, Talley NJ. Dyspepsia consulters and patterns of management: a population‐based study. Aliment Pharmacol Ther. 2005;22(3):251‐9. [DOI] [PubMed] [Google Scholar]
- 70. Westbrook JI, McIntosh J, Talley NJ. Factors associated with consulting medical or non‐medical practitioners for dyspepsia: an australian population‐based study. Aliment Pharmacol Ther. 2000;14(12):1581‐8. [DOI] [PubMed] [Google Scholar]
- 71. Jones MP, Tack J, Van Oudenhove L, Walker MM, Holtmann G, Koloski NA, et al. Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin Gastroenterol Hepatol. 2017;15(7):1014‐20. [DOI] [PubMed] [Google Scholar]
- 72. Hungin AP, Hill C, Raghunath A. Systematic review: frequency and reasons for consultation for gastro‐oesophageal reflux disease and dyspepsia. Aliment Pharmacol Ther. 2009;30(4):331‐42. [DOI] [PubMed] [Google Scholar]
- 73. Castillo EJ, Camilleri M, Locke GR, Burton DD, Stephens DA, Geno DM, et al. A community‐based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin Gastroenterol Hepatol. 2004;2(11):985‐96. [DOI] [PubMed] [Google Scholar]
- 74. Bisschops R, Karamanolis G, Arts J, Caenepeel P, Verbeke K, Janssens J, et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. 2008;57(11):1495‐503. [DOI] [PubMed] [Google Scholar]
- 75. Mullan A, Kavanagh P, O'Mahony P, Joy T, Gleeson F, Gibney MJ. Food and nutrient intakes and eating patterns in functional and organic dyspepsia. Eur J Clin Nutr. 1994;48(2):97‐105. [PubMed] [Google Scholar]
- 76. Pilichiewicz AN, Horowitz M, Holtmann GJ, Talley NJ, Feinle‐Bisset C. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2009;7(3):317‐22. [DOI] [PubMed] [Google Scholar]
- 77. Carvalho RV, Lorena SL, Almeida JR, Mesquita MA. Food intolerance, diet composition, and eating patterns in functional dyspepsia patients. Dig Dis Sci. 2010;55(1):60‐5. [DOI] [PubMed] [Google Scholar]
- 78. Saito YA, Locke GR, 3rd , Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population‐based case‐control study. Am J Gastroenterol. 2005;100(12):2743‐8. [DOI] [PubMed] [Google Scholar]
- 79. Duncanson KR, Talley NJ, Walker MM, Burrows TL. Food and functional dyspepsia: a systematic review. J Hum Nutr Diet. 2018;31(3):390‐407. [DOI] [PubMed] [Google Scholar]
- 80. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. European Helicobacter and microbiota study group and consensus panel. Management of Helicobacter pylori infection – the Maastricht V/Florence consensus report. Gut. 2017;66(1):6‐30. [DOI] [PubMed] [Google Scholar]
- 81. Sugano K, Tack J, Kuipers EJ, Graham DY, El‐Omar EM, Miura S, et al. Faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut;64:1353‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Friedman LS. Helicobacter Pylori and nonulcer dyspepsia. NEJM. 1998;339:1928‐30. [DOI] [PubMed] [Google Scholar]
- 83. Troncon LEA, Bennett RJM, Ahluwalia NK, Thompson DG. Abnormal distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gilja OH, Hausken T, Wilhelmsen I, Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689‐96. [DOI] [PubMed] [Google Scholar]
- 85. Salet GAM, Samsom M, Roelofs JMM, van Berge Henegouwen GP, Smout AJPM, Akkermans LMA. Responses to gastric distention in functional dyspepsia. Gut. 1998;42:823‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boeckxstaens GE, Hirsch DP, van den Elzen BD, Heisterkamp SH, Tytgat GN. Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology. 2001;121(5):1054‐63. [DOI] [PubMed] [Google Scholar]
- 87. Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1(4):264‐72. [PubMed] [Google Scholar]
- 88. Lunding JA, Gilja OH, Hausken T, Bayati A, Mattsson H, Berstad A. Distension‐induced gastric accommodation in functional dyspepsia: effect of autonomic manipulation. Neuro Gastroenterol Motil. 2007;19(5):365‐75. [DOI] [PubMed] [Google Scholar]
- 89. Steinsvik EK, Hausken T, Gilja OH. The ultrasound meal accommodation test in 509 patients with functional gastrointestinal disorders. Scand J Gastroenterol. 2016 Jul;51(7):788‐94. [DOI] [PubMed] [Google Scholar]
- 90. Asano H, Tomita T, Nakamura K, Yamasaki T, Okugawa T, Kondo T, et al. Prevalence of gastric motility disorders in patients with functional dyspepsia. J Neurogastroenterol Motil. 2017;23(3):392‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vanheel H, Carbone F, Valvekens L, Simren M, Tornblom H, Vanuytsel T, et al. Pathophysiological abnormalities in functional dyspepsia subgroups according to the Rome III criteria. Am J Gastroenterol. 2017;112(1):132‐40. [DOI] [PubMed] [Google Scholar]
- 92. Mundt MW, Samsom M. Fundal dysaccommodation in functional dyspepsia: heaD−to‐head comparison between the barostat and three‐dimensional ultrasonographic technique. Gut. 2006;55(12):1725‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caballero‐Plasencia AM, Muros‐Navarro MC, Martín‐Ruiz JL, ValenzuelA−Barranco M, de los Reyes‐García MC, Casado‐Caballero FJ, et al. Dyspeptic symptoms and gastric emptying of solids in patients with functional dyspepsia. Role of Helicobacter pylori infection. Scand J Gastroenterol. 1995;30(8):745‐51. [DOI] [PubMed] [Google Scholar]
- 94. Haag S, Talley NJ, Holtmann G. Symptom patterns in functional dyspepsia and irritable bowel syndrome: relationship to disturbances in gastric emptying and response to a nutrient challenge in consulters and non‐consulters. Gut. 2004;53(10):1445‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pfeiffer A, Aronbayev J, Schmidt T, Wendl B, Pehl C, Kaess H. Gastric emptying, esophageal 24‐hour pH and gastric potential difference measurements in non‐ulcer dyspepsia. Gastroenterol Clin Biol. 1992;16(5):395‐400. [PubMed] [Google Scholar]
- 96. Quartero AO, de Wit NJ, Lodder AC, Numans ME, Smout AJ, Hoes AW. Disturbed soliD−phase gastric emptying in functional dyspepsia: a metA−analysis. Dig Dis Sci. 1998;43(9):2028‐33. [DOI] [PubMed] [Google Scholar]
- 97. Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli‐Labate AM, Monetti N, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110(4):1036‐42. [DOI] [PubMed] [Google Scholar]
- 98. Perri F, Clemente R, Festa V, Annese V, Quitadamo M, Rutgeerts P, et al. Patterns of symptoms in functional dyspepsia: role of Helicobacter pylori infection and delayed gastric emptying. Am J Gastroenterol. 1998;93(11):2082‐8. [DOI] [PubMed] [Google Scholar]
- 99. Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with delayed emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783‐8. [DOI] [PubMed] [Google Scholar]
- 100. Cuomo R, Sarnelli G, Grasso R, Alfieri M, Niccolai E, Pumpo R, et al. Functional dyspepsia symptoms, gastric emptying and satiety provocative test: analysis of relationships. Scand J Gastroenterol. 2001;36:1030‐6. [DOI] [PubMed] [Google Scholar]
- 101. Delgado‐Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127(6):1685‐94. [DOI] [PubMed] [Google Scholar]
- 102. Tominaga K, Higuchi K, Ochi M, Kadouchi K, Kawamura E, Tanigawa T, et al. Concurrent assessment of reservoir and emptying of the stomach for dyspepsia patients. Hepatogastroenterology. 2008;55(82–83):744‐9. [PubMed] [Google Scholar]
- 103. Coffin B, Azpiroz F, Guarner F, Malagelada JR. Selective gastric hypersensitivity and reflex hyporeactivity in functional dyspepsia. Gastroenterology. 1994;107(5):1345‐51. [DOI] [PubMed] [Google Scholar]
- 104. Mertz H, Fullerton S, Naliboff B, Mayer EA. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Holtmann G, Gschossmann J, Neufang‐Huber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non‐consulters with dyspepsia and healthy controls. Gut. 2000;47:332‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rhee PL, Kim YH, Son HJ, Kim JJ, Koh KC, Paik SW, et al. Evaluation of individual symptoms acannot predict presence of gastric hypersensitivity in functional dyspepsia. Dig. Dis. Sci. 2000. 2000;45:1680‐4. [DOI] [PubMed] [Google Scholar]
- 107. Farré R, Vanheel H, Vanuytsel T, Masaoka T, Törnblom H, Simrén M, et al. Functional dyspepsia, hypersensitivity to postprandial distention correlates with meal‐related symptom severity. Gastroenterology. 2013;145(3):566‐73. [DOI] [PubMed] [Google Scholar]
- 108. Simrén M, Törnblom H, Palsson OS, van Tilburg MA, Van Oudenhove L, Tack J, et al. visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. 2018;67(2):255‐62. [DOI] [PubMed] [Google Scholar]
- 109. Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, et al. Non‐ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population‐based case‐control study. Clin Gastroenterol Hepatol. 2007;5(10):1175‐83. [DOI] [PubMed] [Google Scholar]
- 110. Vanheel H, Vicario M, Vanuytsel T, Van Oudenhove L, Martinez C, Keita AV, et al. Impaired duodenal mucosal integrity and low‐grade inflammation in functional dyspepsia. Gut. 2014;63(2):262‐71. [DOI] [PubMed] [Google Scholar]
- 111. Walker MM, Aggarwal KR, Shim LS, Bassan M, Kalantar JS, Weltman MD, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 2014;29(3):474‐9. [DOI] [PubMed] [Google Scholar]
- 112. Cirillo C, Bessissow T, Desmet AS, Vanheel H, Tack J, Vanden Berghe P. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol. 2015;110(8):1205‐15. [DOI] [PubMed] [Google Scholar]
- 113. Vanheel H, Vicario M, Boesmans W, Vanuytsel T, Salvo‐Romero E, Tack J, et al. Activation of eosinophils and mast cells in functional dyspepsia: an ultrastructural evaluation. Sci Rep. 2018;8(1):5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wauters L, Talley NJ, Walker MM, Tack J, Vanuytsel T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69(3):591‐600. 10.1136/gutjnl-2019-318536. [DOI] [PubMed] [Google Scholar]
- 115. Wauters L, Burns G, Ceulemans M, et al. Duodenal inflammation: an emerging target for functional dyspepsia? Expert Opin Ther Targets. 2020;24(6):511‐23. 10.1080/14728222.2020.1752181. [DOI] [PubMed] [Google Scholar]
- 116. Kindt S, van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neuro Gastroenterol Motil. 2009;21(4):389‐98. [DOI] [PubMed] [Google Scholar]
- 117. Kindt S, Tertychnyy A, de Hertogh G, Geboes K, Tack J. Intestinal immune activation in presumed post‐infectious functional dyspepsia. Neuro Gastroenterol Motil. 2009;21(8):832.e56. [DOI] [PubMed] [Google Scholar]
- 118. Collen MJ, Loebenberg MJ. Basal gastric acid secretion in nonulcer dyspepsia with or without duodenitis. Dig Dis Sci. 1989 Feb;34(2):246‐50. [DOI] [PubMed] [Google Scholar]
- 119. Lee KJ, Demarchi B, Demedts I, Sifrim D, Raeymaekers P, Tack J. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol. 2004;99:1765‐73. [DOI] [PubMed] [Google Scholar]
- 120. Bratten J, Jones MP. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J Clin Gastroenterol. 2009;43(6):527‐33. [DOI] [PubMed] [Google Scholar]
- 121. Van den Houte K, Scarpellini E, Verbeure W, Mori H, Schol J, Masuy I, et al. The role of GI peptides in functional dyspepsia and gastroparesis: a systematic review. Front Psychiatry. 2020;11:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103(10):2613‐23. [DOI] [PubMed] [Google Scholar]
- 123. Chua AS, Bekkering M, Rovati LC, Keeling PW. Clinical efficacy and prokinetic effect of the CCK‐A antagonist loxiglumide in nonulcer dyspepsia. Ann N Y Acad Sci. 1994;713:451‐3. [DOI] [PubMed] [Google Scholar]
- 124. Feinle C, Meier O, Otto B, D'Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001 Mar;48(3):347‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Choi YJ, Park YS, Kim N, Kim YS, Lee SM, Lee DH, et al. Gender differences in ghrelin, nociception genes, psychological factors and quality of life in functional dyspepsia. World J Gastroenterol. 2017;23(45):8053‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shindo T, Futagami S, Hiratsuka T, Horie A, Hamamoto T, Ueki N, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non‐erosive reflux disease. Digestion. 2009;79(2):65‐72. [DOI] [PubMed] [Google Scholar]
- 127. Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis. 2014;32(3):222‐9. [DOI] [PubMed] [Google Scholar]
- 128. Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51 (Suppl 1):i54‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116(3):515‐20. [DOI] [PubMed] [Google Scholar]
- 130. Schwartz MP, Samsom M, Smout AJ. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am J Gastroenterol. 2001;96(9):2596‐602. [DOI] [PubMed] [Google Scholar]
- 131. Zhong L, Shanahan ER, Raj A, Koloski NA, Fletcher L, Morrison M, et al. Dyspepsia and the microbiome: time to focus on the small intestine. Gut. 2017;66(6):1168‐9. [DOI] [PubMed] [Google Scholar]
- 132. Nakae H, Tsuda A, Matsuoka T, Mine T, Koga Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol. 2016;3(1).e000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2(6):650‐6. [DOI] [PubMed] [Google Scholar]
- 134. Holtmann G, Goebell H, Jockenhoevel F, Talley NJ. Altered vagal and intestinal mechanosensory function in chronic unexplained dyspepsia. Gut. 1998;42(4):501‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Manabe N, Nakamura K, Hara M, Imamura H, Kusunoki H, Tanaka S, et al. Impaired gastric response to modified sham feeding in patients with postprandial distress syndrome. Neuro Gastroenterol Motil. 2011;23(3):215‐9:e112. [DOI] [PubMed] [Google Scholar]
- 136. Lunding JA, Nordström LM, Haukelid AO, Gilja OH, Berstad A, Hausken T. Vagal activation by sham feeding improves gastric motility in functional dyspepsia. Neuro Gastroenterol Motil. 2008;20(6):618‐24. [DOI] [PubMed] [Google Scholar]
- 137. Hjelland IE, Svebak S, Berstad A, Flatabø G, Hausken T. Breathing exercises with vagal biofeedback may benefit patients with functional dyspepsia. Scand J Gastroenterol. 2007;42(9):1054‐62. [DOI] [PubMed] [Google Scholar]
- 138. Guo WJ, Yao SK, Zhang YL, Du SY, Wang HF, Yin LJ, et al. Impaired vagal activity to meal in patients with functional dyspepsia and delayed gastric emptying. J Int Med Res. 2018;46(2):792‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Van Oudenhove L, Vandenberghe J, Dupont P, Geeraerts B, Vos R, Dirix S, et al. Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O‐PET study. Am J Gastroenterol. 2010;105(4):913‐24. [DOI] [PubMed] [Google Scholar]
- 140. Liu P, Zeng F, Zhou G, Wang J, Wen H, von Deneen KM, et al. Alterations of the default mode network in functional dyspepsia patients: a resting‐state fmri study. Neuro Gastroenterol Motil. 2013;25(6):e382‐8. [DOI] [PubMed] [Google Scholar]
- 141. Nan J, Liu J, Mu J, Dun W, Zhang M, Gong Q, et al. Brain‐based correlations between psychological factors and functional dyspepsia. J Neurogastroenterol Motil. 2015;21(1):103‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tominaga K, Tsumoto C, Ataka S, Mizuno K, Takahashi K, Yamagami H, et al. Regional brain disorders of serotonin neurotransmission are associated with functional dyspepsia. Life Sci. 2015;137:150‐7. [DOI] [PubMed] [Google Scholar]
- 143. Ly HG, Ceccarini J, Weltens N, Bormans G, Van Laere K, Tack J, et al. Increased cerebral cannabinoiD−1 receptor availability is a stable feature of functional dyspepsia: a [F]MK‐9470 PET study. Psychother Psychosom. 2015;84(3):149‐58. [DOI] [PubMed] [Google Scholar]
- 144. Lee IS, Wang H, Chae Y, Preissl H, Enck P. Functional neuroimaging studies in functional dyspepsia patients: a systematic review. Neuro Gastroenterol Motil. 2016;28(6):793‐805. [DOI] [PubMed] [Google Scholar]
- 145. Locke GR, 3rd , Zinsmeister AR, Talley NJ, Fett SL, Melton LJ, 3rd . Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75(9):907‐12. [DOI] [PubMed] [Google Scholar]
- 146. Dai F, Liu Y, Shi H, Ge S, Song J, Dong L, et al. Association of genetic variants in GNβ3 with functional dyspepsia: a metA−analysis. Dig Dis Sci. 2014 Aug;59(8):1823‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Song YZ, You HY, Zhu ZH, Wen ZD, Xu HY, Chen BC, et al. The C825T polymorphism of the G‐protein β3 gene as a risk factor for functional dyspepsia: a metA−analysis. Gastroenterol Res Pract. 2016;2016.5037254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kourikou A, Karamanolis GP, Dimitriadis GD, Triantafyllou K. Gene polymorphisms associated with functional dyspepsia. World J Gastroenterol. 2015;21(25):7672‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129(5):1756‐80. [DOI] [PubMed] [Google Scholar]
- 150. Moayyedi P, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017 Jul;112(7):988‐1013. [DOI] [PubMed] [Google Scholar]
- 151. Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia? J Am Med Assoc. 2006;295(13):1566‐76. [DOI] [PubMed] [Google Scholar]
- 152. Dutta AK, Rebekah G, Chowdhury SD, Gangadharan SK, Subramani Y, Sahu MK, et al. A simple pre‐endoscopy score for predicting risk of malignancy in patients with dyspepsia: a 5‐year prospective study. Dig Dis Sci. 2018 Dec;63(12):3442‐7. [DOI] [PubMed] [Google Scholar]
- 153. Feld L, Cifu AS. Management of dyspepsia. J Am Med Assoc. 2018;319(17):1816‐7. [DOI] [PubMed] [Google Scholar]
- 154. Duggan AE, Elliott CA, Miller P, Hawkey CJ, Logan RF. Clinical trial: a randomized trial of early endoscopy, Helicobacter pylori testing and empirical therapy for the management of dyspepsia in primary care. Aliment Pharmacol Ther. 2009 Jan;29(1):55‐68. [DOI] [PubMed] [Google Scholar]
- 155. Jönsson KA, Gotthard R, Bodemar G, Brodin U. The clinical relevance of endoscopic and histologic inflammation of gastroduodenal mucosa in dyspepsia of unknown origin. Scand J Gastroenterol. 1989;24(4):385‐95. [DOI] [PubMed] [Google Scholar]
- 156. Talley NJ. Functional dyspepsia and the Rome criteria: a success story. Neuro Gastroenterol Motil. 2015;27(8):1052‐6. [DOI] [PubMed] [Google Scholar]
- 157. Carbone F, Holvoet L, Tack J. Rome III functional dyspepsia subdivision in PDS and EPS: recognizing postprandial symptoms reduces overlap. Neuro Gastroenterol Motil. 2015;27(8):1069‐74. [DOI] [PubMed] [Google Scholar]
- 158. Carbone F, Vanuytsel T, Tack J. Analysis of postprandial symptom patterns in subgroups of patients with Rome III or Rome IV functional dyspepsia. Clin Gastroenterol Hepatol. 2020;18(4):838‐46.e3. [DOI] [PubMed] [Google Scholar]
- 159. Suzuki H, Kusunoki H, Kamiya T, Futagami S, Yamaguchi Y, Nishizawa T, et al. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): a multicentre, prospective, randomized, double‐blind, placebo‐controlled clinical trial. United Eur Gastroenterol J. 2013;1(6):445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Xu S, Wan X, Zheng X, Zhou Y, Song Z, Cheng M, et al. Symptom improvement after helicobacter pylori eradication in patients with functional dyspepsia—a multicenter, randomized, prospective cohort study. Int J Clin Exp Med. 2015;6(9):747‐56. [PMC free article] [PubMed] [Google Scholar]
- 161. Hsu YC, Liou JM, Yang TH, Hsu WL, Lin HJ, Wu HT, et al. Proton pump inhibitor versus prokinetic therapy in patients with functional dyspepsia: is therapeutic response predicted by Rome III subgroups? J Gastroenterol. 2011;46(2):183‐90. [DOI] [PubMed] [Google Scholar]
- 162. Kamiya T, Shikano M, Kubota E, Mizoshita T, Wada T, Tanida S, et al. A multicenter randomized trial comparing rabeprazole and itopride in patients with functional dyspepsia in Japan: the NAGOYA study. J Clin Biochem Nutr. 2017;60(2):130‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kraag N, Thijs C, Knipschild P. DyspepsiA−‐how noisy are gallstones? A metA−analysis of epidemiologic studies of biliary pain, dyspeptic symptoms, and food intolerance. Scand J Gastroenterol. 1995;30(5):411‐21. [DOI] [PubMed] [Google Scholar]
- 164. Berger MY, van der Velden JJ, Lijmer JG, de Kort H, Prins A, Bohnen AM. Abdominal symptoms: do they predict gallstones? A systematic review. Scand J Gastroenterol. 2000;35(1):70‐6. [DOI] [PubMed] [Google Scholar]
- 165. Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127(4):1239‐55. [DOI] [PubMed] [Google Scholar]
- 166. Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom H, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108(9):1382‐91. [DOI] [PubMed] [Google Scholar]
- 167. Vijayvargiya P, Jameie‐Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and metA−analysis. Gut. 2019;68(5):804‐13. [DOI] [PubMed] [Google Scholar]
- 168. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and metA−analysis. Gastroenterology. 2019;156(6):1650‐60. [DOI] [PubMed] [Google Scholar]
- 169. Tack J, Caenepeel P, Arts J, Lee KJ, Sifrim D, Janssens J. Prevalence of acid reflux in functional dyspepsia and its association with symptom profile. Gut. 2005;54(10):1370‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gerson LB, Triadafilopoulos G. A prospective study of oesophageal 24‐h ambulatory pH monitoring in patients with functional dyspepsia. Dig Liver Dis. 2005;37(2):87‐91. [DOI] [PubMed] [Google Scholar]
- 171. Xiao YL, Peng S, Tao J, Wang AJ, Lin JK, Hu PJ, et al. Prevalence and symptom pattern of pathologic esophageal acid reflux in patients with functional dyspepsia based on the Rome III criteria. Am J Gastroenterol. 2010;105(12):2626‐31. [DOI] [PubMed] [Google Scholar]
- 172. Du L, Chen B, Kim JJ, Chen X, Dai N. Micro‐inflammation in functional dyspepsia: a systematic review and metA−analysis. Neuro Gastroenterol Motil. 2018;30(4).e13304. [DOI] [PubMed] [Google Scholar]
- 173. Marzio L, Falcucci M, Grossi L, Ciccaglione FA, Malatesta MG, Castellano A, et al. Proximal and distal gastric distension in normal subjects and H. pylori‐positive and ‐negative dyspeptic patients and correlation with symptoms. Dig Dis Sci. 1998;43(12):2757‐63. [DOI] [PubMed] [Google Scholar]
- 174. Hjelland IE, Ofstad AP, Narvestad JK, Berstad A, Hausken T. Drink tests in functional dyspepsia: which drink is best? Scand J Gastroenterol. 2004;39:933‐72. [DOI] [PubMed] [Google Scholar]
- 175. Jones MP, Hoffman S, Shah D, Patel K, Ebert CC. The water load test: observations from healthy controls and patients with functional dyspepsia. Am J Physiol Gastrointest Liver Physiol. 2003;284:G896‐904. [DOI] [PubMed] [Google Scholar]
- 176. Kindt S, Coulie B, Wajs E, Janssens J, Tack J. Reproducibility and symptomatic predictors of a slow nutrient drinking test in health and in functional dyspepsia. Neuro Gastroenterol Motil. 2008;20:320‐9. [DOI] [PubMed] [Google Scholar]
- 177. Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013 Mar;10(3):168‐74. [DOI] [PubMed] [Google Scholar]
- 178. No authors listed . Surveillance of gastro‐oesophageal reflux disease and dyspepsia in adults: investigation and management; 2019. https://www.nice.org.uk/guidance/cg184 [PubMed] [Google Scholar]
- 179. Miwa H, Ghoshal UC, Gonlachanvit S, Gwee KA, Ang TL, Chang FY, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012 Apr;18(2):150‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Miwa H, Kusano M, Arisawa T, Oshima T, Kato M, Joh T, et al. Evidence‐based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015 Feb;50(2):125‐39. [DOI] [PubMed] [Google Scholar]
- 181. Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Liu XG, et al. Effects of proton‐pump inhibitors on functional dyspepsia: a metA−analysis of randomized placebo‐controlled trials. Clin Gastroenterol Hepatol. 2007 Feb;5(2):178‐85. [DOI] [PubMed] [Google Scholar]
- 182. Pinto‐Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017 Nov 21;11(11).CD011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Blum AL, Arnold R, Stolte M, Fischer M, Koelz HR. Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group. Gut. 2000 Oct;47(4):473‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dillon JF, Finch PJ, Baxter G. A comparison of lansoprazole vs ranitidine in the treatment of functional ulcer‐like dyspepsia as defined by the Rome II criteria. Gut. 2004;53(Suppl VI).A285. [Google Scholar]
- 185. Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013 Mar;10(3):187‐94. [DOI] [PubMed] [Google Scholar]
- 186. Iwakiri R, Tominaga K, Furuta K, Inamori M, Furuta T, Masuyama H, et al. Randomised clinical trial: rabeprazole improves symptoms in patients with functional dyspepsia in Japan. Aliment Pharmacol Ther. 2013 Oct;38(7):729‐40. [DOI] [PubMed] [Google Scholar]
- 187. Pittayanon R, Yuan Y, Bollegala NP, Khanna R, Lacy BE, Andrews CN, et al. Prokinetics for functional dyspepsia: a systematic review and meta−analysis of randomized control trials. Am J Gastroenterol. 2019 Feb;114(2):233‐43. [DOI] [PubMed] [Google Scholar]
- 188. Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, et al. Systematic review: cardiovascular safety profile of 5‐HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012 Apr;35(7):745‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. De Nutte N, Van Ganse W, Witterhulghe M, Defrance P. Relief of epigastric pain in nonulcer dyspepsia: controlled trial of the promotility drug cisapride. Clin Ther. 1989;11(1):62‐8. [PubMed] [Google Scholar]
- 190. Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo‐controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354(8):832‐40. [DOI] [PubMed] [Google Scholar]
- 191. Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double‐blind, placebo‐controlled trials. Gut. 2008;57(6):740‐6. [DOI] [PubMed] [Google Scholar]
- 192. Masuy I, Van Oudenhove L, Tack J. Review article: treatment options for functional dyspepsia. Aliment Pharmacol Ther. 2019;49(9):1134‐72. [DOI] [PubMed] [Google Scholar]
- 193. Carbone F, Vandenberghe A, Holvoet L, Piessevaux H, Arts J, Caenepeel F, et al. A double‐blind randomized, multicenter, placebo‐controlled study of itopride in functional dyspepsia postprandial distress syndrome. Submitted for publication 2020 or Carbone F, Vandenberghe A, Holvoet L, Vanuytsel T, Jones MP, Tack JF. 383—The therapeutic outcome of itopride in functional dyspepsia postprandial distress syndrome: a double‐blind randomized, multicenter, placebo‐controlled study. Gastroenterology. 2018;154(6):S1‐91. [Google Scholar]
- 194. Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta‐analysis. Gut. 2017;66(3):411‐42. [DOI] [PubMed] [Google Scholar]
- 195. Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology. 2015. Aug;149(2):340‐9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lacy BE, Saito YA, Camilleri M, Bouras E, DiBaise JK, Herrick LM, et al. Effects of antidepressants on gastric function in patients with functional dyspepsia. Am J Gastroenterol. 2018;113(2):216‐24. [DOI] [PubMed] [Google Scholar]
- 197. Cheong PK, Ford AC, Cheung CKY, Ching JYL, Chan Y, Sung JJY, et al. Low‐dose imipramine for refractory functional dyspepsia: a randomised, double‐blind, placebo‐controlled trial. Lancet Gastroenterol Hepatol. 2018;3(12):837‐44. [DOI] [PubMed] [Google Scholar]
- 198. Tan VP, Cheung TK, Wong WM, Pang R, Wong BC. Treatment of functional dyspepsia with sertraline: a double‐blind randomized placebo‐controlled pilot study. World J Gastroenterol. 2012;18(42):6127‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. van Kerkhoven LA, Laheij RJ, Aparicio N, De Boer WA, Van den Hazel S, Tan AC, et al. Effect of the antidepressant venlafaxine in functional dyspepsia: a randomized, double‐blind, placebo‐controlled trial. Clin Gastroenterol Hepatol. 2008;6(7):746‐52. [DOI] [PubMed] [Google Scholar]
- 200. Tack J, Ly HG, Carbone F, Vanheel H, Vanuytsel T, Holvoet L, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14(3):385‐92.e4. [DOI] [PubMed] [Google Scholar]
- 201. Jiang SM, Jia L, Liu J, Shi MM, Xu MZ. Beneficial effects of antidepressant mirtazapine in functional dyspepsia patients with weight loss. World J Gastroenterol. 2016;22(22):5260‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Miwa H, Nagahara A, Tominaga K, Yokoyama T, Sawada Y, Inoue K, et al. Efficacy of the 5‐HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. Am J Gastroenterol. 2009;104(11):2779‐87. [DOI] [PubMed] [Google Scholar]
- 203. Tack J, van den ElzenB, Tytgat G, Wajs E, van Nueten L, de Ridder F, et al. placebo‐controlled trial of the 5‐HT agonist R‐137696 on symptoms, visceral hypersensitivity and on impaired accommodation in functional dyspepsia. Neuro Gastroenterol Motil. 2009;21(6):619‐26.e23‐4. [DOI] [PubMed] [Google Scholar]
- 204. Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus‐relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10(11):1239‐45. [DOI] [PubMed] [Google Scholar]
- 205. Van Oudenhove L, Kindt S, Vos R, Coulie B, Tack J. Influence of buspirone on gastric sensorimotor function in man. Aliment Pharmacol Ther. 2008;28(11–12):1326‐33. [DOI] [PubMed] [Google Scholar]
- 206. Melzer J, Rösch W, Reichling J, Brignoli R, Saller R. MetA−analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Aliment Pharmacol Ther. 2004;20(11–12):1279‐87. [DOI] [PubMed] [Google Scholar]
- 207. Koretz RL, Rotblatt M. Complementary and alternative medicine in gastroenterology: the good, the bad, and the ugly. Clin Gastroenterol Hepatol. 2004;2(11):957‐67. [DOI] [PubMed] [Google Scholar]
- 208. Li J, Lv L, Zhang J, Xu L, Zeng E, Zhang Z, et al. A combination of peppermint oil and caraway oil for the treatment of functional dyspepsia: a systematic review and meta‐analysis. Evid Based Complement Alternat Med. 2019;2019:7654947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hoshino N, Nishizaki D, Hida K, Obama K, Sakai Y. Rikkunshito for upper gastrointestinal symptoms: a systematic review and meta‐analysis. Complement Ther Med. 2019;42:255‐63. [DOI] [PubMed] [Google Scholar]
- 210. Chu MHK, Wu IXY, Ho RST, Wong CHL, Zhang AL, Zhang Y, et al. Chinese herbal medicine for functional dyspepsia: systematic review of systematic reviews. Therap Adv Gastroenterol. 2018;11.1756284818785573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Tan VP, Liu KS, Lam FY, Hung IF, Yuen MF, Leung WK. Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2017;45(6):767‐76. [DOI] [PubMed] [Google Scholar]
- 212. Calvert EL, Houghton LA, Cooper P, Morris J, Whorwell PJ. Long‐term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002 Dec;123(6):1778‐85. [DOI] [PubMed] [Google Scholar]
- 213. Haug TT, Wilhelmsen I, Svebak S, Berstad A, Ursin H. Psychotherapy in functional dyspepsia. J Psychosom Res. 1994 Oct;38(7):735‐44. [DOI] [PubMed] [Google Scholar]
- 214. Haag S, Senf W, Tagay S, Langkafel M, Braun‐Lang U, Pietsch A, et al. Is there a benefit from intensified medical and psychological interventions in patients with functional dyspepsia not responding to conventional therapy? Aliment Pharmacol Ther. 2007;25(8):973‐86. [DOI] [PubMed] [Google Scholar]
- 215. Kim KN, Chung SY, Cho SH. Efficacy of acupuncture treatment for functional dyspepsia: a systematic review and meta‐analysis. Complement Ther Med. 2015;23(6):759‐66. [DOI] [PubMed] [Google Scholar]
- 216. Zhou W, Su J, Zhang H. Efficacy and safety of acupuncture for the treatment of functional dyspepsia: meta‐analysis. J Altern Complement Med. 2016;22(5):380‐9. [DOI] [PubMed] [Google Scholar]
- 217. Ho RST, Chung VCH, Wong CHL, Wu JCY, Wong SYS, Wu IXY. Acupuncture and related therapies used as add‐on or alternative to prokinetics for functional dyspepsia: overview of systematic reviews and network meta‐analysis. Sci Rep. 2017;7(1):10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zheng H, Xu J, Sun X, Zeng F, Li Y, Wu X, et al. Electroacupuncture for patients with refractory functional dyspepsia: a randomized controlled trial. Neuro Gastroenterol Motil. 2018;30(7).e13316. [DOI] [PubMed] [Google Scholar]
- 219. Aucoin M, Lalonde‐Parsi MJ, Cooley K. Mindfulness‐based therapies in the treatment of functional gastrointestinal disorders: a meta‐analysis. Evid Based Complement Alternat Med. 2014;2014:140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Vandenbroucke K, Kindt S, Demedts I, Tack J. Outcome of percutaneous jejunal feeding tube placement for refractory idophatic severe gatroparesis: a retrospective review. Acta Gastro‐Enterol Belg. 2006;69:D14. (abstract). [Google Scholar]
- 221. Quartero AO, Numans ME, Post MW, de Melker RA, de Wit NJ. One‐year prognosis of primary care dyspepsia: predictive value of symptom pattern, Helicobacter pylori and GP management. Eur J Gastroenterol Hepatol. 2002;14(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 222. Halder SL, Locke GR, 3rd , Schleck CD, Zinsmeister AR, Melton LJ, 3rd , Talley NJ. Natural history of functional gastrointestinal disorders: a 12‐year longitudinal population‐based study. Gastroenterology. 2007;133(3):799‐807. [DOI] [PubMed] [Google Scholar]
- 223. Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Bjornsson E, Thjodleifsson B. Natural history of functional gastrointestinal disorders: comparison of two longitudinal population‐based studies. Dig Liver Dis. 2012;44(3):211‐7. [DOI] [PubMed] [Google Scholar]
- 224. Chang JY, Locke GR, 3rd , McNally MA, Halder SL, Schleck CD, Zinsmeister AR, et al. Impact of functional gastrointestinal disorders on survival in the community. Am J Gastroenterol. 2010;105(4):822‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Fluctuation of gastrointestinal symptoms in the community: a 10‐year longitudinal follow‐up study. Aliment Pharmacol Ther. 2008;28(8):1013‐20. [DOI] [PubMed] [Google Scholar]


