Abstract
Background
Vedolizumab (VDZ), a humanised monoclonal antibody against a4ß7‐integrin, has shown efficacy in inflammatory bowel disease (IBD). It is of importance to assess the mid‐to long‐term efficacy of VDZ using real‐life data.
Objective
Our study aimed to determine the efficacy of VDZ in patients with IBD with and without prior exposure to anti‐tumour necrosis factor (TNF) treatments in a real‐life setting. Furthermore, we investigated confounding factors influencing the remission to VDZ.
Methods
Patients participating in the Swiss IBD Cohort Study were included in this study. Remission was defined as calprotectin less than 200 mg/kg stool and/or mucosal healing determined by endoscopy. End points were determined between Months 4 and 8 (T1) and between Months 12 and 16 (T2) after VDZ induction.
Results
Remission was reported in 50.5% (110/218) of patients in T1 (48.7% Crohn's disease [CD] and 52.5% ulcerative colitis [UC]) and 46.8% (102/218) in T2 (47% CD and 46.5% UC). In UC patients, a significantly higher remission rate was achieved in T2 among anti‐TNF‐naive patients (57.7%) compared to anti‐TNF‐experienced patients (34.7%; p = 0.02; odds ratio = 0.39, 95% confidence interval: 0.17–0.87). In patients with CD, no difference could be seen in either evaluation interval. Multivariable analysis showed that disease duration significantly influenced remission rates among UC patients. A late response to VDZ therapy with an achievement of remission in T2 was seen in a fifth of all patients (CD: 21.7%, UC: 20.8%). VDZ treatment was stopped in a third of all patients (31.8%) due to nonresponse, adverse events or aggravation of extra‐intestinal manifestations.
Conclusion
In a real‐life national cohort setting, VDZ induced remission in more than half of IBD patients. Previous treatment with anti‐TNF agents was associated with a significant lower efficacy of VDZ in UC but not in CD patients.
Keywords: adverse events, anti‐TNF experienced, anti‐TNF naive, Crohn's disease, inflammatory bowel disease, real‐life data, remission, safety, ulcerative colitis, vedolizumab
1. INTRODUCTION
Inflammatory bowel disease (IBD) patients with moderate to severe disease activity refractory to conventional immunosuppressants are frequently treated with biological agents, especially with anti‐tumour necrosis factor (TNF) alpha agents. 1 , 2 Alternative therapies include an anti‐integrin gut‐selective immunomodulatory humanised IgG1 antibody, marketed as vedolizumab (VDZ). It targets a surface glycoprotein called α4β7‐integrin on activated lymphocytes, which prevents them from trafficking into the intestinal wall.
A significant proportion of IBD patients treated with anti‐TNF agents have to stop their biological treatment, due to primary or secondary nonresponse, intolerance or immunogenicity of the anti‐TNF agent. A systematic review found that almost half of all patients experienced secondary nonresponse to infliximab over time. 3 Anti‐drug antibodies and low trough levels are predictive for nonresponse to anti‐TNF treatment. 4 Data from the Swiss IBD Cohort Study (SIBDCS) revealed in a 10‐years follow‐up that early anti‐TNF administration was associated with a more favourable long‐term outcome. 5 A recent head‐to‐head study in patients with moderately to severely active ulcerative colitis (UC) compared the efficacy of VDZ and adalimumab. VDZ was superior to adalimumab with respect to achievement of clinical remission and endoscopic improvement but not corticosteroid‐free clinical remission. 6 However, another study indicated a comparable efficacy of VDZ and anti‐TNF agents among UC patients. 7 Also, compared to anti‐TNF agents, VDZ has a rather limited effect on extraintestinal manifestations of IBD. 8 In summary, it remains unclear if certain classes of medications would be more favourable compared to others as first‐line therapy, since reliable predictive biomarkers are still lacking.
Concerning the efficacy of VDZ stratified by previous anti‐TNF treatment, several trials have indicated a higher efficacy among anti‐TNF naive patients. 9 , 10 Likewise, previous biological therapies were associated with a decreased efficacy of anti‐TNF agents, especially with multiple previous anti‐TNF treatments. 11
Nevertheless, only limited data are available comparing the efficacy of VDZ in anti‐TNF‐experienced and ‐naive patients using objective outcome measurements. Therefore, our study aimed to compare the mid‐to long‐term efficacy of VDZ between these two groups using real‐life data with objective outcome measurements. Additionally, we aimed to reveal the safety profile of VDZ in a real‐life cohort. Regarding the safety of VDZ, a favourable profile of this medication has been shown in several studies. 12 , 13 , 14 , 15 There is also information about the long‐term safety of VDZ in a real‐life setting. 16
2. METHODS
2.1. Study design and patients
This was a real‐life observational study. Patients with Crohn's disease (CD) and UC who had been treated with VDZ were enrolled in our cohort. Data were provided from four different medical centres in Switzerland (Crohn‐Colitis‐Zentrum Bern and Fribourg, Gastroenterology Inselspital Bern, Gastroenterology Beaulieu Lausanne and Gastroenterology USZ Zürich), and the patients were part of the SIBDCS. 17 , 18 The data were collected between July 2017 and October 2018. Remission rates and adverse events (AEs) were determined between Months 4 and 8 (T1) and between Months 12 and 16 (T2) after VDZ induction. Discontinuation rates were evaluated irrespective of the time frame.
Eligible IBD patients were greater than 18 years of age and had been diagnosed with CD and UC for at least 1 year. After screening, patients were eligible for the analysis of safety and VDZ discontinuation rates (safety and discontinuation cohort, N = 245). Of those, patients not having either endoscopy or calprotectin data available in T1 and T2 were excluded for the remission evaluation (remission subcohort, intention to treat [ITT] population, N = 218). In the remission subcohort, endoscopy and/or calprotectin evaluation were mandatory for patients in both time frames (T1 and T2; Figure 1).
FIGURE 1.
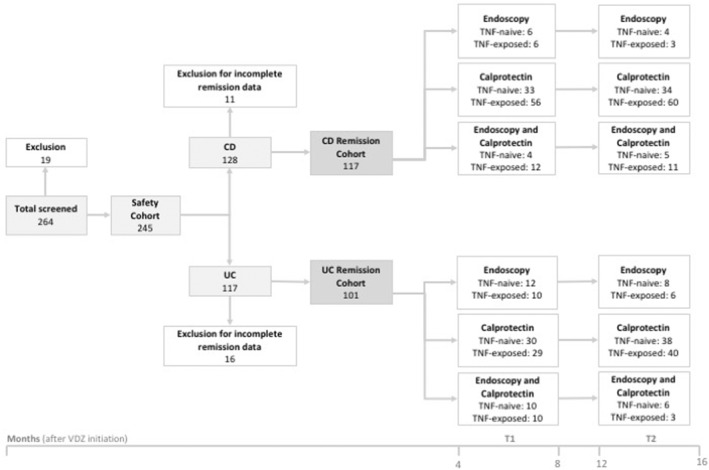
Flow chart describing the number of patients included in the study. In the safety and discontinuation subcohort (N = 245), AEs and reasons for discontinuation of VDZ treatment were investigated. In the remission subcohort, calprotectin and endoscopic remission rates were determined in two time frames after VDZ initiation: Months 4–8 (T1) and Months 12–16 (T2). AEs, adverse events; CD, Crohn's disease; UC, ulcerative colitis; VDZ, vedolizumab
Stable comedication with immunosuppressants and 5‐aminosalicylic acid were permitted. Topical or oral steroids were allowed at any time point of the study, but the exact dose needed to be documented. Patients on a higher dose of steroids at T1 and/or T2 compared to the time point of VDZ initiation were considered as treatment failures. Patients with a current simultaneous treatment of two biologicals, with stoma, colectomy or pregnancy during VDZ treatment were excluded. Additionally, Crohn's patients with isolated disease activity in the upper gastrointestinal tract (Montreal classification L4) were excluded. The study was approved by the ethics committee (SIBDCS, EK1316, 05.02.2007). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and informed consent was obtained from each patient included in the study.
For the stratified analysis of those who were anti‐TNF experienced versus those who were anti‐TNF naive, patients were considered as treatment failures to anti‐TNF agents if they had a primary nonresponse, a loss of response or had to stop their treatment due to side effects.
2.2. Data collection
Data on clinical and demographic characteristics, infusion frequency and premedication of the cohort were collected from the medical records. Calprotectin levels in stool, results of endoscopy, stable coadministration of immunosuppressives and topical or oral corticosteroids were assessed at two evaluation intervals. Moreover, data concerning the frequency and reasons for stopping VDZ treatment as well as side effects were collected. The first evaluation interval (T1, early remission) was defined as being between Months 4 and 8 after VDZ initiation, and the second evaluation interval (T2, late remission) between Months 12 and 16. Remission was defined as biochemical remission with faecal calprotectin less than 200 mg/kg stool and/or mucosal healing determined by endoscopy (UC: Mayo Score = 0, CD: SES‐CD < 3). For patients having both calprotectin levels and endoscopic finding available in an evaluation interval, the patient was considered not to be in remission if inflammation was found upon endoscopy with calprotectin levels less than 200 mg/kg stool. The use of mean calprotectin values was used if more than one sample was available during the specified time frame. If a patient had to discontinue VDZ treatment between T1 and T2, remission data were nevertheless included in our statistical analysis (ITT).
2.3. Statistical analysis
The data were analysed with IBM SPSS Statistics for Windows v25.9 (IBM Corp). Clinical and demographic characteristics are presented as the mean ± standard deviation (SD) or percentages. The efficacy of VDZ treatment was analysed in the remission subcohort. The safety of VDZ treatment and the comparison of infusion frequencies between anti‐TNF‐naive and anti‐TNF‐experienced patients were analysed in the whole cohort (safety and discontinuation cohort; Figure 1).
Univariable and multivariable logistic regression were used to assess the influence of the following variables on VDZ remission in T1 and T2: age, sex, infusion frequency, duration of disease, intestinal involvement, exposure to anti‐TNF agents, concomitant oral steroids and immunosuppressives. Binary outcomes (remission and discontinuation rate, infusion frequency and concomitant therapy) were compared between different subgroups using univariable logistic regression. The effects were reported using odds ratios (ORs) and 95% confidence intervals (CIs). Disease duration was compared between different subgroups using the Mann–Whitney U‐test. A nonparametric test was used due to the small sample in order to avoid normality assumption. AEs and discontinuation characteristics are summarised using descriptive statistics.
2.4. Study aims
The primary outcome of this study was the comparison of the mid‐to long‐term efficacy of VDZ treatment between patients with and without previous treatment with anti‐TNF agents. As a secondary outcome, we aimed to detect factors confounding the remission rate of VDZ‐treated patients. Further secondary outcomes were reasons for stopping treatment and a description of side effects.
3. RESULTS
3.1. Patient characteristics
A total of 264 patients were screened, and 19 patients were excluded (14 colectomies, two pregnancies, one simultaneous treatment with two biologicals and two patients lost to follow‐up). As a result, we analysed the safety data of 245 patients with IBD who received VDZ treatment (128 with CD and 117 with UC). The clinical and demographic characteristics of the patients are described in Table 1. The mean age of the patients was 43.93 years (SD = 15.46), and 53.5% were female. Overall, 57.6% (n = 141, 82 CD/59 UC) of all patients had previous exposure to anti‐TNF treatments, and 42.4% (n = 104, 46 CD/58 UC) were anti‐TNF naive. In T1, 17.2% of all patients were treated with oral steroids, and in T2, 12.8% were treated with oral steroids. Comedication with immunosuppressants was used in 23.8% of the patients in T1 and 19.4% of the patients in T2.
TABLE 1.
Clinical and demographic characteristics of the patients
| CD (N = 128) | UC (N = 117) | CD and UC (N = 245) | Anti‐TNF naive (N = 104) | Anti‐TNF experienced (N = 141) | |
|---|---|---|---|---|---|
| Age (years), M ± SD | 41.98 ± 15.29 | 46.08 ± 15.42 | 43.93 ± 15.46 | 44.71 ± 16.28 | 43.35 ± 14.85 |
| Sex (%) | |||||
| Male | 61 (47.7) | 53 (45.3) | 114 (46.5) | 50 (48.1) | 64 (45.4) |
| Female | 67 (52.3) | 64 (54.7) | 131 (53.5) | 54 (51.9) | 77 (54.6) |
| Duration of disease (years), M ± SD | 12.46 ± 9.22 | 11.03 ± 8.58 | 11.78 ± 8.93 | 9.68 ± 8.44 | 13.34 ± 8.99 |
| Anti‐TNF experienced (%) | |||||
| Yes | 82 (64.1) | 59 (50.4) | 141 (57.6) | 141 | |
| No | 46 (35.9) | 58 (49.6) | 104 (42.4) | 104 | |
| Comedication IS at T0 | 27 (21.1) | 33 (28.2) | 60 (24.5) | 23 (22.1) | 37 (26.2) |
| Comedication oral steroids at T0 | 51 (39.8) | 45 (38.5) | 96 (39.2) | 30 (28.8) | 66 (46.8) |
| Montreal classification CD/UC (%) | |||||
| L1/E1 | 30 (23.4) | 15 (12.8) | |||
| L2/E2 | 26 (20.3) | 39 (33.4) | |||
| L3/E3 | 61 (47.7) | 63 (53.8) | |||
| L1 + L4 | 4 (3.1) | ||||
| L3 + L4 | 7 (5.5) | ||||
Abbreviations: CD, Crohn's disease; IS, immunosuppressives; SD, standard deviation; TNF, tumour necrosis factor; UC, ulcerative colitis.
3.2. Overall efficacy
A calprotectin measurement and/or endoscopy was performed in 218 (117 CD/101 UC) patients in both time frames (remission subcohort; Figure 1). In T1, 50.5% (110/218) of all IBD patients achieved early remission, with 48.7% (57/117) of CD patients and 52.5% (53/101) of UC patients having met the defined remission criteria. In T2, an overall remission rate of 46.8% (102/218) was found, with 47% (55/117) of CD patients and 46.5% (47/101) of UC patients being in late remission (ITT; Figure 2).
FIGURE 2.
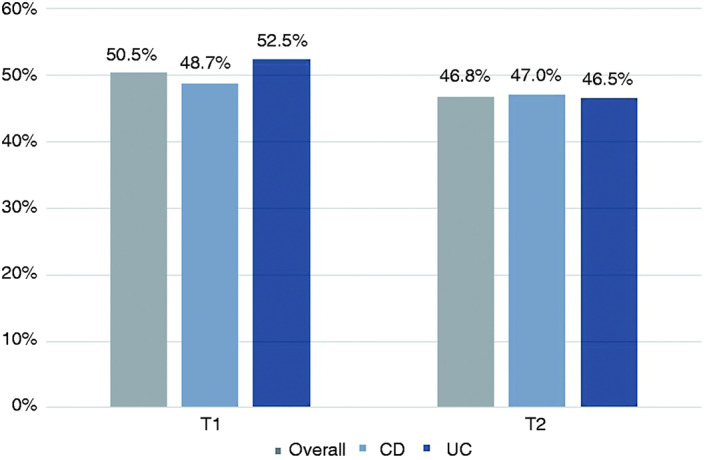
Remission rates of all patients, patients with CD and patients with UC between Months 4 and 8 (T1) and between Months 12 and 16 (T2) after VDZ initiation. T1: n = 110/218, 57/117 and 53/101; T2: n = 102/218, 55/117 and 47/101 for all patients, CD patients and UC patients, respectively. CD, Crohn's disease; UC, ulcerative colitis; VDZ, vedolizumab
3.3. Overall efficacy over time
Among CD patients, 73.7% (42/57) of the early remitters maintained remission through T2. A loss of response in the second evaluation interval was seen in 17.5% (10/57) of the early remitters. The remaining 8.8% (5/57) stopped VDZ treatment due to AEs or due to exacerbation of extra‐intestinal symptoms. However, 21.7% (13/60) of the patients, who did not achieve remission in T1, gained remission in T2. In CD, 40.2% (47/117) of patients were nonresponders at T1 and T2 (Table 2).
TABLE 2.
Remission rates at time points T1 and T2
| CD, n (%) | UC, n (%) | |
|---|---|---|
| Remission at T1 | 57/117 (48.7) | 53/101 (52.5) |
| Maintained remission at T2 | 42/57 (73.7) | 37/53 (69.8) |
| Loss of response between T1 and T2 | 10/57 (17.5) | 12/53 (22.6) |
| VDZ stop due to AEs/extra‐intestinal symptoms | 5/57 (8.8) | 4/53 (7.5) |
| between T1 and T2 among early remitters | ||
| Nonremitters at T1 | 60/117 (51.3) | 48/101 (47.5) |
| Gained remission at T2 | 13/60 (21.7) | 10/48 (20.8) |
Note: A substantial part of patients remained in remission between T1 and T2; greater than >20% of nonremitters at T1 gained remission at T2.
Abbreviations: AEs, adverse events; CD, Crohn's disease; UC, ulcerative colitis; VDZ, vedolizumab.
Among UC patients, 69.8% (37/53) of the remitters in T1 maintained remission through T2. A loss of response in the second evaluation interval could be seen in 12.53 (22.6%) early remitters. VDZ treatment was stopped due to AEs or exacerbation of extraintestinal manifestations in 7.5% (4/53) of the remaining early remitters. Among nonremitters in T1, 20.8% (10/48) gained remission in T2, and in the whole remission subcohort, 37.6% (38/101) never achieved remission (Table 2).
3.4. Remission rates in anti‐TNF‐naive versus anti‐TNF‐experienced patients
In anti‐TNF‐naive UC patients in T1, we found a numerically higher remission rate compared to anti‐TNF‐experienced patients (p = 0.06, OR = 0.47, 95% CI: 0.21–1.04) In T2, the difference was statistically significant (p = 0.02, OR = 0.39, 95% CI: 0.17–0.87; Figure 3a). In patients with CD, no difference could be seen in either evaluation interval (Figure 3b).
FIGURE 3.
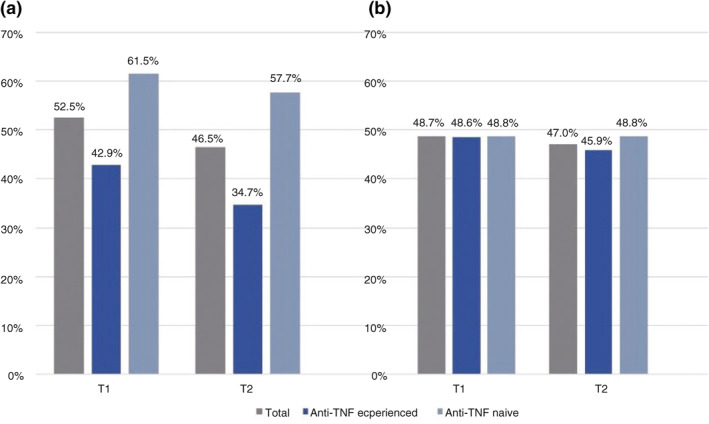
(a) Remission rates of anti‐TNF‐experienced and anti‐TNF‐naive patients between Months 4 and 8 (T1) and between Months 12 and 16 (T2) after VDZ initiation in patients with UC. T1: n = 53/101, 21/49 and 32/52; T2: n = 47/101, 17/49 and 30/52 for all patients, anti‐TNF‐experienced and anti‐TNF‐naive patients, respectively. *p < 0.05. (b) Remission rates of anti‐TNF‐experienced and anti‐TNF‐naive patients between Months 4 and 8 (T1) and between Months 12 and 16 (T2) after VDZ initiation in patients with CD (T1: n = 57/117, 36/74 and 21/43; T2: n = 55/117, 34/74 and 21/43 in all patients, anti‐TNF‐experienced and anti‐TNF‐naive patients, respectively. CD, Crohn's disease; TNF, tumour necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab
To explain this significant difference, we checked for possible confounders such as age, sex, duration of disease, intestinal involvement and comedication with steroids or immunosuppressants. None of these confounders, except disease duration, seemed to be of importance. Among UC patients, nonremitters showed a longer duration of disease compared to remitters (T1: 12.53 ± 9.19 years in nonremitters vs. 9.72 ± 7.82 years in remitters, p = 0.09; T2: 12 ± 8.63 years in nonremitters vs. 9.96 ± 8.44 years in remitters, p = 0.18). This effect was not seen in CD patients (T1: 11.38 ± 7.87 years in nonremitters vs. 13.86 ± 10.74 years in remitters; T2: 11.87 ± 7.58 years in nonremitters vs. 13.40 ± 11.16 years in remitters).
Subgroup analysis stratified for anti‐TNF‐experienced versus anti‐TNF‐naive UC patients revealed a significant difference with respect to disease duration. In UC, anti‐TNF‐experienced remitters showed a significantly longer duration of disease compared to anti‐TNF‐naive remitters in T1 (12.14 ± 7.21 years in experienced vs. 8.13 ± 7.90 years in naive remitters; p = 0.017) and T2 (14.11 ± 9.04 years in experienced vs. 7.6 ± 7.02 years in naive remitters; p = 0.007). A similar trend was seen in CD patients, although with a nonsignificant difference between the two groups (T1: 14.25 ± 10.62 years in experienced vs. 13.19 ± 11.17 years in naive remitters, p = 0.67; T2: 14.56 ± 11.49 years in experienced versus 11.52 ± 10.61 years in naive remitters, p = 0.38).
3.5. Concomitant steroid therapy
Concomitant steroid and immunosuppressive therapy were less frequent among remitters compared to nonremitters. However, this difference did not reach statistical significance in CD patients. In T1, concomitant steroid therapy was 8.8% (5/57) in remitters compared to 18.3% (11/60) in nonremitters.
In T2, concomitant steroid therapy was administered in 7.3% (4/55) of remitters compared to 16.1% (10/62) of nonremitters. These findings were in contrast to UC patients, where a significant difference in concomitant steroid use in T2 was found, with 6.4% (3/47) of steroid use in remitters compared to 22.2% (12/54) in nonremitters (p = 0.03, OR = 0.23, 95% CI: 0.06–0.89).
3.6. Infusion frequency
In T1, 85.3% of patients were treated every 8 weeks, and 14.7% were treated every 4–6 weeks. In T2, VDZ was given every 8 weeks in 76.5% of patients and every 4–6 weeks in 23.5% of patients. Patients, who were anti‐TNF experienced received VDZ significantly more frequently than those who were anti‐TNF naive. The CD subpopulation showed a significant difference at T1 in this regard (p = 0.04, OR = 0.27, 95% CI: 0.07–0.97). In T2, this difference was no longer statistically significant (p = 0.22, OR = 0.54, 95% CI: 0.2–1.44; Figure 4a). The same trend was seen in the UC population but with no significant difference in the first and second evaluation interval (Figure 4b).
FIGURE 4.
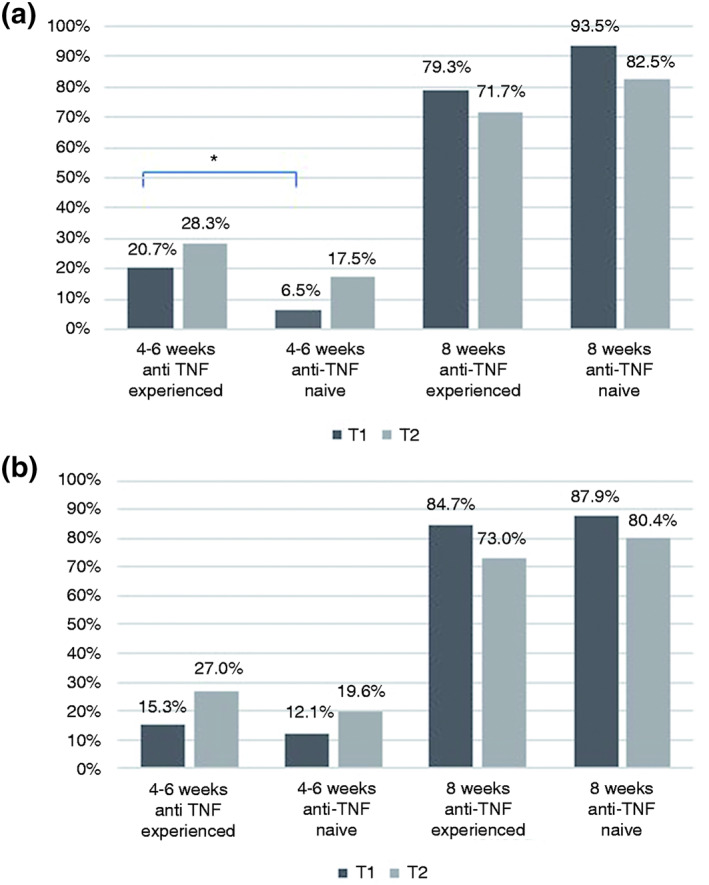
(a) VDZ infusion frequency every 4–6 weeks versus every 8 weeks in patients with CD in anti‐TNF‐experienced and anti‐TNF‐naive patients at time intervals T1 (Months 4–8) and T2 (Months 12–16). Every 4–6 weeks: n = 17/82, 17/60, 3/46 and 7/40; every 8 weeks: n = 65/82, 43/60, 43/46 and 33/40 for anti‐TNF‐experienced and anti‐TNF‐naive patients at T1 and T2, respectively; *p < 0.05). (b) VDZ infusion frequency every 4–6 weeks versus every 8 weeks in patients with UC in anti‐TNF‐experienced and anti‐TNF‐naive patients at time intervals T1 (Months 4–8) and T2 (Months 12–16). Every 4–6 weeks: n = 9/59, 10/37, 7/58 and 9/46; every 8 weeks: n = 50/59, 27/37, 51/58 and 37/46 for anti‐TNF‐experienced and anti‐TNF‐naive patients at T1 and T2, respectively. CD, Crohn's disease; TNF, tumour necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab
3.7. Discontinuation of VDZ treatment
A total of 78 (31.8%) patients stopped VDZ treatment, of whom 29.7% (38/128) were CD patients and 34.2% (40/117) were UC patients. The most frequent reason for discontinuation was nonresponse to VDZ (22.4%; 55/245). VDZ discontinuation was reported in 6.1% (15/245) of all patients due to AEs, in 2.9% (7/245) due to extra‐intestinal symptoms and in one (0.4%) patient due to intolerance of VDZ. There was a statistically significant higher discontinuation rate among anti‐TNF‐experienced UC patients compared to anti‐TNF‐naive UC patients. In this subgroup, 42.4% (25/59) of anti‐TNF‐experienced and 25.9% (15/58) of anti‐TNF‐naive patients discontinued VDZ therapy (p = 0.06, OR = 2.11, 95% CI: 0.96–4.61). With respect to CD, 31.7% (26/82) of anti‐TNF‐experienced patients discontinued VDZ treatment compared to 26.1% (12/46) of anti‐TNF‐naive patients (p = 0.51, OR = 1.32, 95% CI: 0.59–2.95).
3.8. Safety
A total of 112 (45.7%) patients suffered from AEs that were possibly related to the VDZ treatment. The following side effects were observed: upper respiratory infections (n = 26; 10.6%), tiredness (n = 14; 5.7%), exanthema (n = 11; 4.5%), headache (n = 8; 3.3%), pruritus (n = 6; 2.4%), infection of the gastrointestinal tract (n = 3; 1.2%), infection of the middle ear (n = 1; 0.4%) and fever (n = 1; 0.4%). Arthralgia was described among 34 (13.9%) of the patients, and four patients showed neurological AEs. However, the cause of arthralgia did not necessarily have to be a side effect of VDZ, but could also be an untreated extra‐intestinal manifestation. Furthermore, four patients did not tolerate VDZ infusions (Table 3).
TABLE 3.
Adverse events (AEs)
| N = 245 | |
|---|---|
| Respiratory tract infection | 26 (10.6) |
| Fatigue | 14 (5.7) |
| Exanthema | 11 (4.5) |
| Headache | 8 (3.3) |
| Pruritus | 6 (2.4) |
| Intolerance to VDZ | 4 (1.6) |
| Infection of gastrointestinal tract | 3 (1.2) |
| Neurological AE | 4 (1.6) |
| Middle ear infection | 1 (0.4) |
| Fever | 1 (0.4) |
| Arthralgia | 34 (13.9) |
Abbreviation: VDZ, vedolizumab.
4. DISCUSSION
In our real‐life cohort, VDZ therapy was efficacious for induction and maintenance of remission in both UC and CD patients. These findings are consistent with already published trials. 12 , 13 , 19 , 20 , 21 In our cohort, 48.7% (CD) and 52.5% (UC) achieved early remission, and 47.0% (CD) and 46.5% (UC) achieved late remission (ITT analysis). Compared to the randomised, double‐blinded and placebo‐controlled trials GEMINI I and II, a remission rate of 14.5% (CD) and 16.9% (UC) at Week 6% and 39% and 41.8% at Week 52 was achieved. 12 , 13 Possible explanations for our higher remission rates may include longer follow‐up time of VDZ treatment in the SIBDCS, as well as the use of objective criteria: The GEMINI I and II trials determined remission rates using the Mayo score, as well as the more subjective CDAI and HBI scores. In our study, remission rates were measured using objective biochemical and/or endoscopic end points to measure the degree of remission. Furthermore, the hard‐to‐treat population enrolled in the phase III GEMINI study programme might have led to biasing of real‐life remission rates. Finally, a reduction in the treatment intervals, which may improve the treatment results, was possible in our study.
Onset of response and remission to VDZ may vary between different studies. In our study, among patients without early remission, a fifth came into late remission (21.7% CD/20.8% UC). This shows the value of continually treating patients with an early response but without objective remission on VDZ over a longer period of time. In contrast, a proportion of the remitters in T1 lost their response to VDZ in T2 (17.5% CD/22.6% UC). To our knowledge, no comparable data have been reported so far.
With an emphasis on mid‐ and long‐term efficacy, comparable remission data were reported in a systematic review by Schreiber et al. 19 A longer follow‐up was documented in a prospective multicentre cohort study, which indicated that VDZ treatment is able to maintain steroid‐free clinical remission for up to 3 years. 20
It has already been described that previous biological therapies can be associated with decreased efficacy of anti‐TNF agents. 11 Therefore, the question of decreased efficacy of VDZ among anti‐TNF‐experienced IBD patients arose. Within the UC population, our data indicate higher remission rates among anti‐TNF‐naive patients compared to anti‐TNF‐experienced patients. This difference was significant for UC but not for CD. Consistent with our findings, a retrospective cohort study from Belgium determined a higher rate of mucosal healing among UC patients naive to anti‐TNF treatment at Week 14. 9 This is in line with a German real‐world analysis, which showed a higher clinical remission rate at Week 54 among anti‐TNF‐naive compared to anti‐TNF‐experienced UC patients. 10 Therefore, consistent with other studies, the nonresponse to an anti‐TNF agent is an important predictor for the efficacy of VDZ treatment. 22 , 23 An important confounder that may partly explain the better outcome in anti‐TNF‐naive patients may be their shorter duration of disease. The duration of disease was lower in anti‐TNF‐naive compared to anti‐TNF‐experienced remitters, with a statistical significance for patients with UC.
Our results show a lower discontinuation rate among anti‐TNF‐naive patients. Furthermore, the outcome of this study indicated that anti‐TNF‐experienced patients received VDZ more frequently in shorter infusion intervals than anti‐TNF‐naive patients, with a statistical significance for patients with CD. This further supports the finding of a higher efficacy of VDZ among anti‐TNF‐naive patients. Generally, early VDZ drug monitoring may help to identify patients in need for an intensified dosing regimen. 24
Data from another large cohort of IBD patients indicated a reduced efficacy of VDZ in patients with prior anti‐TNF treatment, where reduction of effectiveness increased with the number of anti‐TNF agents previously used. 25 Therefore, a medical history with one or more anti‐TNF medication may reflect a difficult‐to‐treat population and does not necessarily mean that the former anti‐TNF exposure itself may negatively influence the VDZ response. For the decision‐making process of initiating VDZ as a firstline biological therapy, data from the Varsity VDZ versus adalimumab head‐to‐head trial should be taken into consideration. 6 As patients with a longlasting disease responded less favourably to VDZ in our real‐life study, it may be important to use the early therapeutic window in these patients.
The safety profile in our study revealed very limited side effects of VDZ. This is in accordance with the studies by Meserve et al. 26 and Lenti et al. 21 A real‐world experience by Navaneethan et al. 27 indicated a good safety profile and efficacy of VDZ in elderly patients (>60 years old). Comparing the safety of an anti‐TNF agent with VDZ, the Varsity trial showed a lower rate of severe AEs, as well as a lower exposure‐adjusted rate of infections among VDZ compared to adalimumab‐treated patients. 6 However, another study indicated, that VDZ compared to anti‐TNF agents may have a higher risk for certain AEs, including cardiovascular and thromboembolic diseases. 28
VDZ treatment discontinuation was observed in a third of all patients (29.7% in CD and 34.2% in UC). In contrast, another real‐world trial determined a discontinuation rate of 10.7% and 20.3% among CD and UC patients, respectively. 14 Reasons for our high discontinuation rate could be a high rate of patients with a severe IBD disease course. The main reason of discontinuation was nonresponse to VDZ, consistent with the results of Kopylov et al. 14 Furthermore, possible AEs to VDZ were another important reason for treatment discontinuation. In our cohort, the most frequent AE was upper respiratory infection, with a frequency of 10.6%, which corresponds to the results of other published evidence. 13 , 14 Arthralgia was described among 34 (13.9%) patients. Our results do not indicate whether arthralgia occurred due to the gut‐selective effect of VDZ as an untreated extra‐intestinal manifestation or as a side effect. Taken together, 45.7% of all patients (arthralgia included) showed AEs possibly related to VDZ, whereas these AEs could also be symptoms of IBD or AEs of other concomitant medications.
With respect to a network meta‐analysis, the Varsity trial and the low rate of severe side effects, VDZ may be considered to be used as first‐line therapy. 6 , 29 With respect to safety considerations, a literature review suggests VDZ as first‐line therapy, particularly for elderly patients and those with a previous serious or opportunistic infection. 30 Despite the lack of data, it could be conceivable that VDZ will be used as first‐line therapy for all IBD patients in the future.
Our study clearly bears some limitations, as it was a real‐life observational study and was therefore not prospective or placebo controlled. Furthermore, different measures of remission (faecal calprotectin or mucosal healing determined by endoscopy) may have been used in one patient for T1 and T2.
5. CONCLUSION
Our real‐life study confirmed the mid‐to long‐term efficacy of VDZ treatment in inducting and maintaining remission in patients with moderately to severely active IBD. Furthermore, our results revealed a significant higher VDZ efficacy among anti‐TNF‐naive compared to anti‐TNF‐experienced patients among UC but not CD patients. A late response to VDZ was observed in one fifth of patients, who achieved remission in T2 only. Multivariable analysis showed that disease duration influenced remission rates, with significance for UC patients. Reasons for VDZ discontinuation were mainly nonresponse to VDZ therapy, and VDZ showed a favourable safety profile.
CONFLICT OF INTERESTS
Gerhard Rogler has consulted to Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; has received speaker's honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; has received educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller. Frank Seibold has consulted to Abbvie, Janssen, MSD, Pfizer, Roche and Takeda. Pascal Juillerat has consulted to Abbvie, Janssen, MSD, Pfizer, Takeda and Vifor. Luc Biedermann has consulted to Abbvie, Janssen, MSD, Pfizer, Takeda and Vifor. Pierre Michetti has consulted to Abbvie, FAlk, Ferring, Janssen, MSD, Pfizer, UCB, Takeda, Vifor. Petr Hruz has consulted to Abbvie, Janssen, Pfizer and Takeda. The other authors do not have any financial disclosures to declare.
FUNGING
The authors received no financial support for the research, authorship and/or publication of this article.
ETHICS APPROVAL
EK1316, SIBDCS, 05.02.2007.
INFORMED CONSENT
All patients gave written informed consent in order to participate.
References
- 1. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn's disease: medical treatment. J Crohns Colitis. 2020;14:4–22. [DOI] [PubMed] [Google Scholar]
- 2. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- 3. Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta‐analysis. Am J Gastroenterol. 2011;106:644–59. [DOI] [PubMed] [Google Scholar]
- 4. Greuter T, Maillard MH, Juillerat P, et al. Therapeutic drug monitoring to guide clinical decision making in inflammatory bowel disease patients with loss of response to anti‐TNF: a Delphi technique‐based consensus. Digestion . 2020;101:683–91. 10.1159/000501930. [DOI] [PubMed] [Google Scholar]
- 5. Frei R, Fournier N, Zeitz J, et al. Early initiation of anti‐ TNF is associated with favourable long‐term outcome in Crohn's disease: 10‐year‐follow‐up data from the Swiss IBD Cohort Study. J Crohns Colitis. 2019;13:1292–301. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Peyrin‐Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate‐ to‐severe ulcerative colitis. N Engl J Med. 2019;381:1215–26. [DOI] [PubMed] [Google Scholar]
- 7. Davis R, McParland P, Dodd S, et al. Comparative effectiveness of antitumour necrosis factor agents and vedolizumab in ulcerative colitis. Eur J Gastroenterol Hepatol. 2019;31:661–7. [DOI] [PubMed] [Google Scholar]
- 8. Chateau T, Bonovas S, Le Berre C, et al. Vedolizumab treatment in extra‐intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2019;13:1569–77. [DOI] [PubMed] [Google Scholar]
- 9. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1937–46. [DOI] [PubMed] [Google Scholar]
- 10. Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease: a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44:1199–212. [DOI] [PubMed] [Google Scholar]
- 11. Allez M, Vermeire S, Mozziconacci N, et al. The efficacy and safety of a third anti‐TNF monolonal antibody in Crohn's disease after failure of two other anti‐TNF antibodies. Aliment Pharmacol Ther. 2010;31:92–101. [DOI] [PubMed] [Google Scholar]
- 12. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 14. Kopylov U, Ron Y, Avni‐Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease ‐ the Israeli real‐world experience. Inflamm Bowel Dis. 2017;23:404–8. [DOI] [PubMed] [Google Scholar]
- 15. Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn's disease: 4 years of global post‐marketing. J Crohns Colitis. 2020;14:192–204. [DOI] [PubMed] [Google Scholar]
- 16. Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD—lessons from real‐world experience; a systematic review and pooled analysis. J Crohns Colitis. 2018;12:245–57. [DOI] [PubMed] [Google Scholar]
- 17. Pittet V, Michetti P, Mueller C, et al. Cohort profile update: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol. 2019;48:385–6f. [DOI] [PubMed] [Google Scholar]
- 18. Pittet V, Juillerat P, Mottet C, et al. Cohort profile: the Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol. 2009;38:922–31. [DOI] [PubMed] [Google Scholar]
- 19. Schreiber S, Dignass A, Peyrin‐Biroulet L, et al. Systematic review with meta‐analysis: real‐world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53:1048–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amiot A, Serrero M, Peyrin‐Biroulet L, et al. Three‐year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2019;50:40–53. [DOI] [PubMed] [Google Scholar]
- 21. Lenti MV, Levison S, Eliadou E, et al. A real‐world, long‐term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: the Cross Pennine study. Dig Liver Dis. 2018;50:1299–304. [DOI] [PubMed] [Google Scholar]
- 22. Rosario M, French JL, Dirks NL, et al. Exposure‐efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J Crohns Colitis. 2017;11:921–9. [DOI] [PubMed] [Google Scholar]
- 23. Shmidt E, Kochhar G, Hartke J, et al. Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 25. Dulai P, Meserve JD, Hartke JG, et al. Predictors of clinical and endoscopic response with vedolizumab for the treatment of moderately‐severely active ulcerative colitis: results from the US Victory Consortium. Gastroenterology. 2017;152:371. [Google Scholar]
- 26. Meserve J, Aniwan S, Koliani‐Pace JL, et al. Retrospective analysis of safety of vedolizumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Navaneethan U, Edminister T, Zhu X, et al. Vedolizumab is safe and effective in elderly patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:E17. [DOI] [PubMed] [Google Scholar]
- 28. Cross RK, Chiorean M, Vekeman F, et al. Assessment of the real‐world safety profile of vedolizumab using the United States Food and Drug Administration adverse event reporting system. PLoS One. 2019;14:e0225572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vickers AD, Ainsworth C, Mody R, et al. Systematic review with network meta‐analysis: comparative efficacy of biologics in the treatment of moderately to severely active ulcerative colitis. PLoS One. 2016;11:e0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pouillon L, Stappen JV, Bossuyt P, et al. Should we use anti‐tumor necrosis factor agents or vedolizumab as firstline biological therapy in ulcerative colitis? Best Pract Res Clin Gastroenterol. 2018;32‐33:17–25. [DOI] [PubMed] [Google Scholar]


