Abstract
Among its many functions, owing to its oversized effect on colloid oncotic pressure, intravascular albumin helps preserve the effective circulatory volume. Hypoalbuminemia is common in hospitalized patients and is found especially frequently in patients who require KRT either for AKI or as maintenance hemodialysis. In such patients, hypoalbuminemia is strongly associated with morbidity, intradialytic hypotension, and mortality. Intravenous albumin may be administered in an effort to prevent or treat hypotension or to augment fluid removal, but this practice is controversial. Theoretically, intravenous albumin administration might prevent or treat hypotension by promoting plasma refilling in response to ultrafiltration. However, clinical trials have demonstrated that albumin administration is not nearly as effective a volume expander as might be assumed according to its oncotic properties. Although intravenous albumin is generally considered to be safe, it is also very expensive. In addition, there are potential risks to using it to prevent or treat intradialytic hypotension. Some recent studies have suggested that hyperoncotic albumin solutions may precipitate or worsen AKI in patients with sepsis or shock; however, the overall evidence supporting this effect is weak. In this review, we explore the theoretical benefits and risks of using intravenous albumin to mitigate intradialytic hypotension and/or enhance ultrafiltration and summarize the current evidence relating to this practice. This includes studies relevant to its use in patients on maintenance hemodialysis and critically ill patients with AKI who require KRT in the intensive care unit. Despite evidence of its frequent use and high costs, at present, there are minimal data that support the routine use of intravenous albumin during KRT. As such, adequately powered trials to evaluate the efficacy of intravenous albumin in this setting are clearly needed.
Keywords: chronic hemodialysis, dialysis, hemodialysis, hypotension, albumin, intravenous albumin, kidney replacement therapy, intradialytic hypotension, ultrafiltration, acute kidney injury
Introduction
Albumin is an important 66-kD protein that is synthesized solely in the liver and serves many roles within the human body (1,2). The metabolism of albumin is summarized in Figure 1. Although accounting for only approximately half of the plasma protein content, albumin is responsible for generating an even greater proportion (approximately 70%–80%) of intravascular colloid oncotic pressure due to its capacity to attract water (1–3). Beyond that, endogenous albumin serves many other important functions in the human body. These include maintenance of the redox state as well as molecular and drug transport (4,5). Given albumin’s many functions and that its synthesis is dependent on adequate dietary protein intake, it is not surprising that poor outcomes are associated with hypoalbuminemia across a range of medical conditions (6,7). The corollary to this is that administering intravenous albumin could theoretically be beneficial to patients who are hypoalbuminemic or hypotensive due to intravascular volume depletion. To that end, exogenous human albumin products have been used as therapeutic agents since their introduction into the medical field in the 1940s (8).
Figure 1.
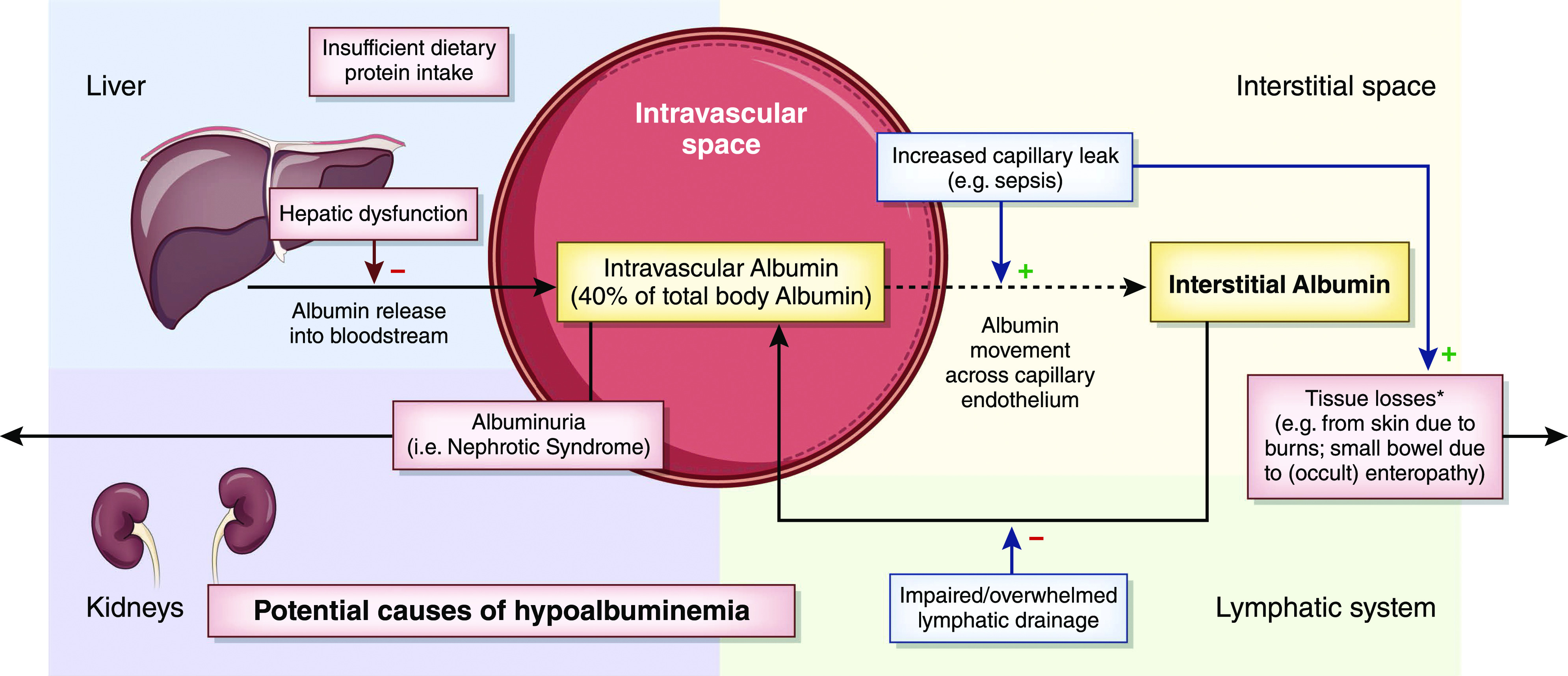
Metabolism of albumin and causes of hypoalbuminemia. Overview of albumin metabolism and selected mechanisms for the development of hypovolemia. Not shown is the normal breakdown of albumin, which has a t 1/2 of approximately 3 weeks. Hypoalbuminemia results from some combination of decreased production, increased losses, acute dilution (not shown), or increased shift out of the vascular space. *Increased skin and gastrointestinal losses typically occur in the context of concurrent, increased capillary leak.
Management of hypotension specifically during KRT (i.e., intradialytic hypotension [IDH]) can be challenging in both critically ill patients on KRT and in patients on maintenance hemodialysis (HD). Prompt identification and reversal of IDH during treatment are important, not only for symptomatic relief but also to mitigate the potential consequences of hypoperfusion (9). Both hyperoncotic (20%–25%) and iso-oncotic (4%–5%) intravenous albumin have been used in place of saline for volume resuscitation in this setting, but this practice is controversial. In the context of hemodynamic instability due to intravascular hypovolemia, hyperoncotic albumin can theoretically be expected to shift water into the vasculature, which may in turn improve hemodynamic stability and then permit greater overall fluid removal via ultrafiltration. There are several other properties of albumin that could theoretically be helpful in the settings of dialysis-requiring AKI and maintenance HD, such as its anti-inflammatory action, antioxidant properties, and role in promoting microvascular circulation (10–12). Nonetheless, not all IDH episodes are due to hypovolemia (13,14), and few studies have specifically addressed the role of intravenous albumin in preventing or treating IDH. The objective of this narrative review is to highlight knowledge gaps in the use of intravenous albumin to prevent or treat IDH and/or facilitate ultrafiltration. In this article, we will summarize the physiology of albumin, explore the implications of hypoalbuminemia, and discuss the potential risks and benefits of intravenous albumin use for these indications.
Incidence and Implications of Hypoalbuminemia
The etiology of hypoalbuminemia in most patients is likely multifactorial. As detailed in Figure 1, several mechanisms are implicated, including increased capillary permeability, exudative losses, breakdown, occult protein wasting enteropathy in critical illness and decreased liver production (4,15). In patients with AKI, the extent to which proteinuria due to tubular damage contributes to hypoalbuminemia is unclear.
Marked hypoalbuminemia (when defined as serum albumin <25 g/L) correlates with a 34% in-hospital mortality rate compared with a 2% mortality rate for hospitalized patients with normal albumin levels of 35–45 g/L (6).The prevalence of hypoalbuminemia in patients on maintenance HD is even greater than in hospitalized patients, reported to be as high as 33% in a study that defined hypoalbuminemia as serum albumin <35 g/L (16). In both patients on incident HD and patients on maintenance HD, hypoalbuminemia is strongly associated with morbidity and mortality (16–18). To a large extent, the correlation between hypoalbuminemia and worse outcomes may simply be due to hypoalbuminemia serving as a marker for poor nutrition and/or inflammation in chronic or severe illness. Notably, the correlation between hypoalbuminemia and increased mortality in patients on maintenance HD is markedly less in those with normal C-reactive protein levels (19). Similarly, hypoalbuminemia is independently associated with IDH (20,21), theoretically due to its effect on oncotic pressure in the circulation; however, residual confounding on the basis of concurrent chronic or severe illness is very likely.
Physiology Relevant to Intravenous Albumin Administration for Intradialytic Hypotension
Endogenous albumin is distributed between the intravascular (40%) and extravascular (60%) space, where it is the most abundant protein in both compartments (3). Serum albumin is responsible for approximately 80% of the colloid oncotic pressure within the vasculature (2). Regarding intravenous albumin, 5% albumin is iso-oncotic with human plasma, whereas 25% albumin is approximately five times more osmotically active.
From a physiologic standpoint, the oncotic action of albumin infusion, which is calculated to attract a total of 18 ml of water per 1 g of protein, would be expected to expand plasma volume to a greater degree than the same volume of normal saline (2). Theoretically, this would be helpful in a setting where a physician desires to minimize volume overload, retaining fluid intravascularly to reduce third spacing. On the basis of its oncotic properties, one would expect that intravenous albumin administration would expand intravascular plasma by a volume that is several times greater than crystalloid alone (3). In intensive care unit (ICU) studies involving critically ill patients, this ratio was found to be much lower than expected, with only approximately 1.4 L saline necessary to result in an equivalent volume of intravascular expansion as 1 L of human albumin product (22,23). Notably, in a trial involving 321 critically ill patients requiring fluid resuscitation, the cumulative volume of resuscitation fluid was reduced by 600 ml over 48 hours through the use of 20% versus 4%–5% albumin fluids, indicating that volume effects depend on the albumin concentration applied (24).
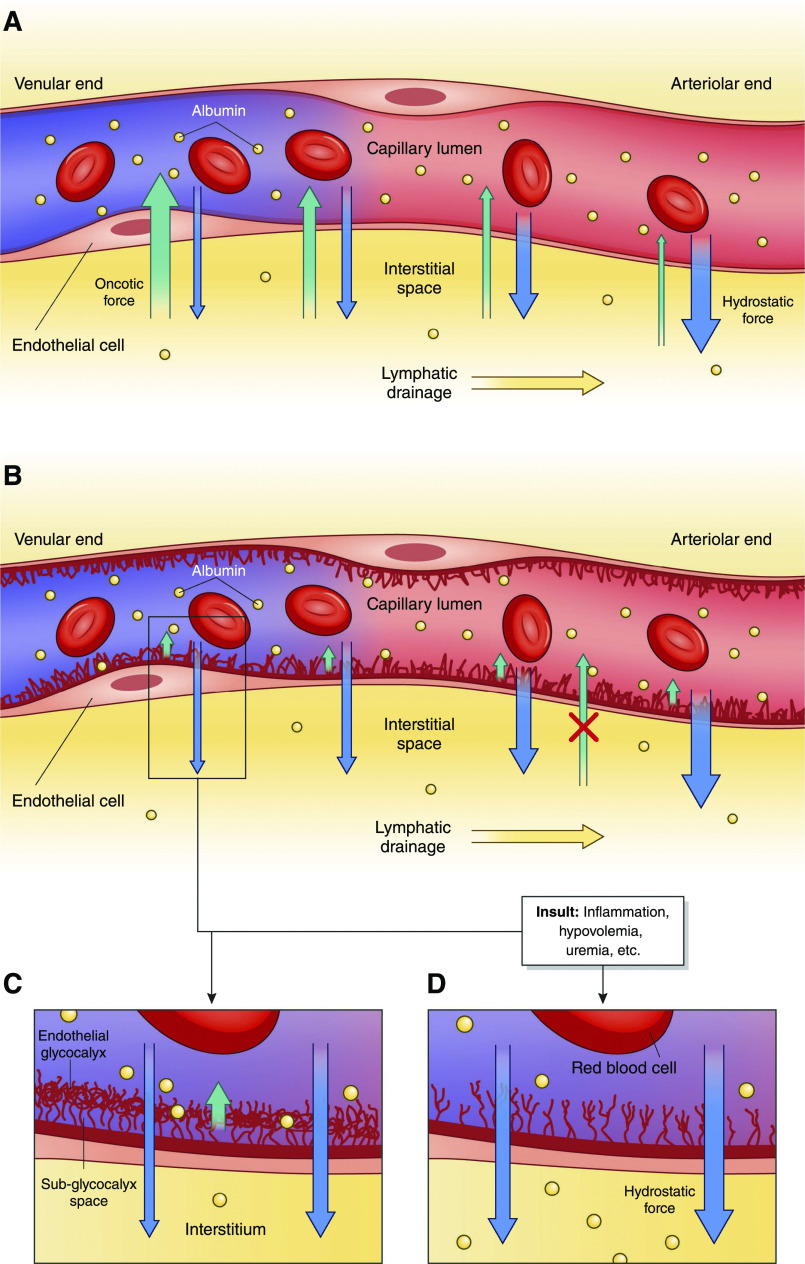
Despite classic teaching, recent studies indicate that fluid movement across the capillary bed into the interstitial space and subsequent reabsorption are not influenced by Starling forces, such as oncotic pressure, in the same manner as previously thought (25). This issue and the role of the endothelial glycocalyx in regulating albumin distribution are detailed in Figure 2 and in Supplemental Material. Supplemental Material also includes additional information regarding the antioxidant and anti-inflammatory roles of albumin in patients with dialysis-requiring AKI or those on maintenance HD.
Figure 2.
Albumin movement across the capillary endothelium in health and disease. (A) In the historical model of capillary fluid exchange on the basis of Starlings forces, net plasma filtration was believed to occur at the arteriolar end of the capillary due to predominant hydrostatic force (blue arrows), which then transitioned to net reabsorption as plasma reached the venular end due to increasing oncotic drive from the interstitium, back into the capillary lumen (green arrows) (additional details are in Supplemental Material). Some research now suggests that there is, in fact, little to no reabsorption at the level of the capillary in the steady state (but it still may occur during acute hypovolemia). (B and C) A simplified version of the endothelial glycocalyx in its role as a selectively permeable barrier for fluid resorption. Hydrostatic pressure is the predominant force in this new model regulated by the glycocalyx, with little to no effect from osmotic forces between the vascular lumen and the interstitium. Instead, the endothelial glycocalyx functions to create an osmotic gradient (orange arrow) between the capillary lumen and the subglycocalyx space that opposes outward shift of fluid but does not reverse the direction of fluid flow. Both the interstitium and the subglycocalyx space have low oncotic content, resulting in negligible oncotic forces between the two spaces and overall net ultrafiltration throughout plasma transit in capillary microcirculation. Fluid that has shifted into the interstitium is resorbed solely through the lymphatic system, with exceptions (including the kidneys). (D) Fluid shift in the setting of a damaged endothelial glycocalyx from stressors, including inflammation, uremia, arteriosclerosis, hyperglycemia, and hypovolemia, among many others. With a damaged endothelial glycocalyx, capillaries have increased permeability to both serum proteins and fluid, leading to increased net fluid movement into the interstitium and interstitial edema when the lymphatic drainage capacity is exceeded.
Albumin Usage for Intradialytic Hypotension
Currently, there are few generally agreed upon indications for intravenous albumin (3). These indications include spontaneous bacterial peritonitis, large volume paracentesis, and hepatorenal syndrome (3). Despite the apparent effectiveness of albumin in the setting of end stage liver disease, it is unclear the extent to which that hinges on pharmacologic cointerventions or other disease-specific factors. Newly reported but not yet published data from a randomized controlled trial (n=828) that assessed albumin normalization using 20% intravenous albumin in hospitalized patients with cirrhosis and serum albumin <30 g/L indicates that this strategy did not significantly improve 28-day mortality. Furthermore, the utility of intravenous albumin products is widely debated for primary volume expansion and specific to nephrology, nephrotic syndrome, enhancement of ultrafiltration/prevention of IDH, or treatment of refractory IDH.
IDH is multifactorial (i.e., not always due to volume depletion) (14), and thus, fluid administration often may not be the most appropriate response. The initial clinical response to IDH is usually to place the patient in Trendelenburg position, pause ultrafiltration, or stop treatment altogether, prior to administering any type of fluid (9,15,26). In a study that included 2559 inpatient HD sessions, of the 433 sessions complicated by IDH, a protocolized approach that used albumin administration as the last line of treatment resulted in reversal of hypotension for 91% of patients who did not receive albumin and in another 2% overall who did get it (27). Currently, normal saline is generally advised as first line if fluid resuscitation is required to restore BP in this setting (28). The potential benefits and risks of using intravenous albumin to enhance ultrafiltration and/or prevent or treat IDH are detailed in Table 1. One unique consideration of the use of albumin in this setting is that its use runs counter to the usual overall goal of net fluid removal with KRT. This is especially the case when considering the use of albumin for prevention of IDH (or to facilitate overall fluid removal) where the alternative would be to not give any fluid. One must also consider that there is an accompanying sodium load as albumin formulations typically have a sodium concentration similar to that of normal saline (as detailed in Table 2). This is a particularly relevant concern given that worse outcomes are found in both critically ill patients and patients on maintenance HD with more fluid overload (29–32). The basis for intravenous albumin use in the setting of KRT (including maintenance HD) is generally extrapolated from the results of studies in critical care but, as further discussed below, is not backed by high-quality trials designed specifically to assess its effect on KRT-related outcomes. Nonetheless, recent data from three large academic medical centers in Ontario, Canada provide ancillary evidence that albumin is frequently used for patients for the prevention or treatment of IDH: approximately 30% of all inpatients who received any form of KRT while hospitalized were also administered albumin (25%) on at least 1 day that they underwent KRT treatment (33).
Table 1.
Potential benefits and risks of intravenous albumin for patients on KRT
| Benefits/Risks | Related Evidence | Comments |
|---|---|---|
| Potential benefits | ||
| Reduced hypotension, enhanced ultrafiltration | No data support use of iso-oncotic albumin. Hyperoncotic albumin trials report hemodynamic benefits (Table 3) | <150 patients included across all trials |
| Anti-inflammatory effects | Patients on maintenance treated with 20% albumin have reduced markers of inflammation (Supplemental Table 1) | Potential effect on patient outcomes is unclear |
| Potential risks | ||
| Unnecessary fluid accumulation | Observational data: less net fluid removal reported for inpatient HD sessions when 25% albumin was given | This finding likely confounded according to indication for albumin use |
| AKI due to hyperoncotic albumin administration | Finding of recent observational studies but hyperoncotic albumin never found to cause AKI across multiple trials | Implications for kidney recovery in AKI, preservation of residual function in HD. Trial data are very reassuring |
| Aluminum toxicity | Historically, trace metals were introduced during manufacturing | Very unlikely given manufacturing improvements |
| Infection | Theoretical risk of transmission of viruses, prions | No cases have been reported |
| Other | Anaphylaxis and hypotension have been reported | Extremely rare relative to other blood products |
HD, hemodialysis.
Table 2.
Fluids used in the treatment or prevention of intradialytic hypotension
| Fluid | Sodium Content, mEq/L | Protein Content, g/L | pH | Cost per 100 ml, US Dollars |
|---|---|---|---|---|
| Iso-oncotic albumin a | 140–145 | 4–5 | 7 | 15.91 |
| Hyperoncotic albumin a | 140–145 | 20–25 | 7 | 108.75 |
| Normal saline | 154 | 0 | 5.5 | 0.10 |
| Hydroxyethyl starch | Contraindicated in AKI and maintenance HD | |||
HD, hemodialysis.
Average values are reported for albumin products (57). Specific manufacturer-reported details for albumin brands available in the United States and Canada are reported in Supplemental Table 1.
Safety of Intravenous Albumin Administration
The safety and efficacy of intravenous albumin administration have been scrutinized heavily in the past due to its cost in comparison with crystalloid solutions and concerns raised by a flawed 1998 metanalysis (34) that included studies done prior to significant quality improvements in the production of albumin (35). Table 1 includes additional details regarding albumin safety. The issue of intravenous iso-osmolar albumin safety, specifically in the setting of fluid resuscitation, was largely resolved with the publication of the Saline versus Albumin Fluid Evaluation (SAFE) study in 2004 (36). A heterogenous group of 6997 patients in the ICU were randomized to receive either 4% albumin or normal saline for intravascular fluid resuscitation, with the primary outcome being all-cause mortality. There was no significant difference in death at 28 days between the two groups. Furthermore, there was no difference in mechanical ventilation, need for KRT, or length of ICU stay. Subgroup and post hoc analyses of 460 patients suggested possible increased risk of death with albumin administration in patients with traumatic brain injury (Relative Risk, 1.63; 95% confidence interval [95% CI], 1.17 to 2.26; P=0.003), as well as increased intracranial pressure in those with intracranial pressure monitoring (36–38).
Some recent studies have suggested a risk of AKI associated with the use of hyperoncotic albumin. A cohort study of 984 patients in the postoperative cardiac surgery period found a dose-dependent increase in AKI with hyperoncotic albumin administration according to a propensity score analysis (39). Similarly, a retrospective cohort study of 11,512 patients in postoperative shock, the majority of whom had undergone cardiac surgery, found a greater risk of AKI (odds ratio [OR], 1.10; 95% CI, 1.04 to 1.17; P=0.002) in those exposed to hyperoncotic albumin (40). Generally, observational studies that have assessed the relationship between hyperoncotic albumin and AKI are significantly limited by the potential for residual confounding according to the indication for hyperoncotic albumin administration. In contrast to the observational data reviewed above, a recent randomized control trial of 220 patients undergoing off-pump coronary artery bypass surgery revealed that preoperative treatment with 20% hyperoncotic albumin for patients with albumin <40 g/L was associated with significantly less postoperative AKI than occurred in patients given the same volume of normal saline (13.7% versus 25.7%, respectively; P=0.05) (41). Furthermore, a 2010 metanalysis evaluating the use of seven trials utilizing hyperoncotic albumin found that albumin was protective against kidney insult (OR, 0.24; 95% CI, 0.12 to 0.48; P<0.001) and mortality (OR, 0.52; 95% CI, 0.28 to 0.95; P=0.05), but the indication for albumin administration in the included studies was quite variable, with many patients with liver cirrhosis included (42). The potential occurrence of renal tubular damage in response to administration of other hyperosmolar fluids is well established (43); however, albumin is not an exogenous colloid and was not found to have accumulated in early kidney autopsy studies of a small number of patients who had received very high volumes of 25% albumin (44). More broadly, a recent network metanalysis including ten studies (6664 patients) with direct comparison could not establish an increased risk for KRT when comparing albumin with crystalloids with moderate certainty (although this included studies of iso-oncotic albumin) (45). In a trial of patients with severe sepsis (n=1818), those randomized to 20% albumin plus crystalloid (targeting albumin >30 g/L) were not at increased risk of severe AKI as a predefined secondary outcome (serum creatinine >300 μmol/L) compared with those randomized to crystalloid alone (21). A post hoc analysis also found no difference between the groups when AKI was defined according to RIFLE criteria.
On balance, we conclude that hyperoncotic intravenous albumin is very unlikely to be directly harmful to the kidneys. This is reassuring with respect to the potential effect on kidney recovery in albumin-treated patients with AKI and preservation of residual kidney function in those on maintenance HD.
Effectiveness of Intravenous Albumin for Patients Receiving Kidney Replacement Therapy
A recent large observational study assessed the use of intravenous 25% albumin for improving ultrafiltration in 238 inpatients (973 HD sessions) at a single large US center (46). Contrary to what might be expected, albumin use (compared with no albumin use) was associated with less ultrafiltration achieved (1242 versus 1899 ml, respectively; P<0.001). Despite adjustment, this result may have been confounded by additional factors related to the indication for which albumin was given, as this study sought to specifically assess instances when albumin had been given to enhance ultrafiltration.
As summarized in Table 3, clinical trials that have specifically assessed the use of intravenous albumin for mitigating hypotension and/or augmenting ultrafiltration (47–51) have generally been small (with all having been conducted at single centers) and are heterogeneous with respect to the intervention (iso- or hyperoncotic albumin, volume, and timing/indications for administration) and outcomes assessed. In the largest trial involving maintenance HD, Knoll et al. (48) sought to assess the use of iso-oncotic (5%) albumin infusion versus normal saline for the acute management of IDH during maintenance HD. Participants were randomized to one of two possible sequences in a crossover study design following three dialysis sessions affected by IDH in a given patient. Individuals in one sequence were treated with 5% albumin infusion for the first dialysis session complicated by IDH, followed by the use of normal saline for the remaining two dialysis sessions where IDH occurred. In the other sequence, treatment was reversed, and patients were treated with normal saline during the first dialysis session complicated by IDH and subsequently, 5% albumin in the remaining sessions. Patients were placed in Trendelenberg position, and ultrafiltration was stopped prior to the administration of study fluid; however, the number of patients whose hypotension resolved prior to any fluid administration was not reported. The primary outcome of the study was percentage of target ultrafiltration achieved on the basis of pre- and postdialysis weights. Approximately 84% and 80% ultrafiltration values were achieved in the albumin and normal saline treatment groups, respectively, with no significant difference detected between the two groups (P=0.14). Secondary outcomes, including postdialysis BP, volume of fluid used in the resuscitation effort, time required to restore BP, or frequency of recurrent hypotensive episodes, were also not significantly different.
Table 3.
Summary of trials assessing intravenous albumin for intradialytic hypotension
| Author [Year] | Study Design a | Study Size | Population | Intervention | Main Results | Study Conclusions |
|---|---|---|---|---|---|---|
| Macedo et al. b | Randomized, crossover | n=65 (249 HD sessions) | Inpatients (maintenance HD or for AKI) with serum albumin <30 g/L | 25% albumin (100 ml) versus NS (100 ml) prior to start of HD session | Albumin resulted in significantly less IDH (defined according to SBP decrease ≥30 or ≥20 mm Hg or nadir SBP <90 mm Hg | Pretreatment with 25% albumin resulted in less frequent IDH |
| Rostoker et al. [2011] (49) | Randomized, single-blind, crossover study | n=10 (HD sessions not specified) | Outpatients on maintenance HD “prone to IDH” | 20% albumin (200 ml) versus 4% gelatin (200 ml) given throughout HD | Fewer episodes of SBP<100 mm Hg in six patients (60%) with albumin | 20% albumin improved hemodynamic parameters |
| Knoll et al. [2004] (48) | Randomized, blinded, crossover study | n=45 (135 HD sessions) | Outpatients on maintenance HD | 5% albumin (250 ml) versus NS (250-ml) boluses (up to 3× per session) for symptomatic IDH | No significant differences in primary outcome (percentage of target UF achieved) or other hemodynamic measures | 5% albumin was not superior to NS for treatment of symptomatic IDH |
| van der Sande et al. [2000] (50) | Randomized, nonblinded, crossover study | n=9 (27 HD sessions) | Outpatients on maintenance HD with recurrent IDH and CHF | 20% albumin (100 ml) versus 3% saline (33 ml) versus 10% HES (100 ml) for BP drop | 20% albumin (and HES) sessions: lower drop in BV and lower drop in end treatment SBP versus 3% saline | 20% albumin was superior to 3% saline for preventing IDH |
| van der Sande et al. [1999] (51) | Randomized, nonblinded, crossover study | n=10 (30 HD sessions) | Outpatients on maintenance HD; CHF excluded | 20% albumin (100 ml) versus NS (100 ml) versus 10% HES (100 ml) for 10% BV decline | Albumin and HES both maintained a significantly smaller change in BV than NS (P=0.05). No significant differences in SBP | 20% albumin was superior to NS for preserving BV during HD |
| Jardin et al. [1982] (47) | Nonblinded, crossover study | n=8 (53 HD sessions) | Inpatients with sepsis and anuric AKI | HD circuit primed with either 300 ml 17.5% albumin or 300 ml NS | Albumin sessions: more UF achieved and higher MAP during treatment versus NS | Priming HD circuit with 17.5% albumin limited IDH, facilitated ultrafiltration |
n, number of patients; HD, hemodialysis; NS, normal saline; IDH, intradialytic hypotension; SBP, systolic BP; UF, ultrafiltration; CHF, congestive heart failure; HES, hydroxyethyl starch; BV, blood volume; MAP, mean arterial pressure.
All single-center studies.
Macedo E, Karl BE, Jacinto LD, Lee E, Mehta RL: A randomized controlled trial of albumin versus saline for the prevention of intradialytic hypotension in hypoalbuminemic patients [Abstract]. J Am Soc Nephrol, 29: 97, 2018
When the Cochrane Group performed a meta-analysis in 2010 to evaluate the use of albumin for IDH in maintenance HD, the study by Knoll et al. (48) in 2004 was the only study to meet inclusion criteria for the analysis (28). No randomized control trials or other randomized crossover studies of sufficient quality were identified, and they concluded that the evidence to support albumin use over normal saline in the setting of IDH was insufficient (28).
In theory, the use of hyperoncotic albumin has a stronger theoretical foundation for use in KRT than iso-oncotic albumin given its potential to result in greater fluid shifts from the interstitium to the intravascular space. In general, the small studies that assessed the use of hyperoncotic albumin did suggest that it led to significant hemodynamic improvements (47,49–51) (see Table 3). The largest trial that assessed the effect of hyperoncotic albumin for prevention (rather than treatment) of IDH included 65 patients starting HD for either AKI or as maintenance HD who were randomized to 100 ml of normal saline versus 25% albumin prior to HD and then alternated between the two fluids for up to six subsequent HD sessions (249 sessions were included). According to some of the IDH definitions assessed (including drop in systolic BP to minimum BP during treatment of >20 mm Hg or nadir systolic BP during treatment <90 mm Hg), albumin administration resulted in significantly fewer episodes of IDH.
There is almost no evidence regarding administration of intravenous albumin for IDH (hemodynamic instability during KRT [14]) in critically ill patients. The only trial in critically ill patients was a study that included eight patients who were septic (47) (Table 3). A 2018 systematic review of interventions to prevent hemodynamic instability during KRT in critically ill patients did not uncover any new studies assessing the use of albumin for IDH (52). The saline versus albumin for extracorporeal fluid removal in slow low-efficiency dialysis pilot study (53) recently completed enrollment of 60 critically ill patients randomized to receive 100 ml of 25% albumin versus normal saline twice during slow low-efficiency dialysis treatments; however, the results have not yet been reported.
Costs of Intravenous Albumin Treatment
Establishing evidence-based indications for intravenous albumin is important given the potential clinical and societal implications, including the high cost of intravenous albumin preparations (Table 2) relative to crystalloid fluids (or relative to not giving anything, if considering its use for prevention of IDH/augmenting ultrafiltration). Analysis over a 3-month period at a single center revealed that the cost associated with albumin use for nonevidence-based indications was US $48,900 (54). In the SAFE trial (described in more detail above), the difference in the cost of treatment was 75-fold higher for patients randomized to 4% albumin versus crystalloid (36), highlighting the need to establish whether the theoretical benefits of albumin use translate into real-world benefits with respect to patient outcomes. One review from 2004 reported that the base cost of a single liter of 5% albumin was $232 compared with <$2.32 for the same volume of normal saline (55). A study published in 2020 estimated that the median cost per patient in the ICU for 25% albumin is $417 (56). Another recently published study evaluated the costs of resuscitation fluids internationally and reported that, after taking into account the greater volume required to achieve an equally effective volume challenge, the cost of normal saline was approximately 27-fold less than for albumin (57). In our opinion, albumin should be thought of as an expensive medication whose routine use should be restricted to evidence-based indications, which do not currently include the prevention or treatment of IDH or facilitation of ultrafiltration during KRT.
Conclusion
More rigorous studies are needed to determine whether there is a role for the routine use of intravenous albumin during KRT. It is possible that hyperoncotic intravenous albumin use during KRT can improve patient outcomes by minimizing fluid overload and limiting cardiac, kidney, and other organ damage potentiated by IDH. Studies to definitively establish if this is true are not only needed, but also, they are readily justifiable on the following basis: there are well-grounded theoretical benefits, and, although generally safe, there are also potential risks. Beyond that, intravenous albumin is very expensive, and there is good evidence that this intervention, tested in fewer than 150 patients in all trials combined, is likely already being given to tens of thousands of patients for these indications on an annual basis. If shown to be ineffective in improving clinically meaningful outcomes, significant resources could be saved, but if shown to be effective, intravenous albumin is widely available, and its use could immediately be more broadly implemented.
Disclosures
J. Callum reports employment at Sunnybrook Health Sciences Centre and has received program support funding from Canadian Blood Services and a clinical trial grant from Octapharma. E. Clark reports receiving research salary support from the University of Ottawa, outside the submitted work. S. Hiremath reports receiving research salary support from the University of Ottawa and serving on the boards for Hypertension Canada and NephJC, outside the submitted work. M. Joannidis reports receiving grants and personal fees from Baxter Healthcare, grants from Fresenius Kabi, and speakers’ fees from CLS Behring, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09670620/-/DCSupplemental.
Supplemental Material. Additional details regarding albumin physiology in patients on KRT.
Supplemental Table 1. Albumin formulations available in the United States and Canada.
References
- 1. Carter DC, Ho JX: Structure of serum albumin. Adv Protein Chem 45: 153–203, 1994. [DOI] [PubMed] [Google Scholar]
- 2. Doweiko JP, Nompleggi DJ: Role of albumin in human physiology and pathophysiology. JPEN J Parenter Enteral Nutr 15: 207–211, 1991. [DOI] [PubMed] [Google Scholar]
- 3. Clarke G, Yan M: Albumin. In: Clinical Guide to Transfusion, edited by Clarke G and Chargé S, Ottawa, Canada, Canadian Blood Services, 2018, pp 1–19 [Google Scholar]
- 4. Cantin AM, Paquette B, Richter M, Larivée P: Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am J Respir Crit Care Med 162: 1539–1546, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Evans TW: Review article: Albumin as a drug--Biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 16[Suppl 5]: 6–11, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Akirov A, Masri-Iraqi H, Atamna A, Shimon I: Low albumin levels are associated with mortality risk in hospitalized patients [published correction appears in Am J Med 133: 646, 2020 10.1016/j.amjmed.2020.02.001]. Am J Med 130: 1465.e11–1465.e19, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Jellinge ME, Henriksen DP, Hallas P, Brabrand M: Hypoalbuminemia is a strong predictor of 30-day all-cause mortality in acutely admitted medical patients: A prospective, observational, cohort study. PLoS One 9: e105983, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McClelland DB: ABC of transfusion. Human albumin solutions. BMJ 300: 35–37, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reilly RF: Attending rounds: A patient with intradialytic hypotension. Clin J Am Soc Nephrol 9: 798–803, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damiani E, Ince C, Orlando F, Pierpaoli E, Cirioni O, Giacometti A, Mocchegiani F, Pelaia P, Provinciali M, Donati A: Effects of the infusion of 4% or 20% human serum albumin on the skeletal muscle microcirculation in endotoxemic rats. PLoS One 11: e0151005, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papatheodorou L, Weiss N: Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal 9: 1941–1958, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Rostoker G, Griuncelli M, Loridon C, Bourlet T, Illouz E, Benmaadi A: Modulation of oxidative stress and microinflammatory status by colloids in refractory dialytic hypotension. BMC Nephrol 12: 58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chou JA, Kalantar-Zadeh K, Mathew AT: A brief review of intradialytic hypotension with a focus on survival. Semin Dial 30: 473–480, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Douvris A, Zeid K, Hiremath S, Bagshaw SM, Wald R, Beaubien-Souligny W, Kong J, Ronco C, Clark EG: Mechanisms for hemodynamic instability related to renal replacement therapy: A narrative review. Intensive Care Med 45: 1333–1346, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assimon MM, Flythe JE: Intradialytic blood pressure abnormalities: The highs, the lows and all that lies between. Am J Nephrol 42: 337–350, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antunes SA, Canziani ME, Campos AF, Vilela RQB: Hypoalbuminemia seems to be associated with a higher rate of hospitalization in hemodialysis patients. J Bras Nefrol 38: 70–75, 2016. [DOI] [PubMed] [Google Scholar]
- 17. Owen WF Jr., Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, Ball A, Stehman-Breen CO: Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int 61: 630–637, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Alves FC, Sun J, Qureshi AR, Dai L, Snaedal S, Bárány P, Heimbürger O, Lindholm B, Stenvinkel P: The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One 13: e0190410, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, Amin A, Kovesdy CP, Sim JJ, Kalantar-Zadeh K: Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant 33: 149–159, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kora M, Tawfeek A, El-zorkany K, El-Mohsen AHA: The relationship between hypoalbuminemia and intradialytic hypotention in hemodialysis patients. Menoufia Med J 33: 110–115, 2020. [Google Scholar]
- 22. Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, Fanizza C, Caspani L, Faenza S, Grasselli G, Iapichino G, Antonelli M, Parrini V, Fiore G, Latini R, Gattinoni L; ALBIOS Study Investigators: Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 370: 1412–1421, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J; SAFE TRIPS Investigators: Resuscitation fluid use in critically ill adults: An international cross-sectional study in 391 intensive care units. Crit Care 14: R185, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mårtensson J, Bihari S, Bannard-Smith J, Glassford NJ, Lloyd-Donald P, Cioccari L, Luethi N, Tanaka A, Crisman M, Rey de Castro N, Ottochian M, Huang A, Cronhjort M, Bersten AD, Prakash S, Bailey M, Eastwood GM, Bellomo R: Small volume resuscitation with 20% albumin in intensive care: Physiological effects: The SWIPE randomised clinical trial. Intensive Care Med 44: 1797–1806, 2018. [DOI] [PubMed] [Google Scholar]
- 25. Levick JR, Michel CC: Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Agarwal R: How can we prevent intradialytic hypotension? Curr Opin Nephrol Hypertens 21: 593–599, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Emili S, Black NA, Paul RV, Rexing CJ, Ullian ME: A protocol-based treatment for intradialytic hypotension in hospitalized hemodialysis patients. Am J Kidney Dis 33: 1107–1114, 1999. [DOI] [PubMed] [Google Scholar]
- 28. Fortin PM, Bassett K, Musini VM: Human albumin for intradialytic hypotension in haemodialysis patients. Cochrane Database Syst Rev CD006758, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettilä V; FINNAKI Study Group: Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: Data from the prospective FINNAKI study. Crit Care 16: R197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang N, Jiang L, Zhu B, Wen Y, Xi XM; Beijing Acute Kidney Injury Trial (BAKIT) Workgroup: Fluid balance and mortality in critically ill patients with acute kidney injury: A multicenter prospective epidemiological study. Crit Care 19: 371, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Chen Z, Diao Y, Yang Y, Fu P: Associations of fluid overload with mortality and kidney recovery in patients with acute kidney injury: A systematic review and meta-analysis. J Crit Care 30: 860.e7–860.e13, 2015. [DOI] [PubMed] [Google Scholar]
- 32. Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F; Lung US in CKD Working Group: Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 24: 639–646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tinmouth A, Chasse M, Heddle NM, Hsia C, Liu Y, Barty R, Eckert K, Kinney J, McIntyre L: Ontario data collection strategy: Albumin proof of concept project: Final Report. Ottawa, ON, Canada, Canadian Blood Services, 2016.
- 34. Patey R, Wilson G, Hulse T, Pinsky M, Grounds R, Bennett E: Albumin controversy continues. Meta-analysis has affected use of albumin. BMJ 318: 464, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matejtschuk P, Dash CH, Gascoigne EW: Production of human albumin solution: A continually developing colloid. Br J Anaesth 85: 887–895, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R; SAFE Study Investigators: A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350: 2247–2256, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Cooper DJ, Myburgh J, Heritier S, Finfer S, Bellomo R, Billot L, Murray L, Vallance S; SAFE-TBI Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group: Albumin resuscitation for traumatic brain injury: Is intracranial hypertension the cause of increased mortality? J Neurotrauma 30: 512–518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S; SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health: Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 357: 874–884, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Frenette AJ, Bouchard J, Bernier P, Charbonneau A, Nguyen LT, Rioux JP, Troyanov S, Williamson DR: Albumin administration is associated with acute kidney injury in cardiac surgery: A propensity score analysis. Crit Care 18: 602, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Udeh CI, You J, Wanek MR, Dalton J, Udeh BL, Demirjian S, Rahman N, Hata JS: Acute kidney injury in postoperative shock: Is hyperoncotic albumin administration an unrecognized resuscitation risk factor? Perioper Med (Lond) 7: 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee EH, Kim WJ, Kim JY, Chin JH, Choi DK, Sim JY, Choo SJ, Chung CH, Lee JW, Choi IC: Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology 124: 1001–1011, 2016. [DOI] [PubMed] [Google Scholar]
- 42. Wiedermann CJ, Dunzendorfer S, Gaioni LU, Zaraca F, Joannidis M: Hyperoncotic colloids and acute kidney injury: A meta-analysis of randomized trials. Crit Care 14: R191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fogo AB, Lusco MA, Najafian B, Alpers CE: AJKD atlas of renal pathology: Osmotic tubular injury. Am J Kidney Dis 69: e11–e12, 2017. [DOI] [PubMed] [Google Scholar]
- 44. Janeway CA, Gibson ST, Woodruff LM, Heyl JT, Bailey OT, Newhouser LR: Chemical, clinical, and immunological studies on the products of human plasma fractionation. VII. Concentrated human serum albumin. J Clin Invest 23: 465–490, 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rochwerg B, Alhazzani W, Gibson A, Ribic CM, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, Mbuagbaw L, Szczeklik W, Alshamsi F, Altayyar S, Ip W, Li G, Wang M, Włudarczyk A, Zhou Q, Annane D, Cook DJ, Jaeschke R, Guyatt GH; FISSH Group (Fluids in Sepsis and Septic Shock): Fluid type and the use of renal replacement therapy in sepsis: A systematic review and network meta-analysis. Intensive Care Med 41: 1561–1571, 2015. [DOI] [PubMed] [Google Scholar]
- 46. Buckley MS, Erstad BL, Lansburg JM, Agarwal SK: Hyperoncotic albumin reduces net fluid loss associated with hemodialysis. Hosp Pharm 55: 130–134, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jardin F, Prost JF, Ozier Y, Margairaz A: Hemodialysis in septic patients: Improvements in tolerance of fluid removal with concentrated albumin as the priming fluid. Crit Care Med 10: 650–652, 1982. [PubMed] [Google Scholar]
- 48. Knoll GA, Grabowski JA, Dervin GF, O’Rourke K: A randomized, controlled trial of albumin versus saline for the treatment of intradialytic hypotension. J Am Soc Nephrol 15: 487–492, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Rostoker G, Griuncelli M, Loridon C, Bourlet T, Illouz E, Benmaadi A: A pilot study of routine colloid infusion in hypotension-prone dialysis patients unresponsive to preventive measures. J Nephrol 24: 208–217, 2011. [DOI] [PubMed] [Google Scholar]
- 50. van der Sande FM, Luik AJ, Kooman JP, Verstappen V, Leunissen KML: Effect of intravenous fluids on blood pressure course during hemodialysis in hypotensive-prone patients. J Am Soc Nephrol 11: 550–555, 2000. [DOI] [PubMed] [Google Scholar]
- 51. van der Sande FM, Kooman JP, Barendregt JN, Nieman FH, Leunissen KM: Effect of intravenous saline, albumin, or hydroxyethylstarch on blood volume during combined ultrafiltration and hemodialysis. J Am Soc Nephrol 10: 1303–1308, 1999. [DOI] [PubMed] [Google Scholar]
- 52. Douvris A, Malhi G, Hiremath S, McIntyre L, Silver SA, Bagshaw SM, Wald R, Ronco C, Sikora L, Weber C, Clark EG: Interventions to prevent hemodynamic instability during renal replacement therapy in critically ill patients: A systematic review. Crit Care 22: 41, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clark EG, McIntyre L, Ramsay T, Tinmouth A, Knoll G, Brown PA, Watpool I, Porteous R, Montroy K, Harris S, Kong J, Hiremath S: Saline versus albumin fluid for extracorporeal removal with slow low-efficiency dialysis (SAFER-SLED): Study protocol for a pilot trial. Pilot Feasibility Stud 5: 72, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dastan F, Jamaati H, Emami H, Haghgoo R, Eskandari R, Hashemifard SS, Khoddami F, Mirshafiei Langari Z: Reducing inappropriate utilization of albumin: The value of pharmacist-led intervention model. Iran J Pharm Res 17: 1125–1129, 2018. [PMC free article] [PubMed] [Google Scholar]
- 55. Primack WA, Estes K: Fluid resuscitation in the intensive care unit. N Engl J Med 351: 1905–1908, 2004. [PubMed] [Google Scholar]
- 56. Torbic H, Bauer SR, Militello M, Welch S, Udeh C, Richardson S: Evaluation of albumin 25% use in critically ill patients at a tertiary care medical center. Hosp Pharm 55: 90–95, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor C, Yang L, Finfer S, Machado FR, YouZhong A, Billot L, Bloos F, Bozza F, Cavalcanti AB, Correa M, Du B, Hjortrup PB, McIntyre L, Saxena M, Schortgen F, Watts NR, Myburgh J, Thompson K, Hammond NE: An international comparison of the cost of fluid resuscitation therapies. Aust Crit Care, 2020, Published ahead of print 10.1016/j.aucc.2020.06.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.