Abstract
Background
Gastroparesis is a condition characterized by epigastric symptoms and delayed gastric emptying (GE) rate in the absence of any mechanical obstruction. The condition is challenging in clinical practice by the lack of guidance concerning diagnosis and management of gastroparesis.
Methods
A Delphi consensus was undertaken by 40 experts from 19 European countries who conducted a literature summary and voting process on 89 statements. Quality of evidence was evaluated using grading of recommendations assessment, development, and evaluation criteria. Consensus (defined as ≥80% agreement) was reached for 25 statements.
Results
The European consensus defined gastroparesis as the presence of symptoms associated with delayed GE in the absence of mechanical obstruction. Nausea and vomiting were identified as cardinal symptoms, with often coexisting postprandial distress syndrome symptoms of dyspepsia. The true epidemiology of gastroparesis is not known in detail, but diabetes, gastric surgery, certain neurological and connective tissue diseases, and the use of certain drugs recognized as risk factors. While the panel agreed that severely impaired gastric motor function is present in these patients, there was no consensus on underlying pathophysiology. The panel agreed that an upper endoscopy and a GE test are required for diagnosis. Only dietary therapy, dopamine‐2 antagonists and 5‐HT4 receptor agonists were considered appropriate therapies, in addition to nutritional support in case of severe weight loss. No consensus was reached on the use of proton pump inhibitors, other classes of antiemetics or prokinetics, neuromodulators, complimentary, psychological, or more invasive therapies. Finally, there was consensus that gastroparesis adversely impacts on quality of life and healthcare costs and that the long‐term prognosis of gastroparesis depends on the cause.
Conclusions and Inferences
A multinational group of European experts summarized the current state of consensus on definition, symptom characteristics, pathophysiology, diagnosis, and management of gastroparesis.
Keywords: consensus, endoscopy, gastric emptying, gastroparesis, guideline, prokinetic
Key Summary
Current knowledge
The epidemiology of gastroparesis is not well known.
Diagnosis and treatment of gastroparesis is challenging due to uncertainties in definition and optimal therapeutic approach.
What is new here
A Delphi panel consisting of 40 experts from 19 European countries established the level of consensus on 89 statements regarding gastroparesis.
The statements reaching consensus serve to guide clinicians in recognizing, diagnosing and treating gastroparesis in clinical practice.
The statements without consensus identify areas in need of future research.
INTRODUCTION
Gastroparesis is a condition characterized by epigastric symptoms (nausea, vomiting, postprandial fullness, early satiation, and epigastric pain) and significantly delayed gastric emptying (GE) rate in the absence of any mechanical obstruction. 1 , 2 Gastroparesis is a complication of diabetes, especially type 1 diabetes, and may also occur following upper gastrointestinal tract surgery. Nevertheless, in the largest subgroup no underlying cause is identified and these patients are referred to as having idiopathic gastroparesis. 1 , 2
The epidemiology of gastroparesis is unknown, as it requires procedures such as GE tests to make a firm diagnosis. In clinical gastroenterology practice, gastroparesis is frequently encountered and considered one of the more challenging conditions, as there are uncertainties in terms of definition, symptom spectrum, diagnosis and optimal therapeutic approach, especially as there is a paucity of interventions with established efficacy. 1 , 2 , 3 , 4
The aim of this project was to develop a European consensus on the definition, clinical characteristics, pathophysiological concepts, diagnosis and management of gastroparesis, with a focus on idiopathic gastroparesis. The results of this consensus can offer the clinician guidance in diagnosing and managing these patients, with the aim to optimizing outcomes.
METHODS
The European Society for Neurogastroenterology and Motility (ESNM) initiated a Delphi process, to develop consensus statements on different aspects of functional dyspepsia (FD) and gastroparesis in collaboration with other European societies. The Delphi approach, which combines the principles of evidence‐based medicine, supported by systematic literature reviews and a voting process, aims to determine consensus for complex problems in medicine for which evidence from controlled trials is lacking. 5
The principal steps in the process were: (1) selection of a working group of eight ESNM members with expertise in FD and gastroparesis and/or Delphi consensus processes; (2) selection of a European Consensus Group consisting of experts in FD and gastroparesis from different European countries, recruited through the ESNM board and through United European Gastroenterology (UEG) Federation Sister Societies; (3) drafting of statements allowing to evaluate the current knowledge on gastroparesis; (4) systematic literature reviews to identify evidence to support each statement; (5) two rounds of repeated voting of the statements and voting discussion until a stable level of consensus voting was reached; and (6) grading of the strength using accepted criteria.
For the Consensus Group, ESNM board members nominated experts from their respective national societies for participation, and the UEG Sister Societies (European Association for Gastroenterology, Endoscopy and Nutrition, European Helicobacter and Microbiota Study Group and the European Society for Primary Care Gastroenterology) nominated additional experts. A total of 40 experts from 19 European countries agreed to participate. Members had a background of expertise in gastroenterology, general practice, gastric physiology, or gastrointestinal motility. All members submitted a conflict of interest statement by December 2018. The ESNM FD guideline was finalized and published separately. 6
The eight‐member Core Group drafted and finalized a list of 65 statements covering several aspects of gastroparesis. The finalized list was evaluated in a first voting round by all members in the second quarter of 2019, where each member indicated the degree of agreement for the statement using a 6‐point Likert scale (Table 1). Participants were blinded to the votes of other participants and also gave feedback on clarity of the statement and made suggestions for adapting or splitting the statements into two or more questions, or for adding additional statements on a given topic. The Core Group adjusted the statement list, generating a total of 91 statements, and subdivided the Guideline Group members into 12 Working Groups with 3–4 members each. Each Working Group was allocated statements for which they needed to conduct a systematic literature search using several relevant keywords and provide narrative substantiation of the statements. The literature review and references were made available on a share‐point server, accessible to all members. This was finalized by the summer of 2019, followed by a voting round in which each statement was presented with the evidence summary, and each member indicated the degree of agreement for the statement using a 6‐point Likert scale (Table 1). Participants were blinded to the votes of other participants. Available members of the Guideline Group met in September 2019 at the ESNM meeting in Lisbon and in October 2019 at the UEG week in Barcelona, to discuss statements and voting outcomes. A final voting round was conducted after these meetings, finalized by early 2020 and focusing on statements that were adapted based on the evaluation at the ESNM meeting. Throughout the process, all votes were mutually anonymous and blinded.
TABLE 1.
6‐Point Likert scale
| Point | Description |
|---|---|
| A+ | Agree strongly |
| A | Agree with minor reservation |
| A− | Agree with major reservation |
| D− | Disagree with major reservation |
| D | Disagree with minor reservation |
| D+ | Disagree strongly |
Consensus was defined as when at least 80% of the Consensus Group agreed (A+ or A) with a statement. The strength of evidence for each statement was scored using the GRADE system (Table 2). 7 After the final voting round, the manuscript was drafted and circulated for final approval by the participants. The references cited in this chapter are only a selection of the articles reviewed in each area, chosen to clarify the discussion. A final meeting planned in October 2020 was canceled because of the COVID‐19 pandemic.
TABLE 2.
Grading of recommendations assessment, development and evaluation system 9
| Code | Quality of evidence | Definition |
|---|---|---|
| A | High | Further research is very unlikely to change our confidence in the estimate of effect
|
| B | Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
|
| C | Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
|
| D | Very low | Any estimate of effect is very uncertain
|
RESULTS
-
1.
Definitions and symptom descriptors
-
1.1Gastroparesis refers to a symptom or set of symptoms that is (are) associated with delayed GE in the absence of mechanical obstruction.STATEMENT ENDORSED, overall agreement 100%: A+ 60%, A 40%, A− 0%, D− 0%, D 0%, D+ 0%. GRADE A
-
1.2Gastroparesis refers to a symptom or set of symptoms that is (are) associated with severely disturbed gastric motor function in the absence of mechanical obstruction.STATEMENT ENDORSED, overall agreement 85%: A+ 48%, A 38%, A− 13%, D− 3%, D 0%, D+ 0%. GRADE B
-
1.3Nausea and vomiting are cardinal symptoms in gastroparesis.STATEMENT ENDORSED, overall agreement 95%: A+ 68%, A 28%, A− 5%, D− 0%, D 0%, D+ 0%. GRADE B
-
1.4Dyspeptic symptoms such as postprandial fullness, early satiation, epigastric pain, as well as bloating in the upper abdomen and belching are often present in gastroparesis patients.STATEMENT ENDORSED, overall agreement 95%: A+ 60%, A 35%, A− 5%, D− 0%, D 0%, D+ 0%. GRADE B
-
1.5Symptoms in gastroparesis patients overlap mainly with PDS and less with EPS symptoms of FD.STATEMENT ENDORSED, overall agreement 90%: A+ 45%, A 45%, A− 8%, D− 0%, D 3%, D+ 0%. GRADE B
-
1.1
The presence of symptomatic delayed GE is mandatory for a patient to be diagnosed with gastroparesis. 1 , 2 , 3 , 4 The delay in GE rate should be based on a well‐established reference range in healthy, asymptomatic controls (usually the 97.5th percentile, reflecting a 95% normal reference range). Asymptomatic delayed GE may be present especially in diabetic patients. 8 However, it has been proposed not to include asymptomatic subjects in the diagnosis of gastroparesis. 4 Mechanical obstruction, which can also lead to a delayed GE, should be excluded. 1 , 2 , 3 , 4
A variable range of symptoms may be present in patients with gastroparesis, including nausea, vomiting, early satiety, bloating, postprandial fullness, abdominal pain/discomfort, and anorexia. 4 , 9 , 10 , 11 , 12 A systematic review and meta‐analysis, which included only studies with a high‐quality emptying test showed significant associations between the presence of delayed GE and symptoms of nausea, vomiting, early satiety, postprandial fullness and epigastric pain. 9 Other studies also confirmed that gastroparesis symptoms have considerable overlap with FD defined by the Rome IV criteria. 4 , 10 , 11
A problem of this definition is that the association between delayed GE and symptoms may suggest that delayed GE is directly causing the symptoms. However, this conclusion should be considered with caution. 4 Symptoms occurring in subjects with delayed GE may also be associated with other types of gastric sensorimotor dysfunction, such as impaired gastric accommodation, hypersensitivity to gastric distention and uncoordinated intense motor activity in proximal small bowel. 12 , 13 , 14
Gastroparesis is a chronic condition and symptoms have to be present for some time before a diagnosis is triggered, and a timeframe of at least 3 months was proposed. 15 Nausea is the most common symptom in gastroparesis, affecting more than 95%, and has been associated with the severity of delay in emptying. 4 , 9 , 10 , 16 Vomiting is associated with nausea and with more severe delay in GE. 8 , 9 , 17 The recently published ESNM FD consensus identified early satiation, postprandial fullness and epigastric pain or burning as cardinal FD symptoms. 6 Nausea and vomiting are not cardinal symptoms of FD, thus allowing the symptom profiles of both entities to be distinguished. 4 , 18 , 19 Based on these observations, nausea and vomiting are considered cardinal symptoms of gastroparesis by this panel, providing a different symptom basis for both conditions. 1 , 4 , 18
Nevertheless, the symptoms of gastroparesis largely overlap with those of FD. A US study of 243 patients with idiopathic gastroparesis showed that 86% of patients fulfilled the ROME III criteria for FD, especially postprandial distress syndrome (PDS), which was present in 91%, compared to epigastric pain syndrome (EPS) in 1.2%. 11 Another study showed that of 357 patients referred for gastroparesis symptoms overlapping FD was found in 90.8%, especially PDS, which was present in 88.3% and EPS in 59.8%. 20 A number of studies have reported the presence of pain in a large subset of gastroparesis patients, although some of these studies included patients taking opioids, which is a major confounder. 21 , 22 In studies that exclude patients on opioids, pain is less prevalent, and it is also inconsistently correlated with GE delay. 4 , 8 , 9 , 10 , 11 , 12 , 13 , 16 , 17 , 20 Bloating is a common symptom of functional digestive disorders including FD and IBS, but its prevalence is high in gastroparesis patients. 9 , 10 , 12 , 16 , 17
-
2.
Epidemiology and risk factors
-
2.1
The epidemiology of gastroparesis is not established, mainly because it requires GE testing which has not been done at the population level.
STATEMENT ENDORSED, overall agreement 93%: A+ 40%, A 53%, A− 8%, D− 0%, D 0%, D+ 0%. GRADE B
-
2.2
Diabetes is a risk factor for development of gastroparesis.
STATEMENT ENDORSED, overall agreement 100%: A+ 85%, A 12%, A− 3%, D− 0%, D 0%, D+ 0%. GRADE A
-
2.3
Acute gastrointestinal infection is a risk factor for development of gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 59%: A+ 18%, A 40%, A− 33%, D− 3%, D 5%, D+ 3%. GRADE B
-
2.4
Partial gastric resection/vagotomy, bariatric surgery, antireflux surgery are risk factors for development of gastroparesis
STATEMENT ENDORSED, overall agreement 85%: A+ 40%, A 45%, A− 13%, D− 0%, D 3%, D+ 0%. GRADE B
-
2.5
Hypothyroidism is a risk factor for development of gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 56%: A+ 23%, A 33%, A− 28%, D− 10%, D 8%, D+ 0%. GRADE C
-
2.6
Some neurological disorders (e.g., Parkinson's disease, multiple sclerosis, amyloid neuropathy) are associated with increased risk for gastroparesis.
STATEMENT ENDORSED, overall agreement 90%: A+ 33%, A 55%, A− 10%, D− 0%, D 3%, D+ 0%. GRADE B
-
2.7
Some connective tissue diseases are associated with increased risk for gastroparesis.
STATEMENT ENDORSED, overall agreement 85%: A+ 43%, A 43%, A− 13%, D− 3%, D 0%, D+ 0%. GRADE B
-
2.8
Some drugs (e.g., opioids) are associated with increased risk for gastroparesis.
STATEMENT ENDORSED, overall agreement 100%: A+ 63%, A 38%, A− 0%, D− 0%, D 0%, D+ 0%. GRADE A
-
2.1
As a diagnosis of gastroparesis requires symptoms, an objective demonstration of delayed GE and absence of a mechanical obstruction, 1 , 2 , 3 , 4 no population‐based studies have addressed its prevalence. Currently, analyses of diagnostic records and procedures suggest a low prevalence of formally diagnosed gastroparesis. In Olmsted county, based on hospital records and population adjustments, the estimated incidence was 6.3 (4.9–7.7) per 100,000 person‐years. The age‐adjusted prevalence of gastroparesis per 100,000 persons was 24.2 (15.7–32.6), with a female predominance (82% female). 23 An analysis of the UK Clinical Practice Research Datalink (CPRD) database generated standardized prevalence of 13.8 (95% confidence interval [CI]: 12.6–15.1) per 100,000 persons in 2016, and standardized incidence of 1.9 (95% CI: 1.4–2.3) per 100,000 person‐years in 2016, with female predominance (64% female). 24
In these cohorts, the idiopathic subgroup is the largest, but a high prevalence of gastroparesis is found among diabetic patients, with 25 and 38% of patients identified in the respective studies. 23 , 24 Gastroparesis occurs more frequently in type 1 diabetes compared to type 2. 2 , 4 In a population‐based, historical cohort study, the cumulative proportions developing gastroparesis over a 10‐year time period were 5.2% in type 1 DM, 1.0% in type 2 DM, compared to 0.2% in controls. 25
There is limited evidence suggesting that (viral) gastrointestinal infections may cause postinfectious gastroparesis. In a single center cohort, 52 out of 143 patients diagnosed with gastroparesis, 52 were idiopathic in origin, with 12 of them considered to be consistent with a postviral etiology. 26 In the NIDDK cohort, of 243 subjects with idiopathic gastroparesis, 19% reported a history suggestive of infectious etiology. 17 Small studies reported viral presence in the mucosa of patients with gastroparesis. 27 , 28
Postsurgical gastroparesis is well established and may occur after gastric surgery, antireflux surgery, lung transplant, pancreaticoduodenectomy and esophagectomy. 29 , 30 Hypothyroidism is more frequent in gastroparesis compared to the general population (14% of patients vs. 8.2% in controls). 31 Several neurological and systemic disorders have also been linked to gastroparesis, including Parkinson's disease, systemic sclerosis, and so on. 23 , 31 , 32 , 33
Several drugs can retard GE, of which opioids have the greatest impact. 1 , 4 Opioids may induce or worsen gastroparesis and its symptoms, and it has been argued that patients on opioids should not be considered as gastroparesis unless the diagnosis is confirmed off opioids. 1 , 4 , 21 , 34
-
3.
Impact of gastroparesis
-
3.1
Gastroparesis is a major source of healthcare costs.
STATEMENT ENDORSED, overall agreement 85%: A+ 35%, A 50%, A− 13%, D− 0%, D 3%, D+ 0%. GRADE A
-
3.2
Gastroparesis is associated with decreased life expectancy.
STATEMENT NOT ENDORSED, overall agreement 18%: A+ 5%, A 13%, A− 38%, D− 18%, D 23%, D+ 3%. GRADE B
-
3.3
Gastroparesis is a major source of self‐costs to patients.
STATEMENT NOT ENDORSED, overall agreement 65%: A+ 18%, A 48%, A− 25%, D− 3%, D 5%, D+ 0%. GRADE B
-
3.4
Gastroparesis is an important source of loss of work productivity.
STATEMENT NOT ENDORSED, overall agreement 78%: A+ 28%, A 50%, A− 20%, D− 0%, D 3%, D+ 0%. GRADE B
-
3.5
Gastroparesis is associated with a significant decrease in quality of life.
STATEMENT ENDORSED, overall agreement 93%: A+ 68%, A 25%, A− 8%, D− 0%, D 0%, D+ 0%. GRADE A
-
3.6
Gastroparesis is associated with psychosocial comorbidities such as anxiety and depression.
STATEMENT ENDORSED, overall agreement 83%: A+ 33%, A 50%, A− 15%, D− 3%, D 0%, D+ 0%. GRADE A
-
3.7
Weight loss can be a consequence of gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 70%: A+ 40%, A 30%, A− 28%, D− 3%, D 0%, D+ 0%. GRADE B
-
3.8
In case of weight loss, eating disorders must be ruled out.
STATEMENT ENDORSED, overall agreement 98%: A+ 40%, A 58%, A− 3%, D− 0%, D 0%, D+ 0%. GRADE B
-
3.9
Healthcare consulting behavior in gastroparesis is driven by symptom severity and impact.
STATEMENT NOT ENDORSED, overall agreement 78%: A+ 20%, A 58%, A− 18%, D− 3%, D 3%, D+ 0%. GRADE B
-
3.10
Healthcare consulting behavior in gastroparesis is driven by psychosocial comorbidity.
STATEMENT NOT ENDORSED, overall agreement 40%: A+ 15%, A 25%, A− 38%, D− 8%, D 15%, D+ 0%. GRADE B
-
3.11
Healthcare consulting behavior in gastroparesis is driven by access to the healthcare system.
STATEMENT NOT ENDORSED, overall agreement 54%: A+ 10%, A 43%, A− 35%, D− 8%, D 5%, D+ 0%. GRADE B
-
3.1
Only a few studies, mainly from the United States, have investigated the impact of gastroparesis on healthcare costs. Based on the National Inpatient Sample Database, which is designed to include approximately 95% of the US population, a remarkable 300% increase in gastroparesis‐related admissions occurred in the United States between 1997 and 2013, with a decrease in average length of stay but an increase in the cost of each hospitalization with time. 35 Over the same time period, emergency department visits for gastroparesis more than doubled, and this was also associated with substantial healthcare costs. 36
In a population‐based study in Olmsted county, overall survival of subjects diagnosed with gastroparesis was significantly lower than the age‐ and sex‐specific expected survival computed for the reference population, and a higher mortality was seen in diabetic versus idiopathic gastroparesis. 23 In the UK CPRD database study, mortality rates were also higher in diabetic compared to idiopathic gastroparesis patients. 24 However, in these studies the excess mortality was largely driven by cardiovascular comorbidity.
No data are available on self‐cost to gastroparesis patients, but they may incur both direct and indirect costs for use of OTC medications (antiemetics, antacids, and H2 receptor antagonists), alternative therapies, medical consultations, and cofinanced treatments, as well as the cost of some dietary adjustments and nutritional support, which is often not reimbursed.
In a small US gastroparesis patient cohort, the majority of patients surveyed (54%) was not employed and considered disabled. 37 Employment rates are even lower in those on opioids. 38 In a survey of almost 500 gastroparesis patients, the symptoms reduced daily activities in 67.5% and lowered annual income in 28.5%; 11% were disabled due to gastroparesis symptoms. 39 Quality of life was significantly decreased in this cohort. Also, in a large online survey of over 1400 self‐reported gastroparesis patients, quality of life as measured by the 36‐item short‐form health survey was decreased, mainly on the physical health component, and this was negatively correlated with increased symptom severity, especially nausea, early satiety, and abdominal pain. 40
In a study of 299 gastroparesis patients enrolled from US referral centers, anxiety and depression scores correlated with the severity of gastroparesis symptoms but did not differ between diabetic and idiopathic gastroparesis and did not correlate with the delay in GE rate. 41 In a systematic review, based on three studies in gastroparesis (n = 378) combined anxiety/depression was present in 24% of patients, severe anxiety in 12.4%, depression in 23%, and somatization in 50%. 42
Although it is often assumed that gastroparesis may lead to weight loss, this is not substantiated in the literature. In large cohorts of patients with dyspeptic symptoms, delayed GE was not associated with weight loss. 8 , 43 Gastroparesis may affect adolescent and young patients. When accompanied by weight loss, an eating disorder should be excluded, especially since delayed GE is a feature of anorexia nervosa. 44 , 45
Healthcare consulting in gastroparesis is likely driven by symptom severity and impact, but no data are available from the literature. Moreover, it is not known whether healthcare consulting is driven by psychosocial comorbidity, although this seems plausible given its correlation with symptom severity. 41 A study in the United States showed regional differences in healthcare assistance in gastroparesis patients, with admissions rates independently predicted by high overall hospitalizations within a state, suggesting that access to healthcare is a factor determining healthcare consulting. 46
-
4.
Pathophysiology of gastroparesis
-
4.1
Delay in GE underlies symptom generation in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 28%: A+ 10%, A 18%, A− 48%, D− 13%, D 10%, D+ 0%. GRADE B
-
4.2
Impaired gastric accommodation contributes to symptom generation in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 75%: A+ 20%, A 55%, A− 20%, D− 3%, D 3%, D+ 0%. GRADE B
-
4.3
Hypersensitivity to gastric distention contributes to symptom generation in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 73%: A+ 18%, A 55%, A− 20%, D− 5%, D 3%, D+ 0%. GRADE B
-
4.4
Duodenal mucosal alterations (low grade inflammation, impaired permeability) are not implicated as pathophysiological mechanisms in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 58%: A+ 10%, A 48%, A− 25%, D− 10%, D 8%, D+ 0%. GRADE C
-
4.5
Loss of interstitial cells of Cajal is a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 60%: A+ 10%, A 50%, A− 33%, D− 0%, D 8%, D+ 0%. GRADE B
-
4.6
Loss of enteric nerves is a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 65%: A+ 5%, A 60%, A− 30%, D− 0%, D 5%, D+ 0%. GRADE B
-
4.7
Primary changes in gastric smooth muscle are a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 53%: A+ 8%, A 45%, A− 38%, D− 5%, D 5%, D+ 0%. GRADE B
-
4.8
Loss of agus nerve function is a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 68%: A+ 8%, A 60%, A− 30%, D− 3%, D 0%, D+ 0%. GRADE B
-
4.9
Helicobacter pylori infection is not a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 60%: A+ 18%, A 43%, A− 23%, D− 3%, D 13%, D+ 3%. GRADE B
-
4.10
Altered gastric acid secretion is not a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 78%: A+ 23%, A 55%, A− 15%, D− 5%, D 3%, D+ 0%. GRADE C
-
4.11
Altered release of peptide hormones is not a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 60%: A+ 5%, A 55%, A‐ 23%, D‐ 8%, D 8%, D+ 3%. GRADE C
-
4.12
Increased sensitivity to duodenal luminal content is not a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 70%: A+ 3%, A 68%, A‐ 18%, D‐ 8%, D 5%, D+ 0%. GRADE C
-
4.13
Altered duodenal microbiota composition is not a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 59%: A+ 10%, A 48%, A− 25%, D− 10%, D 5%, D+ 3%. GRADE C
-
4.14
Anxiety is a pathophysiological factor in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 43%: A+ 10%, A 33%, A− 28%, D− 15%, D 13%, D+ 3%. GRADE B
-
4.15
Depression is a pathophysiological factor in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 43%: A+ 8%, A 35%, A− 28%, D− 13%, D 15%, D+ 3%. GRADE B
-
4.16
Disordered processing of incoming signals from the gastroduodenal region is a pathophysiological mechanism in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 58%: A+ 5%, A 53%, A− 25%, D− 10%, D 5%, D+ 3%. GRADE B
-
4.17
Genetic factors determine susceptibility to gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 55%: A+ 0%, A 55%, A− 30%, D− 8%, D 8%, D+ 0%. GRADE C
-
4.1
Symptoms of gastroparesis, either alone or collectively, have proven to be poor predictors of the rate of GE or response to prokinetic agents. However, a number of observations showed a tendency for more severe symptom burden with more severely delayed emptying, but the association is inconsistent. 1 , 2 , 3 , 4 , 8 , 9 , 10 , 11 , 12 , 13 , 16 , 17 , 20 , 21 , 32 A systematic review and meta‐analysis, including only studies with a high‐quality emptying test showed significant associations between the presence of delayed GE and symptoms of nausea, vomiting, early satiety, postprandial fullness, and epigastric pain. 9 However, the relationship between symptom severity and emptying delay remains poor.
In idiopathic gastroparesis, barostat studies demonstrated that 43% of patients had impaired accommodation, associated with a higher prevalence of weight loss and early satiety, 29% of patients had hypersensitivity to gastric distension, associated with higher rates of early satiety, epigastric pain, and weight loss. 12 In diabetic gastroparesis, gastric accommodation to a meal and sensory thresholds for discomfort were lower compared to healthy controls. 13 Impaired duodenal mucosal integrity, with mucosal infiltration with eosinophils and mast cells, has been reported as a putative pathophysiological mechanism in FD but studies in gastroparesis seem to be lacking. 47
Histopathological studies in an animal model of type 1 diabetes and transmural biopsy specimens from gastroparesis patients showed decreased density of interstitial cells of Cajal, possibly due to switch from anti‐inflammatory M2 macrophages to pro‐inflammatory M1 macrophages. 48 , 49 Conflicting data exist on a possible decrease of electrically coupled platelet‐derived growth factor receptor α‐fibroblast‐like cells in gastroparesis. 50 , 51
In small patient cohort studies, fibrotic and inflammatory changes to gastric smooth muscle were reported in diabetic gastroparesis patients, but this was not confirmed in the larger NIDDK consortium cohort. 48 , 49 , 52
In a study which evaluated heart‐rate variability responses in 41 gastroparesis patients, diabetic gastroparesis was more frequently associated with signs of vagal dysfunction than idiopathic gastroparesis. 53 In a large study of 242 patients, there were signs of both sympathetic and parasympathetic dysfunction, the latter associated with more severe symptoms and more delayed GE. 54
Few studies have evaluated the relationship between H. pylori status and GE rate, but no consistent correlation was found. 55 , 56 , 57 No studies evaluated gastric acid secretion, duodenal sensitivity to acid or lipids or duodenal microbiota composition in gastroparesis. There is also no consistent proof of altered release of gut peptides contributing to gastroparesis pathophysiology. 58
Several authors have reported higher degrees of anxiety and depression in gastroparesis patients. In those studies, anxiety and depression scores were related to gastroparesis symptom severity and hospitalization rates but not to disease etiology and GE rates. 16 , 17 , 21 , 31
Both in idiopathic and in diabetic gastroparesis, hypersensitivity to gastric distension is present and, in the former group, is associated with the symptom pattern and severity (higher rates of early satiety, epigastric pain, and weight loss). 12 , 13 As gastric compliance is not altered in these patients, the pathophysiology is likely to involve altered processing of incoming signals in the central nervous system. One study using evoked potentials showed altered processing of esophageal electrical stimulation signals in the brain in patients with diabetic neuropathy, and this was related to upper gastrointestinal symptom severity and quality of life impact. 59 An fMRI study showed altered connectivity of the insula and a tendency towards decreased insula gray matter volume in gastroparesis patients. 60
Only a few studies have evaluated genetic factors that predispose for the development of gastroparesis. A long repeat polymorphism in the heme oxygenase (HO1) has been associated with worse outcomes in several diseases, including gastroparesis, and the presence of longer polyGT repeats in the HMOX1 promoter results in lower transcriptional activity compared to shorter repeats. 61
-
5.
Diagnosis
-
5.1
Upper gastrointestinal endoscopy is mandatory for establishing a diagnosis of gastroparesis.
STATEMENT ENDORSED, overall agreement 93%: A+ 75%, A 18%, A− 8%, D− 0%, D 0%, D+ 0%. GRADE A
-
5.2
The presence of food in fasting state during endoscopy is diagnostic for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 40%: A+ 10%, A 30%, A− 30%, D− 10%, D 15%, D+ 5%. GRADE B
-
5.3
An abnormal GE test is mandatory for establishing a diagnosis of gastroparesis.
STATEMENT ENDORSED, overall agreement 95%: A+ 80%, A 15%, A− 3%, D− 0%, D 3%, D+ 0%. GRADE A
-
5.4
Scintigraphic GE assessment is a valid test for diagnosing gastroparesis.
STATEMENT ENDORSED, overall agreement 98%: A+ 88%, A 10%, A− 3%, D− 0%, D 3%, D+ 0%. GRADE A
-
5.5
Breath test assessment is a valid test for diagnosing gastroparesis.
STATEMENT ENDORSED, overall agreement 95%: A+ 60%, A 35%, A− 0%, D− 3%, D 3%, D+ 0%. GRADE A
-
5.6
Wireless motility capsule assessment is a valid test for diagnosing gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 33%: A+ 8%, A 25%, A− 48%, D− 5%, D 15%, D+ 0%. GRADE B
-
5.7
Gastric ultrasound assessment is a valid test for diagnosing gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 18%: A+ 5%, A 13%, A− 48%, D− 5%, D 28%, D+ 3%. GRADE B
-
5.8
Small bowel obstruction can be ruled out in gastroparesis through imaging.
STATEMENT ENDORSED, overall agreement 88%: A+ 25%, A 63%, A− 10%, D− 0%, D 3%, D+ 0%. GRADE B
-
5.1
Esophagogastroduodenoscopy (EGD), is a prerequisite for the diagnosis of gastroparesis to be made. Most guidelines dictate that an EGD should be performed before motility testing is considered in patients with upper abdominal symptoms not responding to first‐line therapy to exclude gastric outlet obstruction. 1 , 2 , 19
Food retention in the stomach as seen during EGD after an overnight fast has been used as a probable sign of gastroparesis in epidemiological studies, even in the presence of a normal GE test. 23 Only one retrospective analysis study specifically addressed the relationship between GE and food retention, suggesting that gastric food retention at endoscopy could be a moderately specific sign for gastroparesis, but with poor sensitivity. 62
The definition of gastroparesis implies objective delayed GE in the absence of mechanical obstruction. To date, GE scintigraphy of a solid‐phase meal is considered the gold standard, and by consensus, the test is performed using a 99mTechnetium‐labeled standardized low‐fat, egg meal with imaging at 0, 1, 2, and 4 h after meal ingestion. 63 The scintigraphic emptying test is fairly reproducible between repeated studies in patients with upper gastrointestinal symptoms, with a concordance correlation coefficient of 0.54 for GE assessment at 4 h, and of 0.79 for GE half time. 64 GE breath test (GEBT) is an FDA‐approved method for the evaluation of GE. GEBT incorporates a stable isotope, 13C, in a substrate such as octanoic acid, acetate or spirulina platensis. This non‐invasive method is easy to perform, with similar or lower cost to scintigraphy, and does not involve exposing patients to ionizing radiation. Previous studies have shown high correlation (0.73–0.95) between scintigraphy and breath test GE times. 65
The wireless motility capsule (WMC) is an FDA‐approved device for the evaluation of GE. A systematic review from 2013 that included seven studies found that for the diagnosis of gastroparesis, as compared with gastric scintigraphy, WMC had a sensitivity of 59%–86% and specificity of 64%–81%. 66 The pitfall with WMC is that it is an indigestible solid and, therefore, it empties from the stomach in response to phase 3 migrating motor complexes rather than with the test meal. A further disadvantage is that WMC is relatively expensive and its availability across different centers is limited.
Gastric ultrasonography has been used to assess antral wall motion, patterns of transpyloric flow, and GE based on changes in the cross‐sectional area or diameter of the gastric antrum. Gastric ultrasonography is noninvasive, safe, cheap, widely available, allows for bedside monitoring, does not expose the patient to ionizing radiation which is restricted particularly in children and pregnant women, and shows reasonably good interobserver agreement in the evaluation of liquid GE. 66 However, ultrasonography is unable to distinguish between the solid and liquid components of a meal and therefore is unsuitable to assess emptying of solids. Ultrasonography also requires an experienced technician, is user dependent, may be influenced by the presence of intragastric air or posture and is generally considered impractical for prolonged observations. 67
When small bowel obstruction is suspected, patients generally undergo abdominal radiography which is widely available, inexpensive and has a reported accuracy of 50%–86%. Where small bowel obstruction is strongly suspected, CT scanning is the most accurate examination. 68
-
6.
Treatment
-
6.1
Dietary adjustments are useful for managing gastroparesis patients.
STATEMENT ENDORSED, overall agreement 85%: A+ 35%, A 50%, A− 15%, D− 0%, D 0%, D+ 0%. GRADE B
-
6.2
Proton pump inhibitor (PPI) therapy is the most appropriate first line therapy for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 0%: A+ 0%, A 0%, A− 18%, D− 13%, D 55%, D+ 15%. GRADE C
-
6.3
PPI therapy is effective in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 10%: A+ 0%, A 10%, A− 15%, D− 23%, D 48%, D+ 5%. GRADE C
-
6.4
PPI therapy is only effective for associated reflux symptoms in gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 70%: A+ 15%, A 55%, A− 25%, D− 3%, D 3%, D+ 0%. GRADE C
-
6.5
Antiemetic (anti‐“nauseant”) therapy is the most appropriate first line therapy for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 43%: A+ 5%, A 38%, A− 33%, D− 10%, D 15%, D+ 0%. GRADE B
-
6.6
Antiemetic (anti‐“nauseant”) therapy is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 48%: A+ 8, A 40%, A− 45%, D− 3%, D 5%, D+ 0%. GRADE B
-
6.7
Dopamine‐2 antagonists are effective for gastroparesis.
STATEMENT ENDORSED, overall agreement 86%: A+ 23%, A 63%, A− 15%, D− 0%, D 0%, D+ 0%. GRADE B
-
6.8
5‐HT3 antagonists are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 63%: A+ 5%, A 58%, A− 33%, D− 5%, D 0%, D+ 0%. GRADE B
-
6.9
NK1 antagonists are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 40%: A+ 5%, A 35%, A− 48%, D− 3%, D 10%, D+ 0%. GRADE B
-
6.10
Prokinetic therapy is the most appropriate first line therapy for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 78%: A+ 38%, A 40%, A− 15%, D− 0%, D 8%, D+ 0%. GRADE B
-
6.11
Prokinetic therapy is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 75%: A+ 18%, A 58%, A− 23%, D− 0%, D 3%, D+ 0%. GRADE B
-
6.12
The efficacy of prokinetics is not related to their enhancement of GE rate.
STATEMENT NOT ENDORSED, overall agreement 55%: A+ 10%, A 45%, A− 35%, D− 5%, D 5%, D+ 0%. GRADE B
-
6.13
Itopride is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 35%: A+ 5%, A 30%, A− 23%, D− 23%, D 20%, D+ 0%. GRADE B.
-
6.14
5‐HT4 agonists are effective for gastroparesis.
STATEMENT ENDORSED, overall agreement 85%: A+ 18%, A 68%, A− 15%, D− 0%, D 0%, D+ 0%. GRADE B
-
6.15
Motilin‐receptor agonists are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 58%: A+ 10%, A 48%, A− 40%, D− 0%, D 0%, D+ 3%. GRADE B
-
6.16
Ghrelin‐receptor agonists are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 40%: A+ 8%, A 33%, A− 53%, D− 3%, D 3%, D+ 3%. GRADE B
-
6.17
Tricyclic antidepressants are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 8%: A+ 3%, A 5%, A− 25%, D− 23%, D 40%, D+ 5%. GRADE B
-
6.18
Tricyclic antidepressants are not effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 65%: A+ 8%, A 53%, A− 15%, D− 13%, D 13%, D+ 0%. GRADE B
-
6.19
SSRI are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 10%: A+ 0%, A 10%, A− 18%, D− 20%, D 50%, D+ 3%. GRADE C
-
6.20
SSRI are not effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 63%: A+ 10%, A 53%, A− 13%, D− 13%, D 13%, D+ 0%. GRADE C
-
6.21
SNRI are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 0%: A+ 0%, A 0%, A− 13%, D− 30%, D 55%, D+ 3%. GRADE C
-
6.22
SNRI are not effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 63%: A+ 10%, A 53%, A− 25%, D− 5%, D 8%, D+ 0%. GRADE C
-
6.23
Mirtazapine is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 35%: A+ 5%, A 30%, A− 50%, D− 5%, D 10%, D+ 0%. GRADE B
-
6.24
5‐HT1A agonists (tandospirone, buspirone, ….) are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 15%: A+ 0%, A 15%, A− 20%, D− 30%, D 33%, D+ 3%. GRADE C
-
6.25
5‐HT1A agonists are not effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 40%: A+ 5%, A 35%, A− 33%, D− 5%, D 23%, D+ 0%. GRADE C
-
6.26
Gastric electrical stimulation (GES) is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 38%: A+ 15%, A 23%, A− 38%, D− 15%, D 10%, D+ 0%. GRADE B
-
6.27
Pyloric botulinum toxin injection is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 13%: A+ 0%, A 13%, A− 38%, D− 20%, D 25%, D+ 5%. GRADE B
-
6.28
Pyloric myotomy is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 28%: A+ 5%, A 23%, A− 43%, D− 10%, D 20%, D+ 0%. GRADE B
-
6.29
Partial or subtotal gastrectomy is effective for gastroparesis. STATEMENT NOT ENDORSED, overall agreement 18%: A+ 3%, A 15%, A− 38%, D− 8%, D 33%, D+ 5%. GRADE B
-
6.30
Surgical or pyloric targeting therapies for gastroparesis may induce dumping syndrome.
STATEMENT NOT ENDORSED, overall agreement 35%: A+ 5%, A 30%, A− 40%, D− 8%, D 18%, D+ 0%. GRADE B
-
6.31
H. Pylori‐infected gastroparesis patients should receive eradication therapy.
STATEMENT NOT ENDORSED, overall agreement 5%: A+ 20%, A 55%, A− 18%, D− 0%, D 8%, D+ 0%. GRADE B
-
6.32
Herbal therapies are effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 10%: A+ 0%, A 10%, A− 45%, D− 20%, D 23%, D+ 3%. GRADE B
-
6.33
Herbal therapies are not effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 23%: A+ 3%, A 20%, A− 23%, D− 38%, D 15%, D+ 3%. GRADE B
-
6.34
Iberogast (STW‐5) is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 20%: A+ 8%, A 13%, A− 33%, D− 13%, D 35%, D+ 0%. GRADE B
-
6.35
Hypnotherapy is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 8%: A+ 0%, A 8%, A− 20%, D− 25%, D 43%, D+ 5%. GRADE B
-
6.36
Cognitive behavioral therapy is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 15%: A+ 0%, A 15%, A− 23%, D− 18%, D 40%, D+ 5%. GRADE B
-
6.37
Acupuncture is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 3%: A+ 0%, A 3%, A− 43%, D− 5%, D 45%, D+ 5%. GRADE B
-
6.38
Mindfulness is effective for gastroparesis.
STATEMENT NOT ENDORSED, overall agreement 13%: A+ 0%, A 13%, A− 10%, D− 25%, D 48%, D+ 5%. GRADE B
-
6.39
In case of severe weight loss or intractable vomiting, nutritional support may be needed in the form of enteral or parenteral nutrition.
STATEMENT ENDORSED, overall agreement 98%: A+ 53%, A 45%, A− 3%, D− 0%, D 0%, D+ 0%. GRADE B
-
6.1
A diet composed of small particle size reduces gastroparesis and reflux symptoms in patients with diabetic gastroparesis. 69 In addition, gastroparesis carries an increased risk for a diet deficient in calories, which can be corrected by nutritional support and engagement with dieticians. 17
The efficacy of PPIs in gastroparesis has not been addressed in controlled trials. However, PPI intake is high in gastroparesis patients (70% or more) and more than 50% have an overlapping GERD diagnosis. 17 , 70 It has been suggested that the coexisting GERD rather than gastroparesis is the explanation for the PPI intake.
While they are recommended as first line symptomatic treatment, there are no formal studies establishing efficacy of traditional antiemetic agents in the treatment of nausea and vomiting associated with gastroparesis. 71 In the United States, metoclopramide, a dopamine‐2 receptor antagonist, is approved for the treatment of gastroparesis. However, it carries a black box warning, as it is generally not well‐tolerated and chronic use (>12 weeks) may lead to extrapyramidal side effects and potential irreversible tardive dyskinesia, which has been reported in a small percentage of cases. 71 In a phase 2, randomized controlled trial (RCT) on 285 diabetic patients with gastroparesis, 10 or 14 mg metoclopramide doses, administrated via nasal spray could not reduce the overall symptom scores significantly more than placebo. 72 However, when males and females were analyzed separately, a significant effect was observed in females. Reported adverse events were mainly headache, fatigue, and dysgeusia. Domperidone is a peripherally acting dopamine‐2 antagonist that decreases nausea and increases GE rates. It does not readily cross the blood–brain barrier, making it much less likely to cause extrapyramidal side effects. However, domperidone is associated with prolongation of the cardiac QTc interval which has restricted its use. 73
The 5‐HT3 antagonists, such as ondansetron or granisetron, also have antiemetic properties, but controlled studies in gastroparesis are lacking. An open label study suggested efficacy of granisetron transdermal patches for controlling nausea and vomiting in gastroparesis. 74 Aprepitant, a neurokinin‐1 receptor antagonist approved for use for the treatment of chemotherapy‐induced emesis, was efficacious in the treatment of nausea in some patients with gastroparesis and related disorders. 75
Clinical trials of prokinetic agents report that, as a group, they enhance symptoms and improve GE rate. 76 However, there is disparity, as the relationship between improvement of symptoms and enhancement of GE rate is poor but becomes significant when only high‐quality emptying studies are considered. 76 , 77 The availability of prokinetics is limited in many parts of the world, but new prokinetic agents are in the pipeline for the treatment of gastroparesis. 78 Nevertheless, GE rate enhancement per se cannot be used as a marker of clinical efficacy. 79 Based on these considerations, the panel did not support efficacy of prokinetics as a treatment group. The panel did support the classes of dopamine‐2 antagonists and 5‐HT4 agonists as having efficacy for treating gastroparesis symptoms.
Itopride, available in Asia and to some extent in Eastern Europe, is a combined dopamine‐2 antagonist and cholinesterase inhibitor, which has been extensively studied in FD. A small controlled cross‐over study evaluating the effect of itopride 200 mg t. i.d. in 25 diabetic gastroparesis patients found no significant effect. 80
Cisapride was for a long time the preferred medication for outpatient treatment of gastroparesis but has been withdrawn from the market because of cardiologic side effects. 81 Tegaserod, another 5‐HT4 agonist, showed ability to enhance GE but was temporarily withdrawn from the market and only developed for bowel disorders. 82 Prucalopride, a 5‐HT4 agonist belonging to another chemical class, is approved in most countries for the treatment of chronic constipation. In a recent study of mainly idiopathic gastroparesis patients, prucalopride was efficacious in improving GE rate and symptoms, while a second study in mainly diabetic gastroparesis patients failed to show benefit. 83 , 84
Several motilin‐receptor agonists have been studied as potential treatment of gastroparesis over the past 20 years: Camicinal (GSK962040), RaQualia (RQ‐00201864), Mitemcinal (GM‐611), and so on. 85 Nevertheless, further research, investigating these drugs in RCTs, is needed. Macrolide antibiotics such as erythromycin, which may also act on the motilin receptor, are clinically available and have been studied. 58 , 85 , 86 , 87 The use of antibiotics to increase gastric motility includes risks and side effects, such as resistance or tachyphylaxis. 85 Three Ghrelin‐receptor agonists, TZP‐101, TZP‐102, and RM‐131 (relamorelin), have been studied in gastroparesis. Studies with TZP‐101 and TZP‐102 showed inconsistent efficacy results in diabetic gastroparesis. 85 With regard to relamorelin, two randomized, placebo‐controlled trials, with a significant number of included patients have been published. 88 , 89 A phase 3 program in diabetic gastroparesis was in progress but has recently been discontinued.
Neuromodulators are often used for managing refractory symptoms in functional and motility disorders. 90 The only gastroparesis focused study was performed with nortriptyline in 130 idiopathic gastroparesis patients, showing no gain over placebo. 91 A second multicenter, randomized, double‐blind controlled study compared amitriptyline 50 mg with escitalopram 10 mg in patients with FD. 92 Only amitriptyline appeared beneficial, particularly in patients with ulcer‐like FD who were threefold more likely to report symptom relief. When stratifying patients according to GE rate, no beneficial effect was seen with amitriptyline or citalopram in this subgroup. 91 No studies with selective noradrenaline/serotonin reuptake blockers have been performed in patients with gastroparesis. While there are controlled studies supporting the use of mirtazapine and buspirone in FD, no controlled data are available for gastroparesis. A prospective, open label study of mirtazapine 15 mg in gastroparesis patients demonstrated an improvement in symptoms of nausea and vomiting and perceived loss of appetite. 93
Uncontrolled long‐term cohort studies have reported efficacy ofGES in patients with gastroparesis in terms of improving symptom scores and quality of life. 94 , 95 , 96 However, these results were not further corroborated by RCTs. 97 , 98 , 99 A recent sham‐controlled, multicenter study from France reported improvement on vomiting frequency, regardless of the GE status. 100 Most of studies failed to demonstrate acceleration of GE by GES, and the most recent study demonstrates that delayed GE is not a prerequisite for the antivomiting effect of GES.
Open‐label studies all reported short term (<6 months) efficacy of intrapyloric injection of botulinum toxin both on symptoms and GE in gastroparesis. 101 However, two subsequent controlled trials failed to demonstrate an improvement in symptoms and GE. 102 , 103 Several open‐label studies reported short and midterm (<18 months) efficacy of endoscopic pyloric myotomy on symptoms, quality of life, and GE in gastroparesis. 104 However, there are currently no controlled validating observational studies on the efficacy of pyloric myotomy. In light of disappointing results from several RCTs evaluating intrapyloric injection of botulinum toxin, these open‐label observations warrant further corroboration by RCTs. Whether the preassessment of pyloric physiology in order to improve patient selection to intrapyloric injection of botulinum toxin and/or pyloric myotomy remains uncertain. 105 Only one report mentioned the occurrence of dumping syndrome in two patients after laparoscopic pyloroplasty. 106
Only a few studies in limited numbers of patients evaluated the outcomes of near‐total gastrectomy with Roux‐en‐Y reconstruction 107 , 108 or completion gastrectomy. 109 , 110 , 111 , 112 , 113 Symptomatic improvement was reported in 43%–90% of patients, mainly for nausea, vomiting, or pain, but morbidity occurred in up to 40%.
Very limited data exist on the effect of H. pylori eradication in gastroparesis. Two studies from China suggested that eradication enhanced GE rate but they used nonvalidated GE tests. 114 , 115 A number of herbal therapies have been used for the treatment of FD, including peppermint oil with or without caraway oil, ginger, Iberogast, Rikkunshito, and Artichoke Extract. 116 However, there is a lack of data on their effects in gastroparesis. In a placebo‐controlled study in 106 FD or gastroparesis patients, STW‐5 (Iberogast) did not alter GE rate. 117
For hypnotherapy, cognitive behavioral therapy and mindfulness, there is a lack of specific reports in gastroparesis patients. A few Western and multiple Chinese studies have evaluated the efficacy of acupuncture for diabetic or postoperative gastroparesis. A Cochrane systematic review found overall higher symptom improvement rates with acupuncture compared to usual or medical therapy, but due to the heterogeneity and low quality of studies, and the risk of bias, the conclusion is considered uncertain. 118
Several algorithms propose nutritional support in the form of enteral or parenteral nutrition for refractory gastroparesis with weight loss and/or nutritional deficiencies. 44 , 71 , 119 While short‐term parenteral nutrition may provide rapid weight recovery, its long‐term use should be avoided for risk of catheter‐related sepsis and hepatotoxicity. 119 Jejunal tube feeding has been advocated for long‐term nutritional support in gastroparesis. Case series in diabetic gastroparesis from the Mayo Clinic and idiopathic gastroparesis from Leuven show that percutaneous feeding tube positioning is associated with acceptable morbidity and mortality, allows weight recovery, and that the jejunal tube can be removed after an average period of 20 months. 120 , 121
-
7.
Prognosis of gastroparesis
-
7.1
The long‐term prognosis of gastroparesis is unfavorable in the majority of patients.
STATEMENT NOT ENDORSED, overall agreement 35%: A+ 15%, A 20%, A− 28%, D− 23%, D 15%, D+ 0%. GRADE B
-
7.2
The prognosis of gastroparesis depends on the cause.
STATEMENT ENDORSED, overall agreement 88%: A+ 33%, A+ 54%, A 10%, A− 3%, D− 0%, D 0%, D+ 0%. GRADE B
-
7.3
Life expectancy in gastroparesis is shortened.
STATEMENT NOT ENDORSED, overall agreement 25%: A+ 5%, A 20%, A− 35%, D− 18%, D 23%, D+ 0%. GRADE B
-
7.1
The natural history and outcome of patients with gastroparesis is incompletely understood. Cohort studies from tertiary care centers suggest that symptoms and GE delay persist for several years in the majority of patients. 123 , 124 , 125 In a 262 patient follow‐up cohort from the NIDDK gastroparesis consortium, there was some symptom improvement of symptoms at 48 weeks, but not from 48 to 192 weeks. Factors independently associated with reduced symptoms at 48 weeks included male sex, age above 50, an initial infectious prodrome, antidepressant use, and a 4‐h gastric retention more than 20%. Factors associated with lack of improvement of symptoms included overweight or obesity, a history of smoking, the use of pain modulators, moderate to severe abdominal pain, severe gastroesophageal reflex, and moderate to severe depression. 122 Case series have also reported a higher likelihood of symptom resolution or improvement in patients with a presumed post‐infectious form of gastroparesis. 17 , 26
In terms of the effect of gastroparesis on life expectancy, data are conflicting. Based on 86 diabetic patients who were followed for at least 9 years, delayed GE was not related with mortality after adjustment for comorbidities, 125 but 6‐year follow‐up data from a tertiary care setting observed that 7% had died and 22% needed long‐term parenteral or enteral feeding, suggesting gastroparesis is not a benign condition. 124 Studies conducted in referral populations demonstrate no effect of delayed GE on mortality among patients with diabetes mellitus after 12–25 years of follow‐up. 126 , 127 In the analysis of the UK CPRD database, mortality risk was higher in diabetic compared to idiopathic gastroparesis patients (adjusted hazard ratio: 1.9, 95% CI: 1.2–3.0). 24 A population‐based study compared the observed and expected mortality of patients with gastroparesis, demonstrating a significantly higher death rate in patients compared to age‐and sex‐matched controls, which was largely due to cardiovascular comorbidity in diabetic patients. 23
RECOMMENDATIONS
Based on the statements that achieved consensus, a number of recommendations for understanding and managing of gastroparesis can be made (Table 3), which are summarized in Table 4 and Figure 1. The Delphi process also identified several areas of uncertainty, which require additional evidence or further research.
TABLE 3.
All statements with endorsement and references
| Statement | Endorsement | Grade of evidence | References |
|---|---|---|---|
| 1.1. Gastroparesis refers to a symptom or set of symptoms that is(are) associated with delayed gastric emptying in the absence of mechanical obstruction | Yes | A | 1, 2, 3, 4 |
| 1.2. Gastroparesis refers to a symptom or set of symptoms that is(are) associated with severely disturbed gastric motor function in the absence of mechanical obstruction | Yes | B | 1, 2, 3, 4 |
| 1.3. Nausea and vomiting are cardinal symptoms in gastroparesis | Yes | B | 4, 9, 10, 16, 17, 18, 19 |
| 1.4. Dyspeptic symptoms such as postprandial fullness, early satiation, epigastric pain, as well as bloating in the upper abdomen and belching are often present in gastroparesis patients | Yes | B | 4, 9, 12 |
| 1.5. Symptoms in gastroparesis patients overlap mainly with PDS and less with EPS symptoms of FD | Yes | B | 12, 17, 20 |
| 2.1. The epidemiology of gastroparesis is not established, mainly because it requires gastric emptying testing which has not been done at the population level | Yes | B | 23, 24 |
| 2.2. Diabetes is a risk factor for development of gastroparesis | Yes | A | 23, 24, 25 |
| 2.3. Acute gastrointestinal infection is a risk factor for development of gastroparesis | No | B | 26, 27, 28 |
| 2.4. Partial gastric resection/vagotomy, bariatric surgery, antireflux surgery are risk factors for development of gastroparesis | Yes | B | 29, 30 |
| 2.5. Hypothyroidism is a risk factor for development of gastroparesis | No | C | 29, 30 |
| 2.6. Some neurological disorders (e.g., Parkinson's disease, multiple sclerosis, amyloid neuropathy) are associated with increased risk for gastroparesis | Yes | B | 23, 31, 32, 33 |
| 2.7. Some connective tissue diseases are associated with increased risk for gastroparesis | Yes | B | 23, 31, 32, 33 |
| 2.8. Some drugs (e.g., opioids) are associated with increased risk for gastroparesis | Yes | A | 1, 4, 21, 34 |
| 3.1. Gastroparesis is a major source of healthcare costs | Yes | A | 35, 36 |
| 3.2. Gastroparesis is associated with decreased life expectancy | No | 35, 36 | |
| 3.3. Gastroparesis is a major source of self‐costs to patients | No | B | 35, 36 |
| 3.4. Gastroparesis is an important source of loss of work productivity | No | B | 37, 38, 39 |
| 3.5. Gastroparesis is associated with a significant decrease in quality of life | Yes | A | 37, 38, 39 |
| 3.6. Gastroparesis is associated with psychosocial co‐morbidities such as anxiety and depression | Yes | A | 41, 42 |
| 3.7. Weight loss can be consequence of gastroparesis | No | B | 9, 42 |
| 3.8. In case of weight loss, eating disorders must be ruled out | Yes | B | 44, 45 |
| 3.9. Healthcare consulting behavior in gastroparesis is driven by symptom severity and impact | No | B | 41, 46 |
| 3.10. Healthcare consulting behavior in gastroparesis is driven by psychosocial co‐morbidity | No | B | 41, 46 |
| 3.11. Healthcare consulting behavior in gastroparesis is driven by access to the healthcare system | No | B | 41, 46 |
| 4.1. Delay in gastric emptying underlies symptom generation in gastroparesis | No | B | 1, 2, 3, 4, 8, 9, 10, 11, 12, 13, 16, 17, 20, 21, 32 |
| 4.2. Impaired gastric accommodation contributes to symptom generation in gastroparesis | No | B | 12, 13 |
| 4.3. Hypersensitivity to gastric distention contributes to symptom generation in gastroparesis | No | B | 12, 13 |
| 4.4. Duodenal mucosal alterations (low grade inflammation, impaired permeability) are not implicated as pathophysiological mechanisms in gastroparesis | No | C | 47 |
| 4.5. Loss of interstitial cells of Cajal is a pathophysiological mechanism in gastroparesis | No | B | 48, 49, 50, 51 |
| 4.6. Loss of enteric nerves is a pathophysiological mechanism in gastroparesis | Yes | B | 48, 49, 50, 51 |
| 4.7. Primary changes in gastric smooth muscle are a pathophysiological mechanism in gastroparesis | No | B | 48, 49, 50, 51, 52 |
| 4.8. Loss of vagus nerve function is a pathophysiological mechanism in gastroparesis | No | B | 48, 49, 50, 51, 52 |
| 4.9. HP infection is not a pathophysiological mechanism in gastroparesis | No | B | 55, 56, 57 |
| 4.10. Altered gastric acid secretion is not a pathophysiological mechanism in gastroparesis | No | C | 1, 2, 3, 4 |
| 4.11. Altered release of peptide hormones is not a pathophysiological mechanism in gastroparesis | No | C | 1, 2, 3, 4 |
| 4.12. Increased sensitivity to duodenal luminal content is not a pathophysiological mechanism in gastroparesis | No | C | 1, 2, 3, 4 |
| 4.13. Altered duodenal microbiota composition is not a pathophysiological mechanism in gastroparesis | No | C | 1, 2, 3, 4 |
| 4.14. Anxiety is a pathophysiological factor in gastroparesis | No | B | 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 |
| 4.15. Depression is a pathophysiological factor in gastroparesis | No | B | 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 |
| 4.16. Disordered processing of incoming signals from the gastroduodenal region is a pathophysiological mechanism in gastroparesis | No | B | 59, 60 |
| 4.17. Genetic factors determine susceptibility to gastroparesis | No | C | 59, 60 |
| 5.1. Upper GI endoscopy is mandatory for establishing a diagnosis of gastroparesis | Yes | A | 1, 2, 19 |
| 5.2. The presence of food in fasting state during endoscopy is diagnostic for gastroparesis | No | B | 23, 62 |
| 5.3. An abnormal gastric emptying test is mandatory for establishing a diagnosis of gastroparesis | Yes | A | 1, 2, 3, 4, 63, 64, 65, 66, 67 |
| 5.4. Scintigraphic gastric emptying assessment is a valid test for diagnosing gastroparesis | Yes | A | 63, 64 |
| 5.5. Breath test assessment is a valid test for diagnosing gastroparesis | Yes | A | 65 |
| 5.6. Wireless motility capsule assessment is a valid test for diagnosing gastroparesis | No | B | 66 |
| 5.7. Gastric ultrasound assessment is a valid test for diagnosing gastroparesis | No | B | 66, 67 |
| 5.8. Small bowel obstruction can be ruled out in gastroparesis through imaging | Yes | B | 68 |
| 6.1. Dietary adjustments are useful for managing gastroparesis patients | No | B | 17, 69 |
| 6.2. Proton pump inhibitor (PPI) therapy is the most appropriate first line therapy for gastroparesis | No | C | 17, 70 |
| 6.3. PPI therapy is effective in gastroparesis | No | C | 17, 70 |
| 6.4. PPI therapy is only effective for associated reflux symptoms in gastroparesis | No | C | 17, 70 |
| 6.5. Anti‐emetic (anti‐“nauseant”) therapy is the most appropriate first line therapy for gastroparesis | No | B | 17, 70 |
| 6.6. Antiemetic (anti‐“nauseant”) therapy is effective for gastroparesis | No | B | 17, 70 |
| 6.7. Dopamine‐2 antagonists are effective for gastroparesis | Yes | B | 71, 72, 73 |
| 6.8. 5‐HT3 antagonists are effective for gastroparesis | No | B | 71, 72, 73 |
| 6.9. NK1 antagonists are effective for gastroparesis | No | B | 71, 72, 73 |
| 6.10. Prokinetic therapy is the most appropriate first line therapy for gastroparesis | No | B | 76, 77, 78, 79 |
| 6.11. Prokinetic therapy is effective for gastroparesis | No | B | 76, 77, 78, 79 |
| 6.12. The efficacy of prokinetics is not related to their enhancement of gastric emptying rate | No | B | 76, 77, 78, 79 |
| 6.13. Itopride is effective for gastroparesis | No | B | 76, 77, 78, 79 |
| 6.14. 5‐HT4 agonists are effective for gastroparesis | Yes | B | 81, 82, 83, 84 |
| 6.15. Motilin‐receptor agonists are effective for gastroparesis | No | B | 85, 86, 87 |
| 6.16. Ghrelin‐receptor agonists are effective for gastroparesis | No | B | 88, 89 |
| 6.17. Tricyclic antidepressants are effective for gastroparesis | No | B | 90, 91, 92 |
| 6.18. Tricyclic antidepressants are not effective for gastroparesis | No | B | 90, 91, 92 |
| 6.19. SSRI are effective for gastroparesis | No | C | 90, 92 |
| 6.20. SSRI are not effective for gastroparesis | No | C | 90, 92 |
| 6.21. SNRI are effective for gastroparesis | No | C | 90, 92 |
| 6.22. SNRI are not effective for gastroparesis | No | C | 90, 92 |
| 6.23. Mirtazapine is effective for gastroparesis | No | B | 90, 93 |
| 6.24. 5‐HT1A agonists (tandospirone, buspirone, ….) are effective for gastroparesis | No | C | 90, 93 |
| 6.25. 5‐HT1A agonists are not effective for gastroparesis | No | C | 90, 93 |
| 6.26. Gastric electrical stimulation is effective for gastroparesis | No | B | 94, 95, 96, 97, 98, 99, 100 |
| 6.27. Pyloric botulinum toxin injection is effective for gastroparesis | No | B | 101, 102, 103 |
| 6.28. Pyloric myotomy is effective for gastroparesis | No | B | 104, 105 |
| 6.29. Partial or subtotal gastrectomy is effective for gastroparesis | No | B | 107, 108, 109, 110, 111, 112, 113 |
| 6.30. Surgical or pyloric targeting therapies for gastroparesis may induce dumping syndrome | No | B | 107, 108, 109, 110, 111, 112, 113 |
| 6.31. Helicobacter pylori‐infected gastroparesis patients should receive eradication therapy | No | B | 114, 115 |
| 6.32. Herbal therapies are effective for gastroparesis. | No | B | 114, 115 |
| 6.33. Herbal therapies are not effective for gastroparesis | No | B | 114, 115 |
| 6.34. Iberogast (STW‐5) is effective for gastroparesis | No | B | 114, 115 |
| 6.35. Hypnotherapy is effective for gastroparesis | No | B | 114, 115 |
| 6.36. Cognitive behavioral therapy is effective for gastroparesis | No | B | 114, 115 |
| 6.37. Acupuncture is effective for gastroparesis | No | B | 114, 115 |
| 6.38. Mindfulness is effective for gastroparesis | No | B | 114, 115 |
| 6.39. In case of severe weight loss or intractable vomiting, nutritional support may be needed in the form of enteral or parenteral nutrition | Yes | B | 44, 71, 119, 120, 121 |
| 7.1. The long‐term prognosis of gastroparesis is unfavorable in the majority of patients | No | B | 24, 123, 124, 125, 126 |
| 7.2. The prognosis of gastroparesis depends on the cause | Yes | B | 24, 123, 124, 125, 126 |
| 7.3. Life expectancy in gastroparesis is shortened | No | B | 24, 123, 124, 125, 126, 127, 128 |
TABLE 4.
Summary of the ESNM consensus on gastroparesis
| Recommendations | Based on statement(s) |
|---|---|
| Gastroparesis refers to a symptom or set of symptoms that is (are) associated with delayed gastric emptying in the absence of mechanical obstruction, as well as severely disturbed gastric motor function | 1.1, 1.2 |
| Nausea and vomiting are the cardinal symptoms of gastroparesis. Symptoms in gastroparesis patients overlap mainly with postprandial distress syndrome symptoms such as postprandial fullness, early satiation, epigastric pain, as well as bloating in the upper abdomen | 1.3, 1.4, 1.5 |
| The epidemiology of gastroparesis is not established, mainly because it requires gastric emptying testing which has not been done at the population level | 2.1 |
| Diabetes, upper abdominal surgeries, neurological and connective tissue diseases as well as the use of certain drugs are risk factors for development of gastroparesis | 2.3, 2.4, 2.6, 2.7, 2.8 |
| Gastroparesis is a major source of healthcare costs | 3.1, |
| Gastroparesis is associated with a significant decrease in quality of life and with psychosocial co‐morbidities | 3.5,3.6 |
| In case of weight loss, an eating disorder must be ruled out | 3.8 |
| Upper GI endoscopy to rule out mechanical obstruction and an abnormal gastric emptying test are mandatory for establishing a diagnosis of gastroparesis. Gastric emptying can reliably be assessed by scintigraphy or breath test. Additional radiological imaging can be used to exclude obstruction in case of doubt | 5.1, 5.3, 5.4, 5.5, 5.8 |
| Gastroparesis patients should be explained dietary adjustments. Dopamine‐2 antagonists or 5‐HT4 agonists can be used to manage symptoms | 6.1, 6.7, 6.14 |
| In case of severe weight loss in gastroparesis, nutritional support may be needed | 6.39 |
| The long‐term prognosis of gastroparesis depends on the underlying cause | 7.2 |
Abbreviation: ESNM, European Society for Neurogastroenterology and Motility.
FIGURE 1.
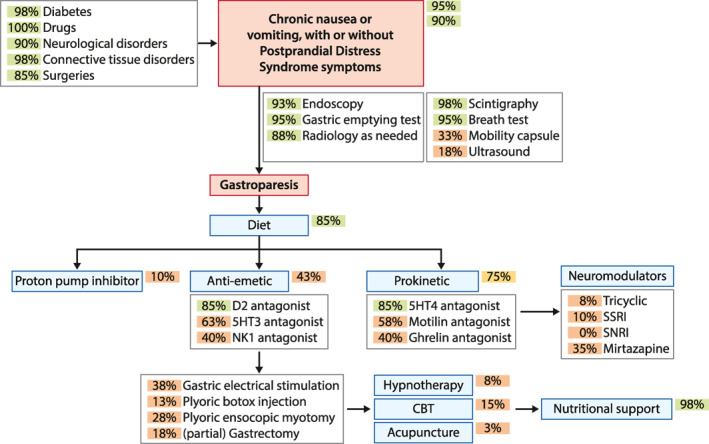
Schematic representation, in an algorithm‐like fashion, of the outcome of the ESNM consensus on gastroparesis management. The blue arrows depict the diagnostic and therapeutic flow of the patient. Green arrows refer to risk or pathophysiological factors. The circles depict the percentage of agreement, using a green color for ≥80% consensus, light orange for consensus between 70% and 80%, and dark orange for lower levels of consensus. ESNM, European Society for Neurogastroenterology and Motility
In line with existing definitions and guidelines, gastroparesis is based on the presence of upper gastrointestinal symptoms and delayed GE in the absence of an obstructive lesion. 1 , 2 , 3 , 4 The panel also agrees that gastroparesis is associated with other manifestations of severely disturbed gastric motor function. 12 , 13 , 14 The European consensus identifies nausea and vomiting as the cardinal symptoms of gastroparesis. While the panel also agrees that there are often coexisting FD/PDS symptoms, the presence of predominant nausea and vomiting offers an approach to differentiate (idiopathic) gastroparesis from PDS, where early satiation or postprandial fullness are dominant symptoms. 2 , 4 , 18 , 19 The overlap with EPS is considered less prevalent. 4 , 17 , 20
There is consensus that the true epidemiology of gastroparesis is not known, but that diabetes, gastric surgery, certain neurological and connective tissue diseases, as well as the use of certain drugs are associated with an increased risk of gastroparesis. 23 , 24 , 25 , 29 , 30 , 31 , 32 , 33 , 34 The panel agreed that gastroparesis has an important impact on quality of life and healthcare costs, and also concurs that gastroparesis is often associated with psychological comorbidities such as anxiety and depression. 35 , 40 , 41 , 42 There is no consensus that gastroparesis may lead to unintended weight loss, but when present, eating disorders must be excluded. 9 , 43 , 44 , 45
In terms of pathophysiological mechanisms that are relevant to gastroparesis, while the panel agreed that severely impaired gastric motor function is present in these patients, there was no consensus for a direct role for delayed emptying, impaired gastric accommodation or gastric hypersensitivity in determining symptom pattern and severity. 1 , 2 , 3 , 4 , 8 , 9 , 10 , 11 , 12 , 13 , 16 , 17 , 20 , 21 , 32 There was also no qualifying majority support for pivotal pathophysiological roles for loss of interstitial cells of Cajal, intrinsic or extrinsic (vagus) nerves, changes in smooth muscle, peptide hormone release, gastric acid secretion, duodenal mucosal changes, psychosocial comorbidity, or altered central processing in the generation of gastroparesis symptoms. 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70
There is consensus that an abnormal GE test as well as an EGD, to rule out mechanical obstruction, are mandatory for establishing a diagnosis of gastroparesis, but that the presence of food at endoscopy is not a reliable diagnostic marker. 1 , 2 , 19 , 23 , 62 Radiological examinations, preferably using a CT scan, can be added in case of uncertainty regarding the absence of a mechanical obstructive factor. Scintigraphy and breath tests are agreed to be reliable diagnostic tests, but there is no support for the WMC or gastric ultrasound to detect delayed GE. 63 , 64 , 65 , 66 , 67
Besides the statements on pathophysiology, the section on treatment approaches is a second one to display a major lack of consensus. In spite of very few literature data, 69 the panel supported dietary intervention in the treatment of gastroparesis. There is no consensus on the efficacy of PPIs, nor for different types of antiemetics. 70 , 71 , 72 , 73 , 74 , 75 There is borderline support (78% agreement) for the use of prokinetics as a group, but the panel agreed on the use of 5‐HT4 receptor agonists as a class. 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 There is also no consensus on the use of other neuromodulators, herbal therapies, acupuncture or psychological therapies in gastroparesis. 90 , 91 , 92 , 93 , 116 , 117 , 118 The same is true for invasive therapies such as botulinum toxin injection, GES, pyloric endoscopic myotomy or (partial) gastrectomy. 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 There is agreement on the use of nutritional support in case of severe weight loss. 44 , 71 , 119 , 120 , 121 Finally, there is consensus that the long‐term prognosis of gastroparesis depends on the cause, but there is no consensus on an unfavorable long‐term prognosis or increased mortality. 123 , 124 , 125 , 126 , 127 , 128
The most important progress with this consensus is the identification of a cardinal symptom pattern for gastroparesis: nausea or vomiting, with or without overlapping PDS. In the past, the separation of gastroparesis from FD has been an issue of controversy, and novel terms such as (diabetic) gastropathy and FD with delayed emptying have been proposed. 128 , 129 The same Delphi panel for the current consensus also generated the ESNM FD guideline, confirming early satiation, postprandial fullness and epigastric pain or burning as cardinal FD symptoms. 6 These different cardinal symptom‐based approaches may allow for a better differentiation of gastroparesis from FD for future research and clinical management. Otherwise, the areas of uncertainty revealed by this consensus are multiple. There is no consensus and hence no clarity on the underlying pathophysiological factors in gastroparesis. The therapy section also sees little agreement, as only dietary therapy and 5‐HT4 receptor agonists are considered appropriate by consensus, even though the available evidence is limited. It is anticipated that advances in our understanding of pathophysiology of FD and gastroparesis, and how symptoms are generated in these conditions, may allow refinement of definitions and identifications of more specific phenotypes with definable treatment response. Moreover, this clearly establishes the need for more therapeutic trials to determine the place of anti‐emetics, other classes of prokinetics, neuromodulators and perhaps even PPIs. For now, the path to regulatory approval for gastroparesis treatments remains unfinished. For some of the older agents, scientific or professional organizations such as ESNM or the Rome Foundation may consider taking the lead.
CONCLUSION
This Delphi process used a multinational and multidisciplinary group of European experts to summarize the current state of consensus on definition, symptom characteristics, pathophysiology, diagnosis, treatment, and prognosis of gastroparesis. The Consensus Group voted on several statements that may guide clinicians in recognizing, diagnosing and treating gastroparesis in clinical practice, whereas the statements without consensus identify areas in need of future research.
1. AUTHOR CONTRIBUTIONS
Jolien Schol drafted consensus questions, drafted literature review sections, participated in voting, summarized voting outcomes, reviewed and corrected the manuscript. Lucas Wauters drafted consensus questions, drafted literature review sections, set up the voting and summaries access, participated in voting, summarized voting outcomes, reviewed and corrected the manuscript. Ram Dickman drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript. Vasile Drug drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript. Agata Mulak drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript. Jordi Serra drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected the manuscript. Paul Enck coinitiated the process, obtained funding, drafted consensus questions, drafted literature review sections, participated in voting, reviewed and corrected manuscript content. Jan Tack coinitiated the process, drafted consensus questions, drafted literature review sections, wrote manuscript sections, participated in voting, wrote manuscript, reviewed and corrected content. All members of the ESNM gastroparesis consensus group (A. Accarino, G. Barbara, S. Bor, B. Coffin, M. Corsetti, H. De Schepper, D. Dumitrascu, A. Farmer, G. Gourcerol, G. Hauser, T. Hausken, G. Karamanolis, D. Kestzhelyi, C. Malagelada, T. Milosavljevic, C. O'Morain, A. Papathanasopoulos, D. Pohl, D. Rumyantseva, G. Sarnelli, E. Savarino, A. Sheptulin, A. Smet, A. Stengel, O. Storonova, M. Storr, H. Törnblom, T. Vanuytsel, M. Velosa, M. Waluga, N. Zarate, and F. Zerbib) drafted consensus questions, wrote manuscript sections, participated in voting, reviewed and corrected content.
ESNM GASTROPARESIS CONSENSUS GROUP
A. Accarino, Digestive System Research Unit, CIBERehd and Departament de Medicina, University Hospital Vall d'Hebron, Barcelona, Spain.
G. Barbara, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy. S. Bor, Division Gastroenterology, School of Medicine, Ege University, Izmir, Turkey. B. Coffin, Université de Paris and AP‐HP Hôpital Louis Mourier, Paris, France. M. Corsetti, NIHR Nottingham Biomedical Research Centre (BRC), Hospitals NHS Trust and the University of Nottingham, Nottingham, UK. H. De Schepper, Department of Gastroenterology and Hepatology, University Hospital Antwerp, Antwerp, Belgium. D. Dumitrascu, second Department of Internal Medicine, Iuliu Hatieganu University of Medicine and Pharmacy Cluj‐Napoca, Romania. A. D. Farmer, School of Medicine, University of Keele, Keele, Staffordshire, UK. G. Gourcerol, Physiology Department, Rouen University Hospital, Rouen, France. G. Hauser, Medical Faculty Rijeka, University of Rijeka and Department of Gastroenterology, Clinical Hospital Centre Rijeka, Rijeka, Croatia. T. Hausken, Department of Gastroenterology, Haukeland University Hospital, Bergen, Norway. G. Karamanolis, Gastroentrology Unit, Second Departement of Surgery, Aretaieio Hospital, National and Kapodistrian University of Athens, Athens, Greece. D. Kestzhelyi, Division of Gastroenterology‐Hepatology, Maastricht University Medical Center, NUTRIM, Maastricht, the Netherlands. C. Malagelada, Digestive System Research Unit, CIBERehd and Departament de Medicina, University Hospital Vall d'Hebron, Barcelona, Spain. T. Milosavljevic, Department of Gastroenterology, Euromedik Hospital Belgrade, Belgrade, Serbia. C. O'Morain, Department of Medicine, Trinity College Dublin and National Clinical Lead for Gastroenterology and Hepatology, Health Service Executive and Royal College Physicians Ireland, Dublin, Ireland. A. Papathanasopoulos, Private Practice, Ioannina, Greece. D. Pohl, Division of Gastroenterology and Hepatology, University Hospital Zurich, Zurich, Switzerland. D. Rumyantseva, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University), Moscow, Russia. G. Sarnelli, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy. E. Savarino, Gastroenterology Unit, Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, Italy. A. Sheptulin, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University), Moscow, Russia. A. Smet, Laboratory of Experimental Medicine and Pediatrics, Faculty of Medicine and Health Sciences and Infla‐Med Research Consortium of Excellence, University of Antwerp, Antwerp, Belgium. A. Stengel, Internal Medicine VI, Department of Psychosomatic Medicine and Psychotherapy, University Hospital Tübingen, Tübingen and Charité Center for Internal Medicine and Dermatology, Department for Psychosomatic Medicine, Charité‐Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt‐Universität zu Berlin and Berlin Institute of Health, Berlin, Germany. O. Storonova, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University), Moscow, Russia. M. Storr, Center of Endoscopy, Starnberg, Germany and Ludwig‐Maximilians‐University, Munich, Germany. H. Törnblom, Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, Gothenburg, Sweden. T. Vanuytsel, Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium. M. Velosa, Neurogastroenterology and Pelvic Floor Unit, Gastroenterology Department, Sheba Medical Center, Tel Hashomer, Israel. M. Waluga, Department of Gastroenterology and Hepatology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland. N. Zarate, Gastrointestinal Physiology Unit, University College London Hospital, London, UK. F. Zerbib, CHU de Bordeaux, Centre Medico‐chirurgical Magellan, Hôpital Haut‐Lévêque and Gastroenterology Department, Université de Bordeaux, INSERM CIC 1401, Bordeaux, France.
Contributor Information
Jan Tack, Email: jan.tack@kuleuven.be.
the ESNM Gastroparesis Consensus Group:
A. Accarino, G. Barbara, S. Bor, B. Coffin, M. Corsetti, H. De Schepper, D. Dumitrascu, A. Farmer, G. Gourcerol, G. Hauser, T. Hausken, G. Karamanolis, D. Kestzhelyi, C. Malagelada, T. Milosavljevic, C. O'Morain, A. Papathanasopoulos, D. Pohl, D. Rumyantseva, G. Sarnelli, E. Savarino, A. Sheptulin, A. Smet, A. Stengel, O. Storonova, M. Storr, H. Törnblom, T. Vanuytsel, M. Velosa, M. Waluga, N. Zarate, and F. Zerbib
DATA AVAILABILITY STATEMENT
Not applicable for a consensus document.
REFERENCES
- 1. Parkman HP, Camilleri M, Farrugia G, McCallum RW, Bharucha AE, Mayer EA, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22(2):113–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tack J, Carbone F, Rotondo A. Gastroparesis. Curr Opin Gastroenterol. 2015;31(6):499–505. [DOI] [PubMed] [Google Scholar]
- 3. Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol. 2018;43:111–7. [DOI] [PubMed] [Google Scholar]
- 4. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63(12):1972–8. [DOI] [PubMed] [Google Scholar]
- 5. Wauters L, Dickman R, Drug V, Mulak A, Serra Pueyo J, Enck P, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. UEG J. 2021. [DOI] [PubMed] [Google Scholar]
- 6. Mullen PM. Delphi: myths and reality. J Health Organ Manag. 2003;17:37–52. [DOI] [PubMed] [Google Scholar]
- 7. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 8. Cherian D, Parkman HP. Nausea and vomiting in diabetic and idiopathic gastroparesis. Neurogastroenterol Motil. 2012;24(3):217–22.e103. [DOI] [PubMed] [Google Scholar]
- 9. Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with delayed emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–8. [DOI] [PubMed] [Google Scholar]
- 10. Vijayvargiya P, Jameie‐Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta‐analysis. Gut. 2019;68(5):804–13. [DOI] [PubMed] [Google Scholar]
- 11. Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ , et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut. 2007;56(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20(6):635–42. [DOI] [PubMed] [Google Scholar]
- 14. Abrahamsson H. Gastrointestinal motility disorders in patients with diabetes mellitus. J Intern Med. 1995;237(4):403–9. [DOI] [PubMed] [Google Scholar]
- 15. Horowitz M, Su YC, Rayner CK, Jones KL. Gastroparesis: prevalence, clinical significance and treatment. Can J Gastroenterol. 2001;15(12):805–13. [DOI] [PubMed] [Google Scholar]
- 16. Parkman HP, Hallinan EK, Hasler WL, Farrugia G, Koch KL, Calles J, et al. Nausea and vomiting in gastroparesis: similarities and differences in idiopathic and diabetic gastroparesis. Neurogastroenterol Motil. 2016;28(12):1902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaffe JK, Paladugu S, Gaughan JP, Parkman HP. Characteristics of nausea and its effects on quality of life in diabetic and idiopathic gastroparesis. J Clin Gastroenterol. 2011;45(4):317–21. [DOI] [PubMed] [Google Scholar]
- 18. Stanghellini V, Talley N, Chan F, Hasler B, Malagelada J, Suzuki H, et al. Functional gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–92. [DOI] [PubMed] [Google Scholar]
- 19. Wauters L, Dickman R, Drug V, Mulak A, Serra J, Enck P, et al. European Society for Neurogastroenterology and Motility (ESNM) consensus on functional dyspepsia. UEG J. 2021. [DOI] [PubMed] [Google Scholar]
- 20. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire complements patient assessment of gastrointestinal symptoms for patients with gastroparesis symptoms. Dig Dis Sci. 2018;63(9):2231–43. [DOI] [PubMed] [Google Scholar]
- 21. Hasler WL, Wilson LA, Nguyen LA, Snape WJ, Abell TL, Koch KL , et al. Opioid use and potency are associated with clinical features, quality of life, and use of resources in patients with gastroparesis. Clin Gastroenterol Hepatol. 2019;17(7):1285–94.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thapa N, Kappus M, Hurt R, Diamond S. Implications of the opioid epidemic for the clinical gastroenterology practice. Curr Gastroenterol Rep. 2019;21(9):44. [DOI] [PubMed] [Google Scholar]
- 23. Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted county, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ye Y, Jiang B, Manne S, Moses PL, Almansa C, Bennett D, et al. Epidemiology and outcomes of gastroparesis, as documented in general practice records, in the United Kingdom. Gut. 2021;70(4):644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Melton LJ, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis—clinical characteristics and long‐term outcomes. Am J Gastroenterol. 1997;92:1501–4. [PubMed] [Google Scholar]
- 27. Barkin JA, Czul F, Barkin JS, Klimas NG, Rey IR, Moshiree B. Gastric enterovirus infection: a possible causative etiology of gastroparesis. Dig Dis Sci. 2016;61(8):2344–50. [DOI] [PubMed] [Google Scholar]
- 28. Naftali T, Yishai R, Zangen T, Levine A. Post‐infectious gastroparesis: clinical and electrogastrographic aspects. J Gastroenterol Hepatol. 2007;22(9):1423–8. [DOI] [PubMed] [Google Scholar]
- 29. Kim DH, Yun HY, Song YJ, Ryu DH, Han HS, Han JH, et al. Clinical features of gastric emptying after distal gastrectomy. Ann Surg Treat Res. 2017;93(6):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng H, Zhou D, Jiang X, Ding W, Lu L. Incidence and risk factors for postsurgical gastroparesis syndrome after laparoscopic and open radical gastrectomy. World J Surg Oncol. 2013;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nassar Y, Richter S. Gastroparesis in non‐diabetics: associated conditions and possible risk factors. Gastroenterol Res. 2018;11(5):340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson's disease. World J Gastroenterol. 2016;22(25):5742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rönnblom A, Andersson S, Hellström PM, Danielsson A. Gastric emptying in myotonic dystrophy. Eur J Clin Invest. 2002;32(8):570–4. [DOI] [PubMed] [Google Scholar]
- 34. Pannemans J, Carbone F, Tack J. Opioids in gastroparesis: bystander or cause? Clin Gastroenterol Hepatol. 2020;18(4):998–9. [DOI] [PubMed] [Google Scholar]
- 35. Wadhwa V, Mehta D, Jobanputra Y, Lopez R, Thota PN, Sanaka MR. Healthcare utilization and costs associated with gastroparesis. World J Gastroenterol. 2017;23(24):4428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirsch W, Nee J, Ballou S, Petersen T, Friedlander D, Lee HN , et al. Emergency department burden of gastroparesis in the United States, 2006 to 2013. J Clin Gastroenterol. 2019;53(2):109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navas CM, Crowell MD, Lacy BE. The willingness of patients with gastroparesis to take risks with medications. Aliment Pharmacol Ther. 2019;49(4):429–36. [DOI] [PubMed] [Google Scholar]
- 38. Jehangir A, Parkman HP. Chronic opioids in gastroparesis: relationship with gastrointestinal symptoms, healthcare utilization and employment. World J Gastroenterol. 2017;23(40):7310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis. J Clin Gastroenterol. 2018;52(1):20–4. [DOI] [PubMed] [Google Scholar]
- 40. Yu D, Ramsey FV, Norton WF, Norton N, Schneck S, Gaetano T, et al. The burdens, concerns, and quality of life of patients with gastroparesis. Dig Dis Sci. 2017;62(4):879–93. [DOI] [PubMed] [Google Scholar]
- 41. Hasler WL, Parkman HP, Wilson LA, Pasricha PJ, Koch KL, Abell TL, et al. Psychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesis. Am J Gastroenterol. 2010;105(11):2357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woodhouse S, Hebbard G, Knowles SR. Psychological controversies in gastroparesis: a systematic review. World J Gastroenterol. 2017;23(7):1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Talley NJ, Locke GR, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55(7):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tack J. The difficult patient with gastroparesis. Best Pract Res Clin Gastroenterol. 2007;21(3):379–91. [DOI] [PubMed] [Google Scholar]
- 45. Stacher G, Kiss A, Wiesnagrotzki S, Bergmann H, Höbart J, Schneider C. Oesophageal and gastric motility disorders in patients categorised as having primary anorexia nervosa. Gut. 1986;27(10):1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bielefeldt K. Regional differences in healthcare delivery for gastroparesis. Dig Dis Sci. 2013;58(10):2789–98. [DOI] [PubMed] [Google Scholar]
- 47. Wauters L, Talley NJ, Walker MM, Tack J, Vanuytsel T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut. 2020;69(3):591–600. [DOI] [PubMed] [Google Scholar]
- 48. Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68(12):2238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone‐Pellegrini MS, Smyrk TC, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–85.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herring BP, Hoggatt AM, Gupta A, Griffith S, Nakeeb A, Choi JN, et al. Idiopathic gastroparesis is associated with specific transcriptional changes in the gastric muscularis externa. Neurogastroenterol Motil. 2018;30(4):e13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grover M, Bernard CE, Pasricha PJ, Parkman HP, Abell TL, Nguyen LA, et al. Platelet‐derived growth factor receptor α (PDGFRα)‐expressing "fibroblast‐like cells" in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24(9):844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ejskjaer NT, Bradley JL, Buxton‐Thomas MS, Edmonds ME, Howard ER , Purewal T, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16(6):488–95. [DOI] [PubMed] [Google Scholar]
- 53. Mohammad MK, Pepper DJ, Kedar A, Bhaijee F, Familoni B, Rashed H, et al. Measures of autonomic dysfunction in diabetic and idiopathic gastroparesis. Gastroenterology Res. 2016;9(4‐5):65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nguyen L, Wilson LA, Miriel L, Pasricha PJ, Kuo B, Hasler WL, et al. Autonomic function in gastroparesis and chronic unexplained nausea and vomiting: relationship with etiology, gastric emptying, and symptom severity. Neurogastroenterol Motil. 2020;32(8):e13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sarnelli G, Cuomo R, Janssens J, Tack J. Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori . Dig Dis Sci. 2003;48:2229–36. [DOI] [PubMed] [Google Scholar]
- 56. Güvener N, Akcan Y, Paksoy I, Soylu AR, Aydin M, Arslan S, et al. Helicobacter pylori associated gastric pathology in patients with type II diabetes mellitus and its relationship with gastric emptying: the Ankara study. Exp Clin Endocrinol Diabetes. 1999;107(3):172–6. [DOI] [PubMed] [Google Scholar]
- 57. Salicru M, Juarez D, Genta RM. Low prevalence of H. pylori infection in patients with gastroparesis. Dig Liver Dis. 2013;45(11):905–8. [DOI] [PubMed] [Google Scholar]
- 58. Van den Houte K, Scarpellini E, Verbeure W, Mori H, Schol J, Masuy I, et al. The role of GI peptides in functional dyspepsia and gastroparesis: a systematic review. Front Psychiatry. 2020;11:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lelic D, Brock C, Simrén M, Frøkjaer JB, Søfteland E, Dimcevski G, et al. The brain networks encoding visceral sensation in patients with gastrointestinal symptoms due to diabetic neuropathy. Neurogastroenterol Motil. 2014;26(1):46–58. [DOI] [PubMed] [Google Scholar]
- 60. Snodgrass P, Sandoval H, Calhoun VD, Ramos‐Duran L, Song G, Sun Y, et al. Central nervous system mechanisms of nausea in gastroparesis: an fMRI‐based case‐control study. Dig Dis Sci. 2019;65:551–6. 10.1007/s10620-019-05766-5 [DOI] [PubMed] [Google Scholar]
- 61. Gibbons SJ, Grover M, Choi KM, Wadhwa A, Zubair A, Wilson LA, et al. Repeat polymorphisms in the Homo sapiens heme oxygenase‐1 gene in diabetic and idiopathic gastroparesis. PloS One. 2017;12(11):e0187772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Coleski R, Baker JR, Hasler WL. Endoscopic gastric food retention in relation to scintigraphic gastric emptying delays and clinical factors. Dig Dis Sci. 2016;61(9):2593–601 [DOI] [PubMed] [Google Scholar]
- 63. Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103(3):753–63. [DOI] [PubMed] [Google Scholar]
- 64. Desai A, O'Connor M, Neja B, Delaney K, Camilleri M, Zinsmeister AR, et al. Reproducibility of gastric emptying assessed with scintigraphy in patients with upper GI symptoms. Neurogastroenterol Motil. 2018;30(10):e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bharucha AE, Camilleri M, Veil E, Burton D, Zinsmeister AR. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neurogastroenterol Motil. 2013;25(1):e60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stein E, Clarke JO, Hutfless S, Boult C, Doggett D, Fawole OA, et al. Wireless motility capsule versus other diagnostic technologies for evaluating gastroparesis and constipation: future research needs: identification of future research needs from comparative effectiveness review no. 110. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Report No.: 13‐EHC018‐EF. [PubMed] [Google Scholar]
- 67. Irvine EJ, Tougas G, Lappalainen R, Bathurst NC. Reliability and interobserver variability of ultrasonographic measurement of gastric emptying rate. Dig Dis Sci. 1993;38(5):803–10. [DOI] [PubMed] [Google Scholar]
- 68. Camilleri M, Shin A. Novel and validated approaches for gastric emptying scintigraphy in patients with suspected gastroparesis. Dig Dis Sci. 2013;58(7):1813–5. [DOI] [PubMed] [Google Scholar]
- 69. Paulson EK, Thompson WM. Review of small‐bowel obstruction: the diagnosis and when to worry. Radiology. 2015;275(2):332–42. [DOI] [PubMed] [Google Scholar]
- 70. Olausson EA, Störsrud S, Grundin H, Isaksson M, Attvall S, Simrén M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109(3):375–85. [DOI] [PubMed] [Google Scholar]
- 71. Koch KL, Hasler WL, Yates KP, Parkman HP, Pasricha PJ, Calles‐Escandon J, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil. 2016;28(7):1001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology . Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parkman HP, Carlson MR, Gonyer D. Metoclopramide nasal spray reduces symptoms of gastroparesis in women, but not men, with diabetes: results of a phase 2B randomized study. Clin Gastroenterol Hepatol. 2015;13(7):1256–63.e1. [DOI] [PubMed] [Google Scholar]
- 74. Cavero‐Redondo I, Álvarez‐Bueno C, Pozuelo‐Carrascosa DP, Díez‐Fernández A, Notario‐Pacheco B. Risk of extrapyramidal side effects comparing continuous vs. bolus intravenous metoclopramide administration: a systematic review and meta‐analysis of randomised controlled trials. J Clin Nurs. 2015;24(23‐24):3638–46. [DOI] [PubMed] [Google Scholar]
- 75. Midani D, Parkman HP. Granisetron transdermal system for treatment of symptoms of gastroparesis: a prescription registry study. J Neurogastroenterol Motil. 2016;22:650–5. 10.5056/jnm15203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pasricha PJ, Yates KP, Sarosiek I, McCallum RW, Abell TL, Koch KL, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology. 2018;154(1):65–76.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Janssen P, Harris MS, Jones M, Masaoka T, Farre R, Tornblom H, et al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108(9):1382–91. [DOI] [PubMed] [Google Scholar]
- 78. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of Promotility agents on gastric emptying and symptoms: a systematic review and meta‐analysis. Gastroenterology. 2019;156(6):1650–60. [DOI] [PubMed] [Google Scholar]
- 79. Tack J, Goelen N, Carbone F, Van den Houte K, Masuy I, Wauters L, et al. Prokinetic Effects and Symptom Relief in the Pharmacotherapy of Gastroparesis. Gastroenterology. 2020;158(6):1841–2. [DOI] [PubMed] [Google Scholar]
- 80. Rayner CK, Jones KL, Horowitz M. Is making the stomach pump better the answer to gastroparesis? Gastroenterology. 2019;156(6):1555–7. [DOI] [PubMed] [Google Scholar]
- 81. Stevens JE, Russo A, Maddox AF, Rayner CK, Phillips L, Talley NJ, et al. Effect of itopride on gastric emptying in longstanding diabetes mellitus. Neurogastroenterol Motil. 2008;20(5):456–63. [DOI] [PubMed] [Google Scholar]
- 82. Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, et al. Systematic review: cardiovascular safety profile of 5‐HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35(7):745–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Degen L, Petrig C, Studer D, Schroller S, Beglinger C. Effect of tegaserod on gut transit in male and female subjects. Neurogastroenterol Motil. 2005;17(6):821–6. [DOI] [PubMed] [Google Scholar]
- 84. Carbone F, Van den Houte K, Clevers E, Andrews CN, Papathanasopoulos A, Holvoet L, et al. Prucalopride in gastroparesis: a randomized placebo‐controlled crossover study. Am J Gastroenterol. 2019;114(8):1265–74. [DOI] [PubMed] [Google Scholar]
- 85. Andrews CN, Woo M, Buresi M, Curley M, Gupta M, Tack J, et al. Prucalopride in diabetic and connective tissue disease‐related gastroparesis: randomized placebo‐controlled crossover pilot trial. Neurogastroenterol Motil. 2021;33:e13958. 10.1111/nmo.13958 [DOI] [PubMed] [Google Scholar]
- 86. Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Curr Opin Pharmacol. 2008;8(6):690–6. [DOI] [PubMed] [Google Scholar]
- 87. Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. N Engl J Med. 1990;322:1028–31. [DOI] [PubMed] [Google Scholar]
- 88. Lembo A, Camilleri M, McCallum R, Sastre R, Breton C, Spence S, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151(1):87–96.e6. [DOI] [PubMed] [Google Scholar]
- 89. Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo‐controlled study. Gastroenterology. 2017;153(5):1240–50.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut−brain interaction): a Rome foundation working team report. Gastroenterology. 2018;154(4):1140–71.e1. [DOI] [PubMed] [Google Scholar]
- 91. Parkman HP, Van Natta ML, Abell TL, McCallum RW, Sarosiek I, Nguyen L, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. J Am Med Assoc. 2013;310(24):2640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology. 2015;149(2):340–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Malamood M, Roberts A, Kataria R, Parkman H, Schey R. Mirtazapine for symptom control in refractory gastroparesis. Drug Des Devel Ther. 2017;11:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anand C, Al‐Juburi A, Familoni B, Rashed H, Cutts T, Abidi N, et al. Gastric electrical stimulation is safe and effective: a long‐term study in patients with drug‐refractory gastroparesis in three regional centers. Digestion. 2007;75(2‐3):83–9. [DOI] [PubMed] [Google Scholar]
- 95. Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125(2):421–8. [DOI] [PubMed] [Google Scholar]
- 96. McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8(11):947–54. [DOI] [PubMed] [Google Scholar]
- 97. McCallum RW, Sarosiek I, Parkman HP, Snape W, Brody F, Wo J, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25(10):815.e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9(4):314–9.e1. [DOI] [PubMed] [Google Scholar]
- 99. Gourcerol G, Chaput U, LeBlanc I, Gallas S, Michot F, Leroi AM, et al. Gastric electrical stimulation in intractable nausea and vomiting: assessment of predictive factors of favorable outcomes. J Am Coll Surg. 2009;209(2):215–21. [DOI] [PubMed] [Google Scholar]
- 100. Ducrotte P, Coffin B, Bonaz B, Fontaine P, Des Varannes SB, Zerbib F, et al. Gastric electrical stimulation reduces refractory vomiting in a randomized cross‐over trial. Gastroenterology. 2020;158(3):506–14.e2. [DOI] [PubMed] [Google Scholar]
- 101. Thomas A, de Souza Ribeiro B, Malespin M, de Melo SW. Botulinum toxin as a treatment for refractory gastroparesis: a literature review. Curr Treat Options Gastroenterol. 2018;16(4):479–88. [DOI] [PubMed] [Google Scholar]
- 102. Arts J, Holvoet L, Caenepeel P, Bisschops R, Sifrim D, Verbeke K, et al. Clinical trial: a randomized‐controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26(9):1251–8. [DOI] [PubMed] [Google Scholar]
- 103. Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103(2):416–23. [DOI] [PubMed] [Google Scholar]
- 104. Podboy A, Hwang JH, Nguyen LA, Garcia P, Zikos TA, Kamal A, et al. Gastric per‐oral endoscopic myotomy: current status and future directions. World J Gastroenterol. 2019;25(21):2581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jacques J, Pagnon L, Hure F, Legros R, Crepin S, Fauchais AL, Palat S, et al. Peroral endoscopic pyloromyotomy is efficacious and safe for refractory gastroparesis: prospective trial with assessment of pyloric function. Endoscopy. 2019;51(1):40–9. [DOI] [PubMed] [Google Scholar]
- 106. Toro JP, Lytle NW, Patel AD, Davis SS, Christie JA, Waring JP, et al. Efficacy of laparoscopic pyloroplasty for the treatment of gastroparesis. J Am Coll Surg. 2014;218(4):652–60. [DOI] [PubMed] [Google Scholar]
- 107. Karlstrom L, Kelly KA. Roux‐Y gastrectomy for chronic gastric atony. Am J Surg. 1989;157(1):44–9. [DOI] [PubMed] [Google Scholar]
- 108. Hinder RA, Esser J, DeMeester TR. Management of gastric emptying disorders following the Roux‐en‐Y procedure. Surgery. 1988;104(4):765–72. [PubMed] [Google Scholar]
- 109. Vogel SB, Woodward ER. The surgical treatment of chronic gastric atony following Roux‐Y diversion for alkaline reflux gastritis. Ann Surg. 1989;209(6):756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McCallum RW, Polepalle SC, Schirmer B. Completion gastrectomy for refractory gastroparesis following surgery for peptic ulcer disease. Long‐term follow‐up with subjective and objective parameters. Dig Dis Sci. 1991;36(11):1556–61. [DOI] [PubMed] [Google Scholar]
- 111. Eckhauser FE, Conrad M, Knol JA, Mulholland MW, Colletti LM. Safety and long‐term durability of completion gastrectomy in 81 patients with postsurgical gastroparesis syndrome. Am Surg. 1998;64(8):711–6. [PubMed] [Google Scholar]
- 112. Forstner‐Barthell A, Murr MM, Nitecki S, Camilleri M, Prather CM, Kelly KA, et al. Near‐total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long‐term results in 62 patients. J Gastrointest Surg. 1999;3(1):15–23. [DOI] [PubMed] [Google Scholar]
- 113. Bhayani NH, Sharata AM, Dunst CM, Kurian AA, Reavis KM, Swanstrom LL. End of the road for a dysfunctional end organ: laparoscopic gastrectomy for refractory gastroparesis. J Gastrointest Surg. 2015;19(3):411–7. [DOI] [PubMed] [Google Scholar]
- 114. Huang J. Analysis of the relationship between Helicobacter pylori infection and diabetic gastroparesis. Chin Med J. 2017;130(22):2680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang C‐L, Geng CH, Yang ZW, Li YL, Tong LQ, Gao P, et al. Changes in patients' symptoms and gastric emptying after Helicobacter pylori treatment. World J Gastroenterol. 2016;22(18):4585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Masuy I, Van Oudenhove L, Tack J. Review article: treatment options for functional dyspepsia. Aliment Pharmacol Ther. 2019;49 (9):1134–72. 10.1111/apt.15191 [DOI] [PubMed] [Google Scholar]
- 117. Braden B, Caspary W, Börner N, Vinson B, Schneider AR. Clinical effects of STW 5 (Iberogast) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterol Motil. 2009;21(6):632–8.e25. [DOI] [PubMed] [Google Scholar]
- 118. Kim KH, Lee MS, Choi TY, Kim TH. Acupuncture for symptomatic gastroparesis. Cochrane Database Syst Rev. 2018;12(12):CD009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bharadwaj S, Meka K, Tandon P, Rathur A, Rivas JM, Vallabh H, et al. Management of gastroparesis‐associated malnutrition. J Dig Dis. 2016;17(5):285–94. [DOI] [PubMed] [Google Scholar]
- 120. Fontana RJ, Barnett JL. Jejunostomy tube placement in refractory diabetic gastroparesis: a retrospective review. Am J Gastroenterol. 1996;91(10):2174–8. [PubMed] [Google Scholar]
- 121. Vandenbroucke K, Kindt S, Demedts I, Tack J. Outcome of percutaneous jejunal feeding tube placement for refractory idiopathic severe gastroparesis: a retrospective review. Acta Gastroenterol Belg. 2006;69:D14. [Google Scholar]
- 122. Pasricha PJ, Yates KP, Nguyen L, Clarke J, Abell TL, Farrugia G, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology. 2015;149(7):1762–74.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med. 2002;113(6):449–55. [DOI] [PubMed] [Google Scholar]
- 124. Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long‐term follow‐up of patients with gastroparesis. Dig Dis Sci. 1998;43(11):2398–404. [DOI] [PubMed] [Google Scholar]
- 125. Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22(3):503–7. [DOI] [PubMed] [Google Scholar]
- 126. Hyett B, Martinez FJ, Gill BM, Mehra S, Lembo A, Kelly CP, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137(2):445–52. [DOI] [PubMed] [Google Scholar]
- 127. Chang J, Rayner CK, Jones KL, Horowitz M. Prognosis of diabetic gastroparesis—a 25‐year evaluation. Diabet Med. 2013;30(5):e185–8. [DOI] [PubMed] [Google Scholar]
- 128. Talley NJ. Diabetic gastropathy and prokinetics. Am J Gastroenterol. 2003;98(2):264–71. [DOI] [PubMed] [Google Scholar]
- 129. Arts J, Caenepeel P, Verbeke K, Tack J. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut. 2005;54(4):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable for a consensus document.


