Abstract
Background
Patients with primary oesophageal squamous cell carcinoma are at risk of developing multiple primary tumours in the upper aero digestive tract. To date, most studies are performed in the Asian population. We aimed to evaluate the risk of multiple primary tumours in the upper aero digestive tract and stomach in patients with oesophageal squamous cell carcinoma in a Western population.
Methods
We performed a nationwide, retrospective cohort study in collaboration with the Netherlands Cancer Registry. Patients with primary oesophageal squamous cell carcinoma, diagnosed between 2000 and 2016, were included. Primary endpoints were synchronous and metachronous multiple primary tumour risk.
Results
The cohort consisted of 9058 patients, diagnosed with oesophageal squamous cell carcinoma (male: 57.3%, median age 67 years). In 476 patients (5.3%), 545 multiple primary tumours have been diagnosed. Most of them were located in the head and neck region (49.5%). Among all multiple primary tumours, 329 (60.4%) were diagnosed synchronously (<6 months after oesophageal squamous cell carcinoma diagnosis) and 216 (39.6%) metachronously (6 months). Patients with oesophageal squamous cell carcinoma had a significantly increased risk of both synchronous (standardised incidence ratio 10.95, 99% confidence interval 9.40–12.53) and metachronous multiple primary tumours (standardised incidence ratio 4.36, 99% confidence interval 3.56–5.10), compared to the general population. The median interval to metachronous second primary tumour diagnosis was 3.0 years (interquartile range 1.8–5.9).
Conclusion
Approximately one in 20 patients with primary oesophageal squamous cell carcinoma have a second primary tumour in the upper aero digestive tract or stomach, either at the time of oesophageal squamous cell carcinoma diagnosis or at a later stage. As second primary tumours occur at an increased risk compared to the general population, prospective studies are necessary to investigate the yield and survival benefit of screening for second primary tumours in patients with oesophageal squamous cell carcinoma.
Keywords: head and neck neoplasms, lung neoplasms, multiple primary neoplasms, oesophagealneoplasms, second primary neoplasms
Key Summary
A minimum of one out of 20 patients with oesophageal squamous cell carcinoma (ESCC) in a Western population develops multiple primary tumours (MPTs).
Most MPTs were detected in the head and neck region, and were detected synchronously with a higher risk than in the general population.
Screening for both synchronous and metachronous MPTs in patients with primary ESCC should be considered.
1. INTRODUCTION
Squamous cell carcinoma is the most common histologic type of oesophageal cancer worldwide, and has the highest incidence in Eastern Asia. 1 , 2 Multiple primary tumours (MPTs) frequently develop in patients with oesophageal squamous cell carcinoma (ESCC), especially in the upper aero digestive tract (UADT). 3 , 4
Since survival of patients with oesophageal cancer has improved over the last years due to better treatment options, the risk of developing MPTs may increase. 5 These MPTs affect the prognosis and survival of patients with ESCC, and the choice of ESCC treatment in case of synchronous second primary tumour (SPT) detection. 6 It is therefore important to detect MPTs at an early stage, when curative treatment is still possible. The development of MPTs in the UADT particularly occurs in patients with squamous cell carcinomas, and can be explained by the ‘field cancerization’ theory. 7 This theory states that premalignant epithelial changes can occur around the primary tumour, due to exposure to common carcinogens. 7 Well‐known carcinogens for the development of both ESCC and MPTs, especially in the head and neck region and lungs, are tobacco and alcohol. 8 , 9
In retrospective studies, up to 19.3% of patients with primary ESCC develop MPTs in the UADT. 3 , 10 , 11 , 12 , 13 Most studies consider the head and neck region, lungs and oesophagus as the UADT, the stomach is another important region to be at risk for MPT development. 3 , 12 , 14 , 15 Most studies about MPT development, however, are performed in Asian populations. There is a lack of Western studies about MPT incidence in ESCC patients.
We therefore conducted a nationwide, retrospective, registry study of patients with primary ESCC to determine the risk of developing MPTs in the UADT and stomach, in a Western country.
2. MATERIALS AND METHODS
2.1. Patient and study design
We conducted a nationwide, retrospective, registry study in collaboration with the Netherlands Cancer Registry (NCR; nationwide registry of all cancers). Adult patients diagnosed with ESCC between 1 January 2000 and 31 December 2016 were selected from the NCR. Patients were excluded when ESCC was not the index tumour; defined as another tumour in the UADT or stomach which was diagnosed >180 days prior to ESCC diagnosis. This study was approved by the Medical Ethical Review Committee of the Erasmus Medical Centre (MEC‐2018‐1631) on 7 January 2019. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's Human Research Committee.
2.2. Data collection
Anonymous patient data were obtained from the NCR. The following data were available and collected: year and age at ESCC diagnosis, sex, ESCC tumour characteristics, the presence of metastases and ESCC treatment. Tumour characteristics included histology, location in the oesophagus (cervical part; <18 cm from the incisors, upper third; 18–24 cm from the incisors, middle third; 24–32 cm from the incisors, lower third of the oesophagus; 32–40 cm from the incisors, or overlapping locations; between two parts of the oesophagus), differentiation grade, clinical and histopathological Tumour, Node, Metastasis (TNM) stage according to the 5th (2000–2002), 6th (2003–2009) and 7th (2010–2016) TNM stage classification. 16 , 17 , 18 For final analysis, we converted all different TNM classifications into the 7th TNM classification. cM1A classification was considered as M0 according to the 7th TNM classification. Information about vital state (alive or not) was collected until 31 January 2018.
We collected the following data of patients with MPTs: MPT location in the UADT (defined as: head and neck region, lungs and oesophagus) and in the stomach, age at MPT diagnosis, year of MPT diagnosis, time between ESCC and MPT diagnosis, tumour characteristics and MPT treatment. No information was available about how MPTs were detected (e.g., CT‐scan, endoscopy). Diagnosis of MPTs was based on information in medical records in accordance with the Warren and Gates criteria: an MPT (a) must be malignant on histological examination, (b) must be separated from the index tumour by normal mucosa, and (c) may not be a metastasis of the index tumour. 19 An MPT was defined as synchronous when it developed within 6 months before or after ESCC diagnosis, and as metachronous when it developed 6 months after ESCC diagnosis. This six month cut‐off value was used in most other studies about MPT development in the UADT. 20 , 21 , 22 Pathology information was available in the NCR to verify that MPTs were not metastases of the primary tumour. All MPTs were identified, also whether patients had more primary tumours (second, third, etc.). The first MPT was also called the SPT. During the study period of 16 years, no MPT screening programmes were performed.
2.3. Study endpoints
The primary endpoint was the risk of MPTs in patients with primary ESCC compared with the general population. Secondary endpoints were; (a) MPT localization in the UADT and stomach, (b) MPT histology, (c) the proportion of synchronous and metachronous MPTs, (d) the cumulative incidence of SPTs, (e) the difference in survival between ESCC patients with low (stage 0/I/II) and high (stage III/IV) stage metachronous SPTs, and (f) risk factors associated with MPT development.
2.4. Statistics
Continues variables were expressed as mean (standard deviation [SD]) and median (interquartile range [IQR] and range) for normally and skewed distributed variables, respectively. For risk factor analysis, a multivariate Cox proportional hazards model was used. Hazard ratios (HRs) and 95% confidence intervals (CIs) with a time‐dependent covariate (follow‐up time until development of an SPT, death or the last date of follow‐up) were calculated in this model.
To assess the MPT risk in patients with primary ESCC, the standardised incidence ratio (SIR) was calculated with 99% CIs, assuming a Poisson probability distribution for the occurrence of SPTs. The SIR was defined as the total number of observed MPTs (the study cohort) divided by the total number of expected cancers in that same group of patients based on the specific age, gender, year and type of cancer incidence in general population. The SIR was calculated for different groups, separately for synchronous or metachronous MPTs, and stratified in accordance with sex, cancer location, age in 20‐year intervals and cancer treatment. Cancer incidence data from the general population were acquired from the NCR. The cumulative incidence of SPTs was estimated with death as competing risk. STATA (v.14) was used for this analysis.
Survival analysis was determined by Kaplan‐Meier analysis, and we performed log‐rank analysis using SPSS. SIRs were calculated with SAS 9.2 software (SAS Institute Inc.), all other analyses were carried out using IBM SPSS version 25. For SIRs, a two‐sided test with a p‐value of <0.01 was considered significant. For all other analysis, a two‐sided test with a p‐value of <0.05 was considered significant.
3. RESULTS
3.1. Patient characteristics
We identified a total of 9810 patients with ESCC diagnosis between 1 January 2000 and 31 December 2016 in the NCR. In total, 752 patients were excluded because ESCC was not the index tumour (Figure 1). The study cohort consisted of 9058 patients with primary ESCC. The median age at ESCC diagnosis was 67 years (IQR 60–75), most patients were male (57.3%) (baseline characteristics are shown in Table 1). The total follow‐up consisted of 17,072 person‐years with a median follow‐up time of 9.8 months (IQR 4.2–23.8 months). The median survival after ESCC diagnosis was 9.9 months (IQR 9.6–10.3). The overall five‐year survival rate of the total cohort was 15.9%.
FIGURE 1.
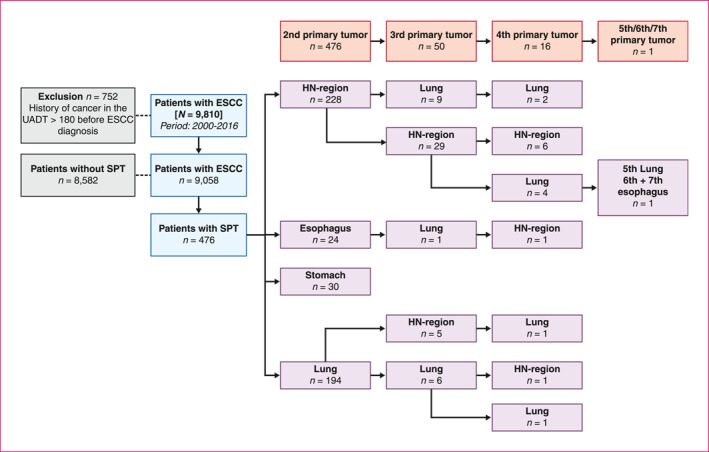
Flow‐chart patient selection and MPT development. ESCC, oesophageal squamous cell carcinoma; HN, head and neck; MPT, multiple primary tumour; SPT, second primary tumour; UADT, upper aero digestive tract
TABLE 1.
Baseline and tumour characteristics of patients with ESCC
| Characteristics | (n = 9058) |
|---|---|
| Gender, n (%) | |
| Male | 5193 (57.3%) |
| Median age (years) (IQR) | 67 (60–75) |
| Follow‐up | |
| Person‐years at risk (years) | 17,072 |
| Median follow‐up (months) (IQR) | 9.8 (4.2–23.8) |
| Vital status (31‐01‐2018), n (%) | |
| Alive | 1358 (15.0%) |
| Dead | 7700 (85.0%) |
| Overall survival after ESCC diagnosis (months) | |
| Median survival (95% CI) | 9.9 (9.6–10.3) |
| Anatomical sub‐location, n (%) | |
| Cervical oesophagus | 276 (3.0%) |
| Upper third oesophagus | 1236 (13.6%) |
| Middle third oesophagus | 3301 (36.4%) |
| Lower third oesophagus | 3501 (38.7%) |
| Oesophagus overlapping or unspecified | 744 (8.3%) |
| ESCC clinical tumour stage, n (%) | |
| 0 | 73 (0.8%) |
| 1 | 591 (6.5%) |
| 2 | 1717 (19.0%) |
| 3 | 2428 (26.8%) |
| 4 | 2620 (28.9%) |
| Missing | 1629 (18.0%) |
| Differentiation grade, n (%) | |
| Grade 1 (good) | 298 (3.3%) |
| Grade 2 (moderate) | 2739 (30.2%) |
| Grade 3 (poor) | 2594 (28.6%) |
| Grade 4 (undifferentiated) | 8 (0.1%) |
| Missing | 3419 (37.8%) |
| Distant metastases at diagnosis (cM stage), n (%) | 2372 (26.2%) |
| ESCC treatment, n (%) | |
| Endoscopic resection | 116 (1.3%) |
| Surgical resection | 734 (8.1%) |
| Chemotherapy | 479 (5.3%) |
| Radiotherapy | 2163 (23.9%) |
| Chemotherapy + radiotherapy | 1812 (20.0%) |
| Neoadjuvant chemotherapy + surgery | 267 (2.9%) |
| Chemotherapy + radiotherapy + surgery | 1021 (11.3%) |
| Surgerya + radiotherapy | 25 (0.3%) |
| Other a | 61 (0.7%) |
| No therapy | 2380 (26.2%) |
Abbreviations: CI, confidence interval; ESCC, oesophageal squamous cell carcinoma; IQR, interquartile range.
Other treatment combinations with either endoscopic or surgical resection combined with (neo)adjuvant chemo and/or radiotherapy.
3.2. ESCC characteristics
The majority of ESCCs were located in the middle (36.4%) and lower third (38.7%) of the oesophagus (Table 1). ESCC tumour stage was low in 26.3% and high in 55.7% of patients. Pathological assessment of ESCCs revealed good or moderate differentiation grade (G1/G2) in 33.5%, and poor or undifferentiated grade (G3/G4) in 28.7%. In total, 2372 patients (26.2%) had distant metastases at time of diagnosis (cM stage). In total, 2163 patients (23.9%) were treated with radiotherapy for ESCC, 1812 patients (20.0%) with chemo‐radiotherapy and 1288 (14.2%) patients received neoadjuvant therapy followed by surgery.
3.3. Multiple primary tumours
A total of 545 MPTs were registered in 476 (5.3%) patients. Of these 476 patients, 50 patients developed a third primary tumour, 16 patients a fourth primary tumour, and one patient developed another fifth, sixth and seventh primary tumour (Figure 1). Of all MPTs (545), 329 (60.4%) were diagnosed synchronously and 216 (39.6%) metachronously (Table 2). The majority of both synchronous and metachronous MPTs were located in the head and neck region (270/545; 49.5%) and lungs (219/545; 40.2%). MPT tumour stage was low (stage 0/I/II) in 39.6%, and high (stage III/IV) in 43.5% of the tumours, which was roughly the same for synchronous and metachronous MPTs. Of all MPTs, 160/545 (29.4%) were treated with radiotherapy and 170/545 MPTs (31.2%) did not receive any treatment. Squamous cell carcinoma (337/545; 61.8%) was the most prevalent histologic MPT type (Table 2).
TABLE 2.
Tumour characteristics of all MPTs (545)
| Characteristics | Total (545) | Synchronous (329) | Metachronous (216) |
|---|---|---|---|
| MPT location, n (%) | |||
| Head and neck region | 270 (49.5%) | 167 | 103 |
| Lung | 219 (40.2%) | 123 | 96 |
| Oesophagus | 26 (4.8%) | 14 | 12 |
| Stomach | 30 (5.5%) | 25 | 5 |
| Histology, n (%) | |||
| Squamous cell carcinoma | 337 (61.8%) | 196 | 141 |
| Adenocarcinoma | 79 (14.5%) | 50 | 29 |
| Carcinoid | 2 (0.4%) | 2 | 0 |
| Neoplasm and carcinoma (unspecified) | 127 (23.3%) | 81 | 46 |
| Tumour stage, n (%) | |||
| 0 | 11 (2.4%) | 6 | 5 |
| 1 | 149 (32.9%) | 86 | 63 |
| 2 | 56 (12.4%) | 37 | 19 |
| 3 | 65 (14.3%) | 38 | 27 |
| 4 | 172 (38.0%) | 94 | 78 |
| Missing | 92 | 68 | 24 |
| MPT treatment, n (%) | |||
| Endoscopic resection | 20 (3.7%) | 8 | 12 |
| Surgical resection | 60 (11.0%) | 32 | 28 |
| Chemotherapy | 45 (8.3%) | 22 | 23 |
| Radiotherapy | 160 (29.4%) | 90 | 70 |
| Chemotherapy + radiotherapy | 72 (13.2%) | 54 | 18 |
| Chemo + surgery | 1 (0.2%) | 1 | 0 |
| Chemo + radio + surgery | 2 (0.4%) | 2 | 0 |
| Surgery + radio | 13 (2.4%) | 5 | 8 |
| Surgery + chemo + radio | 1 (0.2%) | 1 | 0 |
| Endoscopic resection + radio | 1 (0.2%) | 0 | 1 |
| No treatment | 170 (31.2%) | 114 | 56 |
Abbreviation: MPTs, multiple primary tumours.
3.4. Standardised incidence ratios
In total, 545 MPTs were detected during the observation period. Patients with ESCC had a significantly increased risk of both synchronous (SIR 10.95, 99% CI 9.40–12.53) and metachronous MPTs (SIR 4.36, 99% CI 3.56–5.10) compared to the general population (Table 3). Sub‐analyses showed that patients with ESCC had the highest risk of developing synchronous (SIR 36.33, 99% CI 29.44–44.30) and metachronous (SIR 14.17, 99% CI 10.41–17.52) MPTs in the head and neck region. Patients aged 41–60 years at ESCC diagnosis had a highest SIR of 32.07 (99% CI 24.71–40.77) for developing synchronous MPTs, and patients aged 18–40 years at ESCC diagnosis had a highest SIR of 70.87 (99% CI 2.72–329.97) for developing metachronous MPTs. In order to determine whether radiotherapy had influence on MPT development compared to other treatments, we stratified the MPT risk for different treatment groups. The MPT risk was high for all different treatment groups compared to the general population. We were unable to address any influence of previous radiotherapy on MPT development; of patients who developed metachronous MPTs >10 years after ESCC diagnosis (n = 15), only four patients received radiotherapy for ESCCs. For all sub‐analyses, females had the highest SIR compared to males (Table 3).
TABLE 3.
SIR for synchronous and metachronous MPTs
| Characteristics | Observed (n) | Expected (n) | SIR (99% CI) total | SIR (99% CI) male | SIR (99% CI) female |
|---|---|---|---|---|---|
| Synchronous MPTs | |||||
| All cancers | 329 | 30.05 | 10.95 (9.40–12.53) | 9.82 (8.20–11.65) | 14.07 (10.79–17.98) |
| Head and neck | 164 | 4.51 | 36.33 (29.44–44.30) | 34.69 (27.05–43.76) | 41.51 (27.29–60.29) |
| Lungs | 123 | 19.17 | 6.42 (5.02–8.06) | 5.35 (3.90–7.14) | 9.48 (6.29–13.66) |
| Stomach | 25 | 3.16 | 7.92 (4.43–12.99) | 7.75 (3.84013.83) | 8.37 (2.40–20.52) |
| Oesophagus | 17 | 4.04 | 4.21 (2.04–7.63) | 3.14 (1.00–7.30) | 10.64 (3.05–26.08) |
| Age at ESCC diagnosis (years) | |||||
| 41–60 | 110 | 3.43 | 32.07 (24.71–40.77) | 28.26 (20.21–38.35) | 40.86 (26.43–60.09) |
| 61–80 | 202 | 22.57 | 8.95 (7.41–10.70) | 8.15 (6.49–10.09) | 11.56 (8.10–15.95) |
| >80 | 17 | 4.04 | 4.21 (2.04–7.63) | 4.99 (2.21–9.56) | 2.44 (0.25–8.96) |
| Treatment ESCC | |||||
| Chemo + radiotherapy | 100 | 9.85 | 10.15 (7.73–13.08) | 9.25 (6.58–12.61) | 12.53 (7.68–19.21) |
| Chemotherapy | 22 | 2.15 | 10.23 (5.47–17.31) | 7.99 (3.54–15.32) | 20.09 (6.39–46.71) |
| Radiotherapy | 88 | 8.59 | 10.24 (7.75–13.55) | 9.49 (6.69–13.03) | 13.34 (7.56–21.69) |
| No chemo‐ or radiotherapy | 119 | 9.48 | 12.60 (9.98–16.08) | 11.72 (8.65–15.48) | 15.74 (10.00–23.48) |
| Metachronous MPTs | |||||
| All cancers | 216 | 49.51 | 4.36 (3.56–5.10) | 3.84 (3.03–4.79) | 5.25 (3.87–6.93) |
| Head and neck | 106 | 7.48 | 14.17 (10.41–17.52) | 12.85 (9.19–17.44) | 15.55 (9.53–23.85) |
| Lungs | 96 | 31.95 | 3.00 (2.22–3.82) | 2.50 (1.71–3.53) | 3.81 (2.45–5.62) |
| Stomach | 5 | 4.56 | 1.10 (0.23–3.11) | 0.94 (0.10–3.46) | 1.45 (0.06–6.76) |
| Oesophagus | 9 | 5.53 | 1.63 (0.78–4.12) | 1.42 (0.36–3.70) | 3.86 (0.81–10.95) |
| Age at ESCC diagnosis (years) | |||||
| 18–40 | 2 | 0.03 | 70.87 (2.72–329–97) | 157.62 (6.05–733.84) | 0.00 |
| 41–60 | 95 | 11.02 | 8.62 (6.35–10.96) | 6.94 (4.65–9.93) | 11.10 (7.26–16.18) |
| 61–80 | 115 | 36.17 | 3.18 (2.42–3.96) | 2.99 (2.18–4.00) | 3.44 (2.15–5.18) |
| >80 | 4 | 2.30 | 1.74 (0.28–5.50) | 2.12 (0.22–7.79) | 1.13 (0.00–8.46) |
| Treatment ESCC | |||||
| Chemo + radiotherapy | 112 | 24.89 | 4.50 (3.44–5.67) | 3.87 (2.77–5.25) | 5.88 (3.82–8.61) |
| Chemotherapy | 19 | 6.05 | 3.14 (1.47–5.31) | 2.87 (1.22–5.62) | 3.30 (0.69–9.36) |
| Radiotherapy | 26 | 5.68 | 4.58 (2.46–7.22) | 3.58 (1.59–6.88) | 6.18 (2.42–12.82) |
| No chemo‐ or radiotherapy | 59 | 12.89 | 4.58 (3.12–6.26) | 4.45 (2.75–6.78) | 4.58 (2.49–7.67) |
Abbreviations: CI, confidence interval; MPTs, multiple primary tumours; SIR, standardised incidence ratio.
3.5. Metachronous SPT
Of all patients who were alive 6 months after ESCC diagnosis (n = 5715), 191 patients developed metachronous MPTs. Of these patients, 16 had already developed a synchronous SPT. In total, 175 patients developed a metachronous SPT. Figure 2 shows the cumulative incidence of metachronous SPTs. Fifteen years after ESCC diagnosis, the cumulative incidence of metachronous SPTs was 19.7%. Cumulative incidences of different SPT sub‐locations is shown in the Tables S1–S4, Figures S1 and S2. The median time between ESCC diagnosis and metachronous SPT diagnosis was 3.0 years (IQR 1.8– 5.9). The median time between ESCC and metachronous head and neck SPT diagnosis was 2.8 years (IQR 2.2–3.4) and for lung SPT diagnosis 3.2 years (IQR 1.9–4.5). SPT stage was high (stage III/IV) in 57.4% of the patients with metachronous SPTs. These patients had a significantly worse two‐year survival after SPT diagnosis than low stage SPTs (stage 0/I/II) (15.1% vs. 51.9%, p < 0.01) (Figure 3).
FIGURE 2.
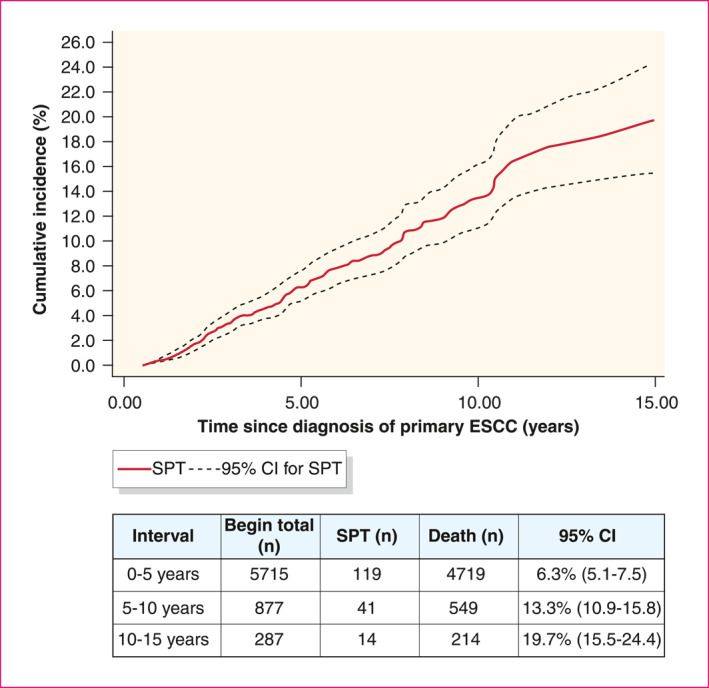
Cumulative incidence of metachronous SPT after ESCC diagnosis. Censored cases: patients in column ‘death (n)’, Number at risk: patients in column ‘Begin total (n)’. CI, confidence interval; ESCC, oesophageal squamous cell carcinoma; SPT, second primary tumour
FIGURE 3.
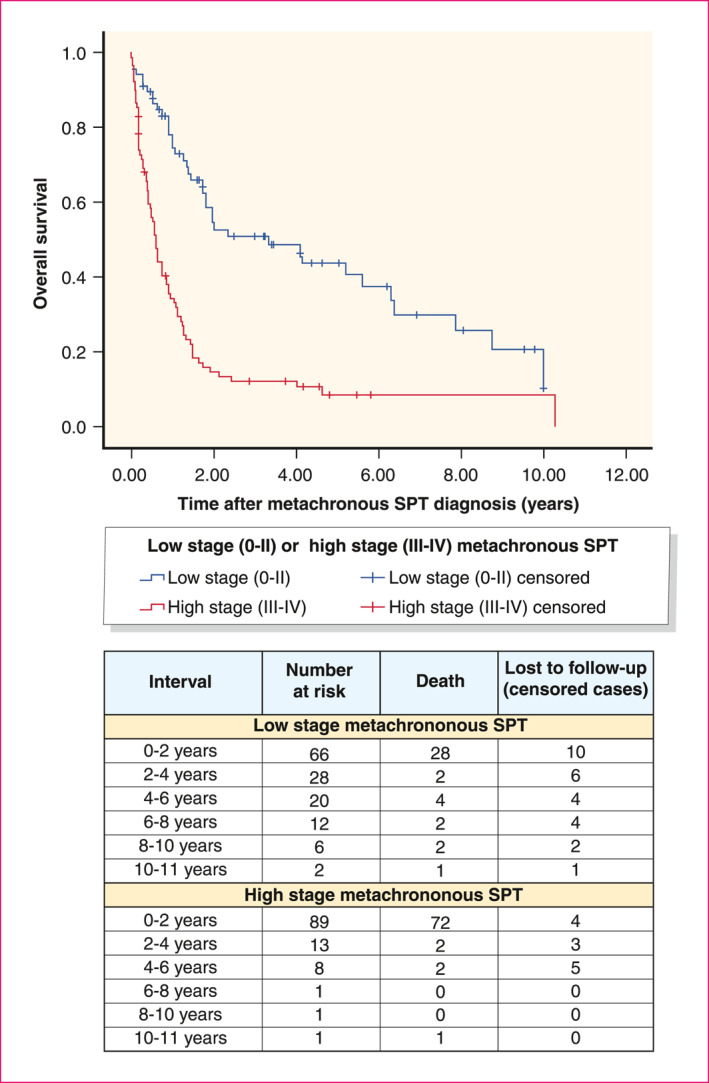
Survival after SPT diagnosis between low‐ and high‐stage metachronous SPTs. *n = 155; 21 patients were excluded for this analysis, as result of missing SPT tumour stages. SPT, second primary tumour
3.6. Cumulative incidence of MPTs in patients with low‐stage ESCC
Subgroup analysis was performed in patients with lowstage ESCC (n = 2381). The 15‐year cumulative incidence for this subgroup was 21.7% (Table S5 and Figure S3).
3.7. Predictive factors for MPTs
Multivariable Cox proportional hazard analysis showed that being male (HR 1.59, 95% CI 1.29–1.97, p < 0.001), age <70 years (HR 1.51, 95% CI 1.19–1.90, p = 0.001), and having a low ESCC tumour stage (stage 0/I/II) at diagnosis (HR 1.50, 95% CI 1.19–1.89, p = 0.001) were independent predictors for MPT development (Table 4). Analysis with age (HR 0.99, 95% CI 0.98–0.10, p = 0.022) and tumour stage (HR 0.73, 95% CI 0.64–0.82, p < 0.001) as continues variables did not alter the outcomes.
TABLE 4.
Multivariable analysis for MPT development using the Cox proportional hazards regression model
| Variables | Multivariate analysis | |
|---|---|---|
| HR (95% CI) | p‐Value | |
| Sex | ||
| Male | 1.593 (1.286–1.974) | <0.001 |
| Female | Reference | |
| Age | ||
| <70 | 1.507 (1.194–1.900) | 0.001 |
| ≥70 | Reference | |
| Metastasis | ||
| Yes | 1.057 (0.791–1.411) | 0.709 |
| No | Reference | |
| ESCC tumour stage | ||
| Low (0/I/II) | 1.497 (1.188–1.887) | 0.001 |
| High (III/IV) | Reference | |
Abbreviations: CI, confidence interval; HR, hazard ratio; MPT, multiple primary tumour.
4. DISCUSSION
We determined the risk of developing MPTs in the UADT and stomach in patients with primary ESCC. Our study shows that a minimum of one out of 20 patients with primary ESCC develops an SPT, with a 15‐year cumulative metachronous SPT incidence of 19.7%. Which means that approximately one in five ESCC patients who survive longer than 6 months will develop an SPT within 15 years. The risk of developing synchronous (SIR 10.95) and metachronous MPTs (SIR 4.36) among patients with primary ESCC was increased compared to the general population, with the highest risk of developing MPTs in the head and neck region. Risk factors associated with MPT development are being male, age <70 years and low ESCC tumour stage.
In retrospective studies, the incidence of MPTs localised the UADT among patients with primary ESCC ranged between 1.9% and 19.3%. 3 , 10 , 11 , 12 , 13 Most studies are performed in the Asian population, where the MPT incidence is reported to be >10%. 3 , 11 , 13 , 23 Studies performed in a Western population reported lower MPT incidences (1.9%–6.3%), which is in accordance with our findings. 12 , 24 , 25 The difference in MPT incidence between Asian and non‐Asian populations could possibly be explained by a difference in aetiology. While the aetiology of ESCC and MPTs in the UADT is clearly linked to smoking and alcohol intake in a Western population, the aetiology in an Asian population is also linked to a poor nutritional status. 26 , 27
Another explanation might be the difference in genetic polymorphisms in alcohol metabolism between Asian and Western populations. 28 , 29
The increased MPT risk among patients with primary ESCC, as reported in our study, has also been reported in other studies. 12 , 15 , 30 , 31 Chuang et al. reported an increased risk of MPTs in the UADT, especially in the head and neck region (SIR 6.68) and lungs (SIR 1.55), among ESCC patients in 13 different countries. 15 Other studies also reported increased MPT risks. 10 , 14 , 24 , 30 , 32 As reported in our study, patients with ESCC diagnosis at a young age showed the highest SIR for developing both synchronous (SIR 32.07) and metachronous MPTs (SIR 70.87). The same results were reported in a study by Chen et al., with a SIR of 36.56 for patients aged between 20 and 39 years at ESCC diagnosis. 30
We found the head and neck region to be the most common region for developing MPTs (synchronous; SIR 36.33, metachronous: SIR 14.17), which is also reported in both Asian (SIR 15.83) and Western (SIR 6.68–8.64) studies. 12 , 14 , 15 , 30 , 31 Studies from Japan and Korea reported the stomach as their most common MPT location. 3 , 4 This could be due to the high incidence of stomach cancer in Japan and Korea. 2 Unfortunately, SIRs were not reported in these studies. 3 , 4 For synchronous MPTs, we found the stomach to be the second most common region for MPT development. Less patients had synchronous or metachronous oesophageal MPT in our cohort. In case a synchronous oesophageal SPT is detected, one could argue that these patients probably had mucosal dysplasia or neoplasia at the time of the primary ESCC diagnosis. Careful inspection of the oesophagus with Lugol chromoendoscopy is therefore very important in cases of curative primary ESCC diagnosis, because early oesophageal SPT might be easily overlooked during routine white light endoscopy. 33
Patients who develop metachronous SPT may potentially benefit from screening programmes. The median time between ESCC diagnosis and metachronous SPT detection was 3 years (IQR 1.8–5.9 years), with a cumulative 15‐year incidence of 19.7%. Most metachronous SPTs, however, had a high tumour stage (57.4%), with a worse two‐year survival compared to low‐stage metachronous SPTs (15.1% vs. 51.9%, p < 0.01). Screening for metachronous SPTs could possibly help to increase survival in these patients by detecting these SPTs at an early stage. Patients with high‐stage ESCC with poor prognosis will possibly not benefit from screening programmes since their prognosis is already determined by the ESCC. Diagnosing MPTs in these patients is probably not clinically relevant. The overall survival of our cohort was 9.9 months, the overall five‐year survival was 15.9%. Prospective studies are necessary to investigate the yield of screening for MPTs, and especially whether screening will lead to survival benefit. One could argue that synchronous SPTs already existed at time of index tumour diagnosis but had not yet been detected at that time. The question arises whether routine screening of especially the head and neck region should be performed in curative ESCC patients prior to ESCC treatment.
Asian screening studies to detect head and neck SPTs have been performed in patients with primary ESCC. A systematic review about active screening for head and neck SPTs in patients with primary ESCC showed a pooled prevalence of 6.7% (range 3.0%– 29.6%). 22 Active screening showed a low SPT tumour stage in most patients (85.7%), and a better survival compared to patients who were not screened. 22 , 34 These studies suggest that active screening contributes to an increase in SPT detection and overall survival. 4 Nowadays, no screening studies in patients with ESCC have been performed in Western countries.
Being male was a predictive factor for MPT development in our study, which is in accordance with previous studies. 24 , 30 , 31 Our study revealed age <70 years as a significant predictive factor, which is also in line with a previous study. 24 Chen et al., however, reported age 60 years as a predictive factor for MPT development. 30 Although higher age is reported as a significant risk factor for cancer development in the UADT or stomach in general, one might hypothesise that when patients survive ESCC at a young age they are expected to live longer and might have an increased risk of MPT development. The same might be true for patients with a low ESCC tumour stage, they have a more favourable course of their disease and are therefore expected to live longer. We reported low ESCC tumour stage as a predictive factor for MPT development, but this has to be interpreted with caution.
Although this is the largest registry study in Europe with more than 9000 patients, some limitations need to be acknowledged. First, this is a retrospective cohort study with limited patient information and a substantial amount of missing data. We could therefore not report on risk factors such as smoking or alcohol which are common risk factors for MPT development. 30 This might have caused confounding in our risk factor analysis. In addition, cause of death was not reported in the NCR and this information could be relevant in this patient cohort with a large number of high‐stage ESCCs and a median survival of only 10 months. Second, since this is a registry study, MPT incidence could be underestimated. Because of the retrospective design of this study, we did not know whether MPTs were diagnosed during regular follow‐up or as a result of patients symptoms. As a consequence, no specific advice for time interval for screening can be drawn from this study.
A minimum of one out of 20 patients with primary ESCC develops an MPT in the UADT or stomach. The majority of these MPTs were detected synchronously, in the head and neck region and in young patients. Survival of patients with ESCC is low. Prospective screening studies are necessary to determine the true MPT incidence and to investigate the yield and benefit of screening for MPTs.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
ETHICS APPROVAL
This study was approved by the Medical Ethical Review Committee of the Erasmus Medical Centre (MEC‐2018‐1631).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the registration team of the NCR for the collection of data. The authors received no financial support for the research, authorship and/or publication of this article.
REFERENCES
- 1. Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–7. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancerstatistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 3. Lee GD, Kim YH, Kim JB, et al. Esophageal cancerassociated with multiple primary cancers: surgical approaches and long‐term survival. Ann Surg Oncol. 2013;20:4260–6. [DOI] [PubMed] [Google Scholar]
- 4. Kagei K, Hosokawa M, Shirato H, et al. Efficacy of intense screening and treatment for synchronous second primary cancers in patients with esophageal cancer. Jpn J Clin Oncol. 2002;32:120–7. [DOI] [PubMed] [Google Scholar]
- 5. van Putten M, de Vos‐Geelen J, Nieuwenhuijzen GAP, et al. Long‐term survival improvement in oesophageal cancer in The Netherlands. Eur J Cancer. 2018;94:138–47. [DOI] [PubMed] [Google Scholar]
- 6. Lee JS, Ahn JY, Choi KD, et al. Synchronous second primary cancers in patients with squamous esophageal cancer: clinical features and survival outcome. Korean J Intern Med. 2016;31:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. [DOI] [PubMed] [Google Scholar]
- 8. Henry MA, Lerco MM, Naresse LE, et al. Outcome ofsuperficial squamous cell carcinoma of the esophagus: a clinicopathological study. Acta Cir Bras. 2013;28:373–8. [DOI] [PubMed] [Google Scholar]
- 9. Abiko S, Shimizu Y, Miyamoto S, et al. Risk assessmentof metachronous squamous cell carcinoma after endoscopic resection for esophageal carcinoma based on the genetic polymorphisms of alcoholdehydrogense‐1B aldehyde dehydrogenase‐2: temperance reduces the risk. J Gastroenterol. 2018;53:1120–30. [DOI] [PubMed] [Google Scholar]
- 10. Utada M, Ohno Y, Hori M, et al. Incidence of multipleprimary cancers and interval between first and second primary cancers. Cancer Sci. 2014;105:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumagai Y, Kawano T, Nakajima Y, et al. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872–6. [DOI] [PubMed] [Google Scholar]
- 12. Zhu G, Chen Y, Zhu Z, et al. Risk of second primarycancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25:505–11. [DOI] [PubMed] [Google Scholar]
- 13. Natsugoe S, Matsumoto M, Okumura H, et al. Multipleprimary carcinomas with esophageal squamous cell cancer: clinicopathologic outcome. World J Surg. 2005;29:46–9. [DOI] [PubMed] [Google Scholar]
- 14. Mitani S, Kadowaki S, Oze I, et al. Risk of second primary malignancies after definitive treatment for esophageal cancer: a competing risk analysis. Cancer Med. 2020;9:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chuang SC, Hashibe M, Scelo G, et al. Risk of secondprimary cancer among esophageal cancer patients: a pooled analysis of 13 cancer registries. Cancer Epidemiol Biomarkers Prev. 2008;17:1543–9. [DOI] [PubMed] [Google Scholar]
- 16. American Joint Committee on Cancer . AJCC cancer staging manual. 5th ed. Philadelphia/New York: Lippincott‐Raven; 1997. p.294. [Google Scholar]
- 17. American Joint Committee on Cancer . AJCC cancer staging manual . 6th ed. New York: Springer; 2002. [Google Scholar]
- 18. American Joint Committee on Cancer . AJCC cancer staging manual . 7th ed. New York: Springer; 2010. [Google Scholar]
- 19. Warren S, Gates O. Multiple primary malignanttumors: a survey of the literature and statistical study. Am J Cancer. 1932;51:1358–414. [Google Scholar]
- 20. Bugter O, van de Ven SEM, Hardillo JA, et al. Earlydetection of esophageal second primary tumors using Lugol chromoendoscopy in patients with head and neck cancer: a systematic review and meta‐analysis. Head Neck. 2019;41:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bugter O, van Iwaarden DLP, Dronkers EAC, et al. Survival of patients with head and neck cancer with metachronous multiple primary tumors is surprisingly favorable. Head Neck. 2019;41:1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van de Ven SEM, Bugter O, Hardillo JA, et al. Screeningfor head and neck second primary tumors in patients with esophageal squamous cell cancer: a systematic review and meta‐analysis. United European Gastroenterol J. 2019;7:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. S, Onda M, Sasajima K, et al. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226–30. [DOI] [PubMed] [Google Scholar]
- 24. Levi F, Randimbison L, Maspoli M, et al. Second neoplasms after oesophageal cancer. Int J Cancer. 2007;121:694–7. [DOI] [PubMed] [Google Scholar]
- 25. Ribeiro Junior U, Cecconello I, Safatle‐Ribeiro AV, et al. Squamous cell carcinoma of the esophagus and multiple primary tumors of the upper aerodigestive tract. Arq Gastroenterol. 1999;36:195–200. [DOI] [PubMed] [Google Scholar]
- 26. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 27. Yokokawa Y, Ohta S, Hou J, et al. Ecological study on the risks of esophageal cancer in Ci‐Xian, China: the importance of nutritional status and the use of well water. Int J Cancer. 1999;83:620–4. [DOI] [PubMed] [Google Scholar]
- 28. Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higuchi S, Matsushita S, Murayama M, et al. Alcoholand aldehydedehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry. 1995;152:1219–21. [DOI] [PubMed] [Google Scholar]
- 30. Chen SC, Teng CJ, Hu YW, et al. Secondary primary malignancy risk among patients with esophageal cancer in Taiwan: a nationwide population‐based study. PLoS One. 2015;10:e0116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsubara T, Yamada K and Nakagawa A. Risk ofsecond primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336–4341. [DOI] [PubMed] [Google Scholar]
- 32. Hu WS, Liu ZJ, Zhang JB, et al. Risk patterns of subsequent primary cancers following esophagectomy in earlystage thoracic esophageal squamous cell cancer patients. Tumori. 2015;101:328–33. [DOI] [PubMed] [Google Scholar]
- 33. van de Ven SEM, Koch AD. When is Lugol still necessary in 2020? Endosc Int Open. 2020;8:E1478–E1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morimoto H, Yano T, Yoda Y, et al. Clinical impact of surveillance for head and neck cancer in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2017;23:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


