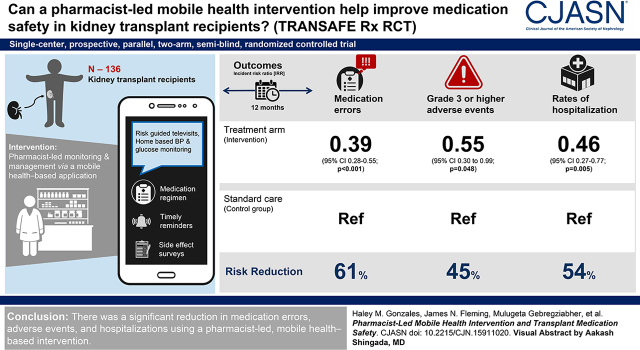
Visual Abstract
Keywords: kidney transplantation, hospitalization, drug interactions, pharmacists
Abstract
Background and objectives
Medication safety events are predominant contributors to suboptimal graft outcomes in kidney transplant recipients. The goal of this study was to examine the efficacy of improving medication safety through a pharmacist-led, mobile health–based intervention.
Design, setting, participants, & measurements
This was a 12-month, single-center, prospective, parallel, two-arm, single-blind, randomized controlled trial. Adult kidney recipients 6–36 months post-transplant were eligible. Participants randomized to intervention received supplemental clinical pharmacist–led medication therapy monitoring and management via a mobile health–based application, integrated with risk-guided televisits and home-based BP and glucose monitoring. The application provided an accurate medication regimen, timely reminders, and side effect surveys. Both the control and intervention arms received usual care, including serial laboratory monitoring and regular clinic visits. The coprimary outcomes were to assess the incidence and severity of medication errors and adverse events.
Results
In total, 136 kidney transplant recipients were included, 68 in each arm. The mean age was 51 years, 57% were male, and 64% were Black individuals. Participants receiving the intervention experienced a significant reduction in medication errors (61% reduction in the risk rate; incident risk ratio, 0.39; 95% confidence interval, 0.28 to 0.55; P<0.001) and a significantly lower incidence risk of Grade 3 or higher adverse events (incident risk ratio, 0.55, 95% confidence interval, 0.30 to 0.99; P=0.05). For the secondary outcome of hospitalizations, the intervention arm demonstrated significantly lower rates of hospitalizations (incident risk ratio, 0.46; 95% confidence interval, 0.27 to 0.77; P=0.005).
Conclusions
We demonstrated a significant reduction in medication errors, adverse events, and hospitalizations using a pharmacist-led, mobile health–based intervention.
Introduction
Kidney transplantation is the preferred treatment option over dialysis for patients with kidney failure, due to its beneficial effect on cost and patient survival. With the total number of kidney transplant recipients alive today approaching 250,000, follow-up care has become more complex due to increasing age and chronic health conditions, coupled with the fragmented health care system in the United States. The use of contemporary immunosuppression has produced a substantial decrease in the incidence of acute rejection, which has dropped to a current 1-year rate of roughly 8%. However, improvements in long-term graft survival have remained relatively stagnant (1 –3).
Immunosuppression adverse events (AEs) and rejection as a result of medication nonadherence are among the chief contributors to suboptimal graft survival (4 –6). Although modern immunosuppressive agents are highly effective, the toxicity burden and relative complexity of these regimens leave transplant recipients vulnerable to developing AEs and medication safety issues, including medication errors (7). Previous research demonstrated that medication errors occur in as many as two thirds of kidney transplant recipients, and result in the hospitalization of one in every eight recipients (2,8). Further, clinically significant medication errors were linked to a considerably higher risk of graft loss, AEs, readmissions, and acute rejections (9). We demonstrated a correlation between medication errors and immunosuppression AEs, with patients experiencing medication errors at 2.3-fold higher risk of developing multiple AEs (P=0.02) (8). These studies highlight the need for innovative approaches to improve medication safety in patients who are high risk, such as kidney transplant recipients. Supplemental support of medical and public health practices using mobile devices, such as mobile phones, has emerged as a new pathway for improving outcomes across several disciplines, including transplant. However, many of these mobile health technologies lack sophisticated integration of health care providers. Clinical pharmacists are in a unique position to apply their skills toward early identification and intervention via mobile health–based approaches to mitigate or prevent downstream consequences of medication safety issues that contribute to poor kidney graft outcomes.
We conducted a prospective, randomized controlled trial that tested a mobile health–based, pharmacist-led telehealth intervention (TRANSAFE Rx) with the primary objective to assess medication safety. The goal of this study was to determine the effect of a collaboration between kidney transplant recipients, pharmacists, and innovative technology on medication-related outcomes and hospitalizations (1,2).
Materials and Methods
Study Design
This was a single-center, 12-month, parallel, two-arm, single-blind, 1:1 randomized controlled clinical trial involving 136 adult kidney transplant recipients (68 in each arm; NCT03247322). Comprehensive details of the study rationale and design have been published elsewhere (1). The primary aims were to assess the efficacy of a pharmacist-led, mobile health–based intervention on improving medication safety and health outcomes in kidney transplant recipients, as compared with usual care. This study was reviewed and approved by the Institutional Review Board at the Medical University of South Carolina.
Study Eligibility and Enrollment
Adult (≥18 years old at time of transplant) kidney recipients 6–36 months post-transplant were eligible for the study. Multiorgan recipients were excluded, as were patients incapable of measuring their own BP and blood glucose (if applicable), self-administering medications; speaking, hearing, and reading English; or utilizing the mobile health application (app) after sufficient training. Patients who were eligible and agreed to study participation were consented and randomized by research personnel using a random number generator in a simple blocked manner (blocks of eight) into one of the two study arms. Only study coordinators and clinical pharmacists assessing medication errors, AEs, and clinical outcomes were blinded to study assignment.
Intervention
Participants randomized to the intervention arm were provided the same usual care as the control cohort. As part of usual care, kidney transplant recipients are seen by pharmacists while in the hospital and during routine clinic visits for the first 6 months post-transplant. After this, pharmacists see patients only when requested by a provider for medication-related issues. In addition to usual care, the intervention group received clinical pharmacist–led supplemental medication therapy monitoring and management, utilizing a smartphone-enabled mobile health app, integrated with risk-driven televisits and home-based BP and blood glucose monitoring (when applicable). The mobile health app, developed by our group, provided participants with an accurate list of their medication regimen, which was automatically updated from the electronic medical record (EMR), timely medication reminders, automated messages triggered by missed doses or scheduled health monitoring, medication side effect tracking, and BP and blood glucose trends (when applicable). Monthly and subject-initiated surveys were delivered through the app regarding the frequency and severity of common side effects. The intervention included clinical pharmacist telemonitoring of medications, medical appointment adherence, weekly BP/glucose readings, and scheduling telehealth visits with participants. The clinical pharmacist was notified of any medication changes and transitions of care by subject self-report or via new medications reported in the EMR, and automatically notified of nonadherence (≥20% missed self-reported medication doses over a week), critical BP or blood glucose values, or alarming trends in readings or symptom assessments from surveys via rule-based algorithms. The pharmacist responded to alerts through communication with the participant and care team, and updated the medication regimen in the EMR as necessary. Televisits enabled the pharmacist to conduct medication reviews to identify any medication safety issues, ensure accurate medications through transitions of care, screen for drug interactions, and provide recommendations to the participant. Full details on the development and validation of the mobile health app and dashboard are published elsewhere (2).
End Points
The coprimary outcomes were (1) the incidence and severity of medication errors and (2) the incidence and severity of AEs, as compared between the intervention and control arms. Medication errors were defined as the participant taking a different medication than intended, on the basis of comparison of the EMR to the participant’s reported regimen (8,10). The type of medication error was recorded and included both administrative and clinical subtypes. Administrative medication errors were defined as discrepancies on the EMR, including drug omissions, additions, dose errors, incorrect drug, incomplete dosage instructions, prescribing errors, and any prescribed drug that the participant was not taking. Clinical medication errors included duplicate therapy, no indication, untreated condition, high/low dose, contraindications, and other. Medication error severity was determined using a previously validated scale (11). AE type and severity were defined using the Common Terminology Criteria for Adverse Events (12). Infections were defined as any diagnosed and treated infection. Hospitalizations were defined as admission to a hospital with at least one overnight stay. Data on medication errors, AEs, and clinical outcomes were collected by blinded study coordinators and clinical pharmacists, who did not have access to the randomization module within the electronic case report form system (REDCap, https://www.project-redcap.org/).
Sample Size
We estimated that approximately 64% of transplant recipients in the control group would experience at least one medication error during the 12-month study. Prior research demonstrates that pharmacist-led interventions can produce up to a 50% reduction in these medication errors. A total sample size of 136 participants was needed to provide at least 80% power, accounting for dropouts, and detect a 50% reduction in medication errors. Given an estimated AE incidence rate of 87%, this sample size was also sufficiently powered to detect a 33% reduction in significant AEs. A comprehensive overview of the sample size calculation is included in the trial protocol (1).
Statistical Analyses
This analysis utilized intent-to-treat methodology. Data are reported using percentages for nominal and ordinal variables, and compared using Fisher’s exact test or Pearson’s chi-squared test as appropriate. Results are reported using means and SDs or medians and interquartile ranges, with statistical comparison using t test or the Mann–Whitney U test. Multivariable modeling was also utilized to assess for the independent effect of the treatment intervention on end points. For count outcomes, we used Poisson or negative binomial regression models, depending on data dispersion and model fit. For the repeated measure outcome of medication errors (measured every 2 months during the 12-month study), we used generalized linear mixed modeling with a random intercept using likelihood methods, and accounting for time and correlation of repeated measures within participants. The effect of the intervention over time was assessed using a time*treatment interaction term within the model. We report both unadjusted and adjusted outcomes. Multivariable models were adjusted for recipient age, sex, race, history of diabetes, years on dialysis, calculated panel reactive antibody, cold ischemic time, induction therapy, delayed graft function, cytomegalovirus (CMV) serostatus, and donor characteristics (donor type, kidney donor risk index). A two-sided P value <0.05 was considered statistically significant. SAS version 9.4 (SAS Institute, Cary, NC) was used for analyses.
Results
Study Population
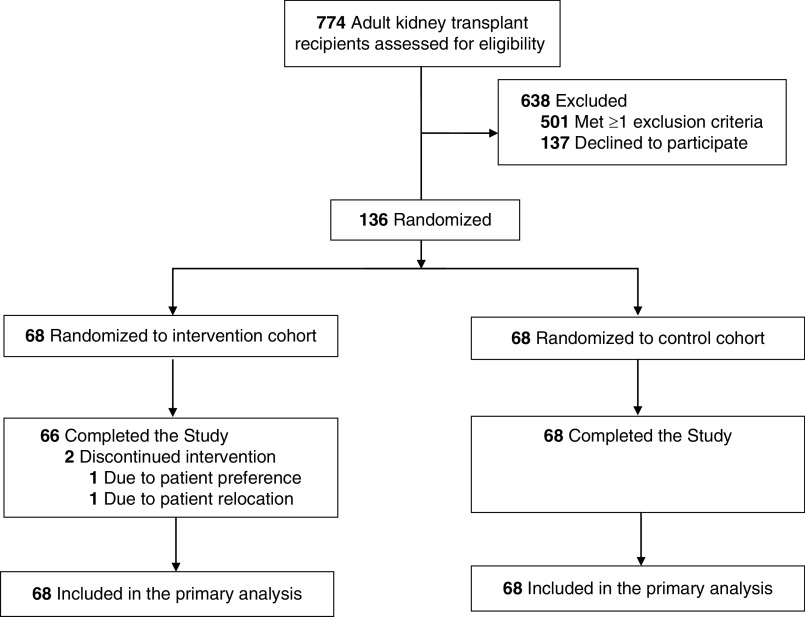
Between October 2017 and January 2019, a total of 774 kidney recipients at the Medical University of South Carolina were screened for eligibility; 273 were approached for consent and 136 kidney transplant recipients agreed to participate, provided informed written consent, and were enrolled in the study (68 in each arm; Figure 1). The most common reason for ineligibility was failure to meet the study window of 6–36 months post-transplant. Patients who were eligible and declined participation primarily were not interested in the study, were uncomfortable with the technology, or felt their medications and comorbidities were already well controlled. Two participants withdrew from the study intervention arm before completing the study for a 99% retention rate; both participants are included in this intent-to-treat analysis.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram: Randomization and patient flow in the TRANSAFE Rx Study.
Baseline characteristics were mostly comparable between the two study arms (Table 1). The mean age was 51 years, 57% of participants were male, and 64% were Black individuals. The primary etiologies of kidney failure were diabetes and hypertension, followed by polycystic kidney disease and lupus. History of hypertension was similar between groups, however, 52% of participants in the control group had a history of diabetes, compared with 28% in the intervention group. On average, participants spent 4 years on dialysis, and 84% of participants were on dialysis at the time of transplant. More participants in the intervention group experienced delayed graft function as compared with the control group (27% versus 13%). In the intervention group, 27% had donor-positive, recipient-negative CMV serostatus (high risk), versus 12% in the control group. The 6-month ambulatory procedure history, hospitalization history, and mean number of clinic visits were comparable between groups.
Table 1.
Baseline characteristics of participants in a randomized controlled trial that tested the effects of a mobile health–based, pharmacist-led intervention on medication errors and adverse events (TRANSAFE Rx)
| Characteristic | All (n=136) | Control (n=68) | Intervention (n=68) |
|---|---|---|---|
| Mean age (SD), yr | 51±13 | 51±14 | 50±12 |
| Male, n (%) | 77 (57) | 42 (62) | 35 (52) |
| Female, n (%) | 59 (43) | 26 (38) | 33 (49) |
| White, n (%) | 46 (34) | 19 (28) | 27 (40) |
| Black, n (%) | 87 (64) | 47 (69) | 40 (59) |
| Hispanic, n (%) | 3 (2) | 2 (3) | 1 (2) |
| Height (SD), cm | 172±11 | 173±11 | 171±11 |
| Mean weight (SD), kg | 89±19 | 88±19 | 90±20 |
| History of diabetes, n (%) | 54 (40) | 35 (52) | 19 (2) |
| History of hypertension, n (%) | 124 (92) | 61 (91) | 63 (93) |
| Dialysis at transplant, n (%) | 114 (84) | 56 (84) | 58 (85) |
| Hemodialysis | 80 (7) | 42 (75) | 38 (66) |
| Peritoneal dialysis | 34 (30) | 14 (25) | 20 (35) |
| Mean years on dialysis (SD), yr | 4.0±2.8 | 4.0±3.0 | 3.9±2.6 |
| Mean cPRA at transplant (SD), % | 49.9±39.7 | 49.7±41.0 | 50.0±38.6 |
| Median HLA mismatch (IQR) | 5 (4–5) | 4 (4–5) | 5 (3–5) |
| Mean cold ischemic time (SD), h | 17.0±8.0 | 17.5±8.0 | 16.4±8.0 |
| Mean warm ischemic time (SD), min | 38.0±13.9 | 38.0±15.8 | 38.1±11.9 |
| Basiliximab induction, n (%) | 51 (38) | 26 (38) | 25 (37) |
| Thymoglobulin induction, n (%) | 85 (63) | 42 (62) | 43 (63) |
| Delayed graft function, n (%) | 26 (20) | 8 (13) | 18 (27) |
| CMV serostatus (D/R), n (%) | |||
| Neg/Neg | 12 (9) | 7 (10) | 5 (7) |
| Neg/Pos | 35 (26) | 22 (32) | 13 (19) |
| Pos/Pos | 59 (43) | 30 (44) | 29 (43) |
| Pos/Neg | 26 (19) | 8 (12) | 18 (27) |
| Unknown | 4 (3) | 1 (2) | 3 (4) |
| Mean donor age (SD), yr | 35±14 | 37±15 | 33±12 |
| Donor sex, n (%) | |||
| Male | 88 (65) | 47 (69) | 41 (60) |
| Female | 47 (35) | 20 (29) | 27 (40) |
| Missing | 1 (1) | 1 (2) | 0 (0) |
| Donor race, n (%) | |||
| White | 81 (60) | 42 (62) | 39 (57) |
| Black | 35 (26) | 16 (24) | 19 (28) |
| Other | 20 (15) | 10 (1) | 10 (15) |
| Living donor, n (%) | 13 (10) | 7 (10) | 6 (9) |
| DCD donor, n (%) | 16 (13) | 11 (18) | 5 (8) |
| Mean KDRI in deceased donors (SD) | 41.6±24.5 | 46.2±24.8 | 37.1±23.4 |
| Acute rejection history, n (%) | 6 (4) | 3 (4) | 3 (4) |
| Hospitalization history, n (%) | 33 (24) | 16 (24) | 17 (25) |
| Mean clinic visit history (SD) | 5.9±4.7 | 5.7±4.3 | 6.1±5.0 |
| Median ambulatory procedures history (IQR) | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) |
| Yr from transplant to enrollment (SD) | 1±1 | 1±1 | 1±1 |
cPRA, calculated panel reactive antibodies; HLA, human leukocyte antigen; IQR, interquartile range; CMV, cytomegalovirus; D, donor; R, recipient; Neg, negative; Pos, positive; DCD, deceased; KDRI, Kidney Donor Risk Index.
Coprimary Outcome 1: Medication Errors
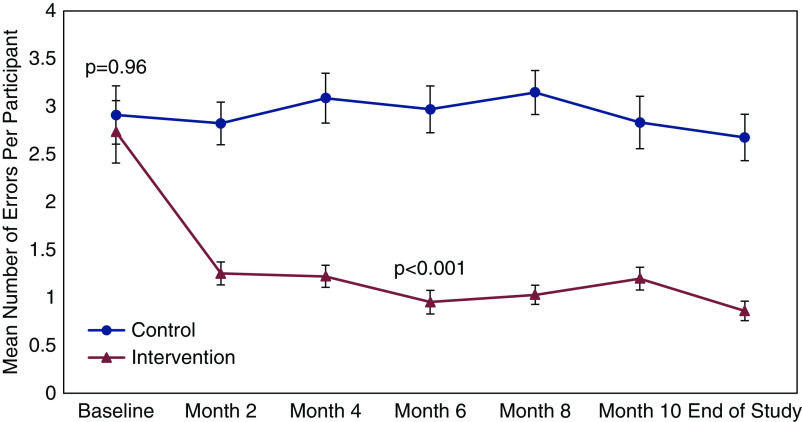
All 68 participants in both arms experienced at least one medication error during the study. Figure 2 displays the unadjusted total number of medication errors identified by blinded research coordinators and pharmacists, compared between the control and treatment arms at each time point across the 12-month study. This included 904 separate assessments in the 136 participants over the 12-month study (of the 952 potential assessments, 48 were missed, 95% completion rate). In total, there were 1385 medication errors in the control arm (mean 20.4±14.0) and 614 in the intervention arm (mean 9.0±5.9), leading to a 56% reduction in medication errors in the treatment arm (Table 2). In the multivariable model, total adjusted medication errors were reduced by an average of 0.11 per month in the intervention arm (95% confidence interval [95% CI], 0.05 to 0.17; P<0.001), as compared with the control arm, leading to a 61% reduction in the risk rate of medication errors over the 12-month study (incident risk ratio [IRR], 0.39; 95% CI, 0.28 to 0.55; P<0.001). Common administrative errors included omissions, additions, and prescribing errors. Clinical errors were largely due to non- or undertreated conditions, primarily electrolyte abnormalities (Supplemental Table 1). Using the Overhage criteria, most medication errors were categorized as significant, but ranged from minor to serious (Supplemental Table 2).
Figure 2.
Coprimary outcome 1: Unadjusted rates of medication errors over the course of the study compared between treatment arms.
Table 2.
Unadjusted event numbers and rates
| Outcomes | Control (n=68) | Intervention (n=68) | P value |
|---|---|---|---|
| Coprimary | |||
| Total number of medication errors | 1385 | 614 | <0.001 |
| Potentially lethal | 0 | 0 | |
| Serious | 1 | 0 | <0.001 |
| Significant | 1320 | 586 | 0.001 |
| Minor | 64 | 28 | <0.001 |
| No error | 0 | 0 | |
| Overall medication error rate, mean (SD) | 20.4 (14.0) | 9.0 (5.9) | |
| Total number of adverse events | 1446 | 1406 | 0.85 |
| Grade 1 | 952 | 949 | 0.98 |
| Grade 2 | 359 | 376 | 0.81 |
| Grade 3 | 107 | 75 | 0.18 |
| Grade 4 | 26 | 5 | <0.001 |
| Grade 5 | 1 | 0 | 1.00 |
| Grade 3 or higher | 134 | 80 | 0.04 |
| Adverse event rate, mean (SD) | 21.26 (25.24) | 20.68 (18.35) | 0.83 |
| Grade 1 | 14.00 (15.10) | 13.96 (1.47) | 0.96 |
| Grade 2 | 5.28 (0.94) | 5.53 (0.72) | 0.93 |
| Grade 3 | 1.57 (0.34) | 1.10 (0.22) | 0.19 |
| Grade 4 | 0.38 (0.15) | 0.07 (0.05) | <0.001 |
| Grade 5 | 0.01 (0.01) | 0 (0) | 1.00 |
| Grade 3 or higher | 1.97 (0.46) | 1.18 (0.25) | 0.04 |
| Secondary | |||
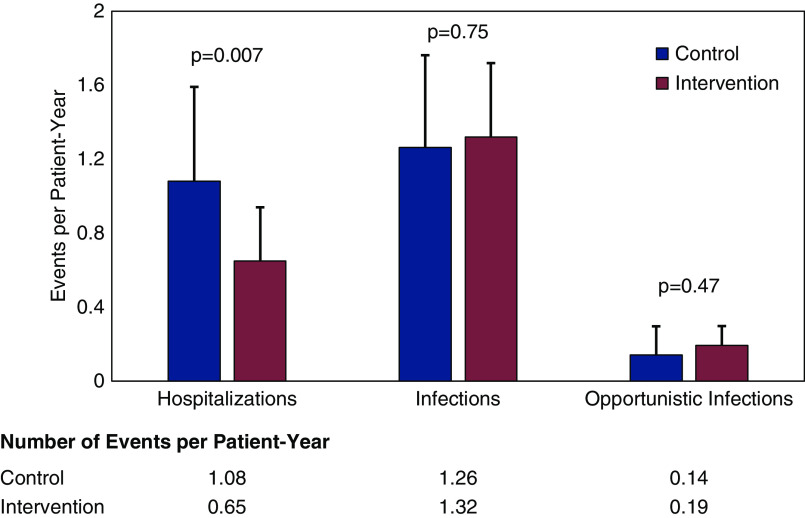
| Total hospitalizations | 74 | 44 | |
| Hospitalization rate (95% CI) | 1.08 (0.60–1.6) | 0.65 (0.40–0.94) | 0.007 |
| Total infections | 86 | 90 | |
| Infection rate (95% CI) | 1.26 (0.76–1.76) | 1.32 (0.92–1.72) | 0.75 |
| Total opportunistic infections | 10 | 13 | |
| Opportunistic infection rate (95% CI) | 0.14 (0.01–0.30) | 0.19 (0.09–0.30) | 0.47 |
95% CI, 95% confidence interval.
Coprimary Outcome 2: Adverse Events
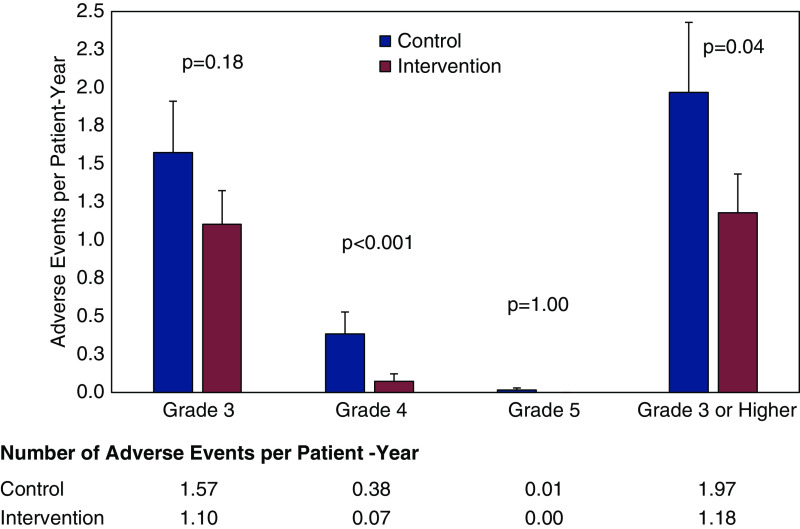
All study participants in the treatment and control arms reported at least one AE. Rates of Grade 1 and Grade 2 AEs were comparable between treatment arms. Participants in the intervention arm experienced numerically lower rates of Grade 3 AEs, but the difference was not statistically significant (1.10 versus 1.57 per patient-year; P=0.19; Table 2). The intervention produced significantly lower rates of Grade 4 AEs, with an overall 12-month risk reduction of 88% (0.07 versus 0.38 per patient-year, adjusted IRR, 0.12; 95% CI, 0.04 to 0.31; P<0.0001; Figure 3). Participants in the intervention group also experienced a 45% lower incidence risk of composite Grade 3 or higher AEs, as compared with the control group (adjusted IRR, 0.55; 95% CI, 0.30 to 0.99; P=0.05; Supplemental Table 3). AEs were most commonly classified as cardiovascular, metabolism, and nutrition disorders, or kidney (Supplemental Table 4). The most common cardiovascular AEs were hypertension (n=124) and anemia (n=81). Metabolism and nutrition disorders were primarily electrolyte imbalances, including hypomagnesemia (n=57), hyponatremia (n=41), and hypercalcemia (n=35). Kidney AEs included proteinuria (n=65), elevated creatinine (n=62), CKD (n=49), and hematuria (n=32).
Figure 3.
Coprimary outcome 2: Unadjusted rates of moderate to severe adverse events compared between treatment arms.
Hospitalizations and Infections
In total, participants receiving intervention experienced fewer hospitalizations as compared with the control arm (44 versus 74). Over the 12-month follow-up, the intervention arm had significantly lower rates of hospitalization (1.08 versus 0.65 hospitalizations per patient-year; P=0.007), with similar rates of clinic visits, procedures, and infections (Figure 4). In multivariable modeling, the intervention produced a 54% reduction in hospitalization, as compared with control (IRR, 0.46; 95% CI, 0.27 to 0.77; P=0.005). The primary causes of hospitalization were infection, AKI, and cardiovascular- or gastrointestinal-related conditions. The most common opportunistic infections were BK viremia and CMV.
Figure 4.
Hospitalization and infection rates compared between treatment arms.
Discussion
In this randomized controlled trial, we demonstrated that a pharmacist-led, mobile health–based intervention improved medication safety in kidney transplant recipients. The treatment produced a significant reduction in medication errors, lower rates of Grade 3 or higher AEs, and reduced hospitalization rates, as compared with controls, during the 12-month study. In terms of AEs, this study demonstrated a significant difference in severity, but was not powered, and it did not demonstrate any difference in type of AEs between the treatment arms.
To our knowledge, this is the first large, randomized controlled trial demonstrating an improvement in medication safety outcomes in organ transplantation using a mobile health–based technology coupled with a pharmacist-led intervention. One previous study with 108 transplant recipients sought to investigate whether a mobile app targeting nonadherence could improve medication adherence. However, the function of the mobile app was limited to medication alerts and participant education, and, ultimately, the study did not improve medication adherence (13). Another study in transplant recipients utilizing the same mobile health platform demonstrated that app users had higher rates of medication recollection, but these findings were not statistically significant (14). Reese et al. conducted a single-center study to investigate the effect of using wireless pill bottles to store and record tacrolimus in kidney transplant recipients. Participants were randomized 1:1:1 to adherence monitoring with customized reminders, customized reminders plus provider notification, or wireless bottle use alone. The study demonstrated significant improvement in adherence in each intervention group as compared with controls (15). In a younger kidney transplant population (median age 15.5 years), participants who received the intervention could elect to receive text messages, emails, and/or visual cue medication reminders, and met with coaches to discuss adherence data in 3-month intervals. This multicomponent intervention led to significantly better medication adherence as compared with controls (16). McGillicuddy et al. conducted a study to assess the sustainability of improvements in medication adherence after a mobile health–based intervention. A total of 18 participants who completed a 3-month randomized controlled trial of a mobile health program designed to improve BP and medication adherence were included in this study. Investigators demonstrated that participants in the intervention group continued to exhibit lower BP as compared with the control group 12 months after completion of the trial (17).
In other conditions, several mobile health–based interventions targeting medication safety issues have demonstrated promising results. In one randomized controlled trial involving 411 adults with poorly controlled hypertension, participants randomized to the intervention utilized a smartphone-enabled app that provided medication lists, medication reminders, and BP tracking using a Bluetooth-enabled monitor. The primary outcome measures were change in self-reported medication adherence and systolic BP at 12 weeks. At week 12, participants in the intervention arm demonstrated a small improvement in mean adherence rates, as compared with control (18). Other studies have demonstrated improvements in clinical outcomes after a mobile health–based intervention, not necessarily related to medication safety issues (19).
We designed our study using a pharmacist as the clinician leading the intervention, recognizing that pharmacists are considered medication safety experts. Previous studies have described the benefits of pharmacist-led interventions on medication safety in the transplant population. Musgrave et al. conducted a prospective observational study to determine if transplant pharmacist involvement in transitions of care would improve medication safety. A prospective cohort of 64 abdominal transplant recipients was matched to a historical cohort of 128 patients. During the prospective period, pharmacists prevented 119 out of 191 errors identified on discharge medication reconciliations. In the retrospective cohort, a total of 430 errors were made and none were prevented at the time of discharge. This study demonstrated a significant reduction in medication errors after transplant pharmacist involvement as compared with a historical control (12). Other studies have described the role of pharmacists during transitions of care outside of the transplant population. One meta-analysis sought to examine the effectiveness of pharmacist-based transitions of care intervention on medication errors after discharge. This study demonstrated that pharmacist involvement in transitions of care leads to a reduction in medication errors and reduces subsequent emergency room visits (20).
The mobile health app developed by our team and utilized in this study addresses multiple levels of medication safety issues beyond nonadherence, and focused on an integrated approach where a pharmacist served as a clinician-coordinator: identifying potential problems and working with the patient and care team to obtain a mutually agreeable solution. We attempted to address many of the previously identified challenges and goals for mobile health platforms, including interoperability between the EMR and app, developing an effective partnership and buy-in between patients and clinicians, ease of use and perceived utility to support durable changes, and provide measurable clinically important changes in care (21). From our results, we believe clinicians should consider integrating these technologies into established clinical treatment pathways to improve medication safety–related outcomes (22). However, it is important to recognize all mobile health–based apps are not created equal. Many existing platforms are narrowly focused on adherence unidirectionally with patients and fail to incorporate clinicians; we believe this inhibits the development of a partnership between patients and clinicians that is a central theory behind the potential effectiveness of mobile health (13 –16). Future research should focus on comprehensive mobile health apps, such as the one utilized in this study, that investigate a more global approach to medication safety. Furthermore, these apps should appropriately involve health care providers, including pharmacists, to adequately mitigate medication safety issues.
There are several limitations to this study. First, we did not use attention control in the control arm, such as general text messaging or education, to minimize risk of bias related to increased attention. Because smartphones were only supplied to intervention arm participants due to cost constraints, this could not be done in all control participants. Thus, it is not possible to delineate which components of this intervention affected outcomes; it may be related to the mobile health application, the increased attention intervention participants received, or the pharmacist-led monitoring and televisits. It is likely all three components affected the outcomes this study achieved. Thus, application of one component of this intervention may not produce similar results. Second, we did not assess level of education, which may have limited our assessment of participant outcomes. Another limitation was that 48 follow-up assessments could not be completed due to lack of ability to contact participants during the assessment windows; however, this represented only 5% of all assessments and did not differ between the treatment and control arms. For completed assessments, medication errors due to electrolyte abnormalities were identified on the basis of center-specific protocols, thus the clinical judgment was not considered, and borderline electrolyte abnormalities were documented as errors. This likely led to a small overestimation of clinical medication errors in both arms but would not affect overall medication error rates. The study duration also represented a limitation, as 12 months was not an adequate amount of time to assess the long-term effect of this intervention. Additionally, this analysis does not address the cost of implementation and maintenance of our mobile health–based intervention; we plan to complete a cost analysis when claims data become available. Finally, the generalizability of these results is limited as this was a single-center study. The key to the success of this intervention was likely the use of technology coupled with the pharmacist-led telemonitoring and management of patients. This intervention can be used at other centers, but both components are important to implement.
This study demonstrated a significant improvement in medication safety using a mobile health, pharmacist-led, telehealth intervention. The intervention arm also had a significant reduction in hospitalizations over the 12-month study period. This system represents a promising mechanism to improve medication safety and outcomes in the kidney transplant population.
Disclosures
D.J. Taber reports employment with Medical University of South Carolina; receiving research funding from Astellas, CareDx, Novartis, and Veloxis; has participated in advisory board meetings with Sanofi-Genzyme; and reports serving as a scientific advisor or member of American Society of Transplantation Community of Practice. J.N. Fleming is employed by CareDx and Transplant Genomics, Inc., but was employed by Medical University of South Carolina during the conduct of this clinical trial. H.M. Gonzales and M.G. Gebregziabher report employment with Medical University of South Carolina. Z. Su reports employment with Medical University of South Carolina and research funding from Novartis. All remaining authors have nothing to disclose.
Funding
This research was performed with support from the Agency for Healthcare Research and Quality grant R18HS023754.
Supplementary Material
Acknowledgments
The Agency for Healthcare Research and Quality had no role in the design or conduct of this study; data collection and analysis; preparation or decision to submit the manuscript for publication. Drs. James Fleming, Mulugeta Gebregziabher, John McGillicuddy, Maria Aurora Posadas-Salas, and David Taber were responsible for the study design and rationale and data review; Drs. James Fleming, John McGillicuddy, and David Taber were responsible for conducting the study; Dr. Haley Gonzales was responsible for data collection; Dr. Mulugeta Gebregziabher, Dr. Haley Gonzales, and Zemin Su were responsible for the data analysis; Dr. John McGillicuddy and Zemin Su were responsible for manuscript editing; Drs. James Fleming, Mulugeta Gebregziabher, Haley Gonzales, John McGillicuddy, Maria Aurora Posadas-Salas, and David Taber were responsible for manuscript preparation; and Zemin Su was responsible for data management.
Data Sharing Statement
Deidentified participant data will be shared immediately after publication when specifically requested. The study protocol, including the statistical analysis plan, have been previously published and are available elsewhere (1). Requests must be reviewed and approved by the local IRR. Data requestors will need to sign a data use agreement.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Twenty-First Century Solutions to Increase Medication Optimization and Safety in Kidney Transplant Patients,” on pages 679–681.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.15911020/-/DCSupplemental.
Supplemental Table 1. Types of medication errors compared between treatment arms.
Supplemental Table 2. Severity of medication errors compared between treatment arms.
Supplemental Table 3. Rates of adverse events by severity, compared between treatment arms.
Supplemental Table 4. Types of adverse events compared between treatment arms.
References
- 1. Fleming JN, Treiber F, McGillicuddy J, Gebregziabher M, Taber DJ: Improving transplant medication safety through a pharmacist-empowered, patient-centered, mHealth-based intervention: TRANSAFE Rx study protocol. JMIR Res Protoc 7: e59, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taber DJ, Pilch NA, McGillicuddy JW, Mardis C, Treiber F, Fleming JN: Using informatics and mobile health to improve medication safety monitoring in kidney transplant recipients. Am J Health Syst Pharm 76: 1143–1149, 2019. [DOI] [PubMed] [Google Scholar]
- 3. Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, Wainright JL, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 20[Suppl s1]: 20–130, 2020. [DOI] [PubMed] [Google Scholar]
- 4. Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Parasuraman R, Abouljoud M, Jacobsen G, Reddy G, Koffron A, Venkat KK: Increasing trend in infection-related death-censored graft failure in renal transplantation. Transplantation 91: 94–99, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Samal L, Bates DW: To err is human: Lessons from patient safety research for transplant care. Clin J Am Soc Nephrol 9: 845–847, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taber DJ, Spivey JR, Tsurutis VM, Pilch NA, Meadows HB, Fleming JN, McGillicuddy JW, Bratton CF, Treiber FA, Baliga PK, Chavin KD: Clinical and economic outcomes associated with medication errors in kidney transplantation. Clin J Am Soc Nephrol 9: 960–966, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taber DJ, Pilch NA, Bratton CF, McGillicuddy JW, Chavin KD, Baliga PK: Medication errors and adverse drug events in kidney transplant recipients: Incidence, risk factors, and clinical outcomes. Pharmacotherapy 32: 1053–1060, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Musgrave CR, Pilch NA, Taber DJ, Meadows HB, McGillicuddy JW, Chavin KD, Baliga PK: Improving transplant patient safety through pharmacist discharge medication reconciliation. Am J Transplant 13: 796–801, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Overhage JM, Lukes A: Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am J Health Syst Pharm 56: 2444–2450, 1999. [DOI] [PubMed] [Google Scholar]
- 12. National Cancer Institute: Common Terminology Criteria for Adverse Events, Version 5.0, Bethesda, MD, National Institutes of Health National Cancer Institute, 2017. [Google Scholar]
- 13. Levine D, Torabi J, Choinski K, Rocca JP, Graham JA: Transplant surgery enters a new era: Increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg 218: 18–20, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Zanetti-Yabur A, Rizzo A, Hayde N, Watkins AC, Rocca JP, Graham JA: Exploring the usage of a mobile phone application in transplanted patients to encourage medication compliance and education. Am J Surg 214: 743–747, 2017. [DOI] [PubMed] [Google Scholar]
- 15. Reese PP, Bloom RD, Trofe-Clark J, Mussell A, Leidy D, Levsky S, Zhu J, Yang L, Wang W, Troxel A, Feldman HI, Volpp K: Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: A randomized trial. Am J Kidney Dis 69: 400–409, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Foster BJ, Pai ALH, Zelikovsky N, Amaral S, Bell L, Dharnidharka VR, Hebert D, Holly C, Knauper B, Matsell D, Phan V, Rogers R, Smith JM, Zhao H, Furth SL: A randomized trial of a multicomponent intervention to promote medication adherence: The teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT) [published correction appears in Am J Kidney Dis 73: 578, 2019 10.1053/j.ajkd.2019.01.009]. Am J Kidney Dis 72: 30–41, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGillicuddy JW, Taber DJ, Mueller M, Patel S, Baliga PK, Chavin KD, Sox L, Favela AP, Brunner-Jackson BM, Treiber FA: Sustainability of improvements in medication adherence through a mobile health intervention. Prog Transplant 25: 217–223, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morawski K, Ghazinouri R, Krumme A, Lauffenburger JC, Lu Z, Durfee E, Oley L, Lee J, Mohta N, Haff N, Juusola JL, Choudhry NK: Association of a smartphone application with medication adherence and blood pressure control: The MedISAFE-BP randomized clinical trial. JAMA Intern Med 178: 802–809, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Meij E, Anema JR, Leclercq WKG, Bongers MY, Consten ECJ, Schraffordt Koops SE, van de Ven PM, Terwee CB, van Dongen JM, Schaafsma FG, Meijerink WJHJ, Bonjer HJ, Huirne JAF: Personalised perioperative care by e-health after intermediate-grade abdominal surgery: A multicentre, single-blind, randomised, placebo-controlled trial. Lancet 392: 51–59, 2018. [DOI] [PubMed] [Google Scholar]
- 20. De Oliveira GS Jr, Castro-Alves LJ, Kendall MC, McCarthy R: Effectiveness of pharmacist intervention to reduce medication errors and health-care resources utilization after transitions of care: A meta-analysis of randomized controlled trials [published online ahead of print June 30, 2017]. J Patient Saf 10.1097/PTS.0000000000000283 [DOI] [PubMed] [Google Scholar]
- 21. Fleming JN, Taber DJ, McElligott J, McGillicuddy JW, Treiber F: Mobile health in solid organ transplant: The time is now. Am J Transplant 17: 2263–2276, 2017. [DOI] [PubMed] [Google Scholar]
- 22. Rowland SP, Fitzgerald JE, Holme T, Powell J, McGregor A: What is the clinical value of mHealth for patients? NPJ Digit Med 3: 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.