Diabetic nephropathy (DN) is a complex disease with microvascular complications, profound changes in metabolism, inflammation, and redox status, leading to structural and functional changes that can culminate in ESKD. Early DN is defined by glomerular hyperfiltration and albuminuria, and histologically, it presents as glomerular hypertrophy, podocyte foot process effacement, and mesangial expansion. By 2045, it is predicted there will be >690 million adults with diabetes, and a third of these will develop CKD, predominantly due to DN.1 Thus, there is significant need to develop safe and effective therapeutic interventions to prevent DN.
Over the past decade, studies have suggested that metabolic derangements, like reduced nicotinamide to NAD+ metabolism, may precede glomerular dysfunction and albuminuria.2 NAD+ is a critical metabolic cofactor involved in >500 enzymatic reactions, including being necessary for the deacetylase function of the sirtuins. It is predominantly synthesized by the salvage pathway, whereby nicotinamide is catalyzed by the enzyme NAMPT to nicotinamide mononucleotide (NMN) that is then catalyzed by NMNAT to NAD+ (Figure 1A). There has been significant progress made in experimental models to show that NMN treatment is antidiabetic, improves energy expenditure and insulin resistance, suppresses inflammation and oxidative stress, and provides beneficial neurologic effects.3,4 Thus, novel approaches to increase kidney NAD+ are attractive to prevent DN, but the mechanism of how NAD+ is renoprotective is still obscure. Are the renoprotective effects of NMN indirect through improving the diabetic milieu, or does NMN act directly in the kidney to ameliorate DN?
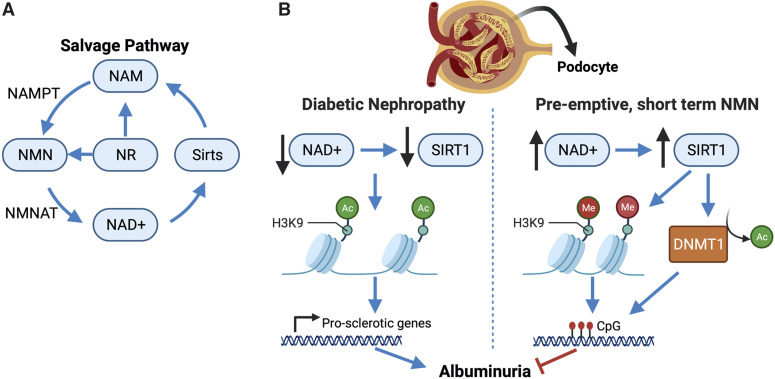
Figure 1.
The NAD+ salvage pathway and effects in diabetic nephropathy. (A) The salvage pathway of NAD+ metabolism. (B) Diabetes causes a decrease in NAD+ and podocyte SIRT1. This leads to acetylation (Ac) of H3K9 and transcription of prosclerotic genes and other genes involved in the pathogenesis of DN. Short-term, preemptive treatment with NMN increases kidney NAD+ and increases Sirt1, which in turn, deacetylates H3K9, allowing H3K methylation (Me) to occur. SIRT1 also deacetylates and activates the DNA methyltransferase, DNMT1, which methylates CpG islands and silences genes.2 Effectively, albuminuria is significantly reduced even 20 weeks after termination of NMN treatment. NAM, nicotinamide; NAMPT, nicotinamide phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyl transferase; DNMT1, DNA methyltransferase 1.
The sirtuins are NAD+-dependent deacetylases, epigenetic regulators of transcription, and key regulators of energy metabolism.5 Sirtuin-1 (SIRT1) is predominantly expressed in the nucleus of cells within the kidney (and other organs). Its expression decreases with age and is suppressed in diabetic rodent models and patients with DN.6 SIRT1 is highly expressed in the podocytes and tubular epithelium.5 Preclinical studies determined that podocyte SIRT1 knockdown exacerbates complications of DN that can be prevented by overexpression of SIRT1.6 Could the DN-dependent decreases in kidney NAD+ be driving SIRT1 insufficiency, leading to the progression of DN? This hypothesis has been tested, and results from these studies have put NMN supplementation at the forefront of interventions to increase endogenous NAD+ levels (e.g., ref. 6). However, there is evidence that NAD+ and SIRT1 are not always benign and can promote tumor growth under some conditions.4 Thus, the deeper mechanism(s) of the NMN/NAD+ benefits and appropriate treatment regimens require rigorous investigation.
In this issue of JASN, Yasuda et al. 7 used a novel treatment regimen of preemptive short-term NMN treatment to determine if this was effective in attenuating DN-associated pathologies. Their model was the db/db mouse, which develops obesity and type 2 diabetes accompanied by some of the clinical manifestations of DN—glomerular hyperfiltration and hypertrophy, mesangial expansion, and albuminuria. At 8 weeks of age, mice were randomly assigned to either saline (herein referred to as db/db) or 500 mg/kg per day NMN groups (500NMN) that received daily intraperitoneal injections for 14 days. Cohorts of mice were studied at 10 weeks of age right after treatment and at 14 and 20 weeks post-treatment to determine if there were legacy effects of the short-term NMN treatment. In contrast to other studies,3,4 NMN treatment was not antidiabetic with this treatment protocol, and the 500NMN mice had similarly high hemoglobin A1c, energy expenditure, and weight gain over the study period. However, despite this, 500NMN mice had lower mortality and significantly improved urinary albumin-creatinine ratios even at 20 weeks post-treatment compared with the db/db mice. This was associated with preserved glomerular foot process density, podocyte SIRT1, and synaptopodin abundance as well as attenuated mesangial expansion, even though glomerular surface area increased and a trend toward hyperfiltration at 10 weeks of age was observed in the 500NMN mice. What is a possible mechanism leading to preserved glomerular structure and attenuation of albuminuria even though glycemia and hyperfiltration were not improved? Impressively, at 14 weeks post-treatment, kidney NAD+ content was still higher than in db/db and control mice, and NMN was reduced. This suggests that the short-term NMN supplementation led to long-term modification of the salvage pathway, resulting in increased kidney NAD+. The mechanism of this legacy effect is proposed to be through the SIRT1 epigenetic regulation of histone marks, such as H3K9 methylation; SIRT1 deacetylates H3K9, leading to DNMT1 methylation of this mark (Figure 1B). One limitation of the study was that formal proof using podocyte-specific SIRT1 knockout db/db mice was not pursued. Future studies determining the gene targets that are significantly affected by modification of H3K9 (or other histone marks) will provide insight into the legacy effects of NMN supplementation.
For the clinical nephrologist, interest lies in the potential for a course of NMN to be safely used in patients to “reprogram” maladaptive epigenetic events within the glomerulus during the early stages of DN and by so doing, induce lasting protection against subsequent glomerulosclerosis and progressive CKD. Attempts to replicate the beneficial effects of NAD+ salvage pathway precursors in the setting of metabolic conditions and aging have generally used the naturally occurring salvage pathway substrate nicotinamide riboside (NR), whereas clinical evaluation of NMN has, thus far, been limited to a single-dose phase 1 study.8 Most notable is a randomized, placebo-controlled trial of NR 1000 mg twice daily for 12 weeks in men with obesity and prediabetic insulin resistance reported by Dollerup et al. 9 In this study, NR was well tolerated, but no significant effects were observed on insulin sensitivity, body composition, and other metabolic parameters.9 However, as Yasuda et al. 7 have demonstrated that salvage pathway supplementation with NMN preserved glomerular structure/function in the absence of antidiabetic effects in obese, type 2 diabetic mice, it remains of interest to determine whether such kidney-specific benefits occur in humans matching this phenotype. Interestingly, NR and NMN may be purchased over the counter as nutritional supplements and are already being promoted to the public as antiaging, life-extending agents. Ideally, therefore, a pipeline of well-conducted clinical trials would test the veracity of such claims. Given the long duration of follow-up that will be necessary to definitively determine whether NMN supplementation prevents the progressive loss of GFR due to DN in a large-scale, randomized clinical trial, mechanistically informed intermediate outcomes will be essential for the next phase of clinical translation. The detailed preclinical experiments of Yasuda et al. 7 suggest that surrogate measures of efficacy in this setting could include urine albumin excretion, measured GFR, and urine assays of shed podocytes and NAD+ pathway metabolites. It is also clinically relevant to ask how any documented benefits or adverse effects of NAD+ precursor therapy will interact with those of other established and emerging therapies, such as the SGLT2 inhibitors. Recent studies have demonstrated that SGLT2 inhibitors restore SIRT1 expression and nutrient-responsive signaling pathways in the diabetic kidney, suggesting the potential for an interplay with the effects of intrarenal NAD+ augmentation.10
In conclusion, therapeutic protocols leading to legacy effects by epigenetic reprogramming of the salvage pathway to sustain or even increase kidney NAD+ show promise for slowing the development of DN. Deeper understanding of these epigenetic pathways and determining how long NMN-dependent legacy effects last will require further rigorous investigation. Finally, translating these findings to our patients to provide a proven safe and effective intervention for DN will require well-conducted, mechanistically informed clinical trials with kidney-specific end points.
Disclosures
M.D. Griffin reports research funding from Randox Laboratories; honoraria from the American Society of Nephrology (ASN), Hebei Medical University in China, and the National Institutes of Health (NIH); and scientific advisor or membership via editorial boards for Frontiers in Renal Pharmacology and Transplantation, section editor of Mayo Clinic Proceedings, and associate editor for JASN. M.D. Griffin is supported by European Commission Horizon 2020 Collaborative Health Project NEPHSTROM grant 634086, Science Foundation Ireland CÚRAM Research Centre grant 13/RC/207, and the European Regional Development Fund. K.A. Hyndman reports scientific advisor or membership via the American Heart Association Membership and Communications committee, the editorial board for American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, the editorial board of Hypertension, and Veterans Affairs Merit Nephrology Study Section; and other interests/relationships as a member of the American Heart Association, a member of the American Physiological Society, a JASN Editorial Fellow, and a member of the Mount Desert Island Biological Laboratory. K.A. Hyndman is supported by the Dr. Priya Nagar Research Award from the University of Alabama at Birmingham-University of California San Diego O’Brien Center and NIH grant P30-DK079337 and National Institute of Diabetes and Digestive and Kidney Diseases of the NIH awards K01DK105038 and R03DK120503.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article “Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy,” on pages 1355–1370.
References
- 1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al.: IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138: 271–281, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Hasegawa K, Wakino S, Simic P, Sakamaki Y, Minakuchi H, Fujimura K, et al.: Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19: 1496–1504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al.: Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab 24: 795–806, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshino J, Baur JA, Imai SI: NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab 27: 513–528, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morigi M, Perico L, Benigni A: Sirtuins in renal health and disease. J Am Soc Nephrol 29: 1799–1809, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhong Y, Lee K, He JC: SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front Endocrinol (Lausanne) 9: 624, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yasuda I, Hasegawa K, Sakamaki Y, Muraoka H, Kawaguchi T, Kusahana E, et al.: Pre-emptive short-term nicotinamide mononucleotide treatment in a mouse model of diabetic nephropathy. J Am Soc Nephrol 32: 1355–1370, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, et al.: Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J 67: 153–160, 2020. [DOI] [PubMed] [Google Scholar]
- 9. Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al.: A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: Safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr 108: 343–353, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Packer M: Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: Implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol 31: 907–919, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]