Abstract
Background
Acute bilirubin encephalopathy (ABE) and the other serious complications of severe hyperbilirubinemia in the neonate occur far more frequently in low‐ and middle‐income countries (LMIC). This is due to several factors that place babies in LMIC at greater risk for hyperbilirubinemia, including increased prevalence of hematologic disorders leading to hemolysis, increased sepsis, less prenatal or postnatal care, and a lack of resources to treat jaundiced babies. Hospitals and clinics face frequent shortages of functioning phototherapy machines and inconsistent access to electricity to run the machines. Sunlight has the potential to treat hyperbilirubinemia: it contains the wavelengths of light that are produced by phototherapy machines. However, it contains harmful ultraviolet light and infrared radiation, and prolonged exposure has the potential to lead to sunburn, skin damage, and hyperthermia or hypothermia.
Objectives
To evaluate the efficacy of sunlight administered alone or with filtering or amplifying devices for the prevention and treatment of clinical jaundice or laboratory‐diagnosed hyperbilirubinemia in term and late preterm neonates.
Search methods
We used the standard search strategy of Cochrane Neonatal to search CENTRAL (2019, Issue 5), MEDLINE, Embase, and CINAHL on 2 May 2019. We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials (RCTs), quasi‐RCTs, and cluster RCTs.
We updated the searches on 1 June 2020.
Selection criteria
We included RCTs, quasi‐RCTs, and cluster RCTs. We excluded crossover RCTs. Included studies must have evaluated sunlight (with or without filters or amplification) for the prevention and treatment of hyperbilirubinemia or jaundice in term or late preterm neonates. Neonates must have been enrolled in the study by one‐week postnatal age.
Data collection and analysis
We used standard methodologic procedures expected by Cochrane. We used the GRADE approach to assess the certainty of evidence. Our primary outcomes were: use of conventional phototherapy, treatment failure requiring exchange transfusion, ABE, chronic bilirubin encephalopathy, and death.
Main results
We included three RCTs (1103 infants). All three studies had small sample sizes, were unblinded, and were at high risk of bias. We planned to undertake four comparisons, but only found studies reporting on two.
Sunlight with or without filters or amplification compared to no treatment for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates
One study of twice‐daily sunlight exposure (30 to 60 minutes) compared to no treatment reported the incidence of jaundice may be reduced (risk ratio [RR] 0.61, 95% confidence interval [CI] 0.45 to 0.82; risk difference [RD] −0.14, 95% CI −0.22 to −0.06; number needed to treat for an additional beneficial outcome [NNTB] 7, 95% CI 5 to 17; 1 study, 482 infants; very low‐certainty evidence) and the number of days that an infant was jaundiced may be reduced (mean difference [MD] −2.20 days, 95% CI −2.60 to −1.80; 1 study, 482 infants; very low‐certainty evidence). There were no data on safety or potential harmful effects of the intervention. The study did not assess use of conventional phototherapy, treatment failure requiring exchange transfusion, ABE, and long‐term consequences of hyperbilirubinemia. The study showed that sunlight therapy may reduce rehospitalization rates within seven days of discharge for treatment for hyperbilirubinemia, but the evidence was very uncertain (RR 0.55, 95% CI 0.27 to 1.11; RD −0.04, −0.08 to 0.01; 1 study, 482 infants; very low‐certainty evidence).
Sunlight with or without filters or amplification compared to other sources of phototherapy for the treatment of hyperbilirubinemia in infants with confirmed hyperbilirubinemia
Two studies (621 infants) compared the effect of filtered‐sunlight exposure to other sources of phototherapy in infants with confirmed hyperbilirubinemia. Filtered‐sunlight phototherapy (FSPT) and conventional or intensive electric phototherapy led to a similar number of days of effective treatment (broadly defined as a minimal increase of total serum bilirubin in infants less than 72 hours old and a decrease in total serum bilirubin in infants more than 72 hours old on any day that at least four to five hours of sunlight therapy was available). There may be little or no difference in treatment failure requiring exchange transfusion (typical RR 1.00, 95% CI 0.06 to 15.73; typical RD 0.00, 95% CI −0.01 to 0.01; 2 studies, 621 infants; low‐certainty evidence). One study reported ABE, and no infants developed this outcome (RR not estimable; RD 0.00, 95% CI −0.02 to 0.02; 1 study, 174 infants; low‐certainty evidence). One study reported death as a reason for study withdrawal; no infants were withdrawn due to death (RR not estimable; typical RD 0.00, 95% CI −0.01 to 0.01; 1 study, 447 infants; low‐certainty evidence). Neither study assessed long‐term outcomes.
Possible harms: both studies showed a probable increased risk for hyperthermia (body temperature greater than 37.5 °C) with FSPT (typical RR 4.39, 95% CI 2.98 to 6.47; typical RD 0.30, 95% CI 0.23 to 0.36; number needed to treat for an additional harmful outcome [NNTH] 3, 95% CI 2 to 4; 2 studies, 621 infants; moderate‐certainty evidence). There was probably no difference in hypothermia (body temperature less than 35.5 °C) (typical RR 1.06, 95% CI 0.55 to 2.03; typical RD 0.00, 95% CI −0.03 to 0.04; 2 studies, 621 infants; moderate‐certainty evidence).
Authors' conclusions
Sunlight may be an effective adjunct to conventional phototherapy in LMIC settings, may allow for rotational use of limited phototherapy machines, and may be preferable to families as it can allow for increased bonding. Filtration of sunlight to block harmful ultraviolet light and frequent temperature checks for babies under sunlight may be warranted for safety. Sunlight may be effective in preventing hyperbilirubinemia in some cases, but these studies have not demonstrated that sunlight alone is effective for the treatment of hyperbilirubinemia given its sporadic availability and the low or very low certainty of the evidence in these studies.
Keywords: Humans; Infant, Newborn; Bias; Exchange Transfusion, Whole Blood; Heliotherapy; Heliotherapy/adverse effects; Heliotherapy/instrumentation; Heliotherapy/methods; Hyperbilirubinemia, Neonatal; Hyperbilirubinemia, Neonatal/epidemiology; Hyperbilirubinemia, Neonatal/prevention & control; Hyperbilirubinemia, Neonatal/therapy; Hyperthermia; Hyperthermia/epidemiology; Hypothermia; Hypothermia/epidemiology; Incidence; Infant, Premature; Jaundice, Neonatal; Jaundice, Neonatal/prevention & control; Jaundice, Neonatal/therapy; Patient Readmission; Patient Readmission/statistics & numerical data; Randomized Controlled Trials as Topic; Treatment Failure
Plain language summary
Sunlight for the prevention and treatment of hyperbilirubinemia in newborns
Review question
How safe and effective is sunlight for treating or preventing jaundice (yellowing of the skin, called hyperbilirubinemia) in newborns?
Background
Babies with jaundice are often treated with phototherapy lamps, which emit blue‐green light that alters the bilirubin (yellow substance found naturally in the baby's blood) so that it can be more easily excreted.
Sunlight emits light in a similar spectrum. However, sunlight also emits harmful ultraviolet rays and infrared radiation, which can cause sunburn and skin cancer. Further, exposing babies to sunlight might mean they could get too warm or too cold, depending on the climate.
In low‐ and middle‐income countries (LMIC) phototherapy is not always available for babies who need it. Further, babies in these countries can be at increased risk for dangerous jaundice, where the bilirubin in their blood reaches levels that allow it to cross the blood‐brain barrier and cause damage to the brain. Babies in LMIC are at increased risk for jaundice for a number of reasons, including poor access to maternal care during pregnancy, increased numbers of blood disorders causing jaundice, and increased risk of infection or birth trauma.
Given that sunlight is readily available, there is an urgent need to determine if sunlight is safe and effective at treating jaundice in babies in LMIC.
Study characteristics
We included three clinical trials containing 1103 infants from two countries. The trials included infants born at or near their due date (35 weeks of gestation or later) who were less than two weeks old. One study evaluated healthy babies, and the other two evaluated babies with jaundice. In one study, the babies received either sunlight therapy or no treatment to assess sunlight for the prevention or reduction of jaundice. In the other two studies, infants with jaundice were randomly assigned to receive treatment with phototherapy machines or to receive sunlight through a light‐filtering tent that blocked ultraviolet light and infrared radiation, and these groups were compared for improvement in their jaundice. One study did not comment on funding. The other two studies were funded by the Thrasher Research Fund. Evidence is current to June 2020.
Key results
Sunlight versus no treatment: babies exposed to sunlight may have a reduced occurrence of jaundice and be jaundiced for fewer days compared to babies who have no preventive treatment for jaundice. There was no reduction in readmission to hospital for jaundice in babies exposed to sunlight compared to babies who were not treated.
Sunlight versus other sources of phototherapy: when compared to babies who were exposed to electric phototherapy treatment, babies exposed to sunlight had a similar rate of decline in bilirubin levels. Using light‐filtering films, babies exposed to sunlight did not have increased rates of sunburn, dehydration, or hypothermia. Babies exposed to sunlight were at an increased risk of hyperthermia. The effectiveness of sunlight might not be inferior to phototherapy, if sunlight can be delivered for at least four hours per day, and electric phototherapy can be delivered at night when needed.
Certainty of the evidence
The certainty of the evidence for outcomes in all three studies was very low to moderate. It was very low for all the main outcomes in each study. We are uncertain whether sunlight is effective for the prevention or treatment of hyperbilirubinemia in term or late preterm neonates.
Summary of findings
Summary of findings 1. Sunlight (with or without filters or amplification) versus no treatment for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates.
| Sunlight (with or without filters or amplification) versus no treatment for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates | ||||||
| Patient or population: term and late preterm neonates Setting: Schenzen Maternity and Child Healthcare Hospital and individual patients' homes in and around Schenzen, China Intervention: sunlight with or without filters or amplification Comparison: no treatment | ||||||
| Outcomesa | Illustrative comparative risksb (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with no treatment | Risk with sunlight with or without filters or amplification | |||||
| Use of conventional phototherapy, if sunlight (with or without filters or amplification) was first used for prevention or early treatmentc | Study population | — | — | — | Not reported | |
| — | — | |||||
| Treatment failure requiring exchange transfusionc,d | Study population | — | — | — | Not reported |
|
| — | — | |||||
| Acute bilirubin encephalopathyc,e | Study population | — | — | — | Neither acute nor chronic bilirubin encephalopathy reported | |
| — | — | |||||
| Deathc | Study population | — | — | — | Not reported | |
| — | — | |||||
| Rate of rehospitalization within 7 days of discharge for treatment for hyperbilirubinemia | Study population | RR 0.55 (0.27 to 1.11) | 482 (1 RCT) | ⊕⊝⊝⊝ Very lowf | — | |
| 83 per 1000 | 46 per 1000 (23 to 93) | |||||
| Incidence of jaundice in participantsg | Study population | RR 0.61 (0.45 to 0.82) | 482 (1 RCT) | ⊕⊝⊝⊝ Very lowf | — | |
| 354 per 1000 | 216 per 1000 (159 to 290) | |||||
| Duration of jaundice (days) | The mean duration of jaundice was 6.5 (SD 2.7) days | MD 2.2 lower (2.6 lower to 1.8 lower) | — | 482 (1 RCT) | ⊕⊝⊝⊝ Very lowf | — |
| CI: confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDuring initial hospitalization unless otherwise noted. bThe basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). cProspectively identified as primary outcome measures. dDefined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life. eDefined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures, or chronic bilirubin encephalopathy or kernicterus, defined as athetoid cerebral palsy, auditory dysfunction, dental‐enamel dysplasia, paralysis of upward gaze, and, occasionally, intellectual and other disabilities. fSingle center, unblinded study of 482 infants; downgraded twice for very serious study limitations (due to high risk of bias) and once for imprecision. gDefined as bilirubin level greater than 257 µmol/L.
Summary of findings 2. Sunlight (with or without filters or amplification) versus other sources of phototherapy for the treatment of hyperbilirubinemia in infants with confirmed hyperbilirubinemia.
| Sunlight (with or without filters or amplification) versus other sources of phototherapy for the treatment of hyperbilirubinemia in infants with confirmed hyperbilirubinemia | ||||||
| Patient or population: infants with confirmed hyperbilirubinemia Setting: Island Maternity Hospital in Lagos, Nigeria and Bowen University Teaching Hospital in Ogbomoso, Nigeria Intervention: sunlight with or without filters or amplification Comparison: other sources of phototherapy | ||||||
| Outcomesa | Anticipated absolute effectsb (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other sources of phototherapy | Risk with sunlight with or without filters or amplification | |||||
| Use of conventional phototherapy, if sunlight (with or without filters or amplification) was first used for prevention or early treatmentc | Study population | — | — | — | Not reported in either study | |
| — | — | |||||
| Treatment failure requiring exchange transfusionc,d | Study population | RR 1.00 (0.06 to 15.73) | 621 (2 RCTs) | ⊕⊕⊝⊝ Lowe | — | |
| 3 per 1000 | 3 per 1000 (0 to 51) | |||||
| Acute bilirubin encephalopathyc,f | Study population | RR not estimable RD 0.00 (−0.02 to 0.02) |
174 (1 RCT) | ⊕⊕⊝⊝ Lowe | — | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Hyperthermia while receiving sunlight or conventional phototherapyg | Study population | RR 4.39 (2.98 to 6.47) | 621 (2 RCTs) | ⊕⊕⊕⊝ Moderateh | — | |
| 87 per 1000 | 382 per 1000 (260 to 564) | |||||
| Deathc | Study population | RR not estimable RD 0.00 (−0.01 to 0.01) |
447 (1 RCT) | ⊕⊕⊝⊝ Lowe | — | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Proportion of days of effective treatmenti | Study population | RR 1.02 (0.97 to 1.06) | 621 (2 RCTs) | ⊕⊝⊝⊝ Very lowj | — | |
| 896 per 1000 | 914 per 1000 (869 to 950) | |||||
| Hypothermia while receiving sunlight or conventional phototherapyk | Study population | RR 1.06 (0.55 to 2.03) | 621 (2 RCTs) | ⊕⊕⊕⊝ Moderateh | — | |
| 52 per 1000 | 55 per 1000 (28 to 105) | |||||
| CI: confidence interval; RCT: randomized controlled trial; RD: risk difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDuring initial hospitalization unless otherwise noted. bThe risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). cProspectively identified as primary outcome measures. dDefined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life. eDowngraded twice for very serious imprecision. fDefined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizure. gDefined as temperature greater than 38 °C. hDowngraded once for serious risk of bias (lack of blinding of intervention). iDefined in individual studies. jDowngraded twice for serious risk of bias (lack of blinding of intervention) and twice for indirectness. kDefined as temperature less than 35.5 °C.
Background
Phototherapy for the treatment of hyperbilirubinemia (jaundice) was first reported in the Lancet in 1958 after the use of the first artificial light sources in Rochford Hospital in Essex, England. Rochford Hospital conducted this experimental treatment after an observation by one of their nurses. Nurse Ward took the babies out to the courtyard regularly for sunlight and fresh air, and noticed that the babies' jaundice faded from the areas of their skin that were exposed to sunlight (Stokowski 2011). Although phototherapy is now given with specialized devices that deliver a certain wavelength of light, sunlight remains a potential source of treatment for hyperbilirubinemia.
Description of the condition
Hyperbilirubinemia is defined as an elevated level of bilirubin in the serum. Pathologic unconjugated hyperbilirubinemia includes jaundice appearing within the first 24 hours of life or a rate of rise of serum bilirubin level exceeding 0.2 mg/dL/hour (AAP 2004). The Bhutani nomogram guides clinicians with absolute values indicating need for phototherapy or exchange transfusion, based on the baby's age and specific risk factors, but these are only established for babies of gestational age 35 weeks and older (Bhutani 1999). Hyperbilirubinemia for these infants is defined as a serum bilirubin level greater than the 95th percentile for age on the Bhutani nomogram. There are no absolute values defining hyperbilirubinemia in preterm neonates, though consensus statements with suggested values exist (Cashore 2000; Maisels 2012; Morris 2008). Regardless, hyperbilirubinemia is to be avoided as severe elevations can lead to bilirubin‐induced neurologic dysfunction (BIND), comprised of acute bilirubin encephalopathy and kernicterus. This occurs most often in cases of severe hyperbilirubinemia, where unconjugated bilirubin levels exceed 20 mg/dL. In several studies, bilirubin levels greater than 20 mg/dL occurred in 0.007% to 2% of neonates (Ebbesen 2012; Kuzniewicz 2014; Manning 2007; McGillivray 2016). However, these studies were all conducted in upper‐middle‐ or high‐income countries (UHIC). One background study of the burden of hyperbilirubinemia at one large urban hospital in Nigeria found that 17% of babies had acute bilirubin encephalopathy and 31.5% received exchange transfusions, far higher numbers than are reported in the literature in UHIC (Emokpae 2016). Etiologies for hyperbilirubinemia in this study included ABO incompatibility, rhesus incompatibility, sepsis, asphyxia, and exposure to hemolytic agents. These findings indicate that hyperbilirubinemia and its sequelae still place a substantial burden on low‐ or middle‐income countries (LMIC).
Neonatal hyperbilirubinemia occurs due to a variety of factors. Bilirubin is a product of heme catabolism, and 80% to 90% of hyperbilirubinemia occurs due to the breakdown of hemoglobin (Wong 2017). Neonates are uniquely predisposed to physiologic hyperbilirubinemia since they have an increased number of red blood cells, and these have a shorter life span than in adults. Additionally, neonates have increased enterohepatic circulation as they often have decreased gastrointestinal tract motility in the first few days of life, and so bilirubin is often reabsorbed. The liver enzyme responsible for conjugating bilirubin, uridine diphosphate glucuronosyltransferase (UDPGT), in neonates has only 1% of the activity of the same enzyme in adults. This is all compounded by a physiologic volume restriction due to the low volumes of breast milk that are available to neonates in the first three to five days of life, leaving them uniquely predisposed to hyperbilirubinemia (Kawade 1981; Maisels 2012; Stokowski 2011).
There are several pathologic mechanisms that can be responsible for hyperbilirubinemia in neonates as well, and these often underlie the severe cases of hyperbilirubinemia necessitating exchange transfusions or leading to BIND. These include red blood cell hemolysis due to blood‐type incompatibility, structural instability, autoimmune hemolysis, and erythrocyte enzymatic defects. Bowel obstruction or ileus, hypothyroidism, or rare conditions like Crigler‐Najjar can also lead to decreased bilirubin clearance.
Many LMIC lie in sub‐Saharan Africa or Southeastern Asia, where higher population rates of illnesses such as glucose‐6‐phosphate dehydrogenase (G6PD) deficiency put their population at increased risk of neonatal hyperbilirubinemia. In addition, there are many barriers to treating hyperbilirubinemia in resource‐limited settings, including lack of medical care surrounding birth and the neonatal period, lack of maternal understanding of jaundice and the associated risks, local herbal or traditional practices that may be harmful, lack of availability of tests for bilirubin levels, shortage or lack of functioning phototherapy machines, and lack of trained clinicians who understand how to use phototherapy correctly (Olusanya 2015). Infants in these countries are also at higher risk for infection, are more likely to be exclusively breastfed (and so volume contracted), and their mothers are less likely to have undergone Rh screening or received Rho(D) Immune Globulin (RhoGAM) to prevent Rh isoimmunization.
It should be stated that in many LMIC, lower cutoff points for the treatment of hyperbilirubinemia than those discussed in the above definitions are prudent, given the higher incidence of severe hyperbilirubinemia as well as the limitations to timely screening, diagnosis, and treatment already described.
Description of the intervention
Since the 1950s, the allopathic medical community has formally recognized phototherapy as an effective therapy for hyperbilirubinemia. However, it was not widely adapted until the landmark study by Lucey and colleagues in Pediatrics in 1968, in which a randomized controlled trial (RCT) of 111 infants showed that exposure to phototherapy safely prevented hyperbilirubinemia in neonates (Lucey 1968). Since that time, phototherapy has been widely adopted in UHIC. Phototherapy reduces hyperbilirubinemia by converting bilirubin to water‐soluble isomers, bypassing liver metabolism (Stokowski 2011). Phototherapy devices should ideally emit light between the wavelengths 460 nm and 490 nm, in the blue‐green spectrum, as this is most readily absorbed and most effective (Bhutani 2011). Phototherapy devices should not emit any ultraviolet (UV) light or infrared (IR) radiation. Phototherapy is provided via blue light‐emitting diodes (LEDs), halogen white lamps, fluorescent blue lamps, or fiber optic light pads at a prescribed dose, or irradiance. The dose commonly ranges from 6 μW/cm²/nm to 30 μW/cm²/nm depending on the desired amount of phototherapy, and can be adjusted by changing the number of lights on the infant, the distance the light is from the infant's skin, and the surface area of skin exposed (Bhutani 2011). However, while phototherapy is a safe and relatively inexpensive treatment, it is not always readily available in resource‐limited settings, where, for example, consistent access to an electrical source may not be available, or economic constraints may not allow for the purchase of an adequate number of phototherapy devices. It is common for infants in LMICs to be forced to go without phototherapy, even when their clinical status would merit treatment. Since phototherapy utilizes light emitted in a spectrum that is naturally emitted by the sun (hence Nurse Ward's discovery in Essex, England), it is worth determining if sunlight itself – a resource that is readily available and free worldwide – can safely be used for the treatment of hyperbilirubinemia in neonates, and if so, how. Safety is a concern as unfiltered sunlight includes harmful IR and UV light, which has been associated with sunburn, hyperthermia, and a long‐term risk of various malignancies of the skin. This may involve technology that amplifies or filters the sunlight to make it safer. One such device is a filtered‐sunlight phototherapy (FSPT) canopy that has imbedded commercial window tinting films that remove most UV and a portion of IR light (Slusher 2014). Potential risks of such devices include possible increased risk of hyperthermia and potential for diminished efficacy of sunlight as a treatment. We are not aware of every device that has been invented or trialed that may be utilized for the administration of sunlight as a treatment for hyperbilirubinemia, but would like to include and assess any that are reported in the literature in this analysis.
How the intervention might work
Sunlight obviously can treat hyperbilirubinemia, as this is how the treatment of phototherapy was discovered, and the sun emits blue‐green light in the spectrum needed to most effectively convert bilirubin to its water‐soluble isomers for excretion. Phototherapy is also often used for the prevention of hyperbilirubinemia in high‐risk but asymptomatic babies. Sunlight can likewise be used to prevent hyperbilirubinemia or jaundice before they present in infants considered at risk. However, there is concern for potential safety issues when using sunlight for the treatment or prevention of hyperbilirubinemia, as sunlight also emits UV light and IR radiation, and there is the potential for the infants to have hyperthermia or sunburn, and to be put at risk for the development of neoplasia of the skin. Also, sunlight cannot be applied at prescribed dosages, as the amount and intensity of sunlight available on any given day cannot be controlled. Nevertheless, if sunlight can be utilized in a safe and controlled manner to provide phototherapy, it could be extremely helpful in mitigating morbidity and mortality from hyperbilirubinemia in LMICs.
Why it is important to do this review
This review is important because it will evaluate an inexpensive and widely available therapy that has the potential to save lives and reduce acute and chronic morbidity from hyperbilirubinemia and its sequelae. It will also determine the potential cost benefit of sunlight therapy (especially valuable in the LMIC setting) and potential benefit to maternal–child interaction provided by sunlight therapy, which could decrease separation between newborns and their mothers and therefore reduce interruptions to maternal–child bonding. This review investigated a therapy that could help deliver the United Nations Sustainable Development Goal to end preventable deaths of newborns and children under five years of age, with all countries aiming to reduce neonatal mortality below 12 per 1000 live births by 2030 (United Nations 2020). Finally, a search of PubMed revealed no systematic reviews of sunlight in the prevention or treatment of hyperbilirubinemia.
Objectives
To evaluate the safety and efficacy of sunlight administered alone or with filtering or amplifying devices for the prevention and treatment of clinical jaundice or laboratory‐diagnosed hyperbilirubinemia in term and late preterm neonates.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include RCTs, quasi‐randomized trials, and cluster RCTs. We excluded crossover RCTs.
Types of participants
We included term (37+0 weeks' gestation or greater) and late preterm infants (35+0 to 36+6 weeks' gestation) enrolled by one‐week postnatal age.
We included studies of either the clinical finding of jaundice or the diagnosis of hyperbilirubinemia to be inclusive of studies performed in extremely resource‐limited settings where hyperbilirubinemia could not be measured.
We performed separate comparisons in four distinct populations:
low‐risk term and late preterm infants (based on risk criteria as defined by Bhutani 1999);
infants identified as 'at risk' for hyperbilirubinemia: isoimmune hemolytic disease, glucose‐6‐phosphate dehydrogenase (G6PD) deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, and albumin less than 3 g/dL (Bhutani 1999);
infants with clinical jaundice (at the discretion of the caregiver);
infants with confirmed hyperbilirubinemia as defined by the Bhutani nomogram and determined by serum bilirubin measurement (Bhutani 1999).
Types of interventions
Sunlight with or without filtering devices or amplification devices compared to no treatment, other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), or other sunlight devices.
We planned to conduct the following comparisons stratified by distinct population type.
-
Comparison 1. Low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999).
Sunlight (with or without filters or amplification) versus no treatment.
Sunlight (with or without filters or amplification) versus other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), and include irradiance if known.
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type (as defined by individual studies).
-
Comparison 2. Infants identified as 'at risk' for hyperbilirubinemia: isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, and albumin less than 3 g/dL (Bhutani 1999)
Sunlight (with or without filters or amplification) versus no treatment.
Sunlight (with or without filters or amplification) versus other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), and include irradiance if known.
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type (as defined by individual studies).
-
Comparison 3. In infants with clinical jaundice (at the discretion of the caregiver)
Sunlight (with or without filters or amplification) versus no treatment.
Sunlight (with or without filters or amplification) versus other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), and include irradiance if known.
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type (as defined by individual studies).
-
Comparison 4. In infants with confirmed hyperbilirubinemia as defined by the Bhutani nomogram and determined by serum bilirubin measurement
Sunlight (with or without filters or amplification) versus no treatment.
Sunlight (with or without filters or amplification) versus other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), and include irradiance if known.
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type (as defined by individual studies).
Types of outcome measures
Primary outcomes
Use of conventional phototherapy (CPT), if sunlight (with or without filters or amplification) was first used for prevention or early treatment.
Treatment failure requiring exchange transfusion (as defined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life) (Bhutani 1999).
Acute bilirubin encephalopathy, defined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures (AAP 2004; Johnson 2002).
Chronic bilirubin encephalopathy or kernicterus, defined as athetoid cerebral palsy, auditory dysfunction, dental‐enamel dysplasia, paralysis of upward gaze, and, occasionally, intellectual and other disabilities (AAP 2004) assessed at 12 to 24 months of age.
Death.
Secondary outcomes
Duration of treatment from initiation to end (hours).
Peak bilirubin levels during hospitalization (mg/dL) (and if known, hour or day of life this bilirubin was measured).
Rate of change of serum bilirubin (mg/dL/hour) from initiation of sunlight or phototherapy to cessation of either treatment.
Days in hospital.
Rate of rehospitalization within seven days of discharge for treatment for hyperbilirubinemia.
Athetoid cerebral palsy assessed at 12 to 24 months of age.
Auditory dysfunction (defined as failed otoacoustic emissions or auditory brainstem response hearing screen, or caregiver report that the child is deaf or has difficulty with hearing), assessed at 12 to 24 months of age.
Total cost (USD).
Sunburn (as defined in individual studies).
Hyperthermia (defined as body temperature greater than 38 °C) while receiving sunlight or CPT.
Maternal satisfaction (as defined in individual studies).
Nursing staff satisfaction (as defined in individual studies).
Need for fluid supplementation (defined as intravenous fluids, nasogastric fluids, or supplemental formula or breast milk in addition to breastfeeding).
Cessation of breast milk feeding.
Additional post‐hoc outcomes
Incidence of jaundice in participants (defined as bilirubin level greater than 257 μmol/L).
Duration of jaundice (days).
Proportion of days of effective treatment (as defined in individual studies).
Hypothermia (defined as body temperature less than 35.5 °C) while receiving sunlight or CPT.
Dehydration.
(Note: all outcomes measured during initial hospital course unless otherwise noted.)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2019, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 2 May 2019); Embase (1980 to 2 May 2019); and CINAHL (1982 to 2 May 2019) (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry [apps.who.int/trialsearch/default.aspx] and Platform, and the ISRCTN Registry [www.isrctn.com/]).
We updated the search on 1 June 2020 (see Differences between protocol and review). We conducted a comprehensive search in CENTRAL (2020, Issue 5) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R); and CINAHL (1 January 2019 to 1 June 2020). We included the search strategies for each database in Appendix 1. We applied no language restrictions.
We re‐searched clinical trial registries for ongoing or recently completed trials.
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
Data collection and analysis
We used the standard methods of Cochrane Neonatal.
Selection of studies
We screened all studies that met our search criteria using Covidence. We identified and excluded duplicates and collated multiple reports of the same study, so that each study rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and Characteristics of included studies and Characteristics of excluded studies tables.
Data extraction and management
Two review authors (DH and DE) screened the title and abstract of all studies identified by the search strategy, and two review authors independently assessed the full articles for all potentially relevant trials. We excluded studies that did not meet all the inclusion criteria, and stated the reason for exclusion in the Characteristics of excluded studies table. We discussed any disagreements until consensus. We used a standard data collection form that was adapted for the purpose of collecting all identified outcome measures.
Assessment of risk of bias in included studies
Two review authors (DH, RFS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool (Higgins 2011), for the following domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
Selective reporting (reporting bias);
any other bias.
We resolved any disagreements by discussion or by a third review author. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We analyzed the treatment effects in the individual trials using Review Manager 5 (Review Manager 2020), and reported risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD; when studies assessed the same outcome using the same method) or standardized mean difference (SMD) (when studies assessed the same outcome but measured it using a different method) for continuous data, with respective 95% confidence intervals (CI). We determined the number needed to treat for an additional beneficial outcome (NNTB), or an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials and the neonatal unit (or subunit) for cluster‐randomized trials.
We considered an infant only once in an analysis. We excluded infants with multiple enrollments as we were unable to address the unit of analysis issues that arose.
If we had identified cluster‐randomized trials, we would have undertaken analyses at the level of the individual while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Dealing with missing data
If there had been missing data, we planned to approach the analysis of missing data as follows per the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020):
we contacted the original study investigators to request the missing data where more than 20% of data were missing (Guyatt 2017);
where possible, we imputed missing standard deviations (SDs) using the coefficient of variation or calculated them from other available statistics including standard errors, CIs, t values and P values;
if the data were assumed to be missing at random, we analyzed the data without imputing any missing values;
if this could not be assumed, then we imputed the missing outcomes with replacement values, assuming all to have a poor outcome. We conducted sensitivity analyses to assess any changes in the direction or magnitude of effect resulting from data imputation.
Assessment of heterogeneity
We assessed clinical heterogeneity by visual inspection of forest plots and statistical heterogeneity using the I² statistic. We calculated the I² statistic for each RR analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may have been due to heterogeneity rather than to sampling error. We defined heterogeneity as:
I² statistic less than 25%: none;
I² statistic 25% to 49%: low;
I² statistic: 50% to 74% moderate;
I² statistic 75% or greater: high.
If we detected 'high' levels of heterogeneity (I² statistic of 75% or greater), we explored the possible causes (e.g. differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
We planned to assess publication bias by visual inspection of funnel plot asymmetry in meta‐analyses with at least 10 studies. If we found significant asymmetry in the funnel plot, we planned to report this in the corresponding results. However, as we only included three studies in the review, we were unable to undertake this.
Data synthesis
We performed meta‐analysis using Review Manager 5 (Review Manager 2020). For categorical outcomes, we calculated the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we calculated the MD or SMD, each with its 95% CI. We used a fixed‐effect model to combine data where it is reasonable to assume that studies were estimating the same underlying treatment effect. If we judged meta‐analysis to be inappropriate, we analyzed and interpreted individual trials separately. If there was evidence of high statistical heterogeneity (I² statistic 75% or greater), we planned to evaluate this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We conducted four separate comparisons on the following populations:
low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999);
infants identified as 'at risk' for hyperbilirubinemia: isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis, and albumin less than 3 g/dL (Bhutani 1999);
infants with clinical jaundice (at the discretion of the caregiver);
infants with confirmed hyperbilirubinemia as defined by the Bhutani nomogram and determined by serum bilirubin measurement (Bhutani 1999).
We judged these populations to be sufficiently different that we did not combine results from these analyses.
For each of the four distinct populations that we stratified for comparison, we analyzed the following:
sunlight (with or without filters or amplification) versus no treatment;
sunlight (with or without filters or amplification) versus other sources of phototherapy (fluorescent blue lights, blue LED lights, halogen white lamp, fiber optic blanket), and include irradiance if known;
sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type (as defined by individual studies).
We conducted no other subgroup analyses.
Sensitivity analysis
If data had been available, we planned to perform sensitivity analyses to determine if the findings were affected by including only studies of adequate methodology (low risk of bias), defined as adequate randomization and allocation concealment, blinding of intervention and measurement, and less than 10% loss to follow‐up.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes:
-
Comparison 1
Use of CPT, if sunlight (with or without filters or amplification) was first used for prevention or early treatment.
Treatment failure requiring exchange transfusion (as defined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life) (Bhutani 1999).
Acute bilirubin encephalopathy, defined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures (AAP 2004; Johnson 2002), or chronic bilirubin encephalopathy or kernicterus, defined as athetoid cerebral palsy, auditory dysfunction, dental‐enamel dysplasia, paralysis of upward gaze, and, occasionally, intellectual and other disabilities (AAP 2004).
Death.
Rate of rehospitalization within seven days of discharge for treatment for hyperbilirubinemia.
Incidence of jaundice in participants (defined as bilirubin level > 257 μmol/L).
Duration of jaundice (days).
-
Comparison 2
Use of CPT, if sunlight (with or without filters or amplification) was first used for prevention or early treatment.
Treatment failure requiring exchange transfusion (as defined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life) (Bhutani 1999).
Acute bilirubin encephalopathy, defined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures (AAP 2004; Johnson 2002).
Death.
Hyperthermia (defined as temperature greater than 38 °C) while receiving sunlight or CPT.
Proportion of days of effective treatment (as defined in individual studies).
Hypothermia (defined as temperature less than 35.5 °C) while receiving sunlight or CPT.
Given the different populations included in each comparison, we considered that slightly different outcomes were most critical for decision‐making related to these different populations and this is reflected in the summary of findings reported for each comparison.
Two review authors (DH; RFS) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create two summary of findings tables to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
See Figure 1.
1.
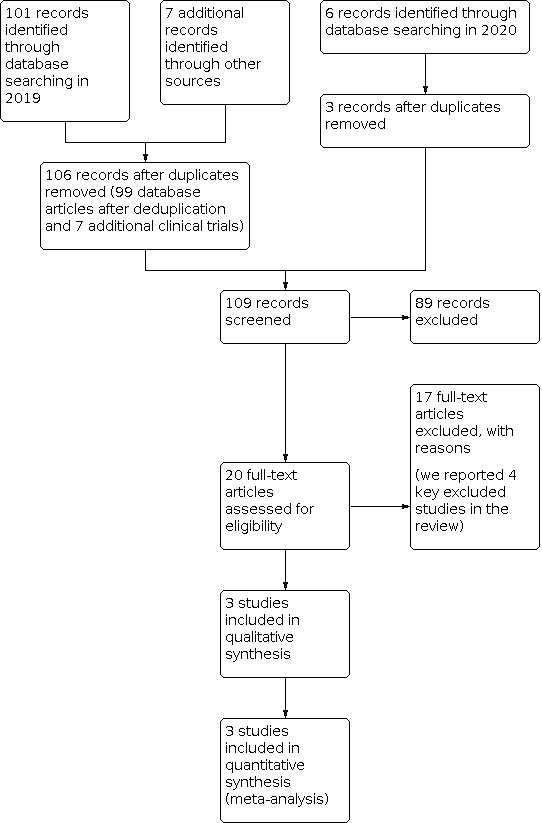
PRISMA study flow diagram.
Results of the search
Our 2019 search yielded 108 records, 106 after we removed duplicates. Our 2020 search yielded six further records, three after duplicates were removed. We screened 109 records at abstract and title stage and found 89 to be irrelevant. We screened 20 records at full‐text screening, of these, we excluded 17 studies and reported on four key studies in the Characteristics of excluded studies table. We included three studies in our review.
Included studies
We included three studies (1103 infants) (see Characteristics of included studies table).
Sunlight (with or without filters or amplification) compared to no treatment for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates
Xiao 2009 conducted an RCT at Shenzhen Maternity and Child Health Care Hospital in Shenzhen City, China. They included term infants weighing 2 kg or more at birth with Apgar 7 to 10, no obvious abnormalities, and with healthy mother's with no history of hepatitis B or liver dysfunction and followed the participants for up to seven days after birth. The primary endpoints were incidence of jaundice, duration of jaundice (in days), and hospitalization rate. There were no other safety measures or long‐term outcomes assessed. Jaundice and management of jaundice were defined as follows: a serum bilirubin level of 257 μmol/L to 291 μmol/L indicated a diagnosis of jaundice, a value above the upper limit (291 μmol/L) required hospital admission, and normal serum bilirubin was 205 μmol/L to 257 μmol/L. Infants had transcutaneous bilirubin measured twice within the first 72 hours of life prior to hospital discharge: once on the forehead and once on the chest. The mean of these two measurements was designated the “serum bilirubin” and was used to determine jaundice.
After discharge, infants were randomized to one of four groups: sunlight exposure, baths with traditional Chinese herbs, sunlight exposure plus baths, or neither sunlight exposure nor baths from their parents at home. Sunlight exposure was defined as placing the infant directly under sunlight, with as much skin exposed as possible. This was to be performed once in the morning and once in the afternoon for 30 minutes to one hour at a time. The bath involved boiling 5000 mL of water with Chinese herbs, letting the water cool until it was warm, then bathing the infant once or twice per day for 15 to 20 minutes at a time for the first three to five days following discharge home.
From March 2004 through September 2007, the study recruited and randomized 960 infants (the randomization procedure not described); 242 participants to sunlight exposure, 238 to baths, 240 to sunlight exposure plus baths, and 240 to neither sunlight exposure nor baths.
Sunlight (with or without filters or amplification) compared to other sources of phototherapy for the treatment of hyperbilirubinemia in infants with confirmed hyperbilirubinemia
Slusher 2015 conducted a randomized, controlled non‐inferiority trial that compared filtered sunlight with CPT for the treatment of hyperbilirubinemia in term and late preterm neonates in a large, urban Nigerian maternity hospital. The study enrolled and randomly assigned 447 infants; 224 to FSPT and 223 to CPT. The primary endpoint was efficacy, defined as a rate of increase in total serum bilirubin of less than 0.2 mg/dL/hour for infants up to 72 hours of age or a decrease in total serum bilirubin for infants older than 72 hours of age who received at least five hours of phototherapy on that day; they prespecified a non‐inferiority margin of 10% for the difference in efficacy rates between groups. The need for an exchange transfusion was a secondary endpoint. In this study they also assessed safety, defined as the absence of the need to withdraw therapy because of hyperthermia, hypothermia, dehydration, or sunburn. Long‐term outcomes were not assessed. FSPT was delivered via one of two tinting films: either the Film canopy Air Blue 80 if the sky was overcast (84% blue light passage), or the Film canopy Gila Titanium if the sky was sunny (39% blue light passage). CPT was provided using locally available materials, with irradiance of 8 μW/cm2/nm to 10 μW/cm2/nm. Sunlight was provided for at least five hours per day. The Thrasher Research Fund, Salt Lake City, and the National Center for Advancing Translational Sciences of the National Institutes of Health funded the study (Clinical Trials.gov number, NCT01434810).
Slusher 2018 conducted a prospective, randomized controlled non‐inferiority trial in Ogbomoso, Nigeria, a simulated rural setting. Participants were near‐term or term infants aged 14 days or younger who were of 35 weeks or more of gestational age, and with total serum bilirubin concentrations at or above the recommended age‐dependent treatment levels for high‐risk neonates. They were randomized (1:1) to FSPT or intensive electric phototherapy (IEPT). The primary outcome was efficacy, which was based on assessable treatment days only (i.e. those on which at least four hours of phototherapy was delivered) and defined as a rate of increase in total serum bilirubin concentrations of less than 3.4 μmol/L/hours in infants aged 72 hours or younger, or a decrease in total serum bilirubin concentrations in those older than 72 hours. Safety was defined as the proportion of all treatment days on which the neonate did not have sustained hypothermia, hyperthermia, dehydration, or sunburn. Long‐term outcomes were not assessed. Analysis was by intention‐to‐treat with a non‐inferiority margin of 10%. Randomization was computer‐generated, and neither clinicians nor the parents or guardians of participants were blinded to group allocation. FSPT was delivered in a transparent polycarbonate room lined with commercial tinting films that transmitted effective phototherapeutic light, blocked UV light, and reduced IR radiation. IEPT was delivered with locally designed and constructed IEPT devices, with irradiance at least 30 μW/cm2/nm. Sunlight was provided for at least four hours per day. The study took place between 31 July 2015 and 30 April 2017. A total of 174 neonates were enrolled and randomly assigned: 87 to FSPT and 87 to IEPT. The Thrasher Research Fund, Salt Lake City, and the National Center for Advancing Translational Sciences of the National Institutes of Health funded the study.
Excluded studies
We reported on four key excluded studies in the Characteristics of excluded studies table.
Emokpae 2016 was not an RCT. Emokpae 2016 reviewed medical records of infants admitted for neonatal hyperbilirubinemia in an inner‐city Children's Hospital in Lagos, Nigeria, between January 2012 and December 2014 to determine the pattern, treatment, and outcomes during the preintervention period. Factors associated with adverse outcomes were identified through multivariable logistic regression.
Slusher 2014 was not an RCT. It was a pilot study prior to the RCT conducted in 2015 (Slusher 2015). Slusher and colleagues evaluated the safety and efficacy of FSPT in term/late preterm infants ages 14 days or less with clinically significant jaundice, assessed by total bilirubin levels. Infants were recruited from a maternity hospital in Lagos, Nigeria. Sunlight was filtered with commercial window‐tinting films that removed most UV and significant levels of IR light, and transmitted effective levels of therapeutic blue light. After placing infants under an FSPT canopy, hourly measurements of axillary temperatures, monitoring for sunburn, dehydration, and irradiances of filtered sunlight were performed. Treatment was deemed safe and efficacious if infants were able to stay in FSPT for five hours or greater, and rate of rise of total bilirubin was less than 0.2 mg/dL/hour for infants ages 72 hours or less or total bilirubin decreased for infants over 72 hours of age. A total of 227 infants received 258 days of FSPT.
Olusanya 2014 was not an RCT. It was a cross‐sectional satisfaction survey conducted among mothers of jaundiced infants treated with FSPT in an inner‐city maternity hospital in Lagos, Nigeria from November 2013 to March 2014. Mothers' experiences during treatment were elicited with a pretested questionnaire consisting of closed and open‐ended items. Satisfaction was rated on a five‐point Likert scale. Correlates of overall maternal satisfaction were explored with descriptive and inferential non‐parametric statistics. A total of 191 mothers were surveyed, 77 (40%) of whom had no prior knowledge of neonatal jaundice.
Vreman 2013 was not an RCT. They evaluated nine semi‐transparent plastic window‐tinting films for their ability to block ultraviolet A (UVA) and IR radiation and transmit therapeutic blue light (wavelength 400 nm to 520 nm) for treating jaundiced newborns. For indoor testing, three light sources (TL/52 special blue fluorescent, Black Light UVA, and IR heat lamps) were positioned above each film and measured successively using a thermocouple thermometer, UVA radiometer, and blue light irradiance meter, placed below each film. Outdoor testing used the same setup with the sun at the zenith and a cloudless sky. Compared with unfiltered radiation, blue light transmission through films ranged from 24% to 83%, UVA transmission was 0.1% to 7.1% and reductions in IR heat were 6 °C to 12 °C for heat lamp and 5 °C to 10 °C for sun. The data suggested that most of the relatively low‐cost window‐tinting films tested could effectively reduce sunlight UV and IR and offered a range of significant attenuations of therapeutic blue light.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
There was no information given on measures taken for allocation concealment or on sequence generation for Xiao 2009 (unclear risk of allocation bias).
Slusher 2015 recorded treatment assignments on sequentially numbered sheets of paper; enclosed in opaque, sealed, matching, numbered envelopes; and shipped from the USA to Lagos and opened sequentially for each enrolled infant. Slusher 2018 printed assignments on sequentially numbered sheets of paper; enclosed in opaque, sealed, sequentially numbered envelopes; and transported to Nigeria by the study regulatory sponsor. When the study nurse enrolled an infant, she opened the envelope and recorded the number and treatment assignment on a case report form. Slusher 2015 randomly assigned sequence generation using a block randomization procedure, with block sizes of two, four, six, eight, and 10. Slusher 2018 assigned sequence generation using a 1:1 randomization procedure with variable block sizes, and assignments were computer generated by the study statistician. Slusher 2015 and Slusher 2018 had low risk of allocation bias.
Blinding
Xiao 2009 did not comment on blinding. Slusher 2015 and Slusher 2018 were not blinded. All studies were at high risk of performance bias due to the inherent nature of the intervention and difficulty in performing a blinded study.
Incomplete outcome data
Xiao 2009 did not report on participants enrolled but lost to follow‐up or otherwise excluded after enrollment, and so attrition bias was difficult to determine (unclear risk).
For Slusher 2015 and Slusher 2018, there were days where outcome data were not included because the infants received less than the prespecified number of hours of sunlight phototherapy.
Selective reporting
Slusher 2015 and Slusher 2018, assessed efficacy based on “treatable days”, therefore they only included days that had the opportunity to be efficacious (at least x number of hours of sunlight phototherapy). This negates the greatest flaw in sunlight as a treatment: the fact that it is not always available (low risk of reporting bias). Xiao 2009 did not comment on reporting bias (unclear risk).
Other potential sources of bias
Xiao 2009 reported no information about participant characteristics (unclear risk of other bias). It was a single center study undertaken at Shenzhen Maternity and Child Healthcare Hospital. There were no comments on ethical approval, Internal Review Board process, whether informed consent was obtained, whether funders had a role in the study, if there was any conflict of interest.
We did not have specific concerns for other sources of bias in Slusher 2015 or Slusher 2018 not already noted above.
Effects of interventions
Comparison 1. Low‐risk term and late preterm infants
Sunlight (with or without filters or amplification) versus no treatment
One study compared sunlight (with or without filters or amplification) versus no treatment in low‐risk term and late preterm infants (Table 1) (Xiao 2009).
Primary outcomes
Use of conventional phototherapy
The study did not assess use of CPT.
Treatment failure requiring exchange transfusion
The study did not assess treatment failure requiring exchange transfusion.
Acute bilirubin encephalopathy
The study did not assess acute bilirubin encephalopathy.
Chronic bilirubin encephalopathy or kernicterus
The study did not assess chronic bilirubin encephalopathy or kernicterus.
Death
The study did not assess death.
Secondary outcomes
Duration of treatment from initiation to end
The study did not assess duration of treatment from initiation to end.
Peak bilirubin levels during hospitalization
The study did not assess peak bilirubin levels during hospitalization.
Rate of change of serum bilirubin
The study did not assess rate of change of serum bilirubin.
Days in hospital
The study did not assess days in hospital.
Rate of rehospitalization within seven days of discharge for treatment for hyperbilirubinemia
There was no evidence of a difference in the rate of hospitalization associated with sunlight therapy (RR 0.55, 95% CI 0.27 to 1.11; RD −0.04, −0.08 to 0.01; 1 study, 482 infants; very low‐certainty evidence; Analysis 1.1) (Xiao 2009).
1.1. Analysis.

Comparison 1: Low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999), Outcome 1: Rate of rehospitalization
Athetoid cerebral palsy
The study did not assess athetoid cerebral palsy.
Auditory dysfunction
The study did not assess auditory dysfunction.
Total cost
The study did not assess total cost.
Sunburn
The study did not assess sunburn.
Hyperthermia
The study did not assess hyperthermia.
Maternal satisfaction
The study did not assess maternal satisfaction.
Nursing staff satisfaction
The study did not assess nursing staff satisfaction.
Need for fluid supplementation
The study did not assess need for fluid supplementation.
Cessation of breast milk feeding
The study did not assess cessation of breast milk feeding.
Additional post‐hoc outcomes
Incidence of jaundice
There may be a reduction in the incidence of jaundice associated with sunlight (26.03% in sunlight exposure group versus 43.7% in no treatment group; RR 0.61, 95% CI 0.45 to 0.82; RD −0.14, 95% CI −0.22 to −0.06; NNTB 7, 95% CI 5 to 17; 1 study, 482 infants; very low‐certainty evidence; Analysis 1.2) (Xiao 2009).
1.2. Analysis.

Comparison 1: Low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999), Outcome 2: Incidence of jaundice
Duration of jaundice
There may be a reduction in the duration of jaundice in infants receiving sunlight therapy (4.3 days, SD 1.7 in sunlight exposure group versus 6.5, SD 2.7 in no treatment group; MD −2.20 days, 95% CI −2.60 to −1.80; 1 study, 482 infants; very low‐certainty evidence; Analysis 1.3) (Xiao 2009).
1.3. Analysis.

Comparison 1: Low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999), Outcome 3: Duration of jaundice (days)
Proportion of days of effective treatment
The study did not assess proportion of days of effective treatment.
Hypothermia (defined as temperature less than 35.5 °C) while receiving sunlight or conventional phototherapy
The study did not assess hypothermia.
Dehydration
The study did not assess dehydration.
Sunlight (with or without filters or amplification) versus other sources of phototherapy
We found no studies comparing sunlight (with or without filters or amplification) versus other sources of phototherapy in low‐risk term and late preterm infants.
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type
We found no studies comparing sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type in low‐risk term and late preterm infants.
Comparison 2. In infants identified as 'at risk' for hyperbilirubinemia
We found no studies in infants identified as 'at risk' for hyperbilirubinemia.
Comparison 3. In infants with clinical jaundice
We found no studies in infants with clinical jaundice.
Comparison 4. In infants with confirmed hyperbilirubinemia
We found two studies in infants with confirmed hyperbilirubinemia (Slusher 2015; Slusher 2018).
Sunlight (with or without filters or amplification) versus no treatment
We found no studies comparing sunlight (with or without filters or amplification) versus no treatment in infants with confirmed hyperbilirubinemia.
Sunlight (with or without filters or amplification) versus other sources of phototherapy
Two studies compared sunlight (with or without filters or amplification) versus other sources of phototherapy (see Table 2) (Slusher 2015; Slusher 2018).
Primary outcomes
Use of conventional phototherapy
Neither study assessed use of CPT as both groups in both studies received CPT at night or on rainy days if they had afternoon bilirubin levels at or above treatment threshold.
Treatment failure requiring exchange transfusion
Both studies reported treatment failure requiring exchange transfusion. No infants required exchange transfusion in Slusher 2015, two infants (one in each group) received exchange transfusions in Slusher 2018. This was not considered treatment failure in that study. There was no effect on the use of exchange transfusion (typical RR 1.00, 95% CI 0.06 to 15.73; typical RD 0.00, 95% CI −0.01 to 0.01; 2 studies, 621 infants; low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 1: Treatment failure requiring exchange transfusion
Acute bilirubin encephalopathy
Slusher 2015 did not assess acute bilirubin encephalopathy.
Slusher 2018 reported that no infant in either group developed acute bilirubin encephalopathy (RR not estimable; RD 0.00, 95% CI −0.02 to 0.02; 1 study, 174 infants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 2: Acute bilirubin encephalopathy
Chronic bilirubin encephalopathy or kernicterus
Neither study assessed chronic bilirubin encephalopathy or kernicterus.
Death
Slusher 2015 assessed death. None of the infants who were withdrawn after enrollment had death reported as the reason for withdrawal (RR not estimable; typical RD 0.00, 95% CI −0.01 to 0.01; 1 study, 447 infants; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 3: Death
Slusher 2018 did not report death.
Secondary outcomes
Duration of treatment from initiation to end
Neither study assessed duration of treatment from initiation to end.
Peak bilirubin levels during hospitalization
Neither study assessed peak bilirubin levels during hospitalization.
Rate of change of serum bilirubin
Both studies reported rate of change of serum bilirubin but used different parameters and units (Slusher 2015: median −0.07 mg/dL/hour, interquartile range [IQR] −0.2 to 0.02 in FSPT group versus median 0.0 mg/dL/hour, IQR −0.14 to 0.09 in CPT group; Slusher 2018: mean −18 µmol/L/hour, SD 24 in FSPT group versus mean −14 µmol/L/hour, SD 31 in IEPT group; MD −4.00 μmol/L/hour, 95% CI −12.24 to 4.24; moderate‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 4: Rate of change of serum bilirubin (μmol/L/hour)
Days in hospital
Neither study assessed days in hospital.
Rate of rehospitalization
Neither study assessed rate of hospitalization.
Athetoid cerebral palsy
Neither study assessed athetoid cerebral palsy.
Auditory dysfunction
Neither study assessed auditory dysfunction.
Total cost
Neither study assessed total cost.
Sunburn
There were no cases of sunburn in either study (typical RR not estimable; typical RD 0.00, 95% CI −0.01 to 0.01; 2 studies, 621 infants; very low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 5: Sunburn
Hyperthermia
Both studies reported an increased risk of hyperthermia (body temperature greater than 38 °C: Slusher 2015: 86/224 [5%] infants in FSPT group versus 15/223 [1%] infants in CPT group; Slusher 2018: 33/87 [38%] infants in FSPT group versus 12/87 [14%] infants in IEPT group; typical RR 4.39, 95% CI 2.98 to 6.47; typical RD 0.30, 95% CI 0.23 to 0.36; NNTH 3, 95% CI 2 to 4; 2 studies, 621 infants; moderate‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 6: Hyperthermia (> 38 °C)
Maternal satisfaction
Neither study assessed maternal satisfaction; however, Slusher 2015 reported that two mothers of infants in the CPT group requested to be switched to FSPT on the last day of treatment.
Nursing staff satisfaction
Neither study assessed nursing staff satisfaction.
Need for fluid supplementation
Neither study reported specifically on the need for fluid supplementation. However, both studies reported “dehydration,” which we added as a post hoc outcome ('Hydration' below).
Cessation of breast milk feeding
Neither study assessed cessation of breast milk feeding.
Additional post‐hoc outcomes
Incidence of jaundice
Neither study assessed incidence of jaundice.
Duration of jaundice
Neither study assessed duration of jaundice.
Proportion of days of effective treatment (%)
Slusher 2015 defined an effective treatment day as a day when the rate of increase of total serum bilirubin was less than 0.2 mg/dL/hour for infants up to 72 hours of age or a decrease in total serum bilirubin for infants older than 72 hours of age, who had received at least five hours of sunlight phototherapy that day. Slusher 2018 defined an effective treatment day as a day when the rate of increase in total serum bilirubin was less than 3.4 µmol/L/hour for neonates less than 72 hours old, or a decrease in serum bilirubin for neonates 72 hours of age or older, who had received at least four hours of sunlight phototherapy that day.
There was no evidence of a difference in proportion of days of effective treatment (effectiveness: Slusher 2015: 232/250 treatment days [93%, 95% CI 89% to 96%] in FSPT group versus 280/311 treatment days [93%, 95% CI 86% to 93%] in CPT group; Slusher 2018: 116/133 [87.2%] treatment days in FSPT group versus 135/152 [88.8%] treatment days in IEPT group [P = 0.8165]; MD −1.6%, 95% CI −9.9 to 6.7; very low‐certainty evidence; Analysis 2.7). The non‐inferiority margin was −10% in both studies.
2.7. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 7: Proportion of days of effective treatment
Hypothermia
There was no evidence of a difference in hypothermia (infants with a body temperature less than 35.5 °C: Slusher 2015: 8/224 (less than 1%) infants in the FSPT group versus 2/223 (less than 1%) infants in the CPT group; Slusher 2018: 9/87 (10%) infants in the FSPT group versus 14/87 (16%) infants in the IEPT group; typical RR 1.06, 95% CI 0.55 to 2.03; typical RD 0.00, 95% CI −0.03 to 0.04; 2 studies, 621 infants; moderate‐certainty evidence; Analysis 2.8).
2.8. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 8: Hypothermia (< 35.5 °C)
Dehydration
Both studies reported dehydration. No infant in either study had signs of dehydration requiring fluid supplementation (typical RR not estimable; typical RD 0.00, 95% CI −0.01 to 0.01; 2 studies, 621 infants; very low‐certainty evidence; Analysis 2.9).
2.9. Analysis.

Comparison 2: Infants with confirmed hyperbilirubinemia, Outcome 9: Dehydration
Sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type
We found no studies comparing sunlight filtering or amplifying device of one type versus sunlight filtering or amplifying device of another type in infants with confirmed hyperbilirubinemia.
Discussion
Summary of main results
We identified three studies including 1103 infants that assessed sunlight therapy for the prevention or treatment of hyperbilirubinemia. These studies showed that it is uncertain if sunlight exposure may be beneficial for infants with hyperbilirubinemia or jaundice. It may decrease the incidence and duration of jaundice. Sunlight may also cause a rate of change in serum bilirubin comparable to that seen in infants receiving CPT, so long as CPT is still provided whenever sunlight is not available. However, these studies demonstrated that sunlight exposure may also put infants at risk for hyperthermia. The potential utility of sunlight to prevent or treat hyperbilirubinemia is severely limited by the low‐certainty evidence and the design of the studies. We are uncertain whether sunlight is effective for the prevention or treatment of hyperbilirubinemia in term or late preterm neonates.
Only one study compared low‐risk term and late preterm infants who received sunlight with infants who received no treatment (Xiao 2009). Xiao 2009 reported a reduction in the incidence of jaundice and the number of days that an infant was jaundiced with between 30 minutes and one hour of sun exposure twice daily when compared with no intervention. There were no data on safety or potential harmful effects of the intervention reported, and the mechanism for sun exposure was not discussed.
Two studies compared infants with known hyperbilirubinemia who received sunlight with infants who received other sources of phototherapy (Slusher 2015; Slusher 2018). Both studies demonstrated that FSPT was non‐inferior to conventional or IEPT, if given for at least four hours per day, and if CPT was available when sunlight was not. Both studies showed an increased risk for hyperthermia with FSPT.
Overall completeness and applicability of evidence
Despite these promising results the applicability of sunlight for the treatment of hyperbilirubinemia is questionable given the design of both Slusher's studies (Slusher 2015; Slusher 2018). Routine phototherapy was available to all infants on rainy days, and at night as needed. The effect of sunlight was only measured on days when an infant received at least four hours of therapy, and there were far more assessable treatment days for infants receiving electric phototherapy in both studies. Can sunlight then be deemed non‐inferior to conventional or intensive phototherapy? It is unclear if sunlight is practical or provides a real benefit if CPT is still required. In Xiao 2009, there were other treatment groups not included in this review (one where infants received a herbal bath and one where infants received a herbal bath plus sunlight exposure, both for prevention of jaundice). These were not included because it is unclear how these infants might have been treated differently based on their treatment group, but the infants receiving the herbal bath had less jaundice than the infants receiving sunlight alone. It is difficult to imagine a physiologic explanation for these findings, and so places doubts on the conclusions of Xiao and colleagues. However, sunlight may have other benefits, including allowing for a rotational use of limited numbers of phototherapy machines during the day and providing a practical way for mothers to avoid the need for CPT or hospitalization for their infants. Further, Olusanya 2014 considered maternal satisfaction with filtered sunlight phototherapy, and found that, overall, mothers were more satisfied with phototherapy treatment when their babies were receiving filtered sunlight therapy. Increasing maternal satisfaction and allowing for family bonding could be compelling reasons to provide FSPT whenever feasible.
Quality of the evidence
The certainty of the evidence in all three studies was moderate to very low. It was very low for all the primary outcomes (main outcomes) in each study. The certainty of the evidence was downgraded due to these being unblinded single‐center studies with risk of bias, indirectness, and imprecision.
These studies were limited by a lack of blinding, and by structured definitions of success which detracted from the applicability of sunlight phototherapy. The review process included a comprehensive review of the literature, but was constrained by the number of studies that have been performed on this subject. The review was also limited to RCTs; however, the search identified no observational studies or other less rigorous studies. These limitations led to a small sample size and imprecision with regard to results.
Potential biases in the review process
This review was limited by the few studies that were identified. Potential biases in the review process include the addition of several post‐hoc outcomes after the included studies were identified. The authors decided to include all these post‐hoc outcomes because they reflected potential safety measures that the authors had not considered, as well as the primary outcomes assessed by two of the three studies. However, there exists the potential for bias in selecting these outcomes for inclusion. Since the review identified very few studies, the data were very limited, and we felt it was important to include all relevant data.
Agreements and disagreements with other studies or reviews
We identified no other reviews.
Authors' conclusions
Implications for practice.
There is some evidence that sunlight may be effective in preventing the development of hyperbilirubinemia requiring hospitalization in low‐risk term or late preterm infants, though this cannot be concluded with confidence as only one study with very low‐certainty evidence demonstrated this. We found no evidence to support or refute the use of sunlight in the treatment of infants with confirmed hyperbilirubinemia. Based on these studies, we are uncertain whether sunlight is effective for the prevention or treatment of hyperbilirubinemia in term or late preterm neonates.
Implications for research.
In resource‐limited settings, sunlight might allow for rotational use of limited numbers of available electric phototherapy devices. It may play a role in promoting maternal/family bonding, and support exclusive breastfeeding. Ongoing research into sunlight for the prevention or treatment of hyperbilirubinemia would be most beneficial if it addressed the implementation and delivery issues facing this potential mode of therapy that has variable availability. Sunlight should be assessed as an independent therapy, without conventional phototherapy provided to study participants, to evaluate sunlight without contamination. Otherwise, research should address operationalizing sunlight therapy in a meaningful way to promote family bonding and resource conservation recognizing the limitations of this therapy (Slusher 2017). A detailed model for delivery of sunlight therapy is included in Slusher and colleagues' two studies (Slusher 2015; Slusher 2018), but needs to be further studied in various settings to be standardized.
History
Protocol first published: Issue 3, 2019
Acknowledgements
The methods section was based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support.
Carol Friesen, Information Specialist for Cochrane Neonatal, designed and ran the literature searches, and Colleen Ovelman peer reviewed the Ovid MEDLINE search strategy.
As a Cochrane Neonatal Associate Editor, Mohan Pammi has peer reviewed and offered feedback for this review.
We would like to thank Bonnie Cheung for translation assistance with Xiao 2009, and Cristiana Riboni and Marco Da Roit for translation assistance with Romagnoli 1994 (not included).
Appendices
Appendix 1. Search methodology
We used Cochrane's highly sensitive search strategies for identifying randomized trials to create the RCT filters (Higgins 2020). The Cochrane Neonatal Information Specialist created and tested the neonatal filters. We ran searches in the following databases in September 2019. We updated the searches in June 2020, running them only in CENTRAL via CRS Web, MEDLINE via Ovid, MEDLINE via PubMed, CINAHL via EBSCOhost, and ISRCTN.
Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) from 1946:
1. exp Heliotherapy/ 2. heliotherapy.mp. 3. exp Sunlight/ 4. sunlight.mp. 5. "sun light".mp. 6. sunshine.mp. 7. "sun shine".mp. 8. sun*.mp. 9. solar*.mp. 10. window*.mp. 11. daylight.mp. 12. "day light".mp. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp hyperbilirubinemia/ or exp hyperbilirubinemia, neonatal/ or exp jaundice, neonatal/ or exp jaundice/ or exp jaundice, obstructive/ or exp kernicterus/ 15. hyperbilirubinemi*.mp. 16. hyperbilirubinaemi*.mp. 17. bilirubinemi*.mp. 18. bilirubinaemi*.mp. 19. jaundice.mp. 20. (jaundices or jaundiced).mp. 21. kernicterus.mp. 22. icter*.mp. 23. (encephalopath* adj2 bilirubin).mp. 24. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. exp infant, newborn/ 26. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 27. 25 or 26 28. randomized controlled trial.pt. 29. controlled clinical trial.pt. 30. randomized.ab. 31. placebo.ab. 32. drug therapy.fs. 33. randomly.ab. 34. trial.ab. 35. groups.ab. 36. or/28‐35 37. exp animals/ not humans.sh. 38. 36 not 37 39. 27 and 38 40. 13 and 24 and 39
MEDLINE via PubMed
((("Heliotherapy"[Mesh] OR "Sunlight"[Mesh] OR heliotherapy[TW] OR sunlight[TW] OR "sun light"[TW] OR sunshine[TW] OR "sun shine"[TW] OR sun*[TW] OR solar*[TW] OR window*[TW] OR daylight[TW] OR "day light"[TW])) AND ((("Hyperbilirubinemia"[Mesh] OR "Hyperbilirubinemia, Neonatal"[Mesh] OR "Jaundice"[Mesh] OR "Jaundice, Obstructive"[Mesh] OR "Jaundice, Neonatal"[Mesh] OR "Kernicterus"[Mesh]) OR (hyperbilirubinemi*[TW] OR hyperbilirubinaemi*[TW] OR bilirubinemi*[TW] OR bilirubinaemi*[TW] OR jaundice[TW] OR jaundices[TW] OR jaundiced[TW] OR kernicterus[TW] OR icter*[TW] OR (encephalopath*[TW] AND bilirubin[TW]))))) AND (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR neonat*[TIAB]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT humans[mh]))) Publication date from 2018/04/01
CINAHL via EBSCOhost
(hyperbilirubinemi* OR hyperbilirubinaemi* OR bilirubinemi* OR bilirubinaemi* OR jaundice OR jaundices OR jaundiced OR kernicterus OR icter* OR (encephalopath* AND bilirubin)) AND (heliotherapy OR sunlight OR "sun light" OR sunshine OR "sun shine" OR sun* OR solar* OR window* OR daylight OR "day light") AND (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Embase via Ovid
1. exp heliotherapy/ 2. heliotherapy.mp. 3. exp sunlight/ 4. sunlight.mp. 5. "sun light".mp. 6. sunshine.mp. 7. "sun shine".mp. 8. sun*.mp. 9. solar*.mp. 10. window*.mp. 11. daylight.mp. 12. "day light".mp. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp hyperbilirubinemia/ or exp jaundice/ or exp eye jaundice/ or exp kernicterus/ or exp newborn jaundice/ or exp obstructive jaundice/ or neonatal hyperbilirubinemia/ 15. hyperbilirubinemi*.mp. 16. hyperbilirubinaemi*.mp. 17. bilirubinemi*.mp. 18. bilirubinaemi*.mp. 19. jaundice.mp. 20. (jaundices or jaundiced).mp. 21. kernicterus.mp. 22. icter*.mp. 23. (encephalopath* adj2 bilirubin).mp. 24. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. exp prematurity/ 26. exp infant/ 27. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or infantile or infancy or neonat*).ti,ab. 28. 25 or 26 or 27 29. (human not animal).mp. 30. (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. 31. 28 and 29 and 30 32. 13 and 24 and 31
Cochrane CENTRAL via CRS Web
1. MESH DESCRIPTOR Heliotherapy EXPLODE ALL AND CENTRAL:TARGET 2. heliotherapy AND CENTRAL:TARGET 3. MESH DESCRIPTOR Sunlight EXPLODE ALL AND CENTRAL:TARGET 4. sunlight AND CENTRAL:TARGET 5. "sun light" AND CENTRAL:TARGET 6. sunshine AND CENTRAL:TARGET 7. "sun shine" AND CENTRAL:TARGET 8. sun* AND CENTRAL:TARGET 9. solar* AND CENTRAL:TARGET 10. window* AND CENTRAL:TARGET 11. daylight AND CENTRAL:TARGET 12. "day light" AND CENTRAL:TARGET 13. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 14. MESH DESCRIPTOR Hyperbilirubinemia EXPLODE ALL AND CENTRAL:TARGET 15. MESH DESCRIPTOR Hyperbilirubinemia, Neonatal EXPLODE ALL AND CENTRAL:TARGET 16. MESH DESCRIPTOR Jaundice, Neonatal EXPLODE ALL AND CENTRAL:TARGET 17. MESH DESCRIPTOR jaundice EXPLODE ALL AND CENTRAL:TARGET 18. MESH DESCRIPTOR Jaundice, Obstructive EXPLODE ALL AND CENTRAL:TARGET 19. MESH DESCRIPTOR kernicterus EXPLODE ALL AND CENTRAL:TARGET 20. hyperbilirubinemi* OR hyperbilirubinaemi* OR bilirubinemi* OR bilirubinaemi* OR jaundice OR jaundices OR jaundiced OR kernicterus OR icter* OR (encephalopath* ADJ2 bilirubin) AND CENTRAL:TARGET 21. #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 22. MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 23. infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 24. #23 OR #22 AND CENTRAL:TARGET 25. #13 AND #21 AND #24
Clinical Trial Registries
Clinicaltrials.gov
sunlight OR heliotherapy | jaundice OR hyperbilirubinemia | Child filter applied
WHO ICTRP
sunlight AND hyperbilirubinemia
ISRCTN.com
sunlight AND hyperbilirubinemia sunlight AND jaundice heliotherapy AND jaundice heliotherapy AND hyperbilirubinemia
Appendix 2. Risk of bias tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (less than 20% missing data);
high risk (20% or greater missing data); or
unclear risk.
Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could have put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Low‐risk term and late preterm infants (based on risk criteria defined by Bhutani 1999).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Rate of rehospitalization | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Sunlight (with or without filters or amplification) versus no treatment | 1 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.27, 1.11] |
| 1.2 Incidence of jaundice | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Sunlight (with or without filters or amplification) versus no treatment | 1 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.45, 0.82] |
| 1.3 Duration of jaundice (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Sunlight (with or without filters or amplification) versus no treatment | 1 | 482 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐2.60, ‐1.80] |
Comparison 2. Infants with confirmed hyperbilirubinemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Treatment failure requiring exchange transfusion | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.73] |
| 2.2 Acute bilirubin encephalopathy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 1 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Rate of change of serum bilirubin (μmol/L/hour) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐12.24, 4.24] |
| 2.5 Sunburn | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6 Hyperthermia (> 38 °C) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.6.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [2.98, 6.47] |
| 2.7 Proportion of days of effective treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 2.8 Hypothermia (< 35.5 °C) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.8.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.03] |
| 2.9 Dehydration | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9.1 Sunlight (with or without filters or amplification) versus other sources of phototherapy | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Slusher 2015.
| Study characteristics | ||
| Methods | Randomized, controlled non‐inferiority trial in which filtered sunlight was compared with CPT for the treatment of hyperbilirubinemia in term and late preterm neonates at Island Maternity Hospital in Lagos, Nigeria, from November 2012 to September 2013. | |
| Participants |
Eligibility: all infants up to 14 days of age at > 35 weeks' gestation (or > 2.2 kg BW if GA unknown), black Africans, with elevated serum bilirubin (defined as 3 mg/dL below the level recommended by the AAP (based on Bhutani 1999), to begin PT in high‐risk infants) Enrollment: 447 term and late preterm neonates enrolled and randomly assigned; 224 to filtered sunlight, 223 to CPT |
|
| Interventions | Filtered sunlight compared with CPT.
At night and on rainy days, all enrolled infants who met criteria for treatment received CPT. |
|
| Outcomes |
Primary
Secondary
Safety monitoring
|
|
| Notes | Funded by the Thrasher Research Fund, Salt Lake City, and the National Center for Advancing Translational Sciences of the National Institutes of Health; Clinical Trials.gov number, NCT01434810. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using a block randomization procedure, with block sizes of 2, 4, 6, 8, and 10. |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were recorded on sequentially numbered sheets of paper, enclosed in opaque, sealed, matching, numbered envelopes, and shipped to Lagos and opened sequentially for each enrolled infant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was non‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was non‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 13/220 infants could not be evaluated (4 had missing time under PT, 6 received < 5 hours of PT, 4 had missing afternoon bilirubin assessments). No infant in either group met criteria for withdrawal due to safety. |
| Selective reporting (reporting bias) | Low risk | Trial formally registered. All outcomes reported. |
| Other bias | Low risk | 2 mothers of infants in CPT group requested their infants be switched to FSPT on the last day of treatment. |
Slusher 2018.
| Study characteristics | ||
| Methods | Prospective, randomized controlled non‐inferiority trial conducted at Bowen University Teaching Hospital in Ogbomoso, Nigeria – a simulated rural setting. Randomization was computer‐generated, and neither clinicians nor the parents or guardians of participants were blinded to group allocation. Study was conducted from July 2015 through April 2017. |
|
| Participants |
Eligibility: neonates aged ≤ 14 days or younger who were ≥ 35 weeks' GA or weighed > 2.2 kg if age not known and had TSB at or higher than high risk curve (> 35 weeks) (based on Bhutani 1999), irrespective of actual GA. Infants meeting exchange transfusion requirements could be included at discretion of treating physician. Enrollment: 174 neonates enrolled and randomly assigned: 87 to FSPT group, 87 to IEPT group. |
|
| Interventions | Randomly assigned (1:1) to either FSPT or IEPT
At night and on rainy days, all enrolled infants who met criteria for treatment received IEPT. |
|
| Outcomes |
Primary
Secondary
Safety
Analysis by intention‐to‐treat with a non‐inferiority margin of 10% |
|
| Notes | Funded by the Thrasher Research Fund, Salt Lake City, and the National Center for Advancing Translational Sciences of the National Institutes of Health. Clinical trials.gov registration number NCT02612727. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned 1:1. Randomization procedure with variable block sizes, assignments were computer generated by study statistician. |
| Allocation concealment (selection bias) | Low risk | Assignments were printed on sequentially numbered sheets of paper, enclosed in opaque envelopes, sealed, sequentially numbered envelopes, transported to Nigeria by regulatory sponsor. When study nurse enrolled infant she opened the envelope and recorded number and treatment assignment on case report form. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was non‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was non‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 12/174 infants could not be evaluated. Of treatment days, 149/434 treatment days could not be evaluated, 145 because infant had < 4 hours of PT, and 110 because infant had missing TSB measurement (most of these days had both < 4 hours of PT and a missing TSB measurement). Additionally, 2 days were not evaluated (in 1 infant) because the infant had direct hyperbilirubinemia and so was subsequently excluded from the study. 9 infants were withdrawn after randomization, 5 in the FSPT group (4 by parental request and 1 needed treatment incompatible with PT) and 4 in the IEPT group (2 had direct hyperbilirubinemia, 1 by parental request, and 1 needed treatment incompatible with PT). |
| Selective reporting (reporting bias) | Low risk | Trial formally registered. All outcomes reported. |
| Other bias | Low risk | 2 infants in the FSPT group received IEPT on 1 treatment day, and 1 infant in the IEPT group received 3 days of FSPT. |
Xiao 2009.
| Study characteristics | ||
| Methods | Randomized controlled trial conducted at Schenzen Maternity and Child Healthcare Hospital from March 2004 through September 2007. | |
| Participants | Term infants born in Schenzen, China to healthy mothers with no history of hepatitis B or liver dysfunction, infants weighing ≥ 2 kg with Apgar 7–10 with no obvious abnormalities. Monitored from day 3 to 6–7 of life. | |
| Interventions | Randomized to 1 of 4 groups: sunlight, bath, sunlight + bath, or neither sunlight nor bath (control) Sunlight
Bath
|
|
| Outcomes |
|
|
| Notes | Jaundice definitions and management criteria: serum bilirubin level 257–291 µmol/L indicated diagnosis of jaundice. Value > 291 µmol/L required hospital admission. Normal serum bilirubin 205–257 µmol/L. Funding source not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not reported in study. |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study did not comment on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study did not comment on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not comment on data completion/loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not identified. |
| Other bias | Unclear risk | None identified. |
AAP: American Academy of Pediatrics; BW: birthweight; CPT: conventional phototherapy; FSPT: filtered‐sunlight phototherapy; GA: gestational age; IEPT: intensive electric phototherapy; PT: phototherapy; TCB: transcutaneous bilirubin; TSB: total serum bilirubin.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Emokpae 2016 | Emokpae and colleagues reviewed medical records of infants admitted for neonatal hyperbilirubinemia in an inner‐city Children's Hospital in Lagos, Nigeria, between January 2012 and December 2014 to determine the pattern, treatment, and outcomes for these infants during the preintervention period prior to the start of enrollment for Slusher 2015. Factors associated with adverse outcomes were identified through multivariable logistic regression. This was not an RCT evaluating sunlight for hyperbilirubinemia. |
| Olusanya 2014 | Cross‐sectional satisfaction survey conducted among mothers of jaundiced infants treated with FSPT in an inner‐city maternity hospital in Lagos, Nigeria from November 2013 to March 2014. Mothers' experience during treatment was elicited with a pretested questionnaire consisting of closed and open‐ended items. Satisfaction was rated on a 5‐point Likert scale. Correlates of overall maternal satisfaction were explored with descriptive and inferential non‐parametric statistics. 191 mothers were surveyed, 77 (40%) of whom had no prior knowledge of neonatal jaundice. Maternal satisfaction was highest for quality of nursing care received (mean 4.72, SD 0.55; median 5, IQR 5 to 5) and lowest for physical state of the test environment (mean 3.85, SD 0.74, median 4, IQR 3 to 4). The overall rating (mean 4.17, SD 0.58; median 4, IQR 4 to 5) and the observed effect of FSPT on the infants (mean 4.34, SD 0.58; median 4, IQR 4 to 5) were satisfactory. FSPT experience was significantly correlated with the adequacy of information received (P < 0.0005), test environment (P = 0.002), and the observed effect of FSPT on the child (P < 0.0005). 98.4% of mothers indicated willingness to use FSPT in future or recommend it to others, although some (30 [15.7%]) disliked the idea of exposing newborns to sunlight. This was not an RCT evaluating sunlight for hyperbilirubinemia. |
| Slusher 2014 | Pilot study prior to the RCT conducted in Slusher 2015. Slusher and colleagues evaluated safety and efficacy of FSPT in term/late preterm infants ≤ 14 days old with clinically significant jaundice, assessed by TB levels. Infants were recruited from a maternity hospital in Lagos, Nigeria. Sunlight was filtered with commercial window‐tinting films that removed most UV and significant levels of IR light and transmitted effective levels of therapeutic blue light. After placing infants under an FSPT canopy, hourly measurements of axillary temperatures, monitoring for sunburn, dehydration, and irradiances of filtered sunlight were performed. Treatment was deemed safe and efficacious if infants were able to stay in FSPT for ≥ 5 hours and rate of rise of TB was < 0.2 mg/dL/hour for infants ≤ 72 hours of age or TB decreased for infants > 72 hours of age. 227 infants received 258 days of FSPT. |
| Vreman 2013 | Vreman and colleagues evaluated 9 semi‐transparent plastic window‐tinting films for their ability to block UVA and IR radiation and transmit therapeutic blue light (400–520 nm) for treating jaundiced newborns. For indoor testing, 3 light sources (TL/52 special blue fluorescent, Black Light UVA, and IR heat lamps) were positioned above each film and measured successively using a thermocouple thermometer, UVA radiometer, and blue light irradiance meter, placed below each film. For outdoor testing, the same setup was used with the sun at zenith and a cloudless sky. Compared with unfiltered radiation, blue light transmission through films ranged was 24–83%, UVA transmission was 0.1–7.1%, and reductions in IR heat were 6–12 °C for the heat lamp and 5–10 °C for the sun. Data suggested that most of the relatively low‐cost window‐tinting films tested can effectively reduce sunlight UV and IR and offer a range of significant attenuations of therapeutic blue light. This was not an RCT evaluating sunlight for the treatment of hyperbilirubinemia. |
FSPT: filtered‐sunlight phototherapy; IQR: interquartile range; IR: infrared; RCT: randomized controlled trial; SD: standard deviation; TB: total bilirubin; UV: ultraviolet; UVA: ultraviolet A.
Differences between protocol and review
We made the following changes to the published protocol (Horn 2019).
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomized controlled trials (RCTs) and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see How CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
From July 2019, Cochrane Neonatal no longer searches for RCTs and CCTs on the following platforms: ClinicalTrials.gov or from The World Health Organization's International Clinical Trials Registry Platform (ICTRP), as records from both platforms are added to CENTRAL on a monthly basis (see How CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN Registry (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
As such for the 2020 updated search, we ran searches in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid, MEDLINE via PubMed, CINAHL via EBSCOhost, and ISRCTN.
We used the Covidence workflow to help assess the search results.
-
We analyzed the following additional post‐hoc outcomes after identification of the included studies to reflect the primary efficacy outcome in two studies as well as to address safety issues not originally identified by the authorship team:
incidence of jaundice in participants (defined as bilirubin level greater than 257 µmol/L);
duration of jaundice;
proportion of days of effective treatment (as defined in individual studies);
hypothermia (less than 35.5 °C);
dehydration.
We added a 'data synthesis' section.
-
Given the different populations included in each comparison, we considered that slightly different outcomes were most critical for decision‐making related to these different populations and this is reflected in the summary of findings reported for each comparison. We updated the summary of findings and assessment of the certainty of the evidence section to reflect that we would focus on the following outcomes.
-
Comparison 1
Use of CPT, if sunlight (with or without filters or amplification) was first used for prevention or early treatment.
Treatment failure requiring exchange transfusion (as defined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life) (Bhutani 1999).
Rate of rehospitalization within seven days of discharge for treatment for hyperbilirubinemia.
Acute bilirubin encephalopathy, defined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures (AAP 2004; Johnson 2002), or chronic bilirubin encephalopathy or kernicterus, defined as athetoid cerebral palsy, auditory dysfunction, dental‐enamel dysplasia, paralysis of upward gaze, and, occasionally, intellectual and other disabilities (AAP 2004).
Death.
Incidence of jaundice in participants (defined as bilirubin level > 257 μmol/L).
Duration of jaundice (days).
-
Comparison 2
Use of CPT, if sunlight (with or without filters or amplification) was first used for prevention or early treatment.
Hyperthermia (defined as temperature greater than 38 °C) while receiving sunlight or CPT.
Treatment failure requiring exchange transfusion (as defined by receiving an exchange transfusion; or bilirubin level greater than 15 mg/dL in the first 24 hours of life, greater than 17 mg/dL in the first 48 hours of life, or greater than 20 mg/dL after 72 hours of life) (Bhutani 1999).
Acute bilirubin encephalopathy, defined as retrocollis and opisthotonus in association with irritability, drowsiness, poor or no feeding, alternating tone, high‐pitched or shrill cry, lethargy, coma, fever, or seizures (AAP 2004; Johnson 2002).
Death.
Proportion of days of effective treatment (as defined in individual studies).
Hypothermia (defined as temperature less than 35.5 °C) while receiving sunlight or CPT.
-
Contributions of authors
DH acted a lead author and participated in the design and drafting of the protocol (Horn 2019) and review, identified included studies from Covidence, extracted study data from included studies, and entered the data in Review Manager 5 (Review Manager 2020).
DE participated in the design and drafting of the protocol (Horn 2019) and review, and identified included studies from Covidence.
RFS participated in the design and drafting of the protocol (Horn 2019) and review, extracted study data from included studies, and entered the data in Review Manager 5 (Review Manager 2020).
KG participated in the design and drafting of the protocol (Horn 2019) and review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
DH: none.
DE is an Associate Editor of Cochrane Neonatal but did not participate in the editorial review of the protocol or review.
KG: is a Co‐Editor of Cochrane Neonatal but did not participate in the editorial review of the protocol or review.
RFS is the Co‐ordinating Editor of Cochrane Neonatal but did not participate in the editorial review of the protocol or review.
New
References
References to studies included in this review
Slusher 2015 {published data only}
- Slusher TM, Olusanya BO, Vreman HJ, Brearley AM, Vaucher YE, Lund TC, et al. A randomized trial of phototherapy with filtered sunlight in African neonates. New England Journal of Medicine 2015;373(12):1115-24. [DOI: 10.1056/NEJMoa1501074] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Slusher TM, Olusanya BO, Vreman HJ, Wong RJ, Brearley AM, Vaucher YE, et al. Treatment of neonatal jaundice with filtered sunlight in Nigerian neonates: study protocol of a non-inferiority, randomized controlled trial. Trials 2013;14:446. [DOI: 10.1186/1745-6215-14-446] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slusher 2018 {published data only}
- Slusher TM, Vreman HJ, Brearley AM, Vaucher YE, Wong RJ, Stevenson DK, et al. Filtered sunlight versus intensive electric powered phototherapy in moderate-to-severe neonatal hyperbilirubinaemia: a randomised controlled non-inferiority trial. Lancet Global Health 2018;10:e1122-31. [DOI: 10.1016/S2214-109X(18)30373-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xiao 2009 {published data only}
- Xiao DM, Wu CL, Liu QL. Traditional Chinese medicine bath with the sun on the prevention of neonatal jaundice. Chinese Medicine Modern Distance Education of China 2009;7(3):19. [CENTRAL: CN-00838198] [Google Scholar]
References to studies excluded from this review
Emokpae 2016 {published data only}
- Emokpae AA, Mabogunje CA, Imam ZO, Olusanya BO. Heliotherapy for neonatal hyperbilirubinemia in southwest, Nigeria: a baseline pre-intervention study. PloS One 2016;11(3):e0151375. [DOI: 10.1371/journal.pone.0151375] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olusanya 2014 {published data only}
- Olusanya BO, Imam ZO, Mabogunje CA, Emokpae AA, Slusher TM. Maternal satisfaction with a novel filtered-sunlight phototherapy for newborn jaundice in Southwest Nigeria. BMC Pediatrics 2014;14:180. [DOI: 10.1186/1471-2431-14-180] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slusher 2014 {published data only}
- Slusher TM, Vreman HJ, Olusanya BO, Wong RJ, Brearley AM, Vaucher YE, et al. Safety and efficacy of filtered sunlight in treatment of jaundice in African neonates. Pediatrics 2014;133(6):e1568-74. [DOI: 10.1542/peds.2013-3500] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vreman 2013 {published data only}
- Vreman HJ, Slusher TM, Wong RJ, Shulz S, Olusanya BO, Stevenson DK. Evaluation of window-tinting films for sunlight phototherapy. Journal of Tropical Pediatrics 2013;59(6):496-501. [DOI: 10.1093/tropej/fmt062] [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2004
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297-316; erratum in: Pediatrics 2004:114(4):1138. [DOI: 10.1542/peds.114.1.297] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutani 1999
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103(1):6-14. [DOI: 10.1542/peds.103.1.6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutani 2011
- Bhutani VK, Committee on the Fetus and Newborn, American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2011;128(4):e1046-52. [DOI: 10.1542/peds.2011-1494] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cashore 2000
- Cashore WJ. Bilirubin and jaundice in the micropremie. Clinical Perinatology 2000;27(1):171-9. [DOI: 10.1016/s0095-5108(05)70012-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebbesen 2012
- Ebbesen F, Bjerre JV, Vandborg PK. Relation between serum bilirubin levels ≥ 450μmol/L and bilirubin encephalopathy; a Danish population-based study. Acta Paediatrica 2012;101(4):384-9. [DOI: 10.1111/j.1651-2227.2011.02565.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 1 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [DOI: 10.1016/j.jclinepi.2017.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Johnson 2002
- Johnson LH, Bhutani VK, Brown AK. System-based approach to management of neonatal jaundice and prevention of kernicterus. Pediatrics 2002;140(4):396-403. [DOI: 10.1067/mpd.2002.123098] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kawade 1981
- Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochemical Journal 1981;196(1):257-60. [DOI: 10.1042/bj1960257] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuzniewicz 2014
- Kuzniewicz MW, Wickremasinqhe AC, Wu YW, McCulloch CE, Walsh EM, Wi S, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics 2014;134(3):504-9. [DOI: 10.1542/peds.2014-0987] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucey 1968
- Lucey J, Ferreiro M, Hewitt J. Prevention of hyperbilirubinemia of prematurity by phototherapy. Pediatrics 1968;41(6):1047-54. [PMID: ] [PubMed] [Google Scholar]
Maisels 2012
- Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. Journal of Perinatology 2012;32(9):660-4. [DOI: 10.1038/jp.2012.71] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manning 2007
- Manning D, Todd P, Maxwell M, Jane Platt M. Prospective surveillance study of severe hyperbilirubinaemia in the newborn in the UK and Ireland. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(5):F342-6. [DOI: 10.1136/adc.2006.105361] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGillivray 2016
- McGillivray A, Polverino J, Badawi N, Evans N. Prospective surveillance of extreme neonatal hyperbilirubinemia in Australia. Pediatrics 2016;168:82-7.e3. [DOI: 10.1016/j.jpeds.2015.08.048] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2008
- Morris BH, Oh W, Tyson JE, Stevenson DK, Phelps DL, O'Shea TM, et al, NICHD Neonatal Research Network. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. New England Journal of Medicine 2008;359(18):1885-96. [DOI: 10.1056/NEJMoa0803024] [DOI: 18971491] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01434810
- NCT01434810. Treatment of neonatal jaundice with filtered sunlight phototherapy: safety and efficacy in African neonates. clinicaltrials.gov/ct2/show/NCT01434810 (first received 15 September 2011).
Olusanya 2015
- Olusanya BO, Ogunlesi TA, Kumar P, Boo NY, Iskander IF, Almeida MF, et al. Management of late-preterm and term infants with hyperbilirubinaemia in resource-constrained settings. BMC Pediatrics 2015;15(39):1-12. [DOI: 10.1186/s12887-015-0358-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Slusher 2017
- Slusher TM, Day LT, Ogundele T, Woolfield N, Owa JA. Filtered sunlight, solar powered phototherapy and other strategies for managing neonatal jaundice in low-resource settings. Early Human Development 2017;114:11-5. [DOI: 10.1016/j.earlhumdev.2017.09.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stokowski 2011
- Stokowski LA. Fundamentals of phototherapy for neonatal jaundice. Advances in Neonatal Care 2011;11(5 Suppl):S10-21. [DOI: 10.1097/ANC.0b013e31822ee62c] [PMID: ] [DOI] [PubMed] [Google Scholar]
United Nations 2020
- United Nations. The Sustainable Development Goals Report 2020. unstats.un.org/sdgs/report/2020/ (accessed prior to 5 June 2021).
Wong 2017
- Wong RJ, Bhutani VK. Pathogenesis and etiology of unconjugated hyperbilirubinemia in the newborn. www.uptodate.com/contents/pathogenesis-and-etiology-of-unconjugated-hyperbilirubinemia-in-the-newborn (accessed 23 October 2017).
References to other published versions of this review
Horn 2019
- Horn D, Ehret D, Suresh G, Soll R. Sunlight for the prevention and treatment of hyperbilirubinemia in term and late preterm neonates. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013277. [DOI: 10.1002/14651858.CD013277] [DOI] [PMC free article] [PubMed] [Google Scholar]


