Abstract
Objectives
Guidelines recommend to initiate anticoagulation within 4-14 days after cardioembolic stroke. Data supporting this did not account for key factors potentially affecting the decision to initiate anticoagulation such as infarct size, hemorrhagic transformation, or high risk features on echocardiography.
Methods
We pooled data from stroke registries of 8 comprehensive stroke centers across the United States. We included consecutive patients admitted with ischemic stroke and atrial fibrillation. The primary predictor was timing of initiating anticoagulation (0-3 days, 4-14 days, or >14 days) and outcomes were recurrent stroke/TIA/systemic embolism, symptomatic intracerebral hemorrhage (sICH), and major extracranial hemorrhage (ECH) within 90 days.
Results
Among 2084 patients, 1289 met the inclusion criteria. The combined endpoint occurred in 10.1% (n = 130) subjects (87 ischemic events, 20 sICH, and 29 ECH). Overall, there was no significant difference in the composite endpoint between the three groups: 0-3 days [10.3% (64/617)], 4-14 days [(9.7%) 52/535)], >14 days [10.2% (14/137), p=0.933]. In adjusted models, patients started on anticoagulation between 4-14 days did not have a lower rate of sICH (vs. 0-3 days) (OR 1.49 95% CI 0.50 – 4.43) neither did they have a lower rate of recurrent ischemic events (vs. > 14 days) (OR 0.76 95% CI 0.36 – 1.62, p = 0.482).
Interpretation
In this multicenter real world cohort, the recommended (4-14 days) time frame to start oral anticoagulation was not associated with reduced ischemic and hemorrhagic outcomes. Randomized trials are required to determine the optimal timing of anticoagulation initiation.
Keywords: stroke, atrial fibrillation, anticoagulation
Introduction
Cardioembolic stroke in the setting of atrial fibrillation (AF) is associated with high risk of mortality and morbidity1 and carries a relatively high recurrence rate in the first 90 days.2 Studies have shown that early anticoagulation relates to an increased risk of hemorrhagic complications without any reduction in ischemic events.3–5 Current guidelines from the American Heart Association recommend to initiate anticoagulation after cardioembolic stroke within 4-14 days from the index event.6 However, the data supporting this suggestion stems from observational studies that did not take into account some of the circumstances that may impact the clinical decision to start anticoagulation after cardioembolic stroke.7–10 These include factors that may lead to delaying initiation such as large ischemic lesion size or early hemorrhagic transformation and conditions that may lead to early initiation such as the presence of a cardiac thrombus.7–10 In addition, because in most of the previous studies, warfarin was the most commonly used anticoagulant it is uncertain whether results can be extrapolated to direct oral anticoagulants (DOAC). 7–10 Therefore, it is of paramount importance to provide real world data on the optimal timing of anticoagulation initiation that are more reflective of current practice parameters.
In this study, we sought to determine the association between the time to start anticoagulation after a cardioembolic stroke and hemorrhagic and ischemic outcomes at 90 days using real world data.
Methods
Study sample
The Initiation of Anticoagulation after Cardioembolic stroke (IAC) study is a multicenter retrospective study that pooled data from stroke registries of 8 comprehensive stroke centers across the United States within the years 2015 and 2018. We included consecutive patients admitted with acute ischemic stroke in the setting of AF. Institutional Review Board approval was obtained from each of the participating centers.
Primary Predictor and Outcome
The primary predictor was timing of initiating anticoagulation (0-3 days, 4-14 days, or >14 days) confirmed by chart review. The primary endpoint was the composite endpoint of recurrent ischemic stroke, TIA, and systemic arterial embolism, as well as symptomatic intracerebral hemorrhage (sICH), or major extracranial hemorrhage (ECH) within 90 days. Symptomatic intracranial hemorrhage was defined as neurological deterioration in the setting of any new or worsening hemorrhage and the hemorrhage being the cause of the neurological deterioration and ECH was defined as major extracranial hemorrhage requiring blood transfusion, associated with a 2 g/dL decrease in hemoglobin level, or symptomatic bleeding in a critical area or organ.7 sICH and ECH occurring prior to anticoagulation initiation were not considered as outcomes. Other outcomes were defined using previous definitions.7
In all participating centers, patients discharged with a diagnosis of stroke were scheduled to have an in-person clinic visit at 90 days. In addition, in 3 out of 8 centers, pre-specified phone calls were performed at the 30-day (in one center) and 90-day (in two centers) time points that assessed for recurrent ischemic and hemorrhagic outcomes. Outcomes were preferentially abstracted from the 90-day patient follow up visit and pre-specified 90-day phone calls. In the sites assessing 90-day outcomes by phone and for patients not showing up to their 90 day visits, three attempts were made on different occasions to contact the patient or health care provider by phone. If unsuccessful, then outcomes were assessed by chart review of hospitalization and other outpatient visit and outside hospital records. All outcomes were abstracted by the study local research assistant and confirmed by the site principal investigator. Multiple queries were sent to the participating sites regarding study outcomes and other variables in our dataset and several data cross-checks were performed to confirm the integrity of the data sent by individual sites.
Co-variates
Clinical co-variates included: demographic factors (age and sex), clinical variables (hypertension, diabetes, hyperlipidemia, prior stroke, congestive heart failure, coronary artery disease, CHA2DS2-Vasc score, and admission NIHSS score).
Ancillary testing covariates included: neuroimaging and vascular imaging variables (presence of intracranial or extracranial stenosis atherosclerosis with ≥ 50% luminal narrowing in the territory of the stroke, largest ischemic stroke lesion volume determined on brain MRI or CT if MRI was not obtained using the a x b x c/2 method11, hemorrhagic transformation on 24 hour brain imaging, MRI or CT, categorized as hemorrhagic infarction or parenchymal hematoma12), and echocardiographic variables (moderate to severe aortic or mitral valvular heart disease and the presence of intracranial thrombus). The choice of brain imaging at baseline (CT vs. MRI) was at the discretion of the treating physician.
We also included in-hospital treatments: type of oral anticoagulant used (warfarin vs. DOAC), whether bridging treatment with heparin or low molecular weight heparin was performed, alteplase treatment, and mechanical thrombectomy treatment.
Analytical plan
Data from sites was pooled and queries were sent to assure accuracy of data, as indicated. Patients who did not meet a study endpoint but died within 90 days were excluded. In addition, patients lost to follow up, who were not started on anticoagulation, and whose time to starting any anticoagulation could not be confirmed were excluded. We then divided patients into two groups (primary outcome vs. no-primary outcome) and compared the rates of the primary endpoint based on time to initiate anticoagulation (0-3 days, 4-14 days, and >14 days). We performed binary logistic regression analyses to determine the association between time to initiate anticoagulation and ischemic events (stroke/TIA/systemic embolism) and sICH, adjusting for potential prespecified confounders based on the outcome of interest. For the ischemic event outcome, we adjusted for factors that could possibly increase the risk of ischemic events such as valvular heart disease13, prior stroke14, cardiac thrombus15, and CHA2DS2Vasc score.16 For the sICH outcome, we adjusted for factors that could possibly increase the risk of sICH such as infarct size, asymptomatic hemorrhage, and bridging with therapeutic heparin or low molecular weight heparin.7, 17 In addition, we included an additional logistic regression model adjusting for baseline differences in the time to initiation of anticoagulation groups. Analysis was done using SPSS version 25.0 (Chicago, IL) and a p-value < 0.05 was considered statistically significant.
Additionally, to account for potential confounding owing to any unmeasured competing risk factors, we performed a sensitivity analysis including participants who died or were lost to follow-up within 90 days. For this, we performed weighted Cox proportional hazards regression analysis with adjustments for potential imbalances between the group assignments to the timing of the start of anticoagulation using inverse probability of treatment weighting (IPTW) and by treating the timing of the initiation of anticoagulation as a time-varying covariate. We applied the cohort propensity score (PS) method in subgroup analyses. The IPTW method was used as the primary strategy to adjust the baseline imbalances. We used SAS version 9.4 (SAS Institute Inc., Cary, NC) to perform IPTW Cox proportional hazards regression.
Results
We included 2084 patients from 8 comprehensive centers in the United States [28.3% (590/2084) received alteplase and 26.2% (547/2084) mechanical thrombectomy, 1289 met the inclusion criteria (369 were excluded from the primary analyses due to non-composite endpoint related death within 90 days, 195 were lost to follow up at 90 days, and 231 were not started on oral anticoagulation (n = 216) or the timing was not reported (n = 15) (Figure 1). Of included patients 872 received DOAC treatment, 399 received warfarin treatment, and 203 received bridging therapy with heparin or low molecular weight heparin. Therapeutic dose anticoagulation was initiated in 0-3 days in 47.9% (617/1289), 4-14 days in 41.5% (535/1289), and >14 days in 10.6% (137/1329). Supplementary table 1 compares baseline characteristics of patients who survived or had a study outcome in the first 90 days with follow-up available and timing of initiation of anticoagulation available (if started) vs. those who were started on anticoagulation and the timing is known but died within 90 days (n = 82) or were lost to follow up (n = 145). Among 216 patients who were not started on anticoagulation, 17 (7.9%) had recurrent ischemic events and 1 (0.5%) had a sICH. Baseline characteristics of patients stratified according to the time of initiation of anticoagulation are shown in Table 1.
Figure 1:
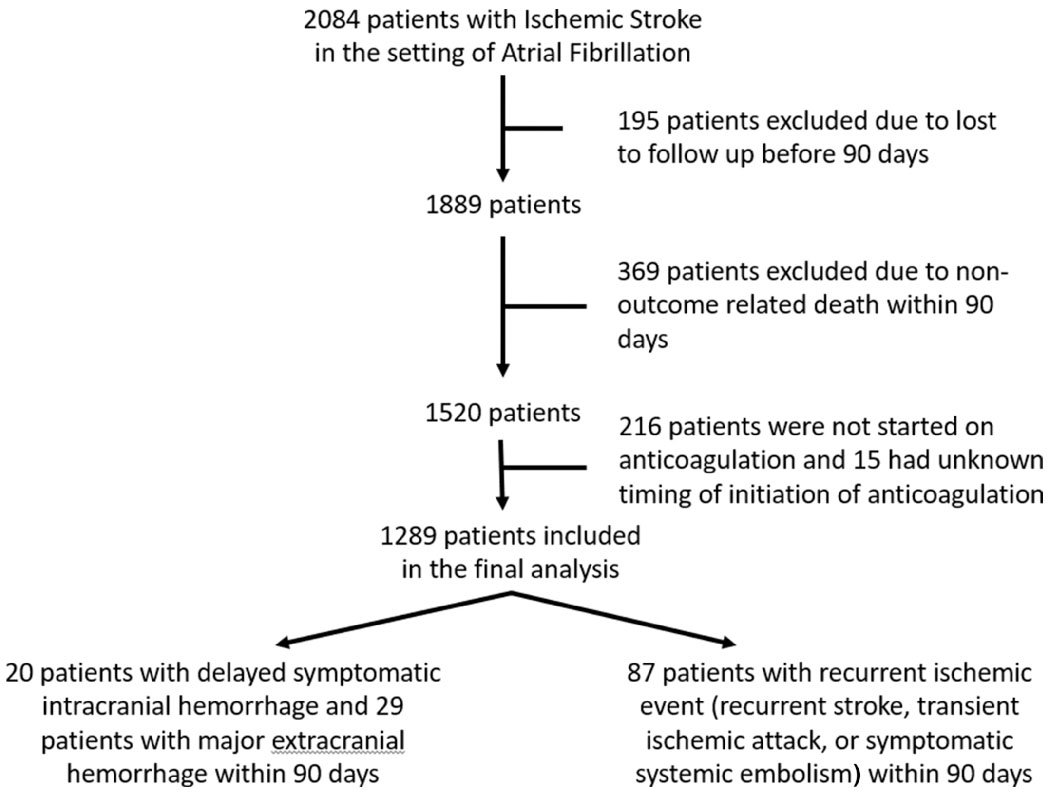
Study flow chart
TABLE 1.
Baseline Characteristics of Patients Based on Time to Initiation of Anticoagulation
| Characteristic | Time to Initiation = 0–3 Days, n = 617 | Time to Initiation 4–14 Days, n = 535 | Time to Initiation >14 Days, n = 137 | p |
|---|---|---|---|---|
| Age, yr, median (IQR) | 77 (17) | 78 (17) | 76 (18) | 0.275 |
| Sex, n (% men) | 310 (50.2%) | 261 (48.8%) | 78 (56.9%) | 0.234 |
| Hypertension, n (%) | 532 (86.2%) | 435 (81.3%) | 112 (81.8%) | 0.064 |
| Diabetes, n (%) | 212 (34.4%) | 174/534 (32.6%) | 46 (33.6%) | 0.817 |
| Hyperlipidemia, n (%) | 369 (59.8%) | 307 (57.4%) | 77 (56.2%) | 0.606 |
| Prior ischemic stroke, n (%) | 191 (31.0%) | 111 (20.8%) | 36 (26.2%) | <0.001a |
| Congestive heart failure, n (%) | 178/610 (29.2%) | 117/528 (22.2%) | 29/135 (21.5%) | 0.014a |
| Coronary artery disease, n (%) | 216 (35.0%) | 151 (28.2%) | 46 (34.1%) | 0.045a |
| CHA2DS2-Vasc score, median (IQR) | 5 (2) | 4 (3) | 4 (3) | 0.072 |
| NIHSS score, median (IQR) | 5 (9) | 10 (13) | 15 (12) | <0.001a |
| Cardiac thrombus, n (%) | 15/578 (2.6%) | 3/508 (0.6%) | 0/134 (0%) | 0.008a |
| Valvular heart disease, n (%) | 148/578 (25.6%) | 100/508 (19.7%) | 23/134 (17.2%) | 0.021a |
| Stroke lesion ≥60ml, n (%) | 33/558 (5.9%) | 88/505 (17.4%) | 44/134 (32.8%) | <0.001a |
| Hemorrhagic transformation, n (%) | 53 (8.6%) | 100 (18.7%) | 67 (48.9%) | <0.001a |
| Direct oral anticoagulant use, n (%) | 360 (58.3%) | 409 (76.4%) | 103 (75.2%) | <0.001a |
Statistically significant.
IQR = interquartile range; NIHSS = National Institutes of Health Stroke Scale.
Univariate analyses
The combined endpoint occurred in 10.1% (n = 130) subjects (87 ischemic events, 20 sICH, and 29 ECH). Overall, there was no significant difference in the composite endpoint between the three groups: 0-3 days [10.3% (64/617)], 4-14 days [(9.7%) 52/535)], >14 days [10.2% (14/137), p=0.933]. When analyzed separately, recurrent ischemic events did not differ across the 3 groups: 0-3 days [7.3% (45/617), 4-14 days [(6.0%) 32/535)], >14 days [7.2% (10/137)], p=0.651. Likewise, there was no significant difference in the occurrence of anticoagulation related sICH between the 3 groups: 0-3 days [1.1% (7/617)], 4-14 days [1.7% (9/535)], >14 days [2.9% (4/137)], p=0.295.
Association of starting anticoagulation at 4-14 days with recurrent ischemic events
The time to initiation of anticoagulation was not significantly different between those with vs. without ischemic events (median, IQR) [3 (8) vs. 4(8), p = 0.358). Figure 2 shows the Kaplan Meier curve of sICH for each of the initiation time categories, showing no significant difference between the three groups.
Figure 2:
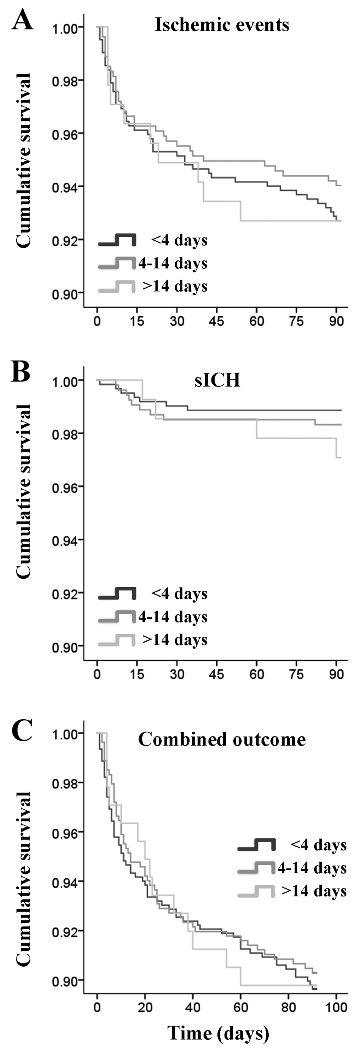
Kaplan Meier survival analysis of time to start anticoagulation categories versus recurrent ischemic event (A), symptomatic intracranial hemorrhagic (B), and combined events (C).
In unadjusted models, anticoagulation initiated at 4-14 days was not associated with a reduction in ischemic events (OR 0.81 95% CI 0.52 – 1.27, p = 0.355). These findings persisted after adjusting for potential confounders (bridging, CHA2DS2-Vasc, cardiac thrombus, prior stroke, and valvular heart disease) (adjusted OR 0.82 95% CI 0.51 – 1.32, p = 0.415) (Table 2). In addition, patients started on anticoagulation between 4-14 days did not have a lower rate of sICH compared to the 0-3 day interval (adjusted OR 1.49 95% CI 0.50 – 4.43) neither did they have a lower rate of recurrent ischemic events as compared to initiation after 14 days (adjusted OR 0.76 95% CI 0.36 – 1.62, p = 0.482).
TABLE 2.
Associations between Ischemic Events, sICH, and Combined Outcomes across Different Time Categories in Univariate or Multivariate Models
| Time Category | Ischemic Event, OR (95% CI), p | sICH, OR (95% CI), p | Combined, OR (95% CI), p |
|---|---|---|---|
| 4–14 days | 0.81 (0.52–1.27), 0.355 | 1.16 (0.48–2.81), 0.749 | 0.93 (0.65–1.35), 0.713 |
| 0.82 (0.51–1.32), 0.415 | 1.24 (0.49–3.13), 0.648 | 0.93 (0.61–1.39), 0.707 | |
| 0.90 (0.54–1.51), 0.693a | 0.91 (0.35–2.37), 0.841 | 0.89 (0.59–1.35), 0.570a | |
| 4–7 days | 0.92 (0.54–1.56), 0.756 | 1.43 (0.54–3.75), 0.470 | 0.99 (0.65–1.53), 0.973 |
| 0.94 (0.54–1.63), 0.815 | 1.54 (0.57–4.20), 0.397 | 0.96 (0.59–1.55), 0.863 | |
| 0.89 (0.49–1.64), 0.717a | 1.30 (0.43–3.89), 0.642 | 0.93 (0.57–1.51), 0.758a | |
| 7–10 days | 0.64 (0.33–1.26), 0.200 | 0.56 (0.13–2.43), 0.439 | 0.64 (0.36–1.11), 0.114 |
| 0.64 (0.31–1.30), 0.217 | 0.72 (0.16–3.30), 0.672 | 0.71 (0.39–1.27), 0.250 | |
| 0.76 (0.37–1.58), 0.463a | 0.59 (0.13–2.70), 0.499 | 0.68 (0.38–1.24), 0.212a | |
| 10–14 days | 0.83 (0.43–1.59), 0.568 | 0.64 (0.15–2.78), 0.550 | 0.92 (0.55–1.56), 0.762 |
| 0.83 (0.42–1.65), 0.597 | 0.66 (0.14–3.04), 0.592 | 0.95 (0.54–1.69), 0.867 | |
| 1.06 (0.52–2.17), 0.872a | 0.47 (0.10–2.18), 0.336 | 0.93 (0.52–1.66), 0.804a |
The first row in each category is unadjusted and the second and third rows are adjusted for covariates.
In the second row, ischemic event is adjusted for moderate or severe aortic or mitral valvular heart disease, prior stroke, cardiac thrombus, and CHA2DS2Vasc. sICH is adjusted for largest ischemic lesion size (<60ml vs ≥60ml), early hemorrhagic transformation, and bridging with heparin or low–molecular-weight heparin. Combined outcome is adjusted for moderate to severe aortic or mitral valvular heart disease, prior stroke, cardiac thrombus, CHA2DS2Vasc, largest ischemic lesion size (<60ml vs ≥60ml), early hemorrhagic transformation, and bridging therapy with heparin or low–molecular-weight heparin.
In the third row, data are adjusted for prior stroke, coronary artery disease, congestive heart failure, National Institutes of Health Stroke Scale score, moderate or severe aortic or mitral valvular heart disease, cardiac thrombus, largest ischemic lesion size (<60ml vs ≥60ml), early hemorrhagic transformation, and direct oral anticoagulant use.
CI = confidence interval; OR = odds ratio; sICH = symptomatic intracerebral hemorrhage.
Association of starting anticoagulation at 4-14 days with symptomatic intracranial hemorrhage
The time to initiation of anticoagulation was not significantly different between those with vs. without sICH (median, IQR) [5 (11) vs. 4 (8), p = 0.279). Figure 2 shows the Kaplan Meier curve of sICH for each of the initiation time categories, showing no significant difference between the three groups.
In unadjusted models, anticoagulation initiated at 4-14 days was not associated with a reduction in sICH (OR 1.16 95% CI 0.48 – 2.81, p = 0.749). These findings persisted after adjusting for potential confounders (bridging, asymptomatic hemorrhage on 24 hour brain imaging, and stroke size) (adjusted OR 1.24 95% CI 0.49 – 3.13, p = 0.648) (Table 2).
Timing of initiation and recurrent ischemic events, sICH, and combined outcome
To better determine whether there is an optimal time to start anticoagulation among patients started 4-14 days from their index stroke, we further divided this category into: time to start anticoagulation 4-7 days, time to start anticoagulation 7-10 days, and time to start anticoagulation 10-14 days. In these analyses, the results remained unchanged with the associations between combined outcome, ischemic events, sICH and all different time intervals for initiating anticoagulation (4-7 days, 7-10 days, and 10-14 days) not achieving statistical significance (Table 3).
TABLE 3.
Inverse Probability of Treatment-Weighted Cox Regression Models for the Primary Outcome Events between Different Time Categories for All Participants (Including Those Who Died or Were Lost to Follow-up Within 90 Days; n = 1,516) and by Treating the Timing of the Initiation of Anticoagulation as a Time-Varying Covariate
| Ischemic Event, HRa (95% CI), p | sICH, HRb (95% CI), p | Combined, HRc(95% CI), p | |
|---|---|---|---|
| 4–14 days | 0.79 (0.51–1.22), 0.291 | 1.23 (0.55–2.79), 0.610 | 0.99 (0.70–1.43), 0.361 |
| 4–7 days | 0.93 (0.55–1.54), 0.752 | 1.39 (0.62–3.09), 0.412 | 0.94 (0.62–1.43), 0.761 |
| 7–10 days | 0.60 (0.30–1.20), 0.154 | 0.61 (0.24–1.34), 0.341 | 0.58 (0.31–1.14), 0.078 |
| 10–14 days | 0.90 (0.48–1.68), 0.761 | 0.71 (0.20–2.68), 0.562 | 0.86 (0.51–1.32), 0.801 |
Probability of assignment to different timing of the start of anticoagulation in each model was calculated using propensity scores accounting for the following.
For ischemic event, moderate to severe aortic or mitral valvular heart disease, prior stroke, cardiac thrombus, CHA2DS2Vasc.
For sICH, large ischemic lesion size (<60ml vs ≥60ml), early hemorrhagic transformation, and bridging therapy with heparin or low–molecular-weight heparin.
For combined outcome, moderate to severe aortic or mitral valvular heart disease, prior stroke, cardiac thrombus, CHA2DS2Vasc, stroke size (<60ml vs ≥60ml), early hemorrhagic transformation, and bridging therapy with heparin or low–molecular-weight heparin.
CI = confidence interval; HR = hazard ratio; sICH = symptomatic intracerebral hemorrhage.
Weighted Cox proportional hazards with propensity score matching
In weighted cox proportional analyses with propensity score matching that included participants who were started on anticoagulation and the timing was known but died or were lost to follow-up within 90 days (n=1,516) the associations between the time to start anticoagulation categories and outcomes remained largely unchanged compared to the primary analyses, except that the association between anticoagulation initiation in the 7-10 day period and combined outcome approached statistical significance (adjusted HR 0.58 95% CI 0.31 – 1.14, p = 0.07) (Table 3).
Sensitivity analyses
In addition, we performed sensitivity analyses to determine whether the time to anticoagulation is affected by certain factors that would drive the decision to start anticoagulation including large infarct size (> 60 mL), hemorrhagic transformation of the infarct prior to starting anticoagulation, and treatment with DOAC. The associations between the time to start anticoagulation categories and outcomes remained largely unchanged; however, these analyses are limited by the relatively small sample size within each category (Supplemental Table 2). Furthermore, in these analysis, DOAC treatment within 7-10 days (vs. no DOAC treatment) was associated with non-significantly lower odds of the combined outcome (odds ratio 0.50 95% CI 0.23 – 1.06, p = 0.070).
Because recurrent ischemic events occurring prior to starting anticoagulation may have influenced the timing to start anticoagulation, we performed sensitivity analyses counting the time to start anticoagulation as the actual time anticoagulation was started in patients without ischemic events or in those whose ischemic event occurred after starting anticoagulation and counting the time to start anticoagulation as the time of the recurrent ischemic event when this event occurred prior to starting anticoagulation. In these analyses, there was trend for lower odds of combined outcome when starting anticoagulation 7-10 days after index event in unadjusted models (OR 0.60 95% CI 0.35 – 1.03, p = 0.061) but the effect is attenuated and the trend disappears when adjusting for potential confounders (adjusted OR 0.70 95% CI 0.40 – 1.22, p = 0.212) (Table 4).
TABLE 4.
Association between Time Categories and Ischemic Events When the Time to Initiation Was Defined as Time to Start Anticoagulation as the Actual Time Anticoagulation Was Started in Patients without Ischemic Events or in Those Whose Ischemic Event Occurred after Starting Anticoagulation and Counting the Time to Start Anticoagulation as the Time of the Recurrent Ischemic Event When This Event Occurred prior to Starting Anticoagulation
| Time Category | Ischemic Event, OR (95% CI), p | Combined Outcome, OR (95% CI), p |
|---|---|---|
| 4–14 days | 0.87 (0.58–1.31), 0.502 | 0.97 (0.68–1.37), 0.842 |
| 0.92 (0.60–1.42), 0.712 | 1.02 (0.69–1.50), 0.923 | |
| 4–7 days | 1.10 (0.69–1.75), 0.694 | 1.11 (0.75–1.66), 0.597 |
| 1.24 (0.77–2.01), 0.376 | 1.26 (0.82–1.92), 0.295 | |
| 7–10 days | 0.59 (0.31–1.11), 0.103 | 0.60 (0.35–1.03), 0.061 |
| 0.66 (0.34–1.26), 0.208 | 0.70 (0.40–1.22), 0.212 | |
| 10–14 days | 0.68 (0.36–1.29), 0.234 | 0.80 (0.48–1.35), 0.401 |
| 0.61 (0.30–1.23), 0.166 | 0.73 (0.41–1.30), 0.285 |
The first row in each category is unadjusted and the second row is adjusted for the following covariates. Ischemic event was adjusted for valvular heart disease, prior stroke, cardiac thrombus, and CHA2DS2-Vasc. Combined outcome was adjusted for valvular heart disease, prior stroke, cardiac thrombus, CHA2DS2-Vasc, stroke size, asymptomatic hemorrhage, and bridging.
CI = confidence interval; OR = odds ratio.
Discussion
In this study, we found that the 4-14 day recommended time frame to start anticoagulation after a cardioembolic stroke was not associated with reduced ischemic, hemorrhagic, nor combined outcomes. These findings did not seem to be affected by infarct burden or the presence of hemorrhagic transformation prior to initiating anticoagulation. In an exploratory analysis, however, it appeared that initiating anticoagulation (particularly a DOAC) between 7-10 days from the index event was associated with lower odds of sICH, ischemic stroke, and combined outcome but these associations were not statistically significant.
Our findings slightly differ from the findings of other studies7–10 and are surprising in that we found no significant benefit of starting anticoagulation in the 4-14 day period. In our study, there appears to be lower odds of the combined outcome when starting anticoagulation 7-10 days after the index event. These association, however, did not achieve statistical significance, but given the relatively small sample size and the low event rate, our study may have been underpowered. The RAF study for instance7, suggested that the 4-14 day period may be the optimal time to start anticoagulation. Further analysis of the RAF results suggested that the risk of combined outcome appears to be the higher in the first 7 days (HR 1.35 95% CI 0.82 – 2.22) as opposed to 7-14 days (HR 0.43 95% CI 0.23 – 0.83). Most importantly, our study cohort differed from the RAF cohort in the type of anticoagulant used (warfarin more common in RAF and DOAC more common in our study) and the patient population, which may have led to different findings. Furthermore, another study suggested that early DOAC use within 7 days was not associated with increased risk of sICH nor the risk of recurrent ischemic events as compared to DOAC initiation after 7 days.10 Moreover, a multicenter study showed that starting oral anticoagulation within 4 days as opposed to after 4 days was not associated with increased risk of the composite outcome of stroke, TIA, or death.9 These studies however, had different study populations and did not specifically study the effect of starting anticoagulation in the intervals we investigated on the risk of recurrent ischemic events, sICH, and combined outcome.
Our data suggests that initiating anticoagulation prior to 7 days was associated with higher rates of hemorrhagic complications, which offset any potential benefits for reducing ischemic events. This could be due to the fact that our study population was comprehensive stroke centers and therefore the patient population may be sicker patients with large strokes, hemorrhagic complications, or alteplase or mechanical thrombectomy treated patients where post-procedural asymptomatic hemorrhage may be seen in up to 33.5%.18 In our study, nearly 26.7% of patients received mechanical thrombectomy and 28.0% received alteplase and therefore it is possible that this may have influenced our findings.
Furthermore, it is important to recognize that the time to start anticoagulation highly varies between patients as the risk of hemorrhage and that of recurrent ischemic events is variable based on the patient’s profile and what anticoagulation strategy is used. In addition, studies have shown that predictors of sICH are different than those of recurrent ischemic events. For instance, the RAF study showed that predictors of recurrent ischemic events were age, left atrial enlargement, and infarct size and these were not associated with hemorrhagic events.19 This may imply that early anticoagulation may be more beneficial in patients with higher risk for recurrent ischemic events and potentially harmful in those with higher risk of hemorrhagic events. Furthermore, anticoagulation strategies altered the risk of early recurrent outcomes. For example, a recent study showed that bridging therapy was associated with higher risk of recurrent stroke and sICH17 whereas another study showed that DOACs had lower risks of the composite endpoint of ischemic and hemorrhagic outcomes as compared to warfarin.20 In fact, the RAF-NOACs study suggested that the time of initiation of DOACs was not associated with recurrent ischemic or hemorrhagic events.21 In our study, the absolute rate of symptomatic intracranial hemorrhage was lowest in the patients started on anticoagulation within 0-3 days and this is likely due to the treating provider’s ability to identify those at the lowest bleeding risk and initiating anticoagulation early in these patients.
Ongoing clinical trials are investigating the optimal timing of initiating anticoagulation. ELAN (NCT03148457), OPTIMAS (NCT03759938), TIMING (NCT02961348), and START (NCT03021928) are planned or ongoing randomized clinical trials that will shed light on the optimal timing of initiating anticoagulation after a cardioembolic stroke.8, 22
Strengths and Limitations
Our study has several limitations. First, since this is a retrospective study it is subject to bias. Although we adjusted for many confounders such as infarct volume and hemorrhage on 24 hour brain imaging, we are unable to account for other unmeasured factors that may have influenced the timing and type of anticoagulation used. In addition, since this study included patients from comprehensive stroke centers, it may not be generalizable to non-comprehensive stroke centers. Furthermore, nearly 10% of patients where lost to follow up. Although this is a similar rate to that in randomized controlled trials and we adjusted for major differences in baseline characteristics in our models, this loss to follow up may have added bias to our findings. Finally, another major limitation of our study is that outcomes were assessed based on vascular neurology clinical visits and record review of hospitalizations, other outpatient visits, and outside hospital records, which may have led us to underestimate the number of outcomes. Nevertheless, the proportion of patients with ischemic and hemorrhagic outcomes was in line with other prospective studies, assuaging concerns regarding major bias.
Strengths of our study include the large sample size and multicenter design to help account for variations in provider practices and patient populations between individual centers. In addition, our study includes a wide range of variables and adjustment for important confounders, allowing more robust analyses.
Conclusion
In this multicenter real world cohort, the recommended (4-14 days) time-frame to start oral anticoagulation was not associated with reduced ischemic and hemorrhagic outcomes. Randomized trials are needed to determine the optimal timing of anticoagulation.
Supplementary Material
Acknowledgements and disclosures:
Dr. Henninger is supported by K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and R44NS076272 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. Dr. Liberman is supported by K23NS107643 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All other authors report no disclosures or acknowledgments.
Footnotes
Potential conflict of interest: all authors have no relevant conflict of interest
References
- 1.Kamel H, Healey JS. Cardioembolic stroke. Circulation research. 2017;120:514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacchetti DC, Furie KL, Yaghi S. Cardioembolic stroke: Mechanisms and therapeutics. Seminars in neurology. 2017;37:326–338 [DOI] [PubMed] [Google Scholar]
- 3.Low molecular weight heparinoid, org 10172 (danaparoid), and outcome after acute ischemic stroke: A randomized controlled trial. The publications committee for the trial of org 10172 in acute stroke treatment (toast) investigators. Jama. 1998;279:1265–1272 [PubMed] [Google Scholar]
- 4.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: A double-blind randomised study. Haest study group. Heparin in acute embolic stroke trial. Lancet (London, England). 2000;355:1205–1210 [DOI] [PubMed] [Google Scholar]
- 5.The international stroke trial (ist): A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet (London, England). 1997;349:1569–1581 [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 7.Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: Effect of anticoagulation and its timing: The raf study. Stroke. 2015;46:2175–2182 [DOI] [PubMed] [Google Scholar]
- 8.Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. The Lancet. Neurology. 2019;18:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson D, Ambler G, Banerjee G, Shakeshaft C, Cohen H, Yousry TA, et al. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: Multicentre cohort study. Journal of neurology, neurosurgery, and psychiatry. 2019;90:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiffge DJ, Traenka C, Polymeris A, Hert L, Peters N, Lyrer P, et al. Early start of doac after ischemic stroke: Risk of intracranial hemorrhage and recurrent events. Neurology. 2016;87:1856–1862 [DOI] [PubMed] [Google Scholar]
- 11.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. Abc/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2017;48:e343–e361 [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA, Dawber TR, Thomas HE, Kannel WB Jr., Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The framingham study. Neurology. 1978;28:973–977 [DOI] [PubMed] [Google Scholar]
- 14.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The swedish atrial fibrillation cohort study. European heart journal. 2012;33:1500–1510 [DOI] [PubMed] [Google Scholar]
- 15.Comess KA, DeRook FA, Beach KW, Lytle NJ, Golby AJ, Albers GW. Transesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: Prevalence of findings and recurrent stroke risk. Journal of the American College of Cardiology. 1994;23:1598–1603 [DOI] [PubMed] [Google Scholar]
- 16.Yaghi S, Kamel H. Stratifying stroke risk in atrial fibrillation: Beyond clinical risk scores. Stroke. 2017;48:2665–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altavilla R, Caso V, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, et al. Anticoagulation after stroke in patients with atrial fibrillation. Stroke. 2019;50:2093–2100 [DOI] [PubMed] [Google Scholar]
- 18.Hao Y, Liu W, Wang H, Zi W, Yang D, Wang W, et al. Prognosis of asymptomatic intracranial hemorrhage after endovascular treatment. Journal of neurointerventional surgery. 2019;11:123–126 [DOI] [PubMed] [Google Scholar]
- 19.Paciaroni M, Agnelli G, Caso V, Tsivgoulis G, Furie KL, Tadi P, et al. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: The alessa score study. Stroke. 2017;48:726–732 [DOI] [PubMed] [Google Scholar]
- 20.Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, et al. Direct oral anticoagulants versus vitamin k antagonists after recent ischemic stroke in patients with atrial fibrillation. Annals of neurology. 2019;85:823–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-k oral anticoagulants (raf-noacs) study. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac Grory B, Flood S, Schrag M, Paciaroni M, Yaghi S. Anticoagulation resumption after stroke from atrial fibrillation. Current atherosclerosis reports. 2019;21:29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


