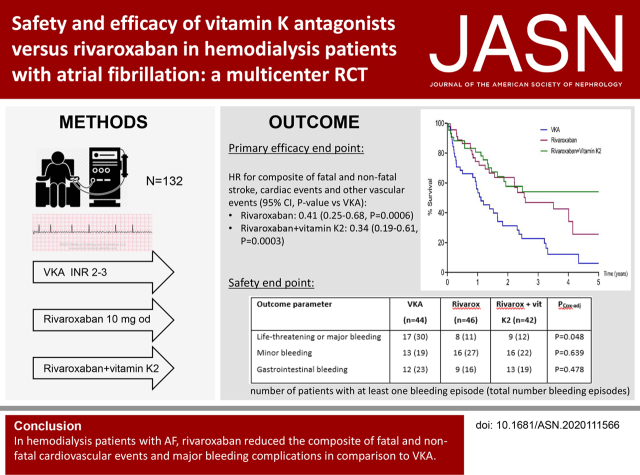
Significance Statement
Direct oral anticoagulants (DOACs) have a superior risk-benefit profile compared with vitamin K antagonists (VKAs) in patients with normal renal function or early stage CKD, but whether this can be extended to the hemodialysis population is unknown. The authors report the first randomized controlled trial of thromboembolic and bleeding risk in patients on hemodialysis with atrial fibrillation on long-term treatment with a VKA or DOAC therapy. After a median follow-up of 1.88 years, the VKA and DOAC groups had a similar risk of stroke. However, the composite outcome of fatal and nonfatal cardiovascular events occurred more frequently with a VKA than with a DOAC, as did major bleeding complications. These findings support a superior risk-benefit profile of DOACs versus VKAs and suggest that VKAs should be avoided in patients on hemodialysis.
Keywords: cardiovascular disease, hemodialysis, randomized controlled trials
Visual Abstract
Abstract
Background
In patients with normal renal function or early stage CKD, the risk-benefit profile of direct oral anticoagulants (DOACs) is superior to that of vitamin K antagonists (VKAs). In patients on hemodialysis, the comparative efficacy and safety of DOACs versus VKAs are unknown.
Methods
In the Valkyrie study, 132 patients on hemodialysis with atrial fibrillation were randomized to a VKA with a target INR of 2–3, 10 mg rivaroxaban daily, or rivaroxaban and vitamin K2 for 18 months. Patients continued the originally assigned treatment and follow-up was extended for at least an additional 18 months. The primary efficacy end point was a composite of fatal and nonfatal cardiovascular events. Secondary efficacy end points were individual components of the composite outcome and all-cause death. Safety end points were life-threatening, major, and minor bleeding.
Results
Median (IQR) follow-up was 1.88 (1.01–3.38) years. Premature, permanent discontinuation of anticoagulation occurred in 25% of patients. The primary end point occurred at a rate of 63.8 per 100 person-years in the VKA group, 26.2 per 100 person-years in the rivaroxaban group, and 21.4 per 100 person-years in the rivaroxaban and vitamin K2 group. The estimated competing risk–adjusted hazard ratio for the primary end point was 0.41 (95% CI, 0.25 to 0.68; P=0.0006) in the rivaroxaban group and 0.34 (95% CI, 0.19 to 0.61; P=0.0003) in the rivaroxaban and vitamin K2 group, compared with the VKA group. Death from any cause, cardiac death, and risk of stroke were not different between the treatment arms, but symptomatic limb ischemia occurred significantly less frequently with rivaroxaban than with VKA. After adjustment for competing risk of death, the hazard ratio for life-threatening and major bleeding compared with the VKA group was 0.39 (95% CI, 0.17 to 0.90; P=0.03) in the rivaroxaban group, 0.48 (95% CI, 0.22 to 1.08; P=0.08) in the rivaroxaban and vitamin K2 group and 0.44 (95% CI, 0.23 to 0.85; P=0.02) in the pooled rivaroxaban groups.
Conclusions
In patients on hemodialysis with atrial fibrillation, a reduced dose of rivaroxaban significantly decreased the composite outcome of fatal and nonfatal cardiovascular events and major bleeding complications compared with VKA.
Clinical Trial registry name and registration number:
Oral Anticoagulation in Hemodialysis, NCT03799822
The use of oral anticoagulation in patients on hemodialysis with nonvalvular atrial fibrillation (AF) is controversial.1 Vitamin K antagonists (VKAs) have long been the standard of care in patients at risk for thromboembolic complications, but physicians are increasingly reluctant to prescribe these drugs. Observational studies failed to convincingly demonstrate that VKAs reduce thromboembolic risk in the hemodialysis population.2,3 Further, patients on hemodialysis treated with VKAs have a disproportionately increased risk of bleeding, in particular of hemorrhagic stroke.3,4 Finally, circumstantial evidence suggests that VKAs accelerate vascular calcification in patients on hemodialysis, although this was not proven by evidence from a recent randomized controlled trial (RCT).5
In the past few years, the use of direct oral anticoagulants (DOACs) has steadily increased, despite few data on their safety and effectiveness and the lack of validated dosing guidelines in the hemodialysis population. Theoretically, DOACs are expected to have a better risk-benefit profile than VKAs. DOACs deliver more predictable and dose-proportional anticoagulation, which is particularly relevant in patients on hemodialysis who are known for having poor anticoagulation control with VKAs. In addition, DOACs are associated with a lower risk of intracerebral bleeding and do not interfere with vascular calcification biology. In patients with AF and normal renal function, DOACs are recommended over VKAs, given their favorable efficacy profile and reduced risk of bleeding.6 In moderate CKD, postanalyses of the key efficacy RCTs in AF also demonstrated equivalent or superior efficacy and safety of DOACs compared with VKAs, provided the recommended dose reductions are made.7–9 Moreover, the risk reduction in major bleeding with apixaban versus VKA seemed to increase as renal function decreased.10,11
Although it is tempting to assume the advantages of DOACs versus VKAs can be extrapolated to the ESKD and hemodialysis populations, robust evidence is currently lacking. Observational studies in patients on hemodialysis reveal that apixaban is associated with a lower risk of major bleeding and a similar risk of stroke compared with VKAs,12,13 and with a higher risk of fatal or intracranial bleeding and a similar risk of stroke compared with no anticoagulation.14 Results from observational studies have to be interpreted with caution because residual confounding due to selective prescribing and healthy user bias cannot be excluded. Three RCTs assessing the safety of apixaban versus VKAs in patients on hemodialysis with AF are currently ongoing or have recently been completed. AXADIA (NCT02933697) intends to randomize 222 patients to VKAs or 2.5 mg apixaban twice daily for 6–24 months.15 SAFE-D (NCT03987711) is a feasibility study of 150 participants, followed over 26 weeks, and compares VKA with 5 mg apixaban twice daily (or 2.5 mg twice daily in patients meeting the criteria for reduced dose) and no anticoagulation. RENAL-AF (NCT02942407) aimed to recruit 760 patients, with a follow-up of 15 months, comparing VKAs with 5 mg apixaban twice daily (or 2.5 mg twice daily in patients meeting the criteria for reduced dose), but this trial was halted prematurely after inclusion of 154 patients.16 Preliminary data did not show a difference with respect to stroke, bleeding, and mortality between the treatment arms. The Valkyrie trial was designed to study the effect of vitamin K status on progression of vascular calcification and randomized 132 patients on hemodialysis with AF who were treated with VKA or qualified for anticoagulation to either VKA, rivaroxaban, or rivaroxaban plus a vitamin K2 supplement for 18 months.5 In this prospective observational follow-up study of this RCT, patients who completed the Valkyrie trial continued the originally assigned treatment, and follow-up was extended for at least 18 months to further record efficacy and safety measures.
Methods
Trial Design and Participants
The Valkyrie study was a randomized, prospective, open-label interventional clinical trial, conducted at three sites in Belgium (AZ Sint-Jan Brugge, OLV Hospital Aalst, ZNA Hospital Antwerpen) from February 2015 to January 2019.5 The study had a three-arm, parallel-group design with a 1:1:1 allocation ratio. A total of 132 adults on chronic hemodialysis with nonvalvular AF and a CHA2DS2-VASc score of two or more were included and followed for 18 months. All patients who completed the Valkyrie study were eligible to enter the extension study and were followed for at least an additional 18 months. The common closeout was on July 23, 2020, when the last enrolled patient achieved a total follow-up of 3 years. All patients provided written informed consent. The study was approved by appropriately authorized ethics committees in all participating sites.
Interventions and Measurements
Patients in the first treatment arm received a VKA, with dose adjustments to achieve an international normalized ratio (INR) of 2–3 on the basis of at least weekly INR measurements. More frequent INR sampling was implemented when the INR was outside the therapeutic range. Patients in the second treatment arm received a daily dose of 10 mg rivaroxaban. The choice of this dose was determined on the basis of a pharmacokinetic and pharmacodynamic analysis, which revealed that a dose of 10 mg in patients on hemodialysis provided a similar exposure than a dose of 20 mg in healthy individuals.17 Patients in the third treatment arm received a daily dose of 10 mg rivaroxaban and 2000 µg menaquinone-7 thrice weekly after dialysis. Concurrent use of antiplatelet agents was at the discretion of the treating physicians. Patients entering the extension study continued the originally assigned treatment without interruption.
Routine clinical and biochemical follow-up was performed. For each patient, the CHA2DS2-VASc score and HAS-BLED score were calculated (Supplemental Appendix 1). In patients treated with a VKA, time in the therapeutic range (TTR) was recorded. The primary efficacy outcome measure was a composite of fatal cardiovascular disease and nonfatal stroke, cardiac events, and other vascular events. Secondary efficacy outcome measures were the individual components of the composite outcome (Supplemental Appendix 1) and all-cause death. Life-threatening bleeding was defined as fatal bleeding; symptomatic intracranial bleeding; a decrease in hemoglobin of ≥5 g/dl; or a requirement for transfusion of four or more units of blood, inotropic agents, or surgery. Major bleeding was defined as a requirement for transfusion of two or more units of blood or a decrease in hemoglobin of ≥2 g/dl and not fulfilling the criteria for life-threatening bleeding. All other bleedings were regarded as minor.
Statistical Methods
Distributions of patient characteristics and outcomes were summarized using means, SDs, medians, interquartile ranges, numbers, proportions, and incidence rates per 100 person-years. Distributions were compared between treatment arms or other subgroups according to the Fisher exact test for categoric variables and the Kruskal–Wallis and Mann–Whitney U tests for continuous variables. Event-free survival times and times to the first bleeding episode occurring during the total observation period were compared between the treatment arms using Cox proportional hazards models. To account for competing noncardiovascular death, the Cox model analyses of the primary efficacy end point and safety outcomes were adapted according to the Fine–Gray method.18 There were no or minimal violations of the proportional hazards assumptions, as assessed visually on the basis of plotted Schoenfeld residuals. A comparison of the pooled rivaroxaban arms with the VKA arm was conducted as an additional post hoc analysis. To assess the net clinical benefit associated with rivaroxaban, the incidence rate of the composite of the primary end point and of life-threatening and major bleeding was expressed as the number of events per 100 person-years. The net clinical benefit was expressed as the hazard ratio (95% CI) from a Cox model comparing this event rate per 100 patient-years in the rivaroxaban group with the similar event rate in the VKA group.19 We considered a two-sided P value <0.05 to indicate statistical significance. All analyses were performed on the basis of the intention-to-treat principle.
Results
Participants
The Valkyrie trial enrolled 132 patients on hemodialysis with documented AF (Figure 1). Baseline demographic, clinical, and biochemical characteristics and baseline maintenance medication have been published previously5 and are summarized in Table 1. The CHA2DS2-VASc score was two or more in all 88 men and three or more in all 44 women. The proportion of patients on aspirin was 34.8% (46 of 132 patients) and not different between the treatment arms. A total of 77 patients completed 18 months follow-up and entered the extension study for at least 18 months or until death. The 132 patients represented 290.5 person-years of observation over the course of the study. Median (interquartile range) follow-up was 1.88 (1.01–3.38) years.
Figure 1.
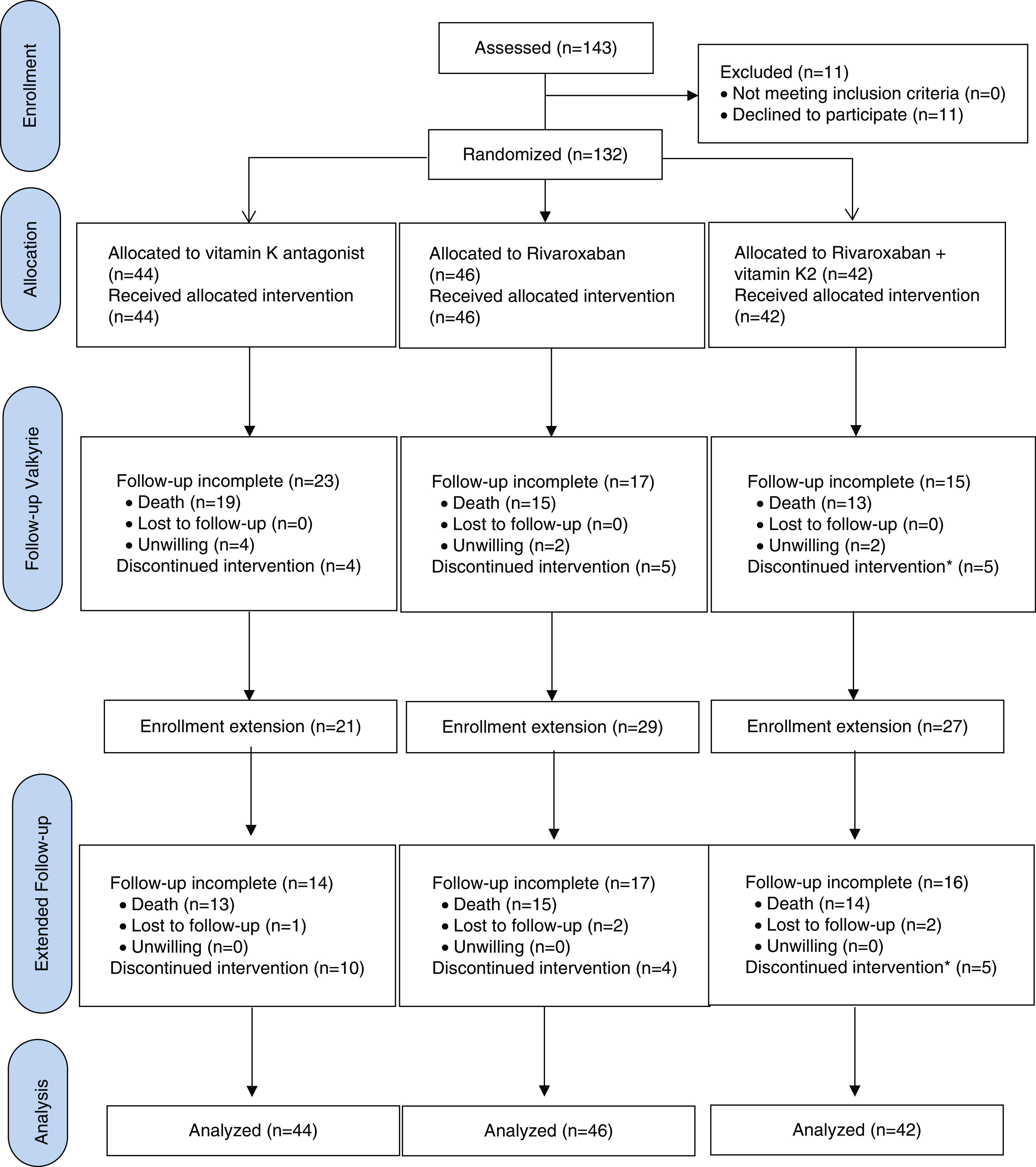
CONSORT diagram. *Patients discontinued rivaroxaban, but continued vitamin K2.
Table 1.
Baseline patient characteristics
| Baseline Characteristics | VKA (n=44) | Rivaroxaban (n=46) | Rivaroxaban and Vitamin K2 (n=42) | P a |
|---|---|---|---|---|
| Age (yr), median (IQR) | 80.3 (71.5–84.3) | 79.9 (74.4–83.9) | 79.6 (73.2–83.1) | 0.92 |
| Male, n (%) | 25 (56.8) | 35 (76.1) | 28 (66.7) | 0.16 |
| History of stroke, n (%) | 16 (36.4) | 15 (32.6) | 9 (21.4) | 0.30 |
| History of gastrointestinal bleeding, n (%) | 12 (27.3) | 9 (19.6) | 16 (38.1) | 0.16 |
| Diabetes, n (%) | 20 (45.5) | 20 (43.5) | 22 (52.4) | 0.74 |
| History of AMI, n (%) | 21 (47.7) | 21 (45.7) | 19 (45.2) | 0.98 |
| Congestive heart failure, n (%) | 9 (20.5) | 17 (37.0) | 15 (35.7) | 0.18 |
| Preexisting vascular disease, n (%) | 28 (63.6) | 25 (54.3) | 17 (40.5) | 0.10 |
| Dialysis vintage (yr), median (IQR) | 1.8 (0.4–5.5) | 2.7 (0.9–5.9) | 2.7 (0.6–5.1) | 0.64 |
| Incident dialysis (<3 mo), n (%) | 9 (20.5) | 4 (8.7) | 9 (21.4) | 0.18 |
| CHAD2DS2-VASc score b | ||||
| Mean (SD) | 4.8 (1.5) | 4.7 (1.4) | 4.5 (1.4) | |
| Median (IQR) | 5 (4–6) | 5 (4–5) | 4 (4–5) | 0.52 |
| Score of ≥2 (men) or ≥3 (women), n (%) | 44 (100) | 46 (100) | 42 (100) | _ |
| HAS-BLED score c | 0.61=P value for median | |||
| Mean (SD) | 4.7 (0.9) | 4.6 (0.8) | 4.7 (0.9) | |
| Median (IQR) | 5 (4–5) | 4 (4–5) | 5 (4–5) | |
| VKA vintage (yr), median (IQR) | 0.9 (0.1–4.7) | 1.1 (0.0–2.8) | 1.5 (0.0–6.3) | 0.46 |
| VKA naive (<3 mo), n (%) | 14 (31.8) | 18 (39.1) | 15 (35.7) | 0.87 |
| Aspirin, n (%) | 14 (31.8) | 15 (32.6) | 17 (40.5) | 0.67 |
| Amiodarone, n (%) | 9 (20.5) | 5 (10.9) | 8 (19.0) | 0.41 |
IQR, interquartile range; AMI, acute myocardial infarction.
According to the Fisher exact test or the Kruskal–Wallis test.
The CHADS2D2-VASc score ranges from two (minimum score to be included) to nine, with higher scores indicating an increased risk.
The HAS-BLED score ranges from one (because patients have renal failure by definition) to eight (because we did not include labile INR), with higher scores indicating an increased risk.
Premature, permanent discontinuation of anticoagulation occurred in 25% (33 of 132) of patients, of which 31.8% (14 of 44) were in the VKA and 21.6% (19 of 88) were in the pooled rivaroxaban groups (P=0.21), mainly as a consequence of a bleeding event (Table 2). The mean ratio between the time on anticoagulation and the total observation time was 0.89 in the VKA group versus 0.88 in the pooled rivaroxaban groups (P=0.47). Overall, 35.6% (47 of 132) of patients were oralanticoagulant naive, meaning they had not previously been exposed to oral anticoagulation or VKA had been initiated <3 months before randomization; this proportion was not different between the treatment arms. Discontinuation of anticoagulation was not significantly different in patients who were stable on oral anticoagulation at randomization (22.4%, 19 of 85) versus those who were anticoagulant naive at randomization (29.8%, 14 of 47) (P=0.40). In patients who were anticoagulant naive, discontinuation was 42.9% (six of 14) in the VKA group, 27.8% (five of 18) in the rivaroxaban group, 20.0% (three of 15) in the rivaroxaban and vitamin K2 groups (P=0.39 for difference between the three arms), and 24.2% (eight of 33) in the pooled rivaroxaban groups (P=0.30 for difference with the VKA arm). Baseline characteristics were similar in patients who discontinued anticoagulant treatment versus those who did not (Supplemental Table 1).
Table 2.
Premature permanent discontinuation of anticoagulation
| Parameter | VKA (n=44) | Rivaroxaban (n=46) | Rivaroxaban and Vitamin K2 (n=42) a | P b |
|---|---|---|---|---|
| Any withdrawal, n (%) | 14 (31.8) | 9 (19.6) | 10 (23.8) | 0.42 |
| Time on anticoagulation/total observation time | 0.89 | 0.88 | 0.87 | 0.71 |
| Bleeding, n | 8 | 8 | 9 | |
| Calciphylaxis, n | 2 | 0 | 0 | |
| Labile INR, n | 3 | — | — | |
| Clinical deterioration, n | 1 | 0 | 0 | |
| Registration on transplant list, n | 0 | 1 | 0 | |
| Wish of the patient, n | 0 | 0 | 1 |
Only rivaroxaban was withdrawn and vitamin K2 supplements were continued.
According to Fisher exact or Kruskal–Wallis test.
In the VKA group, mean TTR was 48.0% during the first 6 months, and this increased progressively over the course of the study (Figure 2). Overall, patients spent more time in the subtherapeutic range than in the supratherapeutic range.
Figure 2.
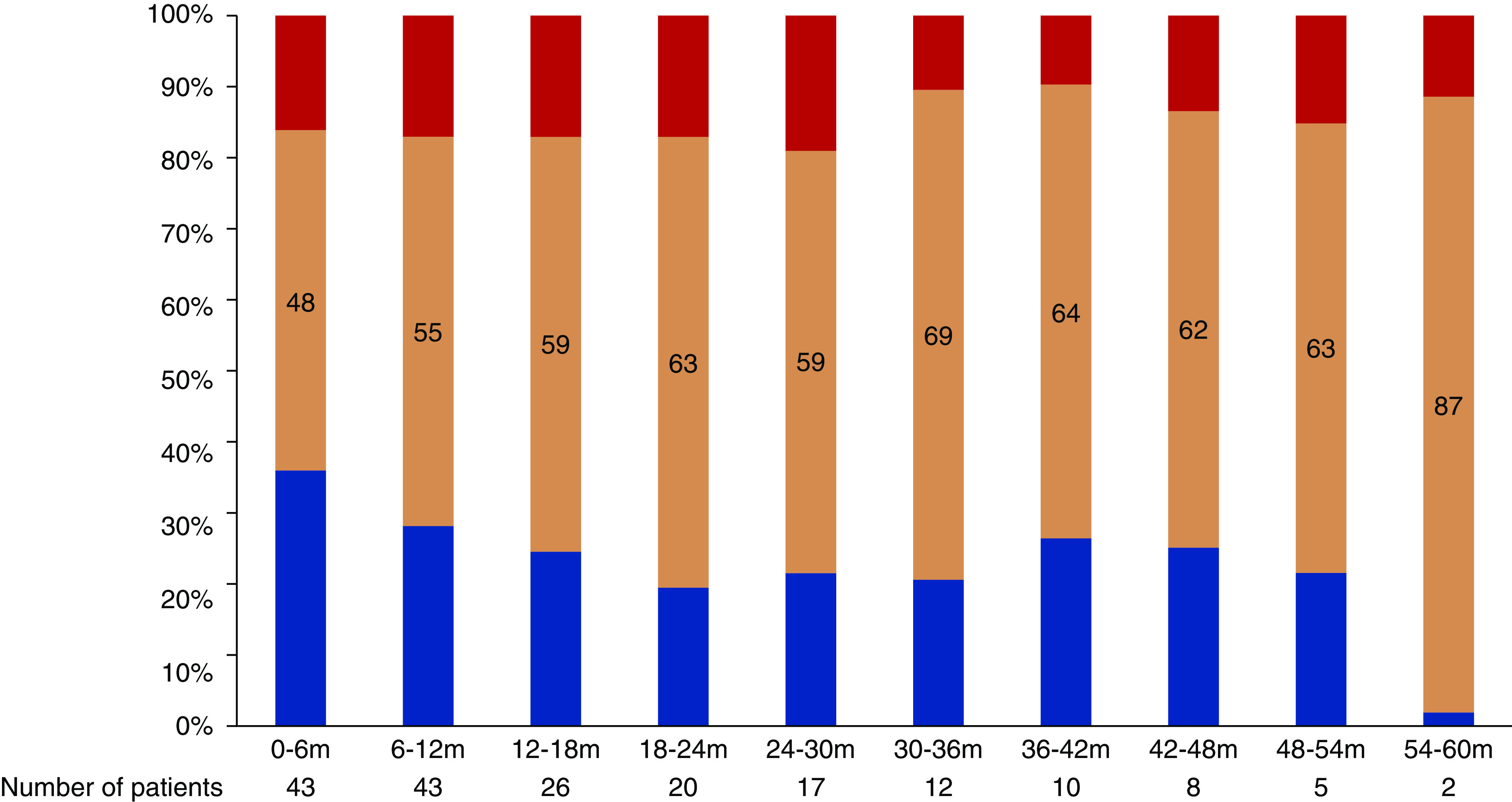
Degree of anticoagulation in the VKA group. Proportion of time in the subtherapeutic range (INR<2, blue bar), within the therapeutic range (INR=2–3, orange bar), and above the therapeutic range (INR>3, red bar) per 6-month interval.
Primary Efficacy Outcome
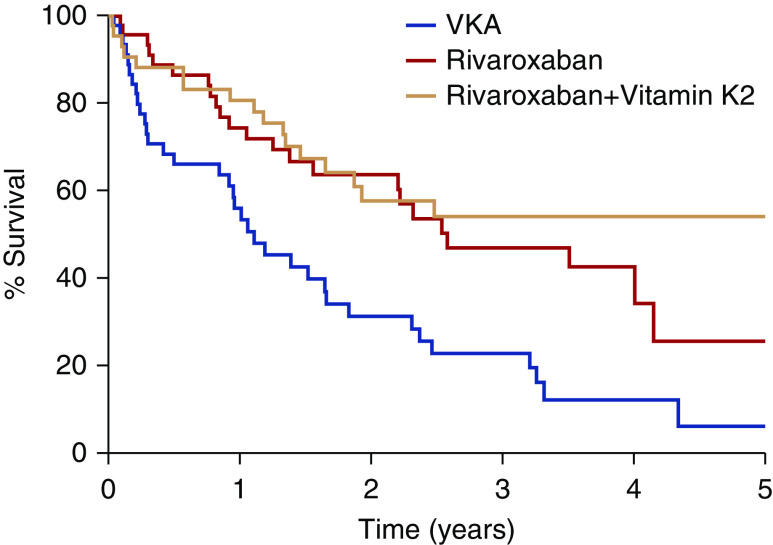
The primary end point was a composite of fatal cardiovascular disease and nonfatal stroke, cardiac events, and other vascular events. This end point occurred in 35 patients in the VKA group (63.8 per 100 person-years), in 23 patients in the rivaroxaban group (26.2 per 100 person-years), and in 17 patients in the rivaroxaban and vitamin K2 group (21.4 per 100 person-years). Kaplan–Meier event-free survival curves are depicted in Figure 3 (for the 3 groups) and Supplemental Figure 1 (for the VKA arm versus the pooled rivaroxaban arms). The estimated competing risk–adjusted hazard ratios in comparison to the VKA group were 0.41 (95% CI, 0.25 to 0.68; P=0.0006) in the rivaroxaban group and 0.34 (95% CI, 0.19 to 0.61; P=0.0003) in the rivaroxaban and vitamin K2 group. The latter groups were not significantly different (P=0.55).
Figure 3.
Kaplan–Meier curve for the primary end point. Survival free of fatal and nonfatal cardiovascular events in the VKA (blue line), rivaroxaban (red line), and rivaroxaban and vitamin K2 (orange line) groups.
Secondary Efficacy Outcomes
Overall, the rate of all-cause death was 30.6 per 100 person-years, with 33.7 per 100 person-years in the VKA group, 28.3 per 100 person-years in the rivaroxaban group, 30.2 per 100 person-years in the rivaroxaban and vitamin K2 group (P=0.66 for difference between the three arms), and 29.2 per 100 person-years in the pooled rivaroxaban groups (P=0.43 for difference with the VKA arm). The main causes of death were withdrawal of dialysis, cardiovascular disease, and sudden death (Supplemental Table 2).
An ischemic or uncertain type of stroke occurred in 13 of 132 patients, corresponding with a stroke rate of 4.7 per 100 person-years. Hemorrhagic stroke was diagnosed in two of 132 patients, corresponding with a stroke rate of 0.7 per 100 person-years. The incidence of stroke was not different between the treatment groups (Table 3 and Supplemental Table 3 for the VKA arm versus the pooled rivaroxaban arms). The mean±SD CHA2DS2-VASc score was not different among patients who developed a stroke (4.9±1.1) versus those who did not (4.7±1.4) (P=0.55). The proportion of patients with a history of stroke was numerically, but not significantly, higher in the patients who subsequently developed a stroke (38.5%, five of 13) versus those who did not (29.4%, 35 of 119) (P=0.53).
Table 3.
Secondary efficacy outcomes
| Outcome Parameter | VKA (n=44) | Rivaroxaban (n=46) | Rivaroxaban and Vitamin K2 (n=42) | P a |
|---|---|---|---|---|
| Death from any cause, n (%) | 32 (72.7) | 30 (65.2) | 27 (64.3) | 0.66 |
| Sudden death, n | 5 | 7 | 4 | 0.75 |
| Stroke or systemic embolism, n | ||||
| Ischemic or uncertain type of stroke | 7 | 4 | 2 | 0.20 |
| Hemorrhagic stroke | 2 | 0 | 0 | 0.21 |
| Systemic embolism | 0 | 0 | 0 | — |
| Cardiac disease, n | ||||
| Acute coronary syndrome | 6 | 9 | 2 | 0.12 |
| Symptom-driven revascularization b | 2 | 6 | 1 | 0.15 |
| Hospitalization for heart failure | 5 | 2 | 2 | 0.47 |
| Symptomatic aortic-valve stenosis | 2 | 0 | 0 | 0.21 |
| Death from cardiac cause | 5 | 4 | 2 | 0.58 |
| Other vascular disease, n | ||||
| Symptomatic lower-limb ischemia | 20 | 10 | 9 | 0.02 |
| Calciphylaxis | 4 | 2 | 0 | 0.14 |
| Bowel ischemia | 1 | 2 | 2 | 0.87 |
According to Fisher exact test.
Including acute coronary syndrome.
Symptomatic limb ischemia occurred significantly more frequently in the VKA than in the rivaroxaban groups (Table 3 and Supplemental Table 3 for the VKA arm versus the pooled rivaroxaban arms). The other cardiovascular end points were not different between the groups.
Safety Outcomes
After adjustment for competing risk of death, the hazard ratio for any bleeding as compared with the VKA group was 0.77 (95% CI, 0.43 to 1.39; P=0.39) in the rivaroxaban group, 0.93 (95% CI, 0.52 to 1.66; P=0.81) in the rivaroxaban and vitamin K2 group, and 0.85 (95% CI, 0.52 to 1.39; P=0.56) in the pooled rivaroxaban groups. A total of 53 life-threatening or major bleeding episodes occurred in the 132 patients, corresponding with a rate of 18.2 per 100 person-years. The number of life-threatening and major bleeding episodes was significantly greater, and the time to first bleeding episode significantly shorter, in the VKA versus the rivaroxaban groups (Table 4 and Supplemental Table 4 for the VKA arm versus the pooled rivaroxaban arms). After adjustment for competing risk of death, the hazard ratio for life-threatening and major bleeding as compared with the VKA group was 0.39 (95% CI, 0.17 to 0.90; P=0.03) in the rivaroxaban group, 0.48 (95% CI, 0.22 to 1.08; P=0.08) in the rivaroxaban and vitamin K2 group, and 0.44 (95% CI, 0.23 to 0.85; P=0.02) in the pooled rivaroxaban groups. The risk of minor bleeding and of gastrointestinal bleeding was not different between the groups.
Table 4.
Bleeding outcomes in the entire patient population
| Outcome Parameter | VKA (n=44) | Rivaroxaban (n=46) | Rivaroxaban and Vitamin K2 (n=42) | P Cox | P Cox-adj |
|---|---|---|---|---|---|
| Total bleeding | 24 (49) | 21 (38) | 22 (34) | 0.03 | 0.19 |
| Life-threatening bleeding | 11 (12) | 3 (3) | 6 (8) | 0.05 | 0.08 |
| Major bleeding | 10 (18) | 6 (8) | 4 (4) | 0.12 | 0.19 |
| Life-threatening or major bleeding | 17 (30) | 8 (11) | 9 (12) | 0.04 | 0.05 |
| Minor bleeding | 13 (19) | 16 (27) | 16 (22) | 0.85 | 0.64 |
| Gastrointestinal bleeding | 12 (23) | 9 (16) | 13 (19) | 0.35 | 0.48 |
Cell entries are number of patients with at least one bleeding episode (total number of bleeding episodes). P Cox, significance of differences in time to first bleeding episode according to Cox proportional hazard model analysis; P Cox-adj, P Cox adjusted for competing risk of death.
The HAS-BLED score did not predict subsequent bleeding: the hazard ratio per unit increase was 1.17 (95% CI, 0.91 to 1.51; P=0.22). A history of gastrointestinal bleeding was predictive for subsequent bleeding with a hazard ratio of 1.84 (95% CI, 1.11 to 3.03; P=0.02). In patients treated with rivaroxaban, the frequency of bleeding episodes was comparable in those treated with amiodarone (46.2%, 6/13) versus those that were not (49.3%, 37/75). However, the average time to first bleeding event was shorter in the 6 patients on amiodarone (1.9 years) compared to the 37 patients not using amiodarone (2.9 years, P=0.060).
Net Clinical Benefit
The incidence rate of the composite of the primary end point and of life-threatening and major bleeding was 84.2 per 100 person-years in the VKA arm and 35.3 per 100 person-years in the pooled rivaroxaban arms. The net clinical benefit for rivaroxaban, expressed as the ratio of these event rates, was 0.45 (95% CI, 0.29 to 0.69; P<0.0001).
Discussion
Our study is the first RCT to report on thromboembolic and bleeding risk in patients on hemodialysis with AF during long-term treatment with VKAs or DOACs. The salient finding is that rivaroxaban significantly reduced the composite outcome of fatal and nonfatal cardiovascular events and major bleeding complications compared with VKAs.
Overall, ischemic stroke risk was 4.7 per 100 person-years in our study population, with a median CHA2DS2-VASc score of five and a 30% history of stroke. Our data are commensurate with those of a racially mixed cohort of 8410 patients on dialysis with AF that had a median CHA2DS2-VASc score of six and a 24% history of stroke; this study revealed stroke rates in patients treated and not treated with oral anticoagulation of 5.3 and 4.0 per 100 person-years, respectively.20 We did not find a statistically significant difference in the incidence of ischemic stroke between the rivaroxaban and VKA groups. In accordance, a retrospective cohort study of 25,523 patients on dialysis with AF and a mean CHA2DS2-VASc score of 5.24 reported stroke rates of 12.4 and 11.8 per 100 person-years in patients treated with apixaban and VKA, respectively, with no difference in survival free of stroke.12
The major finding of our study is that VKAs were associated with a shorter survival free of fatal and nonfatal cardiovascular end points than rivaroxaban. In particular, the incidence of symptomatic lower-limb ischemia was significantly higher in patients on hemodialysis treated with VKAs than in those treated with rivaroxaban. Emerging data bolster a role of DOACs in the prevention of cardiovascular events, in particular of peripheral vascular disease. In the COMPASS trial, the combination of low-dose rivaroxaban and aspirin was associated with a better cardiovascular outcome than aspirin alone among patients with stable atherosclerotic vascular disease,19 a benefit that was preserved in patients with moderate renal dysfunction.21 A subanalysis of the COMPASS trial in patients with symptomatic lower-extremity peripheral artery disease revealed the combination of rivaroxaban and aspirin led to a large absolute reduction in vascular risk compared with aspirin alone.22 An observational study matching 27,552 new DOAC users to 27,552 new VKA users found a lower risk of cardiovascular events and mortality, which was most apparent among patients with CKD.23 Taken together, our findings extend the superiority of DOACs versus VKAs in the prevention of cardiovascular events in the hemodialysis population. Because we did not include a placebo arm, we cannot determine whether our observations result from a deleterious effect of VKAs or from a protective effect of rivaroxaban. However, the absence of an additional beneficial effect of the vitamin K supplements suggests that vitamin K status does not explain the disparity between the VKA and rivaroxaban groups and implies an intrinsic, vasculoprotective effect of rivaroxaban.
In our study, the overall incidence of life-threatening or major bleeding was 18.2 per 100 person-years, taking into account a median HAS-BLED score of five and a 28% history of gastrointestinal bleeding. History of gastrointestinal bleeding, but not the HAS-BLED score, predicted subsequent bleeding in our population.
Severe bleeding complications adjusted for competing risk of death occurred significantly less frequently with rivaroxaban than with VKAs in our patient population. In a large observational study of patients on hemodialysis with AF, the rates of major bleeding were also lower for apixaban than for VKAs (19.7 and 22.9 per 100 person-years, respectively; P<0.001).12 In contrast, a preliminary report of RENAL-AF revealed the proportion of patients with major and clinically relevant, nonmajor bleeding was 25.6% (21 of 82) in the apixaban and 22.2% (16 of 72) in the VKA group after a median follow-up of <1 year.16 However, about a half of the bleeding events were related to the hemodialysis access site. In our view, prolonged access-site bleeding is determined by underlying mechanical problems of the vascular access rather than by the type or degree of anticoagulation. Further, a large majority (71%) of patients in the apixaban group received the standard dose of 5 mg twice daily, which is shown to be associated with supratherapeutic levels in patients on hemodialysis.24 Conversely, TTR was only 44.3% in the VKA group, and patients were three times more likely to be subtherapeutic than supratherapeutic. Taken together, inclusion of vascular access bleeding and an imbalance in the degree of anticoagulation between the treatment arms may have obscured any potential differences in bleeding risk between apixaban and VKAs in RENAL-AF.
In our study, the risk of minor bleeding and gastrointestinal bleeding was not decreased in the rivaroxaban groups. It is tempting to speculate that, in this fragile population with elevated baseline bleeding risk, rivaroxaban causes a shift in bleeding severity from severe to less severe by providing more stable and on-target anticoagulation than VKAs. Indeed, despite our best efforts and at least weekly INR measurements, TTR was only 48% during the first 6 months in the VKA arm. The subsequent increase in TTR likely resulted from discontinuation of VKA in patients with labile INR or after a bleeding episode, and from selective survival of patients with a more stable INR. Patients on hemodialysis characteristically have a low TTR, which is attributed to interference with VKA metabolism by uremia, drug interactions, multiple comorbid conditions, and subclinical vitamin K deficiency. Studies generally report a TTR of around 50%,25–27 with subtherapeutic values more likely in those out of range. Patients with a low TTR generally experience more thrombotic and bleeding complications,25,26 but also have a higher mortality.27 Conversely, rivaroxaban has a lower pharmacokinetic variability and little interaction with food and drugs, leading to more predictable and dose-proportional anticoagulation.17 However, a word of caution should be mentioned for the combination with amiodarone. In the rivaroxaban groups, we found a shorter time to bleeding in patients taking amiodarone, which is in accordance with reports from the general population.28
Premature, permanent discontinuation of oral anticoagulation is characteristically high in patients on hemodialysis, running up to 34%–70% within 1 year of initiation,12,20,29 reflecting the poor tolerability of anticoagulation in this patient population. The relatively low withdrawal rate in our study (overall 25% after a median follow-up of 1.88 years) may have resulted from selection bias, because more than two thirds of patients were stable on oral anticoagulation at the time of randomization. We could not confirm the higher rates of premature, permanent discontinuation in patients randomized to VKAs versus apixaban as observed in the ARISTOTLE trial,30 most likely because of insufficient power.
Effective risk stratification is a prerequisite for adequate prevention of AF-related stroke. Oral anticoagulation is recommended when patients with AF have CHA2DS2-VASc scores of two or more for men and three or more for women.31 Nearly all patients on hemodialysis with AF qualify for oral anticoagulation when these criteria are applied.20 For the general population, the increment of baseline stroke risk is important enough to derive a benefit from anticoagulation, but this may not hold for patients on dialysis. The high risk of mortality due to bleeding32 may outweigh stroke risk in a substantial proportion of patients on dialysis with AF. Risk stratification in patients on dialysis should, therefore, shift from the assessment of stroke risk alone to an integrated evaluation of cardiovascular and bleeding hazard. Compared with VKAs, treatment with rivaroxaban was associated with a substantial net clinical benefit in our patients on hemodialysis.
In conclusion, our findings support a superior risk-benefit profile of DOACs versus VKAs in the hemodialysis population. The differences in major bleeding complications are clinically relevant and suggest VKA should be avoided in patients on dialysis. Because the study had no placebo arm, we cannot make conclusions about the cardiovascular benefits of DOACs versus no anticoagulation in patients on hemodialysis. The differences in symptomatic limb ischemia between VKA and rivaroxaban are notable and may point to a protective effect of rivaroxaban in peripheral vascular disease.
Disclosures
A.S. De Vriese reports being a scientific advisor to Ablynck, Amgen, Alexion, Catenion, and Navigant; receiving consultancy agreements with, and receiving honoraria from, Amgen; being on a speakers bureau for Amgen and Baxter; and receiving research funding from Amgen, Kaydence Pharma, and Nattopharma. All remaining authors have nothing to declare.
Funding
None.
Supplementary Material
Acknowledgments
The authors are indebted to Mirjam Demesmaecker for her invaluable help in the collection of the patient data.
R. Caluwé, A.S. De Vriese, and H. Van Der Meersch designed the study; R. Caluwé, K. De Boeck, A.S. De Vriese, and H. Van Der Meersch collected the data; D. De Bacquer analyzed the data and made the figures; A.S. De Vriese drafted the manuscript; and all authors revised the manuscript and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020111566/-/DCSupplemental.
Supplemental Appendix 1. Supplemental methods.
Supplemental Table 1. Baseline characteristics by anticoagulant discontinuation.
Supplemental Table 2. Causes of death.
Supplemental Table 3. Secondary efficacy outcomes for the VKA arm versus the pooled rivaroxaban arms.
Supplemental Table 4. Bleeding outcomes for the VKA arm versus the pooled rivaroxaban arms.
Supplemental Figure 1. Kaplan-Meier curve for the primary endpoint in the VKA arm versus the pooled rivaroxaban arms.
References
- 1. De Vriese AS, Caluwé R, Raggi P: The atrial fibrillation conundrum in dialysis patients. Am Heart J 174: 111–119, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, et al.: Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: A systematic review and meta-analysis. JAMA Netw Open 3: e202175, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Der Meersch H, De Bacquer D, De Vriese AS: Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. Am Heart J 184: 37–46, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW: Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 131: 972–979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al.: Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: The valkyrie study. J Am Soc Nephrol 31: 186–196, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jame S, Barnes G: Stroke and thromboembolism prevention in atrial fibrillation. Heart 106: 10–17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimachi M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S: Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 11: CD011373, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldberg J, Patel P, Farrell A, Sivarajahkumar S, Cameron K, Ma J, et al.: A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant 34: 265–277, 2019. [DOI] [PubMed] [Google Scholar]
- 9. Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al.: Benefits and harms of oral anticoagulant therapy in chronic kidney disease: A systematic review and meta-analysis. Ann Intern Med 171: 181–189, 2019. [DOI] [PubMed] [Google Scholar]
- 10. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al.: Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Eur Heart J 33: 2821–2830, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, et al.: Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation 141: 1384–1392, 2020. [DOI] [PubMed] [Google Scholar]
- 12. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al.: Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 138: 1519–1529, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H: Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost 2: 291–298, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mavrakanas TA, Garlo K, Charytan DM: Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol 15: 1146–1154, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reinecke H, Jürgensmeyer S, Engelbertz C, Gerss J, Kirchhof P, Breithardt G, et al.: Design and rationale of a randomised controlled trial comparing apixaban to phenprocoumon in patients with atrial fibrillation on chronic haemodialysis: The AXADIA-AFNET 8 study. BMJ Open 8: e022690, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Cardiology : RENal hemodialysis patients ALlocated apixaban versus warfarin in Atrial Fibrillation - RENAL-AF. Available at: https://www.acc.org/latest-in-cardiology/clinical-trials/2019/11/15/17/29/renal-af. Accessed October 24, 2020
- 17. De Vriese AS, Caluwé R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, et al.: Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 66: 91–98, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999. [Google Scholar]
- 19. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al.; COMPASS Investigators: Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 377: 1319–1330, 2017. [DOI] [PubMed] [Google Scholar]
- 20. Pokorney SD, Black-Maier E, Hellkamp AS, Friedman DJ, Vemulapalli S, Granger CB, et al.: Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol 75: 1299–1308, 2020. [DOI] [PubMed] [Google Scholar]
- 21. Fox KAA, Eikelboom JW, Shestakovska O, Connolly SJ, Metsarinne KP, Yusuf S: Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: From the COMPASS trial. J Am Coll Cardiol 73: 2243–2250, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Kaplovitch E, Eikelboom JW, Dyal L, Aboyans V, Abola MT, Verhamme P, et al.: Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: A subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol 6: 21–29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashley J, McArthur E, Bota S, Harel Z, Battistella M, Molnar AO, et al.: Risk of cardiovascular events and mortality among elderly patients with reduced GFR receiving direct oral anticoagulants. Am J Kidney Dis 76: 311–320, 2020. [DOI] [PubMed] [Google Scholar]
- 24. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML: Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol 28: 2241–2248, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinn LM, Richardson R, Cameron KJ, Battistella M: Evaluating time in therapeutic range for hemodialysis patients taking warfarin. Clin Nephrol 83: 80–85, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, et al.: Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 30: 491–498, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Kai B, Bogorad Y, Nguyen LN, Yang SJ, Chen W, Spencer HT, et al.: Warfarin use and the risk of mortality, stroke, and bleeding in hemodialysis patients with atrial fibrillation. Heart Rhythm 14: 645–651, 2017. [DOI] [PubMed] [Google Scholar]
- 28. Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, et al.: Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 318: 1250–1259, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC: Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 66: 677–688, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carnicelli AP, Al-Khatib SM, Xavier D, Dalgaard F, Merrill PD, Wojdyla DM, et al.: Premature permanent discontinuation of apixaban or warfarin in patients with atrial fibrillation [published online ahead of print September 16, 2020]. Heart 10.1136/heartjnl-2020-317229. [DOI] [PubMed] [Google Scholar]
- 31. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al.: 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol 74: 104–132, 2019. [DOI] [PubMed] [Google Scholar]
- 32. Ocak G, Noordzij M, Rookmaaker MB, Cases A, Couchoud C, Heaf JG, et al.: Mortality due to bleeding, myocardial infarction and stroke in dialysis patients. J Thromb Haemost 16: 1953–1963, 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.