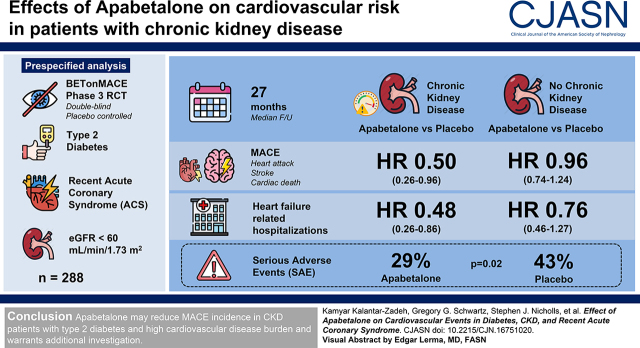
Visual Abstract
Keywords: epigenetic pharmacotherapy, apabetalone, alkaline phosphatase, major adverse cardiovascular events, CKD stage 5, chronic kidney disease, diabetes, diabetes mellitus, cardiovascular
Abstract
Background and objectives
CKD and type 2 diabetes mellitus interact to increase the risk of major adverse cardiovascular events (i.e., cardiovascular death, nonfatal myocardial infarction, or stroke) and congestive heart failure. A maladaptive epigenetic response may be a cardiovascular risk driver and amenable to modification with apabetalone, a selective modulator of the bromodomain and extraterminal domain transcription system. We examined this question in a prespecified analysis of BETonMACE, a phase 3 trial.
Design, setting, participants, & measurements
BETonMACE was an event-driven, randomized, double-blind, placebo-controlled trial comparing effects of apabetalone versus placebo on major adverse cardiovascular events and heart failure hospitalizations in 2425 participants with type 2 diabetes and a recent acute coronary syndrome, including 288 participants with CKD with eGFR <60 ml/min per 1.73 m2 at baseline. The primary end point in BETonMACE was the time to the first major adverse cardiovascular event, with a secondary end point of time to hospitalization for heart failure.
Results
Median follow-up was 27 months (interquartile range, 20–32 months). In participants with CKD, apabetalone compared with placebo was associated with fewer major adverse cardiovascular events (13 events in 124 patients [11%] versus 35 events in 164 patients [21%]; hazard ratio, 0.50; 95% confidence interval, 0.26 to 0.96) and fewer heart failure–related hospitalizations (three hospitalizations in 124 patients [3%] versus 14 hospitalizations in 164 patients [9%]; hazard ratio, 0.48; 95% confidence interval, 0.26 to 0.86). In the non-CKD group, the corresponding hazard ratio values were 0.96 (95% confidence interval, 0.74 to 1.24) for major adverse cardiovascular events, and 0.76 (95% confidence interval, 0.46 to 1.27) for heart failure–related hospitalization. Interaction of CKD on treatment effect was P=0.03 for major adverse cardiovascular events, and P=0.12 for heart failure–related hospitalization. Participants with CKD showed similar numbers of adverse events, regardless of randomization to apabetalone or placebo (119 [73%] versus 88 [71%] patients), and there were fewer serious adverse events (29% versus 43%; P=0.02) in the apabetalone group.
Conclusions
Apabetalone may reduce the incidence of major adverse cardiovascular events in patients with CKD and type 2 diabetes who have a high burden of cardiovascular disease.
Introduction
CKD is found in at least 10% of the general adult population and is associated with a high burden of cardiovascular disease and poor clinical outcomes. A leading cause of CKD is type 2 diabetes mellitus, which in some regions is found in half of all patients with CKD (1). Both diabetes and CKD are strongly associated with higher risk of coronary and cerebrovascular disease, congestive heart failure, and death. The potentiation of cardiovascular risk by CKD, with or without diabetes as its etiology, is associated with abnormal inflammation, dysregulation of the renin-angiotensin system, dyslipidemia, platelet hyper-reactivity, endothelial dysfunction, vascular calcification, and a prothrombotic milieu (2). In addition, alkaline phosphatase is typically elevated in patients with CKD, which may contribute to risk of cardiovascular disease (3). Whereas cholesterol lowering with statins (4) and treatment with some other agents, including sodium-glucose transport protein 2 (SGLT2) inhibitors (5) or glucagon-like peptide-1 agonists (6,7), have reduced cardiovascular risk in patients with moderate CKD, residual risk remains substantial. Thus, there is a major unmet need to lower the residual risk of cardiovascular morbidity and death in patients with CKD.
Epigenetic modulators are novel pharmacologic agents that modify gene transcription. Bromodomain and extraterminal (BET) domain proteins function as epigenetic readers and interact with active chromatin to open regions of DNA containing genes accessible for transcription (8 –11). BET proteins bind acetylated lysine residues on histones, transcription factors, and histone remodelers, forming molecular scaffolds between chromatin and transcriptional machinery to facilitate transcription and mRNA production (8 –11), and, by doing so, they contribute to maladaptive gene expression in various models of cardiovascular disease (12,13). Hence, BET protein inhibition may alter disease-driven cellular responses in persons with high risk of cardiovascular disease, including those with CKD (12 –18). Apabetalone is an oral BET inhibitor with selectivity for binding bromodomain 2 with anti-inflammatory and alkaline phosphatase–lowering properties (14 –18). The phase 3 BETonMACE (Effect of RVX000222 on Time to Major Adverse Cardiovascular Events in High-Risk T2DM Subjects With CAD) trial compared treatment with apabetalone versus placebo in patients with type 2 diabetes, low HDL cholesterol, and recent acute coronary syndrome. Apabetalone treatment resulted in fewer major adverse cardiovascular events (MACE), but the effect did not reach statistical significance (17,18). In this prespecified analysis, we examined the effects of apabetalone or placebo according to the presence of moderate CKD.
Materials and Methods
The design (17) and main results (18) of the phase 3 BETonMACE trial have been reported. Following the Declaration of Helsinki guidelines, the trial was overseen by a blinded, independent, academic steering committee, and safety was continuously assessed by an independent data safety and monitoring committee. BETonMACE was an event-driven, randomized, double-blind, placebo-controlled, multicenter trial performed at 190 sites in 13 countries from November 11, 2015 through July 3, 2019. It included 2425 participants, aged ≥18 years, with type 2 diabetes, low HDL cholesterol levels (<40 mg/dl in men or <45 mg/dl in women), and recent acute coronary syndrome (acute myocardial infarction or unstable angina 7–90 days before randomization) (18). Exclusion criteria included an eGFR <30 ml/min per 1.73 m2, liver transaminase levels >1.5 times the upper limit of normal, and total bilirubin level greater than the upper limit of normal. Participants were classified as having CKD at baseline if their eGFR was <60 ml/min per 1.73 m2 body surface area, calculated with the Cockcroft–Gault equation. Eligible participants were randomized 1:1 to receive 100 mg apabetalone orally twice daily (n=1215) or matching placebo (1210), in addition to intensive statin treatment and other standard care. Treatment allocation was stratified by country and background statin type, using block randomization with a block size of four, and assignment was done using an interactive internet-response system from a computer-generated randomization list (18). The sponsor, academic steering committee, and principal investigators were blinded to treatment allocation, whereas the data-safety monitoring board was unblinded to treatment allocation for safety data only. The primary outcome was time to the first occurrence of cardiovascular death, nonfatal myocardial infarction, or stroke, together referred to as MACE. Hospitalization for heart failure was a secondary outcome. Outcomes were adjudicated by an events committee composed of cardiologists and neurologists who were blinded to treatment assignment. Analyses of the effects of assigned treatment on MACE and on hospitalization for heart failure according to the presence or absence of CKD were prespecified in the supplemental statistical analysis plan (see Supplemental Appendix 1) that was developed and accepted before unblinding the study results. The analysis of effects of treatment on the composite of MACE or hospitalization for heart failure according to CKD status was post hoc and exploratory. Due to the nature of this clinical research, participants of the BETonMACE study were not asked for their data to be shared publicly.
Statistical Analyses
Statistical analyses were conducted in accordance with the prespecified statistical analysis plan (see supplement 1 in Ray et al. [18]) using the full analysis set, i.e., all randomized subjects who received any amount of study therapy and had at least one measurement of the assessment of interest. The CKD and non-CKD subgroups were prespecified subgroups. Baseline characteristics were summarized for CKD and non-CKD subgroups, and by treatment arm for the CKD subgroup, as mean (SD) or median (interquartile range [IQR]) for continuous variables, and counts and percentages for categoric variables. Changes in clinical chemistry variables were analyzed using analysis of covariance models with baseline biomarker value, statin, and country as covariates; the primary coefficient of interest was the between-treatment group difference in change from baseline, referred to herein as the adjusted difference. Adjusted differences were calculated for the CKD and non-CKD groups, and interaction P values were calculated from the analysis of covariance model to assess differences in treatment effect between CKD and non-CKD groups. Time-to-event (MACE or heart failure hospitalization) analyses were conducted using a log-rank test to calculate P values, and a Cox proportional-hazards model to estimate the hazard ratio (HR) with 95% confidence interval (95% CI), with stratification by statin and country and with adjustment for sex and age; event counts and percentages were also summarized. Such analyses were conducted within the placebo group to compare rates between CKD and non-CKD groups, and within the CKD and non-CKD groups, respectively, to assess treatment effects. A Cox model was also used to calculate interaction P values for treatment and CKD/non-CKD group status. Kaplan–Meier analyses assessing time to events by treatment and CKD group were also conducted.
Results
Of a total 2425 participants in the BETonMACE trial, 1215 were assigned to apabetalone and 1210 to placebo (18). A total of 288 participants (12%) had CKD upon study entry, defined as an eGFR of 30–59 ml/min per 1.73 m2. Of these, 124 patients were assigned to apabetalone and 164 to placebo. The aggregate CKD subgroup included 186 participants (65%) with CKD stage 3a (eGFR 45 to <60 ml/min per 1.73 m2) and 102 participants (35%) with CKD stage 3b (eGFR <45 ml/min per 1.73 m2). Mean (SD) eGFR among those with CKD and without CKD was 49 (9) and 111 (35) ml/min per 1.73 m2, respectively.
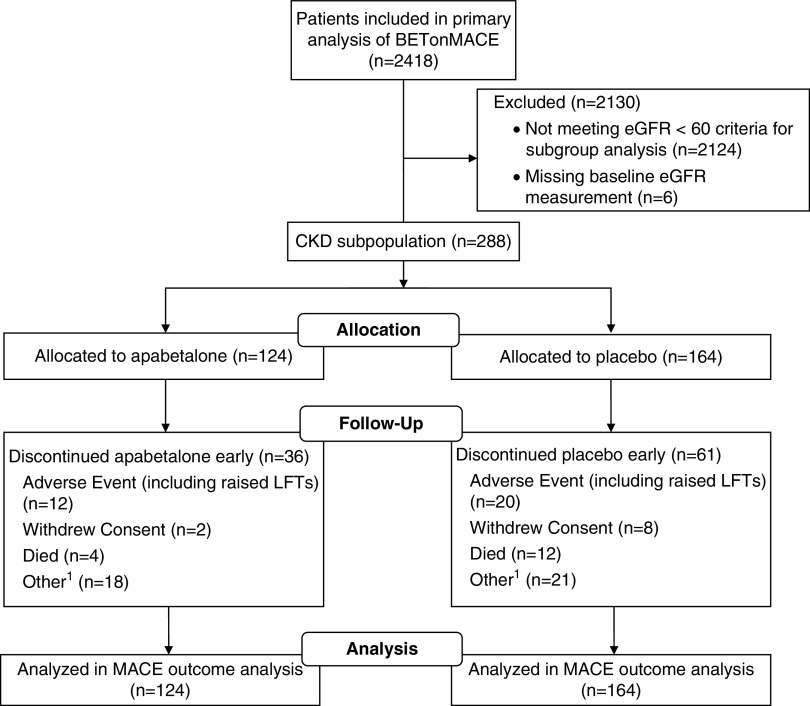
Figure 1 shows patient flow chart in the CKD subgroup of the BETonMACE trial. Table 1 shows the baseline demographic and clinical characteristics of the trial participants according to CKD category. In the full trial cohort and the CKD and no-CKD subgroups, baseline characteristics were generally well balanced between treatment groups (18). Participants with CKD were, on average, 10 years older than those without CKD, were more likely to be female, less likely to be of White race or a current smoker, and had a longer duration of type 2 diabetes. There was no significant difference in proportion of the type of qualifying recent acute coronary syndrome (i.e., acute myocardial infarction versus unstable angina by CKD category), but, among those with myocardial infarction, the proportion without ST elevation was greater in those with CKD.
Figure 1.
Patient flow in the CKD subgroup of the BETonMACE trial of apabetalone for reduction of adverse cardiovascular events in patients with acute coronary syndrome and type 2 diabetes. 1Discontinuation due to patient preference. LFTs, liver function tests; MACE, major adverse cardiovascular events.
Table 1.
Demographic, clinical, pharmacologic, and laboratory characteristics of the BETonMACE trial participants at baseline according to CKD status and assigned treatment group
| Characteristics | Full Study Cohort according to CKD Status a | Patients with CKD by Assigned Treatment Group b | ||
|---|---|---|---|---|
| eGFR ≥60 ml/min per 1.73 m2 (no-CKD) | eGFR <60 ml/min per 1.73 m2 (CKD) | Placebo (eGFR <60 ml/min per 1.73 m2) | Apabetalone (eGFR <60 ml/min per 1.73 m2) | |
| No. of participants | 2125 | 288 | 164 | 124 |
| Age, yr | 61 (54–67) | 71 (65–76) | 71 (66–77) | 70 (65–75) |
| Female, n (%) | 497 (23) | 120 (42) | 72 (44) | 48 (39) |
| White race, n (%) | 1879 (88) | 235 (82) | 137 (84) | 98 (79) |
| Asian race, n (%) | 28 (1) | 11 (4) | 5 (3) | 6 (5) |
| Body mass index, kg/m2 | 30.6 (4.9) | 27.4 (3.9) | 27.5 (4.1) | 27.2 (3.6) |
| Hypertension history, n (%) | 1876 (88) | 263 (91) | 148 (90) | 115 (93) |
| Smoking status, n (%) | 253 (12) | 19 (7) | 11 (7) | 8 (6) |
| Diabetes duration, yr | 8.2 (7.3) | 11.3 (9.1) | 11.9 (9.1) | 10.5 (9.1) |
| Prior myocardial infarction, n (%) | 300 (14) | 49 (17) | 27 (16) | 22 (18) |
| Prior revascularization, n (%) | 442 (21) | 71 (25) | 37 (23) | 34 (27) |
| Index acute coronary syndrome c | ||||
| Myocardial infarction, n (%) | 1560 (74) | 215 (75) | 126 (78) | 89 (72) |
| NSTEMI, n (%) | 718 (46) | 116 (55) | 68 (55) | 48 (54) |
| STEMI, n (%) | 835 (54) | 96 (45) | 56 (45) | 40 (45) |
| Unstable angina, n (%) | 553 (26) | 70 (25) | 36 (22) | 34 (28) |
| Time from index ACS, d | 38 (25–63) | 35 (23–57) | 36 (23–58) | 33 (23–54) |
| Cardiovascular medications, n (%) | ||||
| Atorvastatin | 1084 (51) | 153 (53) | 91 (55) | 62 (50) |
| Rosuvastatin | 1041 (49) | 135 (47) | 73 (45) | 62 (50) |
| Intensive statin therapy | 1929 (91) | 247 (86) | 139 (85) | 108 (87) |
| Ezetimibe | 50 (2) | 17 (6) | 13 (8) | 4 (3) |
| ACE inhibitors or ARB | 1957 (92) | 268 (93) | 154 (94) | 114 (92) |
| β-Blockers | 1925 (91) | 262 (91) | 150 (91) | 112 (90) |
| Antiplatelet agents | 2099 (99) | 287 (99) | 164 (100) | 123 (99) |
| Diabetes medications, n (%) | ||||
| Metformin | 1794 (84) | 200 (69) | 104 (63) | 96 (77) |
| Insulin | 787 (37) | 121 (42) | 74 (45) | 47 (38) |
| Sulfonylureas | 619 (29) | 88 (31) | 46 (28) | 42 (34) |
| DPP4 inhibitors | 307 (14) | 51 (18) | 28 (17) | 23 (19) |
| SGLT2 inhibitors | 279 (13) | 18 (6) | 9 (5) | 9 (7) |
| GLP1 receptor agonists | 81 (4) | 5 (2) | 4 (2) | 1 (0.8) |
| Other d | 90 (4) | 17 (6) | 10 (6) | 7 (6) |
| Biochemical parameters | ||||
| Serum creatinine, mg/dl | 0.90 (0.21) | 1.4 (0.5) | 1.4 (0.5) | 1.4 (0.4) |
| eGFR, ml/min per 1.73 m2 | 111 (35) | 49 (9) | 48 (9) | 49 (9) |
| eGFR 45 to <60 ml/min per 1.73 m 2 | N/A | 186 (65) | 104 (63) | 82 (66) |
| eGFR <45 ml/min per 1.73 m 2 | N/A | 102 (35) | 57 (35) | 41 (33) |
| HbA1c, % | 7.3 (6.4–8.7) | 7.2 (6.4–8.5) | 7.1 (6.4–8.4) | 7.3 (6.5–8.6) |
| Serum glucose, mg/dl | 152 (61) | 149 (66) | 147 (65) | 151 (68) |
| Total cholesterol, mg/dl | 135 (35) | 140 (46) | 145 (49) | 134 (41) |
| LDL cholesterol, mg/dl | 70 (30) | 73 (39) | 77 (42) | 69 (35) |
| HDL cholesterol, mg/dl | 33 (5) | 33 (6) | 34 (6) | 33 (5) |
| Triglycerides, mg/dl | 147 (113–200) | 157 (117–202) | 163 (130–205) | 145 (107–190) |
| Alkaline phosphatase, U/L | 81 (29) | 91 (71) | 90 (61) | 93 (83) |
| Alanine aminotransferase, U/L | 26 (14) | 23 (18) | 24 (22) | 21 (10) |
| Systolic BP (mm Hg) | 129 (15) | 129 (15) | 128 (16) | 131 (14) |
| Diastolic BP (mm Hg) | 77 (9) | 75 (9) | 75 (10) | 75 (9) |
| Total bilirubin, umol/L | 0.6 (0.2) | 0.6 (0.3) | 0.6 (0.3) | 0.6 (0.3) |
| hsCRP, mg/L | 2.7 (1.2–5.9) | 3.2 (1.1–7.6) | 3.9 (1.1–10.1) | 3.0 (1.3–5.7) |
| NLR | 2.5 (1.9–3.3) | 2.9 (2.2–3.9) | 2.9 (2.2–4.0) | 2.8 (2.1–3.7) |
Continuous variables are presented as mean (SD) or median (interquartile range). Categoric variables are presented as n (%). NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; ACS, acute coronary syndrome; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DPP4, dipeptidyl peptidase-4; SGLT2, sodium-glucose cotransporter 2; GLP1, glucagon-like peptide 1; N/A, not applicable; HbA1c, hemoglobin A1C; hsCRP, high-sensitivity C-reactive protein; NLR, neutrophil/lymphocyte ratio.
Data for the full study cohort according to baseline CKD status.
Data for the CKD subgroup according to assigned treatment group.
There was no significant difference in proportion of myocardial infarction versus unstable angina as an index event (P=0.61); but, among those with myocardial infarction as the index event, there were significant differences in proportion of STEMI versus non-STEMI (P=0.03).
Other diabetes medications include acarbose, pioglitazone, and repaglinide.
As shown in Table 1, irrespective of CKD or non-CKD, >90% of participants received inhibitors of the renin-angiotensin pathway, β-blockers, and antiplatelet agents. Fewer participants with CKD than those with non-CKD were treated with metformin (69% versus 84%) or SGLT2 inhibitors (6% versus 13%), while those in the CKD group had a longer mean duration of diabetes (11.3 versus 8.2 years). Among laboratory markers, cholesterol (total, LDL, HDL), triglyceride, and high-sensitivity C-reactive protein levels were similar between the CKD and no-CKD categories; however, mean alkaline phosphatase was 10 U/L higher and mean alanine aminotransferase was 3 U/L lower among those with CKD. Participants with CKD had lower diastolic BP (74.9 [SD, 9.4] versus 76.5 [SD, 8.9] mm Hg) and higher neutrophil/lymphocyte ratio (2.9 [IQR, 2.2–3.9] versus 2.5 IQR, 1.9–3.3]), a marker of inflammation.
The changes in clinical chemistry over the course of the study from baseline to week 24 (week 12 for high-sensitivity C-reactive protein) across CKD status and treatment groups are shown in Table 2. Apabetalone decreased serum alkaline phosphatase compared with placebo, with a greater decrease among participants with CKD (mean, 7.8 U/L) compared with participants without CKD (mean, 1.4 IU/L; interaction P=0.004). Apabetalone produced a modest increase in HDL cholesterol concentration that was of a similar magnitude in CKD and no-CKD groups. No other laboratory parameter showed significant treatment by CKD-category interaction.
Table 2.
Change in clinical chemistry variables by CKD subgroup
| Parameter | eGFR <60 ml/min per 1.73 m2 | eGFR ≥60 ml/min per 1.73 m2 | Interaction P Value b | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Apabetalone | Adjusted Difference (95% CI) a | Placebo | Apabetalone | Adjusted Difference (95% CI) a | ||
| LDL cholesterol, mg/dl | −0.4 (70.0) | 2.6 (70.1) | 2.3 (−3.4 to 8.1) | −2.2 (42.4) | −1.1 (47.1) | −3.4 (−19.5 to 12.7) | 0.51 |
| HDL cholesterol, mg/dl | 10.4 (20.3) | 15.1 (23.6) | 4.7 (2.8 to 6.6) | 14.0 (25.8) | 17.8 (23.8) | 3.9 (−1.5 to 9.3) | 0.79 |
| HbA1c, % c | 0.00 (−0.60–0.60) | 0.00 (−0.80–0.50) | −0.10 (−0.20 to 0.00) | 0.00 (−0.62–0.40) | −0.10 (−0.80–0.50) | −0.00 (−0.30 to 0.20) | 0.35 |
| Serum glucose, mg/dl | 7.4 (67.9) | 9.3 (66.4) | 2.9 (−2.4 to 8.3) | −0.1 (73.1) | 1.1 (82.3) | 2.0 (−13.1 to 17.0) | 0.90 |
| Alkaline phosphatase, U/L | −1.2 (20.8) | −9.3 (22.0) | −7.8 (−9.9 to −5.7) | −6.9 (51.7) | −6.6 (32.5) | 1.4 (−4.5 to 7.3) | 0.004 |
| hsCRP, mg/L c , d | −15.0 (−54.6–26.3) | −25.9 (−61.5–24.5) | −7.8 (−19.6 to 4.1) | −18.8 (−49.0–23.2) | −36.1 (−69.4–15.3) | −11.7 (−48.4 to 25.6) | 0.74 |
| Cholesterol, mg/dl | 0.7 (24.9) | 3.9 (27.7) | 2.7 (0.6 to 4.8) | −1.4 (24.0) | 0.9 (23.3) | −0.7 (−6.5 to 5.2) | 0.29 |
| Triglycerides, mg/dl | 11.8 (64.4) | 15.3 (58.5) | 2.9 (−2.3 to 8.1) | −4.7 (31.1) | 2.8 (35.1) | 4.2 (−10.5 to 18.8) | 0.87 |
| Alanine aminotransferase, U/L | 7.0 (117.0) | 16.1 (73.7) | 8.6 (0.2 to 17.0) | −3.2 (53.2) | 31.5 (84.8) | 27.3 (3.5 to 51.1) | 0.15 |
| Systolic BP, mm Hg | −0.4 (17.8) | 1.4 (16.1) | 1.7 (−0.5 to 7.1) | 0.15 (15.0) | −0.18 (15.1) | 1.3 (−0.38 to 1.9) | 0.02 |
| Diastolic BP, mm Hg | −1.5 (10.2) | 0.4 (10.1) | 2.1 (0.1 to 4.5) | 0.15 (9.3) | −0.18 (9.7) | 0.88 (−0.40 to 1.1) | 0.03 |
| γ-Glutamyl transferase, U/L | 7.4 (59.2) | 5.9 (55.8) | −1.6 (−7.4 to 4.1) | 2.3 (52.4) | 16.9 (145.7) | 14.0 (−2.3 to 30.2) | 0.08 |
| Total bilirubin, mg/dl | 8.6 (35.1) | 27.1 (47.4) | 16.7 (6.3 to 27.0) | 11.1 (42.4) | 22.1 (44.9) | 10.4 (6.7 to 14.1) | 0.26 |
| eGFR, ml/min per 1.73 m2 | 1.7 (16.8) | −0.8 (18.5) | −2.5 (−4.0 to −0.9) | 5.3 (16.6) | 3.8 (13.6) | −1.8 (−6.1 to 2.6) | 0.77 |
Percentage or absolute changes from baseline to 24 wk, except for hsCRP, for which the change is from baseline to 12 wk. Changes are shown as mean (SD) or median (interquartile range). 95% CI, 95% confidence interval; HbA1c, hemoglobin A1C; hsCRP, high-sensitivity C-reactive protein.
Adjusted differences are shown as either mean or Hodges pseudo-median with 95% CI.
Interaction P values indicate whether the observed treatment difference varies with baseline CKD subgroup.
Changes are shown as median (interquartile range).
For hsCRP, 12-wk interval; otherwise 24 wk for all other laboratory values.
Upon study completion at a median follow-up period of 27 months (IQR, 20–32 months), participants in the placebo group with CKD experienced a higher incidence of MACE than those without CKD (35 events in 164 patients [21%] versus 114 events in 1041 patients [11%]; HR, 2.40; 95% CI, 1.67 to 3.44; P<0.001). Similarly, participants in the placebo group with CKD were more likely to be hospitalized for heart failure (14 hospitalizations in 164 patients [9%] versus 34 hospitalizations in 1041 patients [3%]; HR, 3.19; 95% CI, 1.66 to 6.12; P<0.001).
Overall, in the trial, a total of 274 participants experienced a primary MACE end point, including 125 patients (10%) in the apabetalone group and 149 patients (12%) in the placebo group (HR, 0.82; 95% CI, 0.65 to 1.04; P=0.11) (18). Table 3 shows the case mix–adjusted effect of assigned treatment on cardiovascular outcomes according to CKD category. In the CKD subgroup, apabetalone was associated with a reduced hazard for MACE (HR, 0.50; 95% CI, 0.26 to 0.96) and heart failure hospitalization (HR, 0.25; 95% CI, 0.07 to 0.92; P=0.04). In contrast, in the subgroup without CKD, apabetalone’s effect on MACE (HR, 0.96; 95% CI, 0.74 to 1.24) and heart failure hospitalization (HR, 0.76; 95%, CI 0.46 to 1.27) was nonsignificant. The interaction of treatment and CKD category on MACE and MACE plus hospitalization for heart failure was 0.032 and 0.033, respectively. Supplemental Table 1 shows minimally adjusted HRs, i.e., stratified for statin and country, in accordance with the primary analyses of the BETonMACE study (18), suggesting that inclusion or exclusion of multivariable adjustment had little effect on the summary estimates.
Table 3.
Effects of apabetalone versus placebo on primary and secondary outcomes across CKD
| Variable | eGFR <60 ml/min per 1.73 m2 | eGFR ≥60 ml/min per 1.73 m2 | Interaction P Value a | ||||
|---|---|---|---|---|---|---|---|
| Placebo Event/n (%) | Apabetalone Event/n (%) | Adjusted HR (95% CI) | Placebo Event/n (%) | Apabetalone Event/n (%) | Adjusted HR (95% CI) | ||
| Primary outcome | |||||||
| MACE | 35/164 (21) | 13/124 (11) | 0.50 (0.26 to 0.96) | 114/1041 (11) | 112/1084 (10) | 0.96 (0.74 to 1.24) | 0.03 |
| Composite events | |||||||
| MACE and CHF | 41/164 (25) | 16/124 (13) | 0.48 (0.26 to 0.88) | 132/1041 (13) | 123/1084 (11) | 0.91 (0.71 to 1.17) | 0.03 |
| Components | |||||||
| CV death | 17/164 (10) | 6/124 (5) | 0.47 (0.18 to 1.24) | 38/1041 (4) | 39/1084 (4) | 1.02 (0.98 to 1.60) | 0.12 |
| Nonfatal MI | 20/164 (12) | 9/124 (7) | 0.59 (0.26 to 1.33) | 74/1041 (7) | 68/1084 (6) | 0.89 (0.64 to 1.24) | 0.26 |
| Nonfatal stroke | 6/164 (4) | 2/124 (2) | 0.57 (0.11 to 2.97) | 11/1041 (1) | 15/1084 (1) | 1.36 (0.62 to 2.96) | 0.20 |
| CHF hospitalization | 14/164 (9) | 3/124 (2) | 0.25 (0.07 to 0.92) | 34/1041 (3) | 26/1084 (2) | 0.76 (0.46 to 1.27) | 0.12 |
Shown are events and total subjects counts, percentage rates, HRs, and 95% CIs for indicated composite and component end points. All analyses are stratified for statin and country and adjusted for age and sex. See also Supplemental Table 1 for minimally adjusted HRs. HR, hazard ratio; 95% CI, 95% confidence interval; MACE, major adverse cardiovascular events; CHF, congestive heart failure; CV, cardiovascular; MI, myocardial infarction.
Interaction P values indicate differences by CKD status in the effect of apabetalone on event rates.
For the composite of time to first event of hospitalization for MACE or heart failure, the observed HR was 0.48 (95% CI, 0.26 to 0.88), whereas the observed HR was 0.91 (95% CI, 0.71 to 1.17) in the participants without CKD. Treatment HRs were also numerically lower in the CKD subgroup compared with the non-CKD subgroup for cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.
Kaplan–Meier survival plots for MACE, heart failure hospitalization, and their composite are shown in Figure 2 according to CKD subgroup. By log-rank analysis, apabetalone was associated with fewer MACE, hospitalizations for heart failure, and their combination in the CKD subgroup, whereas the apabetalone effects were nonsignificant in the non-CKD subgroup. In participants with CKD, there was early, continued, and sustained curve separation, whereas the curve separation was less pronounced in the participants without CKD.
Figure 2.
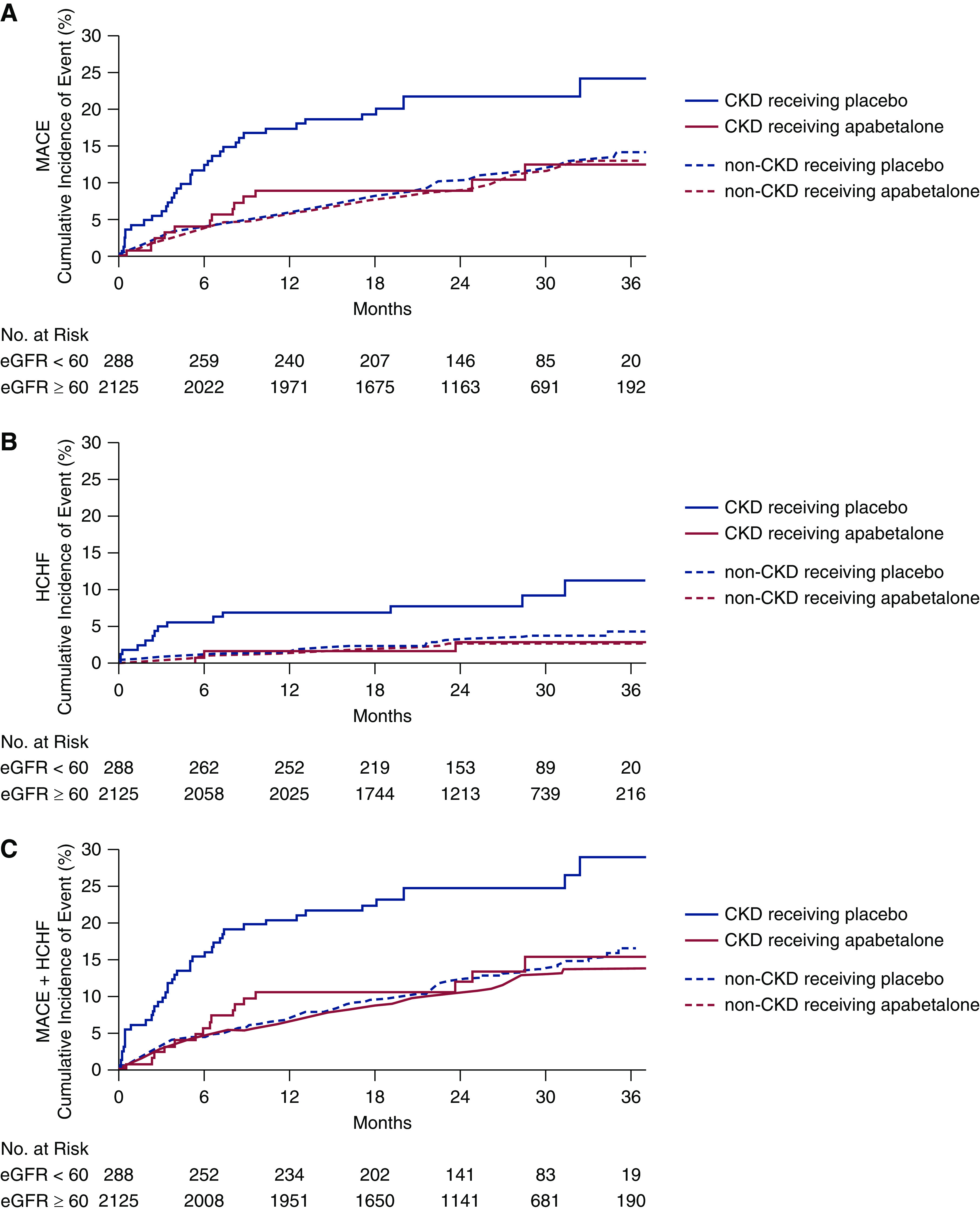
Kaplan–Meier estimates by treatment and CKD/non-CKD group for (A) MACE, (B) HCHF, and (C) the composite MACE plus HCHF. In each panel, four curves are shown: CKD subjects receiving placebo (solid blue) and apabetalone (solid red), and non-CKD subjects receiving placebo (dashed blue) and apabetalone (dashed red). Number of subjects at risk is shown for CKD and non-CKD groups, aggregated over treatment. HCHF, hospitalization for congestive heart failure.
Overall in BETonMACE, more participants allocated to apabetalone than placebo discontinued the study drug (114 [9%] versus 69 [6%] participants) for reasons including liver-enzyme elevations (35 [3%] versus 11 [0.9%] patients), as described elsewhere (18). In the CKD population, more participants allocated to placebo than apabetalone discontinued the study drug (20 [12%] versus 12 [10%] patients). Table 4 shows that, among participants with CKD, similar numbers of participants in the apabetalone and placebo groups experienced adverse events (119 [72%] versus 88 [71%] patients), and fewer participants in the apabetalone group had serious adverse events (29% versus 43%; P=0.02). Only two subjects in each group had a hepatic transaminase level that was greater than the five-fold upper limit of normal on close laboratory monitoring, which required discontinuation of the study therapy in line with the study protocol.
Table 4.
Adverse events in apabetalone versus placebo across CKD status a
| Variable | Patients without CKD by Assigned Treatment Group | Patients with CKD by Assigned Treatment Group | ||
|---|---|---|---|---|
| Placebo (eGFR ≥60 ml/min per 1.73 m2) (n=1041) | Apabetalone (eGFR ≥60 ml/min per 1.73 m2) (n=1084) | Placebo (eGFR <60 ml/min per 1.73 m2) (n=164) | Apabetalone (eGFR <60 ml/min per 1.73 m2) (n=124) | |
| Patients with at least one adverse event (%) a | 699 (67) | 739 (68) | 119 (73) | 88 (71) |
| Frequent adverse events b , c | ||||
| Acute myocardial infarction | 38 (4) | 38 (4) | 12 (7) | 4 (3) |
| Alanine aminotransferase increased | 12 (1) | 60 (6) | 6 (4) | 4 (3) |
| Angina | 65 (6) | 65 (6) | 11 (7) | 9 (7) |
| Anemia | 30 (3) | 32 (3) | 10 (6) | 4 (3) |
| Cardiac failure | 24 (2) | 19 (2) | 14 (9) | 3 (2) |
| Diarrhea | 33 (3) | 36 (3) | 11 (7) | 7 (6) |
| Hypertension | 65 (6) | 61 (6) | 7 (4) | 9 (7) |
| Nasopharyngitis | 47 (5) | 41 (4) | 9 (5) | 5 (4) |
| Pneumonia | 16 (2) | 23 (2) | 10 (6) | 4 (3) |
| Urinary tract infection | 29 (3) | 49 (5) | 11 (7) | 9 (7) |
| Unstable angina | 36 (3) | 56 (5) | 5 (3) | 2 (2) |
| Worsening diabetes mellitus | 55 (5) | 69 (6) | 7 (4) | 7 (6) |
Adverse events were assessed in the safety population, which includes all patients who received at least one dose of study drug medication.
Includes treatment-emergent adverse events only, defined as those occurring after the first dose and within 14 d of the last dose of the study drug.
Defined as occurring with a frequency of ≥5% in any of the CKD or treatment groups.
Discussion
In the BETonMACE trial, assignment to apabetalone or placebo did not significantly affect the primary MACE outcome in 2425 participants with type 2 diabetes, low HDL cholesterol, and recent acute coronary syndrome. However, in a proof-of-concept trial with a novel chemical entity agent, it is important to explore effects in key subgroups that may point to populations most likely to achieve treatment benefit. The observed HR of 0.50 for MACE, 0.25 for heart failure hospitalization, and 0.48 for MACE plus heart failure hospitalization in the participants with CKD is on top of standard of care, including high-intensity statin treatment and other evidence-based procedures and treatments for acute coronary syndrome. The interaction P value tests for difference by CKD status in the effect of apabetalone on event rates were significant for MACE.
Both type 2 diabetes and CKD are independently associated with high risk of cardiovascular disease (19). In addition, patients with coronary disease and either diabetes or CKD have a much higher risk of additional MACE than patients without either of these concomitant conditions (20). The excess risk of cardiovascular disease among patients with CKD may be related to factors beyond hyperlipidemia and hypertension (21). Although statins (4) and glucagon-like peptide-1 receptor agonists reduce MACE, and SGLT2 inhibitors (5) reduce heart failure in patients with type 2 diabetes and CKD, there is a high residual risk of these events. It has been hypothesized that nontraditional factors, including inflammation, endothelial dysfunction, and vascular calcification, may contribute to the residual risk (22) and may be driven, in turn, by a maladaptive epigenetic response (23). It is important to note that the reduction in risk of MACE and heart failure hospitalization with apabetalone occurred without an effect of treatment on kidney function as measured by eGFR, in contrast to SGLT2 inhibitors and some other agents, in which composite end points could be affected by CKD protection or reducing proteinuria (5). Therefore, other mechanisms of potential benefit of apabetalone, which are unique to CKD, may play a role in our findings, as recently described by Wasiak et al. (14), who showed a pronounced correction of the highly perturbed plasma proteome in patients with CKD stages 4–5. Although transcriptional regulation by selective BET inhibition has the trait of multiple small corrections of plasma markers toward normal levels, a noteworthy finding in our analysis pertains to serum alkaline phosphatase, an emerging cardiovascular risk factor in CKD and non-CKD populations (24). Both the expression and circulating level of alkaline phosphatase are elevated in CKD (3). In phase 2 studies, the reduction in serum alkaline phosphatase by apabetalone treatment was consistent (25) and predicted cardiovascular event reduction (16). In the BETonMACE trial, baseline serum alkaline phosphatase was 10 U/L higher in participants with versus without CKD (Table 1). In the CKD subgroup, apabetalone treatment resulted in a more pronounced enzyme reduction than in the non-CKD subgroup. This suggests that alkaline phosphatase may be a biomarker for apabetalone’s effects, potentially through the hypothesized role of alkaline phosphatase in promoting endothelial dysfunction (26) and vascular calcification (15).
Our study is subject to several limitations, including the aforementioned small sample size of the CKD subgroup, comprising 12% of the entire trial population, which led to a suboptimal balance of treatment assignment. Participants with severe CKD (eGFR <30 ml/min per 1.73 m2) were excluded; however, among adults with an eGFR of <60 ml/min per 1.73 m2, >90% of participants were in the 30–60 ml/min per 1.73 m2 range (1). The trial was restricted to patients with type 2 diabetes, so we cannot assess the potential effect of apabetalone in patients with nondiabetic CKD. However, type 2 diabetes is the leading cause of CKD, and evidence is consistent that an association of cardiovascular risk with CKD exists irrespective of the presence or absence of diabetes (27). In BETonMACE, urine samples were not collected, so presence or absence of albuminuria and its modification by apabetalone remains unknown. In BETonMACE, markers of mineral metabolism, including parathyroid hormone, were not collected, so modification of bone turnover in patients with CKD by apabetalone remains unknown. During the BETonMACE trial, no significant change in eGFR in any group or subgroup was detected, which could be related to a mix of participants with likely evolving glomerular hyperfiltration, which often precedes diabetic nephropathy.
In conclusion, in this phase 3, randomized, controlled post–acute coronary syndrome trial in patients with type 2 diabetes, the participants with CKD were highly responsive to apabetalone treatment, with a 50% nominal reduction of MACE over 27 months. BETonMACE is the first cardiovascular outcome trial assessing the effect of epigenetic modification with BET protein inhibition. This prespecified, CKD subgroup analysis of BETonMACE suggests that apabetalone may offer a safe and effective oral pharmacotherapy for reducing cardiovascular risk in participants with CKD, diabetes, and recent acute coronary syndrome. A confirmatory apabetalone trial in patients with CKD and cardiovascular disease is warranted to corroborate the findings in BETonMACE.
Disclosures
K. Buhr reports receiving grants from Amgen, BioCardia, Cytokinetics, GlaxoSmithKline, Pfizer, and The Medicines Company, outside the submitted work; receiving grants from Resverlogix, during the conduct of the study; serving as director of the University of Wisconsin Statistical Data Analysis Center, which contracts with AbbVie, Alnylam, Amgen, BioCardia, Cytokinetics, Goldfinch, GlaxoSmithKline, Novartis, Pfizer, Regeneron, Resverlogix, Sanofi, Theravance, and UCB; being employed by University of Wisconsin–Madison; and receiving personal fees from Resverlogix during the conduct of the study. H. Ginsberg reports receiving research support from Amgen, AstraZeneca, and Pfizer; receiving honoraria from Amgen, AstraZeneca, Kowa, Merck, Pfizer, Regeneron, Resverlogix, and Sanofi; being employed by Columbia University Vagelos College of Physicians and Surgeons; and having consultancy agreements with, honoraria from, and serving as a scientific advisor or member of Resverlogix Corp. J. Johansson reports having ownership interest in, and serving as a scientific advisor or member of, Artery Therapeutics, Inc. and Resverlogix Corp.; and being employed by, and having consultancy agreements with, Resverlogix Corp. K. Kalantar-Zadeh reports receiving honoraria from Abbott, AbbVie, ACI Clinical, Akebia, Alexion, American Society of Nephrology, Amgen, Ardelyx, AstraZeneca, Aveo, B. Braun, Baxter, Cara Therapeutics, Chugai, Cytokinetics, Daiichi Sankyo, DaVita, Dr. Schär, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, Kissei, NIH, National Kidney Foundation (NKF), Novartis, Pfizer, Regulus, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, UptoDate, Vifor, and ZS-Pharma; serving as a scientific advisor or member of Abbott, AbbVie, Amgen, Ardelyx, AstraZeneca, Aveo, Daiichi Sankyo, DaVita, Fresenius, Genetech, Hospira, Keryx, NKF, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma; having consultancy agreements with Abbott, AbbVie, Amgen, Ardelyx, AstraZeneca, Baxter, Daiichi Sankyo, Fresenius, Hospira, Keryx, Otsuka, Resverlogix, Sanofi, Shire, and Vifor; being on a speakers bureau for Akibia, Daiichi Sankyo, Fresenius, Keryx, Relypsa, Sanofi, Shire, and Vifor; serving as a scientific advisor or member of American Journal of Kidney Diseases, American Journal of Nephrology, Cardiorenal Medicine, CJASN, International Urology and Nephrology, JASN, Journal of Cachexia, Sarcopenia and Muscle, Journal of Renal Nutrition, Kidney International, Kidney International Reports, Nature Reviews Nephrology, Nephrology Dialysis Transplantation, Renal and Urology News and Seminars in Dialysis; receiving research funding from Dexcom and National Institutes of Health (NIH); serving as a scientific advisor or member of several NIH study sections; being employed by University of California Irvine; and having patents and inventions for prognostic assays for patients on maintenance hemodialysis. E. Kulikowski reports being employed by, and having ownership interest in, Resverlogix Corp. during the conduct of the study. K. Lebioda reports being employed by, and having ownership interest in, Resverlogix Corp., and being on a speakers bureau for Resverlogix presentations at various bio-partnering conferences. S. Nicholls reports having consultancy agreements with, and receiving honoraria from, Akcea, Anthera, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Merck, Omthera, Resverlogix, Sanofi-Regeneron, and Takeda; receiving research funding from Amgen, Anthera, AstraZeneca, Cerenis, Eli Lilly, Esperion, InfraReDx, LipoScience, Novartis, Resverlogix, Roche, Sanofi-Regeneron, and The Medicines Company; and being employed by Monash University. K. Ray reports having consultancy agreements with AbbVie, Akcea, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly and Company, Esperion, Kowa, New Amsterdam, Novartis, Resverlogix, Sanofi-Regeneron, and Silence Therapeutics; receiving honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Dr. Reddy’s, Novartis, Novo Nordisk, Pfizer, and Sanofi; and receiving research funding from Amgen, Daiichi Sankyo, Regeneron, and Sanofi. G. Schwartz reports receiving research funding from AstraZeneca, Resverlogix, Roche, Sanofi, and The Medicines Company (through the University of Colorado); being employed by Rocky Mountain Regional Veterans Affairs Medical Center and University of Colorado School of Medicine; and being the coinventor of a pending US patent 62/806,313 (“Methods for Reducing Cardiovascular Risk”), assigned in full to the University of Colorado. M. Sweeney reports being employed by, and having ownership interest in, Resverlogix Corp. P. Toth reports having consultancy agreements with Amarin, Amgen, bio89, Kowa, Novartis, Resverlogix, and Theravance; receiving speakers bureau compensation from Amarin, Amgen, Esperion, Merck, and Novo Nordisk; receiving honoraria from Amgen, Amarin, Esperion, Novo Nordisk, and Theravance; and being employed by CGH Medical Center. N. Wong reports serving on speakers bureaus for Abbott, Lilly, Merck, Novo Nordisk, and Valeant; being employed by, having ownership interest in, receiving honoraria from, and having patents and inventions with Resverlogix Corp.; and serving as chief scientific officer of Resverlogix Corp.
Funding
The BETonMACE trial was funded by Resverlogix Corporation.
Supplementary Material
Acknowledgments
Part of these data were presented as a selected oral abstract for the plenary session of the annual congress of the European Renal Association–European Dialysis and Transplant Association on June 10, 2020, and during the American Society of Nephrology Kidney Week on October 24, 2020.
All members of the academic steering committee contributed to the interpretation of the data, including the sponsor coauthors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Novel Therapeutic Options for Cardiovascular Disease with CKD” on pages 682–684.
Contributor Information
Collaborators: BETonMACE Investigators, Kausik K. Ray, Gregory G. Schwartz, Stephen J. Nicholls, Kaymar Kalantar-Zadeh, Peter Toth, Henry Ginsberg, Michael Sweeney, Norman Wong, Jan Johansson, Alberto Lorenzatti, Marisa Vico, Maria Milanova, Goran Milicevic, Zeljko Popovic, Henning Ebelt, Róbert Gábor Kiss, Basil Lewis, Edmundo Bayram Llamas, Maciej Banach, Sergey Tereschenko, Milan Pavlovic, Daniel Pella, Chern-En Chiang, Eva Lonn, Paul Watkins, David Waters, Michael Szarek, Judith Currier, Lawrence Alan Leiter, John McMurray, Mark Petrie, Pardeep Jhund, Matthew Walters, Eugene Connolly, Ninian Lang, Lilia Schiavi, Marisa Vico, Laura Maffei, Anselmo Bordonava, Aldo Prado, Julio Vallejos, Javier Farias, Lucrecia Nardone, Jorge Resk, Orlando Caruso, Alberto Lorenzatti, Natacha Maldonado, Lucio Padilla, Miguel Hominal, Hugo Luquez, Georgina Sposetti, Alberto Caccavo, Jorge Glenny, Virginia Mansilla, Maria Alvarez, Maria Parody, Rodolfo Sarjanovich, Maria Klyver, Maria Eugenia Valdez, Hugo Colombo, Claudia Baccaro, Virginia Visco, Julio Bono, Carlos Cuneo, Fredy Ferré Pácora, Pablo Guzman, Daniel Piskorz, Daniel Vogel, Mladen Grigorov, Plamen Gatzov, Miroslav Stoyanov, Emilya Apostolova, Maria Milanova, Ivo Petrov, Atanas Angelov, Petar Lazov, Valentina Grigorova, Todor Yanev, Emilena Vuchkova, Dobrin Vassilev, Snezhanka Tisheva, Valeri Gelev, Silvia Canecki-Varzic, Zeljko Popovic, Srecko Tusek, Ema Drvodelić Šunić, Goran Milicevic, Marica Jandric-Balen, Nikolina Marinic, Natasa Moser, Henning Ebelt, Mohammed Natour, Peter Schwimmbeck, Karl-Friedrich Appel, Klaus Kleinertz, Béla Merkely, András Papp, Robert Kiss, Zsolt Kovács, András Vértes, Zsolt Sárszegi, Ernö Kis, Amália Benedek, János Takács, Aniko Papp, Andras Matoltsy, Peter Andrassy, Imre Ungi, Michael Shechter, Shaul Atar, Avraham Shotan, Alicia Vazan, Ronny Alcalai, Chaim Lotan, Basil Lewis, Tony Hayek, Mady Moriel, Amos Katz, Idit Liberty, Ilana Harman-Boehm, Elad Schiff, Oscar Kracoff, Yoseph Rozenman, Morris Mosseri, Ladislav Slezak, Muhammad Sabbah, David Zeltser, Khaled Adawi, Muhamed Omary, Rosane Abramof Ness, Faiad Adawi, Jose Garza Ruiz, Jorge Carrillo, Luis Alejandro Nevarez Ruiz, Pedro Garcia Hernandez, Maria Arechavaleta-Granell, Manuel De los Rios Ibarra, Raul Velasco-Sanchez, Hugo Laviada Molina, Lucas Solis Morales, Efrain Montano Gonzalez, Guillermo Llamas Esperón, Edmundo Alfredo Bayram Llamas, Cynthia Mustieles Rocha, Rodrigo Suarez-Otero, Manuel Aguilera Real, Eliud Montes Cruz, Humberto Alvarez Lopez, Susano Lara Vaca, Guillermo Fanghanel-Salmon, Carlos Hernandez Herrera, Joel Rodriguez Saldaña, Elier Pedroza Garcia, Maricela Vidrio Velázquez, Ignacio Rodriguez Briones, Bas Hamer, Ton Slagboom, Aleksander Zurakowski, Marcin Debinski, Iwona Kobielusz-Gembala, Radoslaw Bartkowiak, Marek Rajzer, Adam Witkowski, Alicja Kowalisko, Marek Piepiorka, Karol Stania, Michal Domzal, Janusz Korecki, Romuald Korzeniak, Lukasz Mazurkiewicz, Ewa Mirek-Bryniarska, Maciej Banach, Katarzyna Madziarska, Adam Mlodziankowski, Barbara Rewerska, Zbigniew Gaciong, Maciej Mazurkiewicz, Ivan Maksimov, Yuri Shvarts, Larisa Khaisheva, Vasily Samitin, Svetlana Boldueva, Mikhail Zykov, Olga Barbarash, Victor Kostenko, Elena Kulibaba, Olga Smolenskaya, Nikolay Tarasov, Zaur Shogenov, Leonid Strongin, Anatoly Kuzin, Valeriy Makukhin, Konstantin Nikolaev, Sergey Tereschenko, Natalya Vezikova, Vladimir Miloradovic, Milan Pavlovic, Marina Deljanin Ilic, Dragan Simic, Georgina Pudar-Brankovic, Tanja Jozic, Slobodan Dodic, Arsen Ristic, Sasa Hinic, Vladimir Mitov, Natasa Stokuca-Korac, Edita Stokic, Vera Celic, Nebojsa Despotovic, Dragan Dincic, Biljana Putnikovic Tosic, Aleksandar Selakovic, Daniel Pella, Milan Banik, Jan Fedacko, Karol Micko, Tibor Duris, Martin Kokles, Beata Lachova, Andrej Dzupina, Juraj Mazur, Ingrid Buganova, Milan Behuncik, Silvia Vadinova, Hung-I Yeh, Chern-En Chiang, Cheng-Hen Lee, I-Chang Hsieh, Lin Jiunn-Lee, We-Hsiang Lin, Yen-Wen Wu, Chien Hsun Hsia, and Ping Han Lo
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16751020/-/DCSupplemental.
Supplemental Summary 1. Collaborator information.
Supplemental Appendix 1. Statistical analysis plan.
Supplemental Table 1. Minimally adjusted hazard ratios (HR) for composite and component events in apabetalone versus placebo across CKD status for major adverse cardiovascular events (MACE).
References
- 1. United States Renal Data System : 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020. Available at: https://adr.usrds.org/2020. Accessed February 18, 2021
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med 18: 4, 2008]. N Engl J Med 351: 1296–1305, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P: Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol 13: 429–442, 2017. [DOI] [PubMed] [Google Scholar]
- 4. de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, Monyak JT, Parving HH, Remuzzi G, Sowers JR, Vidt DG: Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): A randomised clinical trial. Lancet Diabetes Endocrinol 3: 181–190, 2015. [DOI] [PubMed] [Google Scholar]
- 5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019. [DOI] [PubMed] [Google Scholar]
- 6. Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB; LEADER Steering Committee and Investigators: Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017. [DOI] [PubMed] [Google Scholar]
- 7. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators: Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM: Structure and ligand of a histone acetyltransferase bromodomain. Nature 399: 491–496, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Zeng L, Zhou M-M: Bromodomain: An acetyl-lysine binding domain. FEBS Lett 513: 124–128, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Devaiah BN, Case-Borden C, Gegonne A, Hsu CH, Chen Q, Meerzaman D, Dey A, Ozato K, Singer DS: BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol 23: 540–548, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanno T, Kanno Y, LeRoy G, Campos E, Sun H-W, Brooks SR, Vahedi G, Heightman TD, Garcia BA, Reinberg D, Siebenlist U, O’Shea JJ, Ozato K: BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat Struct Mol Biol 21: 1047–1057, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown JD, Lin CY, Duan Q, Griffin G, Federation A, Paranal RM, Bair S, Newton G, Lichtman A, Kung A, Yang T, Wang H, Luscinskas FW, Croce K, Bradner JE, Plutzky J: NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell 56: 219–231, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hajmirza A, Emadali A, Gauthier A, Casasnovas O, Gressin R, Callanan MB: BET family protein BRD4: An emerging actor in NFκB signaling in inflammation and cancer. Biomedicines 6: 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wasiak S, Tsujikawa LM, Halliday C, Stotz SC, Gilham D, Jahagirdar R, Kalantar-Zadeh K, Robson R, Sweeney M, Johansson JO, Wong NC, Kulikowski E: Benefit of apabetalone on plasma proteins in renal disease. Kidney Int Rep 3: 711–721, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilham D, Tsujikawa LM, Sarsons CD, Halliday C, Wasiak S, Stotz SC, Jahagirdar R, Sweeney M, Johansson JO, Wong NCW, Kalantar-Zadeh K, Kulikowski E: Apabetalone downregulates factors and pathways associated with vascular calcification. Atherosclerosis 280: 75–84, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Haarhaus M, Ray KK, Nicholls SJ, Schwartz GG, Kulikowski E, Johansson JO, Sweeney M, Halliday C, Lebioda K, Wong N, Brandenburg V, Beddhu S, Tonelli M, Zoccali C, Kalantar-Zadeh K: Apabetalone lowers serum alkaline phosphatase and improves cardiovascular risk in patients with cardiovascular disease. Atherosclerosis 290: 59–65, 2019. [DOI] [PubMed] [Google Scholar]
- 17. Ray KK, Nicholls SJ, Ginsberg HD, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Cummings JL, Sweeney M, Schwartz GG: Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: Rationale, design, and baseline characteristics of the BETonMACE trial. Am Heart J 217: 72–83, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Ray KK, Nicholls SJ, Buhr KA, Ginsberg HN, Johansson JO, Kalantar-Zadeh K, Kulikowski E, Toth PP, Wong N, Sweeney M, Schwartz GG; BETonMACE Investigators and Committees: Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: A randomized clinical trial. JAMA 323: 1565–1573, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. St Peter WL, Khan SS, Ebben JP, Pereira BJ, Collins AJ: Chronic kidney disease: The distribution of health care dollars. Kidney Int 66: 313–321, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, Kalantar-Zadeh K: Outcomes associated with microalbuminuria: Effect modification by chronic kidney disease. J Am Coll Cardiol 61: 1626–1633, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ: Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol 64: 588–597, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26–33, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Fontecha-Barriuso M, Martin-Sanchez D, Ruiz-Andres O, Poveda J, Sanchez-Niño MD, Valiño-Rivas L, Ruiz-Ortega M, Ortiz A, Sanz AB: Targeting epigenetic DNA and histone modifications to treat kidney disease. Nephrol Dial Transplant 33: 1875–1886, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed ML, Wiebe N, Muntner P: Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 120: 1784–1792, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Kulikowski E, Halliday C, Johansson J, Sweeney M, Lebioda K, Wong N, Haarhaus M, Brandenburg V, Beddhu S, Tonelli M, Zoccali C, Kalantar-Zadeh K: Apabetalone mediated epigenetic modulation is associated with favorable kidney function and alkaline phosphatase profile in patients with chronic kidney disease. Kidney Blood Press Res 43: 449–457, 2018. [DOI] [PubMed] [Google Scholar]
- 26. Perticone F, Perticone M, Maio R, Sciacqua A, Andreucci M, Tripepi G, Corrao S, Mallamaci F, Sesti G, Zoccali C: Serum alkaline phosphatase negatively affects endothelium-dependent vasodilation in naïve hypertensive patients. Hypertension 66: 874–880, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA: Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl (37): S24–S31, 2003. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.