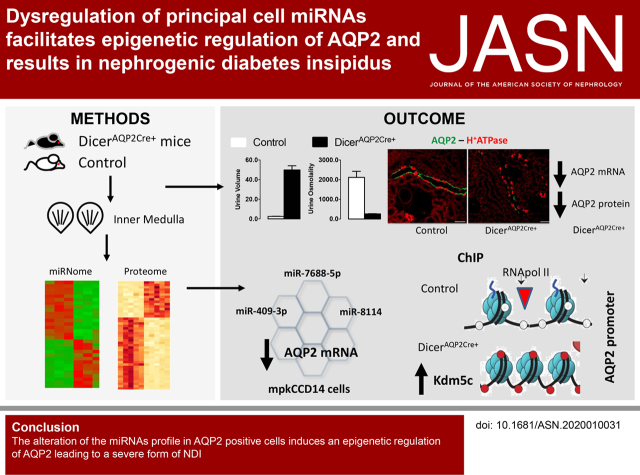
Significance Statement
Water reabsorption along the collecting duct is dependent on the function of aquaporin 2 (AQP2). Currently, information on microRNA (miRNA)-mediated, post-transcriptional regulation of AQP2, which may influence water reabsorption, is limited. In mice, ablation of the Dicer enzyme (crucial for miRNA maturation) in AQP2-expressing cells induces nephrogenic diabetes insipidus (NDI) with dysregulation of the miRNA profile. A major finding is the identification of miRNAs associated with NDI through mediating epigenetic control of AQP2. This study offers novel targets for AQP2 regulation and potential treatment for governing renal water reabsorption.
Keywords: AQP2, Dicer, miRNA, ENaC, DNA methylation, epigenetics
Visual Abstract
Abstract
Background
MicroRNAs (miRNAs), formed by cleavage of pre-microRNA by the endoribonuclease Dicer, are critical modulators of cell function by post-transcriptionally regulating gene expression.
Methods
Selective ablation of Dicer in AQP2-expressing cells (DicerAQP2Cre+ mice) was used to investigate the role of miRNAs in the kidney collecting duct of mice.
Results
The mice had severe polyuria and nephrogenic diabetes insipidus, potentially due to greatly reduced AQP2 and AQP4 levels. Although epithelial sodium channel levels were decreased in cortex and increased in inner medulla, amiloride-sensitive sodium reabsorption was equivalent in DicerAQP2Cre+ mice and controls. Small-RNA sequencing and proteomic analysis revealed 31 and 178 significantly regulated miRNAs and proteins, respectively. Integrated bioinformatic analysis of the miRNAome and proteome suggested alterations in the epigenetic machinery and various transcription factors regulating AQP2 expression in DicerAQP2Cre+ mice. The expression profile and function of three miRNAs (miR-7688-5p, miR-8114, and miR-409-3p) whose predicted targets were involved in epigenetic control (Phf2, Kdm5c, and Kdm4a) or transcriptional regulation (GATA3, GATA2, and ELF3) of AQP2 were validated. Luciferase assays could not demonstrate direct interaction of AQP2 or the three potential transcription factors with miR-7688-5p, miR-8114, and miR-409–3p. However, transfection of respective miRNA mimics reduced AQP2 expression. Chromatin immunoprecipitation assays demonstrated decreased Phf2 and significantly increased Kdm5c interactions at the Aqp2 gene promoter in DicerAQP2Cre+ mice, resulting in decreased RNA Pol II association.
Conclusions
Novel evidence indicates miRNA-mediated epigenetic regulation of AQP2 expression.
Dependent on hydration status, the kidney collecting duct (CD) is the site of reabsorption of approximately 10% of the water filtered by the glomerulus. Arginine vasopressin (AVP) is the main physiologic signal promoting water reabsorption at this site. In CD principal cells (PCs), AVP’s actions on the type 2 AVP receptor (V2R) stimulate gene expression of aquaporin 2 (AQP2) (increasing AQP2 mRNA abundance),1 increase AQP2 levels on the apical membrane,2 and reduce AQP2 degradation.3 Together, these processes maximize AQP2-mediated water reabsorption into the hyperosmotic interstitium. Although several translational and post-translational mechanisms4 regulating this process have been identified, there is limited information on post-transcriptional mechanisms that may influence CD water reabsorption.
MicroRNAs (miRNAs) are small, noncoding, endogenous RNA molecules that are able to predominantly regulate gene expression at a post-transcriptional level. In the nucleus, the microprocessor complex, consisting of Drosha (ribonuclease III enzyme) and Dgcr8 (double-stranded RNA binding protein), cleaves primary miRNA substrates to pre-miRNA. In the cytoplasm, pre-miRNAs are processed to mature miRNAs by the enzyme Dicer. The canonic function of miRNAs is mediated by the RISC complex, which helps binding of miRNAs to a complementary sequence in the untranslated region (UTR) of their target mRNA. This reduces the mRNA t 1/2 or promotes translational repression.5–8 There is also evidence that some miRNAs can regulate gene expression at the epigenetic level, either by controlling DNA methylation or other targets of the epigenetic machinery, the so-called epi-miRNAs.9 Such a process was shown to modulate podocyte damage in the development of diabetic nephropathy.10,11
miRNAs are crucial for the function of several nephron segments.12 We have previously observed that epithelial cell–specific deletion of Dicer in the renal tubule leads to severe polyuria and progressive renal failure in mice.13 Similarly, mice with renal epithelial cell–specific deletion of Dgcr8 suffer from hydronephrosis.14 These findings suggest the integrity of the miRNA system, independent of which step of miRNA maturation is experimentally arrested, is crucial for the function of the renal tubule and renal water reabsorption. However, the role of miRNAs specifically in PCs is unknown. By chromatin immunoprecipitation (ChIP) assay, 19 major miRNAs regulated by 1-desamino-8-D-arginine-vasopressin (dDAVP; an AVP analogue) were identified in rat inner medullary CD (IMCD) tubule suspensions,15 but no direct interaction with AQP2 was detected. Recently, two enhancer regions responsive to the transcription factor C/EBPβ were identified as master regulators of AQP2 gene expression in mpkCCD cells.16 AQP2 gene expression is strongly dependent on protein kinase A (PKA) activity. Using a multiomics approach, a complex network of PKA targets has been described to act transcriptionally and post-transcriptionally to control AQP2 gene expression.17 On the basis of this evidence, we hypothesized miRNAs could modulate renal water reabsorption via genetic and/or epigenetic regulation of the AQP2 gene. In this study, we selectively suppressed Dicer expression in AQP2-positive cells of the mouse CD (DicerAQP2Cre+ mice) to assess potential roles of miRNAs in this cell type. The major finding is that DicerAQP2Cre+ mice have severely reduced AQP2 function and nephrogenic diabetes insipidus (NDI), a likely consequence of miRNA-mediated epigenetic regulation of AQP2.
Methods
Further details are provided in Supplemental Appendix 1.
Animal Experiments
Targeted Inactivation of Dicer1 in the CD
Ablation of the Dicer1 gene in the CD was performed by mating AQP2Cre/+ mice, expressing CRE under the control of the endogenous AQP2 promoter,18 with Dicer1flox/+ mice, in which exons 22–23 of the Dicer1 gene were flanked by loxP sites.19 Dicer1flox/flox; AQP2Cre/+ mice, termed DicerAQP2Cre+, were used as the experimental group, whereas littermate mice with no CRE expression, DicerAQP2Cre− mice, served as controls. Genotyping and confirmation of Dicer excision were performed by PCR analysis using genomic DNA isolated from ear snips and renal tissue. All primers are listed in Supplemental Table 1.
Physiologic Studies
All the procedures involving animals were conducted as indicated in the Italian National Guidelines (D.L. N° 116 G.U., suppl. 40, 18.2.1992, circ. N° 8, G.U. July 1994) and in the appropriate European Directives (EEC Council Directive 86/609, 1.12.1987) under an approved animal license (ID n 547/2017-PR).
dDAVP Administration Test
To investigate their urinary concentrating ability, mice were challenged with the AVP analogue dDAVP (Sigma Aldrich, St. Louis, MO) as described previously.20 Briefly, after voiding the bladder on a cold plate, an intraperitoneal injection of vehicle (0.9% sodium chloride) or dDAVP (1 μg/kg of body wt) dissolved in an equal volume of vehicle was administered. Urine volume and osmolality were evaluated after 5 hours of urine collection in metabolic cages.
Amiloride Administration Test
Mice were housed individually in metabolic cages for 5 days and fed a salt-restricted diet. To assess epithelial sodium channel (ENaC)–dependent sodium reabsorption, mice were challenged with the selective inhibitor of ENaC, amiloride.21 Vehicle or 1.45 mg/kg body wt amiloride hydrochloride (Sigma Aldrich) was injected intraperitoneally for two consecutive days and urine was collected after 6 hours.
cAMP Detection in Urine and Inner Medulla
Tissue and urinary cAMP content was measured using an ELISA (#581001; Cayman Chemical, Ann Arbor, MI). The inner medulla (IM) was homogenized in 5% TCA22 in water and then centrifuged at 1500 × g for 10 minutes for removal of debris. TCA was removed using water-saturated ether and by heating the samples at 70°C for 5 minutes. Total protein concentration was measured using a BCA assay, and cAMP levels were expressed as picomole per milligram of protein. Urine samples were centrifuged for 5 minutes at 2300 × g, diluted in enzyme immunoassay buffer, and assayed directly following the manufacturers’ instructions. cAMP content was expressed in nanomole per milligram of urinary creatinine.
Small-RNA Sequencing and Data Processing
Total RNA was extracted from IM (three control and three DicerAQP2Cre+ mice) with the RNeasy Micro Kit (#74004; Qiagen, Milan, Italy) according to the manufacturer’s protocol. The RNA concentration was assessed by NanoDrop, Qubit, and a Bioanalyzer. The library was generated from 1 µg of RNA using a TruSeq small-RNA protocol (#200-0036; Illumina, San Diego, CA), which was sequenced to obtain 50 bp single reads on the Illumina HiSeq2500 Platform, with 20 million reads per sample. Data quality was checked using FASTQC version 0.11.3 (https://github.com/golharam/FastQC). Data were processed using the iMir tool (https://tools4mirs.org/software/isomirs_identification/imir/) to remove the adapter sequence, and reads with at least 15 nucleotides were retained and mapped to the mouse genome (build mm9) to identify miRNAs according to miRBase version 21 (http://www.mirbase.org/) annotations as previously shown (Supplemental Figure 1).23 Data normalization and differential expression analysis were performed using the DESeq2 R package, removing features with less than three read counts per sample. The raw data of the small-RNA sequencing have been added to the Gene Expression Omnibus database (accession GSE161006) and are accessible at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE161006.
Proteomic Analysis
Dissected mouse IM tissue from both control and DicerAQP2Cre+ mice were processed for liquid chromatography–tandem mass spectrometry analysis. Tandem mass tag labeling, randomly assigned between the two sample sets (as described24), was used to facilitate protein quantification. Mass spectrometry was carried out on a Tribrid Fusion mass spectrometer (Thermo Scientific), using the MS3 collection to allow accurate protein quantification.25 Data were searched using SEQUEST against the UniProt mouse protein database (dated March 25, 2020) and quantified using Proteome Discoverer 2.4. Protein quantification was only based on unique peptides. Proteins were considered significantly regulated when they passed the Benjamini–Hochberg false discovery rate (FDR) of 0.05. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE26 partner repository with the dataset identifier PXD022327.
Bioinformatics
Differentially expressed miRNAs (DicerAQP2Cre+ versus control, FDR-corrected Wald test P value ≤0.05 and expression absolute fold change of two or greater) were assessed by Ingenuity Pathway Analysis software (Qiagen Bioinformatics, Redwood City, CA) to identify potential regulators, relationships, mechanisms, functions, and pathways relevant to the observed changes (an adjusted P≤0.05). TargetScan Mouse 7.1 and mirBase version 21 were used for target prediction and identification of miRNA sequence, respectively. The identification of transcription-factor binding sites was performed by in silico computational analysis of the 1000-bp, 5′-flanking region of the AQP2 gene using the MatInspector software tool (Genomatix database, https://www.genomix4life.com/it/).27 The miRNA/protein expression scatter graph was generated in R by plotting, for each miRNA/target pair, the expression fold change of miRNA (y axis) and the corresponding target protein identified by the proteomic analysis (x axis). All miRNas with absolute log2 ratio ≥0.6 were used as inputs. The predicted target proteins of these miRNAs (using TargetScan, Ingenuity Expert Findings, miRecords, and Tarbase) were compared with the entire proteome. Common identifiers found in the predicted and “real” lists were plotted according their expression fold change. The dots falling in the top/left and in the bottom/right sections of the graph represented the miRNA-up/protein-down (green) and the miRNA-down/protein-up pairs (red), respectively. The cutoff threshold used for up- and downregulated miRNAs and proteins was absolute log2 ratio ≥0.6 and FDR≤0.05.
miRNA-Mimic Transfection in mpkCCD14 Cells
Three miRNAs (miR-7688-5p, miR-8114, and miR-409-3p) were selected for in vitro validation of the software-based prediction on the basis of their predicted targets being involved both in transcription-factor and epigenetic regulation (Supplemental Figure 2). mpkCCD14 cells (subclone 11)27 were routinely cultured as described previously.28 Cells were seeded at a density of 2×105 cells/cm2 on semipermeable filters (0.4-µm pore size; Transwell #3450; Corning). When 80% confluent, they were transfected with 10 nM miRNA mimics (mirVana miRNA mimics; mmu-miR-7688-5p, mmu-miR-8114, mmu-miR-409-3p) and Lipofectamine 2000 (#11668019, Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Transfection efficiency was evaluated by testing different concentrations of lipofectamine and a Cy5-conjugated miRNA-409 (Supplemental Figure 3).29 Cells were cultured for an additional 7 days before incubation in serum-free media for 24 hours, and then for an additional 24 hours with 1 nM dDAVP.15 Total RNA was isolated using an Ambion Ribopure kit (#10107824; Invitrogen). miRNA-mimics expression was evaluated at the end of the experiment.
Constructs and Luciferase Assay
The 3′-UTR regions of AQP2, GATA2, and GATA3 were amplified by PCR from mouse genomic DNA using the following primers: AQP2 forward, AATTTCTAGAGTCGGTTCCCAGTGCAGG; AQP2 reverse, AATTTCTAGAGAAACACGCAGA- GATGGACG; GATA2 forward, ATTTCTAGACCCGCATAAGAGAAGAATCG; GATA2 reverse, AATT- TCTAGAGGGTAGCATACAATTTTTACAGACAA; GATA3 forward, AATTTCTAGACAGGGTCT- CTAGTGCTGTGAAA; and GATA3 reverse, AATTTCTAGAGGCCTAGCCATGACATTCTC. PCR products were cloned downstream of the luc+ gene in the pGL3-control vector (#E1741; Promega Corporation, Milan, Italy) using XbaI. All plasmids were sequenced for confirmation. HEK293 cells in 24-well plates were transiently transfected with the plasmids with siPORT (#AM4510; Ambion Life Technologies, Paisley, United Kingdom) in the presence of mature miRNA-7688-5p, miRNA-8114, and miRNA-409-3p mimics (final concentration of 100 nM) and assessed after 48 hours. To generate the AQP2 promoter construct, an 840-bp fragment containing the mouse AQP2 promoter was amplified by PCR using the primers: AQP2 promoter forward, GTACTAGGTACCTCATGTACACAGGCAGAGCA; and AQP2 promoter reverse, GTACTAGCTAGCCGGAGAGGCTAGACTGTGG. This fragment was cloned into the pGL3-basic vector (#E1751; Promega Corporation) between KpnI and NheI restriction sites, upstream of the luc+ gene. The promoter-containing plasmid was transiently transfected into HEK293 cells alongside the miRNA mimics. Luciferase activity was assayed with a dual luciferase assay system (#E1500; Promega Corporation) as described in the manufacturer’s instructions. Briefly, the activities of Firefly and Renilla luciferases were measured in sequence from each sample and expressed as the Firefly/Renilla ratio. In case of miRNA binding to the 3′ UTR of the putative target gene, a reduction of Firefly luciferase activity (due to the instability of the fused mRNA) compared with the control sample (nontargeting, scrambled oligonucleotide transfection) is detected. In this way, the Firefly luciferase activity serves as a positive control of the miRNA to mRNA interaction. Luminescence was measured for 10 seconds using a 2103 EnVision Multilabel Plate Reader (PerkinElmer).
Quantitative ChIP Analysis
For the protein-DNA binding analysis, mouse IMs were crosslinked as previously described.30 ChIP assays were performed using the ChromaFlash High-Sensitivity ChIP Kit (Epigentek Group Inc., Farmingdale, NY). Antibodies used for protein-DNA immunoprecipitation were: anti-GRC5/PHF2 (#Ab124434; Abcam Inc.) and anti-KDM5C5c (#Ab194288; Abcam Inc.). Nonimmune IgG and anti–RNA polymerase II were used as negative and positive control antibodies, respectively. DNA was subjected to real-time quantitative PCR (RT-qPCR) using iQ SYBR Green PCR Supermix (Bio-Rad). Amplification of the Aqp2 promoter fragment was performed using the following primers: forward AQP2ChF (position from nucleotides −71 to −88), 5′-CACAGGGTTGGCAGGAAC-3′; and reverse AQP2ChR (position from nucleotides −29 to −49), 5′- GGCCTTCCTATCGTAGACCTG-3′. The following primers, distal from the Aqp2 transcription start site, were used in RT-qPCR as a negative control of binding: forward AQP2NCF (position from nucleotides −2725 to −2753), 5′- AAAGCAAACACGGGAGGAT-3′; and reverse AQP2NCR (position from nucleotides −2562 to −2587), 5′- CTTCATGCCAGGGAAGCA-3′. All RT-qPCR signals from immunoprecipitated DNA were normalized to RT-qPCR signals from nonimmunoprecipitated input DNA.
Label-Free Multiphoton Microscopy for Evaluation of Fibrosis
Unstained, paraffin-embedded, 4-μm-thick sections were used. Two-photon images were recorded using an upright Ultima Investigator two-photon microscope (Bruker, Billerica, MS) equipped with a Ti-Sapphire laser (Mai Tai DeepSee, Spectra-Physics) and a 20× objective (XLUMPlanFL20XW) with a numeric aperture of 1.0 (Olympus, Tokyo, Japan). The fibrillar collagen was detectable from the second-harmonic generation signal.31 Second-harmonic generation and two-photon excitation fluorescence were simultaneously excited by tuning the laser to 900 nm. The emitted light between 500 and 550 nm (green channel) and between 435 and 485 nm (blue channel) was recorded using the Hamamatsu H10770PB-40 GaAsP detector and Hamamatsu R3896 multialkali detector, respectively.
Cortical CD Isolation
Tubule isolation was performed as previously detailed.32 Briefly, kidneys from 1-month-old mice were perfused through the abdominal aorta with 1 ml of perfusion solution containing 1 mg/ml Collagenase type IV (#LS0004186; PAN Biotech, Aidenbach, Germany) and 1 mg/ml protease type XIV (#P5147; Sigma Aldrich, Milan, Italy). The whole harvested kidney was minced into 1-mm3 slices and incubated at 37°C with shaking (850 rpm) in 1 ml of digestion solution containing 1 mg/ml collagenase type II (PAN Biotech, Aidenbach, Germany) and 1 mg/ml protease type XIV (#P5147; Sigma Aldrich). CDs were then manually isolated from the different aliquots using a stereo microscope.
Immunohistochemistry, Immunoblotting, and RT-qPCR
Standard procedures were performed according to previous studies for immunohistochemistry,33 immunoblotting,34 and RT-qPCR.34
Statistical Analyses
Values are shown as mean±SEM or mean±SD as stated in the figure legends. Comparison between two groups was made by unpaired t test or one- or two-way ANOVA, as indicated in the figure legends. A value of P<0.05 was considered significant.
Results
Dicer Ablation in CD PCs
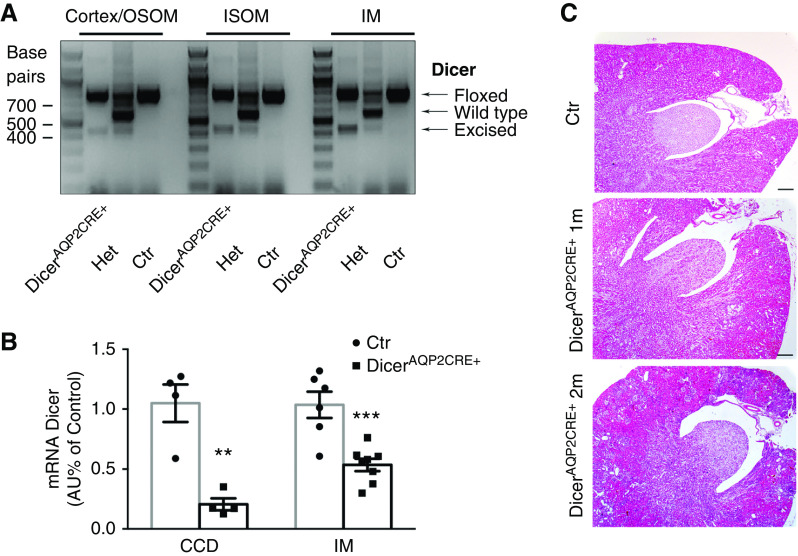
DicerAQP2Cre+ mice were born in a normal Mendelian ratio and were indistinguishable from control mice at birth. PCR of genomic DNA extracted from kidney regions detected a band representing the gene-modified Dicer (excised gene fragment) in all regions from mice bearing Cre recombinase (Figure 1A). In isolated cortical CDs from DicerAQP2Cre+ mice, Dicer mRNA was reduced by about 90% compared with control mice, whereas a 50% reduction of Dicer expression was detected in the whole IM (Figure 1B). There were no gross morphologic alterations of the renal parenchyma in DicerAQP2Cre+ mice at 1 or 2 months of age (Figure 1C), but DicerAQP2Cre+ mice had a more dilated renal pelvis at 2 months of age.
Figure 1.
Efficiency of Dicer ablation in CD PCs. (A) Genotyping for the floxed, wild-type, and excised band of Dicer in renal tissue from Dicerfloxed/floxed to AQP2CRE− (identified as control, Ctr), Dicerfloxed/+ to AQP2CRE+ (Het), and the experimental group, namely Dicerfloxed/floxed to AQP2CRE+ (identified as DicerAQP2CRE+) mice. Excised, but not wild-type, band was detectable in both cortex/OSOM, inner stripe of the outer medulla (ISOM), and IM of DicerAQP2CRE+ mice. (B) RT-qPCR evaluation of Dicer mRNA in isolated cortical CD (CCD; n:4+4) and IM (n:6+8) in DicerAQP2CRE+ (square) and control (Ctr) mice (dots). Data are expressed as mean±SEM. (C) Representative pictures of kidney sections stained with hematoxylin and eosin from 1-month-old Ctr and 1- and 2-month-old DicerAQP2CRE+ mice. At 2 months, DicerAQP2CRE+ mice presented with a pelvis dilation. Ctr mice at 2 months were no different from those at 1 month (data not showed). **P<0.01, ***P<0.001. Het, heterozygous.
DicerAQP2Cre+ Mice Have Reduced AQP2 Levels and NDI
At 1 and 2 months of age, DicerAQP2Cre+ mice had significantly lower body weight relative to control littermates (Table 1). Because Dicer was selectively deleted from water-transporting CD PCs, the renal water-handling ability of DicerAQP2Cre+ was investigated. At 1 month of age, DicerAQP2Cre+ mice had hyposmotic polyuria, with approximately 20-fold higher urine production than their littermate controls (Figure 2A and Table 1). This was paralleled by severe polydipsia (Table 1). Despite this, with free access to water, DicerAQP2Cre+ mice were able to maintain serum electrolyte homeostasis (Table 1). The progressive polyuria likely accounts for the dilation of the pelvis (Figure 1C) associated with tubule dilation and cortical fibrosis at 2 months of age (Supplemental Figure 4). To exclude structural alterations being the basis of the phenotype, we focused the remaining studies on 1-month-old mice with no clear morphologic alterations and no significant increases of serum urea levels compared with controls (Table 1).
Table 1.
Physiological Parameters and Electrolytes
| Parameters | Control | DicerAQP2-CRE | Significance | |||
|---|---|---|---|---|---|---|
| Body weight 1 month old (g) | 21.22±0.49 (7) | 16.77±0.58 (6) | *** | |||
| Body weight 2 month old (g) | 22.99±0.40 (4) | 19.06±0.86 (5) | ** | |||
| Urine | ||||||
| Osmolality 1 M (mOsm/kg H2O) | 2118±309 (5) | 260±14.80 (4) | ** | |||
| Urine volume 1 M (µl/h per gram body weight) | 2.48±0.2 (4) | 49.82±4.3 (4) | **** | |||
| Urine volume 2 M (µl/h per gram body weight) | 2.51±0.6 (4) | 53.91±1.6 (4) | **** | |||
| Water intake 1 M (µl/h per gram body weight) | 15.25±1.4 (4) | 83.31±6.5 (4) | **** | |||
| Water intake 2 M (µl/h per gram body weight) | 17.17±1.4 (4) | 81.94±7.0 (4) | **** | |||
| Serum | ||||||
| Osmolality 1 M (mOsm/kg H2O) | 321.3±8.95 (4) | 333.3±10.65 (3) | ||||
| Na+ (mmol/L) | 145.6±1.17 (5) | 146.5±0.9 (4) | ||||
| K+ (mmol/L) | 4.2±0.2 (5) | 4.9±0.6 (4) | ||||
| Ca2+ (mmol/L) | 1.02±0.07 (5) | 1.06±0.03 (4) | ||||
| Cl− (mmol/L) | 116±1.2 (5) | 115±1.2 (4) | ||||
| Glucose (mmol/L) | 18.3±0.9 (5) | 14.5±1.5 (4) | ||||
| BUN (mg/dl) | 16.43±2.32 (5) | 24.52±3.53 (8) | ||||
**P<0.01; ***P<0.001l ****P<0.0001. n power is in brackets. H2O, water; Na+, sodium; Ca2+, calcium; Cl−, chloride.
Figure 2.
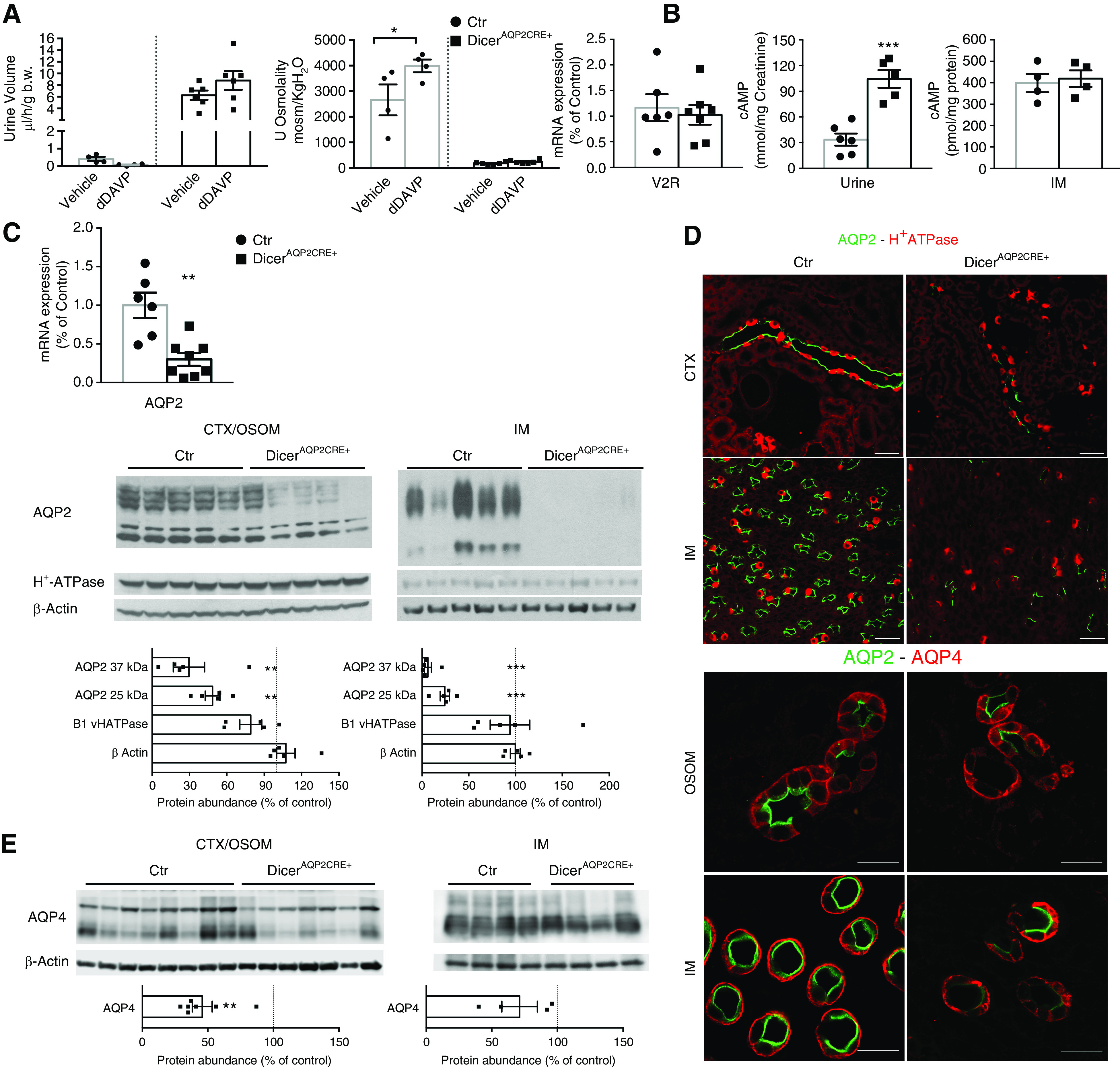
DicerAQP2Cre+ mice have reduced AQP2 levels and NDI. (A) Urine output and osmolality from control (Ctr; dots) and DicerAQP2CRE+ mice (squares) 5 hours after intraperitoneal injection of vehicle (0.9% sodium chloride) or dDAVP. Whereas Ctr mice presented a tendency toward a reduction in urine output and a significant increase in urine osmolality in response to dDAVP, DicerAQP2Cre+ mice were unresponsive (n:4+6; one-way ANOVA). (B) qRT-PCR used to show that V2R mRNA expression is preserved (n:6+7, unpaired t test), along with similar levels of cAMP in the IM (n:4+4, unpaired t test), and even increased levels of urinary cAMP excretion (n:6+5, unpaired t test). (C) AQP2 mRNA is downregulated in IM of DicerAQP2CRE+ mice (squares) compared with Ctr (dots) (n:6+8, unpaired t test). Immunoblotting from cortex (CTX)/OSOM and IM confirms AQP2 downregulation at the protein level in DicerAQP2CRE+ mice, both in CTX/OSOM and IM (n:5+5, unpaired t test). (D) Representative confocal pictures of kidney sections double labeled with anti-AQP2 and anti–H+-ATPase, corroborating that, in DicerAQP2CRE+ mice, there is a very low expression of AQP2 in PCs of CDs, whereas H+-ATPase expression is preserved in intercalated cells. (E) Immunoblotting shows a downregulation of AQP4 in CTX/OSOM (n:7+8, unpaired t test) and only a tendency toward reduction in IM (n:4+4, unpaired t test) of DicerAQP2CRE+ mice compared with Ctr. (F) Representative confocal pictures of double labeling with anti-AQP4 and highly concentrated anti-AQP2 antibodies corroborates that AQP2 expression, more than AQP4, is severely affected in PCs of the CD in DicerAQP2CRE+ mice. All data are expressed as mean±SEM. **P<0.01, ***P<0.001. b.w., body weight; B1 vHATPase, B1-subunit of H+-ATPase.
The hyposmotic polyuria was suggestive of a urinary concentrating defect. To confirm the polyuria was due to renal resistance to AVP, we challenged mice with dDAVP, a V2R agonist. Whereas dDAVP decreased urine volume and increased urine osmolality in control mice (Figure 2, A and B), it had no effect on either of these parameters in DicerAQP2Cre+ mice, suggesting they had NDI. The molecular basis of NDI and dDAVP-resistant polyuria is usually a reduction in expression/localization/activity of the V2R and AQP2. Although there was no significant change in IM V2R mRNA levels or likely activity, as evaluated by cAMP abundance in IM (Figure 2B), DicerAQP2Cre+ mice had a 70% reduction in AQP2 mRNA expression compared with controls (Figure 2C). This reduction in AQP2 mRNA resulted in significantly less AQP2 protein in the cortex/outer stripe of the outer medulla (OSOM) and IM of DicerAQP2Cre+ mice (Figure 2C), as emphasized further by severely reduced AQP2 labeling in PCs throughout the kidney (Figure 2D). Of note, in AQP2-positive cells of DicerAQP2Cre+ mice, there was no obvious alteration in the predominantly apical subcellular distribution of AQP2 and phosphorylated (pSer256) AQP2 (Supplemental Figure 5) at the resolution of immunofluorescence or light microscopy. Finally, no major alteration in the relative abundance of the phosphorylated pSer256 and pSer261 forms of AQP2 (in relation to total AQP2) were observed in the IM, although pSer256-AQP2 was significantly decreased in the cortex/OSOM of DicerAQP2Cre+ mice (Supplemental Figure 6).
Alterations in the ratios of CD PCs and intercalated cells occur in other forms of NDI, such as lithium-induced NDI or during development.35,36 To investigate if such events could underlie the phenotype of DicerAQP2Cre+ mice, the abundance of the intercalated cell–specific, B1-subunit of H+-ATPase was examined. No significant differences in the levels of the B1-subunit of the H+-ATPase were detected in DicerAQP2Cre+ mice (Figure 2C). This was corroborated by immunofluorescence labeling of AQP2 and the B1-subunit of the H+-ATPase in kidney sections from DicerAQP2Cre+ and control mice, where no reduction in the numbers or distributions of PCs and intercalated cells in the cortex and IM were observed (Figure 2D).
To confirm normal PC phenotype, the expression of the basolateral water channel AQP4 was examined. DicerAQP2Cre+ mice had a 50% reduction in the abundance of AQP4 in the cortex, but no significant changes in AQP4 levels were detected in the IM (Figure 2E). Immunofluorescence labeling of kidney sections from control mice demonstrated that all AQP4-positive PCs had distinct AQP2 labeling (Figure 2F). In contrast, although basolateral AQP4 labeling could be observed throughout the kidney in sections from DicerAQP2Cre+ mice, AQP2 labeling in the same cells was weak or not observed (Figure 2F). A few residual PCs from DicerAQP2Cre+ mice had AQP2 and AQP4 labeling of the same intensity as control mice (arrowhead in Figure 2F), suggesting less efficient, Cre-mediated recombination in these cells. In summary, DicerAQP2Cre+ mice have a hyposmotic polyuria that is resistant to dDAVP as a consequence of reduced AQP2 abundance.
Amiloride-Sensitive Sodium Reabsorption Is Preserved in DicerAQP2Cre+ Mice
The amiloride-sensitive ENaC, consisting of αβγ subunits, mediates the majority of electrogenic sodium reabsorption in the PC.37 αENaC, in states of normal salt intake, is mainly expressed in the cortex/OSOM and poorly in the IM.37 Surprisingly, in kidney homogenates isolated from the cortex/OSOM of DicerAQP2Cre+ mice, there was no difference in the abundance of the full-length, 70-kDa form of αENaC compared with the control, but the levels of a 30-kDa, cleaved form (indicative of active αENaC) was reduced in DicerAQP2Cre+ mice compared with controls (Figure 3A). In the IM of DicerAQP2Cre+ mice, there was a two- to three-fold increase in both the full and cleaved forms of αENaC (Figure 3A). αENaC mRNA levels were also increased in IM from DicerAQP2Cre+ mice compared with controls (Figure 3B). Immunofluorescence double labeling of AQP2 and αENaC confirmed an increase in αENaC levels in IM PCs of DicerAQP2Cre+ mice relative to controls (Figure 3C). Although the majority of ENaC-positive cells from DicerAQP2Cre+ mice did not express AQP2 at a detectable level, by using high concentrations of anti-AQP2 antibodies, some AQP2 labeling could be detected in the apical domain of some αENaC-positive cells in the IM.
Figure 3.
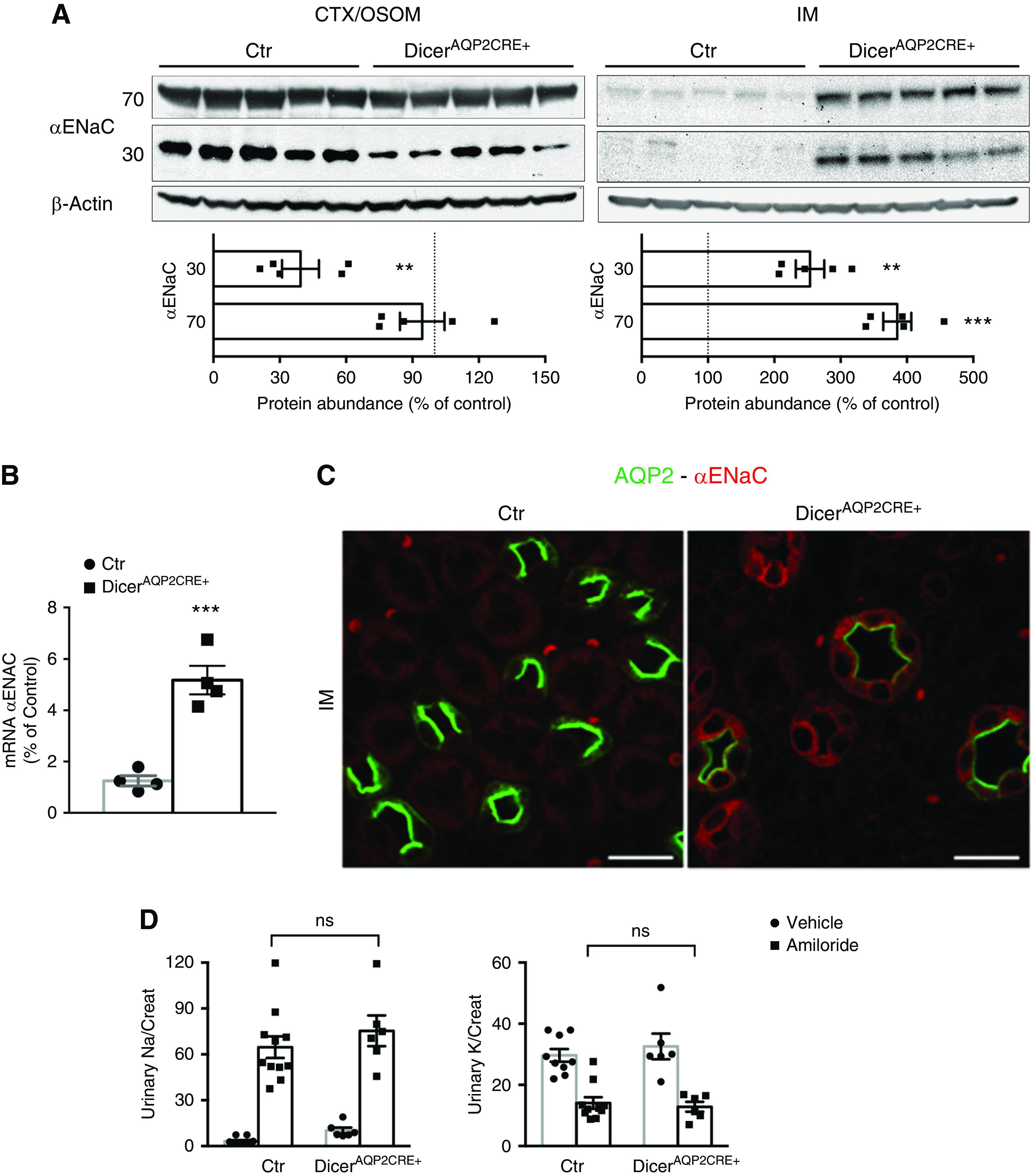
Amiloride-sensitive sodium reabsorption is preserved in DicerAQP2Cre+ mice. (A) Immunoblotting from the cortex (CTX)/OSOM and IM of control (Ctr) and DicerAQP2CRE+ mice. Although the 70-kD fraction of αENaC is similarly expressed in the two groups of mice, the active, 30-kD fraction is downregulated in CTX/OSOM from DicerAQP2CRE+ mice (n:5+5, unpaired t test). However, in the IM, there is a strong, likely compensatory, upregulation of both components of αENaC (n:5+5, unpaired t test). (B) Upregulation of αENaC mRNA in the IM of DicerAQP2CRE+ mice (n:4+4, unpaired t test). (C) Representative confocal pictures showed that, in the IM, αENaC is detectable almost exclusively in PCs of DicerAQP2CRE+ mice. (D) Functional data from the amiloride-inhibition test shows that, both in terms of sodium and potassium excretion, the two groups of mice behave similarly (Ctr, n:10+11; DicerAQP2CRE+, n:6+6; one-way ANOVA; vehicle is represented by dots, amiloride represented by squares), showing that the overall amiloride-sensitive sodium reabsorption is preserved in DicerAQP2CRE+ mice. In both groups, amiloride significantly increases sodium and decreases potassium excretion compared with vehicle (not shown). All data are expressed as mean±SEM. **P<0.01, ***P<0.001.
To examine if the expression profile of αENaC in DicerAQP2Cre+ mice altered their overall ENaC-dependent sodium reabsorption, mice were challenged acutely with the ENaC inhibitor amiloride. No significant differences in urinary sodium or potassium excretion were observed between the genotypes (Figure 3D), suggesting that, although suppression of Dicer in PCs does modify αENaC levels to some extent, electrolyte handling is preserved.
The Use of miRNA and Protein Profiling To Identify Mediators of Water Reabsorption in the IM
To identify miRNAs altered in DicerAQP2Cre+ mice that may associate with the early-onset polyuric phenotype, small-RNA sequencing analysis was initially performed on IM tissue from 1-month-old DicerAQP2Cre+ mice (no structural abnormalities or altered serum BUN) and controls. Two-dimensional principal component analysis of the differentially expressed miRNAs in the IM revealed a distinct distribution pattern between DicerAQP2Cre+ mice and the control group (Supplemental Figure 1B). Out of 56 differentially expressed (FDR-corrected P≤0.05) miRNAs, 31 had at least a two-fold difference in expression (14 upregulated and 17 downregulated) between DicerAQP2Cre+ and control mice (Figure 4A).
Figure 4.
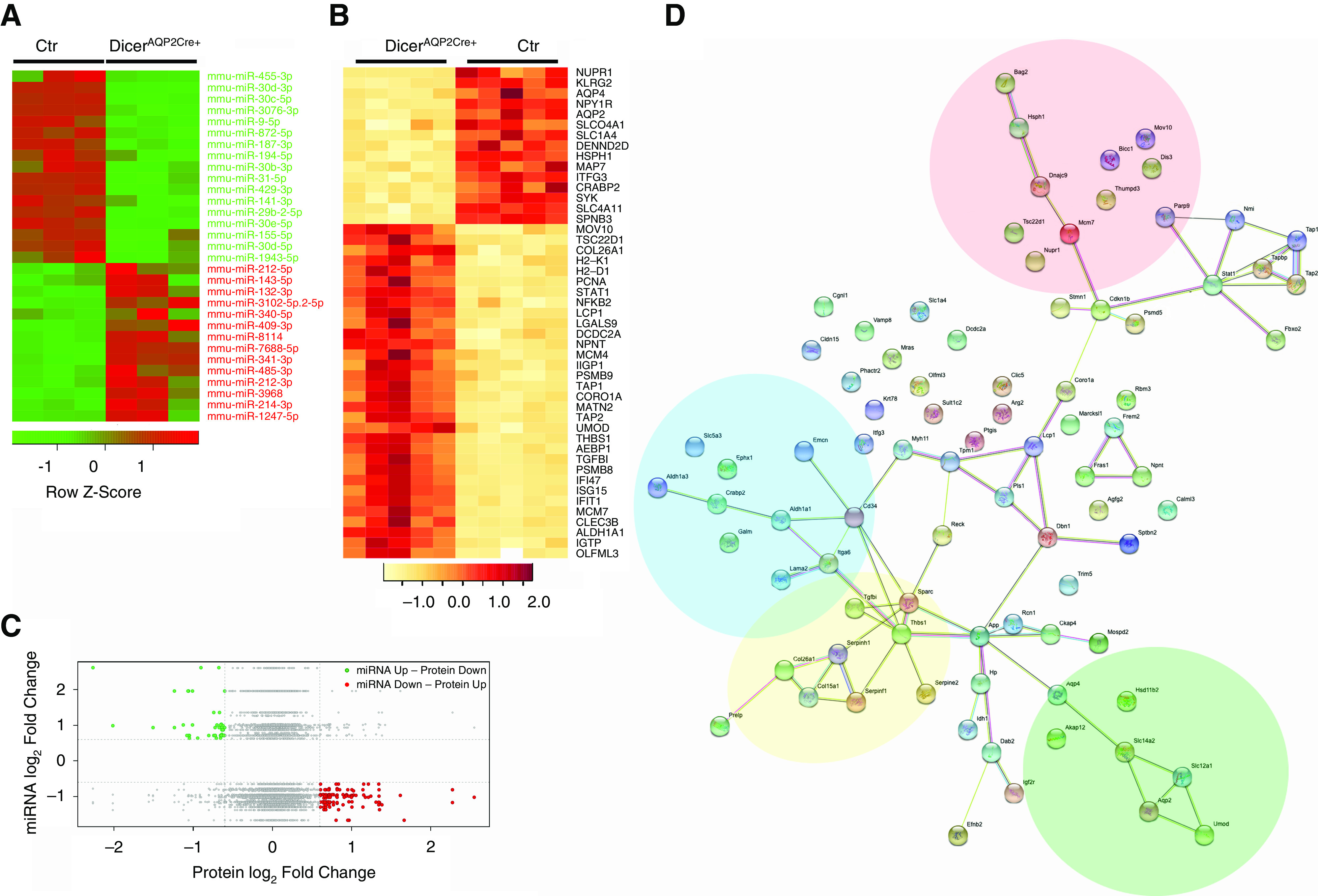
miRNA and protein profiling to identify mediators of water reabsorption in the IM. (A) The heat map shows the list of regulated miRNAs (at least two-fold change and P<0.05) upregulated (red), or downregulated (green) in DicerAQP2CRE+ compared with controls, and their distribution in the single samples (n=3 versus 3). (B) The heat map shows some of the regulated proteins (absolute log2 FC≥1 and FDR≤0.05) in DicerAQP2CRE+ mice. (C) The plot compares the miRNAome and the proteome by identifying each miRNA/target protein with the fold change of miRNA (y axis) and the corresponding target protein abundance (x axis). The green dots indicate miRNAs that are increased (log2 ratio >0.6), and their predicted protein targets are decreased (log2 ratio <−0.6); whereas the red dots represent miRNAs that are decreased (log2 ratio <−0.6), and their predicted protein targets are increased (log2 ratio >0.6). (D) The interaction network arising from the list of significantly changed miRNA/protein pairs (green and red dots in panel (C) or complete list in Supplemental Table 3), showed clusters of proteins involved in water transport (green area), cell metabolism (blue area), DNA and RNA regulation (red area), and cytoskeleton regulation and membrane interaction (yellow area). Ctr, control.
The list of the experimentally validated target genes for these 31 regulated miRNAs (Supplemental Table 2) was built by computing only the genes overexpressed in IMCDs compared with non-IMCDs (http://esbl.nhlbi.nih.gov/IMCD-transcriptome/). The resulting interaction network (Ingenuity Pathways Analysis) (Supplemental Figure 7) highlighted several potential pathways influencing the expression of AQP2, AQP3, and AQP4, and their regulatory transcription factors such as GATA2,38 GATA3,39 and ELF3,40,41 together with several epigenetic regulators (Supplemental Table 2). To create a more complete picture of the effects of Dicer deletion on protein abundance in the IM, we performed a proteomic analysis of the IM. A total of 178 proteins were altered in abundance (log2 fold variation≥0.6 or ≤0.6 and FDR≤ 0.05) relative to controls (Figure 4B, Supplemental Figure 8, Supplemental Table 3). To compare the miRNAome and the proteome, we plotted the expression fold change of miRNA (y axis) and the corresponding target protein abundance (x axis) for each miRNA/target protein pair (real proteomic expression of miRNAs’ predicted targets). Two distinct groups, characterized by the upregulation of the miRNAs and the downregulation of a target protein or vice versa, were identified (Figure 4C, Supplemental Table 4). The interaction network arising from this list of targets showed several clusters of proteins involved in cytoskeletal regulation and membrane interaction, which connected with other clusters of proteins involved in water transport (green), cell metabolism (blue), and DNA-RNA regulation (red) (Figure 4D).
RT-qPCR was used to examine the expression of miR-7688-5p, miR-8114, and miR-409-3p (three miRNAs altered in the IM of DicerAQP2Cre+ mice that could be putative regulators of AQP2 expression) or target transcription factors influencing AQP2 transcription or the epigenetic machinery (Figure 5, Supplemental Figure S2). Confirming the small-RNA sequencing, the levels of miR-7688-5p, miR-8114, and miR-409-3p were significantly increased in DicerAQP2Cre+ mice compared with controls (Figure 5A). This was paralleled by downregulation of three of their predicted target genes, GATA3, GATA2, and ELF3 (Figure 5B), which are transcription factors regulating AQP2 expression. Moreover, expression of three crucial histone demethylases were altered by Dicer ablation, namely Phf2 (histone demethylase plant homeodomain finger 2), which is a histone H3 lysine 9 demethylase; Kdm5c (lysine demethylase 5c), which is a H3K4me2/3 demethylase; and Kdm4a (lysine demethylase 4a), which is a H3K9 and H3K36 demethylase (Figure 5C).
Figure 5.
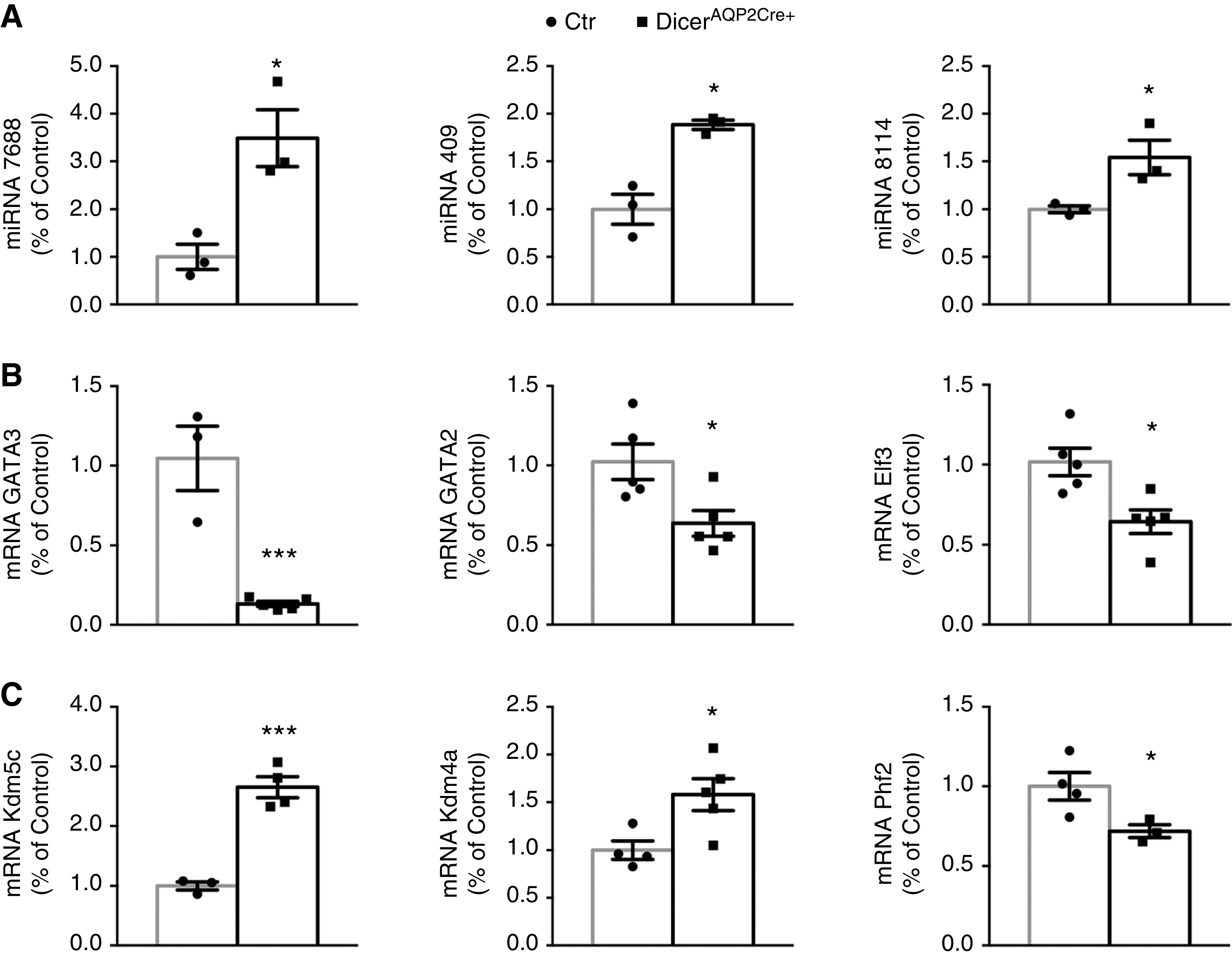
miRNAs and target validation. (A) RT-qPCR evaluation of three miRNAs confirms data from small-RNA sequencing that miR7688-5p, mir409-3p, and miR8114 were increased in the IM of DicerAQP2CRE+ mice (n:3+3, unpaired t test). (B) The expression level of some predicted targets of the regulated miRNAs evaluated by RT-qPCR. GATA3 (n:3 + 5), GATA2 (n:5+5), Elf3 (n:5+5) and (C) Kdm5c (n:3+4), Kdm4a (n:4+5), and Phf2 (n:4+3) confirmed the predicted involvement of RNA and DNA regulation both at transcriptional andepigenetic levels. All data are expressed as mean±SEM; unpaired t test has been used to compare the groups. *P<0.05, **P<0.01, ***P<0.001. Ctr, control.
Reduced AQP2 mRNA Levels in DicerAQP2Cre+ Mice Do Not Likely Occur by Direct Transcriptional Inhibition of AQP2 by miR-7688-5p, miR-8114, and miR-409-3p
To further examine the role of miR-7688-5p, miR-8114, and miR-409-3p in the observed reduction of AQP2 mRNA in DicerAQP2Cre+ mice, an in vitro system was used. RNA mimics of miR-7688-5p, miR-8114, and miR-409-3p or scramble RNA were transfected into a well-characterized CD cell line, mpkCCD14(c11) cells. Seven days later, polarized cells were treated with dDAVP for 24 hours to stimulate AQP2 gene expression. miRNA-mimic transfected cells still had significantly increased levels of the mimics relative to scramble RNA mimics (Figure 6A) 9 days after transfection. All three miRNA mimics induced a significant downregulation of AQP2 mRNA expression (Figure 6A), but not protein levels at the studied time point (Supplemental Figure 9). As predicted by TargetScan, overexpression of the miR-7688-5p and miR-8114 mimics were associated with decreased levels of GATA3 mRNA. In contrast, the miR-409-3p mimic increased both GATA2 and GATA3 mRNA levels. All three miRNAs did not alter Elf3 mRNA expression. miRNAs mainly act by promoting a reduction in mRNA t 1/2 and translational failure due to physical interaction with complementary 3′-UTR regions of target genes.42 To examine whether such a physical interaction occurs between the candidate miRNAs and their predicted target regions, luciferase-based binding assays were performed. No direct interaction of miR-7688-5p and the predicted 3′-UTR of AQP2 was detectable (Figure 6B and Supplemental Figure 10). Similarly, miR-8114 and miR-409-3p did not interact significantly with the 3′-UTR of GATA3 as predicted (Figure 5B). miR-8114 and miR-409-3p were also predicted to potentially bind to regions in the AQP2 promoter, but such physical interactions could not be corroborated when mimics were assessed alone or in combination (Figure 5B). In summary, the in vitro data support that increased levels of three candidate miRNAs can reduce AQP2 mRNA levels, but this is unlikely to occur by direct interaction with AQP2, GATA2, or GATA3 mRNA.
Figure 6.
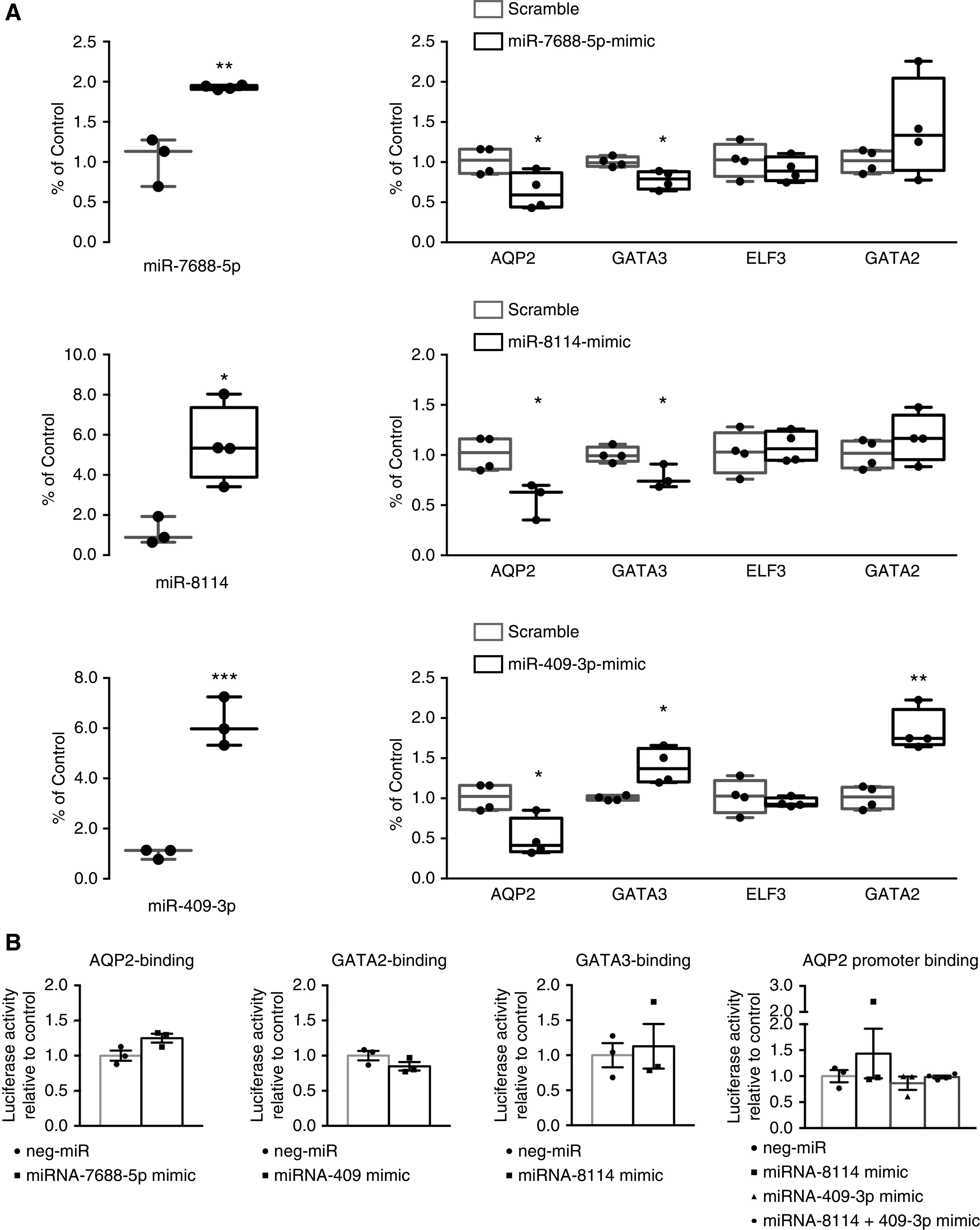
In DicerAQP2Cre+ mice, miR-7688-5p, miR-8114, and miR-409-3p do not induce direct transcriptional inhibition of AQP2. (A) Data from in vitro experiments in mpkCCD14(c11) cells transfected with miRNAs mimics of miR7688-5p, mir409-3p, and miR8114. As shown on the left, the efficiency of the mimic transfection reflects the increased level of specific miRNAs 9 days after transfection. On the right, mpkCCD14(c11) cells transfected with all three miRNA mimics (black square) or scramble oligonucleotides (gray square) were treated with dDAVP to maximize AQP2 expression. The dDAVP-dependent cascade of events and the expression levels of GATA3, GATA2, and ELF3 are reported (each sample indicated by an individual dot). (B) Luciferase-based binding assays reveal the studied miRNAs did not bind their predicted target 3′-UTR regions (see also Supplemental Figure 10) on the transcription factors GATA3, GATA2, and AQP2 (n:3+3 for each). miR-8114 and miR409-3p, either alone or in combination, did not interact with a predicted binding region on the AQP2 promoter (n:3+3 per single mimic; n:3+4 for combined mimics). If the miRNAs (squares) interacted with their putative region, the expected luciferase signal would be reduced compared with the miR-negative scramble oligonucleotide (dots). All data are expressed as mean±SEM; unpaired t test has been used to compare the groups. Neg, negative. *P<0.05; **P<0.01; ***P<0.001.
Reduced AQP2 Levels in DicerAQP2Cre+ Mice Are Associated with Altered Association of Epigenetic Factors with the AQP2 Promoter
The miRNAs miR-7688-5p and miR-8114 are predicted to be regulators of Phf2, Kdm5c, and Kdm4a (Supplemental Figure 2). Kdm5c abundance was significantly increased in the IM of DicerAQP2Cre+ mice (Supplemental Figure 11). Furthermore, the expression level of Phf2 and Kdm5c are higher than Kdm4a, and are predicted to have a biologic interaction with AQP2 in the CD (Kidney System Biology Project; https://hpcwebapps.cit.nih.gov/ESBL/Database). Thus, we focused on a role of Phf2, an activating chromatin modifier (part of a PKA-dependent histone lysine demethylase complex, PHF2-ARID5B),43 and Kdm5c, a repressive modifier of chromatin aggregation,44 in the next set of studies. ChIP assays on IM tissue demonstrated a tendency (P=0.09, two-way ANOVA) for the AQP2 promoter region in DicerAQP2Cre+ mice to interact less with Phf2, but significantly more with Kdm5c relative to the Aqp2 promoter from control mice (Figure 7). This enhanced recruitment of Kdm5c is predicted to result in a closed chromatin conformation, corroborated by a significant displacement of RNA polymerase II from the Aqp2 promoter region of DicerAQP2Cre+ mice (Figure 7). Together, the data suggest that dysregulation of selective miRNAs in PCs of DicerAQP2Cre+ mice results in AQP2 epigenetic regulation, leading to transcriptional repression.
Figure 7.
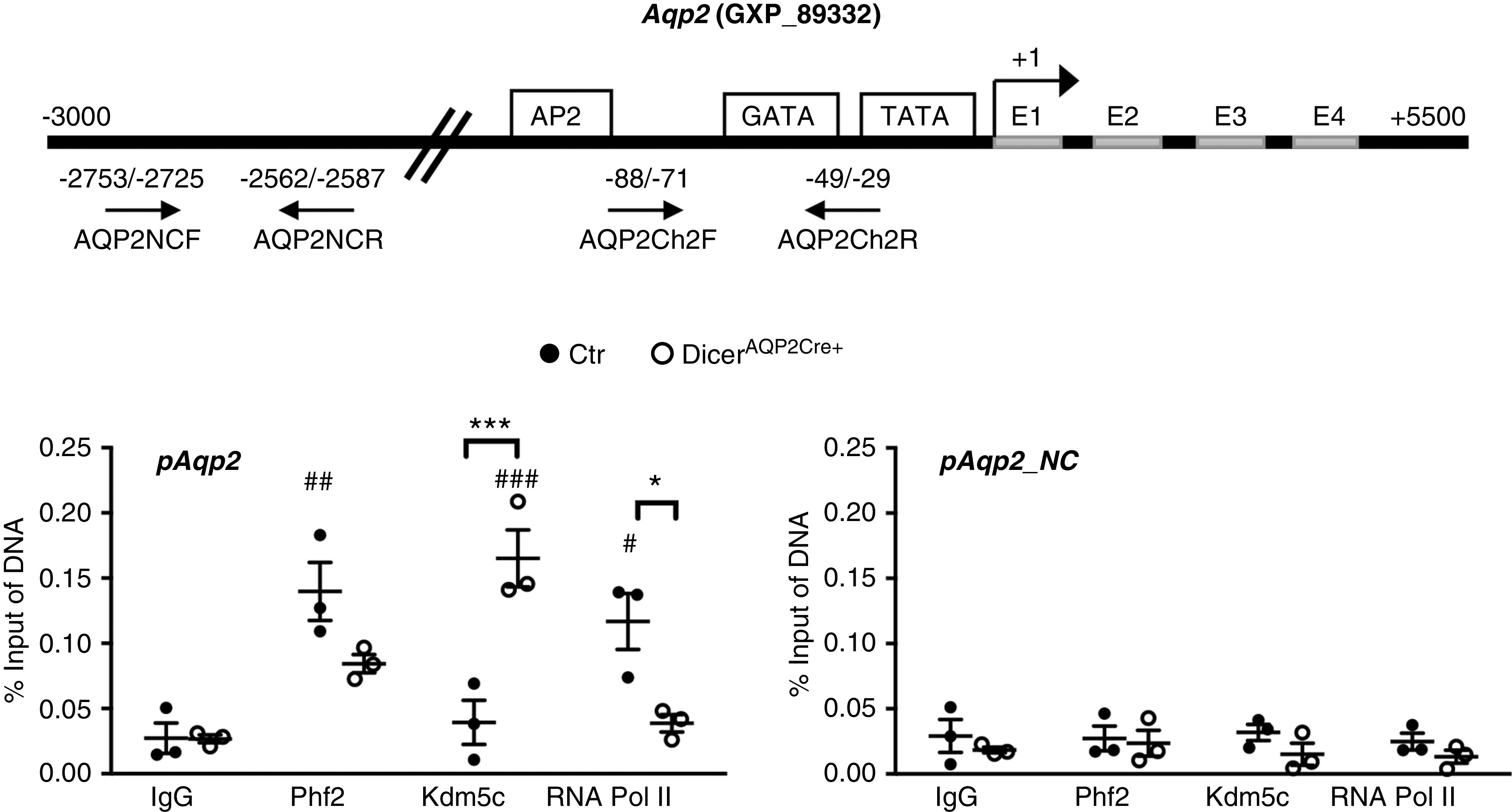
Downregulation of AQP2 in DicerAQP2Cre+ mice is associated with altered association of epigenetic factors with the AQP2 promoter. ChIP assays performed on IM tissue from control (dots) or DicerAQP2Cre+ (squares) mice using anti-Phf2 and anti-Kdm5c antibodies. (A) The location of the used primers (AQP2Ch2F and AQP2Ch2R) for the AQP2 gene promoter region and for a region distal from the AQP2 promoter (AQP2NCF and AQP2NCR; as a negative control). (B) The AQP2 promoter region (pAQP2) in DicerAQP2Cre+ mice had a tendency for less Phf2 interaction, and significantly more Kdm5c interaction, relative to the AQP2 promoter from control mice. This enhanced recruitment of Kdm5c is predicted to result in a closed chromatin conformation, corroborated by a significant displacement of RNA polymerase II from the AQP2 promoter region of DicerAQP2Cre+ mice. No significant binding was detected in the region away from the promoter (pAQP2NC). All data are expressed as mean±SD; two-way ANOVA has been used to compare the groups. n:3+3. *P<0.05 for comparison of control (Ctr) versus DicerAQP2Cre+ mice; # P<0.05 for comparison versus group-related IgG.
Discussion
To investigate the role of miRNAs in the CD PC, we used a Cre-LoxP recombination strategy to generate a mouse model lacking the endoribonuclease Dicer in AQP2-expressing cells. Dicer is a critical regulator of the biogenesis of miRNA, but it is also important for the biogenesis of small-interfering RNAs and the processing of additional endogenous and exogenous substrates.45 Although these additional roles have to be considered in the overall phenotype of the DicerAQP2Cre+ mice, they are not discussed in detail here because it was not the focus of this study.
The DicerAQP2Cre+ mice presented with a progressively severe polyuria that was unresponsive to dDAVP administration, suggestive of NDI. This occurred already at 1 month of age, indicating miRNA integrity is fundamental for the function and homeostasis of CD PCs. Polyuria associated with a defective embryonic development of the CD was also observed in mice with suppression of Dicer in the entire ureteric bud (Hoxb7-Dicer cKO).46 However, in that model, Dicer is ablated at an early stage of development (embryonic day 9.5),46 whereas no developmental abnormalities were detected in DicerAQP2Cre+ mice. This is likely because the AQP2 promoter is only functional later during renal development (embryonic day 16.5). In addition, Dicer suppression in DicerAQP2Cre+ mice is limited to cells that express AQP2 even transiently, whereas recombination under control of the Hoxb7 gene promoter occurs in earlier cellular precursors and affects all cell types of the CD. This is highlighted by the normal abundance and distribution of intercalated cells throughout the entire CD in our DicerAQP2Cre+ mice.
Dysregulation of the normal miRNA pattern by Dicer ablation selectively interfered with the water-reabsorption machinery of CD PCs, leading to a severe reduction in AQP2 levels and, to lesser extent, reduced AQP4. DicerAQP2Cre+ mice had normal ENaC expression and distribution in PCs, and normal amiloride-sensitive sodium reabsorption (assessed by a standardized amiloride administration test21), emphasizing DicerAQP2Cre+ mice do not have an overall dysfunction of the PC.
To uncover potential miRNAs associated with NDI, small-RNA sequencing analysis of the IM was performed. Assessment of the IM, enriched for CDs (non-IMCD tubules account for only 20% of the tissue mass), has been extensively used in systems-biology approaches to examine molecular changes in the medullary CD.20,47–49 Approximately 1200 miRNAs were identified in the IM from DicerAQP2Cre+ mice using this approach. A 70% overlap to miRNAs was identified in isolated CD from Pkhd1-Dicer cKO mice,50 lending credence to the validity of our approach. The common identifiers between the two datasets are even more interesting if we consider the different phenotype of the Pkhd1-Dicer cKO mice, characterized by massive fibrosis in contrast to DicerAQP2Cre+ mice; likely because Dicer deletion is extended to the entire cellular population of CDs.
From our dataset of miRNAs, 31 had log2 ≥ or ≤ two-fold expression differences in DicerAQP2Cre+ mice compared with control mice. The interaction network of their putative targets and the integrated analysis of the miRNAome and proteome connected several water-transport regulatory pathways to both RNA and DNA regulatory pathways, which may provide a basis for the NDI phenotype of DicerAQP2Cre+ mice. Other elements interacting with epigenetic regulatory pathways suggested that some of the miRNAs could serve as epi-miRNAs. Because our focus was to determine how dysregulation of Dicer-dependent miRNAs could interfere with AQP2 transcription, we focused on these two regulatory pathways. Among the most upregulated miRNAs were miR-7688-5p, miR-8114, and miR-409-3p, which had various predicted targets in these canonic pathways. In vitro, despite difficulties in transfecting mpkCCD cells at very high levels, the miRNA mimics miR-7688-5p, miR-8114, and miR-409-3p were able to reduce AQP2 mRNA expression during dDAVP stimulation. To our knowledge, these are the first miRNAs reported to be associated with NDI. Interestingly, none of the homologous rat miRNAs are listed as being regulated by the AVP analogue dDAVP in rat IMCDs.15 Furthermore, miR-341 and miR-143, two miRNAs strongly downregulated by hypertonic stimulation of IMCD cells51 (a condition enhanced by AVP), had a very high expression profile in our polyuric model. In contrast, miR-30 was upregulated in hypertonic-stimulated IMCD cells,51 and downregulated in our polyuric model. Coupling our data to previous reports suggests the abundance of various miRNAs contributes to the regulation of water reabsorption.
We were unable to confirm a direct interaction of miR-7688-5p, miR-8114, and miR-409-3p with predicted regions on AQP2 mRNA or the AQP2 promoter using luciferase-based promoter assays. This may be due to technical limitations, with previous attempts to demonstrate direct interaction between miRNAs and AQP2 mRNAs having similar outcomes.15 Alternatively, the effects of the miRNAs may be indirect. Increased levels of miR-7688-5p and miR-8114 were associated with a downregulation of GATA3, a transcription factor modulating AQP2 promoter activity and gene expression.39 Although this could, at least in part, explain the severe downregulation of AQP2 mRNA, reduced AQP2 via miR-409-3p was independent of the well-described transcription factors GATA3, GATA2, and ELF3, and other mechanisms must exist. A major new mechanism we uncovered is that, by altering the profile of miRNAs in PCs, the levels of the histone demethylases Phf2 and Kdm5c at the AQP2 promoter were altered, resulting in reduced RNA polymerase II binding. Ultimately, this results in epigenetic repression of AQP2 transcription and contributes to the development of NDI in DicerAQP2Cre+ mice. AQP2 has a CpG island about −300 bp from the transcriptional start site and another in the fourth exon,52 suggesting it could be more susceptible to epigenetic regulation relative to, e.g., ENaC, which only has a single predicted CpG island.
To our knowledge, epigenetic regulation of Aqp2 has not previously been demonstrated, but it could have important physiologic and clinically relevant implications. AQP2 expression is a common marker of precursors of both PCs, intercalated cells, and some DCT2 cells of the nephron.47 During embryonic development, this implies its selective inactivation in the non-PC lineage may occur at an epigenetic level. Modulation of this epigenetic lock could occur during CD repair and determine, in adults, a cellular reprogramming from PC to intercalated cells and vice versa, as showed in lineage tracing studies.53,54 Similar changes in DNA methylation are required during intestinal differentiation for the control of gene expression modulated from chromatin accessibility for multiple transcription factors.55 Epigenetic regulation of AQP2 was also suggested by a recent study evaluating the transcriptomic profile of the cortical CD after administration of lithium chloride (LiCl), a well-described model of NDI induced by AQP2 suppression.56 In this study, several epigenetic regulators—such as the demethylase Jmjd6 and the deacetylases Sap30, Hdac7, and Hdac4—were altered during the first 72 hours of LiCl administration. Although this only suggests LiCl-inducing epigenetic regulation of AQP2, it highlights the potential clinical and physiologic relevance of our findings and opens up the possibilities for future perspectives.
In summary, this study suggests that long-term modulation of AQP2 levels in the PC are influenced by various miRNAs, which modulate AQP2 expression by alteration of transcription-factor abundance and other epigenetic factors. These mechanisms, combined with the rapid effects of AVP to modulate AQP2 trafficking,57 are responsible for maintaining body water homeostasis during various physiologic challenges.
Disclosures
R. Fenton reports being an associate editor for the American Journal of Physiology-Renal Physiology, an editorial board member of JASN (since 2008), an editorial board member of Nature Scientific Reports (since 2016), and an editorial board member of PLOS One (since 2011). F. Trepiccione reports being an associate editor of Kidney and Blood Pressure Research. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We are grateful to the European Mouse Mutant Archive for providing the AQP2-CRE mice strain.
Dr. Robert A. Fenton is funded by the Novo Nordisk Foundation, the Lundbeck Foundation, and the Independent Research Fund Denmark.
Dr. Federica Petrillo, Dr. Anna Iervolino, Dr. Tiziana Angrisano, Dr. Sabina Jelen, Dr. Ilaria Guerriero, Dr. Vincenzo Costanzo, Dr. Maria Cristina Mazzarella, and Dr. Alfonso De Falco carried out experiments; Dr. Alfonso De Falco, Dr. Michele Ceccarelli, Dr. Michele Caraglia, Dr. Robert A. Fenton, Dr. Giovambattista Capasso, and Dr. Francesco Trepiccione analyzed data; Dr. Federica Petrillo and Dr. Francesco Trepiccione made the figures; Dr. Federica Petrillo, Dr. Robert A. Fenton, and Dr. Francesco Trepiccione drafted and revised the paper; and all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020010031/-/DCSupplemental.
Supplemental Figure 1. Small RNA sequencing of renal IM, validation parameters.
Supplemental Figure 2. Predicted transcription and epigenetic factors targeted by miR7688-5p, miR-409-3p, and miR-8114.
Supplemental Figure 3. Transfection efficiency.
Supplemental Figure 4. Renal fibrosis evaluation.
Supplemental Figure 5. Localization of phosphorylated (pSer256) AQP2 in inner medulla.
Supplemental Figure 6. Immunoblotting of phosho-forms of AQP2 in mouse kidney.
Supplemental Figure 7. Ingenuity Pathways Analysis interaction network of predicted miRNAs targets.
Supplemental Figure 8. Volcano plot of proteomic analysis output data.
Supplemental Figure 9. Immunoblotting of phosho-forms of AQP2 in mpkCCD14(c11) cells transfected with miRNAs mimics.
Supplemental Figure 10. Predicted interaction sites between miR-7688-5p, miR-409-3p, miR-8114 and their putative 3′UTR regions used in the luciferase assay.
Supplemental Figure 11. Immunoblotting of Kdm5c and Phf2 expression in inner medulla.
Supplemental Table 1. List of primers.
Supplemental Table 2. List of validated targets of the significantly regulated miRNAs.
Supplemental Table 3. List of regulated proteins identified by MS.
Supplemental Table 4. List of the significantly regulated miRNA/protein target pair.
Supplemental Appendix 1. Materials and methods.
References
- 1. Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA: Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep 6: 34863, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marples D, Knepper MA, Christensen EI, Nielsen S: Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol 269: C655–C664, 1995. [DOI] [PubMed] [Google Scholar]
- 3. Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA: Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci U S A 105: 3134–3139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moeller HB, Fenton RA: Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch 464: 133–144, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Carthew RW, Sontheimer EJ: Origins and Mechanisms of miRNAs and siRNAs. Cell 136: 642–655, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim VN, Han J, Siomi MC: Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Chua JH, Armugam A, Jeyaseelan K: MicroRNAs: Biogenesis, function and applications. Curr Opin Mol Ther 11: 189–199, 2009. [PubMed] [Google Scholar]
- 8. Brodersen P, Voinnet O: Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol 10: 141–148, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Wang F, Ma Y, Wang H, Qin H: Reciprocal regulation between microRNAs and epigenetic machinery in colorectal cancer. Oncol Lett 13: 1048–1057, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badal SS, Wang Y, Long J, Corcoran DL, Chang BH, Truong LD, et al.: miR-93 regulates Msk2-mediated chromatin remodelling in diabetic nephropathy. Nat Commun 7: 12076, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY, Chuang PC, et al.: MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol 25: 1698–1709, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrillo F, Iervolino A, Zacchia M, Simeoni A, Masella C, Capolongo G, et al.: MicroRNAs in renal diseases: A potential novel therapeutic target. Kidney Dis 3: 111–119, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iervolino A, Trepiccione F, Petrillo F, Spagnuolo M, Scarfò M, Frezzetti D, et al.: Selective dicer suppression in the kidney alters GSK3β/β-catenin pathways promoting a glomerulocystic disease. PLoS One 10: e0119142, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartram MP, Amendola E, Benzing T, Schermer B, de Vita G, Müller RU: Mice lacking microRNAs in Pax8-expressing cells develop hypothyroidism and end-stage renal failure. BMC Mol Biol 17: 11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JE, Jung HJ, Lee YJ, Kwon TH: Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am J Physiol Renal Physiol 308: F749–F764, 2015. [DOI] [PubMed] [Google Scholar]
- 16. Jung HJ, Raghuram V, Lee JW, Knepper MA: Genome-wide mapping of DNA accessibility and binding sites for CREB and C/EBPβ in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 29: 1490–1500, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, Burg MB, et al.: Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci U S A 114: E8875–E8884, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, et al.: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ: Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A 102: 12135–12140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trepiccione F, Gerber SD, Grahammer F, López-Cayuqueo KI, Baudrie V, Păunescu TG, et al.: Renal atp6ap2/(pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, et al.: Double knockout of the Na+-Driven Cl-/HCO3- exchanger and Na+/Cl- cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. 40th EASD annual meeting of the European association for the study of diabetes: Munich, Germany, 5–9 September, 2004. Diabetologia 47[Suppl 1]: A1–A464, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pellegrini KL, Gerlach CV, Craciun FL, Ramachandran K, Bijol V, Kissick HT, et al.: Application of small RNA sequencing to identify microRNAs in acute kidney injury and fibrosis. Toxicol Appl Pharmacol 312: 42–52, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu A, Wolley MJ, Wu Q, Gordon RD, Fenton RA, Stowasser M: The Cl−/HCO3 − exchanger pendrin is downregulated during oral co-administration of exogenous mineralocorticoid and KCl in patients with primary aldosteronism [published online ahead of print November 10, 2020]. J Hum Hypertens 10.1038/s41371-020-00439-7 [DOI] [PubMed] [Google Scholar]
- 25. Ting L, Rad R, Gygi SP, Haas W: MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 8: 937–940, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al.: The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res 47[D1]: D442–D450, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, et al.: Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci U S A 106: 2441–2446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moeller HB, Slengerik-Hansen J, Aroankins T, Assentoft M, MacAulay N, Moestrup SK, et al.: Regulation of the water channel aquaporin-2 via 14-3-3θ and -ζ. J Biol Chem 291: 2469–2484, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alabdullah AA, Al-Abdulaziz B, Alsalem H, Magrashi A, Pulicat SM, Almzroua AA, et al.: Estimating transfection efficiency in differentiated and undifferentiated neural cells. BMC Res Notes 12: 225, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angrisano T, Pero R, Brancaccio M, Coretti L, Florio E, Pezone A, et al.: Cyclical DNA methylation and histone changes are induced by LPS to activate COX-2 in human intestinal epithelial cells. PLoS One 11: e0156671, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, et al.: The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics 12: 923–933, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hofmeister MV, Fenton RA, Praetorius J: Fluorescence isolation of mouse late distal convoluted tubules and connecting tubules: Effects of vasopressin and vitamin D3 on Ca2+ signaling. Am J Physiol Renal Physiol 296: F194–F203, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Trepiccione F, Altobelli C, Capasso G, Christensen BM, Frische S: Lithium increases ammonium excretion leading to altered urinary acid-base buffer composition. J Nephrol 31: 385–393, 2018. [DOI] [PubMed] [Google Scholar]
- 34. Iervolino A, De La Motte LR, Petrillo F, Prosperi F, Alvino FM, Schiano G, et al.: Integrin beta 1 is crucial for urinary concentrating ability and renal medulla architecture in adult mice. Front Physiol 9: 1273, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trepiccione F, Capasso G, Nielsen S, Christensen BM: Evaluation of cellular plasticity in the collecting duct during recovery from lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 305: F919–F929, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006. [DOI] [PubMed] [Google Scholar]
- 37. Chambrey R, Trepiccione F: Relative roles of principal and intercalated cells in the regulation of sodium balance and blood pressure. Curr Hypertens Rep 17: 538, 2015. [DOI] [PubMed] [Google Scholar]
- 38. Yu L, Moriguchi T, Souma T, Takai J, Satoh H, Morito N, et al.: GATA2 regulates body water homeostasis through maintaining aquaporin 2 expression in renal collecting ducts. Mol Cell Biol 34: 1929–1941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uchida S, Matsumura Y, Rai T, Sasaki S, Marumo F: Regulation of aquaporin-2 gene transcription by GATA-3. off. Biochem Biophys Res Commun 232: 65–68, 1997. [DOI] [PubMed] [Google Scholar]
- 40. Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, et al.: Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23: 1008–1018, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lin ST, Ma CC, Kuo KT, Su YF, Wang WL, Chan TH, et al.: Transcription factor Elf3 modulates vasopressin-induced aquaporin-2 gene expression in kidney collecting duct cells. Front Physiol 10: 1308, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R: Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20: 515–524, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Baba A, Ohtake F, Okuno Y, Yokota K, Okada M, Imai Y, et al.: PKA-dependent regulation of the histone lysine demethylase complex PHF2-ARID5B. Nat Cell Biol 13: 668–675, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Scandaglia M, Lopez-Atalaya JP, Medrano-Fernandez A, Lopez-Cascales MT, Del Blanco B, Lipinski M, et al.: Loss of Kdm5c causes spurious transcription and prevents the fine-tuning of activity-regulated enhancers in neurons. Cell Rep 21: 47–59, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song MS, Rossi JJ: Molecular mechanisms of dicer: Endonuclease and enzymatic activity. Biochem J 474: 1603–1618, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pastorelli LM, Wells S, Fray M, Smith A, Hough T, Harfe BD, et al.: Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome 20: 140–151, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Trepiccione F, Soukaseum C, Iervolino A, Petrillo F, Zacchia M, Schutz G, et al.: A fate-mapping approach reveals the composite origin of the connecting tubule and alerts on “single-cell”-specific KO model of the distal nephron. Am J Physiol Renal Physiol 311: F901–F906, 2016. [DOI] [PubMed] [Google Scholar]
- 48. Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, et al.: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, et al.: Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86: 757–767, 2014. [DOI] [PubMed] [Google Scholar]
- 50. Hajarnis S, Yheskel M, Williams D, Brefort T, Glaudemans B, Debaix H, et al.: Suppression of microRNA activity in kidney collecting ducts induces partial loss of epithelial phenotype and renal fibrosis. J Am Soc Nephrol 29: 518–531, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang W, Liu H, Wang T, Zhang T, Kuang J, Luo Y, et al.: Tonicity-responsive microRNAs contribute to the maximal induction of osmoregulatory transcription factor OREBP in response to high-NaCl hypertonicity. Nucleic Acids Res 39: 475–485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jung HJ, Kwon TH: New insights into the transcriptional regulation of aquaporin-2 and the treatment of X-linked hereditary nephrogenic diabetes insipidus. Kidney Res Clin Pract 38: 145–158, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, et al.: Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, Shin J, et al.: Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest 119: 3290–3300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, et al.: DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28: 652–664, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sung CC, Chen L, Limbutara K, Jung HJ, Gilmer GG, Yang CR, et al.: RNA-Seq and protein mass spectrometry in microdissected kidney tubules reveal signaling processes initiating lithium-induced nephrogenic diabetes insipidus. Kidney Int 96: 363–377, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moeller HB, Praetorius J, Rützler MR, Fenton RA: Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A 107: 424–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.