Abstract
Background: The current study aimed to investigate the impact of asymptomatic or mild severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on female fertility and laboratory and clinical outcomes in assisted reproductive technology (ART) treatments.
Methods: Patients undergoing ART treatments in the Reproductive Medicine Center, Tongji Hospital, Wuhan, from May 2020 to February 2021 were enrolled. Seventy of them were positive for serum SARS-CoV-2 antibodies (IgG and/or IgM), and 3973 patients had negative results. Propensity score matching with a ratio of 1:3 was performed, and there were 65 females in the case group and 195 females in the control group.
Findings: The ovarian reserves and ovarian responses between groups after matching were similar. The proportions of mature oocytes, damaged oocytes, fertilized oocytes, cleavage embryos, high-quality embryos, and available blastocysts were also similar, despite a slight decrease in the blastocyst formation rate in the case group. In addition, there were no significant differences in terms of the biochemical pregnancy rate, clinical pregnancy rate, early miscarriage rate, or implantation rate.
Interpretation: There is no evidence that a history of asymptomatic or mild SARS-CoV-2 infection in females may negatively affect female fertility, embryo laboratory outcomes, or clinical outcomes in ART treatments.
Keywords: COVID 19, SARS CoV-2, Fertility evaluation, IVF outcome
Research in context.
Evidence before this study
The effects of SARS-CoV-2 infection on female fertility and assisted reproductive technology (ART) outcomes remain unclear. Due to the limitation of samples and controls, most of the existing studies on this topic are speculative and have a low grade of the evidence hierarchy. Moreover, these previously relevant studies only compared the fertility evaluation before and after infection. Currently, none of them has investigated the impacts of the virus infection on the fertility function and germ cells by analyzing the in vitro fertilization (IVF) data.
Added value for this study
This study uniquely provided an observational evidence that the impact of the virus infection on the fertility function and germ cells by analyzing the ART data from the largest IVF center in Wuhan. Propensity score matching was performed, and our data suggested no evidence that a history of asymptomatic or mild SARS-COV-2 infection caused impairment of female fertility, embryo laboratory outcomes, or clinical outcomes in ART treatments.
Implication of all the available evidence
The findings suggested no evidence that a history of asymptomatic or mild SARS-CoV-2 infection in females caused impairment of female fertility, embryo laboratory outcomes, or clinical outcomes in ART treatments. To some extent, this study provides an observational evidence that the impact of the SARS-CoV-2 on oocytes and embryos may be limited in females with a history of asymptomatic or mild SARS-CoV-2 infection, while the effects of moderate or severe infection on female fertility need to be considered and further investigated.
Alt-text: Unlabelled box
1. Introduction
The coronavirus disease 2019 (COVID-19) outbreak, mediated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has led to a serious and expanding pandemic and panic worldwide. This virus can not only induce severe respiratory diseases but also a variety of histopathological changes in multiple systems and organs, including the kidney [1], brain [2], and liver [3]. Evidence also showed that the testis [4] and ovary [5] might also be potential targeted organs of the virus infection. Although COVID-19 is not regarded as a sexually transmitted disease, some studies still show an ovarian or testicular functional effect in recovered patients with COVID-19 [6].
During the COVID-19 crisis, there was a heated debate on the impact of virus infection on human fertility. Some studies found that semen parameters were altered in infected males [7] and resulted in a higher serum luteinizing hormone (LH) level [8]. In addition, a recent study indicated that there was no association between the virus infection and obstetric complications [9]. In contrast, many other studies emphasized on the possibility of the SARS-CoV-2 infection impairing sperm and oocyte function [10,11]. However, despite an abundance of recent publications, the effects of SARS-CoV-2 infections on fertility, and the outcomes of in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), and many other assisted reproductive technology (ART) treatments are still not fully elucidated. Moreover, due to the limitation of samples and controls, most of the existing studies on this topic are speculative and have a low grade of the evidence hierarchy. Currently, none of them has investigated the impacts of the virus infection on the fertility function and germ cells by analyzing the IVF data.
Therefore, in the current study, we collected IVF data from our center, the largest IVF center in Wuhan, where the first COVID-19 case was reported, to investigate the impacts of asymptomatic or mild SARS-CoV-2 infection on female fertility and laboratory and clinical outcomes in ART treatments.
2. Methods
2.1. Study population and design
We performed a single-center retrospective cohort study, including women undergoing IVF/ICSI treatment between May 2020 and February 2021. All data were collected from the Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Patients who have all of the following characteristics were included: (a) negative for polymerase chain reaction (PCR) test for detecting SARS-Cov-2 RNA and (b) positive for serum SARS-CoV-2 antibodies. The exclusion criteria were as follows: (a) missed important information; (b) lost to follow up; (c) oocyte donation cycles; (d) oocyte totally or partly freezing cycles; and (e) males with a history of SARS-CoV-2 infection. Data in this study were retrieved from the electronic medical record system. All cycles were categorized into two groups according to the results of the serum SARS-CoV-2 antibody test. To eliminate the imbalance of the number and distribution of samples between groups, a propensity score matching, which is extremely useful for confounding control in observational studies [12], was performed.
2.2. IVF/ICSI protocols and embryo culture
The controlled ovarian stimulation (COS) protocols carried out in our IVF center were categorized into the gonadotropin-releasing hormone (GnRH) agonist protocol, the GnRH antagonist protocol, and other protocols, including mild stimulation and luteal phase stimulation protocols. The details of COS protocols were previously presented and well described [13]. When two to three dominant follicles with a diameter over 18 mm could be observed, 10,000 IU recombinant human chorionic gonadotrophin (HCG) was administered as a trigger. Oocyte retrieval was then performed 36–38 h later.
Oocytes were fertilized through either conventional IVF or ICSI. Pronuclei (PN) evaluation was performed 16–18 h after insemination. The fertilized oocytes were cultured in G1-plus medium (Vitrolife, Gothenburg, Sweden) to cleavage stage embryos until day 3. Then one or two good-quality embryos were selected to be freshly transferred. The surplus embryos continued to be cultured to the blastocyst stage in G2-plus medium (Vitrolife, Gothenburg, Sweden) until day 5 or 6; the available blastocysts were then cryopreserved. The morphologic parameters of the cleavage stage embryos were recorded as previously described [14], and the three main parameters were as follows: (a) the number of blastomeres; (b) the percentage of fragmentation; and (c) the variation in blastomere symmetry. If there were multinucleation or defects in the zona pellucida and cytoplasm, they were emphasized. High-quality embryos (HQE) at the cleavage stage were defined as follows: (a) normally fertilized embryos with 4,5 cells on day 2 or 8–10 cells on day 3; (b) <15% fragmentation; (c) uniform blastomeres; (d) absence of multinucleation; (e) absence of zona pellucida defects; (f) absence of perivitelline space granularity and (g) no inclusions in cytoplasm [14]. Blastocyst morphology evaluation was based on the Gardner scoring system [15]. Available blastocysts were defined as those at stage 3 and above on day 5 or day 6 with a score B and above of inner cell mass. Specifically, the whole IVF operations for patients who tested as positive for serum SARS-CoV-2 antibodies were processed in an independent space with special incubators. The embryos were also cryopreserved in a separate liquid nitrogen tank.
2.3. Serum SARS-CoV-2 antibody test and PCR tests for detecting SARS-CoV-2 RNA
Generally, the patients in IVF cycles have experienced routine serum SARS-CoV-2 antibody (IgG and IgM) tests and PCR tests for detecting SARS-CoV-2 RNA for at least three times, namely in the first visit to our center, before the COS procedure, and before oocyte retrieval. In addition to these two tests, digital chest radiographs were also performed for all the patients to screen for any pulmonary lesions.
Antibodies against both the envelope (E) and nucleocapsid (N) proteins of SARS-CoV-2 in serum samples were measured using chemiluminescence kits purchased from YHLO Biotech, Shenzhen, China, according to the manufacturer's instructions (C86095G, C86095M) [16]. An antibody level ≥ 10 AU/mL was considered positive. The sensitivities of the kits for IgG and IgM tests were 98.5% and 84.3%, respectively. The specificities for IgG and IgM were 100% and 98.5%, respectively.
Nasopharyngeal swabs were performed to collect samples for PCR tests for detecting SARS-CoV-2 RNA. The total viral RNA of the specimen was isolated for SARS-CoV-2 infection detection using real-time PCR (RT-PCR) kits (20203400749) from DA AN GENE, Guangzhou, China. The open reading frame 1ab (ORF1ab) and N genes were the target genes and were simultaneously amplified. The primers were as follows: ORF1ab: 5′-CCCTGTGGGTTTTACACTTAA-3′ (F), 5′-ACGATTGTGCATCAGCTGA-3′ (R); N: 5′-GGGGAACTTCTCCTGCTAGAAT-3′ (F), 5′-CAGACATTTTGCTCTCAAGCTG-3′ (R). The conditions of the RT-PCR assay were 50 °C for 2 min and 95 °C for 2 min, followed by 45 cycles of denaturation at 95 °C for 5 s and extension and fluorescence collection at 60 °C for 10 s [17]. Ct values of less than 30 for both genes were defined as positive. The analytical sensitivity was 500 copies/mL.
2.4. Outcome assessments
The fertility evaluation consists of the ovarian reserve and the ovarian response. For the ovarian reserve, the serum levels of follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH) were measured at days 2–3 of the menstrual periods, and the basal antral follicle count (AFC) were measured by transvaginal ultrasound. For the ovarian response, we included the total dosage and duration of gonadotrophins (Gn) in the process of the stimulation, the number of large follicles on HCG day (>14 mm), the number of oocytes retrieved, and the ovarian response grading based on the POSEIDON classification [18]. AMH and AFC were the primary outcomes. FSH (range of quantitation 0.2–200 mIU/mL, intra-assay variability 3.5%) and AMH (range of quantitation 0.01–23 ng/mL, intra-assay variability 2.9%) were detected using electrochemiluminescent immunoassays (Roche, Switzerland), according to the manufacturer's instructions [19].
For laboratory outcomes, the normal fertilization (2PN) rate and available blastocyst rate were the primary outcomes. The mature oocyte (metaphase II) rate, damaged oocyte rate, cleavage rate, HQE rate, and blastocyst formation rate were the secondary outcomes. The computing methods were also well described previously [13]. In brief, the mature oocyte rate was the number of metaphase II oocytes divided by the number of oocytes retrieved; the damaged oocyte rate was the number of damaged or degenerated oocytes divided by the number of oocytes retrieved; the normal fertilization rate was the number of 2PN oocytes divided by the number of metaphase II oocytes; the cleavage rate was the number of cleavage stage embryos developed from 2PN oocytes divided by the number of 2PN oocytes; the HQE rate was the number of HQE at the cleavage stage divided by the number of cleavage stage embryos; the blastocyst formation rate was the number of blastocysts divided by the number of day 3 embryos for extended culture; the available blastocyst rate was the number of good quality blastocysts for cryopreservation divided by the number of blastocysts formed.
For clinical outcomes, the clinical pregnancy rate was the primary outcome. The implantation rate, biochemical pregnancy rate, and early miscarriage rate were the secondary outcomes. Serum HCG levels were measured 14 days after embryo transfer. The implantation indicated the ultrasound visualization of gestational sacs, and the implantation rate was the number of gestational sacs observed divided by the number of embryos transferred. An active fetal heart in the uterus by transvaginal ultrasound approximately five weeks after embryo transfer was regarded as a clinical pregnancy. Biochemical pregnancy was defined as a positive result of HCG measurement without ultrasonographic visualization of clinical pregnancy. The denominator of both the clinical and biochemical pregnancy rates was the number of embryo transfer cycles. Early miscarriage referred to the loss of fetal heart within the first three months, and the denominator of the early miscarriage rate was the number of clinical pregnancy cycles.
2.5. Statistical analyses
All data analyses were performed using Statistical Package for Social Sciences software (version 26.0; SPSS, IBM, USA). Since none of the continuous variables was normally distributed using Kolmogorov-Smirnova or Shapiro-Wilk normality tests, the continuous variables were presented as medians (first quartile, third quartile), while the categorical variables were presented as% (n). The differences in baseline characteristics between the groups were analyzed using the nonparametric rank-sum test (Mann–Whitney U test) for continuous variables, and the chi-squared test or Fisher's exact test for categorical variables as appropriate.
The propensity scores were estimated using a logistic regression model based on the characteristics including female age (years), female body mass index (BMI, kg/m2), experienced IVF/ICSI times, infertility duration (years), infertility type (primary and secondary), infertility causes (female factor, male factor, both female and male factor, and unexplained), COS protocols (GnRH agonist protocol, GnRH antagonist protocol, and other protocols), and operation types (IVF, ICSI, and rescue ICSI (R-ICSI)). Matching without replacement was performed based on the nearest neighbor random matching algorithm with a match ratio of 1:3 and a match tolerance of 0.02. Two-tailed hypothesis tests were conducted, and a P-value < 0.05 was considered statistically significant.
2.6. Ethical approval
All procedures performed in studies involving human participants followed the standards of the Ethical Committee of Tongji Medical College ([2020]S066), and all enrolled participants provided written informed consent.
2.7. Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, writing of the article, or the decision to submit for publication. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Basal clinical characteristics
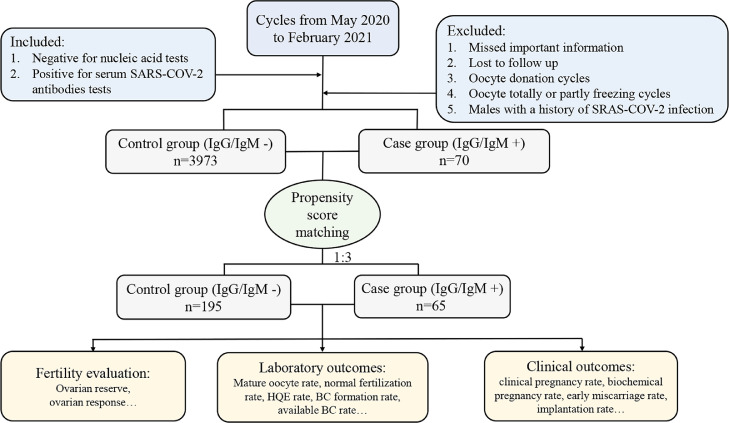
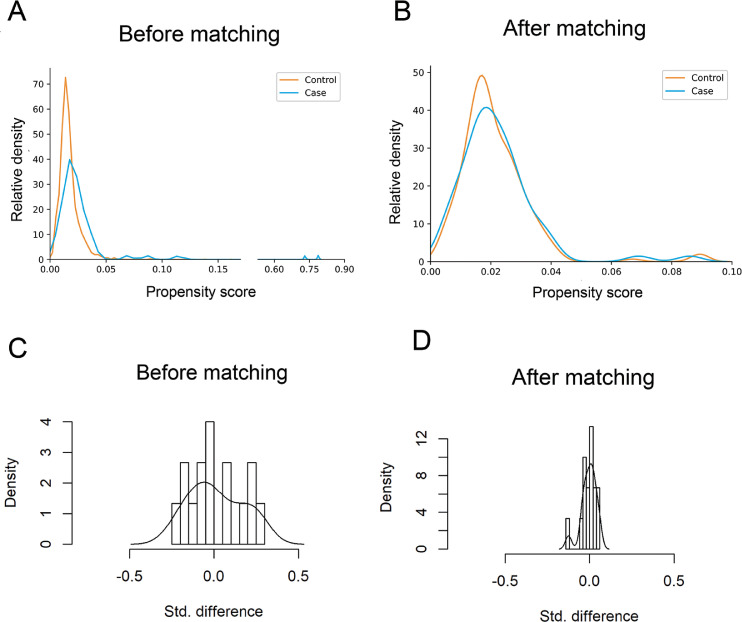
Of all the IVF/ICSI cycles from May 2020 to February 2021 in our IVF center, 4043 cycles were included. According to the results of serum SARS-CoV-2 antibody tests, these patients were subsequently classified into the IgG/IgM - (control, n = 3973) and IgG/IgM + (case, n = 70) groups. (Fig. 1). Overall, compared with the females in the case group, women in the control group had a higher BMI (P = 0.034) and experienced fewer IVF/ICSI procedures (P = 0.021), despite similar results of female ages, infertility durations, infertility types, infertility causes, COS protocols, and operation types (Table 1). Subsequently, propensity score matching was performed to minimize the imbalance of baseline characteristics. One hundred ninety-five couples remained in the control group after matching, and 65 remained in the case group. Among these participants, the majority (n = 45, 69.2%) were asymptomatic patients without any relevant clinical symptoms or radiological changes of the lung during the pandemic. The others (n = 20, 30.8%) had mild SARS-CoV-2 infection (a positive result of PCR test for detecting SARS-CoV-2 RNA and mild symptoms, such as fever, cough, or sore throat, yet without any radiological changes of the lung). These patients were diagnosed between January and March 2020. It was at least four months from the first diagnosis of SARS-CoV-2 infection to ART treatments. Moreover, for the patients with mild infection, there were 10 fresh embryo transfer cycles (10/20, 50.0%), and 4 of them (4/10, 40.0%) became pregnant. For asymptomatic patients, there were 22 fresh embryo transfer cycles (22/45, 48.9%), and 9 of them (9/22, 40.9%) became pregnant. There was no significant difference (P = 1.000) between the patients with mild and asymptomatic infection in terms of the clinical pregnancy rate. The distribution of propensity scores and standard deviations before and after matching are presented in Fig. 2. The distributions of propensity scores of the control group and the case group exhibited a better overlap in densities after matching than before matching. This result indicated that a large group of patients was well matched after matching, revealing a balance between the compared cohorts. The basal characteristics after matching are also presented in Table 1, and none of them demonstrated a significant difference between groups.
Fig. 1.
flowchart of the study. Note: HQE, high quality embryo; BC, blastocyst.
Table 1.
Baseline characteristics before and after matching.
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| Control n = 3973 | Case n = 70 | P value | Control n = 195 | Case n = 65 | P value | |
| Ages (years) | 31.0 (29.0, 35.0) | 32.0 (29.8, 36.0) | 0.374 | 32.0 (29.0, 35.0) | 31.0 (29.0, 34.0) | 0.718 |
| BMI (kg/m2) | 21.9 (21.1, 22.4) | 21.2 (19.4, 23.2) | 0.034 | 21.9 (20.8, 22.4) | 20.6 (19.1, 23.3) | 0.064 |
| ART times | 1 (1, 1) | 1 (1, 2) | 0.021 | 1 (1, 2) | 1 (1, 2) | 0.477 |
| Infertility durations (years) | 3 (1, 4) | 3 (1, 4) | 0.955 | 3 (1, 4) | 3 (1, 4) | 0.845 |
| Infertility types | 0.844 | 0.255 | ||||
| Primary (%) | 66.0 (2623) | 67.1 (47) | 64.6 (126) | 72.3 (47) | ||
| Secondary (%) | 34.0 (1350) | 32.9 (23) | 35.4 (69) | 27.7 (18) | ||
| Infertility causes | 0.340 | 0.731 | ||||
| Female factor (%) | 60.1 (2388) | 57.1 (40) | 53.8 (105) | 53.8 (35) | ||
| male factor (%) | 13.1 (520) | 8.6 (6) | 6.2 (12) | 9.2 (6) | ||
| both female and male factors (%) | 21.5 (853) | 30.0 (21) | 36.9 (72) | 32.3 (21) | ||
| unexplained (%) | 5.3 (212) | 4.3 (3) | 3.1 (6) | 4.6 (3) | ||
| COS protocols | 0.637 | 0.447 | ||||
| GnRH agonist protocol (%) | 37.0 (1469) | 34.3 (24) | 37.9 (74) | 36.9 (24) | ||
| GnRH antagonist protocol (%) | 52.2 (2074) | 51.4 (36) | 47.2 (92) | 53.8 (35) | ||
| Other protocola (%) | 10.8 (430) | 14.3 (10) | 14.9 (29) | 9.2 (6) | ||
| Operation types | 0.159 | 0.830 | ||||
| IVF (%) | 61.7 (2450) | 54.3 (38) | 54.4 (106) | 53.8 (35) | ||
| ICSI (%) | 34.0 (1353) | 44.3 (31) | 42.1 (82) | 44.6 (29) | ||
| R-ICSI (%) | 4.3 (170) | 1.4 (1) | 3.6 (7) | 1.5 (1) | ||
Note: BMI, body mass index; ART, assisted reproductive technique; COS, controlled ovarian stimulation; GnRH, gonadotrophin releasing hormone; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; R-ICSI, rescue intracytoplasmic sperm injection;
Continuous variables were presented as the median (first quartile, third quartile).
Categorical variables were presented as% (n).
P < 0.05 was considered statistically significant.
aOther protocol: including mild stimulation and luteal phase stimulation protocols.
Fig. 2.
Propensity score matching for the control group and the case group. A,B The distribution of propensity scores before and after matching between the groups. C,D The distribution of standard differences before and after matching between the groups.
3.2. Fertility evaluations
Data on the fertility performance are shown in Table 2. For ovarian reserve, the serum levels of FSH, AMH, and the basal AFC at days 2–3 of the menstrual periods were the three most frequent and efficient markers. The matched data showed no differences between groups in terms of FSH, AMH, and AFC. For ovarian response, Gn dosage, Gn duration, the number of large follicles on HCG day, and the number of oocytes retrieved were similar between groups. Moreover, according to the POSEIDON classification, there were also no significant differences in the percentages of adequate and poor ovarian responses between the groups after matching.
Table 2.
Fertility evaluation before and after matching.
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| Control n = 3973 | Case n = 70 | P value | Control n = 195 | Case n = 65 | P value | |
| FSH (IU/L) | 7.5 (6.3, 9.1) | 7.6 (6.5, 9.0) | 0.828 | 7.6 (6.4, 9.3) | 7.5 (6.4, 9.1) | 0.644 |
| AMH (ng/mL) | 2.6 (1.3, 4.6) | 2.7 (1.3, 4.7) | 0.789 | 2.5 (1.1, 4.3) | 2.8 (1.5, 4.9) | 0.247 |
| AFC | 10.0 (6.0, 16.5) | 10.0 (5.0, 18.0) | 0.750 | 10.0 (6.0, 17.0) | 10.0 (7.0, 18.5) | 0.151 |
| Gn duration (days) | 9.0 (8.0, 11.0) | 10.0 (8.0, 11.0) | 0.092 | 10 (9.0, 11.0) | 10.0 (8.0, 11.0) | 0.276 |
| Gn dosage (IU) | 2400.0 (1800.0, 3000.0) | 2475.0 (1873.1, 3084.4) | 0.362 | 2437.5 (1897.5, 3075.0) | 2400.0 (1848.8, 3056.3) | 0.914 |
| NO. of large follicles on HCG day | 9.0 (5.0, 13.0) | 9.0 (5.0, 13.3) | 0.761 | 9.0 (5.0, 13.0) | 9.0 (5.5, 14.0) | 0.322 |
| NO. of oocytes retrieved | 11.0 (6.0, 16.0) | 11.0 (5.0, 14.0) | 0.352 | 11.0 (5.0, 16.0) | 11.0 (6.0, 14.5) | 0.804 |
| Ovarian response grading1 | 0.696 | 0.666 | ||||
| Adequate | 46.6 (1853) | 44.3 (31) | 44.6 (87) | 47.7 (31) | ||
| Poor | 53.4 (2120) | 55.7 (39) | 55.4 (108) | 52.3 (34) | ||
Note:
FSH, follicle stimulation hormone; AMH, anti-Müllerian hormone; AFC, antral follicle counting; Gn, gonadotropin; HCG, human chorionic gonadotrophin.
Continuous variables were presented as the median (first quartile, third quartile).
Categorical variables were presented as% (n).
1 The ovarian response grading was based on the POSEIDON classification.
3.3. Laboratory outcomes
Laboratory outcomes were presented in Table 3. The proportions of mature oocytes (mature oocyte rate) and damaged oocytes (damaged oocyte rate) were similar between groups. Additionally, the normal fertilization ability of mature oocytes (normal fertilization rate) and the cleavage ability of sequent embryos (cleavage rate) showed no differences. Interestingly, women with a history of SARS-CoV-2 infection had a lower blastocyst formation rate (P = 0.020), despite a comparable HQE rate and available blastocyst rate.
Table 3.
Laboratory outcomes before and after matching.
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| Control n = 3973 | Case n = 70 | P value | Control n = 195 | Case n = 65 | P value | |
| No. of oocytes retrieved | 47,104 | 777 | 2205 | 765 | ||
| Mature oocyte rate (%) | 83.9 (39,524) | 83.3 (647) | 0.658 | 83.0 (1831) | 83.3 (637) | 0.911 |
| Normal fertilization rate (%) | 70.6 (27,890) | 71.9 (465) | 0.487 | 68.8 (1259) | 71.9 (458) | 0.147 |
| Damaged oocyte rate (%) | 3.2 (1520) | 2.3 (18) | 0.181 | 3.5 (78) | 2.2 (17) | 0.074 |
| Cleavage rate (%) | 97.8 (27,281) | 98.5 (458) | 0.345 | 98.3 (1238) | 98.7 (452) | 0.669 |
| HQE rate (%) | 48.1 (13,133) | 45.2 (207) | 0.211 | 46.6 (577) | 45.4 (205) | 0.647 |
| Blastocyst formation rate (%) | 70.0 (16,125) | 60.9 (240) | <0.001 | 67.9 (672) | 61.2 (240) | 0.020 |
| Available blastocyst rate (%) | 70.9 (11,438) | 72.9 (175) | 0.520 | 68.3 (459) | 72.9 (175) | 0.192 |
Note:.
HQE, high quality embryo.
Categorical variables were presented as% (n).
P < 0.05 was considered statistically significant.
3.4. Clinical outcomes of embryos transferred
There were 110 (56.4%) fresh ET cycles in the control group and 32 (49.3%) in the case group after matching (Table 4). Although one or two embryos were transferred in each fresh ET cycle as appropriate, the proportion of single-embryo-transfer cycles between the groups was similar. The propensity score match data demonstrated that females with a history of asymptomatic and mild SARS-CoV-2 infection had similar clinical outcomes of embryos transferred with uninfected females. The biochemical pregnancy rate, early miscarriage rate and clinical pregnancy rate, and implantation rate did not significantly differ between the matched cohorts.
Table 4.
Clinical outcomes before and after matching.
| Unmatched |
Matched |
|||||
|---|---|---|---|---|---|---|
| Control n = 3973 | Case n = 70 | P value | Control n = 195 | Case n = 65 | P value | |
| ET cycles | 2134 | 32 | 110 | 32 | ||
| No. of embryo transferred | 2355 | 35 | 126 | 35 | ||
| 1 (%) | 88.1 (1881) | 90.6 (29) | 1.000 | 85.5 (94) | 90.6 (29) | 0.565 |
| 2 (%) | 11.9 (253) | 9.4 (3) | 14.5 (16) | 9.4 (3) | ||
| Biochemical pregnancy rate (%) | 7.0 (149) | 3.1 (1) | 0.722 | 5.5 (6) | 3.1 (1) | 1.000 |
| Early miscarriage rate (%) | 11.4 (114) | 7.1 (1) | 1.000 | 1.9 (1) | 7.1 (1) | 0.372 |
| Clinical pregnancy rate (%) | 46.9 (1000) | 43.8 (14) | 0.726 | 49.1 (54) | 43.8 (14) | 0.595 |
| Implantation rate (%) | 44.0 (1036) | 40.0 (14) | 0.637 | 45.2 (57) | 40.0 (14) | 0.581 |
Note:.
ET, embryo transfer.
Categorical variables were presented as% (n).
4. Discussion
The results of this retrospective cohort study demonstrated that a history of asymptomatic or mild SARS-CoV-2 infection in females might have no significant negative impact on ART treatments for fertility performance, embryo laboratory outcomes, and clinical outcomes, despite a slightly lower blastocyst formation rate. To some extent, this study provides an observational evidence that the impact of the SARS-CoV-2 on oocytes and embryos may be limited in females with a history of asymptomatic or mild SARS-CoV-2 infection.
The first COVID-19 case in Wuhan was reported in December 2019. Because of the Spring Festival, our center, the largest IVF center in Wuhan, was closed on 22 January 2020, the day before the government announced that Wuhan city was locked down. It was not reopened until April, when the pandemic in Wuhan was stable. The first oocyte retrieval surgery in our center after the global COVID-19 outbreak was conducted in May 2020. No previous studies have reported the impact of SARS-CoV-2 infection in females on IVF outcomes. We have collected the IVF data of females with a history of SARS-CoV-2 infection for 10 months, for the first time, compared them with those of uninfected couples in the same period. The fertility performance, embryo laboratory outcomes, and clinical outcomes were compared. No obvious differences were observed between these two groups after matching in terms of FSH, AMH, AFC, mature oocyte rate, damaged oocyte rate, normal fertilization rate, cleavage rate, HQE rate, available blastocyst rate, clinical pregnancy rate, biochemical pregnancy rate, early miscarriage rate, and implantation rate. However, there was a slight decrease in the blastocyst formation rate in the case group.
In the initial stage of the pandemic, the American Society for Reproductive Medicine (ASRM) issued guidance that appealed to suspending most ART treatments except for the most urgent cases, as did the recommendation from the European Society for Human Reproduction and Embryology (ESHRE) [20]. With the recent successful control of the pandemic spread, full reproductive care is advised to resume by these societies. However, there are no sufficient scientific data to ensure the safety of gametes or embryos resulting from a maternal, paternal, or both infections. A cohort study has assessed SARS-CoV-2 in human semen, and their findings suggested that semen parameters were altered after a moderate infection [7]. Similarly, a recent systematic review also demonstrated that potential male fertility issues might be induced by SARS-CoV-2 infection [21], which was reinforced by many other studies [22–24]. However, in regard to females, most studies suggested that SARS-CoV-2 infection was unlikely to have a long-term adverse effect on female reproductive function [25], according to our clinically observed results. A recent cross-sectional study has also demonstrated that sex-related hormones, such as FSH and AMH, showed no significant difference between COVID-19 patients and controls, which confirmed the results of the fertility evaluation in our study [19]. However, until now, limited studies with good designs have been performed to provide direct evidence of the possible effect of the virus on gamete, embryo, and female fertility.
Much about the crisis, including its implications for fertility and IVF outcomes, is still unidentified, making the exact mechanism enigmatic. SARS-CoV-2 gains access to the host cells mainly by attachment to the angiotensin-converting enzyme 2 (ACE2) receptor and further proteolytic cleavage and activation by the transmembrane protease serine 2 (TMPRSS2) [26]. Several studies have reported the absence of these crucial receptors on human spermatozoa [23], oocytes [27], and embryos [28], making gamete infection possible at an extremely low probability during the IVF procedure, which was consistent with the results of our study. However, other studies have also suggested the significant role of oxidative stress in SARS-CoV-2 infection [29,30] at the molecular level. Increased oxidative stress activates pathogenic mechanisms in female fertility [31], alters the epigenetics of oocytes [32], and finally has a negative impact on oocyte quality [33], which may act as one possible explanation of why the blastocyst formation rate was much higher in the control group than in the case group in our study, despite the equivalent results of other parameters. However, ACE2 receptors have also been reported to be present in preovulatory follicles in rats [34], human ovaries [35], and human germ cells. Therefore, a possible adverse impact of the virus on the embryos through an indirect interaction cannot be ruled out [36].
As a serious stressor, infertility diagnosis and treatment can arouse devastating psychological disorders [37] and emotional problems [38] in women who are unable to conceive. The COVID-19 pandemic has also greatly changed the attitudes of infertile couples toward fertility, fertility advisers, and fertility clinics [39,40]. The psychological impact of fertility treatment suspensions during the COVID-19 crisis should also be considered [41]. Numerous fertility centers worldwide were forced to be out of service because of the uncontrolled pandemic [42]. In the post-COVID-19 era, vaccinations against SARS-CoV-2 are likely to become general and essential [43]. Fertility is the fundamental right of women of reproductive age, and concerns about the impact of vaccines on fertility have never ceased for a minute, although currently, no evidence has shown that vaccines can affect fertility [44]. Our findings are promising, indicating that a positive result for the serum IgG and/or IgM test in females has no significant negative impact on IVF outcomes and attenuates negative emotions related to fertility treatment during the pandemic. As fertility health providers, we encouraged infertile or subfertile couples to ask for help from professional fertility institutions as soon as appropriate, once the local pandemic was stable.
Moreover, what should be considered is the subtle difference in the blastocyst formation rate between the groups presented in our study. The embryonic genome is fully activated in the blastocyst stage, and whether this difference may result in a potential effect on epigenetic modifications in females with a history of SARS-CoV-2 infection needs further observation. Therefore, a larger sample size is urgently needed to check our results, and a close follow-up of the outcomes of gravidas and neonates is also necessary.
There are still several limitations in the current study. First, it was a retrospective cohort study conducted in a single-center, which may cause Berkson's bias and limit the generalizability of our conclusions. In addition, although our center is the largest IVF center in Wuhan city, even in Hubei Province, the sample size of 70 was still small. More data from multiple centers worldwide are needed to support our findings. Due to the sample size limitation, the results of subgroup analyses (such as the severity of infection, the time interval between the first diagnosis and ART treatment, etc.) were not accurate nor reliable. Thus, we would like to increase the sample size and perform subgroup analyses in the future. Another limitation that stems from our study was the lack of data on live birth outcomes. In the future, we will follow up with the participants, collect the data on the pregnancy outcomes, and draw a more reliable conclusion. A further limitation was that our participants in the case group were not in the onset period. Most participants had only mild clinical symptoms or were asymptomatic patients, which may not reflect the true effect of a history of SARS-CoV-2 infection on IVF outcomes. Further studies are needed to investigate the long-term consequences of the pandemic among couples receiving infertility treatment. In addition, due to the limitation of the sample sizes, we cannot classify subgroups to investigate the influences of different characteristics (such as the severity, the complications, the time of recovery, etc.) of the virus infection on the IVF outcomes. Moreover, the studied population presented heterogeneity in the baseline characteristics, such as infertility etiology and COS protocols, which could represent confounding factors and jeopardize the final outcomes.
In conclusion, the findings suggested no evidence that a history of asymptomatic or mild SARS-CoV-2 infection in females caused impairment of female fertility, embryo laboratory outcomes, or clinical outcomes in ART treatments. To some extent, this study provides an observational evidence that the impact of the SARS-CoV-2 on oocytes and embryos may be limited in females with a history of asymptomatic or mild SARS-CoV-2 infection, while the effects of moderate or severe infection on female fertility need to be considered and further investigated.
Funding
This study was supported by the National Key Research and Development Project (2018YFC1002103) and the Health Commission of Hubei Province Scientific Research Project (WJ2021M110).
Authors’ contributions
QX, LZ, and LJ conceived the study and have full access to the raw data; MW wrote the paper; MW, QY, and XR analyzed the data; JH, ZL, and RL collected the data.
Declaration of Competing Interest
All authors have no conflicts of interest to declare.
Data sharing statement
Request for access to the data should be made to the corresponding author at leijintjh@163.com. Data could be made available provided the applicant has appropriate ethics approval and approval from the authors.
Acknowledgments
We would like to express heartfelt gratitude to our colleagues in Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
References
- 1.Fanelli V., Fiorentino M., Cantaluppi V., Gesualdo L., Stallone G., Ronco C. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. 2020;24:155. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A., Skriabine S. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei H., Ding Y., Nie K., Dong Y., Xu J., Yang M. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardona Maya W., Du Plessis S., Velilla P. SARS-CoV-2 and the testis: similarity with other viruses and routes of infection. Reprod Biomed Online. 2020;40:763–764. doi: 10.1016/j.rbmo.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh B., Gornet M., Sims H., Kisanga E., Knight Z., Segars J. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its effect on gametogenesis and early pregnancy. Am J Reprod Immunol. 2020;84:e13351. doi: 10.1111/aji.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tur-Kaspa I., Tur-Kaspa T., Hildebrand G., Cohen D. COVID-19 may affect male fertility but is not sexually transmitted: a systematic review. F&S Rev. 2021;2:140–149. doi: 10.1016/j.xfnr.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtmann N., Edimiris P., Andree M., Doehmen C., Baston-Buest D., Adams O. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L., Xie W., Li D., Shi L., Ye G., Mao Y. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93:456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egerup P., Fich Olsen L., Christiansen A., Westergaard D., Severinsen E., Hviid K. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies at delivery in women, partners, and newborns. Obstet Gynecol. 2021;137:49–55. doi: 10.1097/AOG.0000000000004199. [DOI] [PubMed] [Google Scholar]
- 10.Anifandis G., Messini C.I., Daponte A., Messinis I.E. COVID-19 and fertility: a virtual reality. Reprod Biomed Online. 2020;41:157–159. doi: 10.1016/j.rbmo.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetto U., Head S., Angelini G., Blackstone E. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- 13.Wang M., Xi Q., Yang Q., Li Z., Yang L., Zhu L. The relationship between a novel evaluation parameter of premature luteinization and IVF outcomes. Reprod Biomed Online. 2021;42:323–331. doi: 10.1016/j.rbmo.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Braga D., Setti A., Figueira R., Iaconelli A., Borges E. The importance of the cleavage stage morphology evaluation for blastocyst transfer in patients with good prognosis. J Assist Reprod Genet. 2014;31:1105–1110. doi: 10.1007/s10815-014-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner D., Schoolcraft W. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Xie J., Ding C., Li J., Wang Y., Guo H., Lu Z. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020;92:2004–2010. doi: 10.1002/jmv.25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1930–1934. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conforti A., Esteves S., Picarelli S., Iorio G., Rania E., Zullo F. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med. 2019;61:24–29. doi: 10.23736/S0031-0808.18.03511-5. [DOI] [PubMed] [Google Scholar]
- 19.Li K., Chen G., Hou H., Liao Q., Chen J., Bai H. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42:260–267. doi: 10.1016/j.rbmo.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga A., Gianaroli L., Ory S., Horton M., Feinberg E., Penzias A. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Fertil Steril. 2020;114:484–485. doi: 10.1016/j.fertnstert.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalili M.A., Leisegang K., Majzoub A., Finelli R., Panner Selvam M.K., Henkel R. Male fertility and the COVID-19 pandemic: systematic review of the literature. World J Mens Health. 2020;38:506–520. doi: 10.5534/wjmh.200134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omolaoye T.S., Adeniji A.A., Cardona Maya W.D., du Plessis S.S. SARS-COV-2 (Covid-19) and male fertility: where are we? Reprod Toxicol. 2021;99:65–70. doi: 10.1016/j.reprotox.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younis J., Abassi Z., Skorecki K. Is there an impact of the COVID-19 pandemic on male fertility? The ACE2 connection. Am J Physiol Endocrinol Metab. 2020;318:E878–E880. doi: 10.1152/ajpendo.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W., Liu X., Feng L., Xiong S., Li Y., Chen L. Impact of SARS-CoV-2 on Male reproductive health: a review of the literature on male reproductive involvement in COVID-19. Front Med. 2020;7 doi: 10.3389/fmed.2020.594364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley K., Thomas E., Leaver M., Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 2020;114:33–43. doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashour H., Elkhatib W., Rahman M., Elshabrawy H. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barragan M., Guillén J., Martin-Palomino N., Rodriguez A., Vassena R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum. Reprod. 2021;36:390–394. doi: 10.1093/humrep/deaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mali A.S., Magdum M., Novotny J. COVID-19 impact on reproduction and fertility. JBRA Assist Reprod. 2020;25:310–313. doi: 10.5935/1518-0557.20200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39:644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51:384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A., Gupta S., Sharma R. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol RB&E. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menezo Y., Silvestris E., Dale B., Elder K. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online. 2016;33:668–683. doi: 10.1016/j.rbmo.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Prasad S., Tiwari M., Pandey A., Shrivastav T., Chaube S. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honorato-Sampaio K., Pereira V., Santos R., Reis A. Evidence that angiotensin-(1-7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol. 2012;97:642–650. doi: 10.1113/expphysiol.2011.061960. [DOI] [PubMed] [Google Scholar]
- 35.Reis F., Bouissou D., Pereira V., Camargos A., dos Reis A., Santos R. Angiotensin-(1-7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 2011;95:176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Yan L., Yang M., Guo H., Yang L., Wu J., Li R. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20:1131–1139. doi: 10.1038/nsmb.2660. [DOI] [PubMed] [Google Scholar]
- 37.Maroufizadeh S., Karimi E., Vesali S., Omani Samani R. Anxiety and depression after failure of assisted reproductive treatment among patients experiencing infertility. Int J Gynaecol Obstet. 2015;130:253–256. doi: 10.1016/j.ijgo.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 38.Gana K., Jakubowska S. Relationship between infertility-related stress and emotional distress and marital satisfaction. J Health Psychol. 2016;21:1043–1054. doi: 10.1177/1359105314544990. [DOI] [PubMed] [Google Scholar]
- 39.Boivin J., Harrison C., Mathur R., Burns G., Pericleous-Smith A., Gameiro S. Patient experiences of fertility clinic closure during the COVID-19 pandemic: appraisals, coping and emotions. Hum Reprod. 2020;35:2556–2566. doi: 10.1093/humrep/deaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Kimhy R., Youngster M., Medina-Artom T., Avraham S., Gat I., Marom Haham L. Fertility patients under COVID-19: attitudes, perceptions and psychological reactions. Hum Reprod. 2020;35:2774–2783. doi: 10.1093/humrep/deaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon J.L., Balsom A.A. The psychological impact of fertility treatment suspensions during the COVID-19 pandemic. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0239253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleicher N. The COVID-19 pandemic through eyes of a NYC fertility center: a unique learning experience with often unexpected results. Reprod Biol Endocrinol. 2020;18:105. doi: 10.1186/s12958-020-00663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappuoli R., De Gregorio E., Del Giudice G., Phogat S., Pecetta S., Pizza M. Vaccinology in the post-COVID-19 era. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2020368118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iacobucci G. Covid-19: no evidence that vaccines can affect fertility, says new guidance. BMJ. 2021;372:n509. doi: 10.1136/bmj.n509. [DOI] [PubMed] [Google Scholar]