Abstract
Background
Pseudomonas fluorescens (P. fluorescens) has been detected in respiratory samples from patients. However, no previous reports have been published about these P. fluorescens cultures from lung tissues.
Case presentation
Here, we report a case of pneumonia caused by P. fluorescens. P. fluorescens was identified from lung biopsy specimens for the first time in this case. According to the antibiotic susceptibility testing (AST) of P. fluorescens, the patient was given ciprofloxacin treatment. The temperature of the patient then returned to normal. Chest CT examination revealed improvements in pulmonary inflammation.
Conclusions
These findings suggest that the patients with pneumonia caused by P. fluorescens should be treated in a timely manner according to the AST results.
Keywords: Pseudomonas fluorescens, Pneumonia, Fever, Lung biopsy, Case report
Background
Pseudomonas fluorescens (P. fluorescens) is a ubiquitous bacterium commonly found in moist environments, such as soil, leaves, and water [1, 2]. As a Gram-negative psychrophile with an optimum growth temperature at 25–30 °C, it is also able to grow at the human body temperature of 37 °C and can present with its virulence factors [3].
P. fluorescens is significantly less virulent than P. aeruginosa and is a rare cause of invasive hospital-acquired infections, with most common site of infection being the bloodstream [4–15]. It has been isolated in respiratory samples from patients with lung transplants [16–18], ventilator-associated pneumonia (VAP) [19], cystic fibrosis (CF) [20–22] and rice-field drowning-associated pneumonia [23].
While P. fluorescens has been identified in human bronchoalveolar lavage fluid (BALF), sputum specimens or throat swabs, its role in pneumonia pathogenesis is unclear. It has been previously suspected of being an aetiologic agent of pneumonia in several reports [19, 24–26], however, the clinical characteristics and drug susceptibility pattern of P. fluorescens pneumonia have rarely been reported [25].
In this case, we report a patient with pneumonia caused by P. fluorescens. By presenting the clinical and antibiotics susceptibility characteristics of this patient, we will provide significant value for basic and clinical research on P. fluorescens infection in the future.
Case presentation
A 67-year-old man was hospitalized in our hospital complaining of a 10-day history of fever, with a temperature up to 38.8˚C. He denied cough, dyspnoea, chills, shivers or chest pain. Before hospitalization, azithromycin was given orally for 5 days and intravenously for 3 days, and levofloxacin was given intravenously for 1 day. However, the patient still had a fever. He had a past medical history of tuberculosis and gastritis. He was allergic to penicillin, cephalosporin and sulfonamide. He had smoked 10 cigarettes a day for more than 20 years. He retired from an office work and had no pneumotoxic exposure.
At admission, physical examination revealed bilateral reduced breath sounds. The patient was conscious but in a poor state of mind. He was emaciated, with a BMI of 16. The remainder of his physical examination was normal. The results of routine blood examination showed that the white blood cell (WBC) count (10,180/mm3 [normal range 3500–95,000/mm3]) and neutrophil (NEU) count (8600/mm3 [normal range 1800–6300/mm3]) were elevated. The erythrocyte sedimentation rate (ESR) (58 mm/h [normal range 0–15 mm/h]) and C-reactive protein (CRP) were increased (96.7 mg/L [normal range 5–10 mg/L]). Albumin was decreased (34.8 g/L [normal range 40–50 g/L]). The chest computed tomography (CT) scan showed scattered patchy high-density nodules with blurred edges in the bilateral lungs. Pleural effusion was present on the right side (Fig. 1A, B).
Fig. 1.
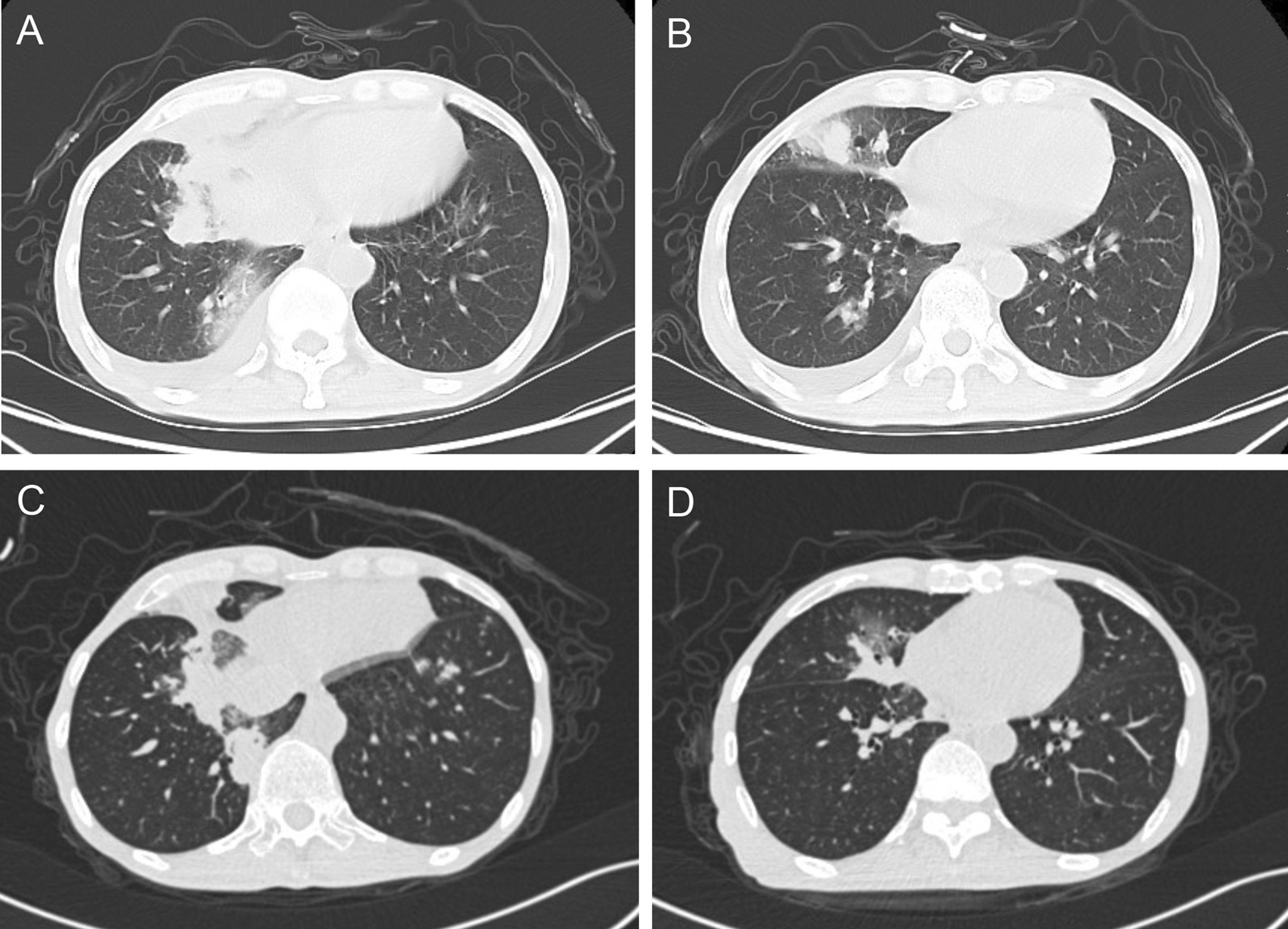
Images of chest computed tomography (CT). A, B Chest CT images during hospitalization showed high-density infiltrate in bilateral lungs and pleural effusion on the right side. C, D Chest CT showed the absorption of the pleural effusion and inflammatory sites in the lungs after treatment
Anti-mycoplasma pneumonia test and T cell spot test of tuberculosis infection (SPOT. TB) were negative. Thyroid function, rheumatism series, immunoglobulin, complement, respiratory system tumour markers, vasculitis antibodies series, and other laboratory results were normal. The rheumatism series included anti-u1RNP Ab, anti-Smith Ab, anti-SSA Ab, anti-SSB Ab, anti-ScL-70 Ab, anti-PM-SCL Ab, anti-Jo-1 Ab, anti-CENP-B Ab, anti-PCNA Ab, anti-HHT Ab, anti-ZDB Ab, anti-HTT Ab, anti-M2 Ab, ANA, anti-dsDNA Ab, ASL, RF, CRP. The respiratory system tumour markers included CEA, CYFRA21-1, SCC, PRO-GRP, CA-125, NSE. The vasculitis antibodies series included cANCA, PR3-cANCA, pANCA, MPO-pANCA, anti-GBM Ab. In addition, cultures of sputum smears were negative for bacteria, fungi or acid-fast bacilli.
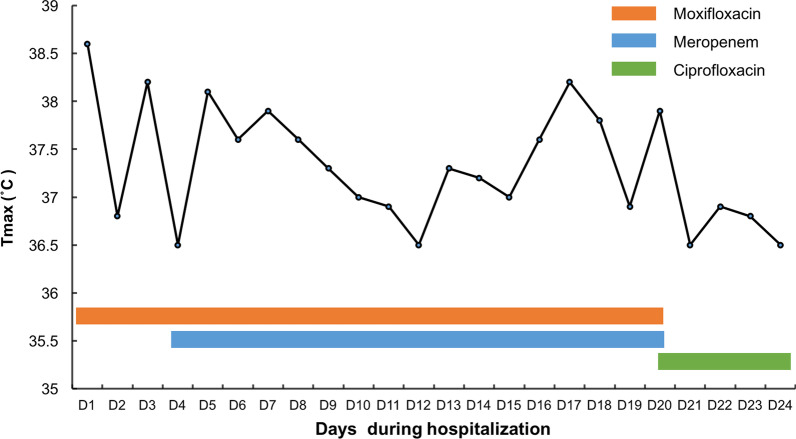
After empiric combination treatment with intravenous moxifloxacin (0.4 g, qd) and meropenem (1 g, q8 h) for approximately 2 weeks, the patient still had a fever (Fig. 2). During this period, hydrotalcite (1 g, tid) was given orally, ambroxol (30 mg, bid) and lansoprazole (30 mg, qd) were also given intravenously for dissolving sputum and protecting stomach. Thymopentin was given intramuscularly (20 mg, qd) for improving immunity. On two occasions, dexamethasone (5 mg, st) was given by intravenous injection under a fever in evening. The patient was in poor nutritional status, with difficulties in sputum excretion, making him at high risk for bronchoscopy. Considering these situations, we decided to perform CT-guided lung puncture biopsy for diagnosis.
Fig. 2.
Temperature and medication timeline. Changes in temperature under antibiotic therapy during hospitalization. Tmax, Temperature maximum
The percutaneous lung puncture biopsy was then performed under the guidance of CT. The lung tissue pathological features displayed acute and chronic inflammation, the proliferation of alveolar cells and fibrous tissue, and the existence of multinucleated giant cells (Fig. 3A). Silver staining of the tissue showed round foreign bodies in foam cells (Fig. 3B). The identification of the organism types in lung tissue was performed by standard biochemical tests using a standard method on a Vitek 2-GN ID card (bioMerieux, Marcy l'Etoile, France). P. fluorescens was identified, and Vitek 2 antibiotic susceptibility testing (AST) of P. fluorescens was performed. The results of AST showed that P. fluorescens was susceptible to ceftazidime, ciprofloxacin, cefepime, amikacin, gentamicin, tobramycin, piperacillin-tazobactam, and levofloxacin. Meanwhile, the P. fluorescens was resistant to ampicillin, cefazolin, imipenem, sulfamethoxazole/trimethoprim, ampicillin/sulbactam, cefotetan, and ceftriaxone (Table 1). According to the susceptibility pattern, moxifloxacin and meropenem were discontinued, and ciprofloxacin (0.4 g, bid) was administered for 4 days. The patient had no fever during treatment with ciprofloxacin (Fig. 2). Then, the patient was discharged and continued to use oral ciprofloxacin.
Fig. 3.
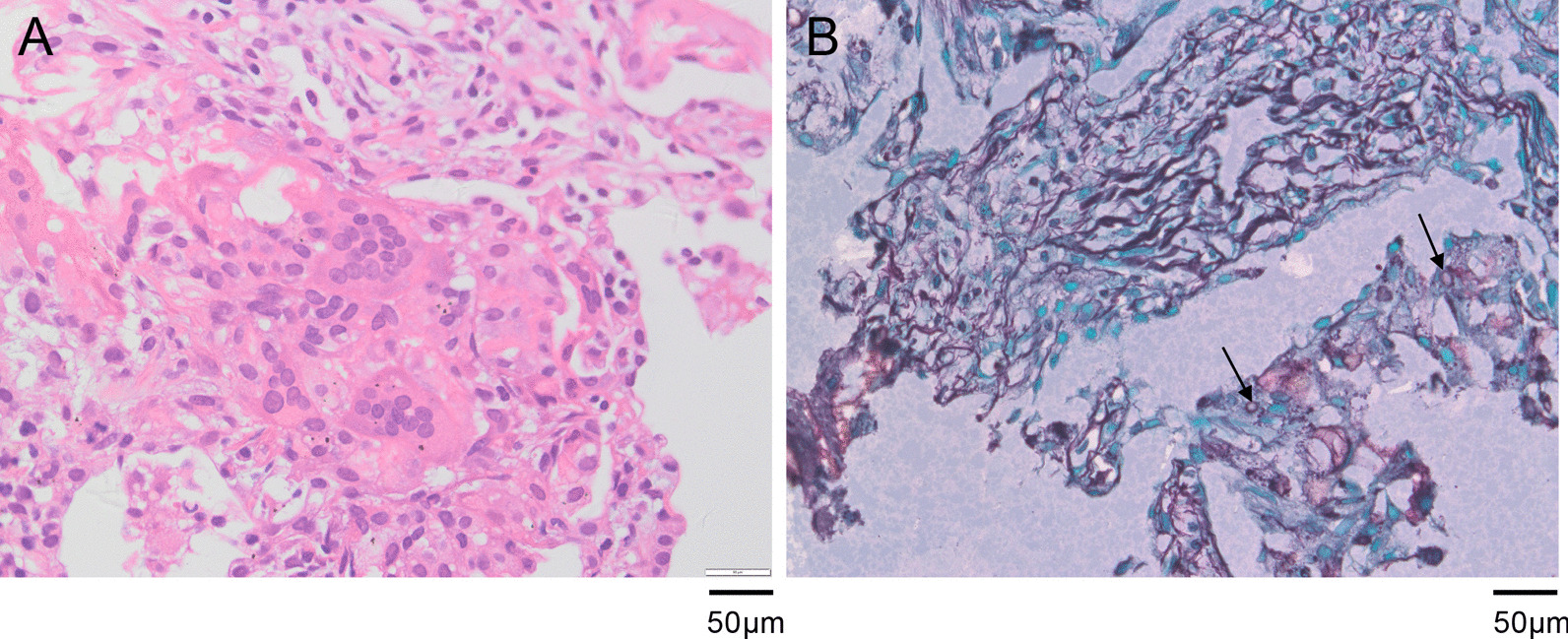
Pathological histology of the biopsy lung tissue. A Haematoxylin–eosin staining of lung tissue pathological features showed acute and chronic inflammation, the proliferation of alveolar cells and fibrous tissue, and the existence of multinucleated giant cells. B Silver staining of the tissue showed round foreign bodies in foam cells (original magnification, × 400)
Table 1.
The antimicrobial susceptibility testing results of P. fluorescens
| Antibiotics | MIC values (μg/ml) | Interpretation |
|---|---|---|
| Ampicillin | ≥ 32 | R |
| Cefazolin | ≥ 64 | R |
| Ceftazidime | 8 | S |
| Ciprofloxacin | ≤ 0.25 | S |
| Imipenem | ≥ 16 | R |
| Sulfamethoxazole/trimethoprim | 80 | R |
| Cefepime | 4 | S |
| Amikacin | ≤ 2 | S |
| Ampicillin/sulbactam | ≥ 32 | R |
| Cefotetan | ≥ 64 | R |
| Ceftriaxone | ≥ 64 | R |
| Gentamicin | ≤ 1 | S |
| Tobramycin | ≤ 1 | S |
| Piperacillin-tazobactam | ≤ 4 | S |
| Levofloxacin | 0.5 | S |
Susceptibility cards were inoculated and interpreted according to the Clinical and Laboratory Standards Institute (CLSI) breakpoints.
P. fluorescens Pseudomonas fluorescens, MIC minimum inhibitory concentration, R resistant, S sensitive.
The patient had a return visit one month later. He denied clinical symptoms. The WBC and NEU counts of his blood samples were in the normal range. Chest CT showed the absorption of the pleural effusion and the inflammatory sites in the lungs (Fig. 1C, D). Six months later, the patient returned again and bronchoscopy was performed. No bacteria, acid-fast bacilli, fungi or spores was found in the bronchial brushings and BALF specimen.
Discussion and conclusion
Here, we report a case of pneumonia caused by P. fluorescens. P. fluorescens was cultured from the biopsy lung tissue of this patient. Based on the AST results of P. fluorescens, the condition of this patient improved in response to ciprofloxacin therapy. In previous studies of P. fluorescens, the clinical samples included sputum, BALF or throat swabs. In contrast to previous reports, we report P. fluorescens cultured from lung biopsy specimens for the first time.
The roles of P. fluorescens in pneumonia or other respiratory diseases pathogenesis are undefined. The clinical features of P. fluorescens-associated pneumonia have rarely been reported [25]. In the case of a myasthenic patient [25], during recovery from a recent polymicrobial peritonitis, he developed clinical evidence of pneumonia, with sputum cultures that were positive for P. fluorescens. Prior to the pneumonia, this 55-year-old patient received the treatment with intravenous cefuroxime and metronidazole, and mechanical ventilation. The P. fluorescens was sensitive to amikacin, gentamicin, tobramycin, netilmicin, piperacillin, ticarcillin, latamoxef and ceftazidime. He recovered after the therapy of metronidazole supplemented with intravenous ceftazidime. P. fluorescens was also mentioned in the aetiology of community-acquired pneumonia in a single case, and the P. fluorescens did not respond to therapy with ceftriaxone, but the clinical details were lacking [26].
In most previous studies, it is unclear whether the positive sputum or BALF culture results reflected acute infection or benign colonization of patients. Using amplification of bacterial 16S rRNA genes, P. fluorescens and other bacteria was detected in the BALF acquired from a single patient with VAP [19]. In another study of over 1,000 respiratory cultures acquired from patients with CF, the authors identified P. fluorescens in approximately 2% of samples and considered the organism a colonizer rather than an acute pathogen [22]. P. fluorescens was also identified in a single patient with CF and new lower airways infection [20]. In a survey of lung transplant recipients, P. fluorescens was frequently identified in BALF samples of asymptomatic recipients by pyrosequencing, but not detected via standard bacterial culture [18].
In their survey [18], researchers also searched the database of bacterial culture isolates from the University of Michigan Clinical Microbiology Laboratory. Over an 11-year period, P. fluorescens was cultured from over 240 distinct respiratory specimens, including sputum, throat swabs, and bronchoscopically-obtained specimens (BALF or brushings). Among patients with positive P. fluorescens respiratory cultures, the most common underlying pulmonary condition was CF (38.8% of all isolates), followed by other chronic airway diseases (COPD, asthma, and non-CF bronchiectasis [16.1%]) and lung transplantation (7.4%). In addition, 26 of these specimens were obtained from patients with suspected acute pneumonia, and 22 of these patients were chronically immunosuppressed or had recent healthcare exposures meeting criteria for healthcare-associated pneumonia.
In our report, the patient had none known risk factor for P. fluorescens colonization or infection, including ventilator usage, lung transplantation, CF, immunosuppression, or other chronic airway diseases. However, it is worth noting that this eldly patient was thin and had low albumin level, with a smoking history of more than 20 years. And he received the treatment with multiple antibiotics before lung biopsy.
The patient presented with a fever and radiographic lung infiltrate. Laboratory examinations revealed elevated WBC and NEU counts. He was in poor nutritional status, with difficulties in sputum excretion, making him at high risk for bronchoscopy. Therefore, the CT-guided lung puncture biopsy was performed for diagnosis. P. fluorescens was cultured from lung biopsy specimens. The clinical symptoms, CT and laboratory test results, pathologic findings, and treatment response to ciprofloxacin provide evidence of P. fluorescens infection in our case. The pathohistological diagnosis of the biopsy provided meaning guidance for a clinical diagnosis, including the exclusion of tumours, granulomatous diseases, TB infection, fungal infection, etc. However, pathological observation cannot identify the type of bacterial pathogens. More importantly, the results of tissue culture and drug sensitivity tests played an important role in guiding the use of antibiotics to treat this patient.
In the AST, P. fluorescens was resistant to multiple antibiotics, which may be the reason for the poor efficacy of initial empirical therapy. Notably, the antimicrobial susceptibility results of our P. fluorescens isolates were in agreement with known findings as described above [25, 26]. They were resistant to ceftriaxone, and sensitive to amikacin, gentamicin, tobramycin, ceftazidime and piperacillin. However, the previous studies relating antimicrobial susceptibility characteristics were early and few, the coverage of antibiotics was relatively narrow. The antimicrobial susceptibility patterns of P. fluorescens pneumonia need to be further summarized and clarified.
In summary, P. fluorescens can cause acute pneumonia, with fever as the main clinical symptom. When encountering patients with pneumonia presenting with poor efficacy of empiric antibiotic treatment, we should consider the possibility of P. fluorescens infection. In addition, it is important to perform the bacterial culture and AST in a timely manner. Antibiotic therapy under the guidance of the P. fluorescens antimicrobial spectrum is significant for such patients. However, more research is needed to study the pathogenesis of P. fluorescens and to establish diagnostic criteria and effective treatment of these cases.
Acknowledgements
Not applicable.
Abbreviations
- P. fluorescens
Pseudomonas fluorescens
- CF
Cystic fibrosis
- VAP
Ventilator-associated pneumonia
- BALF
Bronchoalveolar lavage fluid
- WBC
White blood cell
- NEU
Neutrophil
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- SPOT.TB
T cell spot test of tuberculosis infection
- CT
Chest computed tomography
- Ab
Antibody
- u1RNP
U1-ribonucleoprotein
- SSA
Sjögren’s syndrome A
- SSB
Sjögren’s syndrome B
- ScL-70
Topoisomerase I
- PM-SCL
Polymyositis-sclerosis
- Jo-1
Cytoplasmic aminoacyl-tRNA synthetase
- CENP-B
Centromere protein B
- PCNA
Proliferating cell nuclear antigen
- HHT
Nucleosome
- ZDB
Histone
- HTT
Ribosomal P protein
- M2
Mitochondrial antibody subtype M2
- ANA
Anti-nuclear Ab
- dsDNA
Double-stranded DNA
- ASL
Anti-streptolysin O
- RF
Rheumatoid factors
- CRP
C-reactive protein
- CEA
Carcinoembryonic antigen
- CYFRA21-1
Cytokeratin 19 fragment antigen 21-1
- SCC
Squamous cell carcinoma antigen
- PRO-GRP
Pro-gastrin releasing peptide
- CA-125
Carbohydrate antigen 125
- NSE
Neuron-specific enolase
- cANCA
Cytoplasmic anti-neutrophil cytoplasmic antibody
- PR3
Proteinase-3
- pANCA
Perinuclear anti-neutrophil cytoplasmic antibody
- MPO
Myeloperoxidase
- GBM
Glomerular basement membrane
- COPD
Chronic obstructive pulmonary disease
Authors' contributions
YQ conceived and designed the work. XL integrated the data and wrote the manuscript. LX performed the histological examination of the lung tissue. YY collected the CT images of the case. HL, DM and YQ critically revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Consent for publication
Written informed consent for publication was obtained from the participant.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Silby MW, Cerdeño-Tárraga AM, Vernikos GS, Giddens SR, Jackson RW, Preston GM, et al. Genomic and genetic analyses of diversity and plant interactions of Pseudomonas fluorescens. Genome Biol. 2009;10(5):R51. doi: 10.1186/gb-2009-10-5-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci USA. 2009;106(38):16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin Microbiol Rev. 2014;27(4):927–948. doi: 10.1128/CMR.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibaud M, Martin-Dupont P, Dominguez M, Laurentjoye P, Chassaing B, Leng B. Pseudomonas fluorescens septicemia following transfusion of contaminated blood. Presse Med (Paris, France: 1983) 1984;13(42):2583–2584. [PubMed] [Google Scholar]
- 5.Hsueh PR, Teng LJ, Pan HJ, Chen YC, Sun CC, Ho SW, et al. Outbreak of Pseudomonas fluorescens bacteremia among oncology patients. J Clin Microbiol. 1998;36(10):2914–2917. doi: 10.1128/JCM.36.10.2914-2917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khabbaz RF, Arnow PM, Highsmith AK, Herwaldt LA, Chou T, Jarvis WR, et al. Pseudomonas fluorescens bacteremia from blood transfusion. Am J Med. 1984;76(1):62–68. doi: 10.1016/0002-9343(84)90751-4. [DOI] [PubMed] [Google Scholar]
- 7.Murray AE, Bartzokas CA, Shepherd AJ, Roberts FM. Blood transfusion-associated Pseudomonas fluorescens septicaemia: is this an increasing problem? J Hosp Infect. 1987;9(3):243–248. doi: 10.1016/0195-6701(87)90120-4. [DOI] [PubMed] [Google Scholar]
- 8.Scott J, Boulton FE, Govan JR, Miles RS, McClelland DB, Prowse CV. A fatal transfusion reaction associated with blood contaminated with Pseudomonas fluorescens. Vox Sang. 1988;54(4):201–204. doi: 10.1111/j.1423-0410.1988.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 9.Benito N, Mirelis B, Luz Gálvez M, Vila M, López-Contreras J, Cotura A, et al. Outbreak of Pseudomonas fluorescens bloodstream infection in a coronary care unit. J Hosp Inf. 2012;82(4):286–289. doi: 10.1016/j.jhin.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Pseudomonas bloodstream infections associated with a heparin/saline flush--Missouri, New York, Texas, and Michigan, 2004–2005. MMWR Morb Mortal Wkly Rep. 2005;54(11):269–72. [PubMed]
- 11.Update: Delayed onset Pseudomonas fluorescens bloodstream infections after exposure to contaminated heparin flush--Michigan and South Dakota, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55(35):961–3. [PubMed]
- 12.Gershman MD, Kennedy DJ, Noble-Wang J, Kim C, Gullion J, Kacica M, et al. Multistate outbreak of Pseudomonas fluorescens bloodstream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin Inf Dis. 2008;47(11):1372–1379. doi: 10.1086/592968. [DOI] [PubMed] [Google Scholar]
- 13.Sarubbi FA, Jr, Wilson B, Lee M, Brokopp C. Nosocomial meningitis and bacteremia due to contaminated amphotericin B. JAMA. 1978;239(5):416–418. doi: 10.1001/jama.1978.03280320032015. [DOI] [PubMed] [Google Scholar]
- 14.Arega B, Wolde-Amanuel Y, Adane K, Belay E, Abubeker A, Asrat D. Rare bacterial isolates causing bloodstream infections in Ethiopian patients with cancer. Inf Agents Cancer. 2017;12:40. doi: 10.1186/s13027-017-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SS, Kagen J, Lautenbach E, Bilker WB, Matro J, Dominguez TE, et al. Bloodstream infections after median sternotomy at a children's hospital. J Thorac Cardiovasc Surg. 2007;133(2):435–440. doi: 10.1016/j.jtcvs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 16.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol. 2014;52(10):3605–3613. doi: 10.1128/JCM.01028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Cell-associated bacteria in the human lung microbiome. Microbiome. 2014;2:28. doi: 10.1186/2049-2618-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, et al. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS ONE. 2014;9(5):e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahrani-Mougeot FK, Paster BJ, Coleman S, Barbuto S, Brennan MT, Noll J, et al. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007;45(5):1588–1593. doi: 10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heirali A, McKeon S, Purighalla S, Storey DG, Rossi L, Costilhes G, et al. Assessment of the microbial constituents of the home environment of individuals with cystic fibrosis (CF) and their association with lower airways infections. PLoS ONE. 2016;11(2):e0148534. doi: 10.1371/journal.pone.0148534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scales BS, Erb-Downward JR, Huffnagle IM, LiPuma JJ, Huffnagle GB. Comparative genomics of Pseudomonas fluorescens subclade III strains from human lungs. BMC Genom. 2015;16:1032. doi: 10.1186/s12864-015-2261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger JD, Thomassen MJ. Occurrence and antimicrobial susceptibility of gram-negative nonfermentative bacilli in cystic fibrosis patients. Diagn Microbiol Inf Dis. 1985;3(2):149–158. doi: 10.1016/0732-8893(85)90025-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamawaki S, Nakashima K, Suzuki F, Otsuki A, Watanabe J, Takai M, et al. Rice-field drowning-associated pneumonia in which Pseudomonas spp., Aspergillus fumigatus, and Cunninghamella sp. are isolated. Internal Med (Tokyo, Japan). 2016;55(7):825–829. doi: 10.2169/internalmedicine.55.4454. [DOI] [PubMed] [Google Scholar]
- 24.Redding PJ, McWalter PW. Pseudomonas fluorescens cross-infection due to contaminated humidifier water. Br Med J. 1980;281(6235):275. doi: 10.1136/bmj.281.6235.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thangkhiew I. Successful treatment with ceftazidime of a Pseudomonas fluorescens chest infection in a myasthenic patient. J Antimicrob Chemother. 1986;18(3):428–429. doi: 10.1093/jac/18.3.428. [DOI] [PubMed] [Google Scholar]
- 26.Zervos M, Nelson M. Cefepime versus ceftriaxone for empiric treatment of hospitalized patients with community-acquired pneumonia. The Cefepime Study Group. Antimicrob Agents Chemotherapy. 1998;42(4):729–733. doi: 10.1128/AAC.42.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.