Significance Statement
Apical polarity proteins are key regulators of podocyte function, particularly with respect to slit diaphragm (SD) integrity. However, no experimental evidence demonstrates basolateral polarity proteins regulate SDs, suggesting apicobasal polarity in itself may not be important. Using Drosophila nephrocyte SDs as a model, this study reports the basolateral polarity module promotes SD integrity through roles in endocytic trafficking. These findings suggest apicobasal polarity proteins are cooperative regulators of SDs, and connect these polarity proteins to other pathways important for SD integrity.
Keywords: cytoskeleton, endocytosis, glomerular disease, glomerular filtration barrier, podocyte
Abstract
Background
Podocyte slit diaphragms (SDs) are intercellular junctions that function as size-selective filters, excluding most proteins from urine. Abnormalities in SDs cause proteinuria and nephrotic syndrome. Podocytes exhibit apicobasal polarity, which can affect fundamental aspects of cell biology, including morphology, intercellular junction formation, and asymmetric protein distribution along the plasma membrane. Apical polarity protein mutations cause nephrotic syndrome, and data suggest apical polarity proteins regulate SD formation. However, there is no evidence that basolateral polarity proteins regulate SDs. Thus, the role of apicobasal polarity in podocytes remains unclear.
Methods
Genetic manipulations and transgenic reporters determined the effects of disrupting apicobasal polarity proteins in Drosophila nephrocytes, which have SDs similar to those of mammalian podocytes. Confocal and electron microscopy were used to characterize SD integrity after loss of basolateral polarity proteins, and genetic-interaction studies illuminated relationships among apicobasal polarity proteins.
Results
The study identified four novel regulators of nephrocyte SDs: Dlg, Lgl, Scrib, and Par-1. These proteins comprise the basolateral polarity module and its effector kinase. The data suggest these proteins work together, with apical polarity proteins, to regulate SDs by promoting normal endocytosis and trafficking of SD proteins.
Conclusions
Given the recognized importance of apical polarity proteins and SD protein trafficking in podocytopathies, the findings connecting basolateral polarity proteins to these processes significantly advance our understanding of SD regulation.
Podocytes develop from cuboidal epithelial cells that are polarized along an apicobasal axis (Figure 1A). Apicobasal polarity is characterized by the asymmetric distribution of apical and basolateral polarity proteins to distinct domains of the plasma membrane (PM). Apical polarity proteins restrict the localization of basolateral polarity proteins to the basolateral domain, and vice versa. In epithelial cells, apicobasal polarity complexes help stabilize and position adherens and tight junctions, which themselves form a boundary between apical and basolateral domains. Loss of apical polarity proteins can result in expansion of the basolateral domain and loss or mislocalization of intercellular junctions.1 Similarly, the basolateral polarity module—which includes the proteins Discs large (Dlg), Scribble (Scrib), and Lethal giant larvae (Lgl)—defines the basolateral domain of the PM and limits spreading of the apical domain (Figure 1A).2
Figure 1.
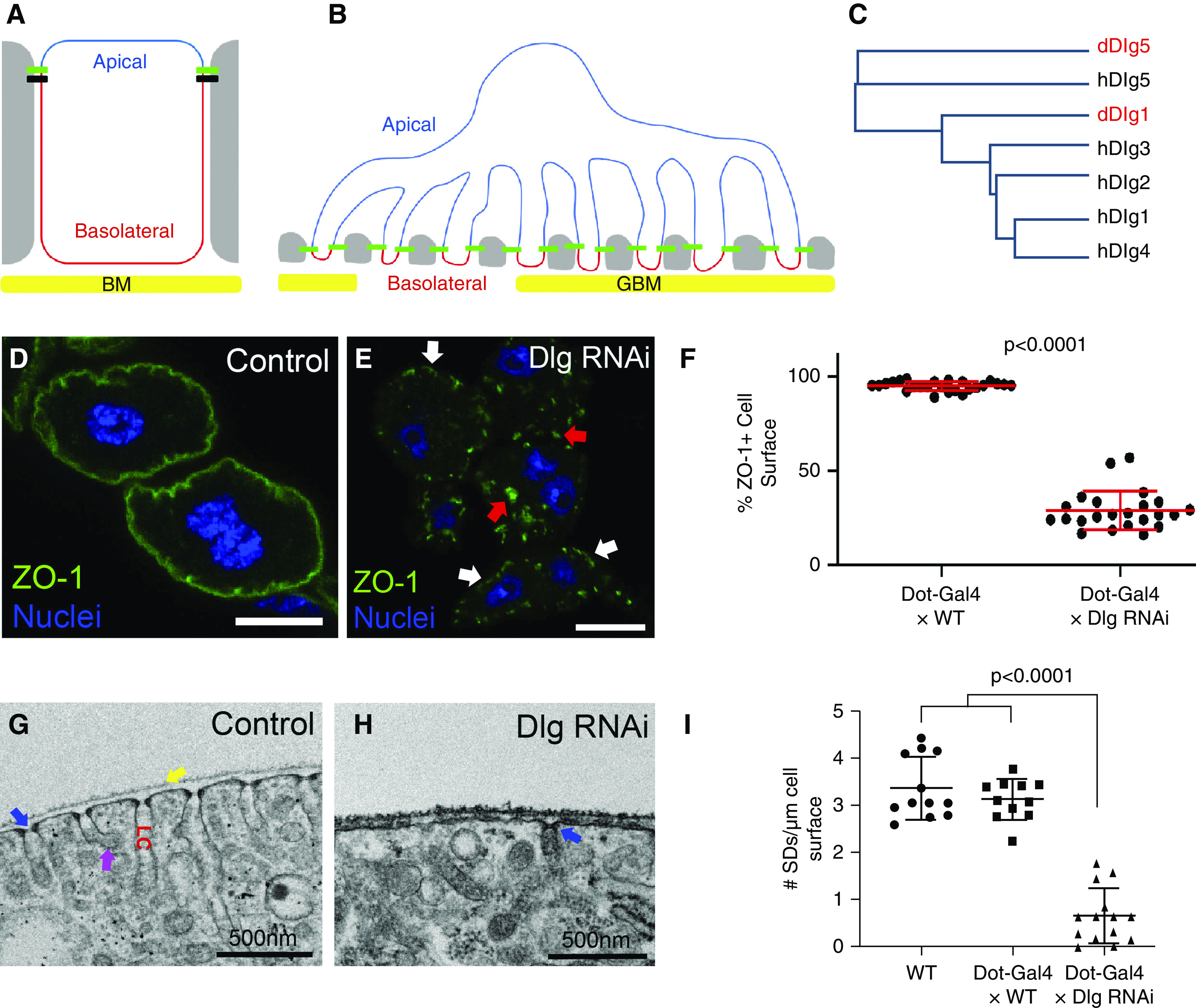
Basolateral polarity proteins promote SD formation. (A) Diagram of a classic polarized epithelial cell, such as an immature podocyte precursor, highlighting the apical domain (blue) and the basolateral domain (red), which abuts the basement membrane (BM; yellow). Tight junctions (green) and adherens junctions (black) connect to neighboring cells (gray). The apical domain in epithelia typically contains polarity proteins such as Crb, aPKC, Par3, and Par6; whereas the basolateral polarity module (Dlg, Scrib, and Lgl) localizes to the basolateral domain. (B) The complex architecture of a mature podocyte retains some aspects of apicobasal polarity, although the apical domain (blue) is now greatly expanded and the basolateral domain (red) is restricted to the region below the SDs (green), adjacent to the GBM (yellow). SDs form the intercellular junctions connecting foot processes of the featured podocyte to foot processes of neighboring podocytes (gray). (C) Dendrogram based on protein sequence alignment of the five human and two Drosophila Dlg proteins; fly Dlgs indicated in red. Human Dlg1 to 4 proteins are most similar to fly Dlg1. Both human and fly Dlg5 proteins cluster separately. (D) Control nephrocyte (Dot-Gal4×WT) stained for the SD protein ZO-1 (green) shows uniform localization along the entire surface of the cell (95.3% of cell surface stains positive for ZO-1; n=25). Note that garland nephrocytes are binucleate (blue). (E) KD of fly Dlg (Dot-Gal4×Dlg RNAi) significantly disrupts ZO-1 localization along the surface of the nephrocyte (only 29.1% of cell surface is ZO-1+; n=23; P<0.001 compared with control); graphed in (F). Small regions of cell surface retain ZO-1 localization (white arrows), whereas numerous internal aggregations can be found in the cytoplasm (red arrows). (G) TEM showing a cross-sectional view of a small surface region from a control nephrocyte. SDs are regularly spaced across the entire surface of the cell; one SD is highlighted by the blue arrow. Nephrocytes are covered with a basement membrane (yellow arrow). Internal to the SDs are invaginations of the PM known as labyrinthine channels (LC). The membrane along these channels is highly endocytic, with many clathrin-coated vesicles budding off (magenta arrow). (H) Consistent with our ZO-1+ surface localization analysis, TEM images of Dlg-KD cells show significant reduction in the number of SDs (blue arrow); quantified in (I). Error bars represent mean and standard deviation. Scale bars, 10 μm.
During podocyte maturation, apical adherens and tight junctions migrate basally and are replaced by SDs.3 This junctional remodeling occurs concurrently with podocyte arborization. Mature SDs contain the transmembrane proteins Nephrin and Neph1, which connect to the actin cytoskeleton through adaptors such as ZO-1 and CD2AP.4–6 Many genetic causes of nephrotic syndrome affect SD-associated proteins or actin regulators.7–9 Mouse studies suggest apicobasal polarity is retained in mature podocytes, with the apical domain greatly expanded and the basolateral aspect restricted to narrow regions of foot process PM adjacent to the glomerular basement membrane (GBM) (Figure 1B).3 Polarization of mature podocytes appears critical to their function. For example, SDs are positioned basally, adjacent to the GBM, where they can participate in filtration. Furthermore, basally restricted adhesion proteins can interact with their GBM partners, whereas proteins, such as Podocalyxin, are apically enriched, which promotes foot process separation.10
Previous studies of apicobasal polarity in podocytes provide important insights. The apical polarity proteins Partitioning defective 3 (Par3), Par6, Crumbs2 (CRB2), and atypical protein kinase C (aPKC) are associated with, or are directly bound to, SD proteins.11–15 Functional studies suggest key roles for CRB2, aPKC, Pals, and Par3 proteins in podocyte polarity and SD formation.13–17 (preprint),18 Mutations in CRB2 are causal in some patients with steroid-resistant nephrotic syndrome,19,20 and genetic variants in aPKC were recently identified as contributors to albuminuria.21 These findings demonstrate the importance of apical polarity proteins in podocyte SDs and glomerular filtration; however, ascribing these defects to disruption of podocyte polarity is complicated by studies in other cell types demonstrating that apical polarity proteins also serve functions that appear polarity independent, such as regulation of cell signaling, proliferation, planar cell polarity, endocytosis, and metabolism.22–28 Notably, some data indicate that aPKC and Par3 promote SD integrity by regulating intracellular trafficking of SD proteins.29 In addition, CRB2 localizes basally in mature mouse podocytes, suggesting divergence from a classic apical polarity function.30 Convincing support for the importance of apicobasal polarity in podocyte SD formation and function could be provided by demonstration of a role for the basolateral proteins; however, studies examining a possible role for basolateral proteins in podocyte SD formation found no such evidence.31,32 Most notably, mouse podocytes lacking Scrib do not have obvious defects in SD formation, morphology, or filtration function, despite the fact that Scrib is expressed in podocytes and localizes to the basolateral domain.32 Consequently, identifying a role for basolateral polarity, if it serves one, remains elusive, precluding our ability to define the relationships between cell polarity, SD formation, and podocyte function.
One possible explanation for the lack of phenotype in Scrib-deficient podocytes is genetic redundancy. In mammalian cells, many polarity proteins localize to the expected polarized domains; however, functional studies have largely been inconclusive as to whether their function in apicobasal polarity is conserved.33 A recent study in colon cancer cells demonstrated it is necessary to simultaneously delete three Scrib protein family members (Scrib, Lano, and Erbin) to disrupt cell polarity.34 In Drosophila epithelial cells, which often have a single protein family member, severe polarity defects can be observed with loss of a single polarity protein (e.g., Scrib).35 Similarly, humans and mice possess five Dlg proteins, whereas flies have only two. The most thoroughly characterized is fly Dlg1, which is homologous by protein sequence to four of the five human or mouse Dlgs (hDlg1 to hDlg4; Figure 1C), underscoring the possibility of genetic redundancy in mammals. In fact, genetic redundancy has already been demonstrated for the apical polarity proteins aPKC and Par3 in mouse podocytes—the most severe defects requiring deletion of two family members. 15,17 (preprint)
Thus, although numerous studies reveal the importance of apical polarity proteins in podocyte development and disease, it is not clear if this reflects polarity or polarity-independent functions. To help resolve this, we asked: (1) do any basolateral polarity proteins function in SD formation, and (2) if so, is it through their role in cell polarity? We addressed these questions using the Drosophila model system for three reasons. First, their simplified genome has limited genetic redundancy. Second, flies provide an unparalleled system for manipulation of gene expression and function. Third, the fly nephrocyte is an excellent model of podocyte SDs. The nephrocyte SD contains homologs of podocyte SD proteins, including Nephrin, Neph1, and ZO-1,36,37 and has been used to study SD regulation by apical polarity proteins Crb, Par3, and aPKC.17 (preprint),21,38 In this study, we identify roles for the basolateral polarity proteins Dlg, Scrib, Lgl, and Par-1 in SD formation. We also provide evidence of a functional relationship between the basolateral and apical polarity complexes in SD regulation. Mechanistically, our data suggest basolateral proteins facilitate SD protein trafficking. These findings are relevant to several podocytopathies and provide new insights into the complex regulation of SDs.
Methods
Drosophila Genetics and Stock Information
The following fly stocks were attained from the Bloomington Drosophila Stock Center: yellow white (yw; #1495) was used as wild type (WT), UAS Dlg RNA interference (RNAi) was used in most experiments (#36771), UAS Dlg RNAi second line (#33620), dlg2 (#36278), UAS Scrib RNAi (#35748), UAS Lgl RNAi (#35773), UAS Par-1 RNAi (#32410), UAS Crb RNAi (#34999), UAS Sec61B:tdTomato (#64747), Golgi:eYFP (#7193), endoplasmic reticulum (ER):eYFP (#7195), UAS Par3:mCherry (#65844), deficiency (df) uncovering scrib (#8105), df uncovering lgl (#24626), UAS Megalin RNAi (#33940), and rab52 (#42702). We used the following additional RNAi lines from the Vienna Drosophila Resource Center: UAS Nephrin (fly Sns) RNAi (#109442), UAS Neph1 (fly Duf/Kirre) RNAi (#109585 and #3111), and UAS Par3 (fly Bazooka) RNAi (#2915). We also used the following additional stocks: Dot-Gal4 (Dr. Deborah Kimbrell, University of California [UC], Davis), Sns-Gal4 (Dr. Susan Abmayr, Stowers Institute), par3xi106 (Dr. Mark Peifer, University of North Carolina [UNC] at Chapel Hill), UAS Dlg:GFP (Dr. Bingwei Lu, Stanford University), scrib2,39 and lgl4 (Dr. Teresa Bonello, UNC; Dr. David Bilder, UC Berkeley). Flies were fed standard Bloomington formulation food and maintained at 25°C, unless otherwise noted.
Microscopy Sample Preparation, Imaging, and Statistical Analyses
Third-instar larvae of indicated genotypes were hemi-dissected and inverted in 1× PBS, and then fixed for 20 minutes in 4% paraformaldehyde (PFA). We then washed three times in 0.1% PBT for 10 minutes per wash. Samples were blocked for 1 hour to overnight in PBT with 1% normal goat serum before overnight incubation in primary antibodies at 4°C. Samples were washed again in PBT before nephrocytes and associated proventriculi were isolated and mounted for imaging. The primary antibody information is provided in Supplemental Table 1. Secondary antibodies were diluted at 1:500 (Invitrogen). Nuclei were labeled with 4′,6-diamidino-2-phenylindole (Sigma) or RedDot2 (Biotium). Confocal images were acquired on Zeiss 700, 710, and 880 and Pascal microscopes. Image adjustments and analyses were performed with ImageJ/Fiji, Adobe Photoshop, Invitrogen Celleste 5.0, or Zeiss ZEN software.
Quantification of ZO-1–positive surface staining was performed in ImageJ, using the following procedure. A central, cross-sectional z-slice was selected from a z-stack of confocal images. The total perimeter of the cell was measured, and line segments were then drawn over the portion of the cell surface with clear enrichment of ZO-1. The percentage of the total perimeter that was positive for ZO-1 was calculated, and the means of the indicated genotypes were statistically compared using ANOVA with the Tukey multiple comparisons test (GraphPad 8.1.2). The adjusted P values are shown in the figures. In the different genetic-interaction studies, the same data are used for control (Sns-Gal4×WT) and Dlg RNAi (Sns-Gal4×Dlg RNAi).
Quantification of internal SDs was performed by calculating the total number of internal SDs relative to the total number of normal, external SDs present in several (n≥6) transmission electron microscopy (TEM) images of each indicated genotype. The numbers of internal versus external SDs were statistically compared by chi-squared test using GraphPad 8.1.2.
For TEM, hemi-dissected larvae were incubated in 4% PFA for 20 minutes, and then washed briefly with 1× PBS. The nephrocytes and attached proventriculi were isolated and placed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, and stored at 4°C before processing. After three washes in 0.1 M sodium cacodylate buffer, the samples were postfixed for 30 minutes in 1% osmium tetroxide/0.1 M sodium cacodylate buffer. The nephrocytes were rinsed with deionized water and dehydrated through increasing concentrations of ethanol (30%, 50%, 75%, 90%, and three times at 100%, for 10 minutes each), followed by two changes of propylene oxide (10 minutes each). Samples were infiltrated for 2 hours in a 1:1 mixture of propylene oxide and PolyBed 812 resin, and infiltrated overnight in 100% PolyBed 812 resin (Polysciences, Warrington, PA). After resin polymerization at 60°C for 24 hours, ultrathin sections (70–80 nm) were cut using a Leica Ultracut UCT ultramicrotome (Leica Microsystems, Buffalo Grove, IL) and a diamond knife, mounted on 200-mesh copper grids. Sections were stained with 4% aqueous uranyl acetate for 12 minutes and lead citrate for 8 minutes. Samples were observed with a JEOL JEM-1230 transmission electron microscope operating at 80 kV (JEOL USA, Peabody, MA), and digital images were acquired using a Gatan Orius SC1000 CCD camera and Gatan Microscopy Suite 3.0 software (Gatan Inc., Pleasanton, CA).
Assays of Nephrocyte Function
For the dextran uptake assays, nephrocytes of the indicated genotypes were dissected in room temperature Grace’s Insect Media (Gibco), and then incubated for 10 minutes together with fixable 10-kD dextran conjugated with 488 nm dye or fixable 70-kD dextran conjugated with Texas Red dye (0.5 mg/ml Grace’s Media) (Invitrogen). Cells were washed three times with Grace’s Insect Media, fixed in 4% PFA, mounted, and imaged by confocal microscopy. The same acquisition settings were applied to both the control and knockdown (KD) nephrocytes. The mean gray value for each cell was calculated in ImageJ. These values were then compared in GraphPad using ANOVA with the Dunnett multiple comparisons test. The number of puncta in the 10-kD dextran experiment was determined in ImageJ by first applying the same pixel intensity threshold to all images, and then using the analyze particles function to calculate the number of puncta per 100 μm2. Statistical comparisons were performed in GraphPad using ANOVA with the Dunnett multiple comparisons test. The number of lysosomes/α-vacuoles in control and Dlg-KD cells was determined from ten TEM images per genotype, standardized by area. Statistical significance was determined in Excel using the unpaired t test.
To compare adult survival in control and KD animals, we transferred newly eclosed flies of the indicated genotypes into food vials (maximum density of 20 flies per vial). The number of living flies was measured every 1–3 days, and flies were transferred to fresh food vials every 2–3 days. Survival graphs were generated in GraphPad Prism 7.04, and statistical significance was determined using the Mantel–Cox log-rank test. For silver nitrate (AgNO3) experiments, ten first-instar larvae of the indicated genotypes were placed on Nutri-Fly Instant Food (Genesee Scientific), with or without 0.005% AgNO3 (Fisher). For each feeding experiment, three to five replicates were performed. The number of eclosed flies and dead pupae/larvae were scored and statistically compared in GraphPad using the Fisher exact test.
Human and Drosophila Dlg Protein Dendrogram
The dendrogram comparing the relationships of the known human Dlg proteins (hDlg1 to hDlg5) and the two known fly Dlg proteins (dDlg1 and dDlg5) was generated by UniProt, using the Clustal multiple sequence alignment tool.40 For esthetics, we modified the appearance of the UniProt dendrogram, while maintaining relative branch lengths. The same multisequence alignment of the five mouse Dlg proteins and the two fly Dlgs produced a dendrogram with the same relationships as the human-fly Dlg alignment.
Results
Basolateral Polarity Proteins Regulate Nephrocyte SDs
To determine if basolateral polarity proteins contribute to SD formation, we used the molecularly and functionally conserved SDs in Drosophila garland nephrocytes.7,36,37,41,42 Nephrocytes filter the blood, reducing systemic damage caused by toxic molecules.43–45 To label SDs, we used antibodies against Polychaetoid, the fly homolog of ZO-1; for simplicity, we use mammalian protein names. ZO-1 links the SD to the actin cytoskeleton, and loss of ZO-1 in podocytes or nephrocytes leads to significant loss of SDs.5,37,46 In WT nephrocytes, cross-sectional confocal imaging of anti–ZO-1 staining shows ZO-1 is specifically and uniformly enriched along the periphery of the cell (Figure 1, D and F). High-resolution imaging of the nephrocyte surface indicates ZO-1 is specifically localized to the SD (Supplemental Figure 1, A and B).17 (preprint),47 TEM reveals nephrocyte SDs form between the many small membrane protrusions repetitively decorating the surface of the same cell (Figure 1G).
We initially focused on Dlg because it is a central component of the basolateral polarity module and was reported to have reduced endocytic capacity in nephrocytes, which can indicate defects in SD integrity.48 Dlg KD in nephrocytes was accomplished by crossing a Dlg1 (hereafter, Dlg) RNAi line to Dot-Gal4, which drives high expression in nephrocytes.49 This led to dramatic mislocalizations of ZO-1 from the cell surface into cytoplasmic aggregations (Figure 1, E and F). We verified the ZO-1 mislocalization phenotype using a different nephrocyte Gal4 driver, a second Dlg RNAi transgene, and a temperature-sensitive mutation in the dlg locus (Supplemental Figure 2, B–F). Dlg KD was dose dependent because animals homozygous for both the Gal4 driver and RNAi transgenes had a more severe phenotype (Supplemental Figure 2, A–D); for the remaining experiments in this study, animals were heterozygous for indicated transgenes. We also examined the potential role of the fly Dlg5 homolog in nephrocyte SD integrity, but observed no defects in ZO-1 localization in Dlg5-KD nephrocytes (Supplemental Figure 2G). To confirm ZO-1 mislocalization reflects SD disruption, we compared the ultrastructure of WT and Dlg-KD nephrocytes. Indeed, Dlg KD significantly reduced the number of SDs (Figure 1, H and I). We also costained Dlg-KD nephrocytes for ZO-1 and the fly homolog of Neph1, and found significant mislocalization of both ZO-1 and Neph1 (Figure 2, A and B). This costaining also revealed these proteins colocalized, even when mislocalized away from the SD, consistent with recent data suggesting these proteins remain in complex even when not localized to SDs.46,50 We also examined fly Nephrin in Dlg-KD cells and observed similar mislocalizations (Supplemental Figure 2, H and I). These experiments identify Dlg as a novel regulator of the nephrocyte SD.
Figure 2.
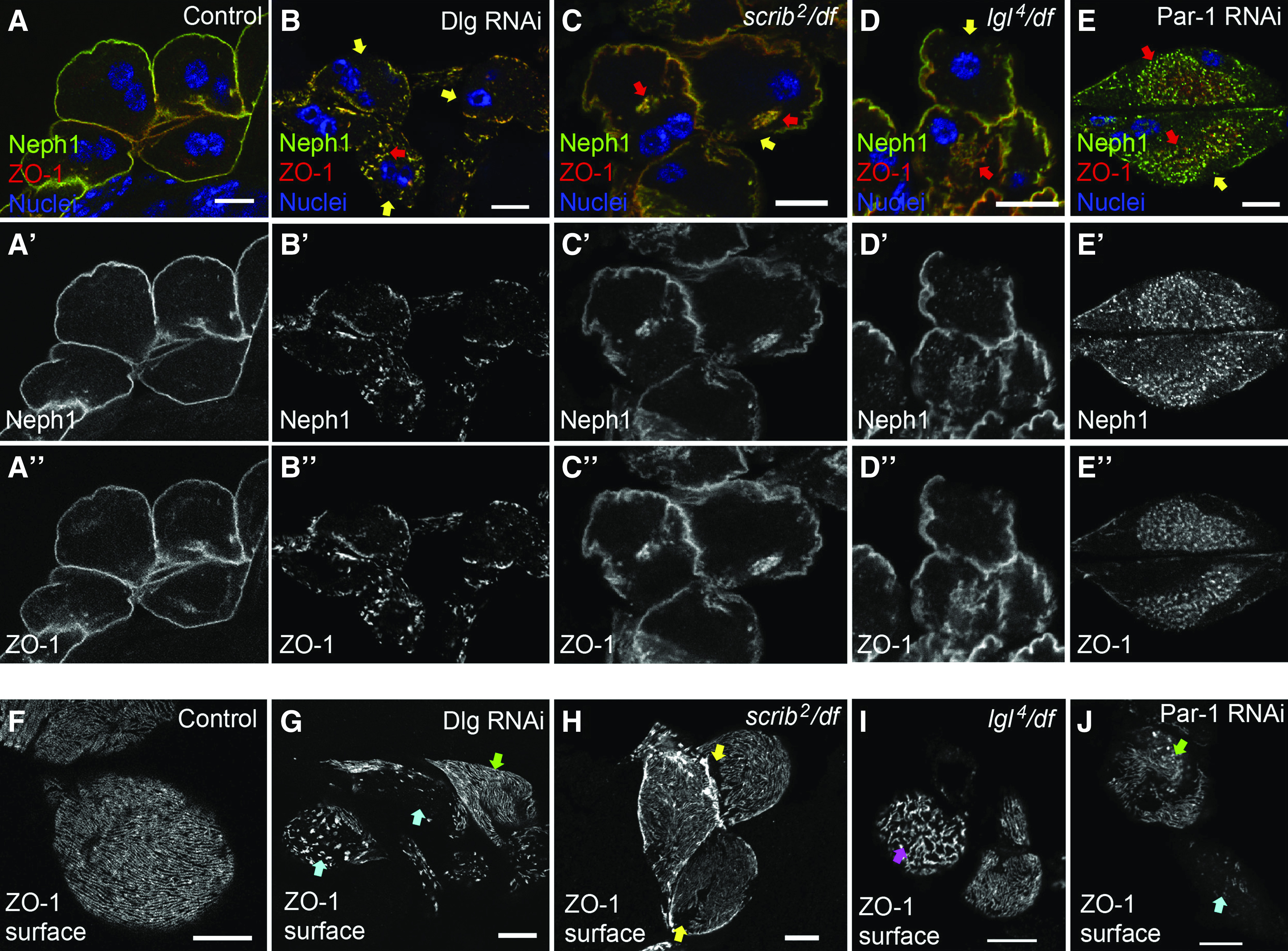
Multiple basolateral polarity proteins contribute to SD protein localization. (A-A'') In control nephrocytes (Sns-Gal4 alone), ZO-1 (red) and the core SD protein Neph1 (green) colocalize along the entire cell surface. (B-B'') In Dlg-KD cells (Sns-Gal4×Dlg RNAi), both ZO-1 and Neph1 are mislocalized from the surface, resulting in gaps at the surface (yellow arrows) and internal aggregations where these proteins are comislocalized (red arrows). (C-C'') Loss of scrib (scrib2 null mutant in trans to a df lacking the entire scrib locus), also causes loss of ZO-1 from the cell surface (yellow arrow) (77.1%; n=29; P<0.001 compared with WT), and into internal aggregations (red arrows). (D-D'') Similar, although less penetrant, phenotypes were observed in lgl4/df null mutant nephrocytes. (E-E'') Par-1 KD (Sns-Gal4×Par-1 RNAi) also caused ZO-1 mislocalization from the cell surface (58.8%; n=15; P<0.001 compared with control) into cloud-like accumulations of ZO-1 and Neph1 puncta (red arrows). (F) A confocal slice at the cell surface reveals the fingerprint-like pattern of the curvilinear SDs in normal nephrocytes. (G) In Dlg-KD cells, the en face view shows defects ranging from mild (green arrow) to severe (blue arrows). In agreement with the cross-sectional views, surface images of (H) scrib and (I) lgl mutant nephrocytes show a largely normal appearance of SDs, although some cells possess small regions lacking SDs (yellow arrows) or truncated, disorganized SDs (pink arrow). (J) Similar to Dlg KD, Par-1 KD was fairly severe with defects ranging from mild (green arrow) to near complete loss of SDs (blue arrow). Scale bars, 10 μm.
We then asked if the Dlg-KD phenotype reflected its canonic role in the basolateral polarity module, or stemmed from a polarity-independent function, which has been reported for Dlg in other contexts.51 To test this, we first examined whether loss of other basolateral polarity proteins also disrupted SD formation. Indeed, scrib null mutants had decreased ZO-1 and Neph1 at the cell surface and increased cytoplasmic accumulations (Figure 2C). lgl null nephrocytes displayed similar defects in ZO-1 and Neph1 localization (Figure 2D), but were less penetrant than depletion of other basolateral proteins (not quantified). Par-1 kinase is not a core member of the basolateral polarity module, but is a key mediator of the basolateral module, and other polarity-independent events.52 Par-1 KD also led to significant loss of ZO-1 and Neph1 from the surface (Figure 2E); however, the pattern of SD protein mislocalization in Par-1 KD was qualitatively different, showing numerous puncta confined to a centralized region of the cytoplasm (described further below). Again, the costaining for ZO-1 and Neph1 in Scrib-, Lgl-, and Par-1–deficient nephrocytes indicates broad effects on SD protein localization, and indicates these proteins mislocalize together, suggesting they remain in complex away from SDs (Figure 2, B–E).46,50 We also examined the surface pattern of SDs in these various genetic backgrounds. Consistent with our TEM data, the variability of the Dlg-KD phenotype was evident, even within the same animal: some nephrocytes retained regions of intact linear SDs, whereas other cells lacked them almost completely, and there were various states in between (Figure 2E). In agreement with the medial views, scrib and lgl mutants had fairly well-formed SDs, although missing patches and irregular, truncated SDs could be observed on some cells (Figure 2, H and I). Par-1 KD was again similar to Dlg, displaying more severe defects (Figure 2J).
Mislocalization of SD proteins in nephrocytes deficient for Dlg, Scrib, Lgl, or Par-1 suggest they may work together to regulate SDs. To test this, we performed genetic-interaction studies of the basolateral proteins by simultaneous KD of Dlg and a second basolateral polarity protein, and then compared double KDs to single KDs. We opted to use Dlg RNAi driven by Sns-Gal4 because it has an intermediate ZO-1 mislocalization phenotype that is amenable to detection of enhancement or suppression: 51.9% of the cell surface is ZO-1 positive (ZO-1+) (Figure 3, B and G). Scrib KD by RNAi resulted in cells with 83.6% of their surface retaining ZO-1 signal (Figure 3, C and G). Strikingly, codepletion of Dlg and Scrib dramatically enhanced ZO-1 mislocalization: only 20.2% of the nephrocyte surface retained ZO-1 (Figure 3, D and G). This finding suggests Dlg and Scrib work in concert to regulate SD formation. Genetic-interaction experiments with Lgl also revealed significant enhancement of ZO-1 mislocalization when Dlg and Lgl are codepleted (92.9% for Lgl KD alone, compared with 38.3% for Dlg+Lgl KD; Figure 3, E–G). These studies suggest the basolateral polarity module likely works in concert to promote SD formation.
Figure 3.
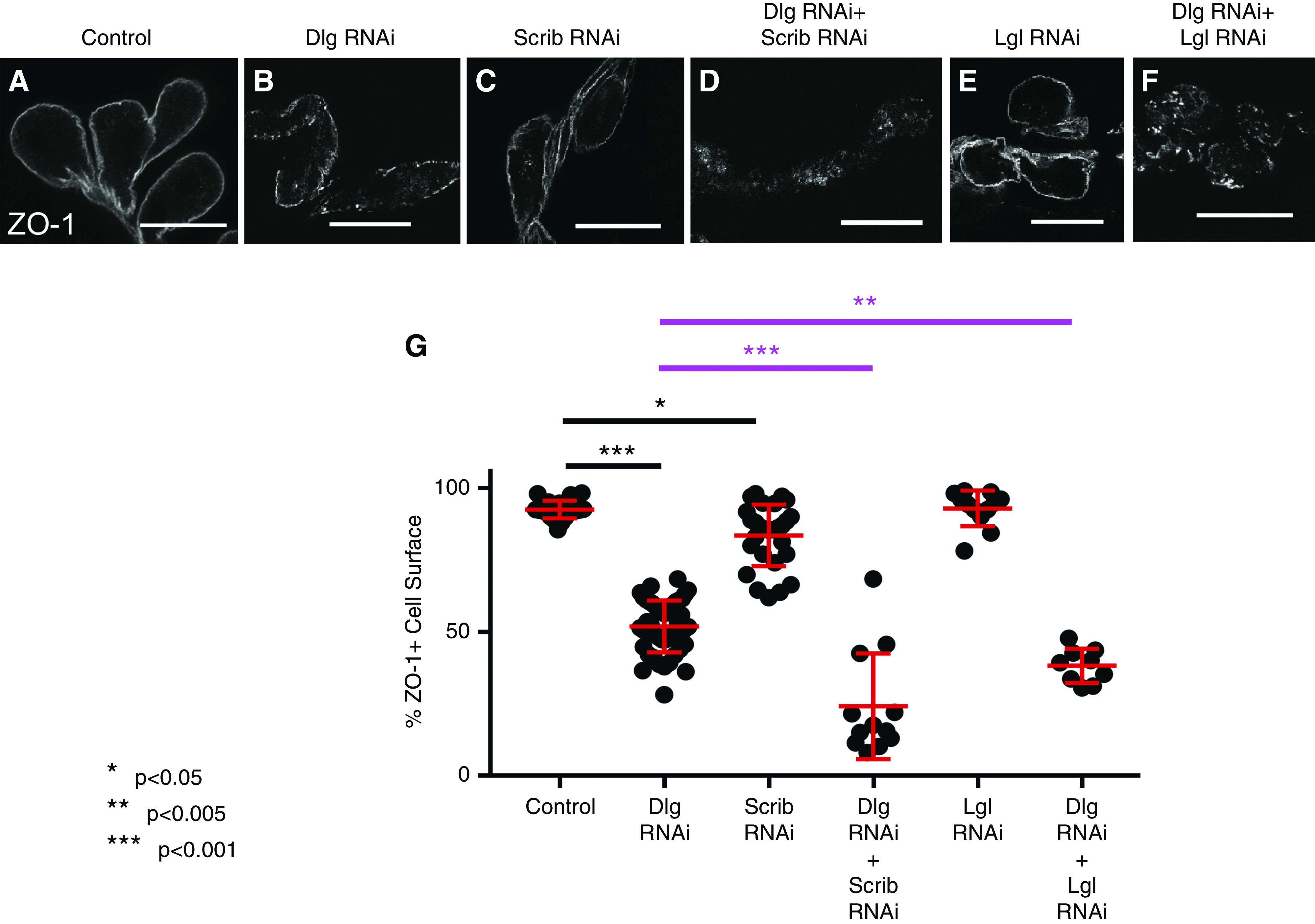
Basolateral polarity proteins cooperatively regulate SD formation. (A and G) In control nephrocytes (Sns-Gal4×WT), 92.6% of the cell surface is ZO-1+ (n=23). (B and G) Dlg RNAi alone results in a significant reduction in ZO-1+ surface (51.9%; n=49; ***P<0.001 compared with control). (C and G) Compared with control, Scrib RNAi alone leads to a modest but significant reduction in ZO-1+ perimeter (83.6%; n=28; *P=0.013). (D and G) KD of both Dlg and Scrib significantly increases ZO-1 mislocalization compared with either single KD genotype (24.2%; n=12; ***P<0.001); the key comparisons of double KDs to Dlg alone are highlighted in magenta. (E and G) Compared with control, Lgl RNAi alone does not significantly disrupt ZO-1 surface localization (92.9%; n=12); (F and G) however, the KD of Dlg and Lgl together significantly enhances ZO-1 mislocalization (***P<0.001 compared with Lgl KD alone and **P=0.0019 compared with Dlg KD alone). For clarity of presentation, some statistical comparisons are not highlighted in the figure (e.g., double KDs compared with control, which are clearly different; P<0.001). Error bars represent mean and standard deviation. Scale bars, 20 μm.
Interestingly, although Par-1 KD alone also led to a significant reduction in ZO-1+ surface staining (58.8%), we detected no significant difference in the amount of ZO-1+ cell surface relative to single KDs in Dlg+Par-1 double KD; however, the unique, punctate phenotype of ZO-1 mislocalization in Par-1 KD (Figure 2E) dominated the Dlg+Par-1 double-KD cells (data not shown). This suggests a more complex relationship between Par-1 and the basolateral proteins, and/or that Par-1 may have polarity-independent functions, upstream of Dlg, in SD formation. This seems plausible, given that Par-1 can phosphorylate many different substrates.53
Basolateral Proteins Are Not Enriched to Specific Membrane Domains, but Function with Apical Proteins
In polarized epithelial cells, apical and basolateral proteins are enriched at their respective PM domains. Previous studies in nephrocytes suggest apical polarity proteins Crb and Par3 localize along labyrinthine channels and SDs,17 (preprint),38 which we also confirmed (Figure 4, A and C). Thus, the PM lining labyrinthine channels may represent the apical domain in nephrocytes. This would imply the basolateral domain may be the region of PM exterior to the SDs, adjacent to the basement membrane. However, antibody staining of Dlg in nephrocytes was diffuse, sometimes punctate, with moderate overall enrichment in the subcortical region of the cytoplasm, perhaps to labyrinthine channels or associated trafficking vesicles (Figure 4, F and I). This pattern is lost in Dlg RNAi nephrocytes (Figure 4G). We verified Dlg’s localization pattern using GFP-tagged Dlg (Figure 4H). Similarly, Par-1 and Scrib had diffuse/punctate localization patterns, with no obvious PM enrichment (Figure 4, J and L). Lgl was also diffuse through the cytoplasm, although it did show weak to moderate enrichment at the cell surface (Figure 4K). Costaining for Dlg and Lgl did not indicate significant colocalization near the periphery of the cells; however, in some nephrocytes, colocalization did occur at dense accumulations of these proteins near the cell center (82% of WT nephrocytes contained the dense accumulations of Dlg [n=31 of 38]; 71% of those also contained Lgl [n=22 of 31]; Figure 4M). This pattern was not apparent with Scrib or Par-1 staining. On the basis of this localization pattern, we considered these Dlg and Lgl aggregations may be associated with the ER or Golgi, however, they did not localize to either organelle (Supplemental Figure 3, A and B). Thus, based solely on their localization patterns in nephrocytes, it is difficult to assign functionality to the basolateral polarity proteins.
Figure 4.
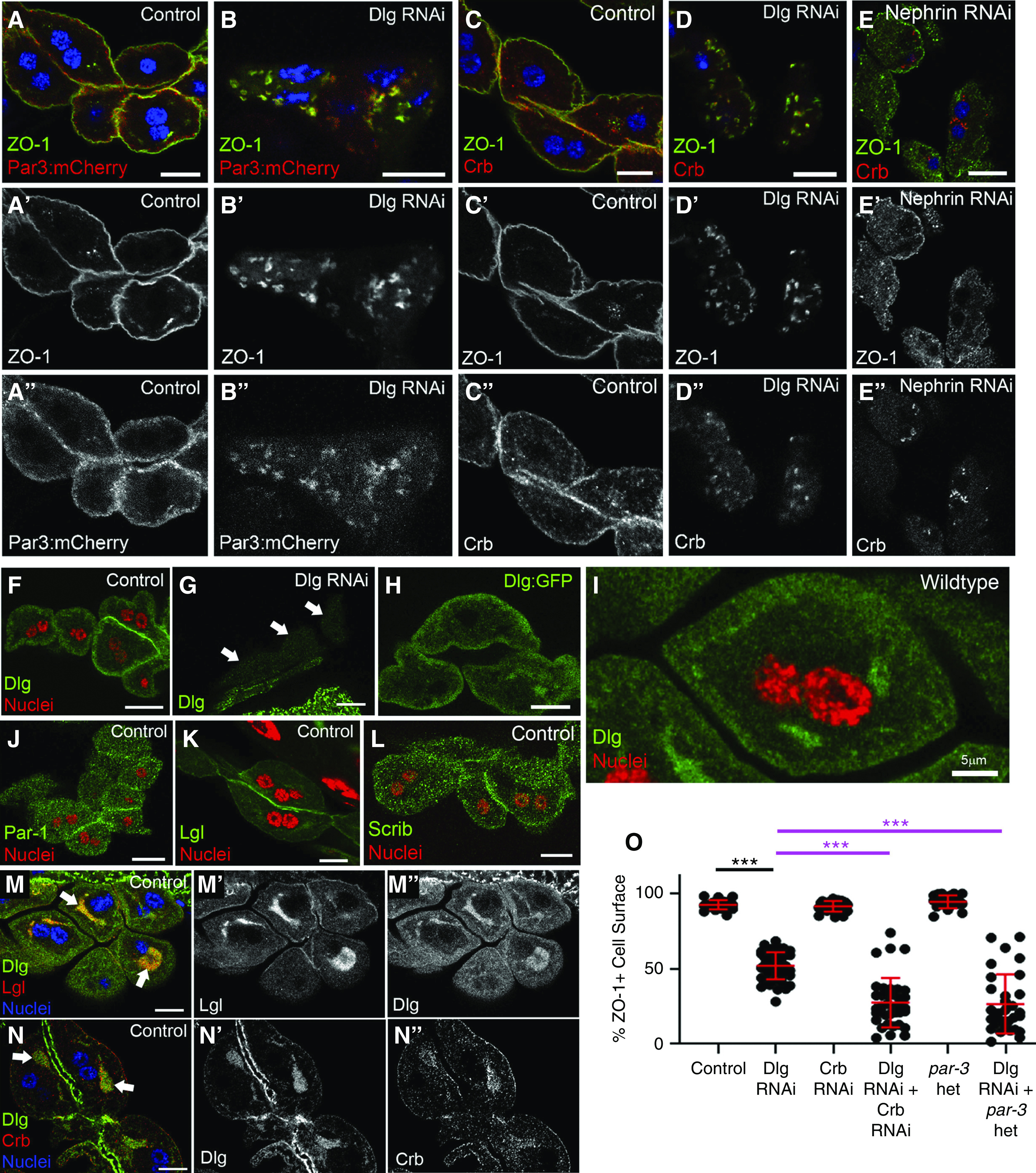
Basolateral proteins are not enriched at specific membrane domains, but cooperate with apical proteins to regulate SDs. (A-A'') In control cells, expression of Par3:mCherry (red) reveals fairly uniform cortical to subcortical localization, with some overlap with the SD protein ZO-1 (green). (B-B'') In contrast, Par3 is strongly mislocalized in Dlg-KD cells to internal aggregates that colocalize with ZO-1. (C-C'') In controls, Crb (red) shows cortical enrichment, with some internal puncta. (D-D'') In Dlg-KD cells, Crb is mislocalized to irregular cytoplasmic structures with ZO-1 (green). (E-E'') Crb and ZO-1 were also mislocalized in Nephrin-KD nephrocytes. However, note that, in Nephrin-KD cells, Crb and ZO-1 do not comislocalize, as seen in Dlg KD (D). (F) In WT nephrocytes, anti-Dlg staining (green) shows a diffuse pattern with some subcortical enrichment; a higher magnification view of Dlg localization is shown in (I). (G) Anti-Dlg staining in Dlg-KD cells shows dramatic loss of signal (white arrows point to three nephrocytes), which suggests the antibody is specific for Dlg protein, confirming that the localization pattern (F and I) is not an artifact. The brighter GFP signal below the nephrocytes is from adjacent cells of the proventriculus and muscle, where Dlg RNAi is not expressed. (H) A similar diffuse, subcortical pattern is observed for GFP-tagged Dlg. Similar localization patterns are observed for (J) Par-1 and (L) Scrib. (K) Lgl is cytoplasmic with some localization to the surface. (M-M'') In a subset of nephrocytes, Dlg and Lgl colocalized in larger internal aggregations. (N-N'') Crb also localized with Dlg at these structures. (O) Quantification of ZO-1+ perimeter in genetic-interaction studies between Dlg and apical polarity proteins. The control (Sns-Gal4×WT) and Dlg RNAi are significantly different from one another (92.6% versus 51.9%; n=23 versus n=49). Crb RNAi alone did not significantly affect ZO-1 localization (91.6%; n=25). However, KD of both Dlg and Crb dramatically reduced the amount of surface ZO-1 (27.6%; n=37). Nephrocytes heterozygous (het) for a par3 loss-of-function allele appear normal (94.6%; n=21). However, Dlg RNAi in a par3 heterozygous background dramatically enhanced the ZO-1 mislocalization phenotype (26.6%; n=31) caused by Dlg KD alone. The most relevant statistical comparisons are highlighted by bars, with magenta bars comparing the interaction groups to Dlg single KD. Error bars represent mean and standard deviation. Scale bars, 10 μm. ***P<0.001.
One function of apical and basolateral polarity complexes in epithelia is regulating localization of the other complex. Thus, we asked if basolateral polarity proteins affect apical polarity protein localization in nephrocytes. Dlg KD perturbed the localization of both Par3 and Crb (Figure 4, B and D). However, it is important to note the disruption of SDs due to loss of Nephrin also disrupted Crb localization (Figure 4E); thus, mislocalization of apical polarity proteins in Dlg-KD cells may be an indirect consequence of SD disruption. It is worth noting that, in Dlg-KD cells, Par3 and Crb comislocalized with ZO-1 at internal aggregates, consistent with the hypothesis that Crb and Par3 may be in complex with SD proteins.12,38 However, in Nephrin-KD cells, mislocalized ZO-1 no longer colocalized with Crb, indicating Nephrin is required to maintain ZO-1’s association with Crb.
To further examine the relationship between basolateral and apical polarity complexes in SD formation, we performed genetic-interaction studies by simultaneous KD of Dlg and Crb. In our hands, KD of Crb alone did not significantly disrupt ZO-1 localization, however, codepletion of Dlg and Crb led to a significant increase in ZO-1 mislocalization, compared with Dlg KD alone (Figure 4O). Because Par3 KD alone causes significant disruption of SDs, which can confound the assessment of genetic interactions, we instead examined ZO-1 localization in Dlg-KD nephrocytes that were also heterozygous for a par3 mutant allele. Although loss of a single copy of par3 did not affect SD integrity, as measured by surface localization of ZO-1, we did observe a significant enhancement of the Dlg-KD phenotype in the par3 heterozygous background (Figure 4O). These data are consistent with a model in which basolateral and apical proteins operate collectively to regulate SD formation. However, alternative interpretations are worth noting; for example, reducing Crb or Par3 levels may affect a process that sensitizes cells to unrelated defects in a separate process caused by Dlg depletion.
Intriguingly, we found that Crb, but not Par3, localized to the cytoplasmic aggregations containing Dlg in WT nephrocytes (79% of Dlg aggregates also contained Crb [n=27 of 34]; Figure 4N, Supplemental Figure 3C). Although the functional significance of these structures remains to be determined, the fact that some apical and basolateral proteins localize here is notable.
To test the hypothesis that basolateral polarity regulators, such as Dlg and Par-1, might affect Crb protein levels, either positively or negatively, we compared the mean fluorescence intensity in control, Dlg-KD, and Par-1–KD cells stained for Crb (Supplemental Figure 4, A–D). We observed a significant reduction in the mean Crb signal intensity in both Dlg- and Par-1–KD cells. The effect was most prominent in Dlg-KD cells. These findings indicate Dlg and Par-1 may promote Crb protein stability. It is worth noting, however, that Crb was also mislocalized in Nephrin-KD cells, thus reduction in Crb protein levels might be an indirect consequence of SD disruption in Dlg- or Par-1–KD cells. Interestingly, we also noted the pattern of Crb mislocalization in Dlg- and Par-1–KD cells was qualitatively different. Dlg-KD cells accumulated Crb in larger cytoplasmic aggregates, whereas Par-1–KD nephrocytes mislocalized Crb from the cell cortex to a more uniform cytoplasmic distribution, which may suggest mechanistic differences in how Dlg and Par-1 affect Crb localization.
Basolateral Polarity Proteins Promote Endocytic Trafficking
Apicobasal polarity is linked to protein trafficking through complex, reciprocal relationships.28 The intracellular aggregations of SD proteins in basolateral polarity–deficient nephrocytes suggest trafficking defects. Therefore, we sought to determine if these proteins were accumulating in a particular organelle or trafficking vesicle. We first examined the sites of protein synthesis and vesicular packaging by labeling the ER and Golgi in Dlg-KD cells, however, mislocalized ZO-1 was not associated with either (Supplemental Figure 5, A–C). Surprisingly, we did notice disorganization of both the Golgi and ER in Dlg-KD cells (Supplemental Figure 5, B and C). Thus, whereas basolateral proteins do not affect movement of SD proteins through the ER or Golgi, they are important for the organization of those organelles. However, it is important to note the organization of the ER and Golgi was also perturbed in nephrocytes depleted of the SD proteins Nephrin or Neph1 (Supplemental Figure 5, D–F), indicating these organelles appear to rely generally on intact SDs.
Endocytic trafficking of SD proteins, like Nephrin, is of great interest in podocyte biology.54 Nephrocytes are highly endocytic cells, and recent studies suggest endocytosis regulates SDs in normal and pathologic contexts.46,47,55 To determine if SD proteins are mistrafficked during endocytosis in Dlg KD, we costained for ZO-1 and Rab5, Rab7, or Rab11; markers of early, recycling, and late endosomes, respectively.56 Interestingly, mislocalized SD protein aggregates were interspersed with, and adjacent to, Rab5-positive vesicles (Figure 5B). We observed no specific association of SD proteins with Rab7 or Rab11 vesicles in Dlg-KD cells (Supplemental Figure 6, C, D, and F); however, the distribution of Rab5, Rab7, and Rab11 vesicles was abnormal in Dlg KD (Figure 5, A and B, Supplemental Figure 6, A–F), suggesting broad disruption of endocytic pathways. Similar defects in Rab5 and Rab7 distributions were recently reported in nephrocytes depleted of Disabled, a regulator of clathrin-mediated endocytosis.57 The Rab5 association with internalized ZO-1 was also observed in scrib mutants (Figure 5C), and was most pronounced in the cloudlike aggregations found in Par-1 KD (Figure 5D). Although mislocalized ZO-1 specifically associated with Rab5 vesicles, they did not perfectly colocalize, suggesting ZO-1 aggregates are adjacent to early endocytic vesicles. This pattern is readily observable in a deconvolved image of Par-1 KD (Figure 5E). Further analysis of these aggregates in Par-1 KD shows that both Rab7 and Rab11 can also be present in these cloudlike aggregations of ZO-1, however, unlike Rab5, they are not specifically enriched there (Supplemental Figure 7, A and B). We observed similar defects and associations between these Rab proteins and the SD proteins Nephrin or Neph1 (Supplemental Figure 8, A–E). These findings suggest mistrafficking of SD proteins in basolateral mutants, leading to their accumulation in Rab5-adjacent structures.
Figure 5.
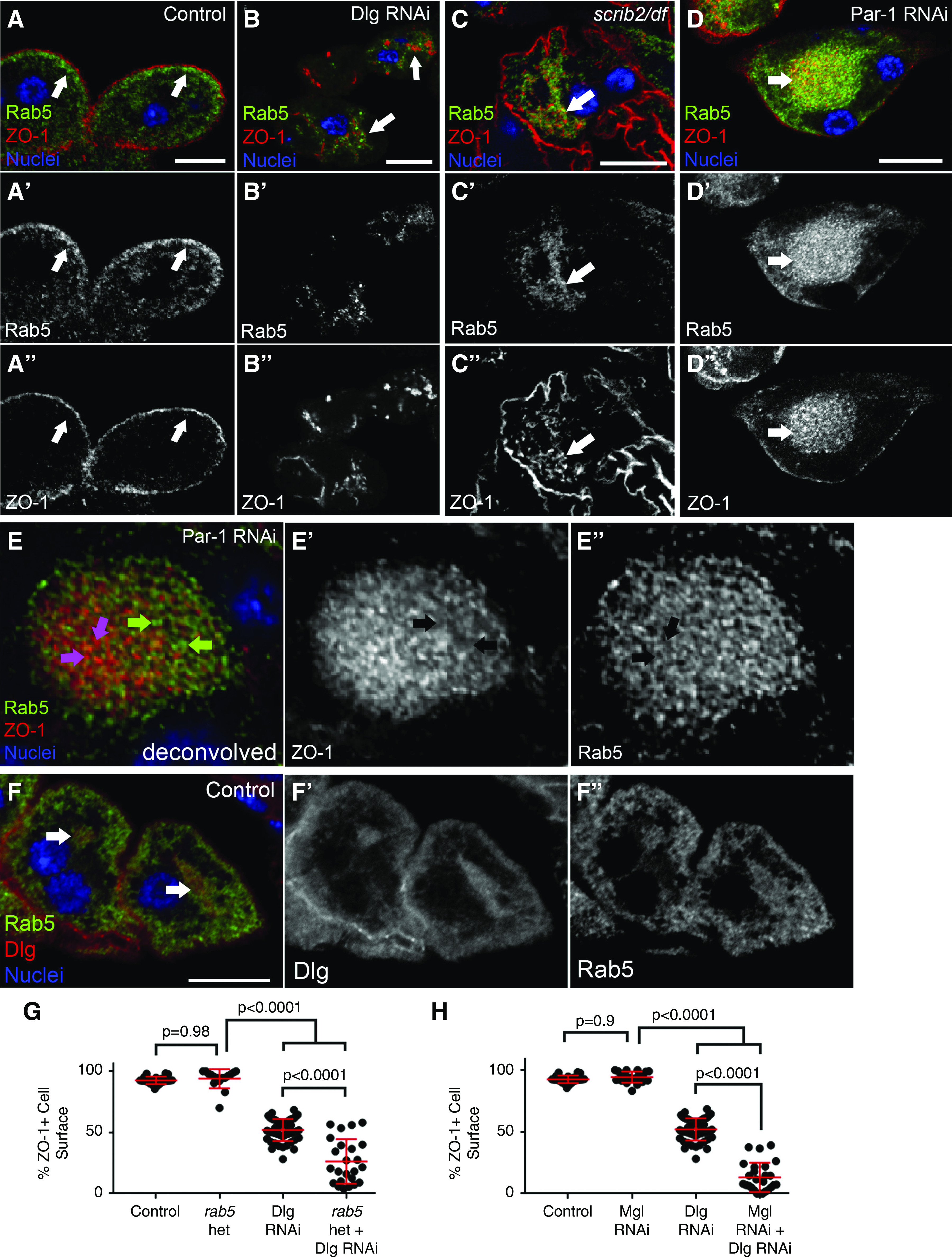
Basolateral polarity proteins are important for endocytic trafficking of SD proteins and nephrocyte function. (A-A'') In control nephrocytes (Sns-Gal4×WT), the early endocytic vesicle marker Rab5 (green) has a punctate pattern concentrated in the subcortical region of the cell (white arrows). (B-B'') In Dlg-KD cells, internally mislocalized ZO-1 (red) associates with Rab5 accumulations (white arrow). (C-C'') Similar associations were observed in scrib null nephrocytes. (D-D'') The enrichment of Rab5+ structures with mislocalized ZO-1 was most obvious in Par-1–KD cells. (E-E'') Deconvolution of a Par-1–KD cell indicates that, although they are tightly associated, Rab5 (green) and ZO-1 (red) do not directly colocalize. Green arrows in (E) highlight Rab5 puncta that do not colocalize with ZO-1; see black arrows in (E′). Conversely, magenta arrows in (E) show ZO-1 puncta that do not contain Rab5; see black arrows in (E″). (F-F'') In some WT nephrocytes, Rab5 can be found in the internal Dlg aggregations (white arrows). (G) Quantification of ZO-1 surface localization reveals that animals heterozygous (het) for rab5 have no defects in ZO-1 localization (93.9%; n=16), whereas Dlg KD in the rab5 heterozygous background (26.3%; n=24) is significantly worse than Dlg KD alone (51.9%; n=49). (H) Nephrocytes depleted of Megalin (Mgl) do not display defects in ZO-1 surface localization (94.3%; n=21); however, double KD of Dlg and Mgl dramatically reduced ZO-1 localization (13.2%; n=26), compared with single KD. Error bars represent mean and standard deviation. Scale bars, 10 μm.
To further explore the relationship between Rab5 and the basolateral polarity machinery, we tested whether reducing the levels of Rab5 affected the ZO-1 mislocalization phenotype observed in Dlg-KD cells. Indeed, in animals heterozygous for a rab5 mutant allele, the severity of the ZO-1 mislocalization caused by Dlg KD is significantly enhanced (Figure 5G). These findings suggest Dlg’s role in promoting SD integrity depends on proper levels of Rab5 protein. Interestingly, closer examination of Rab5 staining in WT nephrocytes showed a weak, but consistent, colocalization between Dlg and Rab5 in the cytoplasmic aggregations described above (Figure 5F), which we have now found to contain Dlg, Lgl, Crb, and Rab5. Again, although the role of these structures is unclear, the pattern of associated localization among these important proteins is consistent with the possibility that they function in related processes.
Previous studies in nephrocytes have shown that endocytosis and SD integrity requires the Cubilin-Amnionless (Cubn-Amn) complex,7,58 which is known to regulate endocytosis in renal tubular cells.59 Given our data suggesting the basolateral polarity proteins affect endocytic trafficking in nephrocytes, we sought to investigate a potential relationship by performing genetic interaction studies between Dlg and Cubn-Amn complex members. Unfortunately, KD of either Cubn or Amn alone causes severe defects that preclude assessment of genetic interaction with Dlg KD. In tubular cells, Cubn functions alongside Megalin to regulate endocytosis.59 However, in nephrocytes, Megalin does not appear as important because Megalin depletion does not significantly disrupt nephrocyte function7; we also found no significant disruption of ZO-1 localization in Megalin-KD nephrocytes (Figure 5H). Therefore, we examined ZO-1 localization in nephrocytes depleted of both Megalin and Dlg, and found a highly significant enhancement of the Dlg single-KD phenotype (Figure 5H). These data suggest Dlg promotes normal trafficking of SD proteins in a manner dependent on correct levels of Megalin. Taken together, our data indicate that basolateral proteins are important for the organization of multiple endosomal compartments and endocytic trafficking of SD proteins. Although these data are consistent with a model in which Dlg’s effect on SD integrity is mediated by a role in endocytic trafficking, we cannot exclude other possibilities, including that Dlg may function in an unrelated cellular process, which, when perturbed in Dlg-KD cells, indirectly affects other pathways, such as endocytosis.
Basolateral Polarity Proteins Are Critical for Nephrocyte Function
On the basis of the defects in endocytic organization described above, and a previous report of reduced endocytosis in Dlg-KD nephrocytes,48 we performed a fluid-phase dextran uptake assay to determine rates of endocytosis. As expected, Dlg or Par-1 KD dramatically reduced 10-kD dextran uptake (Figure 6, A–D). Similar results were observed in scrib mutant nephrocytes (Supplemental Figure 9, A–C). The reduction in endocytosis in Dlg- and Par-1–KD cells can also be observed by comparing the number of dextran-positive vesicles in the fluid-phase uptake experiment (Figure 6F). Similarly, TEM images from Dlg-KD nephrocytes suggest fewer trafficking vesicles compared with controls (Figure 6, G and H). Analysis of TEM images also revealed significantly fewer lysosomes/α-vacuoles in Dlg-KD cells (mean of 0.36/μm2 versus 0.93/μm2 in controls; P<0.001). These data, in addition to the effects on the organization of the endocytic machinery described above, demonstrate the importance of basolateral polarity proteins to proper endocytic trafficking in nephrocytes.
Figure 6.
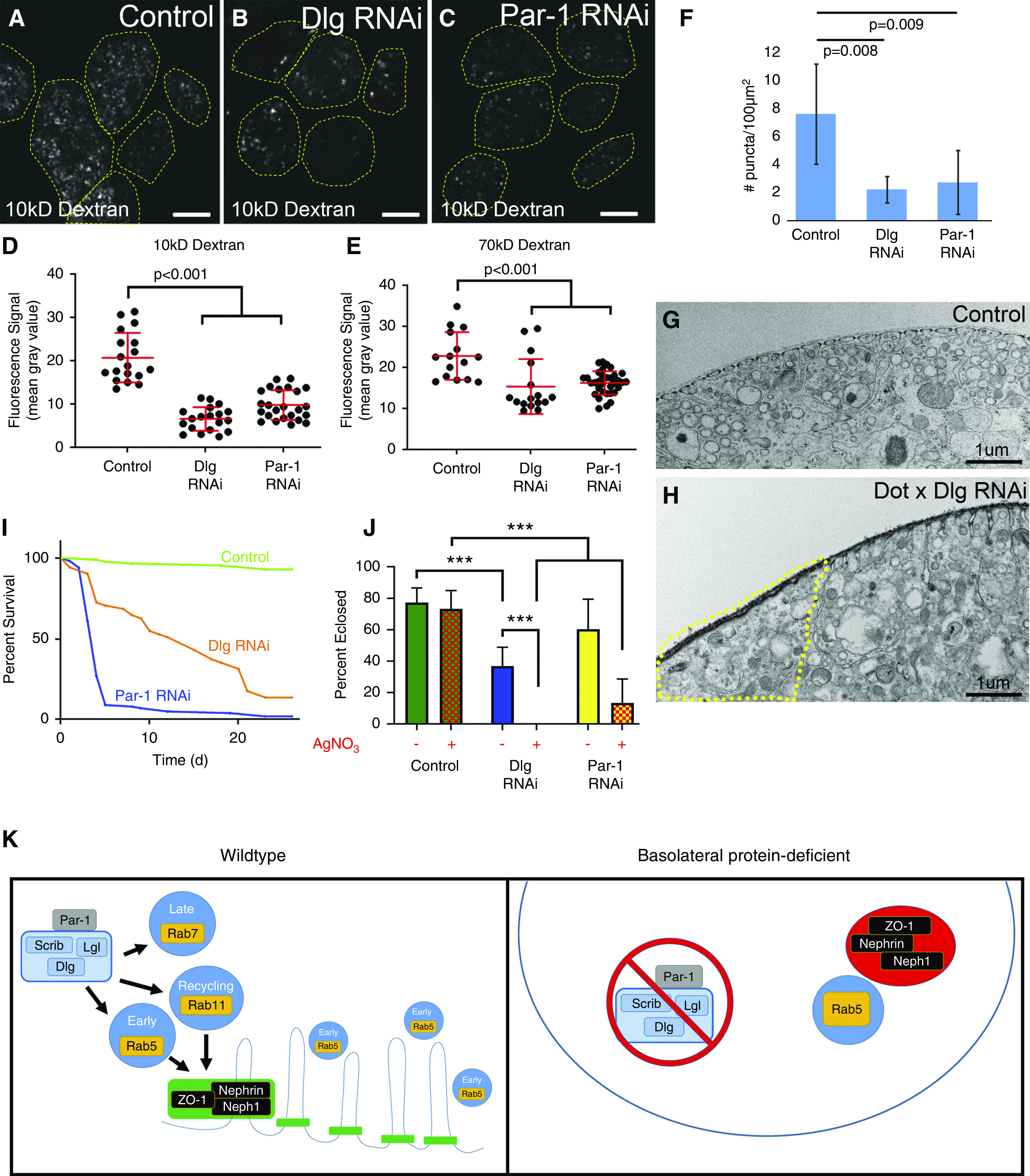
Nephrocyte function relies on the basolateral polarity proteins. (A) In control nephrocytes (Dot-Gal4 alone), there is robust uptake of fluorescently conjugated 10-kD dextran (a fluid-phase marker) into numerous endocytic vesicles (dashed lines depict cell boundaries). In (B) Dlg KD and (C) Par-1 KD, the uptake of 10-kD Dextran is noticeably diminished. (D) Quantification of fluorescence intensity demonstrates reduced levels of endocytosis in Dlg- or Par-1–depleted cells. (E) Similar results were observed with the larger 70-kD dextran. (F) Comparison of the number of puncta per area in control versus Dlg- or Par-1–depleted cells shows a marked decrease in the number of endocytic vesicles. (G) TEM image of a control cell reveals numerous endocytic and other intracellular vesicles, and the regularly interspersed SDs and their associated labyrinthine channels. (H) In comparison, Dlg-KD cells have noticeably fewer intracellular vesicles. This effect is most pronounced in regions of the Dlg-KD cell lacking SDs and labyrinthine channels (area within yellow dashed line); this may be a consequence of the reduced surface area due to absence of labyrinthine channels in this area. (I) Survival curves of control adult flies (Dot-Gal4 alone; n=85) compared with Dlg KD (n=51) and Par-1 KD (n=101). Survival in both KD groups is significantly worse than controls (P<0.001). (J) Comparison of larval survival to eclosure in control, Dlg-KD, and Par-1–KD animals fed diets with (+) or without (−) AgNO3. Although control animals showed no sensitivity to AgNO3, significantly fewer Dlg-KD or Par-1–KD animals survived to adulthood when challenged with AgNO3. Notably, even on regular diet without toxin, fewer Dlg-KD flies survived. Nevertheless, toxin exposure significantly reduced survival of Dlg-KD flies. (K) Models depicting the putative relationships between the basolateral polarity proteins, endocytic machinery, and SDs. In WT nephrocytes (left panel), basolateral proteins promote proper organization and function of the endocytic pathway, which, in turn, is required for the integrity of SDs and labyrinthine channels. Note that it is not clear if these effects are direct or indirect. Par-1 is depicted as outside the basolateral polarity module because it remains to be determined if Par-1 functions as part of that complex in nephrocytes. In nephrocytes lacking basolateral polarity proteins (right panel), particularly Dlg or Par-1, there is a disruption of the endocytic machinery (fewer endocytic vesicles and aberrant distributions), and fewer SDs and labyrinthine channels, leading to mislocalization of SD proteins into cytoplasmic vesicles adjacent to Rab5+ vesicles. Error bars represent mean and standard deviation. Scale bars, 10 μm.
Because nephrocytes and their SDs are also important for hemolymph filtration, we investigated the filtration function of basolateral polarity–deficient nephrocytes by first performing the dextran uptake assay with a larger dextran (70 kD). Nephrocytes depleted of Dlg or Par-1 exhibited significantly reduced uptake (Figure 6E), consistent with nephrocyte studies of known SD regulators.7,41 Previous studies suggested nephrocyte filtration is important for adult survival in flies,41 and we did observe a significant reduction in longevity in flies with nephrocytes depleted of Dlg or Par-1 (Figure 6I). However, other data indicate nephrocytes are not required for adult survival, and such effects may be due to KD in other tissues42,45; this would not be surprising in the case of Dlg and Par-1, which serve critical roles in many other cell types. Thus, a preferred test of nephrocyte filtration function is comparison of survival rates after exposure to toxins, such as AgNO3, which normally accumulate in functional nephrocytes.41 Indeed, compared with control animals, flies with nephrocytes depleted of Dlg or Par-1 had significantly reduced survival during larval/pupal stages when their food was supplemented with 0.005% AgNO3 (Figure 6J). Together, these data suggest basolateral polarity proteins, such as Dlg and Par-1, are critical regulators of nephrocyte function, including endocytosis and filtration of the hemolymph.
Discussion
Growing evidence suggests apicobasal polarity contributes to SD regulation. However, our understanding of the cellular mechanisms underlying important players, such as Crb, Par3, and aPKC, has been restricted by a lack of data supporting a role for basolateral proteins. Genetic redundancy has been reported for some apical polarity proteins, 15,17 (preprint) which may explain why loss of a key basolateral protein, such as Scrib in mouse podocytes, does not perturb cell polarity, SD formation, or renal function.32 Scrib is a LAP family protein that, in mammals, contains four proteins (Scrib, Erbin/ERBB2IP, Lano/LRRC1, and Densin-180/LRRC7), and recent data demonstrate significant functional redundancy in apicobasal polarity.34 Transcriptomic and proteomic studies in mammalian podocytes indicate they express all four LAP family proteins,60,61 consistent with potential functional redundancy. Interestingly, Densin-180 was also found in complex with Nephrin in podocytes.62 A similar potential for redundancy may occur with Dlg because humans and mice possess five Dlgs. A genome-wide association study of patients with FSGS identified Dlg5 as a risk allele, however, Dlg5 KD in mouse podocytes did not cause obvious defects31; we also detected no defects in SD integrity in Dlg5-KD nephrocytes. Our current study in fly nephrocytes provide the first demonstration that basolateral polarity proteins contribute to SD formation. Although challenging, it will now be important to generate vertebrate animal models that simultaneously disrupt multiple genes within basolateral protein families to examine their function in podocytes.
Mechanistically, our data suggest basolateral polarity proteins regulate intracellular trafficking of SD proteins (Figure 6K). Loss of basolateral polarity proteins led to SD protein mislocalization into cytoplasmic vesicles associated with the early endocytic marker Rab5. Notably, Rab5 was recently shown to be a key regulator of SD formation in nephrocytes.47,55 Our genetic-interaction studies between Dlg and Rab5 or Megalin, indicate Dlg’s role in promoting SD integrity relies on proper levels of these known endocytic regulators. We also observed general defects in the organization of the endocytic trafficking machinery in nephrocytes depleted of Dlg. Endocytic defects were also reported for Crb-deficient nephrocytes,38 again consistent with a shared mechanism for the apicobasal polarity machinery in SD regulation. Precisely how disruption of endocytic trafficking causes defects in SDs remains uncertain. Maintenance of SDs may require regular internalization and remodeling of SDs and/or key transmembrane proteins of the SD (i.e., Nephrin and Neph1), as has been found for cadherins at adherens junctions.63 Disruption of recycling endosomes may also prevent return of these proteins to the cell surface. In the context of basolateral polarity proteins, because they can regulate the apical polarity complex, members of which are in complex with SD proteins and regulate protein trafficking, it is possible that the basolateral module affects the SD by regulating apical players that, in turn, facilitate SD protein trafficking.16,28 Alternatively, in basolateral polarity–deficient nephrocytes, the disruption of endocytosis, particularly in highly endocytic cells such as nephrocytes, may lead to multiple forms of cell stress that disrupt SD integrity. Future studies in both nephrocytes and podocytes will be required to unravel these complex relationships.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by UNC Kidney Center endowment funds.
Supplementary Material
Acknowledgments
The authors are grateful to our colleagues for generously sharing reagents: Dr. Susan Abmayr (Stowers Institute), Dr. Mark Peifer and Dr. Teresa Bonello (UNC Chapel Hill), Dr. David Bilder (UC Berkeley), Dr. Deborah Kimbrell (UC Davis), and Dr. Bingwei Lu (Stanford). The authors would like to acknowledge the excellent technical assistance provided by Vicky Madden, Kristen White, and Dr. Pablo Ariel at UNC’s Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine (which is supported, in part, by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center) and Tony Perdue at UNC’s Department of Biology Imaging Core. The authors would also like to thank Dr. Ron Falk, Dr. Mark Peifer, Dr. Charles Jennette, Dr. Tom Hostetter, and members of Dr. Ron Falk’s laboratory for helpful comments on the project and manuscript. The authors are also grateful to the Bloomington Drosophila Stock Center, Developmental Studies Hybridoma Bank, and Vienna Drosophila Resource Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071050/-/DCSupplemental.
Supplemental Figure 1. ZO-1 localizes specifically to nephrocyte SDs.
Supplemental Figure 2. Dlg1, but not Dlg5, is required for correct localization of ZO-1 and Nephrin.
Supplemental Figure 3. Cytoplasmic aggregates of Dlg and Lgl do not colocalize with the ER, Golgi, or Par3.
Supplemental Figure 4. Dlg and Par-1 promote normal Crb localization and protein levels.
Supplemental Figure 5. Mislocalized ZO-1 in Dlg KD cells does not accumulate in the ER or Golgi, but their organization is perturbed by loss of Dlg, Nephrin, or Neph1.
Supplemental Figure 6. In Dlg KD nephrocytes, mislocalized ZO-1 does not colocalize with Rab7 or Rab11, despite their aberrant subcellular distributions.
Supplemental Figure 7. Endocytic markers Rab7 and Rab11 are not specifically enriched in the aberrant ZO-1 aggregations caused by Par-1 KD.
Supplemental Figure 8. In nephrocytes depleted of Dlg or Par-1, mislocalized Nephrin and Neph1 are adjacent to Rab5 structures, while only loosely associated with Rab11 and Rab7.
Supplemental Figure 9. Scrib is required for normal rates of endocytosis.
Supplemental Table 1. List of antibodies used in this study.
References
- 1. Tepass U: Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol 177: 217–225, 1996. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez-Boulan E, Macara IG: Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15: 225–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simons M, Hartleben B, Huber TB: Podocyte polarity signalling. Curr Opin Nephrol Hypertens 18: 324–330, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Palmén T, Lehtonen S, Ora A, Kerjaschki D, Antignac C, Lehtonen E, et al.: Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J Am Soc Nephrol 13: 1766–1772, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Itoh M, Nakadate K, Horibata Y, Matsusaka T, Xu J, Hunziker W, et al.: The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS One 9: e106621, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG: Slit diaphragms contain tight junction proteins. J Am Soc Nephrol 20: 1491–1503, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F: Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol 28: 1521–1533, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cil O, Perwad F: Monogenic causes of proteinuria in children. Front Med (Lausanne) 5: 55, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen JS, McNagny KM: The role of podocalyxin in health and disease. J Am Soc Nephrol 20: 1669–1676, 2009. [DOI] [PubMed] [Google Scholar]
- 11. Takamura S, Fukusumi Y, Zhang Y, Narita I, Kawachi H: Partitioning-defective-6-ephrin-B1 interaction is regulated by nephrin-mediated signal and is crucial in maintaining slit diaphragm of podocyte. Am J Pathol 190: 333–346, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Hartleben B, Schweizer H, Lübben P, Bartram MP, Möller CC, Herr R, et al.: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, et al.: An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PLoS One 4: e4194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, et al.: Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol 20: 798–806, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartleben B, Widmeier E, Suhm M, Worthmann K, Schell C, Helmstädter M, et al.: aPKCλ/ι and aPKCζ contribute to podocyte differentiation and glomerular maturation. J Am Soc Nephrol 24: 253–267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, et al.: A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol 334: 1–9, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Koehler S, Odenthal J, Jess DU, Höhne M, Jüngst C, Grawe F, et al.: Par3A and Par3B orchestrate podocyte architecture by regulating RhoA levels. bioRxiv 10.1101/2020.02.10.933671 (Preprint posted February 10, 2020)
- 18. Weide T, Vollenbröker B, Schulze U, Djuric I, Edeling M, Bonse J, et al.: Pals1 haploinsufficiency results in proteinuria and cyst formation. J Am Soc Nephrol 28: 2093–2107, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebarasi L, Ashraf S, Bierzynska A, Gee HY, McCarthy HJ, Lovric S, et al.: Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am J Hum Genet 96: 153–161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slavotinek A, Kaylor J, Pierce H, Cahr M, DeWard SJ, Schneidman-Duhovny D, et al.: CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am J Hum Genet 96: 162–169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teumer A, Li Y, Ghasemi S, Prins BP, Wuttke M, Hermle T, et al.: Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat Commun 10: 4130, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, et al.: The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A 107: 15810–15815, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, et al.: The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to expanded. Proc Natl Acad Sci U S A 107: 10532–10537, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Djiane A, Yogev S, Mlodzik M: The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121: 621–631, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al.: The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res 68: 8201–8209, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farese RV: Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab 283: E1–E11, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Moscat J, Rennert P, Diaz-Meco MT: PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ 13: 702–711, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Shivas JM, Morrison HA, Bilder D, Skop AR: Polarity and endocytosis: Reciprocal regulation. Trends Cell Biol 20: 445–452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Satoh D, Hirose T, Harita Y, Daimon C, Harada T, Kurihara H, et al.: aPKCλ maintains the integrity of the glomerular slit diaphragm through trafficking of nephrin to the cell surface. J Biochem 156: 115–128, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamano S, Nishibori Y, Hada I, Mikami N, Ito-Nitta N, Fukuhara D, et al.: Association of crumbs homolog-2 with mTORC1 in developing podocyte. PLoS One 13: e0202400, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu H, Artomov M, Brähler S, Stander MC, Shamsan G, Sampson MG, et al.: A role for genetic susceptibility in sporadic focal segmental glomerulosclerosis. J Clin Invest 126: 1067–1078, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, et al.: Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One 7: e36705, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonello TT, Peifer M: Scribble: A master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J Cell Biol 218: 742–756, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi J, Troyanovsky RB, Indra I, Mitchell BJ, Troyanovsky SM: Scribble, Erbin, and Lano redundantly regulate epithelial polarity and apical adhesion complex. J Cell Biol 218: 2277–2293, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bilder D, Perrimon N: Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680, 2000. [DOI] [PubMed] [Google Scholar]
- 36. Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al.: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM: Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development 136: 2335–2344, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hochapfel F, Denk L, Mendl G, Schulze U, Maaßen C, Zaytseva Y, et al.: Distinct functions of Crumbs regulating slit diaphragms and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci 74: 4573–4586, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeitler J, Hsu CP, Dionne H, Bilder D: Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol 167: 1137–1146, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. UniProt Consortium: UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res 47[D1]: D506–D515, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fu Y, Zhu JY, Richman A, Zhao Z, Zhang F, Ray PE, et al.: A Drosophila model system to assess the function of human monogenic podocyte mutations that cause nephrotic syndrome. Hum Mol Genet 26: 768–780, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Helmstädter M, Huber TB, Hermle T: Using the Drosophila nephrocyte to model podocyte function and disease. Front Pediatr 5: 262, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Troha K, Nagy P, Pivovar A, Lazzaro BP, Hartley PS, Buchon N: Nephrocytes remove microbiota-derived peptidoglycan from systemic circulation to maintain immune homeostasis. Immunity 51: 625–637.e3, 2019. [DOI] [PubMed] [Google Scholar]
- 44. Hartley PS, Motamedchaboki K, Bodmer R, Ocorr K: SPARC-dependent cardiomyopathy in Drosophila. Circ Cardiovasc Genet 9: 119–129, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ivy JR, Drechsler M, Catterson JH, Bodmer R, Ocorr K, Paululat A, et al.: Klf15 is critical for the development and differentiation of Drosophila nephrocytes. PLoS One 10: e0134620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carrasco-Rando M, Prieto-Sánchez S, Culi J, Tutor AS, Ruiz-Gómez M: A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms. J Cell Biol 218: 2294–2308, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wen P, Zhang F, Fu Y, Zhu JY, Han Z: Exocyst genes are essential for recycling membrane proteins and maintaining slit diaphragm in Drosophila nephrocytes. J Am Soc Nephrol 31: 1024–1034, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kimbrell DA, Hice C, Bolduc C, Kleinhesselink K, Beckingham K: The Dorothy enhancer has Tinman binding sites and drives hopscotch-induced tumor formation. Genesis 34: 23–28, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Tutor AS, Prieto-Sánchez S, Ruiz-Gómez M: Src64B phosphorylates Dumbfounded and regulates slit diaphragm dynamics: Drosophila as a model to study nephropathies. Development 141: 367–376, 2014. [DOI] [PubMed] [Google Scholar]
- 51. Roberts S, Delury C, Marsh E: The PDZ protein discs-large (DLG): The ‘Jekyll and Hyde’ of the epithelial polarity proteins. FEBS J 279: 3549–3558, 2012. [DOI] [PubMed] [Google Scholar]
- 52. Tassan JP, Le Goff X: An overview of the KIN1/PAR-1/MARK kinase family. Biol Cell 96: 193–199, 2004. [DOI] [PubMed] [Google Scholar]
- 53. Ebnet K, editor: Cell Polarity 1: Biological Role and Basic Mechanisms, Berlin, Springer International Publishing, 2015. [Google Scholar]
- 54. Swiatecka-Urban A: Endocytic trafficking at the mature podocyte slit diaphragm. Front Pediatr 5: 32, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fu Y, Zhu JY, Zhang F, Richman A, Zhao Z, Han Z: Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes. Cell Tissue Res 368: 615–627, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, et al.: Thirty-one flavors of Drosophila rab proteins. Genetics 176: 1307–1322, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schütte-Nütgen K, Edeling M, Mendl G, Krahn MP, Edemir B, Weide T, et al.: Getting a Notch closer to renal dysfunction: Activated Notch suppresses expression of the adaptor protein Disabled-2 in tubular epithelial cells. FASEB J 33: 821–832, 2019. [DOI] [PubMed] [Google Scholar]
- 58. Zhang F, Zhao Y, Chao Y, Muir K, Han Z: Cubilin and amnionless mediate protein reabsorption in Drosophila nephrocytes. J Am Soc Nephrol 24: 209–216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nielsen R, Christensen EI, Birn H: Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int 89: 58–67, 2016. [DOI] [PubMed] [Google Scholar]
- 60. Rinschen MM, Gödel M, Grahammer F, Zschiedrich S, Helmstädter M, Kretz O, et al.: A multi-layered quantitative in vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Rep 23: 2495–2508, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kann M, Ettou S, Jung YL, Lenz MO, Taglienti ME, Park PJ, et al.: Genome-wide analysis of Wilms’ tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J Am Soc Nephrol 26: 2097–2104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahola H, Heikkilä E, Aström E, Inagaki M, Izawa I, Pavenstädt H, et al.: A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol 14: 1731–1737, 2003. [DOI] [PubMed] [Google Scholar]
- 63. Kowalczyk AP, Nanes BA: Adherens junction turnover: Regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell Biochem 60: 197–222, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


