Abstract
Background:
In 2013, the Texas legislature passed House Bill 2 (HB2) restricting use of medication abortion to comply with FDA labeling from 2000. The FDA updated its labeling for medication abortion in 2016, alleviating some of the burdens imposed by HB2.
Objective:
Our objective was to identify the impact of HB2 on medication abortion use by patient travel distance to an open clinic and income status.
Study Design:
In this retrospective study, we collected patient zip code, county of residence, type of abortion, family size, and income data on all patients who received an abortion (medication or aspiration) from seven Texas abortion clinics in three time periods: pre-HB2 (July 1, 2012-June 30, 2013), during HB2 (April 1, 2015-March 30, 2016), and post-FDA labeling update (April 1, 2016-March 30, 2017). Patient driving distance to the clinic where care was obtained was categorized as 1–24, 25–49, 50–99, or 100+ miles. Patient’s county of residence was categorized by availability of a clinic during HB2 (open clinic), county with an HB2-related clinic closure (closed clinic), or no clinic any time period. Patient income was categorized as ≤110% federal poverty level (low-income) and >110% federal poverty level. Change in medication abortion use in the three time periods by patient driving distance, residence in a county with an open clinic, and income status were evaluated using chi-squared tests and logistic regression. We used geospatial mapping to depict the spatial distribution of patients who obtained medication abortion in each time period.
Results:
Among 70,578 abortion procedures, medication abortion comprised 26%, 7%, and 29% of cases pre-HB2, during HB2, and post-FDA labeling update, respectively. During HB2, patients traveling 100+ miles compared to 1–24 miles were less likely to use medication abortion (OR 0.21, 95% CI 0.15, 0.30), as were low compared to higher-income patients, (OR 0.76, 95% CI 0.68, 0.85), and low-income, distant patients (aOR 0.14, 95% CI 0.08, 0.25). Similarly, post-FDA labeling update, rebound in medication abortion use was less pronounced for patients traveling 100+ miles compared to 1–24 miles (OR 0.82, CI 0.74, 0.91), lower compared to higher income patients (OR 0.77, 95% CI 0.72, 0.81), and low-income, distant patients (aOR 0.80, 95%CI 0.68, 0.94). Post-FDA labeling update, patients residing in counties with HB2-related clinic closures were less likely to receive medication abortion as driving distance increased (52% traveling 25–49 miles, 41% traveling 50–99 miles, and 26% traveling 100+ miles, p<0.05). Geospatial mapping demonstrated that patients traveled from all over the state to receive medication abortion pre-HB2 and post-FDA labeling update, whereas during HB2, only those living in or near a county with an open clinic obtained medication abortion.
Conclusions:
Texas state law drastically restricted access to medication abortion and disproportionately impacted low-income patients and those living farther from an open clinic. After the FDA labeling update, medication abortion use rebounded, but disparities in use remained.
Keywords: abortion rate, epidemiology, ethics, income, induced abortion, legislation, rural population, spatial analysis, Texas, United States
Introduction
While the overall U.S. abortion rate is declining, medication abortion use has increased steadily.1 In 2014, approximately 31% of abortions in the U.S. were induced via medication, compared to 6% in 2001.2 Patients choose medication abortion because of a desire to avoid an aspiration procedure, a belief that it is more natural given its similarity to miscarriage, and because the process can occur in the privacy and comfort of one’s home.3
Despite the increasing demand for medication abortion, legislation in some states requiring adherence to the U.S. Food and Drug Administration (FDA) labeling limited its use.4 In 2000, the FDA approved used of mifepristone, along with misoprostol administered at a healthcare facility 48 hours later, to end pregnancy up to 7 weeks gestation.5 Since its initial approval, evolving research on medication abortion demonstrated that effectiveness in inducing complete abortion was maintained up to 10 weeks gestation and patients could safely self-administer misoprostol between 24 to 48 hours after taking mifepristone.3 As a result, a newer evidence-based medication abortion regimen was widely used across the U.S. that diverged from initial FDA labeling.
In July 2013, the Texas state legislature passed House Bill 2 (HB2) requiring physicians to provide medication abortion in accordance with the 2000 FDA label.6 All patients seeking care between 7 to 10 weeks gestation were prevented from obtaining medication abortion. Additionally, for most patients in Texas seeking medication abortion, this law required an increase from three to four in-person visits for 1. state-mandated counseling and sonographic confirmation of gestational age, 2. administration of mifepristone at least 24 hours later, 3. administration of misoprostol in the clinic 48 hours after mifepristone use, and 4. an in-person follow-up visit 7–14 days later. Patients living greater than 100 miles from any open clinic could complete the process in three visits if they received state-mandated counseling via telephone 24 hours prior to receiving mifepristone. Medication abortion became more difficult to obtain compared to aspiration abortion which could be completed in two visits (one visit for patients living greater than 100 miles from any clinic who also completed the state-mandated telephone counseling 24 hours in advance).
Simultaneously, other components of HB2 that required physicians providing abortion to have hospital admitting privileges and all abortion clinics to meet the facility requirements of an ambulatory surgical center (ASC) led to closure of over half of the state’s abortion clinics and long delays to obtain an initial appointment in clinics that remained open.7 Previous research demonstrated that while there was a statewide decrease in the abortion rate by 13%, use of medication abortion decreased by 70% as a result of this restrictive Texas law.2,4,8,9
In March 2016, the FDA updated its labeling allowing mifepristone use up to 10 weeks gestation and home administration of misoprostol 24 to 48 hours later,5 thus minimizing the impact of Texas legislative restrictions and resulting in a rebound of medication abortion use statewide.9
Legislative restrictions against abortion may disproportionately prevent rural and low-income patients from accessing abortion, yet there is limited research on the impact of these laws among these groups.10,11 While the statewide impact of HB2 has been described, our objective was to identify the impact of this law on medication abortion use by patient travel distance to an open clinic and income status.
Materials and Methods
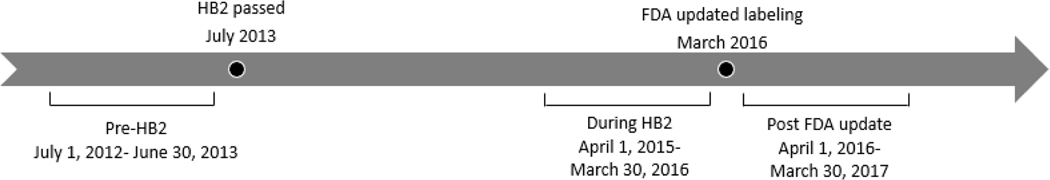
In this retrospective study, we collected data from seven abortion clinics that were open in three time periods: pre-HB2 (July 1, 2012 to June 30, 2013), during HB2 (April 1, 2015 to March 30, 2016), and after the FDA labeling update (April 1, 2016 to March 30, 2017). Data were not collected for the year immediately following passage of HB2 given the flux in abortion clinic availability resulting from a rapidly evolving legal climate. (Figure 1) Six of the seven clinics included in this study began providing medication abortion consistent with the updated FDA label on April 1, 2016, the other began the following month.
Figure 1.
Study period timeline and corresponding policy changes.
When we began this study, the ASC requirement enacted in HB2 was in legal limbo in a series of injunctions blocking enforcement of this requirement and lifting of injunctions allowing it to go in effect.12 We aimed to include data from clinics that met ASC requirements, six of the 41 existing Texas abortion clinics, that would be open in all three study periods if this component of the law was upheld.13 We ultimately collected data from five clinics that met ASC requirements, as well as their two affiliated clinics, including two abortion clinics in Dallas and one each in Fort Worth, Austin, San Antonio, Houston, and McAllen. We were unable to collect data from the sixth clinic meeting ASC requirements which was located in Houston. In June 2016, the U.S. Supreme Court ruled both the ASC and admitting privilege requirements unconstitutional. Therefore, the seven abortion clinics included in this study were among 18 that were open at some point during the latter two study periods, representing approximately 50% of abortions performed in the state and including all cities with an open ASC during HB2.
Each clinic generated a de-identified list of all patients who obtained an abortion (medication or aspiration) during each time period along with the patient zip code, patient county of residence, family size and income, type of abortion, and weeks’ gestation at time of care from their electronic medical record databases. We calculated whether each patient met criteria for funding assistance for abortion defined as living at or below 110% of the federal poverty level from all clinics except Houston which did not keep an electronic record of patient income.
The study protocol was approved by The University of Texas at Austin institutional review board as well as by the research department of participating clinics, if in existence, prior to data collection.
Statistical Analysis
To evaluate the association between patient travel distance and medication abortion use by time period, we calculated the driving distance between the centroid latitude and longitude coordinates of the patient’s zip code and that of the clinic where care was received using Google API. Patient driving distances were then categorized as 1–24, 25–49, 50–99, or 100+ miles from the clinic. Patients residing outside of Texas were excluded from this analysis.
For the Houston clinic in the pre-HB2 time period, only county code information was available for each patient. We identified counties containing zip codes that fell into disparate driving distance categories as described above. Of these, the greatest number of patients came from Harris county (77%), in which the Houston clinic is located. We assigned patient travel distances for these counties to align with the distribution seen during HB2 (e.g. 80% of patients from Harris county resided in zip codes that were 1–24 miles and 20% lived 25–49 miles from the clinic). Given the potential misclassification of driving distances, we also analyzed our results when patients traveling from these counties were categorized into the shortest and longest travel distances to establish a lower and upper range of possible values, respectively.
During HB2, medication abortion use was likely impacted directly by legislative restrictions and indirectly by clinic closures related to the physician admitting privileges and ASC requirement components of the law. To evaluate the association between clinic closures and medication abortion use, we categorized patients as living in a county with an open clinic in all three time periods (Bexar, Dallas, El Paso, Harris, Hidalgo, Tarrant, and Travis counties) and those living in a county with an HB2-related clinic closure (Bell, Brazos, Cameron, Jefferson, Lubbock, McLennan, Midland, Nueces, and Tom Green counties). We also compared medication abortion use in the during HB2 and post-FDA update time periods, when the number of clinics in Texas was relatively stable, to isolate the impact of legislative restrictions on medication abortion from clinic closures.
To examine differences in medication abortion use in each of the three time periods we calculated the number of patients who received medication abortion among all abortion patients and presented the raw numbers and proportions overall and by patient driving distance to the clinic, residence in a county with an open or closed clinic, and federal poverty level. To account for differences in medication abortion use related to legislative restrictions, separate from clinic closures, we reported the number of medication abortion users among all abortion patients, by patient county of residence, and by patient federal poverty level in the during-HB2 and post-FDA update periods stratified by patient driving distance. We conducted chi-squared tests of association to evaluate the relationship between medication abortion use and patient driving distance to a clinic, residence in a county with a clinic that remained open, and federal poverty level by time period. We also calculated odds ratios and 95% confidence intervals to evaluate medication abortion use among those traveling a short compared to longer distances, living in a county with compared to without an open clinic, and higher compared to low-income patients for each time period. We then created logistic regression models to examine the relationship between medication abortion use and patient county of residence, as well as income status, adjusted for patient driving distance in the latter two time periods. Quantitative analyses were conducted using STATA version 14.
To depict the spatial distribution of patients who received medication abortion from our study clinics during each of the three time periods, we used ArcGIS version 10.6. Choropleth maps were created for each time period in which counties were shaded in proportion to the total number of patients per county who received medication abortion. Overlaying these maps, are the cities in which there was an open abortion clinic or HB2-related clinic closure.
Finally, we used the updated FDA label for mifepristone use up to 10 weeks’ gestation to determine how many patients were eligible by gestational age at time of care for this abortion method. We calculated the proportion of medication abortion users among all patients who obtained care at or less than 10 weeks’ gestation in the during HB2 and post-FDA label update time periods. We used these calculations to estimate the proportion of patients who were unable to use medication abortion because of HB2-imposed restrictions.
Results
We collected data from 21,626 abortion cases from the pre-HB2 period, 23,126 during HB2, and 25,826 in the year post-FDA updated labeling for medication abortion.
Among patients who presented for abortion care at our study clinics, 26% received medication abortion pre-HB2, compared to 7% during HB2, and 29% after the FDA label update. (Table 1) When evaluating the association of patient travel distance on medication abortion use, we found a similar pattern of reduced medication abortion use during HB2 compared to pre-HB2 in each patient driving distance category. Yet, patients traveling greater than 100 miles were significantly less likely to obtain medication abortion compared to those traveling 1–24 miles for care during HB2. In each driving distance category, medication abortion use increased in the post-FDA label update period compared to during HB2, yet again, this increase was less pronounced for patients traveling 100+ miles for care compared to those traveling 1–24 miles.
Table 1.
Number, proportion, and odds ratios of patients receiving medication abortion per all abortion patients in pre-HB2, during HB2, and post-FDA label update periods by patient driving distance to clinic, residence in a county with an open clinic, and income status.*
| Pre-HB2 (July 1, 2012- June 30, 2013) | OR (95% CI) | During HB2 (April 1, 2015- March 30, 2016) | OR (95% CI) | Post-FDA update (April 1, 2016- March 30, 2017) | OR (95% CI) | |
|---|---|---|---|---|---|---|
| Total | 5571/21,626 (26) | 1631/23,126 (7) | 7490/25,826 (29) | |||
| Patient Driving Distance to Clinic** | ||||||
| 1–24 miles | 3769/13,258 (28) | ref | 1046/13,716 (8) | ref | 4251/14,977 (28) | ref |
| 25–49 miles | 1189/5337 (22) | 0.72 (0.67,0.78) | 418/5631 (7) | 0.97 (0.86, 1.09) | 1941/6429 (30) | 1.09 (1.02, 1.16) |
| 50–99 miles | 323/1517 (21) | 0.68 (0.60, 0.77) | 136/1964 (7) | 0.90 (0.75, 1.08) | 791/2358 (34) | 1.27 (1.16, 1.39) |
| 100+ miles | 290/1514 (19) | 0.60 (0.52, 0.68) | 31/1815 (2) | 0.21 (0.15, 0.30) | 507/2062 (25) | 0.82 (0.74, 0.91) |
| Patient County of Residence | ||||||
| Open clinic | 3994/15,037 (27) | ref | 1093/15,364 (7) | ref | 4826/16,799 (29) | ref |
| Closed clinic | 138/504 (27) | 1.04 (0.85, 1.27) | 93/1188 (8) | 1.11 (0.89, 1.38) | 488/1379 (35) | 1.36 (1.21, 1.52) |
| No Clinic | 1439/6085 (24) | 0.86 (0.80, 0.92) | 445/6574 (7) | 0.95 (0.85, 1.06) | 2176/7648 (28) | 0.99 (0.93, 1.05) |
| Federal Poverty Level*** | ||||||
| ≤110% | 1768/7683 (23) | 0.98 (0.89, 1.07) | 658/8505 (8) | 0.76 (0.68, 0.85) | 2685/9777 (27) | 0.77 (0.72, 0.81) |
| >110% | 872/3725 (23) | ref | 772/7785 (10) | ref | 3689/11,157 (33) | ref |
P-values in each row to test for differences in medication abortion use comparing all three time periods together are <0.05.
When Houston clinic pre-HB2 data were recategorized into the shortest possible driving distance, the 25–49 mile category is no longer significantly different from the referent group (OR 0.93, 95%CI 0.86, 1.00). When recategorized into the longest possible driving distance, the 25–49 mile and 50–99 mile categories are no longer significantly different from the referent group (OR 0.97, 95% 0.91, 1.04 and OR 0.91, 95%CI 0.81, 1.01, respectively).
Houston clinic excluded since no federal poverty level data available.
When evaluating the combined impact of medication abortion restrictions and clinic closures, we found a similar reduction in the proportion of medication abortion users during HB2 compared to pre-HB2 among patients residing in a county where a clinic remained open during HB2 and those living in a county with an HB2-related closure (73% reduction vs. 72% reduction, respectively). Yet, among those living in a county with an HB2-related clinic closure, patients traveling 100+ miles from the clinic were significantly less likely to use medication abortion during HB2 compared to those traveling 25–49 miles (aOR 0.04, 95%CI 0.02, 0.09). There was no significant difference in medication abortion use during HB2 among patients who lived in a county with an open clinic and traveled 100+ miles compared to 25–49 miles (aOR 1.22, 95% CI 0.65, 2.29).
Comparing medication abortion use in the during HB2 and post-FDA label update periods, when the number of open clinics was stable, allowed us to evaluate the impact of the legislative restriction on medication abortion separate from clinic closures. While medication abortion use increased in the latter period for both patient county categories, those residing in counties with an HB2-related closure were significantly more likely to obtain medication abortion compared to those living in a county with a clinic that remained open (OR 1.36, 95% CI 1.21, 1.52). (Table 1) However, among patients residing in a county with an HB2-related clinic closure, the proportion who received medication abortion in the post-FDA label update period decreased as distance to the clinic increased (52% for those living 25–49 miles from an open clinic, 41% living 50–99 miles away, and 26% for those living 100+ miles, p<0.05). (Table 2)
Table 2.
Impact of patient driving distance on number and proportion of patients receiving medication abortion per all abortion patients during HB2 and post-FDA label update periods by patient residence in a county with an open clinic and income status.*
| During HB2 (April 1, 2015- March 30, 2016) | Post-FDA update (April 1, 2016- March 30, 2017) | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient Driving Distance | 1–24 miles | 25–49 miles | 50–99 miles | 100+ miles | 1–24 miles | 25–49 miles | 50–99 miles | 100+ miles |
| Total | 1046/13,716 (8) | 418/5631 (7) | 136/1964 (7) | 31/1815 (2) | 4251/14,997 (28) | 1941/6429 (30) | 791/2358 (34) | 507/2062 (7) |
| Patient County of Residence | ||||||||
| Open clinic | 909/12,012 (8) | 166/2978 (6) | 7/208 (3) | 11/166 (1) | 3716/13,018 (29) | 991/3405 (29) | 89/256 (35) | 30/120 (25) |
| Closed Clinic | - | 35/154 (23) | 52/469 (11) | 6/565 (1) | - | 88/170 (52) | 228/550 (41) | 172/659 (26) |
| No Clinic | 137/1704 (8) | 217/2499 (9) | 77/1287 (6) | 14/1084 (1) | 535/1959 (27) | 862/2854 (30) | 474/1552 (31) | 305/1283 (24) |
| Federal Poverty Level** | ||||||||
| ≤110% | 410/4794 (9) | 169/1962 (9) | 68/887 (8) | 11/862 (1) | 1526/5556 (27) | 655/2309 (28) | 288/983 (29) | 216/929 (23) |
| >110% | 508/4698 (11) | 205/2059 (10) | 44/496 (9) | 15/532 (7) | 2090/6443 (32) | 944/2776 (34) | 396/994 (40) | 259/944 (27) |
P-values in each row to test for differences in medication abortion use between two time periods areall <0.05.
Houston clinic excluded since no federal poverty level data available.
By patient income level, the reduction in medication abortion use during HB2 was more pronounced for those living at ≤110% of the federal poverty level compared to >110%. Compared to low-income patients traveling 1–24 miles for care, those who traveled 100+ miles were far less likely to use medication abortion during HB2 (aOR 0.14, 95% CI 0.08, 0.25). Medication abortion use rebounded for both income groups after the FDA updated its labeling, but less so among the lower compared to higher income group and among lower income patients traveling 100+ miles compared to 1–24 miles for care (aOR 0.80, 95%CI 0.68, 0.94).
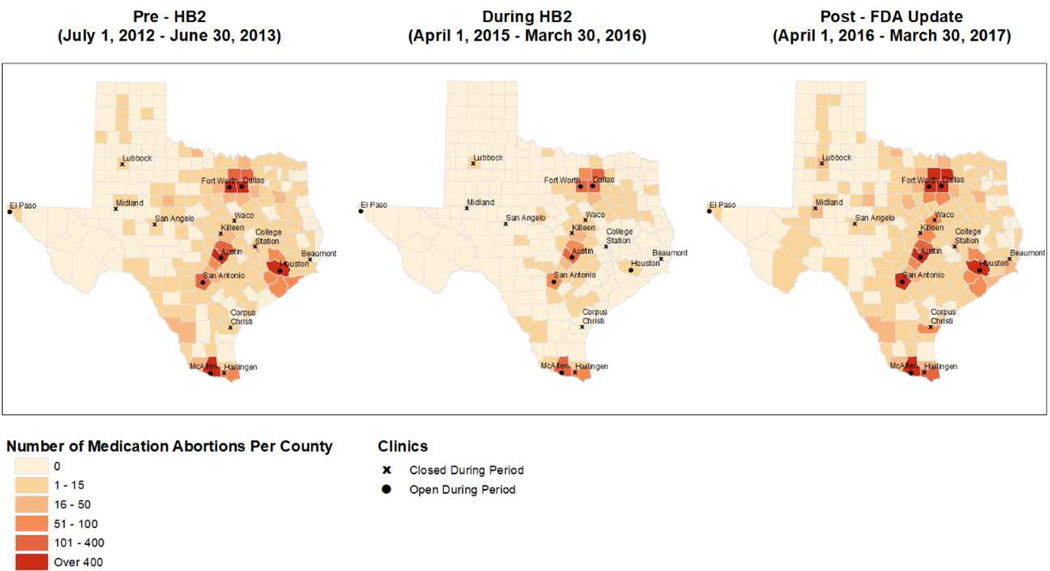
Geospatial mapping demonstrated that in the pre-HB2 period, the highest number of patients obtaining medication abortion lived within or next to a county in which study clinics were located, yet patients from all over the state traveled to the study clinics to obtain this method. During HB2, receipt of medication abortion was limited to those living within close proximity of a study clinic. No patients traveled from western Texas and Gulf Coast counties, which incurred HB2-related clinic closures, to obtain medication abortion from a study clinic during HB2. After the FDA labeling update, medication abortion was again obtained by patients living all throughout the state (Figure 2).
Figure 2.
Spatial distribution of the total number of patients per county receiving medication abortion from a study clinic in pre-HB2, during HB2, and post-FDA label update periods.*
*Study clinics include two abortion clinics in Dallas and one each in Fort Worth, Austin, San Antonio, Houston, and McAllen which were open pre-HB2, during HB2, and post-FDA label update. Data not collected from El Paso, nor Lubbock, Midland, San Angelo, Killeen, Waco, College Station, Beaumont, Corpus Christi, and Harlingen clinics which were open in pre-HB2 period, but then closed.
In the post-FDA label update period, 37% of patients who presented for care at or below 10 weeks gestation used medication abortion (7490/20,233). During HB2, only 9% of those eligible for medication abortion by a 10 week gestational age cut-off used this method (1630/17,431), indicating that as many as 28% of patients seeking abortion care were unable to use medication abortion due to legislative restrictions.
Discussion
Principal Findings
House Bill 2 restricted access to medication abortion among all Texas patients seeking access to safe abortion care, but disproportionately burdened low-income patients and those living far from an open clinic. After the FDA updated it’s labeling for medication abortion, use increased among all patients seeking care at our study clinics. The use of medication abortion post-FDA label update was even higher for those residing in counties with an HB2-related clinic closure compared to those living in counties with an open clinic, suggesting a strong preference for this method particularly among those who no longer had close access to it. Yet, the rebound in medication abortion use was dampened in low-income patients compared to their higher income counterparts, among those who lived farther from an open clinic, and among those doubly hampered by financial and geographic barriers.
Results in the Context of What is Known
Several studies have reported on the statewide decline in medication abortion use in Texas after implementation of HB2.2,4,8 Also, a recent study analyzing medication abortion use in Texas for a six month period after the FDA labeling update found a rebound in use similar to what we report.9 Yet, there are limited data on how the increasing number of state-based abortion restrictions differentially impact access to medication abortion among low-income patients and those who have to travel far distances to obtain care.11,14
Research and Clinical Implications
The impact of the FDA-updated label for use of mifepristone on overriding state-based regulations, imposed under the guise of patient safety, represent a clear victory for the role of science and advocacy in promoting best practices in patient care.15 The safety of medication abortion has been well established in the two decades since initial FDA approval, by additional intervening research, and in a comprehensive review conducted by the National Academies of Science, Engineering, and Medicine.16 Further research into the safety of medication abortion is unwarranted. Despite extensive evidence supporting the safety of this method reflected in the FDA-updated label, many challenges to medication abortion access remain in Texas and other states in the U.S. which are particularly harmful to low-income and rural patients who prefer this method. Specifically, clinic closures caused by undue legislative restrictions, prohibition of use of state funds to cover abortion, mandated administration of mifepristone by a physician, and prevention of telemedicine for distribution of medication abortion disproportionately prevent low-income patients and those living far from an open clinic from obtaining this method. The strong evidence supporting provision of medication abortion by advanced practice clinicians (such as nurse practitioners, physician assistants, and certified nurse mid-wives) and through telemedicine,17–19 as well as repeal of federal regulations that impose funding restrictions on abortion20,21 should be the basis for future legislation.
Strengths and Limitations
Our study takes advantage of a large sample size, linking where patients lived relative to where they obtained care, and abstracting data on patient income which are not included in publicly available state-based vital statistic data. Thus, we were able to extend the existing literature and quantify the negative impact of this restrictive law particularly among low-income and rural patients.
Despite the strengths of our study, there are some limitations. While we did collect data from all cities with an open ASC, we did not collect data from all clinics that were open during the three study periods, including one ASC in Houston and one clinic in El Paso. Additionally, we were unable to collect data for the pre-HB2 period from clinics that closed as a result of this law. Consequently, we are likely underestimating the use of medication abortion among patients living in west Texas and Gulf Coast counties in the pre-HB2 time period and the proportional decrease in use of this method during HB2. Similarly, we were unable to capture unmet demand among those who desired medication abortion but could not overcome logistical, financial, and social hurdles to obtain care at our study clinics. By examining a study population that had a completed aspiration or medication abortion procedure, we also cannot comment on the outcomes (e.g. self-managed abortion, unsafe abortion, continued pregnancies) of those who desired medication abortion, but were not able to obtain it. Altogether, the negative impact of HB2 on access to medication abortion is likely higher than what our results show.
Conclusion
In spite of the strong evidence regarding the safety of medication abortion use in less restrictive settings, multiple states throughout the U.S. have imposed or are proposing additional restrictions on medication abortion.22 Our findings demonstrate how deviation from evidence-based practices are particularly harmful to patients who would prefer to use medication abortion and are low-income or have to travel far distances to obtain care.
AJOG at a Glance:
A. Why was this study conducted?
To evaluate the impact of a Texas legislative restriction on medication abortion among low-income patients and those living far from an open clinic.
B. What are the key findings?
Compared to the year prior to implementation of the law, while the law was in effect, medication abortion use declined for all patients, but more so for low-income patients and those driving 100+ miles for care. After the FDA updated labeling for medication abortion, use of this method increased overall, but less so for low-income patients and those driving 100+ miles for care.
C. What does this study add to what is already known?
Our findings of reduced medication abortion use in Texas during implementation of the law with a rebound after FDA updated labeling are consistent with findings from one other study. We expand upon existing literature by quantifying the impact on low-income patients and those who had to travel far distances to obtain care.
Acknowledgements:
The authors would like to thank Lina Palomares for managing this study, Melissa Farrell, RN, BSN, CCRC for assistance with data collection, and all participating study clinics.
Funding: This work was supported by the Society of Family Planning Research Fund (grant number SFPRF 10–4) and by grant, P2CHD042849, awarded to the Population Research Center at The University of Texas at Austin by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Paper Presentation Information: These data were presented as an oral abstract at the 2018 North American Forum on Family Planning in New Orleans, LA on October 20–22, 2018 and as a clinical seminar at the 2018 American College of Obstetricians and Gynecologists Annual Clinical and Scientific Meeting in Austin, TX on April 27–30, 2018.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of Planned Parenthood Federation of America, Inc.
Condensation: Texas legislative restrictions on medication abortion disproportionately prevented low-income patients and those living far from open clinics from using this method.
Conflict of Interest Statement: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones RK, Boonstra H. The Public Health Implications of the FDA Update to the Medication Abortion Label. Guttmacher Institute; 2016. [Google Scholar]
- 2.Jones RK, Jerman J. Abortion Incidence and Service Availability In the United States, 2014. Perspect Sex Reprod Health 2017;49:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medical management of first-trimester abortion. Contraception 2014;89:148–61. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon WR, Winikoff B. Mifepristone label laws and trends in use: recent experiences in four US states. Contraception 2015;92:182–5. [DOI] [PubMed] [Google Scholar]
- 5.Mifeprex (mifepristone) Information. Silver Spring, MD: U.S. Food & Drug Administration, February 2018. Available at https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111323.htm. Retrieved January 9, 2019. [Google Scholar]
- 6.House Bill Number 2 (HB2) Enrolled. Austin, TX: Texas State Legislature, July 2013. Available at https://capitol.texas.gov/tlodocs/832/billtext/html/HB00002F.htm. Retrieved January 9, 2019. [Google Scholar]
- 7.Wait Time to Obtain an Abortion is Increasing in Texas as Clinics Close. Austin, TX: Texas Policy Evaluation Project; October 2015. Available at https://liberalarts.utexas.edu/txpep/releases/wait-times-release.php. Retrieved January 9, 2019. [Google Scholar]
- 8.Grossman D, Baum S, Fuentes L, et al. Change in abortion services after implementation of a restrictive law in Texas. Contraception 2014;90:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum SE, White K, Hopkins K, Potter JE, Grossman D. Rebound of medication abortion in Texas following updated mifepristone label. Contraception 2019;99:278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bearak JM, Burke KL, Jones RK. Disparities and change over time in distance women would need to travel to have an abortion in the USA: a spatial analysis. Lancet Public Health 2017;2:e493–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finer L, Fine JB. Abortion law around the world: progress and pushback. Am J Public Health 2013;103:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timeline: Family Planning and Abortion Legislation in Texas 2011–2017. December 2017. Available at sites.utexas.edu/txpep/files/2017/12/Abortion-and-Family-Planning-in-Texas-Timeline-update-12-5-17.pdf. Retrieved December 3, 2019.
- 13.Rapidly Changing Access to Abortion in Texas. Austin, TX: Texas Policy Evaluation Project, June 2014. Available at https://repositories.lib.utexas.edu/bitstream/handle/2152/65123/Rapidly-Changing-Access-to-Abortion-in-TX-18Jul2014.jpg?sequence=2&isAllowed=y. Retrieved January 9, 2019. [Google Scholar]
- 14.Last Five Years Account for More than One-quarter of All Abortion Restrictions Enacted Since Roe. New York, NY: Guttmacher Institute, January 2016. Available at https://www.guttmacher.org/article/2016/01/last-five-years-account-more-one-quarter-all-abortion-restrictions-enacted-roe. Retrieved January 9, 2019. [Google Scholar]
- 15.Greene MF, Drazen JM. A New Label for Mifepristone. N Engl J Med 2016;374:2281–2. [DOI] [PubMed] [Google Scholar]
- 16.Henney JE, Gayle HD. Time to Reevaluate U.S. Mifepristone Restrictions. N Engl J Med 2019;381:597–8. [DOI] [PubMed] [Google Scholar]
- 17.Weitz TA, Taylor D, Desai S, et al. Safety of aspiration abortion performed by nurse practitioners, certified nurse midwives, and physician assistants under a California legal waiver. Am J Public Health 2013;103:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman D, Grindlay K. Safety of Medical Abortion Provided Through Telemedicine Compared With In Person. Obstet Gynecol 2017;130:778–82. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyay UD. Innovative models are needed for equitable abortion access in the USA. Lancet Public Health 2017;2:e484–e5. [DOI] [PubMed] [Google Scholar]
- 20.Jones RK, Upadhyay UD, Weitz TA. At what cost? Payment for abortion care by U.S. women. Womens Health Issues 2013;23:e173–8. [DOI] [PubMed] [Google Scholar]
- 21.Boonstra H. The Heart of the Matter: Public Funding of Abortion for Poor Women in the United States. Guttmacher Policy Review 2007;10:12–6. [Google Scholar]
- 22.Medication Abortion. New York, NY: Guttmacher Institute, November 2018. Available at https://www.guttmacher.org/state-policy/explore/medication-abortion. Retrieved January 9, 2019. [Google Scholar]