Abstract
Although legacy-building is a priority for quality palliative care, research has rarely examined effects of legacy interventions in children, particularly their impact on parent-child communication.We examined the impact of a web-based legacy intervention on parent-child communication. We hypothesized that compared to usual care, legacy-making would improve quality of parent-child communication.Between 2015 and 2018, Facebook advertisements were used to recruit families of children (ages 7-17) with relapsed/refractory cancer. Parent-child dyads were randomly assigned to the intervention or usual care group. The intervention website guided children to create digital storyboards over 2 weeks by directing them to answer legacy questions about themselves and upload photographs, videos, and music. Families received a copy of the child’s final digital story. Children and parents completed the Parent-Adolescent Communication Scale pre- (T1) and post-intervention (T2). Linear regressions tested for differences in change from T1 to T2 between the groups controlling for T1 values using an alpha of p < .05. Intervention effects were measured using Cohen’s d. Ninety-seven parent-child dyads were included for analysis. Changes in parent-child communication were not statistically significantly different between the groups, yet meaningful intervention effects were observed. The strongest effects were observed for improving father-child communication (Cohen’s d = −0.22-0.33). Legacy-making shows promise to facilitate improved parent-child communication, particularly for fathers. Future studies should include fathers and measure expression of feelings and parent-child interaction. Providers should continue to facilitate family communication for children with advanced disease and realize that legacy interventions may impact mother-child versus father-child communication differently.
Keywords: parent-child relationship, father-child relationship, family relationships, parent-child communication, web-based intervention, pediatric, palliative care, cancer
Introduction
Approximately 15,800 children ages 0 to 19 years are newly diagnosed with cancer each year in the United States.1Despite survival rates exceeding 80%,1 the burden of cancer and its treatment for children and their caregivers is substantial.2–9Despite evidence supporting the value and feasibility of ill children being able to self-report on their cancer-related symptoms and experience,10 research has shown that parents may avoid cancer-related discussions with their child and do not seek children’s input about treatment options to protect their child from additional distress.11,12 The avoidance occurs even though parents and their ill child may spend more time together due to frequent appointments and hospitalizations, providing opportunities for discussion of physical and emotional effects from treatment andvery difficult topics related to the worry about their child’s mortality.13Some parents have described engaging in more intimate communication and purposefully maintaining an open relationship with their child after diagnosis.13
The quality of parent-child communication following relapse may predict children’s adjustment over time.14Higher openness and fewer problems in communication between children and their parents have predicted lower withdrawn/depression scores for children with advanced cancer.14 Communication quality between children and their parents near the time of relapse has also been found to strongly predict future adjustment a year later for children with advanced cancer. A commonly reported barrier to parent-child communication involves avoiding conversations about making necessary healthcare decisions as the child’s health declines near the end of life.15,16 Parents often feel responsible for choosing how and when to disclose potentially upsetting information in an attempt to protect their child from emotional distress.13 However, some parents have described increased openness in communication when the child’s diagnosis is definitively terminal.15
Although parents may initiate communication about their child’s cancer and prognosis, children often use avoidance or adjust their communication with others, particularly about their prognosis, to minimize parental sadness.17–19 Children may outwardly mask physical symptoms of illness or emotional distress by portraying a positive and cheerful mood.18 Some parents have described children openly sharing plans for the future, even when children recognize such plans are unlikely to occur.15Expressiveness in communication is important to adaptation, but both parents and their children recognize barriers to communication and a strong desire to protect each other against emotional distress.
Legacy-making (i.e., actions or behaviors aimed at being remembered20) is a palliative care intervention to improve communication outcomes for children with serious illnesses and their families. Legacy-making has been explored in both adult and pediatric populations.20–32Legacy-making in adults has been shown to increase patients’ sense of dignity, purpose, meaning, and will to live, while decreasing suffering and depressive symptoms.22,28,32 In our previous work, hospital staff and parents reported that legacy-making improved quality of life (QOL), communication, and coping among children with cancer (ages 7-17).21,24 Research has rarely examined effects of legacy-making on parent-child communication outcomes, and randomized controlled trials in this area are particularly lacking. Thus, this paper examines the impact of a web-based legacy intervention on parent-child communication among children with relapsed or refractory cancer. We hypothesized that compared to customary care, legacy-making would improve the quality of communication between children and their parents from pre- to post-intervention.
Methods
Conceptual Approach
We developed the conceptual framework based on synthesis of components from existing theories and evidence22,25,33to guide the proposed study (See Figure 1). Briefly, this conceptual framework provided a basis for examining the direct effects of our legacy intervention on the quality of communication between children living with refractory or relapsed cancer and their parent caregivers. This paper reports results related to the study outcome of parent-child communication.
Figure 1.
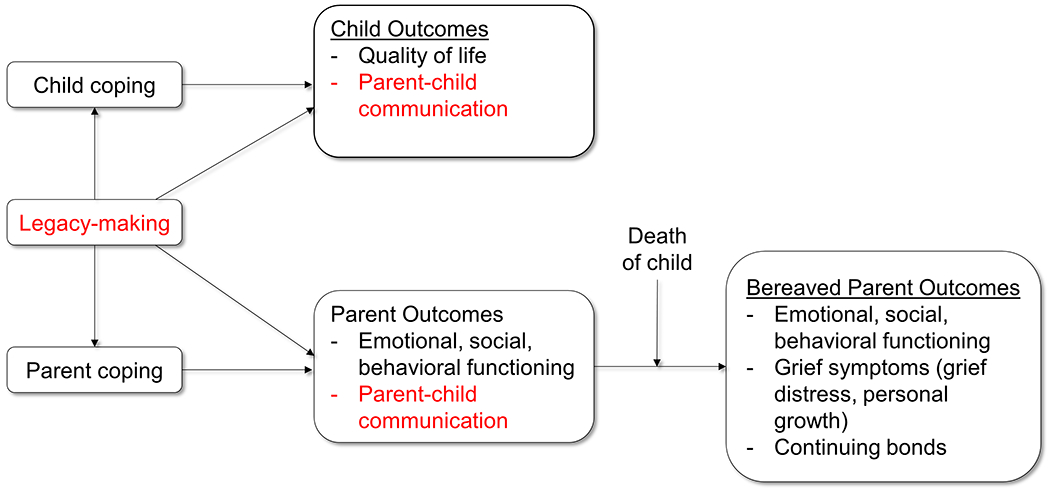
Conceptual framework (red font notes outcomes reported in this paper)
Procedures
This single-site research was part of a larger randomized clinical trial examining the effects of a legacy intervention on child and parent outcomes. Institutional review board approval was obtained prior to participant enrollment using Facebook advertisements. Because Facebook users must be at least 13 years of age to have an account, our advertisements targeted parents of children with cancer. Advertisements were placed on Facebook over 3 years, targeting parents who (a) were 18 years of age or older; (b) were located in the United States; (c) were any gender; and (d) had interest in pediatric oncology (liked other Facebook pages related to childhood cancer, cure childhood cancer, etc.). Parents could click on an electronic REDCap link in the Facebook ad that briefly described the study and included initial screening questions. Potentially eligible parents were then asked to complete basic demographic questions, including their names and contact informationto receive more details about the study. The study coordinator contacted interested parents via phone or email within 1 week to describe the study and confirm eligibility. Eligible participants were (a) children 7 to 17 years of age, (b) children with relapsed or refractory cancer determined by parent self-report, (c) able to speak, understand, read, and type English, (d) those with internet access, and (e) without cognitive impairment as determined by the coordinator during the consent process. Primary parent caregiversincluded the legal parent guardian who spent the most number of hours per week with the eligible child. For all eligible child-parent dyads, the coordinator obtained verbal parent consent and child assent and confirmed contact information to send study documents.
Legacy Intervention
Upon consent, child and parent dyads were randomly assigned to either the web-based intervention (n = 75) or usual care (n = 75) group by using a computer-generated randomization approach with a permuted block scheme. The coordinator emailed an electronic link to an intervention website to families in the intervention group within one week after children and parents completed baseline (T1) measures. Detailed description of the legacy intervention is presented in another article.34 In brief, each child-parent dyad created a username and password for the website. The website guided children to create a personal digital storyboard by directing them to: (a) answer legacy-making questions, (b) upload photographs, (c) upload video, and (d) upload music. Children were asked to complete the intervention within two weeks. When children completed the storyboard, the website generated a unique electronic link that the coordinator emailed to the child or parent. The control group received usual care. The intervention was offered to children in the control group after each child-parent dyad completed T2 measures.
Data Collection
Data collection occurred over three years (2015-2018). Participants in the intervention group completed T1 baseline and T2 post-intervention questionnaires related toparent-child communication, child quality of life, and child and parent coping outcomes. The median time between T1 and T2 was 69 days (range 7-176) for the children, and 68 days (range 7-176) for parents.At the end of the study, parents were asked to complete a T3 concludingsurvey about the intervention process, including what they liked and did not like (Akard et al.34). Participants completed all the study measures electronically online via REDCap, a secure web-based application for building and managing online surveys and databases. The study coordinator conducted reminder calls or sent reminder emails to enrolled participants for any surveys that were not completed within one week. This paper presents T1 and T2 quantitative data related to parent-child communication. The length of time to complete T1 and T2 child assessments was approximately 30 minutes.
Measurement Tools
Parents completed a background survey to report child and parent demographic characteristics and child clinical characteristics. Children and parents completedthe Parent-Adolescent Communication Scale (PACS) to assess parent-child communication.35Children completed 20 items about communication in relation to each parent (mother and father), and parents answered 20 items about communication with their ill child. Although child and parent participants were asked to self-report independently, younger children may have received parent assistance to complete the measure electronically.Responses were rated on a 5-point Likert scale from “strongly disagree” to “strongly agree.” Summary scores reflect open communication, problems in communication, and overall communication. Higher scores for open and overall communication, and lower scores for problems in communication, reflect better quality of parent-child communication. The internal consistency of the scores used in this study ranged from 0.67 – 0.80 for the parental scales and 0.73 – 0.92 for the child scales.
Analysis
Data analyses were conducted using IBM SPSS Statistics (Version 25). Frequency distributions were generated for summarizing the nominal and ordinal variables. Means and standard deviations were used to summarize the normally distributed continuous variables; medians and inter-quartile ranges (IQR) described the skewed distributions. Independent sample t-tests and Chi-Square Tests of Independence were conducted to compare the demographic and clinical characteristics of the participants in the two study data analysis groups. Comparisons between the study groups in the amount of change in each of the study outcome variables from baseline were conducted using generalized linear models that included the respective baseline scores as a covariate. Cohen’s d statistics quantifying the effect of the intervention on PACS outcome variables were generated by transforming the regression coefficient for the group effect after controlling for the baseline value. Interpretations of statistical significance maintained a maximum alpha of 0.05 (p < .05). Given that effect sizes were generated and presented, no adjustment for multiple outcomes to the critical alpha level was made.
Results
Two hundred seventy-three child-parent dyads were screened for eligibility (See Consort Diagram Figure 2). Of those, 150 dyads (55%) were deemed eligible and enrolled in the study. Forty-two (28%) dyads dropped out, including deaths, prior to completing T2 (control=31, experimental=11). Ten (7%) completed T2 more than 6 months after enrollment (control=7, experimental=3). One additional dyad in the control group was excluded from analysis after noting a discrepancy during analysis on parent report of child age compared to date of birth, making the child participant ineligible. Therefore, 97dyads were included in analyses. Summaries of the demographic and clinical characteristics of those completing the study and those excluded have been previously published.34 Region of the country and marital status were the only statistically significant differences observed (p < 0.05).
Figure 2.
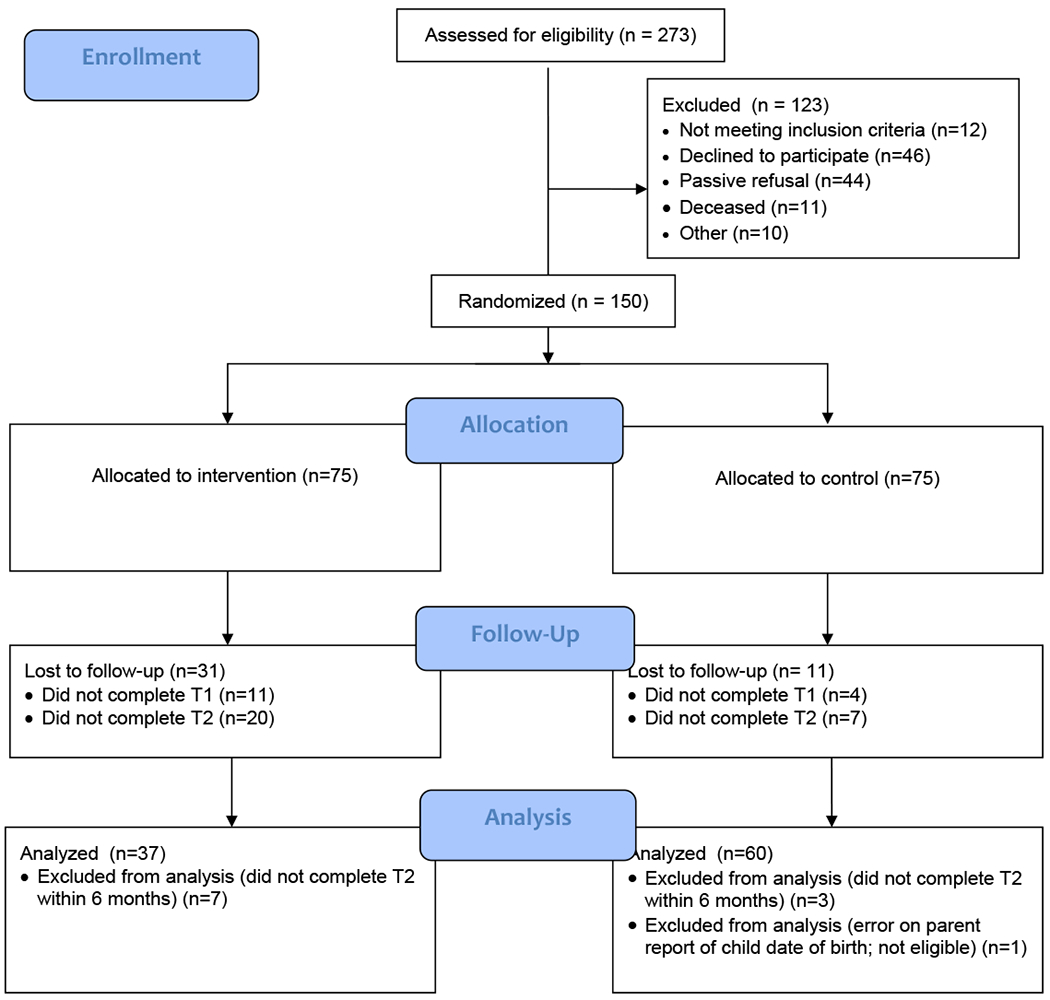
CONSORT Flow Diagram
Other than home region, no statistically significant differences in demographic or clinical characteristics were observed between the groups (experimental versus control group) of participants who completed the study (see Tables I and II). The sample of 97 ill children averaged 10 years of age. The majority were female (n = 57, 59%) and Caucasian (n = 81, 85%). Most had experienced a cancer relapse (n = 69, 71%). Few had (a) been notified that their cancer was terminal (n = 7, 7%), (b) a DNR in place (n = 2, 2%), (c) received a hospice referral (n = 2, 2%), or (d) received a palliative care consult (n = 7, 7%). The majority of caregivers were biological parents (n = 91, 94%), female (n = 88, 93%), Caucasian (n = 88, 93%), living in the Midwest (n = 53, 56%), married (n = 49, 52%), and college educated (n = 64, 67%), and had an annual family income of >$25,000 (n = 53, 56%) (see Tables I and II).
Table I.
Demographic characteristics by study group (N=97).
| Characteristic | Overall (N=97) | Control (n=60) | Experimental (n=37) | p-value |
|---|---|---|---|---|
| Child with Cancer | Mean (SD) | Mean (SD) | Mean (SD) | |
| N=94 | n=57 | |||
| Age (years) | 10.4 (3.0) | 10.6 (3.0) | 10.1 (3.0) | 0.435 |
| N (%) | N (%) | N (%) | ||
| Gender | 0.753 | |||
| Male | 40 (41.2) | 24 (40.0) | 16 (43.2) | |
| Female | 57 (58.8) | 36 (60.0) | 21 (56.8) | |
| Race | N=95 | N=59 | N=36 | 0.059 |
| White | 81 (85.3) | 51 (86.4) | 30 (83.3) | |
| Black or African American | 4 (4.2) | 4 (6.8) | 0 (0.0) | |
| Asian | 2 (2.1) | 2 (3.4) | 0 (0.0) | |
| American Indian or Alaska Native | 2 (2.1) | 1 (1.7) | 1 (2.8) | |
| Other | 6 (6.3) | 1 (1.7) | 5 (13.9) | |
| Ethnicity | N=96 | N=59 | 0.617 | |
| Hispanic or Latino | 11 (11.5) | 6 (10.2) | 5 (13.5) | |
| Not Hispanic or Latino | 85 (88.5) | 53 (89.8) | 32 (86.5) | |
| Primary Language | 0.727 | |||
| English | 95 (97.9) | 59 (98.3) | 36 (97.3) | |
| Spanish | 2 (2.1) | 1 (1.7) | 1 (2.7) | |
| Caregiver | ||||
| Relationship to Child | 0.659 | |||
| Biological parent | 91 (93.8) | 55 (91.7) | 36 (97.3) | |
| Adoptive parent | 4 (4.1) | 3 (5.0) | 1 (2.7) | |
| Foster parent | 1 (1.0) | 1 (1.7) | 0 (0.0) | |
| Grandparent | 1 (1.0) | 1 (1.7) | 0 (0.0) | |
| Gender | N=95 | N=59 | N=36 | 0.057 |
| Male | 7 (7.4) | 2 (3.4) | 5 (13.9) | |
| Female | 88 (92.6) | 57 (96.6) | 31 (86.1) | |
| Race | N=95 | N=59 | N=36 | 0.238 |
| White | 88 (92.6) | 54 (91.5) | 34 (94.4) | |
| Black or African American | 4 (4.2) | 4 (6.8) | 0 (0.0) | |
| American Indian or Alaska Native | 1 (1.1) | 0 (0.0) | 1 (2.8) | |
| Other | 2 (2.1) | 1 (1.7) | 1 (2.8) | |
| Ethnicity | N=90 | N=55 | N=35 | 0.958 |
| Hispanic or Latino | 5 (5.6) | 3 (5.5) | 2 (5.7) | |
| Not Hispanic or Latino | 85 (94.4) | 52 (94.3) | 33 (94.3) | |
| Home Region | N=95 | N=59 | N=36 | 0.019 |
| Northeast | 12 (12.6) | 10 (16.9) | 2 (5.6) | |
| Southeast | 18 (18.9) | 16 (27.1)a | 2 (5.6)b | |
| Middle West | 53 (55.8) | 26 (44.1)a | 27 (75.0)b | |
| Southwest | 10 (10.5) | 6 (10.2) | 4 (11.1) | |
| West | 2 (2.1) | 1 (1.7) | 1 (2.8) | |
| Primary Language | N=94 | N=58 | N=36 | 0.165 |
| English | 91 (96.8) | 55 (94.8) | 36 (100.0) | |
| Spanish | 3 (3.2) | 3 (5.2) | 0 (0.0) | |
| Highest Grade Completed | N=95 | N=59 | N=36 | 0.587 |
| Grade School (K-8) | 1 (1.1) | 1 (1.7) | 0 (0.0) | |
| High School (9-12) | 27 (28.8) | 15 (25.4) | 12 (33.3) | |
| GED | 3 (3.2) | 1 (1.7) | 2 (5.6) | |
| College (Undergraduate) | 52 (54.7) | 35 (59.3) | 17 (47.2) | |
| Graduate School | 12 (12.6) | 7 (11.9) | 5 (13.9) | |
| Current Marital Status | N=95 | N=59 | N=36 | 0.201 |
| Never Married | 24 (25.3) | 12 (20.3) | 12 (33.3) | |
| Married | 49 (51.6) | 32 (54.2) | 17 (47.2) | |
| Divorced | 11 (11.6) | 7 (11.9) | 4 (11.1) | |
| Separated | 1 (1.1) | 0 (0.0) | 1 (2.8) | |
| Widowed | 6 (6.3) | 6 (10.2) | 0 (0.0) | |
| Other | 4 (4.2) | 2 (3.4) | 2 (5.6) | |
| Current Annual Family Income | N=95 | N=59 | N=36 | 0.319 |
| Under $25,000 per year | 42 (44.2) | 25 (42.4) | 17 (47.2) | |
| $25,001-$50,000 per year | 20 (21.1) | 11 (18.6) | 9 (25.0) | |
| $50,001-$75,000 per year | 11 (11.6) | 7 (11.9) | 4 (11.1) | |
| $75,001-$100,000 per year | 11 (11.6) | 10 (16.9) | 1 (2.8) | |
| $100,001 or more per year | 11 (11.6) | 6 (10.2) | 5 (13.9) |
Table II.
Clinical characteristics by study group (N=97).
| Characteristic | Overall (N=97) | Control (N=60) | Experimental (N=37) | p-value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Advanced Cancer Type | 0.543 | |||
| Refractory disease | 28 (28.9) | 16 (26.7) | 12 (32.4) | |
| Relapsed | 69 (71.1) | 44 (73.3) | 25 (67.6) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| If relapsed, months since | 8.6 (3.7, 19.4) | 8.7 (4.5, 14.0) | 8.1 (3.3, 19.6) | 0.694 |
| Time (years since first cancer diagnosis | 4.1 (2.2, 6.0) | 3.9 (2.3, 6.2) | 4.2 (1.8, 5.3) | 0.891 |
| N (%) | N (%) | N (%) | ||
| Type of cancer | N=94 | N=59 | N=35 | 0.063 |
| Hematologic | 47 (50.0) | 31 (52.5) | 16 (45.7) | |
| Solid tumor | 28 (29.8) | 13 (22.0) | 15 (42.9) | |
| Central Nervous System | 19 (20.2) | 15 (25.5) | 4 (11.4) | |
| Secondary Cancer | 0.262 | |||
| No | 95 (97.9) | 58 (96.7) | 37 (100.0) | |
| Yes | 2 (2.1) | 2 (3.3) | 0 (0.0) | |
| Surgery to Remove Tumor | 0.565 | |||
| No | 56 (57.7) | 36 (60.0) | 20 (54.1) | |
| Yes | 41 (42.3) | 24 (40.0) | 17 (45.9) | |
| Chemotherapy | 0.302 | |||
| No | 5 (5.2) | 2 (3.3) | 3 (8.1) | |
| Yes | 92 (94.8) | 58 (96.7) | 34 (91.9) | |
| Radiation | 0.754 | |||
| No | 28 (28.9) | 18 (30.0) | 10 (27.0) | |
| Yes | 69 (71.1) | 42 (70.0) | 27 (73.0) | |
| Bone Marrow Transplant | N=96 | N=36 | 0.248 | |
| No | 83 (86.5) | 50 (83.3) | 33 (91.7) | |
| Yes | 13 (13.5) | 10 (16.7) | 3 (8.3) | |
| Phase I Study | 0.395 | |||
| No | 63 (64.9) | 37 (61.7) | 26 (70.3) | |
| Yes | 14 (14.4) | 8 (13.3) | 6 (16.2) | |
| Unsure | 20 (20.6) | 15 (25.0) | 5 (13.5) | |
| Notified Cancer is Terminal | 0.588 | |||
| No | 90 (92.8) | 55 (91.7) | 35 (94.6) | |
| Yes | 7 (7.2) | 5 (8.3) | 2 (5.4) | |
| DNR Order in Place | 0.069 | |||
| No | 95 (97.9) | 60 (100.0) | 35 (94.6) | |
| Yes | 2 (2.1) | 0 (0.0) | 2 (5.4) | |
| Hospice | 0.692 | |||
| No | 94 (96.9) | 58 (96.7) | 36 (97.3) | |
| Yes | 2 (2.1) | 1 (1.7) | 1 (2.7) | |
| Unknown | 1 (1.0) | 1 (1.7) | 0 (0.0) | |
| Palliative Care | 0.195 | |||
| No | 85 (87.6) | 51 (85.0) | 34 (91.9) | |
| Yes | 7 (7.2) | 4 (6.7) | 3 (8.1) | |
| Unknown | 5 (5.2) | 5 (8.3) | 0 (0.0) |
Descriptive summaries of the PACS scores at baseline (T1) and changes in those scores between T1 and end of study (T2) are shown in Table III. Communication scores were generally moderate to good at baseline with no statistically significant differences between the groups reported at T1. As reported by the child, communication was generally better with mother than with father (Table III). Tests of differences between the groups in the amount of change from T1 to T2 (after controlling for T1 scores) revealed no statistically significant differences (p > .05, Cohen’s d ranged from -0.20 to 0.33). The strongest effects were observed for improving open communication (child report related to father: d = 0.33; parent report: d = 0.21), decreasing child report of problems with mother (d = −0.20), and overall communication (child report related to father: d = 0.20; parent report: d = 0.25) (Table III).
Table III.
Summaries of change in communication scores from baseline (T1) to study end (T2)
| Measure | Baseline |
Change |
p-value | Effect size* |
|---|---|---|---|---|
| Median [IQR] | Median [IQR] | |||
| PACS (child reports) | ||||
| Open communication with mother | 0.623 | −0.09 | ||
| Control (n=57) | 4.3 [3, 5] | 0.1 [−0.3, 0.2] | ||
| Intervention (n=32) | 4.2 [4, 5] | 0.0 [−0.1, 0.2] | ||
| Problems in communication with mother | 0.341 | −0.20 | ||
| Control (n=57) | 2.5 [2, 3] | 0.0 [−0.3, 0.5] | ||
| Intervention (n=32) | 2.6 [2, 3] | −0.1 [−0.4, 0.2] | ||
| Overall communication with mother | 0.637 | 0.10 | ||
| Control (n=57) | 3.9 [3, 5] | −0.1 [−0.3, 0.3] | ||
| Intervention (n=32) | 3.8 [3, 5] | 0.1 [−0.1, 0.2] | ||
| Open communication with father | 0.248 | 0.33 | ||
| Control (n=27) | 4.0 [3, 5] | −0.1 [−0.6, 0.4] | ||
| Intervention (n=17) | 3.7 [3, 5] | 0.2 [−0.2, 0.4] | ||
| Problems in communication with father | 0.877 | 0.05 | ||
| Control (n=27) | 3.0 [2, 4] | 0.0 [−0.4, 0.4] | ||
| Intervention (n=17) | 2.1 [2, 4] | −0.1 [−0.3, 0.5] | ||
| Overall communication with father | 0.502 | 0.20 | ||
| Control (n=27) | 3.5 [3, 4] | 0.1 [−0.4, 0.3] | ||
| Intervention (n=17) | 3.7 [3, 4] | 0.1 [−0.4, 0.5] | ||
| PACS (parent reports) | ||||
| Open communication with child | 0.260 | 0.21 | ||
| Control (n=60) | 3.9 [3, 5] | 0.0 [−0.3, 0.3] | ||
| Intervention (n=37) | 3.9 [3, 5] | 0.0 [−0.2, 0.3] | ||
| Problems in communication with child | 0.372 | −0.18 | ||
| Control (n=60) | 2.7 [2, 3] | 0.1 [−0.1, 0.4] | ||
| Intervention (n=37) | 2.6 [2, 3] | 0.0 [−0.2, 0.2] | ||
| Overall communication with child | 0.201 | 0.25 | ||
| Control (n=60) | 3.6 [3, 4] | −0.1 [−0.3, 0.2] | ||
| Intervention (n=37) | 3.6 [3, 4] | 0.0 [−0.1, 0.2] | ||
Cohen’s d estimate transformed from regression coefficient, after controlling for baseline values
Discussion
This study is the first of its kind to examine changes in parent-child communication following a web-based legacy intervention for children with advanced cancer. Using a two-group (legacy intervention versus usual care) randomized design, we examined the effects of the intervention on the quality of communication between children with relapsed or refractory cancer and their parents. Compared to usual care, legacy-making showed trends for improving the quality of communication between children and their parents across time, especially father-child communication.
Results revealed positive trends in intervention effects on the quality of communication between children and fathers but not for the mothers. This difference could be due to low quality father-child communication in the presence of advanced pediatric cancer at baseline. Studies have shown that fathers may not be adequately equipped to communicate well with children. For example, fathers of children whose mothers were diagnosed with breast cancer have been shown to sometimes misinterpret children’s distress as ‘bad behavior’ and benefit from more information about the relationship between their spouse’s illness and how children may react emotionally.36Although fathers are typically involved in the day-to-day care of children with life-threatening conditions, fathers’ perspectives are often not sought or only measured via mother proxy-reports due to recruitment and retention challenges.37 For example, our study sample of primary parent caregivers included 7.3% fathers. Fathers have offered rich and unique insights in pediatric oncology studies.25,38,39Although the American Academy of Pediatrics emphasizes fathers’ perspectives as critical components in the care of seriously-ill children,40 pediatric oncology research that includes fathers’ reports remains lacking.
Although PACS results lacked statistically significant intervention effects for changes in parent-child communication between T1 and T2 and displayed only small to moderate effect sizes, additional qualitative data collected post-intervention suggested different intervention effects. In the T3 concluding survey, 72% (n = 57) of parents reported that the intervention improved parent-child communication,and 86% (n = 70) reported that the intervention improved children’s expression of feelings.34Such findings highlighted the potential for legacy interventions to facilitate opportunities to acknowledge and document the “child’s voice” within parent-child communication during palliative and end-of-life care.10The contrast in quantitative and qualitative data was also seen in our pilot study.21These results could be due in part to the PACS not being sensitive to intervention effects related to communication. Rather than quality of communication, the intervention may influence other components of parent-child interactions, such as parenting, relationships between family members, or family environment, such as adaptability or cohesion.
Demographic results of our study are important to note. Although few children with cancer and their parents oppose palliative care services,41 only 8.2% of children with advanced cancer in our sample were receiving palliative care services at the point of relapse or refractory cancer. It’s possible that families with little access to palliative care services, or those who have not yet been referred to palliative care, may be more likely to respond to Facebook recruitment and participate in web-based activities. Our intervention appeared to especially attract White, highly educated families living in the Midwest. Researchers can use this knowledge about potential participants, who may be attracted to such interventions, to guide future studies.
Limitations
We acknowledge that study results only generalize to children with advanced cancer and their parents. Participants reflected a volunteer sample, including only those with access to Facebook. Parents included 92.7% mothers and 7.3% fathers, thus, parent reports are likely biased towards mothers’ perceptions. A major study limitation was the significant attrition in the intervention group(41%) compared to usual care (15%).As discussed in more detail in our previous publication,34this participant drop-out likely somewhat reflects intervention feasibility issues (e.g., user-friendliness of the website) experienced early in the study that were later resolved. However, greater drop out at T1 was also seen for participants allocated to the intervention group (15%) compared to the control group (5%). Differences in demographic and clinical characteristics by sample status could explain this in part.34For example, a higher percent of participants who dropped out of the study had a child with a bone marrow transplant (35%) compared to 14% of participants who completed the study (P = .007). Further, the intervention allowed children to complete the activity alone or with assistance from others. Parents reported that more than half (62%) of children completed the activity alone; thus,the lack of structured parent-child interaction during the intervention could have decreased potential effects on parent-children communication.
Implications for Research and Practice
Future pediatric palliative care studies will benefit from considering fathers as important participants to enroll. Web-based legacy interventions may be a good strategy to facilitate improved father-child communication, thus future studies should include fathers as participants and specific recruitment strategies to enroll and retain fathers. In addition to the quality of parent-child communication, family relationships and cohesion may be important outcome variables for researchers to consider when testing interventions that include parent-child dyads. Observational methods may be an alternative strategy to measure parent-child communication. Secondary analyses from our data will examine potential correlations among demographic characteristics (e.g., child age and gender) and parent-child communication.
Providers should remain ever mindful that differences may exist in the quality of communication between children with cancer and their mothers compared to fathers.Nurses are ideally positioned to help identify families who may be at risk for poor quality of communication. Development of a family communication assessment tool and formal integration of such an assessment into nursing practice would provide a standardized mechanism for such identification to occur. Creation of family communication aids designed to facilitate communication, especially between fathers and children, is indicated. Nurses can also help guide families with communication challenges to appropriate resources or referrals when needed.
Conclusion
Children with advanced cancer receiving standard treatments are at a high risk of receiving limited communication about elements of palliative and end-of-life care support from their providers and parents.42Future research is needed to better understandhow family-based legacy activities may impact parent-child communication patterns and preferences to develop interventions to assist parents in initiating open and clear discussions with their child with cancer. Such research would help provide a strong foundation for future development of standardized guidelines for parents related to parent-child communication patterns during a child’s cancer care.
Acknowledgements:
We thank the children and parents who generously participated in this study. We acknowledge Sodium Halogen for their design and hosting of the intervention website and Paramore Digital for assisting in Facebook advertising.
Funding: This work was supported by the National Institute of Nursing Research under grant number 1RO1NR015353; and the NIH/National Center for Advancing Translational Sciences under grant number UL1 TR000445. This project collaborated with the Palliative Care Research Cooperative Group, supported by the National Institute of Nursing Research under grant number U24NR014637.
Biographies
Progress in Palliative Care Author Bios
Dr. Terrah Foster Akard is an Associate Professor at Vanderbilt University School of Nursing. She completed her Masters of Science in Nursing in 2001 from Vanderbilt and Bachelor of Science in 1999 from Jacksonville State University. With a clinical background as a pediatric nurse practitioner, Dr. Akard’s nationally recognized program of research is in pediatric palliative care, specifically focusing on behavioral interventions to enhance life and decrease suffering of children living with life-threatening conditions and their families. Her work has been funded by the National Institutes of Health, Robert Wood Johnson Foundation, and American Cancer Society.
Dr. Mary S. Dietrich is a Professor of Statistics and Measurement at Vanderbilt University, bringing a diverse background of statistical collaborations in academic computing and psychiatric neuroimaging. Her exposure to research began as an undergraduate research assistant. As a Master’s student, she worked as a consultant to researchers using statistical software in mainframe computing environments. Those skills developed and expanded to providing consultations to the entire Vanderbilt research community on advanced statistical methods and further expanded into formal interdisciplinary research collaborations.
Dr. Debra L. Friedman is a pediatric oncologist and senior investigator whose research focuses on cancer prevention, care delivery, control and outcomes. Her research has contributed to psychosocial, functional, and physiologic long-term outcomes for childhood and adult cancer survivors. She has led outcome and cancer care delivery studies through NCI and foundation-funded research. Dr. Friedman has published over 150 manuscripts, focusing on outcomes for cancer survivors. She is the Director for the Division of Pediatric Hematology/Oncology, Director of the Cancer Survivorship Program, the co-leader for the Cancer Health Outcomes and Control Program at the Vanderbilt Ingram Cancer Center.
Dr. Cynthia A. Gerhardt is the recipient of the Janet Orttung-Morrow, MD and Grant Morrow, III, MD Endowed Chair in Pediatric Behavioral Health. She is Director of the Center for Biobehavioral Health at The Research Institute at Nationwide Children’s Hospital and a Professor of Pediatrics and Psychology at The Ohio State University. Her primary focus is research on child and family adjustment to cancer and bereavement.
Dr. Barbara Given has a distinguished research career, she has been funded by NIH for over 30 years. For the last 35 years cancer patients and their family caregivers has been the focus of her research. She believes in giving back to science and has mentored many doctoral students and junior faculty. She is working with AARP to ensure that family members are included in hospital discharge instructions as part of the CARE Act currently enacted in 43 states. She is Interim Associate Dean for Research at Michigan State University College of Nursing, and a University Distinguished Professor.
Dr. Hendricks-Ferguson is the Irene Riddle Endowed Professor at Saint Louis University School of Nursing. Her program of research has focused on making significant contributions to advancing palliative and end-of-life communication practices by pediatric oncology providers with parents of children with poor prognosis cancer. She has published research articles focused on PC/EOL pediatric provider communication practices and parental preferences to receive palliative care and end of life support during the care of children with cancer. Her research has been funded by organizations including the National Institutes of Health, Oncology Nursing Society Foundation, and Alex Lemonade Children’s Cancer Research Foundation.
Dr. Pamela S. Hinds is the Executive Director of the Department of Nursing Science, Professional Practice, and Quality, the William and Joanne Conway Endowed Chair in Nursing Research, and the Research Integrity Officer at Children’s National Health System in Washington, D.C., and a Professor of Pediatrics at the George Washington University, School of Medicine and Health Sciences in Washington, D. C. She is adjunct professor for the University of Pennsylvania, School of Nursing, Johns Hopkins University, School of Nursing, the University of Maryland, College of Nursing, and the Fudan University of Shanghai, China.
Dr. Sheila Ridner is the Martha Rivers Ingram Professor of Nursing at Vanderbilt University School of Nursing. She completed her PhD in Nursing Science (2003), Postdoctoral Fellowship (2005), and Masters of Science in Nursing (2000) from Vanderbilt. She received her Masters of Health Services Administration from the College of St. Francis Graduate School in Joliet, Illinois in 1988. Her Bachelor of Science in Nursing degree was from the University of Kentucky in Lexington, Kentucky in 1978. Dr. Ridner has an extensive background in symptom management, oncology, psych/mental health and substance abuse, healthcare administration, and quality improvement.
Dr. Nicole Beckmann is a pediatric nurse practitioner and nurse scientist at the University of Minnesota Masonic Children’s Hospital within the pediatric blood and marrow transplant program. Nicole completed her master’s degree from Duke University and her doctor of philosophy from Vanderbilt University. Nicole’s research interests focus on family psychosocial care and support following pediatric hematopoietic stem cell transplant, especially among parent caregivers.
Dr. Mary Jo Gilmer is a Professor of Nursing at the Vanderbilt University School of Nursing. She earned her PhD in nursing at the University of North Carolina-Chapel Hill and completed a MBA at Queens University. Prior to that, she worked as a Clinical Nurse Specialist in Pediatric Cardiovascular surgery. Dr. Gilmer has received numerous awards for her research and teaching expertise and has been a leader in several international health care projects. Her focus is enhancing care of children with life-threatening conditions. Research interests include parent-sibling bereavement, parent-child communication, and behavioral interventions to reduce suffering in children with cancer and other serious conditions.
Footnotes
Disclosure Statement: The authors declare that there is no conflict of interest.
Clinical Trials Registry: Number NCT04059393
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Late effects of childhood cancer treatment. American Cancer Society. https://www.cancer.org/treatment/children-and-cancer/when-your-child-has-cancer/late-effects-of-cancer-treatment.html. Published 2017. Accessed December 4, 2019
- 3.Momani TG, Hathaway DK, Mandrell BN. Factors affecting health-related quality of life in children undergoing curative treatment for cancer: A review of the literature. J Pediatr Oncol Nurs. 2016;33(3):228–240. [DOI] [PubMed] [Google Scholar]
- 4.Neu M, Matthews E, King NA, Cook PF, Laudenslager ML. Anxiety, depression, stress, and cortisol levels in mothers of children undergoing maintenance therapy for childhood acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2014;31(2):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olagunju AT, Sarimiye FO, Olagunju TO, Habeebu MY, Aina OF. Child’s symptom burden and depressive symptoms among caregivers of children with cancers: An argument for early integration of pediatric palliative care. Ann Palliat Med. 2016;5(3):157–165. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg AR, Dussel V, Kang T, et al. Psychological distress in parents of children with advanced cancer. JAMA Pediatr. 2013;167(6):537–543. PMC4263253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherief LM, Kamal NM, Abdalrahman HM, et al. Psychological impact of chemotherapy for childhood acute lymphoblastic leukemia on patients and their parents. Medicine (Baltimore). 2015;94(51):e2280. PMC4697977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoshani A, Mifano K, Czamanski-Cohen J. The effects of the make a wish intervention on psychiatric symptoms and health-related quality of life of children with cancer: A randomised controlled trial. Qual Life Res. 2016;25(5):1209–1218. PMC5018253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326–333. [DOI] [PubMed] [Google Scholar]
- 10.Hinds PS, Menard JC, Jacobs SS. The child’s voice in pediatric palliative care and end-of-life care. Progress Pall Care. 2012;20(6):337–342. [Google Scholar]
- 11.Adduci A, Jankovic M, Strazzer S, Massimino M, Clerici C, Poggi G. Parent-child communication and psychological adjustment in children with a brain tumor. Pediatr Blood Cancer. 2012;59(2):290–294. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KP, Pyke-Grimm K, Stewart JL, Hinds PS. Hypothesis generation for childhood cancer communication research: Results of a secondary analysis. West J Nurs Res. 2014;36(4):512–533. [DOI] [PubMed] [Google Scholar]
- 13.Williams LK, McCarthy MC, Eyles DJ, Drew S. Parenting a child with cancer: Perceptions of adolescents and parents of adolescents and younger children following completion of childhood cancer treatment. J Fam Stud. 2013;19(1):80–89. [Google Scholar]
- 14.Keim MC, Lehmann V, Shultz EL, et al. Parent–child communication and adjustment among children with advanced and non-advanced cancer in the first year following diagnosis or relapse. J Pediatr Psychol. 2017;42(8):871–881. PMC5896621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kars MC, Grypdonck MH, van Delden JJ. Being a parent of a child with cancer throughout the end-of-life course. Oncol Nurs Forum. 2011;38(4):E260–271. [DOI] [PubMed] [Google Scholar]
- 16.Viola A, Taggi-Pinto A, Sahler OJZ, Alderfer MA, Devine KA. Problem-solving skills, parent-adolescent communication, dyadic functioning, and distress among adolescents with cancer. Pediatr Blood Cancer. 2018;65(5):e26951. PMC5867217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildenbrand AK, Clawson KJ, Alderfer MA, Marsac ML. Coping with pediatric cancer: Strategies employed by children and their parents to manage cancer-related stressors during treatment. J Pediatr Oncol Nurs. 2011;28(6):344–354. [DOI] [PubMed] [Google Scholar]
- 18.Kamper R, Van Cleve L, Savedra M. Children with advanced cancer: Responses to a spiritual quality of life interview. J Spec Pediatr Nurs. 2010;15(4):301–306. PMC3016439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lou HL, Mu PF, Wong TT, Mao HC. A retrospective study of mothers’ perspectives of the lived experience of anticipatory loss of a child from a terminal brain tumor. Cancer Nurs. 2015;38(4):298–304. [DOI] [PubMed] [Google Scholar]
- 20.Akard TF, Gilmer MJ, Friedman DL, Given B, Hendricks-Ferguson VL, Hinds PS. From qualitative work to intervention development in pediatric oncology palliative care research. J Pediatr Oncol Nurs. 2013;30(3):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akard TF, Dietrich MS, Friedman DL, et al. Digital storytelling: An innovative legacy-making intervention for children with cancer. Pediatr Blood Cancer. 2015;62(4):658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chochinov HM, Hack T, Hassard T, Kristjanson LJ, McClement S, Harlos M. Dignity therapy: A novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520–5525. [DOI] [PubMed] [Google Scholar]
- 23.Coyle N The hard work of living in the face of death. J Pain Symptom Manage. 2006;32(3):266–274. [DOI] [PubMed] [Google Scholar]
- 24.Foster TL, Dietrich MS, Friedman DL, Gordon JE, Gilmer MJ. National survey of children’s hospitals on legacy-making activities. J Palliat Med. 2012;15(5):573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster TL, Gilmer MJ, Davies B, et al. Bereaved parents’ and siblings’ reports of legacies created by children with cancer. J Pediatr Oncol Nurs. 2009;26(6):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akard TF, Duffy M, Hord A, et al. Bereaved mothers’ and fathers’ perceptions of a legacy intervention for parents of infants in the nicu. J Neonatal Perinatal Med. 2018;11(1):21–28. [DOI] [PubMed] [Google Scholar]
- 27.Bernat JK, Helft PR, Wilhelm LR, et al. Piloting an abbreviated dignity therapy intervention using a legacy-building web portal for adults with terminal cancer: A feasibility and acceptability study. Psychooncology. 2015;24(12):1823–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dose AM, McCabe PJ, Krecke CA, Sloan JA. Outcomes of a dignity therapy/life plan intervention for patients with advanced cancer undergoing chemotherapy. J Hosp Palliat Nurs. 2018;20(4):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy K, Grant PC, Depner RM, et al. The photographs of meaning program for pediatric palliative caregivers: Feasibility of a novel meaning-making intervention. Am J Hosp Palliat Care. 2019;36(7):557–563. [DOI] [PubMed] [Google Scholar]
- 30.Rolbiecki AJ, Washington K, Bitsicas K. Digital storytelling: Families’ search for meaning after child death. J Soc Work End Life Palliat Care. 2017;13(4):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer MR, Spencer SK, Barnett M, Reynolds NC, Madan-Swain A. Legacy artwork in pediatric oncology: The impact on bereaved caregivers’ psychological functioning and grief. J Palliat Med. 2019;22(9):1124–1128. [DOI] [PubMed] [Google Scholar]
- 32.Vuksanovic D, Green HJ, Dyck M, Morrissey SA. Dignity therapy and life review for palliative care patients: A randomized controlled trial. J Pain Symptom Manage. 2017;53(2):162–170 e161. [DOI] [PubMed] [Google Scholar]
- 33.Klass D, Silverman PR, Nickman SL. Continuing bonds: New understandings of grief. Philadelphia, PA, US: Taylor & Francis; 1996. [Google Scholar]
- 34.Akard TF, Wray S, Friedman DL, et al. Transforming a face-to-face legacy intervention to a web-based legacy intervention for children with advanced cancer. J Hosp Palliat Nurs. 2020;22(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes HL, Olson DH. Parent-adolescent communication scale. In. St. Paul: Family Social Science, University of Minnesota; 1985. [Google Scholar]
- 36.Forrest G, Plumb C, Ziebland S, Stein A. Breast cancer in young families: A qualitative interview study of fathers and their role and communication with their children following the diagnosis of maternal breast cancer. Psychooncology. 2009;18(1):96–103. [DOI] [PubMed] [Google Scholar]
- 37.Macfadyen A, Swallow V, Santacroce S, Lambert H. Involving fathers in research. J Spec Pediatr Nurs. 2011;16(3):216–219. [DOI] [PubMed] [Google Scholar]
- 38.Chesler MA, Parry C. Gender roles and/or styles in crisis: An integrative analysis of the experiences of fathers of children with cancer. Qual Health Res. 2001;11(3):363–384. [DOI] [PubMed] [Google Scholar]
- 39.Foster TL, Gilmer MJ, Davies B, et al. Comparison of continuing bonds reported by parents and siblings after a child’s death from cancer. Death Studies. 2011;35(5):420–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman WL, Garfield C. Fathers and pediatricians: Enhancing men’s roles in the care and development of their children. Pediatrics. 2004;113(5):1406–1411. [DOI] [PubMed] [Google Scholar]
- 41.Levine DR, Mandrell BN, Sykes A, et al. Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol. 2017;3(9):1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassam A, Skiadaresis J, Alexander S, Wolfe J. Differences in end-of-life communication for children with advanced cancer who were referred to a palliative care team. Pediatr Blood Cancer. 2015;62(8):1409–1413. [DOI] [PubMed] [Google Scholar]


