Abstract
Background
Endoscopically defined mucosal healing in Crohn's disease is associated with improved outcomes. Panenteric capsule endoscopy enables a single non‐invasive assessment of small and large bowel mucosal inflammation.
Aims and Methods
This multicentre observational study of patients with suspected and established Crohn's disease examined the feasibility, safety and impact on patient outcomes of panenteric capsule endoscopy in routine clinical practice. The potential role in assessment of disease severity and extent by a comparison with existing clinical and biochemical markers is examined.
Results
Panenteric capsule endoscopy was performed on 93 patients (71 with established and 22 with suspected Crohn's disease). A complete examination occurred in 85% (79/93). Two cases (2.8%) of capsule retention occurred in patients with established Crohn's disease. Panenteric capsule resulted in management change in 38.7% (36/93) patients, including 64.6% (32/48) of those with an established diagnosis whose disease was active, and all three patients with newly diagnosed Crohn's disease. Montreal classification was upstaged in 33.8% of patients with established Crohn's disease and mucosal healing was demonstrated in 15.5%. Proximal small bowel disease upstaged disease in 12.7% and predicted escalation of therapy (odds ratio 40.3, 95% confidence interval 3.6–450.2). Raised C‐reactive protein and faecal calprotectin were poorly sensitive in detecting active disease (0.48 and 0.59 respectively).
Conclusions
Panenteric capsule endoscopy was feasible in routine practice and the ability to detect proximal small bowel disease may allow better estimation of prognosis and guide treatment intensification. Panenteric capsule endoscopy may be a suitable non‐invasive endoscopic investigation in determining disease activity and supporting management decisions.
Keywords: a novel panenteric capsule endoscope, endoscopy, patient management, PillCam Crohn's, proximal small bowel Crohn's disease
Key Summary
Summarise the established knowledge on this subject
Biochemical and clinical markers poorly predict active disease and need for treatment escalation;
Panenteric capsule endoscope is feasible, safe and has the potential to non‐invasively assess patients with Crohn's Disease.
What are the significant and/or new findings of this study?
Panenteric capsule endoscope can upstage disease in one‐third of patients with a threefold increase in the identification of proximal small bowel disease;
Identification of proximal small bowel disease predicted treatment intensification.
1. INTRODUCTION
Endoscopically defined mucosal healing is associated with a reduced need for steroid treatment, hospitalisation and surgery in patients with Crohn's disease. Therefore, investigation by ileocolonoscopy is recommended in those with suspected Crohn's disease and in those with established disease who need reassessment. 1 Ileal disease is associated with complicated disease phenotypes and the Inflammatory Bowel Disease Genetics Consortium suggests that the association of jejunal disease with stricturing and multiple surgeries is greater still. 2 Therefore, guidelines also recommend routine small bowel assessment in patients with new diagnoses of Crohn's disease in order to assess prognosis. 1 Historically this has involved radiological imaging, which is less sensitive than capsule endoscopy in identifying early mucosal disease, particularly in the jejunum. 3 , 4 , 5 Capsule endoscopy is more acceptable to patients than conventional endoscopy. 6 , 7 Capsule retention occurs in 1.5% of those with suspected, and 213% with established, Crohn's disease, although can be minimised by excluding those known to have strictures or following the failure of passage of a patency device. 1
The PillCam Crohn's (Medtronic) is a novel panenteric capsule endoscope (PCE) developed to identify inflammatory activity in both small and large bowel. It has cameras at both ends and acquires up to 35 frames per second depending on transit speed (Figure 1a). 8 , 9 The platform provides a facility to localise and grade disease activity and quantify extent as well as compare successive examinations to allow assessment of disease progression and response to therapy (Figure 1b). Feasibility studies have suggested that the panenteric capsule is safe and has a greater diagnostic yield than ileocolonoscopy; however, effect on management decisions and correlation with biochemical markers of disease in adult patients has not been studied. 8 , 10
FIGURE 1.
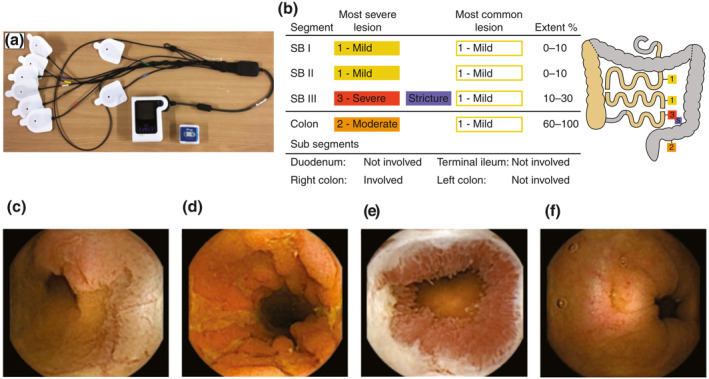
(a) PillCam Crohn's capsule, DR3 data recorder and wireless sensors. (b) A representative graphic of a patient with active Montreal L3 B2 disease and images of small bowel (SB) lesions (c and d), SB stricture (e) and colonic lesion (f). RAPID™ Reader Software breaks down small bowel and colonic segments based on identified anatomical landmarks. The reader classifies the most severe and most common lesion (none, mild, moderate and severe), presence or absence of stricture and extent of disease (0%–10%, 10%–30%, 30%–60%, 60%–100% of segment)
2. OBJECTIVES
The impact of PCE in clinical practice was investigated. The feasibility, safety and effect of a panenteric capsule examination on defining patients' disease phenotype was studied by: (i) examining completion and capsule retention rates, (ii) the effect of panenteric capsule on escalation or de‐escalation of treatment, (iii) comparing the extent of disease (Montreal classification) before and after examination and (iv) comparing disease activity assessed by capsule endoscopy with clinical and biochemical markers of activity.
3. METHODS
Six centres were involved in the study: Sheffield Teaching Hospitals and South Tyneside NHS Foundation Trusts, Sheba Medical Centre, Mater Dei Hospital Ospedale Maggiore Policlinico and Complejo Hospitalario de Navarra. Capsule endoscopy was performed at the discretion of the clinician after consultation with the patient as part of routine practice. Ileocolonoscopy and small bowel radiology or PCE were discussed with patients with established Crohn's or symptoms which the referring clinician suspected as possibly being due to Crohn's disease. Patients who preferred capsule endoscopy were included in the study.
Data collected from patients' case records undergoing PCE between July 2017 and May 2019 included age, sex, disease duration, medication history, indication, blood test parameters, faecal calprotectin (FCP), Harvey Bradshaw Index and Montreal classification. Blood tests and FCP were requested in the month prior to capsule endoscopy. A C‐reactive protein (CRP) of greater than 5 mg/L and FCP of over 200 mg/kg were considered to be elevated.
PCE was performed according to the protocols of individual units based on previous published experience. 8 , 9 The PillCam Crohn's platform includes a software reporting system which divides the gastrointestinal tract into small bowel tertiles (SB1, 2 and 3: according to time and speed of capsule transit) and colon, so that inflammation in each region can be graded (with a score of 1–3) according to ulcer size and depth. The reader records the most common (MCL) and most severe lesion (MSL) in each region and the extent (as a percentage) of the region involved. In addition, an activity score previously designed and validated for small bowel capsule endoscopy, the Lewis score, can also be assigned following small bowel assessment. 11
Disease phenotype was assessed using the Montreal classification (ileal, L1; colonic, L2; ileocolonic, L3 and proximal small bowel disease, L4—defined on Capsule endoscopy [CE] as disease in the first and second tertiles). Active disease was defined as the presence of at least mild ulceration (MCL 1) affecting at least 10% of the segment or the presence of moderate or severe ulceration (MSL 2 or 3) of any extent. An upstage in disease classification was considered if L1 or L2 progressed to L3 disease, or if L4 disease was identified in addition, or if B1 (inflammatory) progressed to B2 (stricturing) disease.
A comprehensive formal report was provided for referring clinicians who had sole responsibility for therapeutic interventions. Effect on patient outcomes was determined by reviewing management decisions made at the post‐capsule endoscopy consultation between clinician and patient.
Continuous and categorical variables are reported as mean (±standard error of mean) and frequency (%) respectively. Differences in groups are compared using independent Student t‐test and chi squared tests (significance level p < 0.05). Logistic regression is used to determine associations between clinical, biochemical and endoscopic measures of disease activity, with escalation of therapy. The performance of CRP and FCP in the prediction of disease activity is reported as area under the receiver operating characteristic curve (AUROCC), sensitivity and specificities.
3.1. Ethical considerations
The service evaluation involved patients seen as part of routine clinical practice. All identifiable medical information was removed and all analyses performed using anonymised data. The data collection was in line with good clinical practice policies and with the General Data Protection Regulation UE 2016/679.
4. RESULTS
PCE was performed in 22 patients with suspected, and 71 patients with established, Crohn's disease, mean age 36.6 (±1.39) years, 36.6% male. Of those with established Crohn's disease, PCE was performed in 58 (81.7%) to assess symptoms, in 12 (16.9%) to assess response to treatment after symptomatic remission and in one (1.4%) to assess the post‐operative risk of disease recurrence. The median time between last disease reassessment and PCE was 23 (interquartile range 36) months (Table 1).
TABLE 1.
Investigations prior to panenteric capsule endoscopy
| n (%) | |
|---|---|
| Suspected Crohn's disease | 22 (23.7) |
| Previous endoscopy | |
| Complete colonoscopy | 10 (45.5) |
| Incomplete colonoscopy | 6 (27.3) |
| No previous endoscopy | 6 (27.3) |
| Established Crohn's disease | 71 (76.3) |
| Previous investigations | |
| Ileocolonoscopy | 51 (71.8) |
| Colonoscopy | 11 (15.5) |
| CE | 19 (26.8) |
| MRI | 30 (42.3) |
| CTE | 16 (22.5) |
| BaFT | 3 (4.2) |
| Colonoscopy alone | 22 (30.1) |
| Colonoscopy and SB investigations | 19 (26.8) |
| SB investigations alone | 28 (39.4) |
| Unknown | 2 (2.8) |
| Had treatment between last assessment and panenteric capsule endoscopy n,(%) Yes | 30 (42.3) |
| Biologics | 20 (28.2) |
| Immunomodulators | 14 (19.7) |
| Surgery | 1 (1.4) |
Abbreviations: BaFT, barium follow through; CE, capsule endoscopy; CTE, computed tomography enterography; MRI, magnetic resonance imaging.
4.1. Disease activity and extent
Active disease was present in 48 of 71 (67.6%) of those with established and three of 22 (13.6%) of those with suspected Crohn's disease. Two of three new cases were confirmed histologically. Active disease was seen in 41 of 58 (70.7%) of symptomatic patients and seven of 13 (53.8%) asymptomatic patients with established Crohn's disease (p = 0.24). Mean Lewis score was 923 (±169) and 321 (±197) respectively for patients with Crohn's disease with and without symptoms (p = 0.09). Figure 1c,d shows images of active disease.
Disease extent was upstaged in 24 of 71 (33.8%) patients. This included patients with upper gastrointestinal or proximal small bowel disease (L4 notification) which increased from five (7.0%) to 14 (19.7%, p < 0.05) following panenteric examination. This represents 24.1% of symptomatic patients who had more proximal small bowel involvement than asymptomatic patients (24.1% vs. 0%, p = 0.05). Disease extent was downstaged in 19 of 71 (26.8%) patients where examination demonstrated complete mucosal healing in 12 of 71 (16.9%) patients, and in the remaining 29 of 71 (40.8%) disease classification remained unchanged (Table 2).
TABLE 2.
Extent of suspected Crohn's disease before and after investigation based on Montreal classification
| Before capsule n (%) | After capsule n (%) | |
|---|---|---|
| No disease | ‐ | 12 (16.9) |
| L1 | 34 (47.9) | 26 (36.6) |
| L2 | 7 (9.9) | 7 (9.9) |
| L3 | 29 (40.8) | 25 (35.2) |
| +L4 | 5 (7.0) a | 14 (19.7) |
| B1 | 54 (76.1) | 38 (53.5) |
| B2 | 13 (18.3) | 17 (23.9) |
| B3 | 4 (5.6) | ‐ |
One patient had only proximal small bowel L4 disease.
4.2. Comparison with non‐invasive markers
Patients with active disease had a higher mean CRP (11.4 ± 21.2 vs. 4.0 ± 9.2, p = 0.003), FCP (812 ± 145.8 vs. 55.8 ± 21.1, p = 0.02) and Harvey Bradshaw Index (5.0 ± 0.49 vs. 4.2 ± 0.88, p = 0.02) than patients with inactive disease. Active disease was associated with a raised CRP level (odds ratio [OR] 11.6, 95% confidence interval [CI] 3.1–43.3) and FCP (OR 3.5, 95% CI 1.1–10.9) but not with changes in Harvey Bradshaw Index.
CRP and FCP results were available in 89% and 60% of cases. CRP and FCP had an AUROCC of 0.73 (95% CI 0.58–0.87) and 0.75 (95% CI 0.60–0.90) respectively. CRP levels above 5 mg/L had 48% sensitivity and 85% specificity in detecting active disease identified by PCE in patients known to have Crohn's disease, of whom 23 patients (47%) had values within normal range. Patients with active disease had FCP levels less than 50 mg/kg in four (14%), between 50 mg/kg and 200 mg/kg in nine (31%) and over 200 mg/kg in 16 (55%). FCP levels of greater than 200 mg/kg had a 59% sensitivity and 65% specificity in detecting active disease.
4.3. Change in management
Management was changed in 36 (38.7%): 33 of 71 (46.5%) with established Crohn's and all three patients in whom a new diagnosis was made. Overall, 32 of 48 (64.6%) patients with an established diagnosis of Crohn's whose disease was active had a change in drug therapy. This included a step up in treatment comprising the addition or class change of biologics in 24.6%, the addition of immunomodulators and steroids in 15.5% and 5.6%, respectively. Endoscopic stricture dilatation occurred in two cases (when retained capsules were also removed). Of the remaining 16 of the 48 patients with active disease, 10 were continued on the same biological or immunomodulator therapy, three were offered further treatment but opted for observation only and complete follow‐up data could not be obtained for three patients. The presence of proximal small bowel involvement and moderate to severe small bowel disease (a Lewis score of over 790) increased the likelihood of therapy escalation (Table 3). De‐escalation of biologics occurred following a normal examination.
TABLE 3.
Predictors of escalation of drug therapy in all patients undergoing panenteric capsule endoscopy
| No escalation n (%) | Drug escalation n (%) | Odds ratio | 95% CI | |
|---|---|---|---|---|
| Raised Harvey Bradshaw Index, >6 | 20 (35.7) | 17 (45.9) | 1.5 | 0.66–3.57 |
| Raised C‐reactive protein, >5 mg/L | 9 (18.8) | 20 (57.1) | 5.8 | 2.2–15.5 |
| Raised faecal calprotectin, >200 mg/L | 10 (28.6) | 13 (61.9) | 4.1 | 1.3–12.8 |
| Lewis score | ||||
| 0–135 | 30 (53.6) | 9 (24.3) | Reference | ‐ |
| 135–790 | 18 (32.1) | 12 (32.4) | 1.6 | 0.5–5.0 |
| >790 | 8 (14.3) | 16 (43.2) | 3.4 | 1.0–12.1 |
| Disease location | ||||
| No disease | 11 (28.9) | 2 (6.1) | Reference | ‐ |
| L1 | 13 (34.2) | 7 (21.2) | 4.7 | 0.49–45.2 |
| L2 | 2 (5.3) | 4 (12.1) | 22.0 | 1.5–314.3 |
| L3 | 8 (21.1) | 10 (30.3) | 15.1 | 1.6–142.2 |
| +L4 | 4 (10.5) | 10 (30.3) | 40.3 | 3.6–450.2 |
Abbreviation: CI, confidence interval.
4.4. Completeness of examinations
Complete small bowel and colon examination was achieved in 94.6% (88/93) and 84.9% (79/93), respectively. Eighty‐eight (94.6%) patients had a patency examination or small bowel imaging (68/71 established, 20/22 suspected) prior to examination. In three with established and two with suspected Crohn's disease, no imaging or patency evaluation was performed. Incomplete examinations included two capsule retentions, amounting to 2.8% (2/71) of patients with established Crohn's disease. Capsule retentions occurred behind one small bowel and one colonic stricture, both of which were treated with endoscopic stricture dilatation and capsule retrieval. In eight patients (8.6%) where the colon was incompletely examined (excluding a colonic stricture), five were due to loss of battery power, two due to loss of capsule signal (2.2%) and one, inadequate bowel preparation (1.1%).
5. DISCUSSION
Active Crohn's disease identified by PCE was not reliably predicted by symptoms, Harvey Bradshaw Index or biochemical markers. The panenteric examination upstaged the Montreal classification in one‐third of patients with a threefold increase in a diagnosis of an L4 phenotype which predicted treatment intensification. PCE provided a complete, single test, small and large bowel assessment in 84.9% of patients. There was a 2.8% capsule retention rate in patients known to have Crohn's disease and both were subsequently removed endoscopically following stricture dilatation. Management was changed overall in 39%, in 47% of those with established disease and in 65% of patients with Crohn's disease who had symptoms.
A more advanced Montreal classification in one‐third of patients with Crohn's disease is not unexpected in a population which included symptomatic patients, but PCE increased the recognition of L4 disease threefold, which is consistent with that of Leighton et al., who showed that PCE detected additional disease proximal to the terminal ileum in 45% of 66 adult patients. 8 Evidence that proximal bowel disease is a poor prognostic factor would explain the association with therapy escalation demonstrated. 2
CRP, FCP level, but not Harvey Bradshaw Index predicted escalation in therapy. A raised CRP >5 mg/L was moderately specific (85%) and FCP >200 mg/kg less so (65%) in the diagnosis of active Crohn's disease. These findings are consistent with the demonstration of better mucosal healing in the tight control arm of the CALM study, which used both biomarkers as part of a protocol to signal the need for escalation of therapy. 12 However, the disappointing sensitivities in detecting activity suggest that this approach might miss patients who could benefit from further treatment. Previous studies have also found that CRP and FCP are poor predictors of disease activity and should not be used alone as surrogate markers for mucosal healing. 13 , 14 The Harvey Bradshaw Index did not correlate with findings. This is unsurprising given that clinical scores do not correlate with endoscopic activity. 15 , 16
Historically, patients having a panenteric examination undergo both small bowel radiology or capsule endoscopy, and ileocolonoscopy. Studies comparing diagnostic accuracy of PCE against ileocolonoscopy and magnetic resonance enterography (MRE) are pending. If we assume that MRE would identify any small bowel disease and that ileocolonoscopy would identify any ileocolonic disease, ileocolonoscopy would have identified active Crohn's disease in 97.6%, although this would fall to 46.3% if ileal intubation were unsuccessful. A small bowel examination in addition would therefore be necessary to identify the remaining patients with active disease. Of 41 patients with active disease, 53 segments of active disease were observed. Colonoscopy alone, ileocolonoscopy alone and MRE alone would have missed 64.2% (34/53), 30.2% (16/53) and 35.8% (19/53) of segments with active disease. Therefore, if presence of proximal small bowel disease helps prognostication and management, up to one‐third may be incompletely assessed with ileocolonoscopy alone. These scenarios are likely to be an underestimate. Although Crohn's disease has a predilection towards the terminal ileum, in reality ileocolonoscopy does not examine the whole distal small bowel tertile as was assumed. Nevertheless, it is possible that repeating conventional investigations might have demonstrated the same upstage in disease classification; however, PCE is a single test (rather than separate small and large bowel assessments) and radiology appears less sensitive in recognising uncomplicated L4 disease. 3 , 17
Shorter intervals between endoscopic assessments are associated with better outcomes. 18 However, in a comparison of acceptability of tests, patients with inflammatory bowel disease (IBD) found flexible endoscopy 6 and MRE 19 less acceptable than capsule endoscopy. Furthermore, Ferreira et al. calculated a 12.7% lifetime risk of serious endoscopic adverse events in patients with IBD in a time prior to the ‘treat to target’ era, after which more frequent assessment is likely to increase rates of complications. 20 Capsule endoscopy may therefore offer a safer, as well as more acceptable, approach to the assessment of mucosal healing.
Studies which associate proximal disease with poor prognosis are based on radiological imaging and therefore the association might be with more advanced disease, rather than uncomplicated mucosal disease detected by capsule endoscopy. However, recent evidence suggests that jejunal lesions identified using capsule endoscopy appear to increase the risk of disease recurrence 21 and a high small bowel capsule endoscopy disease activity score predicts both short‐ and long‐term disease exacerbations. 11 , 22 Furthermore, mucosal disease detected by PCE used to monitor response to treatment intensification in a ‘treat to target’ strategy has been shown to achieve mucosal healing in children. 9
The experience of capsule retention behind small and large bowel strictures in this study is the first reported with PCE: no retentions occurred in the 66 patients examined by Leighton et al., nor in a paediatric study. 8 , 9 In the first capsule retention in our study, no patency device was administered; however, a MRE showed non‐stricturing distal ileitis. In the second case of retention, the capsule was retained behind a colonic stricture following a patency capsule test. A positive radiofrequency signal 30 h post‐ingestion of the device was followed by a targeted computed tomography (CT) 23 which revealed that it was in the ascending colon and correctly concluded that there was no small bowel stricture. Care should therefore be taken in those with a history of colonic disease. Yadav et al. suggest that radiological imaging and the patency device were equally sensitive at detecting significant strictures, although the majority of patients in the study underwent CT rather than magnetic resonance imaging. 1 , 24 Nevertheless other studies suggest that MRE is poorly specific at detecting significant strictures. 25
The study has limitations. This was a pragmatic study of patients in routine clinical practice. Not all patients had their blood or faecal tests within 1 month of capsule endoscopy because of delays in performing the procedure or a failure to provide a faecal sample, which limits the comparison of biochemical markers with capsule endoscopy. Not all centres were able to provide reliable information about patients' use of non‐steroidal anti‐inflammatory drugs. The definition of active disease in PCE was arbitrary. It is possible that any identified inflammatory change was due to active Crohn's disease. On the other hand, there is significant interobserver variability in lesion recognition 26 and it is well established that minor abnormalities are found in 10%–15% of healthy volunteers. 27 , 28 Therefore, the definition of active disease in this study is aimed to recognise at least mild active disease whilst minimizing the chances of including what some might consider variations of normal. Recent efforts to validate a scoring system for PCE are welcome. 29 Finally, clinicians may have chosen a panenteric examination because of a clinical suspicion of small bowel disease, thereby making this a non‐representative population; were this the case, however, much of it was previously unidentified as the panenteric study still had a major impact on the diagnosis of L4 disease. Finally, assessment of small and large bowel was variable in terms of both completeness and proximity in time prior to PCE. Incomplete examination and/or disease progression might also explain why Montreal classification was upstaged in some patients. However, it does also suggest that while the current recommended panenteric assessment is important, using two different tests 1 may be impractical, perhaps due to patient inconvenience or clinician concern about the longer investigative pathway.
6. CONCLUSION
Capsule endoscopy provided an adequate single test, panenteric examination for patients with Crohn's disease leading to escalation in therapy in two‐thirds of those with active disease and with a 2.8% capsule retention rate, both of which were resolved endoscopically. One in five patients had disease proximal to the terminal ileum and this was associated with escalation of therapy. Clinical assessment was unreliable in assessing disease activity and levels of CRP and FCP were insensitive in detecting active disease. A PCE examination would be a suitable alternative to combined ileocolonoscopy and small bowel radiological imaging for those with normal or indeterminate biomarker levels or in whom raised levels might have an alternative explanation. Controlled studies comparing the diagnostic yield of PCE against ileocolonoscopy and MRE are required to determine the utility of this approach.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
The data collection was in line with good clinical practice policies and with the General Data Protection Regulation UE 2016/679.
INFORMED CONSENT
No consent was obtained in this observational study.
REFERENCES
- 1. Maaser C, Sturm A, Vavricka SR, et al. ECCO‐ESGAR Guideline for diagnostic assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 2. Lazarev M, Huang C, Bitton A, et al. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross‐sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Böcker U, Dinter D, Litterer C, et al. Comparison of magnetic resonance imaging and video capsule enteroscopy in diagnosing small‐bowel pathology: Localization‐ dependent diagnostic yield. Scand J Gastroenterol. 2010;45:490–500. [DOI] [PubMed] [Google Scholar]
- 4. Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn's disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–9. [DOI] [PubMed] [Google Scholar]
- 5. Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small‐bowel Crohn's disease: A meta‐analysis. Am J Gastroenterol. 2010;105:1240–8. quiz 1249. [DOI] [PubMed] [Google Scholar]
- 6. Buisson A, Gonzalez F, Poullenot F, et al. Comparative acceptability and perceived clinical utility of monitoring tools: A nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1425–33. [DOI] [PubMed] [Google Scholar]
- 7. Ojidu H, Palmer H, Lewandowski J, et al. Patient tolerance and acceptance of different colonic imaging modalities: An observational cohort study. Eur J Gastroenterol Hepatol. 2018;30:520–5. [DOI] [PubMed] [Google Scholar]
- 8. Leighton JA, Helper DJ, Gralnek IM, et al. Comparing diagnostic yield of a novel pan‐enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn's disease: A feasibility study. Gastrointest Endosc. 2017;85:196–205.e1. [DOI] [PubMed] [Google Scholar]
- 9. Oliva S, Aloi M, Viola F, et al. A treat to target strategy using panenteric capsule endoscopy in pediatric patients with Crohn's disease. Clin Gastroenterol Hepatol. 2018;17:2060–7. [DOI] [PubMed] [Google Scholar]
- 10. Eliakim R, Spada C, Lapidus A, et al. Evaluation of a new pan‐enteric video capsule endoscopy system in patients with suspected or established inflammatory bowel disease ‐ feasibility study. Endosc Int Open. 2018;6:E1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dias de Castro F, Boal Carvalho P, Monteiro S, et al. Lewis score‐prognostic value in patients with isolated small bowel Crohn's disease. J Crohns Colitis. 2015;9:1146–51. [DOI] [PubMed] [Google Scholar]
- 12. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 13. Henriksen M, Jahnsen J, Lygren I, et al. C‐reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population‐based study. Gut. 2008;57:1518–23. [DOI] [PubMed] [Google Scholar]
- 14. Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–31. [DOI] [PubMed] [Google Scholar]
- 15. Tajra JB, Calegaro JU, de Paula AP, et al. Correlation and concordance measures between clinical, endoscopic and histological scores activity in Crohn's disease under treatment. Scand J Gastroenterol. 2019;54:441–5. [DOI] [PubMed] [Google Scholar]
- 16. de Jong MJ, Huibregtse R, Masclee AAM, Jonkers DMAE, Pierik MJ. Patient‐reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: A systematic review. Clin Gastroenterol Hepatol. 2018;16:648–63.e3. [DOI] [PubMed] [Google Scholar]
- 17. Greener T, Klang E, Yablecovitch D, et al. The impact of magnetic resonance enterography and capsule endoscopy on the re‐classification of disease in patients with known Crohn's disease: A prospective Israeli IBD Research Nucleus (IIRN) study. J Crohns Colitis. 2016;10:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouguen G, Levesque BG, Pola S, Evans E, Sandborn WJ. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12:978–85. [DOI] [PubMed] [Google Scholar]
- 19. Lahat A, Kopylov U, Amitai MM, et al. Magnetic resonance enterography or video capsule endoscopy ‐ what do Crohn's disease patients prefer? Patient Prefer Adherence. 2016;10:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira J, Akbari M, Gashin L, et al. Prevalence and lifetime risk of endoscopy‐related complications among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:1288–93. [DOI] [PubMed] [Google Scholar]
- 21. Flamant M, Trang C, Maillard O, et al. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn's disease. Inflamm Bowel Dis. 2013;19:1390–6. [DOI] [PubMed] [Google Scholar]
- 22. Ben‐Horin S, Lahat A, Amitai MM, et al. Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short‐term and long‐term risk of Crohn's disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol. 2019;4:519–28. [DOI] [PubMed] [Google Scholar]
- 23. Assadsangabi A, Blakeborough A, Drew K, Lobo AJ, Sidhu R, McAlindon ME. Small bowel patency assessment using the patency device and a novel targeted (limited radiation) computed tomography‐based protocol. J Gastroenterol Hepatol. 2015;30:984–9. [DOI] [PubMed] [Google Scholar]
- 24. Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc. 2011;74:834–9. [DOI] [PubMed] [Google Scholar]
- 25. Rozendorn N, Klang E, Lahat A, et al. Prediction of patency capsule retention in known Crohn's disease patients by using magnetic resonance imaging. Gastrointest Endosc. 2016;83:182–7. [DOI] [PubMed] [Google Scholar]
- 26. Rondonotti E, Soncini M, Girelli CM, et al. Can we improve the detection rate and interobserver agreement in capsule endoscopy? Dig Liver Dis. 2012;44:1006–11. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. [DOI] [PubMed] [Google Scholar]
- 28. Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis oEf NSAID‐induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–8. [DOI] [PubMed] [Google Scholar]
- 29. Eliakim R, Yablecovitch D, Lahat A, et al. A novel PillCam Crohn's capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn's disease. United European Gastroenterol J. 2020. 10.1177/2050640620913368. [DOI] [PMC free article] [PubMed] [Google Scholar]


