Visual Abstract
Keywords: postmortem, kidney biopsy, acute kidney injury, SARS-CoV-2, COVID-19
Abstract
Background and objectives
AKI in coronavirus disease 2019 (COVID-19) is associated with higher morbidity and mortality. The objective of this study was to identify the kidney histopathologic characteristics of deceased patients with diagnosis of COVID-19 and evaluate the association between biopsy findings and clinical variables, including AKI severity.
Design, setting, participants, & measurements
Our multicenter, observational study of deceased patients with COVID-19 in three third-level centers in Mexico City evaluated postmortem kidney biopsy by light and electron microscopy analysis in all cases. Descriptive and association statistics were performed between the clinical and histologic variables.
Results
A total of 85 patients were included. Median age was 57 (49–66) years, 69% were men, body mass index was 29 (26–35) kg/m2, 51% had history of diabetes, 46% had history of hypertension, 98% received anticoagulation, 66% were on steroids, and 35% received at least one potential nephrotoxic medication. Severe AKI was present in 54% of patients. Biopsy findings included FSGS in 29%, diabetic nephropathy in 27%, and arteriosclerosis in 81%. Acute tubular injury grades 2–3 were observed in 49%. Histopathologic characteristics were not associated with severe AKI; however, pigment casts on the biopsy were associated with significantly lower probability of kidney function recovery (odds ratio, 0.07; 95% confidence interval, 0.01 to 0.77). The use of aminoglycosides/colistin, levels of C-reactive protein and serum albumin, previous use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, antivirals, nonsteroid anti-inflammatory drugs, and anticoagulants were associated with specific histopathologic findings.
Conclusions
A high prevalence of chronic comorbidities was found on kidney biopsies. Nonrecovery from severe AKI was associated with the presence of pigmented casts. Inflammatory markers and medications were associated with specific histopathologic findings in patients dying from COVID-19.
Introduction
The disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; coronavirus disease 2019 [COVID-19]), in addition to the known tropism to the respiratory epithelium, can also affect the kidneys, heart, and the digestive tract, among others (1). Initially, the literature reported a low incidence of AKI (2). However, in a recent meta-analysis, AKI was found in 28% (22%–34%) of hospitalized patients and in 46% (35%–57%) of critically ill patients (3), contributing to a significant higher mortality (4 –6). Interestingly, some studies have suggested that only AKI stages 2 and 3 are associated with a higher mortality (7).
Several abnormalities have been identified in the kidney tissue of deceased patients with AKI and COVID-19, mostly diffuse acute tubular injury (ATI) with cytoplasmic vacuoles (8,9). In electron microscopy (EM), virus-like particles were mainly identified in cytoplasm of proximal tubular epithelium and in podocytes (9,10), with foot process effacement and detachment of podocytes, clathrin-coated structures, tubular epithelium expressing SARS-CoV-2 nucleoprotein by indirect fluorescence (9), diffuse erythrocyte aggregation and obstruction in the capillary lumen, glomerular ischemia, endothelial cell injury, and myoglobin casts or cellular debris casts in cases of rhabdomyolysis (9,11). It is difficult to conclude whether these lesions are direct consequences of the virus, sepsis, and/or multiple organ failure (11).
The objective of this study was to identify the histopathologic characteristics of patients who died from COVID-19 and evaluate the association with clinical variables, including AKI severity.
Materials and Methods
Study Design
This is a multicenter, observational cohort study. We prospectively included adult critically ill patients with diagnosis of COVID-19 by positive PCR for SARS-CoV-2 or with high clinical suspicion, including COVID-19 Reporting and Data System 5 (predominant basal and peripheral multifocal ground glass opacities with or without consolidations) by computed tomography, who died between March and June 2020 in three hospitals in Mexico City. Clinical characteristics were retrospectively obtained from medical records. AKI was defined according to Kidney Disease Improving Global Outcomes guidelines only on the basis of serum creatinine, and AKI stages 2 and 3 were considered as severe AKI. Kidney function recovery prior to death was clinically defined as returning to baseline creatinine or minimum serum creatinine, having lower than half of peak serum creatinine, and being free from KRT, all of them at the end of follow-up prior to their fatal outcome. The research was approved by the ethics committee of each institution (DI/20/105 B/03/60). Informed consent was obtained from the responsible guardians for all patients.
Biopsy Procedure
Biopsies were performed in the autopsy room in one institution within the facilities of the pathology department, with a median time from death to biopsy of 4 (4–8) hours, and inside the intensive care unit in the other two centers, having a median time from death to biopsy of 1 (1–2) hour. In all cases, they were performed by a trained nephrologist by ultrasound scanning and a BARD MONOPTY Disposable Core Biopsy Instrument 16 gauge × 22 cm, placing the specimen in a sterile bottle with 4% formaldehyde. The use of personal protective equipment was carried out by World Health Organization recommendations and supervised by the institution.
Histopathologic Analyses
Kidney specimens were processed for paraffin block fixation; findings were described by direct light microscopy. Four techniques were used (hematoxylin and eosin, Masson trichrome, periodic acid–Schiff, and silver methenamine) using the Olympus BX51 Microscope and the VF Evolution C camera (Media-Cybernetics). Analysis was also carried out by EM. The nephropathologist was blinded to the clinical characteristics of the patients (Supplemental Material).
Statistical Analyses
The data were captured in Microsoft Office Excel, maintaining the confidentiality of the participants by assigning an internal code for each institution. Variables were expressed as numbers and proportions for categorical data and as medians with 25th to 75th percentiles for continuous variables. Patients with inadequate tissue or without serum creatinine were excluded. Other data on continuous variables for clinical characteristics were imputed by their median value if <20% were missing; otherwise, these variables were not considered. Categorical missing values were not imputed. Serum albumin, d-dimer, creatin kinase, and C-reactive protein had <20% of missing values, whereas IL-6 had >50%; therefore, it was excluded. Analyses by groups (severe AKI) were obtained through chi-squared and t tests or the Mann–Whitney U test according to their distribution. Receiver operating characteristic curves were constructed to analyze cutoff points for continuous variables. Unadjusted and multivariable logistic regression analyses were performed. Forest plots were constructed for displaying odd ratios between clinical and histopathologic characteristics. No sample size was calculated; we analyzed the full available population with these criteria in the period. The SPSS program version 23.0 was used.
Results
Six hundred and twenty-three patients with confirmed or suspected COVID-19 died in all three hospitals in the time interval of the study. Limited information from the mortality registries available showed a median age of 58 (49–65) years, that 76% were men, and that 28% had diabetes. Eighty-nine patients were included in the study and underwent kidney biopsy; three patients were excluded due to their degree of autolysis, and one was excluded due to missing data.
Clinical Characteristics
A total of 85 patients were analyzed. Clinical data are provided in Supplemental Table 1. Median age was 57 (50–67) years, 59 (69%) were men, body mass index was 29 (26–35) kg/m2, 43 (51%) had diabetes, and 39 (46%) had chronic hypertension. All patients required invasive mechanical ventilation, 70 (82%) received vasopressors, 83 (98%) were treated with anticoagulants (43% prophylactic and 53% therapeutic dose), 56 (66%) were treated with steroids, and 30 (35%) received at least one potential nephrotoxic medication. In-hospital superimposed bacterial infections were present in 45 (53%) patients, and 40 (47%) patients fulfilled septic shock criteria. Main final diagnosis was acute respiratory distress syndrome due to COVID-19, and median hospital length of stay was 11 (7–17) days.
AKI was present in 85% of patients, whereas 54% had severe AKI and 31% had AKI stage 1. A total of 18% required KRT. Among patients with severe AKI, 83% had no kidney function recovery, whereas 9% showed partial recovery and 9% showed complete recovery at their last evaluation.
Histopathologic Characteristics
Biopsy findings are summarized in Tables 1 and 2. A median of 26 (13–51) glomeruli were observed per patient, and 6% (0–11) had global sclerosis. Pathology descriptions included 25 (29%) with FSGS, 17 of them (68%) having the perihilar variant (with no cases of collapsing glomerulopathy); 23 (27%) with diabetic nephropathy; 24 (28%) with glomerulomegaly; and 69 (81%) with arteriosclerosis. Additionally, eleven (13%) had interstitial fibrosis/tubular atrophy grades 2–3, five (6%) showed amyloid deposits, one (1%) had IgA nephropathy, and one (1%) had neoplastic infiltration from leukemia.
Table 1.
Tubulointerstitial light microscopy
| Tubulointerstitium | Total, n=85 | Severe AKI, n=46 | No Severe AKI, n=39 |
|---|---|---|---|
| Acute tubular injury | |||
| Mild | 36 (42) | 17 (37) | 19 (49) |
| Moderate | 27 (32) | 13 (28) | 14 (36) |
| Severe | 15 (18) | 11 (24) | 4 (10) |
| Bacterial foci | 4 (5) | 3 (6) | 1 (3) |
| Protein casts | 52 (61) | 27 (59) | 25 (64) |
| Molding casts | 21 (25) | 12 (26) | 9 (23) |
| Pigment casts | 46 (54) | 24 (52) | 22 (56) |
| Cytoplasmatic pigments | 50 (59) | 25 (54) | 25 (64) |
| Syncytium | 24 (28) | 15 (33) | 9 (23) |
| Regeneration | |||
| Mild | 48 (56) | 25 (54) | 23 (59) |
| Moderate | 28 (33) | 14 (30) | 14 (36) |
| Severe | 6 (7) | 4 (9) | 2 (5) |
| Isometric vacuolation | 66 (78) | 38 (83) | 28 (72) |
| Microthrombosis | 2 (2) | 1 (2) | 1 (3) |
| TA/IF | |||
| 0 | 32 (38) | 19 (41) | 13 (33) |
| 1 | 38 (45) | 17 (37) | 21 (54) |
| 2 | 9 (11) | 7 (15) | 2 (5) |
| 3 | 2 (2) | 0 (0) | 2 (5) |
| Tubulointerstitial nephritis | 6 (7) | 4 (9) | 2 (5) |
Data are expressed as n (%). TA/IF, tubular atrophy/interstitial fibrosis.
Table 2.
Glomerular light microscopy and electron microscopy
| Histopathological Findings | Total, n=85 | Severe AKI, n=46 | No Severe AKI, n=39 |
|---|---|---|---|
| Glomeruli | |||
| No. of glomeruli | 26 (13–51) | 20 (13–41) | 32 (13–60) |
| Global sclerosis (%) | 6 (0–10) | 6 (0–13) | 4 (2–8) |
| Segmental sclerosis (%) | 0 (0–7) | 0 (0–0) | 0 (0–13) |
| Focal segmental sclerosis variant | |||
| Perihilar | 17 (20) | 9 (20) | 8 (20) |
| Tip lesion | 4 (5) | 0 (0) | 4 (10) |
| Not otherwise specified | 4 (5) | 1 (2) | 3 (8) |
| Hypoperfusion | 46 (54) | 21 (46) | 25 (64) |
| Diabetic nephropathy | |||
| DGP class IIA | 5 (6) | 1 (2) | 4 (10) |
| DGP class IIB | 11 (13) | 7 (15) | 4 (10) |
| DGP class III | 7 (8) | 3 (6) | 4 (10) |
| Glomerulomegaly | 24 (28) | 11 (24) | 13 (33) |
| Thrombotic microangiopathy | 6 (7) | 2 (4) | 4 (10) |
| Arteriosclerosis | 69 (81) | 38 (83) | 31 (79) |
| Electron microscopy | |||
| Loss of tubular epithelium | 50 (59) | 25 (54) | 25 (64) |
| Mitochondrial edema | 63 (74) | 37 (80) | 26 (67) |
| Multivesicular bodies | 37 (43) | 18 (39) | 19 (49) |
| Clathrin-coated structures | 28 (33) | 11 (24) | 17 (44) |
| Podocyte foot processes edema | 13 (15) | 7 (15) | 6 (15) |
| Endothelial edema | 15 (18) | 8 (17) | 7 (18) |
| GBM thickening | 20 (23) | 9 (20) | 11 (28) |
| Mesangial sclerosis | 17 (20) | 9 (20) | 8 (20) |
Data are expressed as median (25th to 75th) or as n (%). DGP, diabetic glomerulopathy; GBM, glomerular basement membrane.
On light microscopy (Figure 1), ATI grades 2–3 were observed in 42 (49%), casts were found in 65 (77%), cytoplasmic pigments were found in 50 (59%), isometric vacuolation was found in 66 (78%), and glomerular hypoperfusion was found in 46 (54%). Acute tubulointerstitial nephritis (AIN) was seen in six (7%), interstitial microthrombosis was seen in two (2%), and thrombotic microangiopathy (TMA) was seen in six (7%) patients.
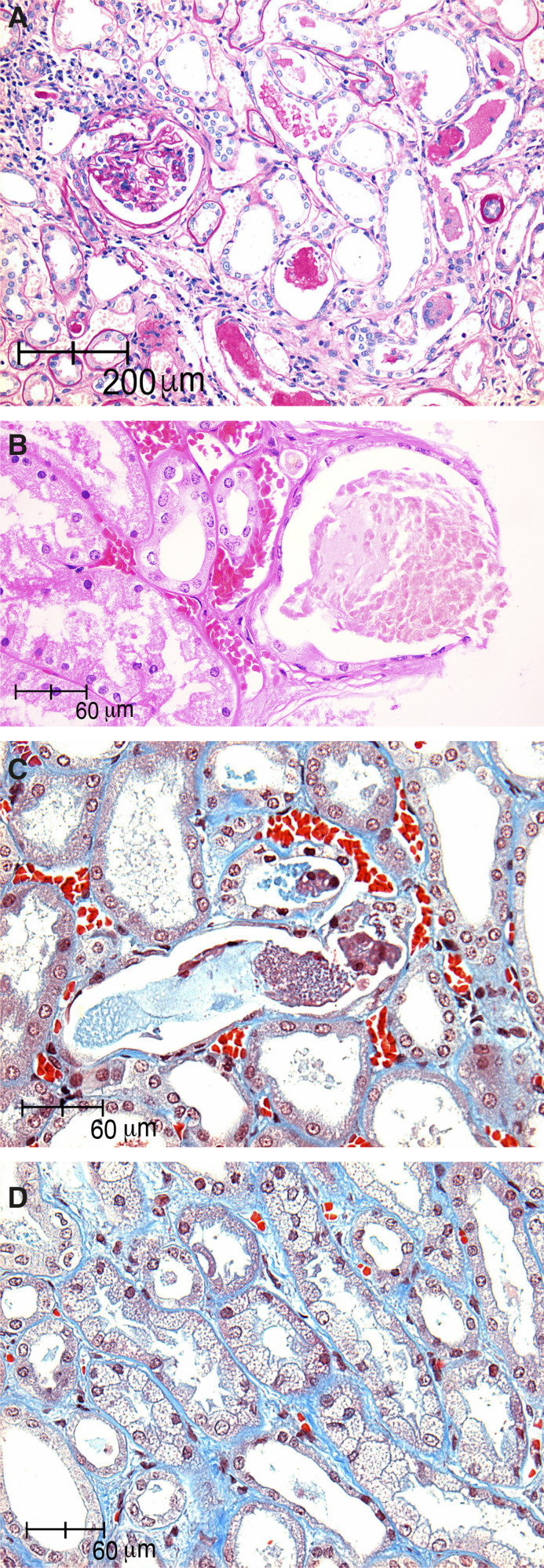
Figure 1.
Acute tubular injury in kidney biopsies of patients with COVID-19. (A) Acute tubular injury with retraction of glomerular capillaries, tubular lumen with proteinaceous cylinders, and regenerative changes in the tubular epithelium and syncitial cells (periodic acid–Schiff, ×10). (B and C) Tubular lumen with pigmented casts and loss of brush borders (hematoxylin and eosin, ×40 and Masson trichrome, ×40). (D) Tubular isometric vacuolization with debris in tubular lumen (Masson trichrome, ×40).
On EM (Figure 2), the most frequent findings were loss of tubular epithelium (59%), mitochondrial edema (74%), and multivesicular bodies (44%); also, 33% had clathrin-coated structures.
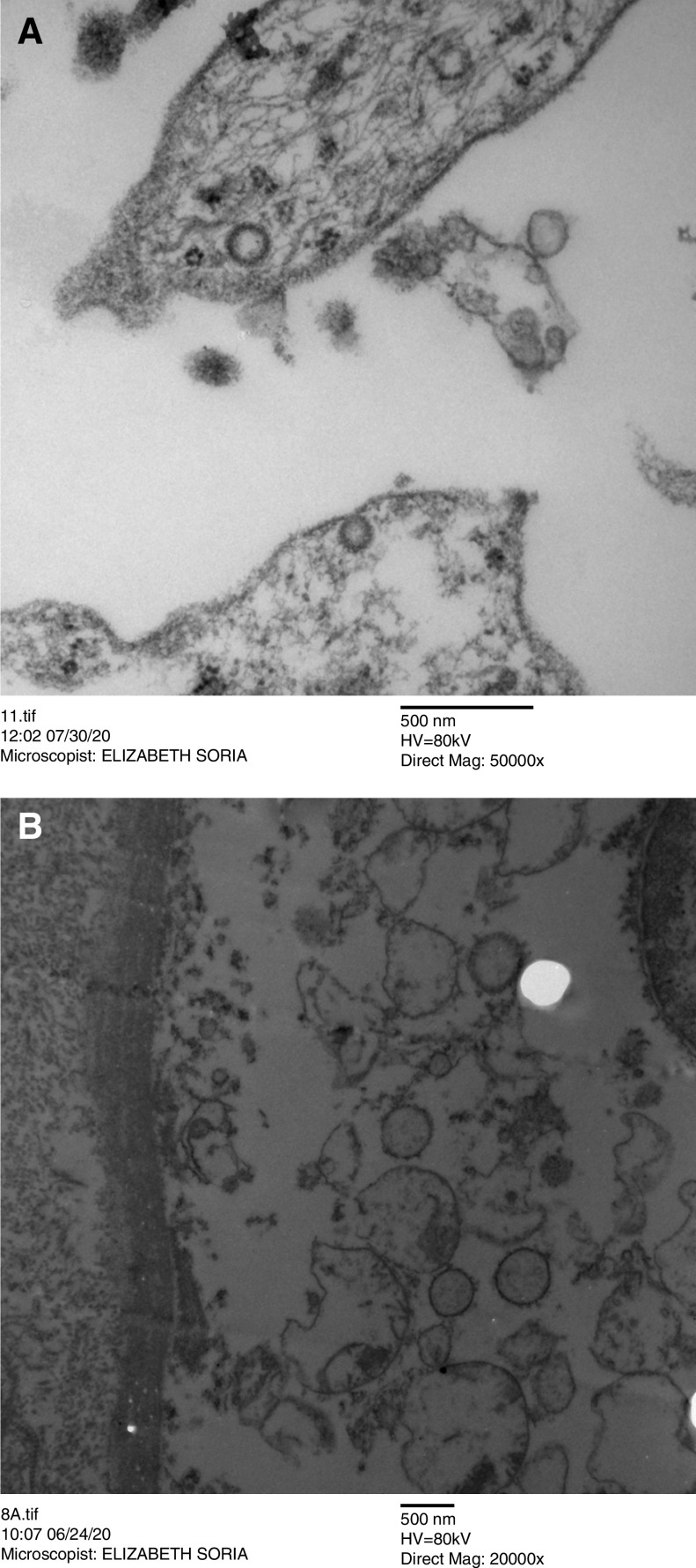
Figure 2.
Ultraestructural features on electron microscopy in kidney biopsies of patients with COVID-19. Multivesicular bodies and clathrin-coated vesicles into endothelial cells of the kidney (transmission electron microscopy: magnification, (A) ×5000 and (B) ×20,000).
Clinical and Histopathologic Characteristics Associated with Severe AKI
Medications, such as steroids, vancomycin, and vasopressors, and superimposed bacterial infections were associated with severe AKI (P=0.05) (Supplemental Table 1). Tables 1 and 2 show the association with biopsy findings, where only the percentage of segmental sclerosis was related to severe AKI. In multivariable models, only superimposed bacterial infections (odds ratio [OR], 2.48; 95% confidence interval [95% CI], 0.94 to 6.57), vancomycin (OR, 3.56; 95% CI, 0.86 to 14.78), and vasopressors (OR, 3.58; 95% CI, 0.94 to 13.62) showed a trend for risk of severe AKI. In the multivariable analysis for kidney function recovery before death, pigment casts were associated with a significantly lower probability of kidney recovery (OR, 0.07; 95% CI, 0.01 to 0.77).
Clinical Characteristics Associated with Histopathologic Findings
Figure 3 shows the forest plots for relevant ORs. The use of amikacin, gentamycin, or colistin showed a trend toward increasing the presence of molding casts, pigment casts, and ATI on light microscopy and loss of tubular epithelium on EM. High levels of C-reactive protein and low serum albumin were associated with ATI, glomerular hypoperfusion, and clathrin-coated structures on EM. Previous use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers was associated with loss of tubular epithelium on EM, and full dose of anticoagulants showed a reduction in ATI on light microscopy and a reduction in clathrin-coated structures on EM.
Figure 3.

Forest plots for add ratios between clinical variables and biopsy findings. (A) Molding casts; (B) pigment casts; (C) Clathrin-coated structures; (D) glomerular hypoperfusion; (E) acute tubular injury; (F) loss of tubular epitelium. AMK, amikacin; CLS, colistin; EM, electron microscopy; GNT, gentamicin.
Finally, all cases with AIN were present in subjects receiving antiviral medication (either lopinavir/ritonavir or oseltamivir), and all patients who were treated with nonsteroid anti-inflammatory drugs showed glomerular hypoperfusion (data not shown).
Discussion
We present the largest postmortem kidney biopsy series to date (Table 3). Santoriello et al. (11) analyzed 42 autopsies, although only ten of them underwent EM. In our cohort, the prevalence of AKI was 84%; this cannot be compared with the reported epidemiology of AKI in COVID-19 (4,12,13), as our findings correspond to those critically ill with a fatal outcome. As a reference, Remmelink et al. (14) and Santoriello et al. (11) showed prevalence rates of AKI of 82.3% and 94%, respectively, in deceased patients, similar to our cohort; however, Su et al. (9) in China and Bradley et al. (15) in the United States reported prevalence rates of only 34.6% and 42.9%, respectively.
Table 3.
Published studies on kidney histopathology analysis in coronavirus disease 2019
| Histopathological Findings | Study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akilesh et al. (19) | Bradley et al. (15) | Bryce et al. (unpublished observations) | Golmai et al. (8) | Hanley et al. (24) | Kudose et al. (21) | Nasr et al. (20) | Remmelink et al. (14) | Santoriello et al. (11) | Sharma et al. (22) | Su et al. (9) | |
| Country | USA | USA | USA | USA | UK | USA | USA | Belgium | USA | USA | China |
| Sample | Biopsies | Autopsies | Autopsies | Biopsies | Autopsies | Biopsies | Biopsies | Autopsies | Autopsies | Biopsies | Autopsies |
| N | 17 | 14 | 25 | 12 | 9 | 17 | 13 | 17 | 42 | 10 | 26 |
| Age, yr | 54 (34–77) | 73 (67–77) | 69 (34–94) | 75 (57–77) | 73 (52–79) | 54 (22–72) | 52 | 72 (62–77) | 71 (38–97) | 66 (60–69) | 69 (39–87) |
| Hypertension, % | 82 | 71 | 63 | 75 | 40 | 65 | 85 | 59 | 73 | 70 | 55 |
| Diabetes, % | 29 | 36 | 40 | 33 | 20 | 18 | 46 | 53 | 42 | 30 | 15 |
| CKD, % | NA | 57 | 27 | 8 | 10 | NA | 44 | NA | 29 | 0 | 10 |
| AKI, % | 88 | 43 | NA | 100 | NA | 88 | 100 | 82 | 94 | 100 | 35 |
| FSGS, % | 65 | 7 | 0 | NA | 0 | 35 | 62 | NA | 3 | 10 | 8 |
| Diabetic nephropathy, % | 12 | 21 | 8 | 8 | 0 | NA | 23 | NA | 17 | 10 | 8 |
| Arteriosclerosis, % | NA | 79 | NA | 83 | 83 | 50 | 77 | NA | 85 | 80 | 69 |
| ATI, % | 82 | 79 | 24 | 100 | 100 | 71 | 100 | NA | 62 | 100 | 100 |
| GN, % | 18 | NA | 0 | 0 | 0 | 71 | 31 | NA | NA | 10 | 0 |
| TMA, % | 35 | NA | 0 | 0 | 17 | NA | 0 | 0 | 14 | 20 | 12 |
USA, United States; UK, United Kingdom; N, number; NA, not available; ATI, acute tubular injury; TMA, thrombotic microangiopathy.
Our findings revealed no evidence of collapsing or postinfectious glomerulopathies. Series of case reports from one to six patients have described collapsing glomerulopathy in COVID-19; however, most of them linked to the Black population with a high-risk allele genotype for apoL1 or in the context of transplant recipients, which may explain why these findings were not prevalent in other reports, including our Hispanic patients (16 –18). Akilesh et al. (19) reported 17 patients with mild COVID-19, 88% had AKI and 65% with heavy proteinuria as criteria for biopsy, seven patients (41%) had collapsing glomerulopathy, six of them with Black race and one of them had this diagnosis 3 years before. Nasr et al. (20) identified 13 patients with COVID-19 (only one required intubation) who underwent biopsy due to AKI (92% severe) and nephrotic-range proteinuria (85%); eight (61%) had collapsing glomerulopathy, and all of them were Black patients. Although direct immunofluorescence analysis was not performed, the EM allowed us to confirm an absence of immune complexes. Kudose et al. (21) found a prevalence of 58.8% glomerulopathies; however, 47.1% of patients had mild COVID-19, and there were other indications for biopsy, including 52.9% had nephrotic-range proteinuria and 17.6% had kidney transplantation. Additionally, Sharma et al. (22) found just one patient with glomerulopathy in ten patients with AKI as inclusion criteria, and 40% had nephrotic-range proteinuria. The autopsy studies have not reported significant glomerulopathies, and therefore, we suggest considering a coexisting glomerulopathy in patients with COVID-19 only in case of a clinical glomerular syndrome (nephrotic, nephritic, or rapid progressive GN) or a suggestive urine sediment, as usual.
The presence of TMA in diverse organs, including kidneys, has been reported (23). We identified TMA in only 7% of patients; however, there is a possibility that the hypoperfusion found in 50% could be linked to thrombotic events not identified by our samples. We considered that the use of anticoagulants in 98% of our patients could explain the reduction in TMA, and also progressively in other reports from 0% to 16.7% (8,9,11,14). Akilesh et al. (19) found TMA in six of 17 (35%) patients with mild COVID-19, with a sampling range from 3 days up to 7 weeks from COVID-19 diagnosis; however, patients had severe hypertension, and four of six had clinically overt TMA, which was part of the criteria for kidney biopsy. Finally, 75% of the latter had additional conditions that may have contributed to TMA (cytotoxic or illicit drugs) as stated by the authors (19).
We believe the presence of FSGS in our population could be associated with comorbidities and not necessarily with the virus. In a subanalysis, we explored the association between FSGS excluding tip variant and the rest of histopathologic and clinical characteristics. Although diabetes, diabetic glomerulopathy, arteriosclerosis, and glomerular basement membrane thickening seemed more prevalent in the FSGS group, they did not reach statistical significance; however, glomerulomegaly was present in 52% with FSGS versus 20% (P=0.005), and the interstitial fibrosis/tubular atrophy score was also higher in the FSGS group (P=0.05) (Supplemental Table 2). Interestingly, excluding Kudose et al. (21), Akilesh et al. (19), and Nasr et al. (20), who had other indications for biopsy (19 –21), our cohort is the youngest adult histopathologic report so far with a median of 57 years, and it has one of the lowest prevalence rates of hypertension (aside from Hanley et al. [24] in the United Kingdom with 40%) and lowest known self-reported CKD; nevertheless, we found a similar prevalence of moderate to severe arteriosclerosis (around 80%) and a higher prevalence of FSGS (29% versus 0%–10% in the rest). Possible explanations are the high prevalence of diabetes and diabetic glomerulopathy and potential subclinical preexisting kidney disease in the context of a well-known spectrum of metabolic syndrome in the Mexican population. All of these factors also mirror the epidemiology of the susceptible population to COVID-19 (7,25,26).
In the EM, mitochondrial edema and loss of the tubular epithelium were observed in the vast majority, similar to other series (9,11,22). The presence of virions in kidney tissues has been a matter of debate. Clathrin-coated structures were found in 33% of our patients, and other studies have varied from 0% to 100%; however, micrographs do not support that these particles are indeed virus (27). By immunohistochemistry, studies from kidney biopsies could not find viral particles, but Bradley et al. (15) and Su et al. (9) found positive results in 14.3% and 50%, respectively. By in situ hybridization, Kudose et al. (21) reported positivity in 11.8%, whereas two other studies could not found any. Finally, by RT-PCR, Remmelink et al. (14), Hanley et al. (24), and Bradley et al. (15) found viral RNA in 58.8%, 60%, and 100%, respectively, in selected cases. With our limitations, we cannot suggest that structures shown by EM in our study correspond to viral particles.
Until now, studies have focused on the histopathologic description (9,22), and therefore, we additionally evaluated the association of these findings with clinical characteristics. Although ATI was highly prevalent, this was not different between patients with or without severe AKI; the evidence of structural damage with no clinical criteria could be explained by the limitations of using serum creatinine in a sarcopenic highly inflamed population, which has been included in the concept of subclinical AKI (28). AIN was found in only 7%, limited to patients receiving antivirals; in other series, AIN has also been rarely described between 0% and 8.3%. The risk for ATI and glomerular hypoperfusion were nine times greater when patients received nephrotoxic agents (aminoglycosides and colistin) and had high levels of C-reactive protein. These nephrotoxic and inflammatory factors are usually present in non–COVID-19 critically ill patients, so this information might be useful in the context of other forms of AKI, including septic AKI. Other well-known contributors for AKI as invasive mechanical ventilation and hemodynamic instability could not be evaluated as they were common denominators in our population (7,29 –31). To be noticed, we found 91.8% of ATI, 49.4% being moderate to severe, similar to other COVID-19 studies with 70.6%–100%; contrary to this, in patients who are septic, some reports account for 20%–30% of ATI (32,33). In this sense, the coparticipation of cytokine storm, nephrotoxic medication, and, possibly, a deeper and more prolonged hypoxia contributing to hypoperfusion could explain the higher incidence of ATI, without taking into account any direct viral-contributing action so far. Our clinical and histopathologic associations are subject to bias, due to sample size, inclusion criteria, and unadjusted analysis for many variables, but they might contribute to generating some hypotheses.
Our study has some other limitations. First, our patients were all critically ill and required mechanical ventilation; therefore, our findings may not be generalizable to other cohorts with COVID-19. Second, we did not have any other valuable method for identification of the virus in kidney tissue, such as antibodies against SARS-CoV-2. Finally, we do not have measurements of proinflammatory cytokines and viral load in most of the cases. Our main strength relies on our sample size and having EM in all cases.
In conclusion, kidney biopsies in deceased patients with COVID-19 in the Mexican population showed a higher than expected prevalence of chronic histopathologic disease, and inflammatory markers and medications might be associated with some acute biopsy findings.
Disclosures
M. Madero reports employment with Instituto Nacional de Cardiologia Ignacio Chavez; receiving research funding from Abbvie, AstraZeneca, Bayer, and Boehringer; receiving honoraria from AstraZeneca, Bayer, and Fresenius Medical Center; serving as a scientific advisor or member of American Journal of Kidney Disease, the International Society of Nephrology, and the Kidney Disease Improving Global Outcomes Executive Committee; and serving on advisory boards of Abbvie, AstraZeneca, and Bayer. G. Rojas reports employment with Instituto Nacional de Cardiología Ignacio Chávez; consultancy agreements with Boehringer Ingelheim and Eli Lilly; research funding from Eli Lilly; honoraria from Boehringer Ingelheim; and speakers bureau for Boehringer Ingelheim and Eli Lilly. R. Valdez-Ortiz reports employment with Hospital General de Mexico Dr. Eduardo Liceaga; consultancy agreements with Alexion, Amgen, AstraZeneca, Bayer, and Novartis; receiving research funding from Alexion, AstraZeneca, and Bayer; speakers bureau for Alexion, AstraZeneca, and Bayer; and other interests/relationships with the Colegio Mexicano de Nefrología, Instituto Mexicano de Investigaciones Nefrológicas, and the International Society of Nephrology. A. Vazquez-Rangel reports consultancy agreements with Baxter, BBraun, and Medtronic; honoraria from Baxter, BBraun, and Medtronic; and speakers bureau for Baxter, BBraun, and Medtronic. C. Zabal reports serving as a consultant to Abbott. All remaining authors have nothing to disclose.
Funding
Publication fee was funded by the Instituto Nacional de Cardiología-Ignacio Chávez.
Supplementary Material
Acknowledgments
The authors appreciate all of the hospital staff members for their efforts in recruiting and treating patients and thank all patients involved in this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16281020/-/DCSupplemental.
Supplemental Table 1. Clinical characteristics of the study population.
Supplemental Table 2. Comparison of patients with and without FSGS.
Supplemental Material. Histopathologic criteria for common descriptions.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang L, Li X, Chen H, Yan S, Li D, Li Y, Gong Z: Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 51: 343–348, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T, Meraz-Munoz A, Wald R, Harel Z: The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: A systematic review and meta-analysis. Kidney Med 3: 83–98.e1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network: Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323: 1574–1581, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W, Ghahramani N: Incidence of acute kidney injury and its association with mortality in patients with COVID-19: A meta-analysis. J Investig Med 68: 1261–1270, 2020. [DOI] [PubMed] [Google Scholar]
- 6. Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L: Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 46: 1339–1348, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, Bijol V, Rosenstock JL: Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 31: 1944–1947, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR: Electron microscopy of SARS-CoV-2: A challenging task. Lancet 395: e99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D’Agati V, Markowitz G: Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 31: 2158–2167, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA 323:2098, 2020 10.1001/jama.2020.7681]. JAMA 323: 2052–2059, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O’Donnell MR: Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 395: 1763–1770, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Remmelink M, De Mendonça R, D’Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I: Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care 24: 495, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA: Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington state: A case series. Lancet 396: 320–332, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR: COVID-19-associated collapsing focal segmental glomerulosclerosis: A report of 2 cases. Kidney Med 2: 493–497, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, Lhopitallier L, Sadallah S, Fakhouri F: Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasr SH, Kopp JB: COVID-19-associated collapsing glomerulopathy: An emerging entity. Kidney Int Rep 5: 759–761, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, Kambham N, Bracamonte E, Houghton D, Ahmed NI, Chong CC, Thajudeen B, Rehman S, Khoury F, Zuckerman JE, Gitomer J, Raguram PC, Mujeeb S, Schwarze U, Shannon MB, De Castro I, Alpers CE, Najafian B, Nicosia RF, Andeen NK, Smith KD: Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 77: 82–93.e1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nasr SH, Alexander MP, Cornell LD, Herrera LH, Fidler ME, Said SM, Zhang P, Larsen CP, Sethi S: Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis 77: 465–468, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, D’Agati VD: Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V; Northwell Nephrology COVID-19 Research Consortium: COVID-19-associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis): High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med 46: 1089–1098, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M: Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 1: e245–e253, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, Wei J, Gong Z, Zhou C, Yu H, Yu M, Lei H, Cheng F, Zhang B, Xu Y, Wang G, Dong W: Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect 26: 767–772, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W: Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 81: e16–e25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller SE, Brealey JK: Visualization of putative coronavirus in kidney. Kidney Int 98: 231–232, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haase M, Kellum JA, Ronco C: Subclinical AKI--An emerging syndrome with important consequences. Nat Rev Nephrol 8: 735–739, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Husain-Syed F, Slutsky AS, Ronco C: Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med 194: 402–414, 2016. [DOI] [PubMed] [Google Scholar]
- 31. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 32. Aslan A, van den Heuvel MC, Stegeman CA, Popa ER, Leliveld AM, Molema G, Zijlstra JG, Moser J, van Meurs M: Kidney histopathology in lethal human sepsis. Crit Care 22: 359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rameshkumar R, Krishnamurthy S, Ganesh RN, Mahadevan S, Narayanan P, Satheesh P, Jain P: Histopathological changes in septic acute kidney injury in critically ill children: A cohort of post-mortem renal biopsies. Clin Exp Nephrol 21: 1075–1082, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.