Abstract
A fundamental question in neuropsychiatry is whether a neurobiological continuum accompanies the behavioral continuum between subclinical and clinical traits. Impulsivity is a trait that varies in the general population and manifests severely in disorders like psychopathy. Is the neural profile of severe impulsivity in psychopathy an extreme but continuous manifestation of that associated with impulsivity in the general population (different by degree)? Or is it discontinuous and unique (different by kind)? Here, we compare systematic reviews of the relationship between impulsivity and gray matter in psychopathy and in the general population. The findings suggest that the neural profile associated with extreme impulsivity in psychopathy (increased gray matter in rostral and ventral striatum and prefrontal cortexes) is distinct from that associated with impulsivity in the general population (decreased gray matter in rostral and ventral prefrontal cortexes). Severe impulsivity in psychopathy may therefore arise from a pathophysiological mechanism that is unique to the disorder. These findings prompt the need for future studies to directly test the effect of group on the impulsivity–gray matter relationship in samples comprised of healthy individuals and individuals with psychopathy. The results caution against the use of community samples to examine impulsive psychopathic traits in relation to neurobiology.
Keywords: impulsivity, gray matter, prefrontal cortex, striatum, psychopathy, healthy
Introduction
Impulsive decisions are those that are made in haste, spurred by the prospect of an immediate reward or by impatience and/or made without sufficient regard for their long-term consequences. For individuals with a consistent propensity for impulsive decision-making—and especially for individuals with psychiatric conditions featuring clinical levels of impulsivity (Moeller et al., 2001)—everyday functioning can become impaired to the point that quality of life suffers. In the general population, impulsivity has been associated with poorer academic outcomes (Duckworth and Seligman, 2005), substance abuse (De Wit, 2009), risky sexual behavior (Charnigo et al., 2013) and a myriad of other negative life outcomes. What is more, the most severe manifestations of impulsive behavior, such as those present in disorders like psychopathy, substance abuse disorder and attention-deficit/hyperactivity disorder (ADHD), are estimated to cost nearly one trillion dollars annually in the USA alone from criminal social costs, medical care costs and losses in economic productivity (Kiehl and Hoffman, 2011). Given the negative impact that impulsivity has on both individual and societal well-being, it has therefore been of interest to elucidate the neurobiological underpinnings of impulsive behavior, with the hope that this knowledge can assist in identifying individuals who are at-risk for exhibiting impulsive behavior, as well as lead to better treatment options.
Substantial progress has been made over the past few decades in identifying the neural substrates whose integrity is related to individual differences in impulsivity. Across numerous species, and in both clinical and healthy samples, studies have highlighted a distinct set of relevant brain regions, connections and neurotransmitter systems in reward-processing and decision-making circuitry whose integrity relates to levels of impulsivity (Buckholtz et al., 2010; Dalley et al., 2011; Dalley and Robbins, 2017). This circuitry involves gray matter nodes in the ventral midbrain, basal ganglia, thalamus, motor cortices and prefrontal cortex, the white matter connections between these nodes and the monoamine neurotransmitter systems (Buckholtz et al., 2010; Dalley et al., 2011; Dalley and Robbins, 2017).
Yet, despite the identification of a network of neural substrates whose integrity is associated with individual differences in impulsivity, there remains a lack of consensus regarding the direction of these relationships and the precise nature of aberrance associated with impulsive behavior (e.g. hyper- or hypo-connectivity? Excessive or diminished gray matter? etc.) (Colzato et al., 2009; Lee et al., 2009; Ghahremani et al., 2012; Kayser et al., 2012; Robertson et al., 2015; Angelides et al., 2017; Korponay et al., 2017a). A further unresolved question is whether the nature of this aberrance is different by degree or by kind when comparing relatively high, but subclinical, impulsivity in the general population to clinical impulsivity in neuropsychiatric disorders. For instance, if high levels of impulsivity in the general population are associated with low levels of gray matter in the prefrontal cortex, would severe levels of impulsivity in a clinical population be associated with even lower levels of gray matter volume in the prefrontal cortex? Or are the neural correlates of severe impulsivity in clinical populations distinct from those in the general population?
To address this question, here we conduct systematic reviews of the literature studies on the relationship between impulsivity and gray matter in the general population and in severely impulsive individuals with psychopathy. Psychopathy is a mental health disorder characterized by callous lack of empathy and impulsive-antisocial behavior. Present in roughly one-fourth of adult prison inmates, psychopathy is associated with a disproportionately high incidence of violent crime, substance abuse and recidivism (Smith and Newman, 1990; Hare, 2003). The detrimental behaviors committed by psychopathic individuals, estimated to result in $460 billion annual costs in the USA alone (Kiehl and Hoffman, 2011), have been linked to the impulsive-antisocial aspects of the disorder (Walters, 2003; Edens et al., 2008).
Comparing the measurement of impulsivity in the general population and in psychopathy
Different instruments have been used in the general population and psychopathy literature studies to measure impulsivity with respect to neuroimaging assessments of gray matter structure. This section will detail the most common instrument used in each literature—the Barratt Impulsiveness Scale (BIS-11) (Patton and Stanford, 1995) and the Factor 2 dimension of the Psychopathy Checklist-Revised (PCL-R) (Hare, 2003)—and establish a basis with which to compare them.
General population.
Several self-report instruments and a host of task-based measurements are used in the literature to measure impulsivity and its different dimensions in healthy samples. The instrument that has been most frequently used in conjunction with structural neuroimaging in healthy samples is the BIS-11, which, as such, is the instrument of focus in this review. The BIS-11 is a self-report questionnaire containing 30 questions, each of which requires the subject to choose between ‘Rarely/Never’, ‘Occasionally’, ‘Often’ and ‘Almost Always’. Items are scored from 1 to 4. The BIS-11 yields a total score and three subscale scores derived by factor analysis: attentional impulsivity (e.g. ‘I am restless at the theatre or lectures‘), motor impulsivity (e.g. ‘I do things without thinking’) and non-planning impulsivity (e.g. I am more interested in the present than the future;) (Patton et al., 1995). Higher scores indicate higher levels of impulsivity.
Psychopathy.
While several instruments exist for the measurement of psychopathy, the most commonly used in forensic populations with pathological levels of impulsivity is the PCL-R. The PCL-R includes both a structured clinical interview and a file review, which raters use to score an individual as either a 0, 1 or 2 on 20 trait items. As such, total scores on the PCL-R can range from 0 to 40. Individuals scoring between 0 and 20 are considered to be non-psychopathic, those scoring between 21 and 29 are considered to be intermediate, and those scoring 30 and above are considered to have psychopathy (Hare, 2003). Importantly, the PCL-R’s 20 trait items can be separated into two factors that reflect the callous/unemotional (Factor 1) and impulsive/antisocial (Factor 2) dimensions of the disorder (Harpur et al., 1989). Therefore, Factor 2 score on the PCL-R is the most commonly used measurement to gauge impulsivity in individuals with psychopathy.
It is important to underscore that Factor 2 score on the PCL-R is not a ‘pure’ measure of impulsivity. The items that comprise the Factor 2 dimension of the PCL-R are determined by factor analysis of the PCL-R items (Harpur et al., 1989), and index a broad array of antisocial traits and behaviors that include some more explicitly related to impulsivity (e.g. ‘impulsivity’, ‘poor behavioral control’ and ‘need for stimulation’) and some more complex behaviors and life events that may indirectly index impulsivity (e.g. ‘sexual promiscuity’, ‘juvenile delinquency’, ‘revocation of conditional release’, ‘criminal versatility’, ‘lack of realistic long-term goals’, ‘irresponsibility’ and ‘many short-term marital relationships’). Furthermore, unlike the BIS-11, the PCL-R is not a self-report instrument.
Comparing BIS-11 scores with PCL-R Factor 2 scores.
Despite their differences, a study measuring both BIS-11 and PCL-R Factor 2 scores within the same sample demonstrates a significant positive correlation between the two (Snowden and Gray, 2011). This study of an incarcerated sample found that Factor 2 score was significantly positively correlated with BIS-11 total score (R = 0.26), BIS-11 motor impulsivity subscale score (R = 0.28) and BIS-11 non-planning impulsivity subscale score (R = 0.31), but not with BIS-11 attentional impulsivity subscale score (R = 0.05).
To establish further basis to be able to compare BIS-11 and PCL-R Factor 2 scores, we corroborated the correspondence between these measures by administering the PCL-R and BIS-11 to a subset of an incarcerated sample (n = 49) used in our group’s recent prior studies of psychopathy (Korponay et al., 2017b,c). Correlation analyses confirmed a significant positive relationship between a subject’s Factor 2 score and his/her BIS-11 total score of modest effect size (Table 1; Figure 1). In line with the prior study cited above, Factor 2 score was significantly correlated with BIS-11 total score, motor impulsivity subscale score and non-planning impulsivity subscale score, but it did not significantly correlate with attentional impulsivity subscale score.
Table 1.
Relationship between PCL-R Factor 2 score and BIS-11 scores
| BIS-11 Total | BIS-11 Motor | BIS-11 Non-Planning | BIS-11 Attentional | |
|---|---|---|---|---|
| Factor 2 | R = 0.333 (P = 0.019) | R = 0.296 (P = 0.039) | R = 0.342 (P = 0.016) | R = 0.147 (P = 0.315) |
Fig. 1.
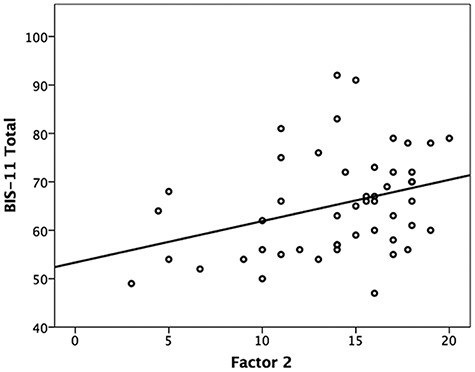
The within-subject relationship between PCL-R Factor 2 score and BIS-11 total score.
Overall, prior work and the current data provide support to treat the two instruments as related measures of the construct of trait impulsivity, with PCL-R Factor 2 gauging more severe manifestations of impulsiveness than the BIS-11.
Methods
Systematic reviews
A systematic review of literature on the relationship between impulsivity and gray matter was first conducted for samples of the healthy, general population. The literature search aimed to identify all publications that met the following criteria: (i) study must report on the relationship between BIS-11 scores and a gray matter metric, and (ii) this relationship must be reported about a sample of healthy adults from the general population. Figure 2 shows the logical operators that were entered into PubMed to achieve initial filtering for these constraints.
Fig. 2.
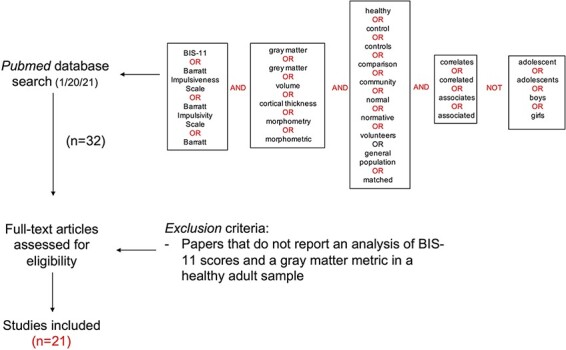
Systematic review search pipeline for identifying studies that examine the relationship between BIS-11 scores and gray matter in adults in the general population.
The initial search yielded 32 qualifying peer-reviewed publications. From these 32, 11 publications were excluded after assessment of the full text, because they did not report an analysis of BIS-11 scores in relation to a gray matter metric in a healthy adult sample. This left a total of 21 publications for subsequent examination of findings in the general population literature.
A systematic review was then conducted on the literature examining the relationship between impulsivity and gray matter in psychopathy. The literature search aimed to identify all publications that met the following criteria: (i) study must report on the relationship between Factor 2 scores on either the PCL-R or Psychopathy Checklist Screening Version (PCL-SV) and a gray matter metric, and (ii) this relationship must be reported in a sample of adults. Figure 3 shows the logical operators that were entered into PubMed to achieve initial filtering for these constraints.
Fig. 3.
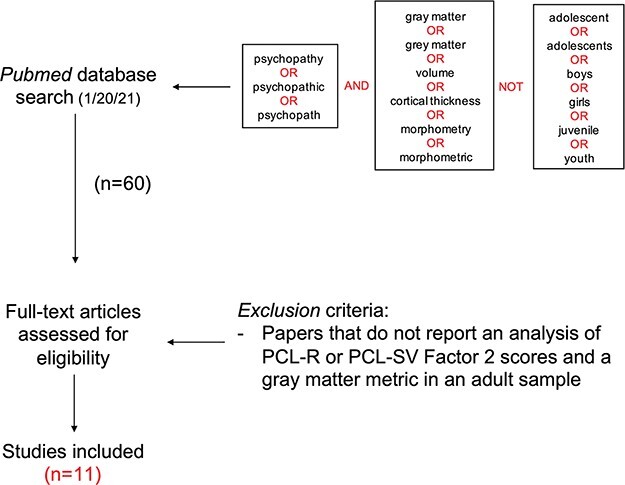
Systematic review search pipeline for identifying studies that examine the relationship between PCL-R Factor 2 scores and gray matter in adults with psychopathy.
The initial search yielded 60 qualifying peer-reviewed publications. From these 60, 49 publications were excluded after assessment of the full text, because they did not report an analysis of either PCL-R or PCL-SV Factor 2 scores in relation to a gray matter metric in a sample of adults. This left a total of 11 publications for subsequent examination of findings in the psychopathy literature.
Results
The relationship between gray matter volume and impulsivity in the general population
Twenty-one studies were found in the literature that examined the relationship between gray matter and impulsivity as measured by BIS-11 scores in samples of healthy adults from the general population. This literature converges on one modestly consistent pattern of findings that self-reported impulsivity in the general population as measured by the BIS-11 is associated with decreases in gray matter in the prefrontal cortex. This relationship is reported by eight studies (Matsuo et al., 2009; Schilling et al., 2012; Holmes et al., 2016; Grodin et al., 2017; Liu and Feng, 2017; Tu et al., 2017; Korponay et al., 2017a; Ai et al., 2019), two of which also replicated the finding in independent samples (Holmes et al., 2016; Liu and Feng, 2017). Eleven studies did not report a relationship between prefrontal gray matter and BIS-11 scores (Bjork et al., 2009; Sala et al., 2011; Deserno et al., 2015; Mei et al., 2015; Ide et al., 2017; Qiu et al., 2017; Kubera et al., 2018; Besteher et al., 2019; Li et al., 2019; Rosenthal et al., 2019; Zsido et al., 2019), and two studies reported a positive relationship (Lee et al., 2011; Cho et al., 2013) (Table 2). The average sample size of the 10 samples reporting a negative impulsivity–gray matter relationship was 173, whereas the average sample size of the two samples reporting a positive relationship was 26. Within prefrontal cortex, the most consistent findings across studies were decreased gray matter in rostral and ventral prefrontal regions, including the orbitofrontal cortex. No relationships were observed between impulsivity and striatal gray matter.
Table 2.
BIS-11 and gray matter associations in general population (healthy adults)
| Study | n | Age | Impulsivity measure | Gray matter measure | Analysis | Relationship | Region |
|---|---|---|---|---|---|---|---|
| (Matsuo et al., 2009) | 62 (38 F) | 35.4 ± 12.1 | BIS-11 | Volume | Regression | Negative | R, L OFC (total; motor and non-planning subscales) |
| (Bjork et al., 2009) | 29 (11 F) | 37.4 ± 11.0 | BIS-11 | Volume | Regression | None | n/a |
| (Sala et al., 2011) | 15 (11 F) | 34.2 ± 8.1 | BIS-11 | Volume | Regression | None (only hippocampus and dlPFC assessed) | n/a |
| (Lee et al., 2011) | 18 (0 F) | 40.5 ± 7.5 | BIS-11 | Volume | Regression | Negative | L STG (attentional; motor) |
| Positive | L OFC, L Lateral Frontopolar Cortex (non-planning) | ||||||
| (Schilling et al., 2012) | 32 (18 F) | 35.2 ± 10.5 | BIS-11 (German version) | Cortical thickness | Regression | Negative | L MFG (total, attentional, motor and non-planning subscales) OFC; R MFG (total and motor subscale) |
| (Cho et al., 2013) | 34 (11 F) | 23.4 ± 4.3 | BIS-11 | Volume | Regression | Positive | L ACC; L DLPFC; R OFC; R, L medial PFC; R, L middle Cingulate (total; non-planning and attentional subscales) |
| (Deserno et al., 2015) | 24 (12 F) 26 (13 F) | 27.29 ± 3.7 27.58 ± 3.7 | BIS-11 | Density | Group (high-impulsive vs low-impulsive) | None | n/a |
| (Mei et al., 2015) | 41 (17 F) | 29.7 ± 10.1 | BIS-11 | Volume | Regression | None (only amygdala and hippocampus examined) | n/a |
| (Holmes et al., 2016) | 1015 (537 F) | 21.4 ± 3.1 | BIS-11 | Cortical Thickness | Regression | Negative | R, L MFG; R, L ACC (motor subscale) |
| 219 (133 F) | 21.2 ± 3.3 | BIS-11 | Cortical Thickness | Regression | Negative | R, L MFG; R, L ACC (motor subscale) | |
| (Ide et al., 2017) | 113 (66 F) | 32 ± 14 | BIS-11 | Volume | Regression | Positive | R, L parieto-occipital sulcus (total; attentional and non-planning subscales) |
| (Qiu et al., 2017) | 18 (0 F) | 24.0 ± 3.08 | BIS-11 | Cortical thickness and subcortical volume | Regression | None | n/a |
| (Korponay et al., 2017c) | 105 (65 F) | 48.6 ± 10.9 | BIS-11 | Volume | Regression | Negative | R Mofc (total; motor and non-planning) R, L paracingulate gyrus (non-planning subscale) |
| (Liu and Feng, 2017) | 85 (55 F) | 20.5 ± 2.07 | BIS-11 | Volume | Regression | Negative | L dlPFC, L calcarine gyrus (total) |
| Positive | R STG (total) | ||||||
| 84 (51 F) | 19.5 ± 1.4 | BIS-11 | Volume | Regression | Negative | L dlPFC, L MFG (total) | |
| (Tu et al., 2017) | 56 (34 F) | 33.9 ± 7.4 | BIS-11 (Chinese version) | Cortical Thickness | Regression | Negative | L Inferior, middle and medial PFC (total) |
| (Grodin et al., 2017) | 49 (26 F) | 38.8 ± 10.9 | BIS-11 | Volume Cortical thickness |
Regression Regression |
Negative Negative |
L ACC (total) R, L ACC (total) |
| (Kubera et al., 2018) | 54 (38 F) | 24.9 ± 4.02 | BIS-11 | Cortical thickness | Regression | Negative | L lingual gyrus; L superior temporal gyrus; R cuneus; R superior parietal gyrus (total) R, L pericalcarine gyrus (non-planning subscale) |
| (Ai et al., 2019) | 84 (43 F) | 21.3 ± 1.7 | BIS-11 (Chinese version) | Volume | Regression | Negative | R supplementary motor area; R paracentral lobule (total) |
| (Besteher et al., 2019) | 85 (57 F) | 24.1 ± 3.0 | BIS-11 (German version) | Volume | Regression | Positive | R inferior parietal gyrus; R postcentral gyrus; R supramarginal gyrus (total) |
| (Li et al., 2019) | 40 (2 F) | 24.4 ± 3.6 | BIS-11 | Sulcal depth, gyrificationand cortical thickness | Regression | None | n/a |
| (Rosenthal et al., 2019) | 62 (23 F) | 42.7 ± 11.69 | BIS-11 | Volume | Regression | None | n/a |
| (Zsido et al., 2019) | 29 (15 F) | 21.9 ± 2.1 | BIS-11 | Cortical thickness | Regression | Positive | R, L middle temporal cortex; L transverse temporal cortex; (total) |
The relationship between gray matter and impulsivity in psychopathy
Eleven studies (two using the same sample) were found in the literature that examined the relationship between gray matter and impulsivity as measured by PCL-R or PCL-SV Factor 2 score in samples of adults. This literature converges on two consistent patterns of findings. The first is that impulsive-antisocial psychopathic traits are associated with increased gray matter in the striatum—five studies (Glenn et al., 2010; Schiffer et al., 2011; Cope et al., 2012; Leutgeb et al., 2015; Korponay et al., 2017b) report this relationship (one of which found a local decrease in conjunction with a local increase (Cope et al., 2012). This is consistent with the findings of a recent quantitative meta-analysis of gray matter in psychopathy (Deming and Koenigs, 2020). Within the striatum, the most consistent findings across studies are increased gray matter in the rostral caudate and the ventral striatum. The second pattern of findings is that impulsive-antisocial psychopathic traits are associated with widespread increases in gray matter throughout the prefrontal cortex—four studies (Cope et al., 2012; Leutgeb et al., 2015; Contreras-Rodriguez et al., 2015b; Korponay et al., 2017c) report this relationship (Table 3). Within prefrontal cortex, the most consistent findings across studies were increased gray matter in rostral and ventral regions, including the orbitofrontal cortex. The aforementioned quantitative meta-analysis did not reveal a consistent frontal cortical area where gray matter correlated with Factor 2 score (Deming and Koenigs, 2020). This is likely because the peak coordinates identified in the studies analyzed are spread throughout different areas of the frontal cortex, rather than concentrated in the same areas.
Table 3.
PCL-R Factor 2 and gray matter associations in samples that include individuals with psychopathy
| Study | Population | N | Age | Psychopathy Severity | Impulsivity Measure | Gray Matter Measure | Analysis | Relationship | Region |
|---|---|---|---|---|---|---|---|---|---|
| (De Oliveira-souza et al., 2008) | Antisocial personality disorder (APD) patients | 15 (7 F) | 32 ± 14 | Mean PCL-SV Score: 17.8 ± 3.8 | PCL-SV Factor 2 | Concent-ration | Regression (within the APD group) | None | n/a |
| Healthy non-patients | 15 (7 F) | 32 ± 13 | Mean PCL-SV Score: 0.4 ± 1.0 | ||||||
| (Muller et al., 2008) | Psychopathy inpatients | 17 (0 F) | 33.0 ± 5.8 | Mean PCL-R Score: 33.4 ± 4.1 | PCL-R Factor 2 | Volume | Regression | None (in temporal lobe) | Analysis for frontal cortical or striatal regions not reported |
| Healthy non-patients | 17 (0 F) | 30.6 ± 5.9 | Mean PCL-R Score: 0.5 ± 1.1 | ||||||
| (Glenn et al., 2010) | Temporary employment agency sample | 44 (4 F) | 31.1 ± 6.7 | Mean PCL-R Score: 20.1 ± 8.2 | PCL-R Factor 2 | Volume | Regression | Positive | Total striatum; L, R lenticular nucleus |
| (Schiffer et al., 2011) | Non-offenders without SUDs | 14 (0 F) | 36.7 ± 11.4 | Mean PCL-SV Score: 4.4 ± 2.6 | PCL-SV Factor 2 | Volume | Regression (across all subjects) | Quadratic: Negative for low scorers, Positive for high scorers | L nucleus accumbens; L insula |
| Non-offenders with SUDs | 13 (0 F) | 37.3 ± 7.9 | Mean PCL-SV Score: 6.5 ± 2.6 | ||||||
| Violent Offenders without SUDs | 12 (0 F) | 37.4 ± 10.6 | Mean PCL-SV Score: 9.3 ± 2.9 | ||||||
| Violent Offenders with SUDs | 12 (0 F) | 36.4 ± 5.5 | Mean PCL-SV Score: 12.8 ± 2.8 | ||||||
| (Cope et al., 2012) | Community substance abuse sample | 66 (30 F) | 36.9 ± 7.9 | Mean PCL-R Score: 18.4 ± 8.0 | PCL-R Factor 2 | Volume | Regression | Positive | Medial, inferior, middle, superior frontal gyri; ACC; postcentral gyrus; caudate head |
| Negative | Temporal lobe; parahippocampal, fusiform gyri; insula; inferior parietal lobule; medial frontal gyrus; caudate tail | ||||||||
| (Pujara et al., 2014) | Incarcerated non-psychopaths | 23 (0 F) | 32.4 ± 8.0 | Mean PCL-R Score: 14.1 ± 3.5 | PCL-R Total | Volume | Regression (separate for each group) | None for non-psychopaths, | R nucleus accumbens |
| Incarcerated psychopaths | 18 (0 F) | 32.2 ± 6.5 | Mean PCL-R Score: 31.7 ± 1.7 | Positive for psychopaths | R nucleus accumbens | ||||
| (Ermer et al. 2013) | Incarcerated adult males | 296 (0 F) | 33.9 ± 9.5 | Mean PCL-R Score: 21.3 ± 7.0 | PCL-R Factor 2 | Volume; Concentration | Regression | Negative | R Temporal Pole |
| (Contreras-Rodriguez et al., 2015a) | Males with history of severe criminal offense | 22 (0 F) | 39.8 ± 9.2 | Mean PCL-R Score: 27.8 ± 4.5 | PCL-R Factor 2 | Volume | Regression | Positive | Medial-dorsal PFC; medial PFC; R lateral PFC; operculum; cerebellum |
| (Leutgeb et al., 2015) | Incarcerated adult males | 40 (0 F) | 38.1 ± 12 | Mean PCL-R Score: 20.6 ± 7.7 | PCL-R Factor 2 | Volume | Regression | Positive | L, R OFC; L, R insula; R SMA; R putamen; L pallidum |
| (Korponay et al., 2017c) and (Korponay et al., 2017a) | Incarcerated adult males | 124 (0 F) | 31.6 ± 7.3 | Mean PCL-R Score: 24.8 ± 7.1 | PCL-R Factor 2 | Volume | Regression | Positive | R mOFC; L middle and superior frontal gyri; L, R ventral striatum |
A third observation is also suggested by this literature. Schiffer and colleagues (Schiffer et al., 2011) report a quadratic relationship such that Factor 2 score is positively related to nucleus accumbens and insula volume in high PCL-SV scoring individuals but is negatively related to volume in these regions in low PCL-SV scoring individuals. Similarly, Pujara and colleagues (Pujara et al., 2014) report that PCL-R total score is significantly positively related to nucleus accumbens volume within individuals with psychopathy, but not within individuals without psychopathy. These results are intriguing because they reflect the same overall difference in findings between the general population and psychopathy literature studies. Namely, they support the idea that the neural underpinnings of impulsivity in individuals with clinically significant levels of psychopathy and impulsive-antisocial traits may be distinct from those with high, but subclinical, levels of impulsive traits.
Figure 4 shows the peak coordinates reported by all included studies that conducted whole-brain voxel-wise analyses of the relationship between gray matter and either BIS-11 total score (for healthy subjects) or PCL-R/PCL-SV Factor 2 score (for subjects with psychopathy). While these peak coordinates do not fully illustrate the findings from these literature studies (some studies do not conduct voxel-wise whole-brain analyses and thus do not report peak coordinates), they nonetheless reflect the overall pattern of findings. Of 11 frontal cortex peak coordinates reported in the healthy adult literature, the majority (seven) reflected a negative relationship between gray matter and BIS-11 total score. Of 22 frontal cortex peak coordinates reported in the psychopathy literature, all reflected a positive relationship between gray matter and PCL-R/PCL-SV Factor 2 score. Furthermore, while the healthy adult literature does not report any peak coordinates in the striatum, the psychopathy literature reports five (four positive and one quadratic).
Fig. 4.
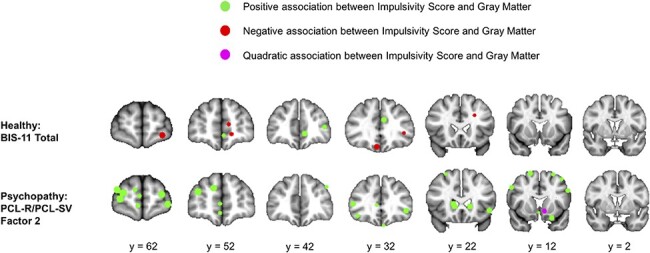
Peak coordinates reported by all included studies that conducted whole-brain voxel-wise analyses of the relationship between gray matter and either BIS-11 total score (for healthy subjects) or PCL-R/PCL-SV factor 2 score (for subjects with psychopathy).
Verifying effects specific to psychopathy within broader forensic samples.
An important caveat to these analyses is that, in nearly all studies in the psychopathy literature, the relationship between Factor 2 scores and gray matter volume is evaluated within samples that include lower PCL-R scoring, non-psychopathic individuals. As such, there is question as to whether the observed positive relationship between frontostriatal gray matter and impulsive-antisocial traits is truly specific to psychopathy. To address this, we cite several prior empirical observations, and present new analyses, that provide confidence that the positive gray matter-Factor 2 findings reported on in this review reflect, and are largely driven by, high PCL-R-scoring individuals with psychopathy.
First, the average PCL-R scores in the samples examined in this review are substantial: 33.4 ± 4.1, 20.1 ± 8.2, 18.4 ± 8.0, 31.7 ± 1.7, 21.3 ± 7.0, 27.8 ± 4.5, 20.6 ± 7.7 and 24.8 ± 7.1. This means that the examined samples are largely comprised of high PCL-R scoring individuals with scores close to or within the PCL-R scoring ranges used to classify psychopathy (PCL-R > 23; PCL-R > 26; PCL-R > 30) or intermediate/moderate psychopathy (30 > PCL-R > 20). As such, findings from analyses of these samples largely reflect relationships within moderate-to-high psychopathic individuals, despite the inclusion of a contingent of lower PCL-R scoring individuals. Furthermore, even forensic samples comprised of individuals with relatively low PCL-R scores (e.g. mean PCL-R = 14.5) (Gonsalves et al., 2013) have significantly higher psychopathic traits than the general population (Blonigen et al., 2003).
This notion is further supported, first, by the prior studies that allow separate dissection of effects for low PCL-R/SV scoring and high PCL-R/SV scoring individuals. As discussed, one such study found a quadratic relationship between PCL-SV Factor 2 score and nucleus accumbens volume, whereby the relationship was positive for high scorers and negative for low scorers. Similarly, in another study, PCL-R total score was significantly positively correlated with nucleus accumbens volume in high PCL-R scoring individuals with psychopathy but not in low PCL-R scoring individuals without psychopathy.
A re-analysis of data from Korponay and colleagues (Korponay et al., 2017b,c) presented here provides stronger evidence that the positive frontostriatal GMV–Factor 2 relationship observed in forensic samples reflects, and is driven most strongly by, the high-scoring individuals with psychopathy. For each significant (P < 0.05) relationship reported in these studies between regional frontostriatal GMV and Factor 2 score, we re-analyzed the regression analysis separately within low PCL-R scoring non-psychopathic individuals (PCL-R < 20; n = 35) and within high PCL-R scoring individuals with psychopathy (PCL-R > 30; n = 41). Table 4 displays the results.
Table 4.
Relationships between factor 2 score and gray matter volume in psychopathic and non-psychopathic individuals
| Region | Group | Standardized Beta | Relationship | P-value |
|---|---|---|---|---|
| Superior Frontal Gyrus (L) | Psychopathic | 0.446 | Positive | 0.004** |
| Non-psychopathic | 0.188 | Positive | 0.316 | |
| Superior Frontal Gyrus (R) | Psychopathic | 0.334 | Positive | 0.013* |
| Non-psychopathic | 0.120 | Positive | 0.425 | |
| Middle Frontal Gyrus (R) | Psychopathic | 0.412 | Positive | 0.002** |
| Non-psychopathic | 0.077 | Positive | 0.602 | |
| Middle Frontal Gyrus (L) | Psychopathic | 0.465 | Positive | 0.005** |
| Non-psychopathic | 0.269 | Positive | 0.103 | |
| Medial Orbitofrontal Cortex (R) | Psychopathic | 0.186 | Positive | 0.092 |
| Non-Psychopathic | 0.136 | Positive | 0.350 | |
| Putamen (R) | Psychopathic | 0.342 | Positive | 0.032* |
| Non-psychopathic | 0.429 | Positive | 0.030* | |
| Caudate (R) | Psychopathic | 0.369 | Positive | 0.040* |
| Non-psychopathic | 0.298 | Positive | 0.112 | |
| Nucleus Accumbens (L) | Psychopathic | 0.225 | Positive | 0.192 |
| Non-psychopathic | 0.172 | Positive | 0.405 | |
| Nucleus Accumbens (R) | Psychopathic | 0.228 | Positive | 0.168 |
| Non-psychopathic | 0.253 | Positive | 0.229 |
As in the original analyses, these analyses control for age, race, substance use, brain volume and PCL-R factor 1 scores. Bolding indicates significance at the
P < 0.01 or
P < 0.05 level.
Nine frontostriatal regions displayed a significant relationship between regional gray matter volume and Factor 2 score across the whole forensic sample. Among these, the positive GMV–Factor 2 relationship was significant in the psychopathy group for six regions. In contrast, the GMV–Factor 2 relationship was only significant in the non-psychopathy group for one region. These findings demonstrate that the positive frontostriatal GMV–Factor 2 relationships observed in the larger forensic sample are primarily driven by and reflect the relationships as they exist in high PCL-R scoring individuals with psychopathy. Together with the two prior studies that demonstrate a similar effect in forensic samples, and the high average PCL-R scores across all forensic samples in this literature, there is appreciable evidence to suggest that the positive GMV–Factor 2 relationship observed in forensic samples throughout the literature reflects the relationship as it exists in psychopathy.
Discussion
Is severe impulsivity in psychopathy associated with a neural profile that is an extreme but continuous manifestation of the neural profile associated with high impulsivity in the general population (i.e. different by degree)? Or is it associated with a neural profile that is discontinuous and unique (i.e. different by kind)? Here, we conducted two systematic reviews—examining the relationship between impulsivity and gray matter in psychopathy and in the general population, respectively—and compared their findings. The resulting pattern of findings from these literature studies suggests that the neural profile associated with extreme impulsivity in psychopathy (increased gray matter in rostral and ventral areas of the striatum and prefrontal cortexes) is different by kind from that associated with high impulsivity in the general population (decreased gray matter in the rostral and ventral prefrontal cortexes). These results suggest that severe impulsivity in psychopathy may arise from a pathophysiological mechanism that is unique to the disorder and which is separable from the mechanisms resulting in relatively high, but subclinical, impulsivity in the general population. The results caution against the use of community samples to examine impulsive psychopathic traits in relation to neural correlates, where the relationship between impulsivity and underlying neurobiology may be an inaccurate model of the relationship in clinical-level cases of psychopathy. These findings also prompt the need for future studies designed to directly test the effect of group on the relationship between impulsivity and gray matter volume in samples comprised of healthy individuals and individuals with psychopathy.
An important question for future research is to determine the neurobiological mechanisms by which greater gray matter volume may underlie impulsivity in psychopathic individuals, whereas reduced gray matter may underlie impulsivity in the general population. Intra-individual changes in gray matter levels have been attributed to a range of prenatal, perinatal and postnatal developmental processes including neuron development, synaptic pruning, myelination, dendritic length changes and changes in capillary and glial cell levels (Gogtay et al., 2004; Courchesne et al., 2007; Giorgio et al., 2010; Anderson, 2011; Gilmore et al., 2012). Aberrance in one or more developmental processes could therefore lead to gray matter levels that are either greater (e.g. via deficient synaptic pruning) or lesser (e.g. via insufficient neuron development) than what is optimal for efficient neural processing, leading to cognitive deficit in either case. Our findings suggest that impulsivity may arise from distinct neurodevelopmental cascades in psychopathy and in the general population.
At present, the literature on the relationships between impulsivity and other neurobiological metrics (e.g. functional connectivity, dopaminergic functioning, etc.) in healthy adults and psychopathy is too small to permit a meaningful comparison of findings. As studies on these metrics grow, it will be of interest to examine whether there is a similar ‘difference by kind’ distinction in these metrics as well. Nonetheless, at least one example of disorder-specific, ‘different by kind’ functional neural correlates of impulsivity can be found in the literature on ADHD. A meta-analysis of fMRI data found that while the ventral striatum shows decreased activity during reward anticipation in individuals with ADHD, ventral striatal activity during reward processing is increased in subjects in the healthy population with high levels of trait impulsivity (Plichta and Scheres, 2014). However, as the psychopathy literature itself demonstrates, there is not necessarily correspondence between the neural correlates of a disorder as a whole and the neural correlates of a particular subcategory of symptoms of the disorder. Nearly all group analyses in the psychopathy literature that compare gray matter in a psychopathy group to a non-psychopathy group find decreased prefrontal gray matter in the psychopathy group (Yang et al., 2005; De Oliveira-souza et al., 2008; Muller et al., 2008; Ly et al., 2012; Contreras-Rodriguez et al., 2015a), despite the findings highlighted here of increased prefrontal gray matter with increasing impulsive-antisocial traits in psychopathy. Indeed, one study reported both of these findings within the same sample (Contreras-Rodriguez et al., 2015a). The discrepancy may be attributable to the callous/unemotional (Factor 1) dimension of psychopathy being typically associated with gray matter decreases (De Oliveira-souza et al., 2008; Contreras-Rodriguez et al., 2015a). Overall, this demonstrates the need for greater future examination of the neural correlates specifically of impulsivity in individuals with disorders such as ADHD, before being able to reliably compare findings to those in the present report.
Limitations
As previously discussed, the instruments used to measure impulsivity in the general population and in psychopathy are different. The BIS-11 is a self-report instrument, while the PCL-R involves a structured interview and file review. Factor 2 of the PCL-R is also not a pure measurement of impulsivity. Future studies on neural correlates of BIS-11 scores within psychopathy samples could help standardize comparisons to findings in the general population. For now, prior reports (Morgan et al., 2011; Snowden and Gray, 2011) and the new data presented here on the significant positive correlation between BIS-11 and PCL-R Factor 2 score provide a provisional basis to compare findings across the two literature studies (See also Supplementary Data).
A second limitation is that both of these literature studies are still somewhat small. Furthermore, only a subset of studies in these small literature studies reports peak coordinates from voxel-wise analysis, precluding the ability to perform a quantitative meta-analysis. And despite the convergence of findings in both literature studies around several identifiable patterns, the findings are not unanimous amongst all studies in each literature. As such, more studies of neural correlates of impulsivity within both populations are needed to further verify whether this relationship is indeed distinct in each population.
Third, whereas all the studies considered here from the general population literature include samples that are close to evenly split between males and females, the majority of the psychopathy literature contains predominantly male samples. While the authors are not aware of any reports detailing distinct neural correlates of impulsivity for males and females, this is an important consideration to keep in mind when comparing the two literature studies.
Conclusion
Overall, this study suggests that there may be important neurobiological signatures that distinguish high, but subclinical, levels of impulsivity from extreme, clinical levels of impulsivity. In particular, subclinical impulsivity in the general population and clinical impulsivity in psychopathy appear to have underlying neural correlates that are different by kind, rather than by degree. The results caution against the use of community samples to examine impulsive psychopathic traits in relation to neural correlates, where the relationship between impulsivity and underlying neurobiology may be an inaccurate model of the relationship in clinical-level cases of psychopathy.
Supplementary Material
Contributor Information
Cole Korponay, Basic Neuroscience Division, McLean Hospital, Belmont, MA 02478, USA; Department of Psychiatry, Harvard Medical School, Cambridge, MA 02215, USA.
Michael Koenigs, Department of Psychiatry, University of Wisconsin–Madison, Madison, WI 53719, USA.
Funding
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism to M.K. (R01AA026290) and from the National Institute on Drug Abuse to C.K. (F32DA048580).
Conflict of interest
The authors report no conflicts of interest.
Supplementary data
Supplementary data are available at SCAN online.
References
- Ai H., Xin Y., Luo Y.J., Gu R., Xu P. (2019). Volume of motor area predicts motor impulsivity. European Journal of Neuroscience, 49(11), 1470–6. doi: 10.1111/ejn.14339 [DOI] [PubMed] [Google Scholar]
- Anderson B.J. (2011). Plasticity of gray matter volume: the cellular and synaptic plasticity that underlies volumetric change. Developmental Psychobiology, 53(5), 456–65. [DOI] [PubMed] [Google Scholar]
- Angelides N.H., Gupta J., Vickery T.J. (2017). Associating resting-state connectivity with trait impulsivity. Social Cognitive and Affective Neuroscience, 12(6), 1001–8.doi: 10.1093/scan/nsx031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteher B., Gaser C., Nenadic I. (2019). Brain structure and trait impulsivity: a comparative VBM study contrasting neural correlates of traditional and alternative concepts in healthy subjects. Neuropsychologia, 131, 139–47.doi: 10.1016/j.neuropsychologia.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Momenan R., Hommer D.W. (2009). Delay discounting correlates with proportional lateral frontal cortex volumes. BiologicalPsychiatry, 65(8), 710–3.doi: 10.1016/j.biopsych.2008.11.023 [DOI] [PubMed] [Google Scholar]
- Blonigen D.M., Carlson S.R., Krueger R.F., Patrick C.J. (2003). A twin study of self-reported psychopathic personality traits. Personality and Individual Differences, 35(1), 179–97. [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., et al. (2010). Dopaminergic network differences in human impulsivity. Science, 329(5991), 532.doi: 10.1126/science.1185778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnigo R., Noar S.M., Garnett C., Crosby R., Palmgreen P., Zimmerman R.S. (2013). Sensation seeking and impulsivity: combined associations with risky sexual behavior in a large sample of young adults. The Journal ofSex Research, 50(5), 480–8.doi: 10.1080/00224499.2011.652264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.S., Pellecchia G., Aminian K., et al. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26(3), 479–87.doi: 10.1007/s10548-012-0270-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L.S., van den Wildenberg W.P., van Wouwe N.C., Pannebakker M.M., Hommel B. (2009). Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. ExperimentalBrain Research, 196(3), 467–74.doi: 10.1007/s00221-009-1862-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodriguez O., Pujol J., Batalla I., et al. (2015a). Functional connectivity bias in the prefrontal cortex of psychopaths. BiologicalPsychiatry, 78(9), 647–55.doi: 10.1016/j.biopsych.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O., Pujol J., Batalla I., et al. (2015b). Functional connectivity bias in the prefrontal cortex of psychopaths. Biological Psychiatry, 78(9), 647–55.doi: 10.1016/j.biopsych.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Cope L.M., Shane M.S., Segall J.M., et al. (2012). Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Research: Neuroimaging, 204(2–3), 91–100.doi: 10.1016/j.pscychresns.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Pierce K., Schumann C.M., et al. (2007). Mapping early brain development in autism. Neuron, 56(2), 399–413.doi: 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69(4), 680–94.doi: 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Robbins T.W. (2017). Fractionating impulsivity: neuropsychiatric implications. Nature Reviews Neuroscience, 18(3), 158–71.doi: 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- de Oliveira-souza R., Hare R.D., Bramati I.E.. et al. (2008). Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. NeuroImage, 40(3), 1202–13.doi: 10.1016/j.neuroimage.2007.12.054 [DOI] [PubMed] [Google Scholar]
- de Wit H. (2009). Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology, 14(1), 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming P., Koenigs M. (2020). Structural and functional neural correlates of psychopathy: a meta-analysis of MRI data. Translational Psychiatry, 10(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deserno L., Wilbertz T., Reiter A., et al. (2015). Lateral prefrontal model-based signatures are reduced in healthy individuals with high trait impulsivity. TranslationalPsychiatry, 5, e659.doi: 10.1038/tp.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth A.L., Seligman M.E. (2005). Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychological Science, 16(12), 939–44.doi: 10.1111/j.1467-9280.2005.01641.x [DOI] [PubMed] [Google Scholar]
- Edens J.F., Poythress N.G. Jr., Lilienfeld S.O., Patrick C.J. (2008). A prospective comparison of two measures of psychopathy in the prediction of institutional misconduct. Behavioral Sciences & theLaw, 26(5), 529–41.doi: 10.1002/bsl.823 [DOI] [PubMed] [Google Scholar]
- Ermer E., Cope L.M., Nyalakanti P.K., Calhoun V.D., Kiehl K.A. (2013). Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry, 52(1), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani D.G., Lee B., Robertson C.L., et al. (2012). Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. Journal of Neuroscience, 32(21), 7316–24.doi: 10.1523/JNEUROSCI.4284-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Shi F., Woolson S.L., et al. (2012). Longitudinal development of cortical and subcortical gray matter from birth to 2 years. CerebralCortex, 22(11), 2478–85.doi: 10.1093/cercor/bhr327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., et al. (2010). Longitudinal changes in grey and white matter during adolescence. NeuroImage, 49(1), 94–103.doi: 10.1016/j.neuroimage.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Glenn A.L., Raine A., Yaralian P.S., Yang Y. (2010). Increased volume of the striatum in psychopathic individuals. BiologicalPsychiatry, 67(1), 52–8.doi: 10.1016/j.biopsych.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174–9.doi: 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves V.M., McLawsen J.E., Huss M.T., Scalora M.J. (2013). Factor structure and construct validity of the psychopathic personality inventory in a forensic sample. International Journal ofLaw and Psychiatry, 36(2), 176–84.doi: 10.1016/j.ijlp.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Grodin E.N., Cortes C.R., Spagnolo P.A., Momenan R. (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug and Alcohol Dependence, 179, 100–8.doi: 10.1016/j.drugalcdep.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D. (2003). The Hare Psychopathy Checklist-revised, 2nd edn, Toronto: Multi-Health Systems. [Google Scholar]
- Harpur T.J., Hare R.D., Hakstian A.R. (1989). Two-factor conceptualization of psychopathy: construct validity and assessment implications. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 1(1), 6–17. [Google Scholar]
- Holmes A.J., Hollinshead M.O., Roffman J.L., Smoller J.W., Buckner R.L. (2016). Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. The Journal of Neuroscience, 36(14), 4038–49.doi: 10.1523/JNEUROSCI.3206-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Tung H.C., Yang C., Tseng Y., Li C.R. (2017). Barratt impulsivity in healthy adults is associated with higher gray matter concentration in the parietal occipital cortex that represents peripheral visual field. Frontiers in Human Neuroscience, 11, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser A.S., Allen D.C., Navarro-Cebrian A., Mitchell J.M., Fields H.L. (2012). Dopamine, corticostriatal connectivity, and intertemporal choice. Journal of Neuroscience, 32(27), 9402–9.doi: 10.1523/JNEUROSCI.1180-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Hoffman M.B. (2011). The criminal psychopath: history, neuroscience, treatment, and economics. Jurimetrics, 51, 355–97. Available: http://www.ncbi.nlm.nih.gov/pubmed/24944437. [PMC free article] [PubMed] [Google Scholar]
- Korponay C., Dentico D., Kral T., et al. (2017a). Neurobiological correlates of impulsivity in healthy adults: lower prefrontal gray matter volume and spontaneous eye-blink rate but greater resting-state functional connectivity in basal ganglia-thalamo-cortical circuitry. NeuroImage, 157, 288–96.doi: 10.1016/j.neuroimage.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C., Pujara M., Deming P., et al. (2017b). Impulsive-antisocial dimension of psychopathy linked to enlargement and abnormal functional connectivity of the striatum. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(2), 149–57.doi: 10.1016/j.bpsc.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay C., Pujara M., Deming P., et al. (2017c). Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Social Cognitive and Affective Neuroscience, 12(7), 1169–78.doi: 10.1093/scan/nsx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera K.M., Schmitgen M.M., Maier-Hein K.H., Thomann P.A., Hirjak D., Wolf R.C. (2018). Differential contributions of cortical thickness and surface area to trait impulsivity in healthy young adults. BehaviouralBrain Research, 350, 65–71.doi: 10.1016/j.bbr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Lee A.K., Jerram M., Fulwiler C., Gansler D.A. (2011). Neural correlates of impulsivity factors in psychiatric patients and healthy volunteers: a voxel-based morphometry study. Brain Imaging and Behavior, 5(1), 52–64. [DOI] [PubMed] [Google Scholar]
- Lee B., London E.D., Poldrack R.A., et al. (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience, 29(47), 14734–40.doi: 10.1523/JNEUROSCI.3765-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb V., Leitner M., Wabnegger A., et al. (2015). Brain abnormalities in high-risk violent offenders and their association with psychopathic traits and criminal recidivism. Neuroscience, 308, 194–201.doi: 10.1016/j.neuroscience.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Li M., Hua K., Li S., et al. (2019). Cortical morphology of chronic users of codeine-containing cough syrups: association with sulcal depth, gyrification, and cortical thickness. European Radiology, 29(11), 5901–9.doi: 10.1007/s00330-019-06165-0 [DOI] [PubMed] [Google Scholar]
- Liu P., Feng T. (2017). The overlapping brain region accounting for the relationship between procrastination and impulsivity: a voxel-based morphometry study. Neuroscience, 360, 9–17. [DOI] [PubMed] [Google Scholar]
- Ly M., Motzkin J.C., Philippi C.L., et al. (2012). Cortical thinning in psychopathy. American Journal ofPsychiatry, 169(7), 743–9.doi: 10.1176/appi.ajp.2012.11111627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Nicoletti M., Nemoto K., et al. (2009). A voxel-based morphometry study of frontal gray matter correlates of impulsivity. HumanBrain Mapping, 30(4), 1188–95.doi: 10.1002/hbm.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S., Xu J., Carroll K.M., Potenza M.N. (2015). Self-reported impulsivity is negatively correlated with amygdalar volumes in cocaine dependence. Psychiatry Research: Neuroimaging, 233(2), 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F.G., Barratt E.S., Dougherty D.M., Schmitz J.M., Swann A.C. (2001). Psychiatric aspects of impulsivity. American Journal ofPsychiatry, 158(11), 1783–93.doi: 10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- Morgan J.E., Gray N.S., Snowden R.J. (2011). The relationship between psychopathy and impulsivity: a multi-impulsivity measurement approach. Personality and Individual Differences, 51, 429–34. [Google Scholar]
- Muller J.L., Sommer M., Dohnel K., Weber T., Schmidt-Wilcke T., Hajak G. (2008). Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behavioral Sciences and the Law, 26(1), 131–50.doi: 10.1002/bsl.796 [DOI] [PubMed] [Google Scholar]
- Patton J., Stanford M. (1995). Barratt impulsiveness scale, version 11 [BIS 11]. Sourcebook of Adult Assessment, 361–4. [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–74. Available: http://www.ncbi.nlm.nih.gov/pubmed/8778124. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. (2014). Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews, 38, 125–34.doi: 10.1016/j.neubiorev.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujara M., Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. (2014). Neural correlates of reward and loss sensitivity in psychopathy. Social Cognitive and Affective Neuroscience, 9(6), 794–801.doi: 10.1093/scan/nst054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.W., Lv X.F., Jiang G.H., et al. (2017). Potential gray matter unpruned in adolescents and young adults dependent on dextromethorphan-containing cough syrups: evidence from cortical and subcortical study. Brain Imaging and Behavior, 11(5), 1470–8. [DOI] [PubMed] [Google Scholar]
- Robertson C.L., Ishibashi K., Mandelkern M.A., et al. (2015). Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. The Journal of Neuroscience, 35(15), 5990–7.doi: 10.1523/JNEUROSCI.4850-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Beck A., Zois E., et al. (2019). Volumetric prefrontal cortex alterations in patients with alcohol dependence and the involvement of self-control. Alcoholism, Clinical and Experimental Research, 43(12), 2514–24.doi: 10.1111/acer.14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M., Caverzasi E., Lazzaretti M., et al. (2011). Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. Journal of Affective Disorders, 131(1–3), 417–21. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Muller B.W., Scherbaum N., et al. (2011). Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Archives of GeneralPsychiatry, 68(10), 1039–49.doi: 10.1001/archgenpsychiatry.2011.61 [DOI] [PubMed] [Google Scholar]
- Schilling C., Kuhn S., Romanowski A., Schubert F., Kathmann N., Gallinat J. (2012). Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage, 59(1), 824–30.doi: 10.1016/j.neuroimage.2011.07.058 [DOI] [PubMed] [Google Scholar]
- Smith S.S., Newman J.P. (1990). Alcohol and drug abuse-dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology, 99(4), 430–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/2266219. [DOI] [PubMed] [Google Scholar]
- Snowden R.J., Gray N.S. (2011). Impulsivity and psychopathy: associations between the barrett impulsivity scale and the psychopathy checklist revised. Psychiatry Research, 187(3), 414–7.doi: 10.1016/j.psychres.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Tu P.C., Kuan Y.H., Li C.T., Su T.P. (2017). Structural correlates of trait impulsivity in patients with bipolar disorder and healthy controls: a surface-based morphometry study. Psychological Medicine, 47(7), 1292–9.doi: 10.1017/S0033291716003299 [DOI] [PubMed] [Google Scholar]
- Walters G.D. (2003). Predicting institutional adjustment and recidivism with the psychopathy checklist factor scores: a meta-analysis. Law and Human Behavior, 27(5), 541–58. Available: http://www.ncbi.nlm.nih.gov/pubmed/14593797. [DOI] [PubMed] [Google Scholar]
- Yang Y.L., Raine A., Lencz T., Bihrle S., LaCasse L., Colletti P. (2005). Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry, 57(10), 1103–8.doi: 10.1016/j.biopsych.2005.01.021 [DOI] [PubMed] [Google Scholar]
- Zsido A.N., Darnai G., Inhof O., et al. (2019). Differentiation between young adult Internet addicts, smokers, and healthy controls by the interaction between impulsivity and temporal lobe thickness. Journal of Behavioral Addictions, 8(1), 35–47.doi: 10.1556/2006.8.2019.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


