Introduction
In an increasingly diverse nation of Black, Hispanic, Asian, Native American, Alaska Native, Native Hawaiian, Pacific Islander, and White individuals, health disparities among racial and ethnic groups are prevalent, pervasive, and persistent. Largely brought to attention in 1985 by the Secretary’s Task Force on Black and Minority Health and reaffirmed in the Institute of Medicine’s report to Congress on Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care in 2003, health and health care equity remains elusive, as recognized in the most recent 2018 National Health Interview Survey and the National Healthcare Quality and Disparities Report. Minorities rate their health status as fair or poor more often than White individuals, and minorities receive worse care than White patients for about 40% of 250 quality measures (1).
Kidney disease is not immune to health and health care disparities. Kidney failure rates are improving but remain up to two times higher for Black individuals and other minorities, with kidney failure occurring up to 5 years earlier (2). Studies show Black individuals have a steeper decline in kidney function starting at an earlier age. When Black individuals and other minorities acquire kidney failure, they are often less likely to have knowledge of different therapies prior to dialysis initiation and be treated with home dialysis or transplantation.
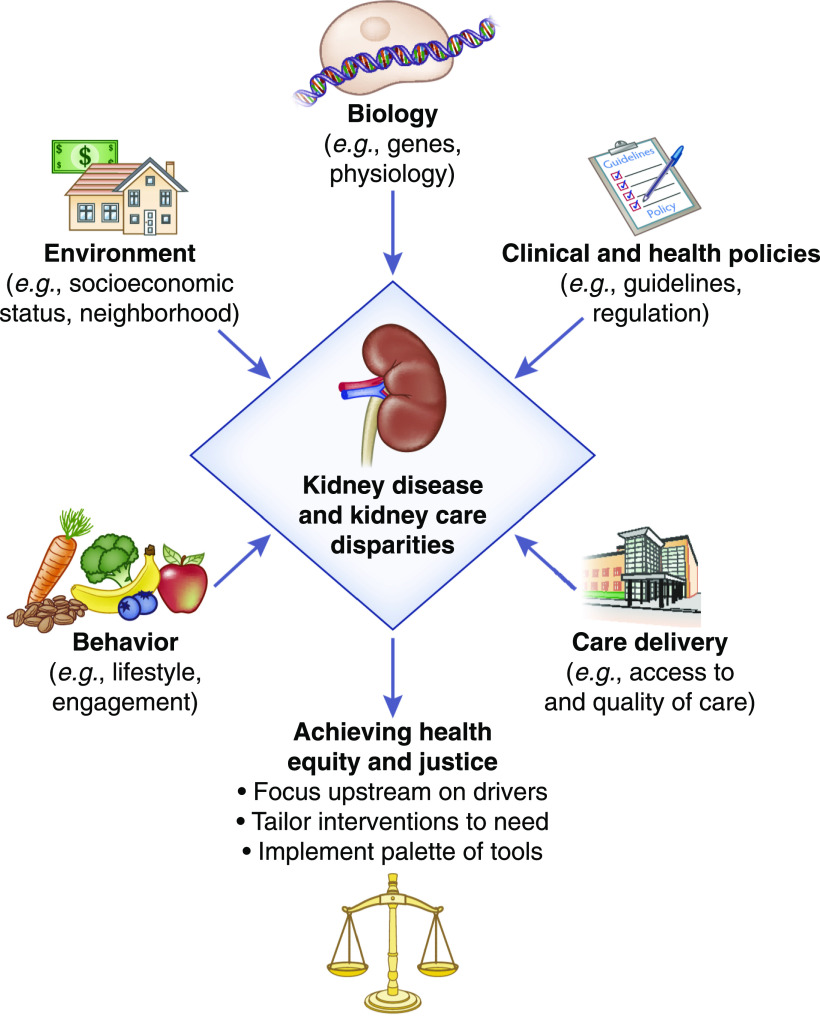
Interest in the genesis of these pathologic disparities in kidney disease has been the subject of an increasing body of research in search of susceptibility, initiation, and progression factors contributing to disparities, referred to as the human exposome. This article provides examples of some of the possible exposome contributors that drive disparities and offers possible targets to achieve health equity (Figure 1).
Figure 1.
Drivers of race and e thnic d isparities. Elements of the human exposome driving health disparities that are possible targets for interventions to achieve health equity and justice.
Biology
Driven by data showing that Black patients starting dialysis are more likely to have a relative who is also being treated for kidney failure, the search for genetic factors revealed that the presence of two variants in the APOL1 gene conferred a higher risk of nondiabetic kidney disease, FSGS, and HIV-associated nephropathy. These variants, present in 10%–15% of the Black population, likely arose by natural selection in West Africa and sub-Saharan Africa due to these variant alleles conferring defense against Trypanosoma brucei rhodesiense, the cause of African sleeping sickness. Furthermore, longitudinal studies demonstrate that persons with two copies of APOL1 risk variants are more likely to progress to kidney failure.
APOL1 genetic variation is unlikely to be solely responsible for greater progression to kidney disease in Black patients because there is considerable overlap in the distributions of eGFR decline by race and APOL1 status (3). Thus, other “second-hit” factors may be operative in contributing to kidney failure seen with APOL1 variants. We do not know whether interventions can modify the effect of APOL1 risk status, perhaps by targeting “second-hit” factors.
Environment
The role of environment is evident in many disparities, especially the socioeconomic milieu in which one is born, raised, and resides over a life span. Presence of CKD was associated with poverty but was more profound among Black patients in research conducted in a racially and socioeconomically diverse urban population (4). Socioeconomic influences could start as early as fetal development (e.g., low birth weight and nephron number) and affect susceptibility to disease in later life (e.g., high BP and dysglycemia). Housing, the built environment, neighborhood stressors, and toxins are other important environmental exposures.
Behavior
Health beliefs may affect behaviors and lifestyles, such as eating practices, physical activity, engagement with providers, and organ donation. For example, diets with high acid load (poor in fruits and vegetables) that are more common in Black patients are associated with progression to kidney damage and failure (5,6). Food insecurity influenced by socioeconomic status is associated with progression to kidney failure that is partially mediated by nutritional factors, such as dietary acid load (7).
Care Delivery
The quality of BP lowering, reduction of proteinuria, prevention of AKI through avoidance of nephrotoxins, and glycemic control in diabetes can contribute to initiation and progression of kidney disease. Minorities have been found to have less control of BP, higher urine albumin excretion, and more dysglycemia (7).
Studies show that minorities also often seem to be more poorly prepared when they develop kidney failure. Predialysis nephrology care is absent more often in Black and Hispanic patients than in White patients and has been shown to affect long-term outcomes such as mortality (8). Black patients are less likely to obtain access to the kidney transplant wait list and less likely to receive kidney transplants. When on dialysis, they are less likely to use home therapies that may offer a better quality of life. Less knowledge about alternative therapies may derive from inadequate time for, or lack of, education and patient-provider shared decision making. Paradoxically, Black patients on dialysis experience better survival than White patients, a phenomenon poorly understood despite explorations into differences in health status.
Clinical and Health Policies
Policies in health care have been spotlighted for reinforcing institutional racism. They are structural edifices sculpted into laws, clinical practice, and culture, as opposed to personal ethnicism due to overt prejudice, oppression, privilege, and beliefs. An example is the structure of the national kidney allocation system and the change in 2014 that removed institutionalized disadvantages for Black patients.
Another recently popularized notion is that use of race in estimation of GFRs since 1999 and its incorporation into clinical practice guidelines are institutionalized practices triggering a decrease in referrals to specialists, as well as decreased access to the transplant wait list, for Black patients with kidney disease (9). By inference, elimination of race would correct disparities. Interestingly, disparities in specialist referral and wait listing were revealed in the decade before the introduction of race into eGFR equations in 1999. These disparities have not been eliminated over time for Black patients, nor have they been eliminated for Hispanic patients for whom the race coefficient is not incorporated into eGFR equations (8).
In derivation and validation of the Modification of Diet in Renal Disease and the Chronic Kidney Disease Epidemiology Collaboration Consortium equations, race was identified as a factor that led to greater accuracy in the prediction of measured GFR from serum creatinine. Research has shown that the proportion of genetic African ancestry correlates with serum creatinine levels. The practice of assigning the non-Black coefficient to Black individuals institutionalizes the exclusion of information on Black individuals in equation derivation. Whether the elimination of race, or substitution of better markers, in estimating equations is able to make progress in eliminating referral or wait-listing disparities, without creating new disparities in other aspects of care (e.g., Food and Drug Administration metformin-prescribing regulations), awaits more empirical evidence (10).
Achieving Health Equity and Justice
What must be done to rectify the disparities we have seen for so long? First, we must recognize that treating disease at end stage is costly, personally, financially, and societally. Second, the spectrum of contributors in the human exposome that conspire to compromise health differentially for ethnic minorities is complex, often varying for different outcomes. Therefore, providing everyone the same threshold for initiating interventions to address health challenges may represent equality, but giving everyone interventions that they need (i.e., equity) will be far more effective. A palette of molecular knowledge and frontier therapeutics coupled with implementation science tools combining environmental, economic, behavioral, care delivery, and policy interventions are all part of the armamentarium. Even more powerful is justice through eliminating the systemic causes of inequity, thereby pre-empting disparities.
Finally, there is tension between ignoring diversity in clinical decisions in order to be race neutral and recognizing differences by race to offer more precise medicine. Ignoring diversity is a lost opportunity to understand the exposome that leads to disease or health. Science on disparities, clinical care learning from diverse patients, and education about health equity inform and enhance all of medicine and human health.
Disclosures
The author has nothing to disclose.
Funding
None.
Acknowledgments
This manuscript was adapted from a presentation at the 2020 Alison Norris Symposium on Social Determinants of Kidney Health held on January 15, 2020, at the New York Academy of Medicine, New York, New York. The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Perspectives, “Social Determinants of Health in People with Kidney Disease: An Introduction,” “Social Determinants of Kidney Health: Focus on Poverty,” “The Seen and the Unseen: Race and Social Inequities Affecting Kidney Care,” “Reducing the Burden of CKD among Latinx: A Community-Based Approach,” and “Personal Experiences of Patients in the Interaction of Culture and Kidney Disease,” on pages 803–805, 809–811, 812–814, 815–817 and 818–819, respectively.
References
- 1. Agency for Healthcare Research and Quality : 2018 National Healthcare Quality and Disparities Report, 2020. Available at: https://www.ahrq.gov/research/findings/nhqrdr/nhqdr18/index.html. Accessed October 23, 2020
- 2. United States Renal Data System (USRDS) : 2018. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Available at: https://www.usrds.org/media/1736/v2_c01_incprev_18_usrds.pdf. Accessed on October 23, 2020
- 3. Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J: Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR: Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55: 992–1000, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crews DC, Banerjee T, Wesson DE, Morgenstern H, Saran R, Burrows NR, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. Am J Nephrol 47: 174–181, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee T, Crews DC, Wesson DE, Dharmarajan S, Saran R, Ríos-Burrows N, Saydah S, Powe NR; CDC CKD Surveillance Team: Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis 70: 38–47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smalls BL, Ritchwood TD, Bishu KG, Egede LE: Racial/ethnic differences in glycemic control in older adults with type 2 diabetes: United States 2003-2014. Int J Environ Res Public Health 17: 950, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Purnell TS, Bae S, Luo X, Johnson M, Crews DC, Cooper LA, Henderson ML, Greer RC, Rosas SE, Boulware LE, Segev DL: National trends in the association of race and ethnicity with predialysis nephrology care in the United States from 2005 to 2015. JAMA Netw Open 3: e2015003, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Tuot DS: Improving equity in medication use through better kidney function measurement. J Am Soc Nephrol 31: 1657–1658, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]