Abstract
Objective:
Neonates are more susceptible to infections, as well as medication toxicities. This study, therefore, sought to describe the clinical characteristics, medication prescription pattern, and treatment outcomes for neonates admitted to the neonatal intensive care unit (NICU) of a tertiary health-care facility in Ghana.
Methods:
A retrospective cross-sectional study was conducted to ascertain clinical records, conditions for admission, spectrum of medications prescribed, and treatment outcomes from neonatal patient folders.
Findings:
Of 667 folders reviewed (51.4% males and 48.6% female), 61.8% were preterm (mean gestational age: 34.2 ± 3.6 weeks), 64.6% had low birth weight (LBW) (mean birth weight: 2.1 ± 0.9 kg), 90.6% were delivered through spontaneous vaginal delivery, and 57.4% delivered at the tertiary health-care facility. Of the 667 neonates, 70%, 27.1%, and 2.9% were queried with one, two, or three medical conditions, respectively. Respiratory distress, preterm, and pyrexia were common single queried conditions (88.5%). LBW, hypothermia, and single queried medical conditions were associated (P ≤ 0.0001) with preterm male neonates. The mean duration of stay of preterm neonates was 3.5 ± 3.2 days (term babies: 1–2 days [P = 0.0085]). Of 1,565 medications prescribed to the 667 neonates, 67.5% were antibacterial, with gentamicin (53.0%) being the most prescribed. 98.4% of neonates were prescribed at least one medication (i.e., 67.5% were prescribed antibacterial medications, 14.6% supplements, 11.0% bronchodilators, and 7.0% antiseizure); mean medication combination 2.6 ± 0.8 per neonate. Majority (75.4%) of the cases reviewed had treatment success.
Conclusion:
Respiratory distress and preterm deliveries are predominant presenting conditions, with antibacterial medication, mainly gentamicin and ampicillin, on prescription. Treatment success is significantly high at the NICU.
KEYWORDS: Gentamicin, neonatal intensive care unit, neonates, preterm delivery, respiratory distress
INTRODUCTION
The neonatal period (≤28 days old) is a critical phase of human development with the newborn having to adjust considerably to various physiological states in its new environment.[1] This presents both the preterm and term neonates with a myriad of health conditions necessitating specialized intervention in intensive care units. Estimates from the United Nation Inter-agency Group for Child Mortality indicate that about 2.5 million of about 130 million newborns die within the first 28 days of their life with the majority of these preventable deaths occurring within the 1st week of life.[2] Considering the peculiarity of the neonate with respect to anatomical and physiological development, inapt use of medication could be detrimental with a spiraling effect as it grows.
Rational prescribing is, therefore, one of the means of reducing neonatal morbidity and mortality. This is important in achieving Goal 3 of the Sustainable Development Goals,[3] aimed, among other things, at improving the quality of life and survival of neonates.[4] Although prescribing medication to neonates in neonatal intensive care units (NICUs) worldwide is regulated by guidelines,[5] it is thought that half of the medications used in neonatal care facilities may have been prescribed and/or dispensed improperly. Due to limited report on medication prescription patterns in newborn units in Ghana, there is a paucity of data in this area of specialization. This study, therefore, was conducted to describe the patterns of medications prescribed to neonates admitted to the NICU of a tertiary health-care facility, Ghana.
METHODS
This study was conducted at a NICU of a tertiary health-care facility in Ghana. The facility is the only tertiary referral hospital in the northern part of Ghana and also serves as a training center for health-care institutions in that part of the country. It is the only health-care facility that provides advanced and specialized care to neonates. The facility has six clinical departments including the Paediatrics and Child Health Department, with the NICU being part of the Paediatrics and Child Health Department. The NICU is a 100-bed capacity unit with one specialist pediatrician, two medical officers, three pediatric nurses, and 37 general nurses and has all infrastructure necessary to run the unit.[6]
The study population comprised neonates (≤28 days), with complete medical history, admitted to the NICU from July 2017 to June 2018. Neonates with congenital abnormalities and those with incomplete medical records were excluded from the study. Ethical approval for the study was obtained from the Ethical Review Committee (ERC) of the Tamale Teaching Hospital, Tamale, Ghana (TTH/ERC/25/06/19/03). A thumbprinting consent to participate in the study was obtained from all mothers/guardians.
Folders of all neonates within the study period, i.e., 743, were purposively reviewed for possible enrollment in this study, of which 23 folders had congenital abnormalities, while 53 had incomplete information. In all, 667 folders met the inclusion criteria and were hence used in this study.
A retrospective descriptive cross-sectional study using a self-designed questionnaire was used to extract data from the medical folders of neonates who had been admitted to the NICU during the study period. Neonatal clinical records such as duration of hospitalization, gestational age, the axillary temperature on admission, clinical presentation, and diagnosis were extracted. Details of medicines that were prescribed and administered were categorized as antibiotics, supplements, bronchodilators, antiseizure, antipyretics, and others and were recorded. Routes of administration and treatment outcomes were also extracted.
Gestational age was defined as preterm if the baby was delivered before 37 completed weeks of gestation and term if the birth occurred ≥37 completed weeks of gestation as defined by the American College of Obstetricians and Gynecologists.[7] Birth weights of neonates were classified as low birth weight (LBW) (<2.5 kg), normal birth weight (2.5–4.0 kg), and high birth weight (>4.0 kg).[8] Mode of delivery was classified as spontaneous vaginal delivery (SVD) if there was the active onset of labor followed by active labor delivery and cesarean section if the baby was delivered through an incision in the abdominal wall and uterus of the mother.[9] Place of delivery of neonates was categorized as home if neonate was delivered in the house, primary if delivery occurred in a primary health center, secondary if it occurred at a secondary health facility, and tertiary if it occurred in a tertiary health-care facility. The axillary temperature of neonates on admission was classified as hypothermia (<36.5°C), normal (36.5–37.5°C), and hyperthermia (>37.5°C).[10] Fevers and sepsis were recategorized as pyrexia; neonatal distress and asphyxia were recategorized as respiratory distress; vomiting, swollen abdomen, distended abdomen, constipation, upper gastrointestinal obstruction, intussusception, omphalocele, and Hirschsprung's disease were recategorized as gastrointestinal disorders (GIDs) and clinical presentations; and queries recorded as cough, hypoglycemia, severe dehydration, right femoral fracture were recategorized as others. Queried clinical presentations, treatment patterns, and their outcomes were also recorded.
Data obtained were entered and analyzed using Statistical Package for the Social Sciences (SPSS) version 23 (IBM, Chicago, Illinois, USA). The mean and standard deviation was reported for continuous variables, and for categorical variables, the frequency and percentages were reported. Unpaired t-test and Chi-square were used to test the association between study variables where appropriate. Graphs were drawn using GraphPad Prism Version 7.00 (GraphPad Software, San Diego, California, USA). For all statistical tests, P ≤ 0.05 was set as the level of significance.
RESULTS
For the 667 neonates whose folders were reviewed, 412 (61.8%) were preterm, while 255 (38.2%) were term with the mean gestational age of all the neonates being 34.2 ± 3.6 weeks. Most (343 [51.4%]) of the neonates were male. The mean birth weight of all neonates was 2.1 ± 0.9 kg, but the majority (431 [64.6%]) were born with LBW. A greater number of these neonates (604 [90.6%]) were delivered through SVD with majority of these deliveries (383 [57.4%]) occurring at the tertiary health-care facility. The average axillary temperature on admission was 36.1 ± 1.5°C. LBW, hypothermia, and single queried medical conditions were found to be significantly associated (P ≤ 0.0001) with preterm male neonates. For preterm neonates, the mean duration on admission at the NICU was 3.5 ± 3.2 days, while for term babies, the period was 1–2 days (P = 0.0085) [ Table 1].
Table 1.
Spectrum of medications prescribed to neonates and their routes of administration (n=656)
| Variable | n (%) |
|---|---|
| Class of medication prescribed | |
| Antibacterial | 443 (67.5) |
| Supplements | 96 (14.6) |
| Bronchodilators | 72 (11.0) |
| Antiseizure medications | 24 (3.7) |
| Antipyretics | 1 (0.2) |
| Others | 20 (3.0) |
| Medication combinations prescribed per neonate | |
| 1 | 2 (0.3) |
| 2 | 333 (50.8) |
| 3 | 228 (34.7) |
| ≥4 | 93 (14.2) |
| Routes of administration | |
| Intravenous | 627 (95.6) |
| Intramuscular | 26 (3.9) |
| Rectal | 2 (0.3) |
| Oral | 1 (0.2) |
Of the 667 neonates, 467 (70.0%), 181 (27.1%), and 19 (2.9%) were queried with one, two, or three medical conditions, respectively. For neonates who were admitted with a single queried condition, majority, i.e., 196 (42.0%), presented with respiratory distress, followed by preterm 126 (27.0%) and pyrexia 91 (19.5%). The remaining queried conditions contributed <11.5% (54) to the total [Table 2].
Table 2.
Clinical characteristics of neonates
| Variables | Total (n=667), n (%) | Preterm (n=412), n (%) | Term (n=255), n (%) | P |
|---|---|---|---|---|
| Gender | 0.0072 | |||
| Male | 343 (51.4) | 195 (47.3) | 148 (58.0) | |
| Female | 324 (48.6) | 217 (52.7) | 107 (42.0) | |
| Birth weight (kg) | <0.0001 | |||
| Mean±SD | 2.1±0.9 | 1.7±0.8 | 2.7±0.7 | |
| LBW | 431 (64.6) | 333 (80.8) | 98 (38.4) | |
| NBW | 234 (35.1) | 79 (19.2) | 155 (60.8) | |
| HBW | 2 (0.3) | 0 | 2 (0.8) | |
| Mode of delivery | 0.6019 | |||
| SVD | 604 (90.6) | 375 (91.0) | 229 (89.8) | |
| Cesarean section | 63 (9.4) | 37 (9.0) | 26 (10.2) | |
| Place of delivery | 0.0731 | |||
| Home | 8 (1.2) | 4 (1.0) | 4 (1.6) | |
| 1° facility | 78 (11.7) | 55 (13.3) | 23 (9.0) | |
| 2° facility | 198 (29.7) | 131 (31.8) | 67 (36.3) | |
| 3° facility | 383 (57.4) | 222 (53.9) | 161 (63.1) | |
| Duration of stay (days) | 0.0085 | |||
| Mean±SD | 3.5±3.2 | 2.9±2.3 | 3.0±1.4 | |
| 1-3 | 483 (72.4) | 330 (80.1) | 183 (71.8) | |
| 4-6 | 130 (19.5) | 64 (15.5) | 64 (25.1) | |
| ≥7 | 54 (8.1) | 18 (4.4) | 8 (3.1) | |
| Axillary temperature (°C) | <0.0001 | |||
| Mean±SD | 36.1±1.5 | 35.7±1.5 | 36.6±1.4 | |
| Hypothermic | 350 (52.5) | 254 (61.7) | 96 (37.6) | |
| Normal | 250 (37.5) | 137 (33.2) | 113 (44.3) | |
| Hyperthermic | 67 (10.0) | 21 (5.1) | 46 (18.0) | |
| Number of queried medical conditions | 0.0080 | |||
| Mean±SD | 1.4±0.6 | 1.4±0.6 | 1.5±0.6 | |
| 1 | 403 (60.4) | 268 (65.0) | 135 (52.9) | |
| 2 | 235 (35.2) | 128 (31.1) | 107 (42.0) | |
| ≥3 | 29 (4.3) | 16 (3.9) | 13 (5.1) |
LBW=Low birth weight, NBW=Normal birth weight, HBW=High birth weight, SD=Standard deviation
Of a total of 1565 medications prescribed to the 667 neonates during the study period, 1055 (67.5%) were antibacterial, with gentamicin (559 [53.0%]) being the most prescribed. Out of the total number of 667 neonates whose records were reviewed, 656 (98.4%) were prescribed at least one medication, of these 443 (67.5%) were prescribed antibacterial, 96 (14.6%) prescribed supplements, and 72 (11.0%) bronchodilators. Approximately 55 (7.0%) of the neonates were prescribed antiseizure medications, antipyretic, and medications classified as others. The mean medication combination prescribed per neonate was 2.6 ± 0.8 medications. While only 2 (0.3%) neonates were prescribed to a one-medication, 333 (50.8%) were prescribed to a combination of a two-medication, 228 (34.7%) to a three-medication, and 93 (14.2%) to four- or more-medication regimen, with the mean number of medications prescribed to a neonate being 2.6 ± 0.8 medications. Most of the medications prescribed to the neonates were administered intravenously [Table 1]. Of 443 neonates who were prescribed antibacterial, 235 (53.0%) were prescribed gentamicin, while the remaining 178 (40.1%) were prescribed ampicillin. Ceftriaxone, flucloxacillin, metronidazole, and cefuroxime contributed 6.9% (30) of the total number of antibacterial prescribed [Figure 1].
Figure 1.
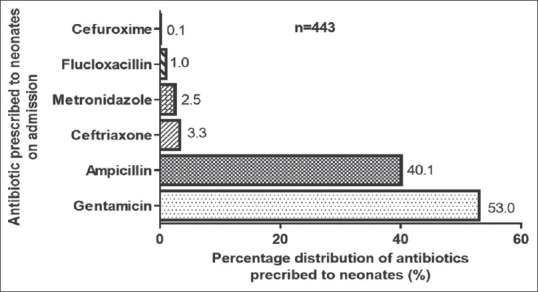
Commonly prescribed antibacterial medications to neonates at the neonatal intensive care unit of the tertiary health-care facility
Of the 667 neonates whose medical records were reviewed, 467 were queried with a single medical condition on admission. Of these, majority, i.e., 196 (42.0%), presented with conditions recategorized as respiratory distress, 91 (19.5%) with pyrexia, 126 (27) admitted as preterm, 20 (4.3%) with GID, 17 (3.6%) with conditions reclassified as others, 15 (3.2%) with neonatal jaundice, and 2 (0.4%) as seizures [Figure 2].
Figure 2.
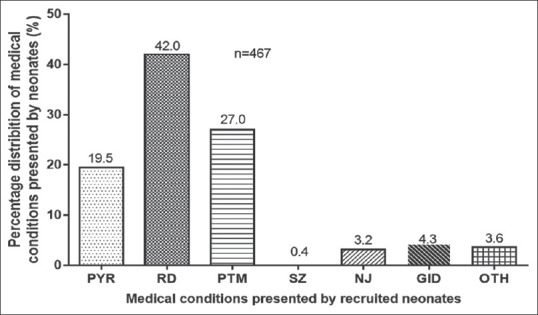
Distribution of single queried medical conditions in neonates. PYR = Pyrexia, RD = Respiratory distress, PTM = Preterm, SZ = Seizures, NJ = Neonatal jaundice, GID = Gastrointestinal disorders, OTH = Others
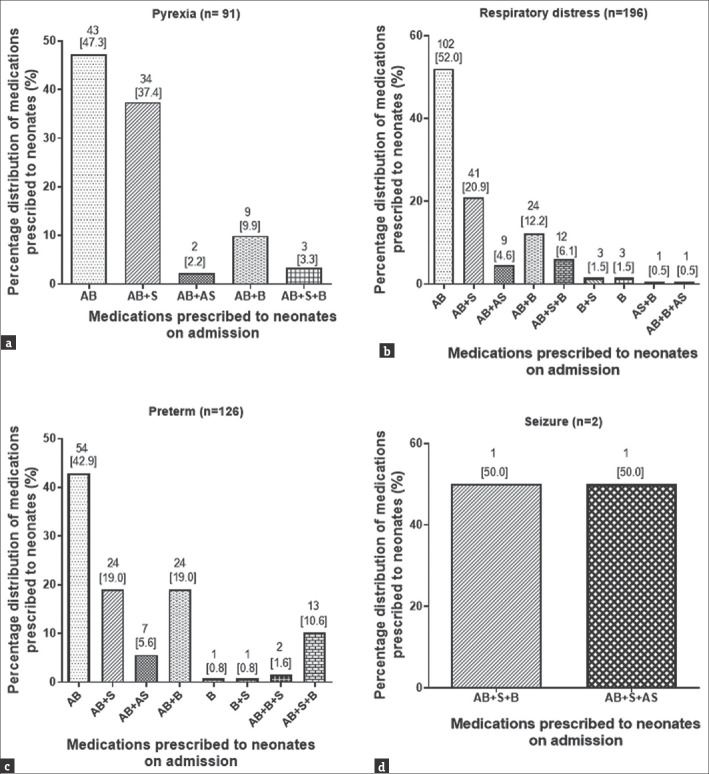
For neonates queried with pyrexia, 43 (47.3%) were treated with only antibacterial, while 34 (37.4%) were treated with a combination of antibacterial and supplements (e.g., Vitamin K). The remaining were treated with a combination of antibacterial, bronchodilators (e.g., aminophylline), antiseizure medications, or a supplement [Figure 3a]. For neonates (196/467) who were queried with only respiratory distress on admission, 102 (52.0%) were treated with only antibacterial, 41 (20.9%) treated with a combination of antibacterial and supplement (Vitamin K), 24 (12.2%) with an antibacterial and bronchodilator (aminophylline), and 12 (6.1%) with a combination of an antibacterial, a supplement (Vitamin K), and a bronchodilator (aminophylline) [Figure 3b].
Figure 3.
Medication combination regimen prescribed for pyrexia (a), respiratory distress (b), preterm birth (c) and siezure (d) presented at the neonatal intensive care unit. AB = Antibacterial alone, AB + S = Antibacterial and supplement, AB + AS = Antibacterial and antiseizure, AB + B = Antibacterial and bronchodilator, B = Bronchodilator alone, B + S = Bronchodilator and supplement, AB + S + B = Antibacterial and supplement and bronchodilator, AB + B + AS = Antibacterial and bronchodilator and antiseizure, AB + AP = Antibacterial and antipyretic
Of the neonates who were admitted as preterm, 54 (42.9%) were treated with only an antibacterial, 24 (19.0%) treated with both an antibacterial and supplement (Vitamin K), 24 (19.0%) treated with both an antibacterial and bronchodilator (aminophylline), 13 (10.3%) with a combination of an antibacterial, supplement, and bronchodilator, and 7 (5.6%) treated with a combination of antibacterial and an antiseizure [Figure 3c and d]. For neonates who were queried and admitted as having a single diagnosis of neonatal jaundice, 8 (53.3%) were prescribed antibacterial with 4 (26.7%) being prescribed a regimen of an antibacterial and a bronchodilator with 13.3% (2) and 1 (6.7%) being prescribed a medication combination of an antibacterial + supplement and an antibacterial + antipyretic, respectively. Majority, i.e., 11/20 (55.0%), of neonates who presented with conditions classified as GIDs were prescribed an antibacterial, with 10/17 (58.8%) of neonates who were queried with conditions categorized as others being prescribed antibacterial [Figure 4a and b]. The most prescribed antibacterial was gentamicin followed by ampicillin [Figure 4c].
Figure 4.
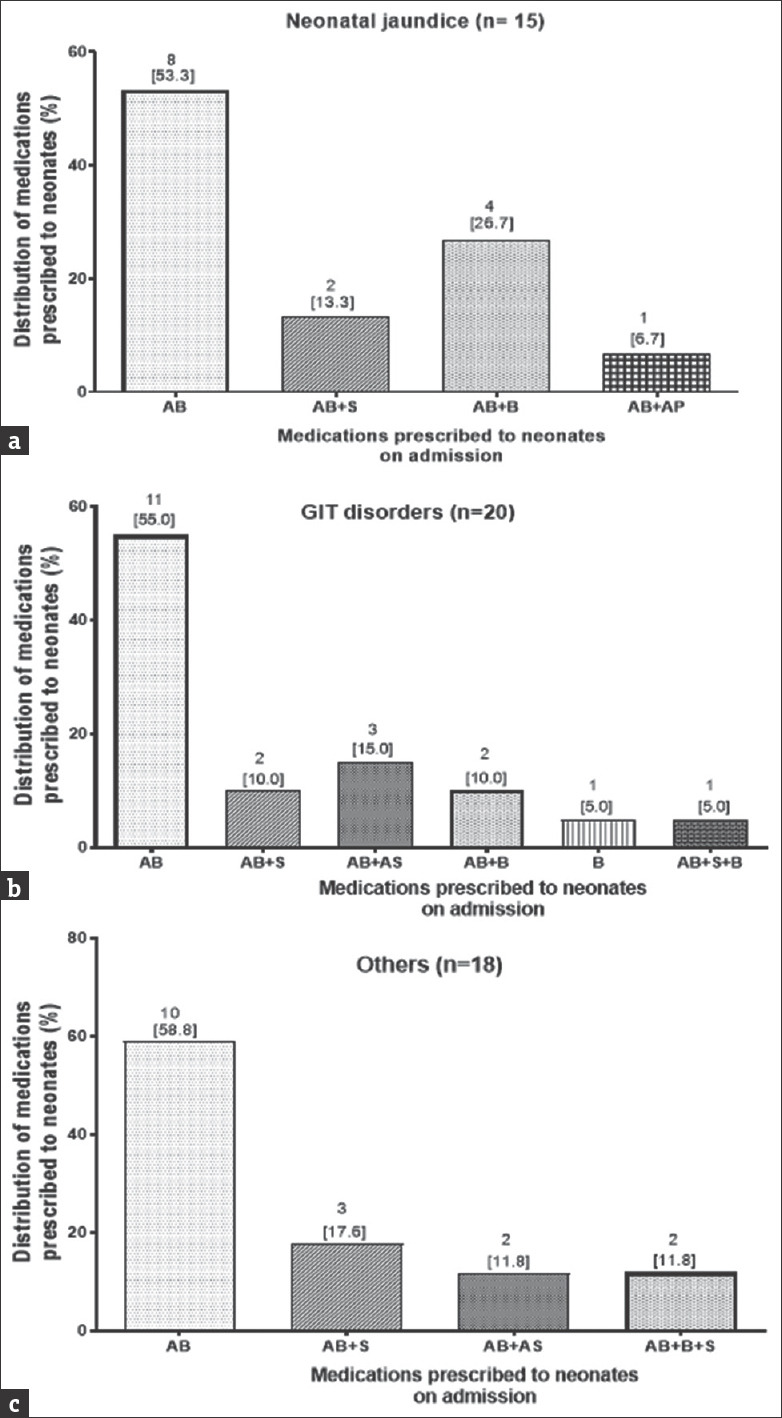
Medication combination regimen prescribed for neonatal jaundice (a), GIT disorders (b), as well as other conditions (c) presented at the neonatal intensive care unit. AB = Antibacterial alone, AB + S =Antibacterial and supplement, AB + AS = Antibacterial and antiseizure, AB + B = Antibacterial and bronchodilator, B = Bronchodilator alone, B + S = Bronchodilator and supplement, AB + S + B = Antibacterial and supplement and bronchodilator, AB + B + AS = Antibacterial and bronchodilator and antiseizure, AB + AP = Antibacterial and antipyretic
Majority (510 [75.4%]) of the cases were treated successfully; while the remaining 157 (23.5%) ended up in mortality.
DISCUSSION
The prescription patterns of medication usage and the high exposure of neonates to medications have not been well documented in Ghana, and without the regular description of such trends in medication usage among neonatal patients, discrepancies in the treatment regimen may not be noticed.
Multiple medication usage, often referred to as polypharmacy, is a common practice in neonatal population presenting with multiple morbidities.[11] Findings from this study point to the extensive practice of polypharmacy among the study participants. The practice of polypharmacy at NICU has been reported by Fadare et al. in Nigeria[12] and Suryawanshi et al. in India.[13] Polypharmacy in neonates is beneficial in multiple disease states or complex disease conditions. However it may lead to drug-drug interactions that are associated with adverse drug reactions which could overburden the healthcare system.[14,15] Bakaki et al. emphasized the greater challenge faced by neonates who are exposed to such polypharmacy and this is further worsened when prescribed to in-patients.[16]
This study provides evidence to support the empirical treatment of sick neonates with antibacterial at admission as recommended by the WHO established guidelines.[17] The prescription of antibacterial for neonates admitted to NICUs [Figure 1] was congruent with previous studies among neonates in similar Ghanaian settings as documented by Acquah et al.[18] in Tamale and Labi et al. in Accra.[19] This empirical use of antibacterial is mostly justified in neonates because of an immature immunological apparatus and also because they present with nonspecific fever and are at a higher risk of sepsis with associated increased risk of complication. In most cases, they receive these antibacterial medications before any laboratory confirmation of culture samples.[20,21]
The high prescription rate of gentamicin (aminoglycoside) to neonates seen in this study was consistent with earlier findings by Aku et al. in Ghana[22] and Mabila et al. in South Africa.[23] Gentamicin has high antimicrobial efficacy and with low cost and thus making it a popular medication of choice for clinicians.[24] Despite its wide use and availability, it is known to cause ototoxicity and a common cause of bilateral vestibulopathy.[25,26]
The findings from this study in which cases of neonatal respiratory distress and preterm delivery with its associated complications [Figures 2 and 3b, c] are presented to NICUs with antibacterial prescription being essential in their management were consistent with Fadare et al.'s findings, 2015, in Nigeria,[12] and also with Girardi et al.'s reports, 2017, on medication prescription among newborns in an Italian setup.[27] The prescription of antibacterial medications such as the aminoglycosides and beta-lactam penicillin in treating neonates has been widely reported in clinical practice and consistent with the WHO guidelines on the management of neonates.[12,28] The use of regimens that combine antibiotics and supplements [Figure 4] for care in NICU in various jurisdictions has been reported earlier[29,30] and was confirmed in this study. These medications have broad-spectrum activity, readily available, and less expensive. Although the high exposure of neonates to multiple medications, including antibiotics may come with treatment success, it may also lead to long term damage to organs and organ systems such as the renal system.[27]
Respiratory distress and preterm deliveries are predominant presenting conditions, with antibacterial, mainly gentamicin and ampicillin, on prescription. Treatment success is significantly high. On the basis of these findings, it is recommended that a facility-specific predictive model be developed to identifying possible risk factors for prompt attention to improve neonatal treatment outcomes. The current study has provided a clear overview of prescription patterns among neonates in a tertiary health of a middle-income country. The high prescription of antibiotics to otherwise neonates with nonbacterial infection should be a major concern for neonatal care givers. Policies and practices that resulted in the relatively good treatment success rate at the facility should be strengthened.
AUTHORS’ CONTRIBUTION
Kwame Opare-Asamoah and George Asumeng Koffuor brought up the concept and definition of intellectual content. Kwame Opare-Asamoah, George Asumeng Koffuor, Alhassan Abdul-Mumin, Baba Mohammed Sulemana, Majeed F. Saeed, and Lawrence Quaye designed the study. Kwame Opare-Asamoah did the literature search. All authors were involved with the clinical studies and data acquisition. Data analysis was done by Kwame Opare-Asamoah and George Asumeng Koffuor. All authors were involved with statistical analysis of data, manuscript preparation, manuscript editing, and manuscript review.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the valuable contribution of staff of the Neonatal Intensive care Unit, Tamale Teaching Hospital.
REFERENCES
- 1.Mesek I, Nellis G, Lass J, Metsvaht T, Varendi H, Visk H, et al. prescription pattern in European neonatal units. Int. J. Clin. Pharm. 2019;41:1578–91. doi: 10.1007/s11096-019-00923-2. [DOI] [PubMed] [Google Scholar]
- 2.Hug L, Alexander M, You D, Alkema L. UN Inter-agency Group for Child Mortality Estimation. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: A systematic analysis. Lancet Glob Health. 2019;7:e710–20. doi: 10.1016/S2214-109X(19)30163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gbagbo FY, Nkrumah J. Implications of self-medication in pregnancy for Safe Motherhood and Sustainable Development Goal-3 in selected Ghanaian communities. Public Health Pract. 2020;1:1–6. doi: 10.1016/j.puhip.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golding N, Burstein R, Longbottom J, Browne AJ, Fullman N, Osgood-Zimmerman A, et al. Mapping under-5 and neonatal mortality in Africa, 2000-15: A baseline analysis for the sustainable development goals. Lancet. 2017;390:2171–82. doi: 10.1016/S0140-6736(17)31758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathish D, Bahini S, Sivakumar T, Thiranagama T, Abarajithan T, Wijerathne B, et al. Drug utilization, prescription errors and potential drug-drug interactions: An experience in rural Sri Lanka. BMC Pharmacol Toxicol. 2016;17:27. doi: 10.1186/s40360-016-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.THF. Tertiary Health Facility Annual Report. 2018 [Google Scholar]
- 7.ACOG Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol. 2013;122:1139–40. doi: 10.1097/01.AOG.0000437385.88715.4a. [DOI] [PubMed] [Google Scholar]
- 8.Poon LC, Tan MY, Yerlikaya G, Syngelaki A, Nicolaides KH. Birth weight in live births and stillbirths. Ultrasound Obstet Gynecol. 2016;48:602–6. doi: 10.1002/uog.17287. [DOI] [PubMed] [Google Scholar]
- 9.Barbara G, Pifarotti P, Facchin F, Cortinovis I, Dridi D, Ronchetti C, et al. Impact of mode of delivery on female postpartum sexual functioning: Spontaneous vaginal delivery and operative vaginal delivery vs.cesarean section. J Sex Med. 2016;13:393–401. doi: 10.1016/j.jsxm.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien EA, Colaizy TT, Brumbaugh JE, Cress GA, Johnson KJ, Klein JM, et al. Body temperatures of very low birth weight infants on admission to a neonatal intensive care unit. J Matern Fetal Neonatal Med. 2019;32:2763–6. doi: 10.1080/14767058.2018.1446076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy.A systematic review of definitions? BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadare J, Olatunya O, Oluwayemi O, Ogundare O. Drug prescribing pattern for under-fives in a paediatric clinic in South-Western Nigeria. Ethiop J Health Sci. 2015;25:73–8. doi: 10.4314/ejhs.v25i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suryawanshi S, Pandit V, Suryawanshi P, Panditrao A. Antibiotic prescribing pattern in a tertiary level neonatal intensive care unit. J Clin Diagn Res. 2015;9:FC21–4. doi: 10.7860/JCDR/2015/14764.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children's hospitals. Pediatrics. 2015;135:e99–108. doi: 10.1542/peds.2014-2015. [DOI] [PubMed] [Google Scholar]
- 15.Rashed AN, Wong IC, Cranswick N, Tomlin S, Rascher W, Neubert A. Risk factors associated with adverse drug reactions in hospitalised children: International multicentre study. Eur J Clin Pharmacol. 2012;68:801–10. doi: 10.1007/s00228-011-1183-4. [DOI] [PubMed] [Google Scholar]
- 16.Bakaki PM, Horace A, Dawson N, Winterstein A, Waldron J, Staley J, et al. Defining pediatric polypharmacy: A scoping review. PLoS One. 2018;13:e0208047. doi: 10.1371/journal.pone.0208047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatr Infect Dis J. 2016;35:e191–8. doi: 10.1097/INF.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 18.Acquah SE, Quaye L, Sagoe K, Ziem JB, Bromberger PI, Amponsem AA. Susceptibility of bacterial etiological agents to commonly-used antimicrobial agents in children with sepsis at the Tamale Teaching Hospital. BMC Infect Dis. 2013;13:89. doi: 10.1186/1471-2334-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labi AK, Obeng-Nkrumah N, Bjerrum S, Enweronu-Laryea C, Newman MJ. Neonatal bloodstream infections in a Ghanaian Tertiary Hospital: Are the current antibiotic recommendations adequate? BMC Infect Dis. 2016;16:598. doi: 10.1186/s12879-016-1913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman EA, McCulloh RJ, Myers AL, Aronson PL, Neuman MI, Bradford MC, et al. Empiric Antibiotic Use and Susceptibility in Infants With Bacterial Infections: A Multicenter Retrospective Cohort Study. Hosp Pediatr. 2017;7:427–35. doi: 10.1542/hpeds.2016-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzialla C, Borghesi A, Serra G, Stronati M, Corsello G. Antimicrobial therapy in neonatal intensive care unit. Ital J Pediatr. 2015;41:27. doi: 10.1186/s13052-015-0117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aku FY, Akweongo P, Nyarko K, Sackey S, Wurapa F, Afari EA, et al. Bacteriological profile and antibiotic susceptibility pattern of common isolates of neonatal sepsis, Ho Municipality, Ghana-2016. Matern Health Neonatol Perinatol. 2018;4:2. doi: 10.1186/s40748-017-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabila N, Schellack N, Gous A. Antibiotic prescribing patterns among healthcare professionals at Van Velden Memorial Hospital in Tzaneen, Limpopo Province, South Africa. AJPHES. 2016;22:79–97. [Google Scholar]
- 24.Kushner B, Allen PD, Crane BT. Frequency and demographics of gentamicin use. Otol Neurotol. 2016;37:190–5. doi: 10.1097/MAO.0000000000000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Barbary MN, Ismail RI, Ibrahim AA. Gentamicin extended interval regimen and ototoxicity in neonates. Int J Pediatr Otorhinolaryngol. 2015;79:1294–8. doi: 10.1016/j.ijporl.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 26.Aladag I, Guven M, Songu M. Prevention of gentamicin ototoxicity with N-acetylcysteine and Vitamin A. J Laryngol Otol. 2016;130:440–6. doi: 10.1017/S0022215116000992. [DOI] [PubMed] [Google Scholar]
- 27.Girardi A, Galletti S, Raschi E, Koci A, Poluzzi E, Faldella G, et al. Pattern of drug use among preterm neonates: Results from an Italian neonatal intensive care unit. Ital J Pediatr. 2017;43:37. doi: 10.1186/s13052-017-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Bowman C, Alladin-Karan B, Singh N. Antibiotic prescribing patterns in the pediatric emergency department at Georgetown Public Hospital Corporation: A retrospective chart review. BMC Infect Dis. 2016;16:170. doi: 10.1186/s12879-016-1512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumari A, Prasad PL. Drug utilization pattern in neonatal intensive care unit of a tertiary care hospital with particular emphasis on off-label drug use. J Clin Neonatol. 2019;8:15. [Google Scholar]
- 30.Obiero CW, Seale AC, Berkley JA. Empiric treatment of neonatal sepsis in developing countries. Pediatr Infect Dis J. 2015;34:659–61. doi: 10.1097/INF.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]