Abstract
To perform their functions, the kidneys maintain stable blood perfusion in the face of fluctuations in systemic BP. This is done through autoregulation of blood flow by the generic myogenic response and the kidney-specific tubuloglomerular feedback (TGF) mechanism. The central theme of this paper is that, to achieve autoregulation, nephrons do not work as single units to manage their individual blood flows, but rather communicate electrically over long distances to other nephrons via the vascular tree. Accordingly, we define the nephrovascular unit (NVU) to be a structure consisting of the nephron, glomerulus, afferent arteriole, and efferent arteriole. We discuss features that require and enable distributed autoregulation mediated by TGF across the kidney. These features include the highly variable topology of the renal vasculature which creates variability in circulation and the potential for mismatch between tubular oxygen demand and delivery; the self-sustained oscillations in each NVU arising from the autoregulatory mechanisms; and the presence of extensive gap junctions formed by connexins and their properties that enable long-distance transmission of TGF signals. The existence of TGF synchronization across the renal microvascular network enables an understanding of how NVUs optimize oxygenation-perfusion matching while preventing transmission of high systemic pressure to the glomeruli, which could lead to progressive glomerular and vascular injury.
Keywords: renal autoregulation, tubuloglomerular feedback, chronic kidney disease, synchronization, oxygenation-perfusion, nephron
The intrinsic mechanisms matching blood flow to metabolic demand have been recognized in many organs. In the kidney, autoregulation stabilizes renal blood flow (RBF) and GFR, despite naturally occurring variations in BP. To satisfy the functional and metabolic needs of the tissue, the kidneys self-regulate their blood flow by altering preglomerular vascular resistance through two components: the ubiquitous myogenic response (MR) and the kidney-specific tubuloglomerular feedback (TGF) response. TGF is a mechanism in which the luminal sodium-chloride (NaCl) concentration is sensed at the macula densa, and a signal is relayed to the afferent arteriole to alter preglomerular resistance to restore distal tubular NaCl concentration. This review focuses on the TGF mechanism.
Historically, and even today, it is considered that the nephron (which supplies TGF), and its afferent and efferent arterioles, is responsible for the autoregulation of its own blood flow so that whole kidney autoregulation is the sum of all nephrons, i.e., the average of a massively parallel array of units. However, it is difficult to reconcile this idea with the complex renal arterial organization that creates considerable variation in afferent arteriolar origins and lengths, resulting in variability in preglomerular resistance. It is equally difficult to reconcile this idea with the intricate postglomerular-vascular-tubular relationship, which involves the fact that one nephron’s tubule is perfused by several other nephrons’ efferent arterioles. Hence, we define the nephron plus its afferent and efferent arterioles as the minimum structure, or “nephrovascular unit” (NVU), capable of autoregulation.
We propose that groups of NVUs, synchronized with each other in a network, represent an accurate depiction of the distributed nature of autoregulation within the kidney. The following arguments are consistent with our proposition. First, the anatomic irregularity of the renal arterial tree requires that microcirculation must be arranged so that perfusion in one NVU, which dictates its metabolic demand, adjusts to optimize oxygen delivery to other NVUs.1–4 Second, self-sustained oscillations within each NVU are generated by TGF and transmitted to upstream vascular segments using endothelial gap junctions formed by connexin (Cx) proteins. The self-sustained oscillations of multiple TGF signals from neighboring NVUs enable the interaction of these signals at vascular branch points, allowing NVUs to self-organize into synchronized ensembles. As we will discuss below, shared regulation in a branched arterial tree effectively optimizes resistance to smooth blood flow in time and space.5,6 Structural, or functional, damage to the arterial tree would be expected to degrade performance of the communication network and to exacerbate the consequences of the original insult.
These arguments align to support our line of thinking that NVUs are synchronized in functional networks by interactions among their oscillatory TGF responses. This review examines the evidence that NVUs are coupled. Furthermore, we explore how pathophysiologic processes can affect several components of synchronization.
Renal Arterial Organization Supports Distributed Renal Autoregulation
The main physiologic distinction between the kidney and other tissue beds is that increases in RBF cause parallel increases in GFR in normal conditions, thereby augmenting the supply of oxygen to the organ, but also generating an increased metabolic demand by increasing tubular reabsorption.7 The challenges for the kidneys are (1) to ensure tightly regulated glomerular capillary pressure (PGC) and GFR in the face of fluctuating BP, and (2) to ensure the metabolic demand of one NVU does not become mismatched from oxygen delivery which is supplied by three to five other NVUs.1,2 For 90% of NVUs, oxygenation-perfusion matching is an active process enabled by synchronization of TGF; the renal microvasculature forms macroscopic networks that support communication to coordinate the behavior of NVUs and optimize perfusion.
The organization of the renal arterial tree demonstrates irregular topology.3,4,8–10 In addition to originating at terminal branches, a quarter of afferent arterioles originate from nonterminal arteries that continue to branch into additional arteries; a quarter arise from unpaired, lower-order, single vessels that terminate in pairs of afferent arterioles; and the remaining half are present in pairs, triplets, and quadruplets at the tops of vascular trees and at branching points of vessels.3,10,11 This structural variability creates differences in the preglomerular pressure drop between NVUs that would be further exacerbated when autoregulation responds to a BP transient. NVUs with a larger, versus smaller, pressure drop in front of their glomeruli are capable of adjusting their afferent arteriolar tone in response to the pressure transient. However, some amount of interdependence of local perfusion pressure is present because of shared blood flow affecting nephrons downstream of arterial branch points.4 A simulation study of coupling among NVUs supplied by the same cortical radial artery (CRA) predicted that, without synchronization in the network, the fraction of CRA blood flow received by each NVU was more variable with time (i.e., variable PGC), which could cause highly variable GFR.12 Lapses such as this could cause pressure-mediated damage to the glomeruli, impair filtration, and lead to highly variable distal delivery of tubular fluid because of the absence of communication between NVUs to regulate vascular resistance in more proximal vessel segments. Coordinated behavior of NVUs through synchronization allows integration of communication upstream in the renal vasculature, so that every branch downstream can adjust its resistance to maintain appropriate perfusion.
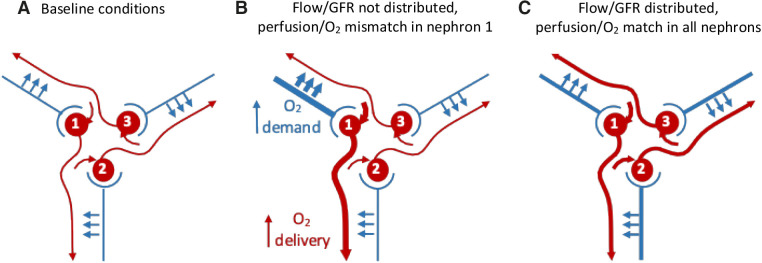
In addition to preglomerular circulation variability, there also exists an anatomic separation of postglomerular (efferent arteriolar) blood flow from tubular flow. Beeuwkes et al. 1,2,13 described the separation between efferent arterioles and tubules in a series of papers that studied multiple species. Only in the superficial cortex does an efferent arteriole perfuse its own tubule; this is about 10% of all NVUs in the kidney. Most efferent arterioles perfuse other tubules and, on average, a proximal tubule travels through three capillary beds. This has been shown in humans,1 dogs,2 marsupials,14 and rodents (W. Kriz, personal communication to W.A.C.). Although the bulk of renal oxygen consumption occurs in the S1 and S2 regions of the proximal tubule, the S3 segment and medullary thick ascending limb are the most susceptible to hypoxia because of their location in the outer medulla, where the PO2 is low. Inadequate supply of oxygen to the outer medulla would affect the operation of the Na+-K+-ATPase, which would alter distal NaCl delivery. Therefore, separation between the postglomerular vasculature and the tubules strongly suggests the network optimizes matching of tubular perfusion and oxygen consumption through synchronization, whereby each NVU contributes to the distribution of flow among groups of NVUs (Figure 1). This ensures homogenous perfusion of multiple glomeruli so that postglomerular blood flow is matched with tubular reabsorption of Na+.
Figure 1.
Distributed control of blood flow results in appropriate oxygen supply-demand matching through coordinated regulation of vascular resistance. In this simplified diagram, three glomeruli receive blood from their respective afferent arteriole. Efferent blood flow and tubular flows from the glomeruli are arranged so that each tubule receives its blood supply from another glomerulus; in vivo it may be up to five other glomeruli. If flow and filtered Na+ increase in NVU 1, reabsorption will generate a greater oxygen demand. If autoregulation is local, a mismatch between oxygen supply and demand would occur. NVU 1 needs to communicate with NVU 3, whose efferent vessel perfuses its tubule. This communication occurs through TGF-induced synchronization and leads to distributed autoregulation; the resistance upstream of every glomerulus is regulated partly by itself and partly by signals coming from all of the other NVUs in the arterial tree. O2, oxygen.
The complexity of the renal microvasculature suggests the requirement of a distributed mechanism that regulates perfusion on a scale that may involve multiple CRAs. TGF-induced synchronization is the most likely solution to the challenges posed by the irregular anatomic arrangement of the renal microvasculature.
TGF Generates Self-Sustained Oscillations within NVUs
Autonomous TGF (and MR) activity generates glomerular blood-flow oscillations that produce measurable oscillations in proximal tubular pressure and proximal tubular flow within individual NVUs.15,16 Oscillations in intratubular pressure were observed as early as 1983 by Leyssac and Baumbach17 in halothane-anesthetized Sprague-Dawley rats. These oscillations subsequently entrain and synchronize between clusters of NVUs, permitting their interaction, which was demonstrated first by Holstein-Rathlou.16
In rat cortical nephrons, fluctuations in PGC change GFR and take approximately 20 seconds to travel to the macula densa. Thus, tubule length is a strong determinant of the TGF period.18 Further delays (approximately 5–10 seconds) occur due to the time it takes for the TGF mediator to be released from the macula densa and alter afferent arteriolar resistance.18 Such delays in the regulatory mechanism can affect the frequency of oscillation, whereas a sufficiently high gain, enabling TGF to be effective, will allow it to oscillate. In the context of autoregulation, “effective” means that in response to a BP change, the preglomerular resistance is adjusted so that the PGC is maintained essentially constant. The overall oscillation of TGF takes about 25–40 seconds for completion in rats,19,20 and about 50 seconds in dogs.21
The self-sustained oscillations of TGF and MR (which is a ubiquitous autoregulatory mechanism intrinsic to vascular myocytes) each reflect operation of nonlinear systems.22,23 MR and TGF interact because they act on the same target: afferent arteriolar vascular smooth muscle cells (VSMCs). In rat kidneys, TGF oscillates at a frequency of 0.015–0.05 Hz, and MR at 0.09–0.25 Hz.24,25 When two oscillating systems interact or “couple,” they adjust their rhythms and can oscillate with a common frequency so that they may become synchronized. To facilitate synchronization, both MR and TGF undergo frequency modulation, and the ratio of their frequencies can form a fixed integer ratio within each NVU; two oscillators whose natural frequencies are close to an integer ratio (e.g., 1:2) will tend to entrain, with slight shifts of frequency, to bring them into an exact integer ratio.23 The most frequent ratio is 5:1, but 4:1 and 6:1 are also observed.26,27 MR, with its higher frequency, stabilizes local fluctuations in flow resulting from BP fluctuations; TGF, with its lower frequency, modulates the amplitude and frequency of MR to stabilize larger fluctuations of BP and thereby stabilizes NaCl delivery to the macula densa.26,27 Several studies have confirmed the interactions of TGF and MR within individual NVUs in the rat kidney.28–30
In sum, measurable, self-sustained oscillations occur in both MR and TGF due to the delays, high gain, and nonlinear properties of the systems, and MR and TGF oscillations interact.
Vascular Conducted Responses Enable Communication in the Arterial Tree
TGF is a property of individual NVUs; increased NaCl reabsorption at the macula densa causes release of ATP and adenosine that diffuse to extraglomerular mesangium, where they activate P2X (ATP) and A1 (adenosine) receptor signaling.31 This initiates depolarization that traverses the extraglomerular mesangium to the afferent arteriole, resulting in upstream vasomotor responses, a vascular conducted response (VCR).15,32–34 The depolarization is a nonregenerative electrical signal that is transmitted along the afferent arteriole, into the CRA, and into nearby afferent arterioles via axial endothelial gap junctions.35,36 Although every NVU adjusts its own perfusion, upstream electrical communication optimizes regional perfusion by exchange of information at branch points regarding the state of TGF and MR in the communicating NVUs. There are two important components to this system: connexins (Cxs) and VCRs.
Connexins
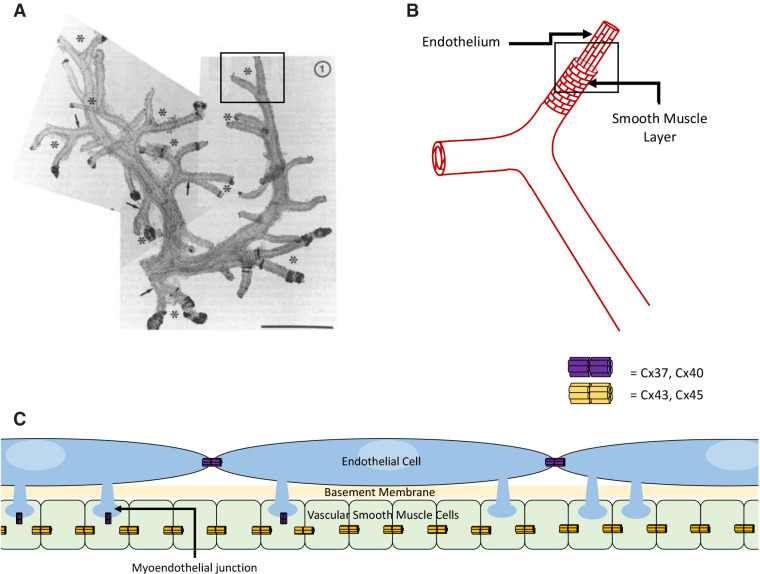
Gap junctions formed by multiple, transmembrane Cx proteins create axial and radial communication pathways, enabling TGF signal transmission by passage of current. It should be noted that gap junctions are absent from macula densa cells.37 The mesangium (both extra- and intraglomerular) is effectively an electrical syncytium due to its high density of gap junction coupling,37–42 and connects to afferent arteriolar endothelial cells (ECs) and renin-secreting cells. Cx37, Cx40, and Cx45 are seen in the mesangium.36,43 Endothelial Cx37 and Cx40 form the axial communication pathway (Figure 2).43–48 Studies with Cx40-knockout mice have shown the dominant role of Cx40 in the kidney49–51 and systemically.6,52–54 VSMCs show prominent expression of Cx4536,55,56 and variable expression of Cx43, mostly in cultured VSMCs.35 Radial communication from ECs to VSMCs occurs via myoendothelial gap junctions (MEGJs) (Figure 2).40,57 At MEGJ sites, ECs physically contact VSMCs by club-shaped cellular protrusions through the internal elastic lamina.57–59 The Cx isoforms in MEGJs are not fully elucidated; Cx37, Cx40, and Cx43 have been found in MEGJs of various vascular beds. Cx37 in MEGJs seems central in controlling the spreading gap-junctional calcium (Ca2+) signal.60,61
Figure 2.
The organization of the renal arterial tree and its various components. (A) A composite low-power micrograph of two CRAs and their afferent arterioles depicts the high variability in the arterial organization. The asterisks point to afferent arterioles organized as pairs, and the arrows point to connecting arteries branching from the CRA. The box encloses an afferent arteriolar pair that is looked at closer in panel B. (B) The vascular layers comprising this pair of arterioles includes the axially oriented cells of the endothelium and radially oriented smooth muscle cell layer. The box encloses a section of the vessel that is analyzed further in panel C. (C) Shown are the myoendothelial junctions (MEJs) protruding from ECs toward the VSMCs. This panel also illustrates the presence of MEGJs comprised of Cxs present at MEJs. Gap junctions enable depolarizing TGF signals to transmit upstream of the afferent arteriole to neighboring afferent arterioles and into the larger artery, where similar signals from other afferent arterioles can interact to regulate vascular resistance. Panel (A) reprinted from ref. 10, with permission.
Vascular Conducted Responses
Measured length constants of TGF-mediated VCRs are in the region of 0.3–0.6 mm.32,33,62 Presumably, VCRs result from the passive electrotonic spread of depolarization, which is initiated by the macula densa upon TGF activation, and spreads upstream from the extraglomerular mesangium through ECs.41,58,63 Macula-densa stimulation in mice resulted in elevated intracellular Ca2+ and depolarization of the mesangium, both of which propagated into the afferent arteriole and the CRA, and then into neighboring afferent arterioles.41,42,63
We focus our discussion on vasoconstriction because preferential transmission of depolarization has been observed in the kidney (K. Endlich, personal communication to W.A.C.).33,62 The slow velocity of conducted Ca2+ waves (0.1 mm/s) excludes them from propagation of VCRs.42 The transformation of transient electrical events into conducting voltage responses is thus enabled by gap junctions.
Longitudinal conduction of VCRs along the endothelium is aided by structural and electrical properties of ECs. ECs are oriented parallel to the long axis of vessels, and their extensive coupling by Cx40 results in a low input resistance and a low impedance pathway (1.5–3 mΩ), and also prevents charge accumulation in the endothelium.47,63 On the other hand, VSMCs are oriented circumferentially and have much higher input resistance than ECs (90 mΩ), which makes the smooth muscle layer an unlikely VCR conduction pathway. Radial communication between ECs and VSMCs occurs through MEGJs.64–66 MEGJs are localized distally in the smallest arterioles, with less expression in the CRA, and little to none in the arcuate and larger arteries.40,57,58,67 Increased localization of MEGJs in the smallest afferent arteriole bolsters conduction, considering local vasoconstriction induced by TGF takes place in the distal afferent arterioles, whereas the absence of MEGJs in these larger arteries reduces current leakage out of the endothelium, enabling long-distance transmission. Because current travels in the endothelium, although its effect is assessed in VSMCs either as constriction or as membrane depolarization, the actual EC length constant in larger arteries may be considerably longer than currently thought.68
VCR transmission is optimal when approximately a third of VSMCs have active MEGJs, which minimizes current drain from ECs into the smooth muscle layer while smoothing out local variations in membrane voltage of VSMCs so they all achieve similar levels of activation.63,65,69,70 Renal arteries, down to and including the CRA, have thinner walls than skeletal muscle arteries of a similar diameter. This may help to limit current drain into the media.71 The presence of glycocalyx on the surface of ECs may also serve as an insulator at the luminal side of the endothelium.72
Factors affecting the conductive pathway in the kidney are not well elucidated; a description of what is known was recently reviewed by Welsh et al. 63 Computational-modeling studies of VCRs show endothelium-initiated conduction and parallel changes in membrane voltage occurring in VSMCs; however, these assume uniform axial MEGJ distribution, which is not the case.69,73 It is becoming increasingly clear that components of VCRs, the communication pathway and the vasomotor responses, are subject to considerable regulation, and this is an active area of research.6,48,61,63
Of note, the kidney uses the same machinery to establish communication in the descending vasa recta, as described in a series of papers by Pallone et al.75–78 The pattern of Cx expression is somewhat different from that seen in the cortex, with ECs showing linear staining with both Cx40 and Cx43, and staining of Cx37 seen only in pericytes.74 Similar to the organization we describe for ECs and VSMCs, pericyte and EC layers are electrical syncytia74,75 with very limited connectivity between the two layers.75–77 Therefore, the vasa recta exhibit a well-developed, independent communication system, providing a potential means for adjusting blood flow among the different medullary compartments.78–80
Taken together, gap junctions allow depolarizing TGF signals to transmit into upstream arteries. Cx40 is the main constituent of endothelial gap junctions and is highly important for renal VCR transmission. The axially oriented ECs provide the “electrical cable,” whereas the radially oriented MEGJs permit an optimal amount of current into the VSMC layer to induce constriction of VSMCs and enable VCR transmission.
TGF Oscillations Enable Synchronization among Groups of NVUs
There is ample evidence of synchronization among NVUs. Holstein-Rathlou16 and Yip et al. 15 identified pairs of nephrons with synchronized tubular-pressure oscillations using micropuncture. Källskog and Marsh34 observed that increasing perfusion in one nephron reduced stop-flow pressure in that nephron and in the adjacent coupled nephron, indicating a constructive interaction. Chen et al. 32 detected interactions between two nephrons at distances as far apart as 1.5 mm along the vasculature in rat kidneys.
TGF-initiated VCRs from multiple afferent arterioles interact at branching points of the vascular tree. Holstein-Rathlou16 demonstrated interactions between nephrons sharing the same CRA: 29 of 33 nephron pairs whose afferent arterioles originated from the same CRA had identical frequency and phase of oscillations. Yip et al. 15 found synchronization in pairs of nephrons that branched from a common CRA, as determined by vascular casts. Both studies showed that, when one of the tubules was perfused with furosemide, the amplitude of tubular-pressure oscillations diminished in coupled nephrons, whereas no changes occurred in the oscillations of noncoupled nephrons. When furosemide perfusion was halted, coupling of the two nephrons’ oscillations was restored.15,16 At the time, the authors recognized these results strongly implicated a vascular, electrical communication route in regulation of PGC and GFR.
These aforementioned studies were constrained by the limitations of micropuncture: the assessment of the behavior of a few NVUs cannot achieve a sample size large enough to estimate variance of the process of TGF synchronization in multiple NVUs. Laser speckle contrast imaging (LSCI) is a noninvasive, full-field technique used to assess real-time tissue perfusion that offers high spatial and temporal resolution.81–83 The use of LSCI is innovative for analyzing large-scale cortical perfusion to provide crucial insight into network dynamics. Because it employs the same surgical preparation as micropuncture and assessment of total RBF autoregulation, the cumulative changes associated with surgery-induced stress and anesthetics are relatively familiar.
The first study assessing synchronization on a large scale assessed hierarchic coupling of TGF dynamics in >50 identified efferent arterioles simultaneously.84 The results were interpreted conservatively to show local (i.e., within a lobule) synchronization of clusters of two to three NVUs that were not always nearest neighbors. The data in this study also showed temporal and spatial variation of synchronization.84
Mitrou et al. 85 used the same laser speckle imager with lower spatial and higher temporal resolution to also capture the MR. Therefore, they treated the image as a flow field and assessed synchronization between all possible pixel pairs. Under control conditions, assessment of the MR was largely uninformative. In contrast, TGF tended to synchronize in large clusters (≥1 mm2 diameter), albeit with variable coupling strength. However, their analysis would have averaged out any temporal variation of synchronization. Thus, both groups demonstrated TGF synchronization on scales larger than can be addressed by micropuncture; Holstein-Rathlou et al. 84 demonstrated temporal and spatial variation of synchronization which is undoubtedly correct (we have unpublished data to that effect). Where they disagreed was on the spatial scale of synchronization. This divergence of opinion has not been explicitly addressed and is an important open question.
In a separate study, Mitrou et al. 86 showed a link between VCRs and autoregulation. Intrarenal infusion of carbenoxolone (a gap junction blocker) but not the control (glycyrrhizic acid) increased spatial and temporal heterogeneity of surface perfusion, reduced coupling, and impaired both dynamic and steady-state autoregulation.86 This finding links autoregulatory effectiveness to synchronization of microcirculatory dynamics mediated by gap junctional intercellular communication.
The nephrovascular network is dynamic and the size of clusters can change.85 However, it is not known how network size or cluster-formation patterns are regulated, and this is an active area of research.48,87–90 Recent experimental and modeling studies suggest post-translational modification of proteins involved in the communication (Cx) and contraction (actin and myosin) components of VCRs regulate network radii across time and space.48,87–90 There are also indications of this in the older literature.58,59 The physical organization of the arterial network and the length of tubules would certainly affect the clustering patterns that emerge because arterial arrangement determines proximity of NVUs to each other. The nonregenerative nature and electrical transmission of VCRs suggest the TGF signal decays with increasing distance. However, most glomeruli are separated (via the vascular tree) by distances less than measured length constants.3,4 Thus it is likely that, as depolarization proceeds up a CRA, the more proximal NVUs act as amplifiers, influencing the pattern and size of the clusters that form.
There is a major unresolved problem about whether, or how, long-looped juxtamedullary NVUs synchronize with the bulk of “short-looped” NVUs. Oscillations of subsurface NVUs are difficult to ascertain because techniques to probe subsurface NVU populations are limited. However, they likely exist because TGF and MR dynamics are routinely captured in whole kidney blood flow, and TGF-MR interactions have been captured in whole kidney blood flow.91 We know that TGF operates in long-looped nephrons92,93 and, from the fitting of those data, one can infer that gain is high enough to support oscillation.94 As noted previously, nephron length is a major determinant of the oscillation frequency,18 but so too is axial fluid velocity, and the longest-looped nephrons tend to have large glomeruli, presumably supporting a high single-nephron GFR.95 So, although we presume long-looped NVUs have a noticeably lower TGF frequency than seen in short-looped NVUs, relevant data are lacking. Current thinking suggests at least three possibilities. One is that there may be an “m/:n” coupling between long- and short-looped NVUs, in which they are coupled in a frequency ratio other than 1:1 (e.g., 3:2 or 2:1). It is also possible that long-looped NVUs remain uncoupled from the remainder of NVUs. In this case, it is possible that signals of incommensurate frequencies “quench,”96 limiting spread of coupling. This appears inconsistent with conclusions drawn from studies treating surface perfusion as a flow field,85,86,97 but has not been addressed by studies of perfusion of individual star vessels (efferent arterioles). Finally, Christensen et al.’s95 data of segmental lengths of 158 nephrons in a rat kidney suggest only a small number of “long-looped” NVUs may have loops of Henle long enough to preclude effective coupling with the bulk of NVUs in the cortex. This is a difficult problem that will remain unsolved until techniques are developed to assess nephron dynamics in deep nephrons.
To summarize, all afferent arterioles have autonomous oscillations arising from autoregulatory dynamics. When VCRs from afferent arterioles reach each other, the information exchange among signals generated by multiple NVUs results in synchronization of the dynamics of those NVUs. Recent studies using LSCI show synchronization that occurs in macroscopic regions across the kidney.85
There is ongoing development of imaging techniques, including algorithms for analyzing their output. A recent study addressed long-standing questions concerning the most appropriate model for interpretation of LSCI (and laser Doppler flowmetry) signals.83 It explored two factors that can affect interpretation. LSCI generates information about perfusion from the scatter of coherent laser light by moving particles (largely red cells). But that is complicated by scattering of light from static sources that introduces a signal, which is not related to flow and can be substantial. Certainly, the kidney is a rich source of static scatter; consider the proximal tubule with its basal infolding, luminal brush border, and large, numerous mitochondria. Equally, vessels of different sizes form “dynamic scattering regimes” that have different scatter properties. By default, the properties of small arteries and veins (diameter of approximately 40–150 µm) have been used. However, smaller arteries and arterioles dominate vasomotor activity in the kidney and their scatter properties more closely match those of parenchyma. This could introduce discordance between LSCI and RBF signals that are expected to be most important at low RBF. The design of first-generation commercial instruments was derived from a less well-informed understanding of the measurement theory; more sophisticated studies, with better perfusion imaging in arterioles and parenchyma, are needed.
Is the Function of the Nephrovascular Network Disturbed in Disease States?
Disrupted network dynamics impair distributed autoregulation and increase susceptibility of the renal parenchyma to ischemia. This is a critical feature underlying the pathophysiology of diabetic nephropathy, AKI, and progression to CKD. The emerging paradigm shift regarding renal autoregulation and synchronization of NVUs has substantial potential to influence renal disease management and treatment.
Microvascular dysfunction is present in several disease states, including sepsis,98 hypertension,99 and diabetes mellitus (Figure 3).100,101 Pathologic processes may affect multiple aspects of synchronization, most of which have not yet been characterized. Communication, and thus smoothing of blood flow in time and space, can be reduced in several ways (Figure 4). Loss of NVUs via glomerulosclerosis or tubular injury can have variable effects on TGF, glomerulotubular (GT) balance, and the “effector” (the wall of the microvasculature in the remaining NVUs). The TGF system itself might sense a change in macula-densa fluid composition differently; the disease may affect the formation and release of the mediator and the ability of the mediator to reach the afferent arteriole (e.g., due to mesangial damage). Meanwhile, adaptations in GT balance could affect the individual nephron’s handling of solutes proximal to the macula densa and either enhance or unbalance TGF activity. Further modulation of both the TGF system and GT balance will happen due to neurohumoral adjustments. At this moment, it would be highly speculative to talk about directions of changes, in the absence of data; yet, the currently available knowledge would make it highly unlikely that the network function would remain untouched by progressive injury to the NVUs and the microvascular architecture.
Figure 3.
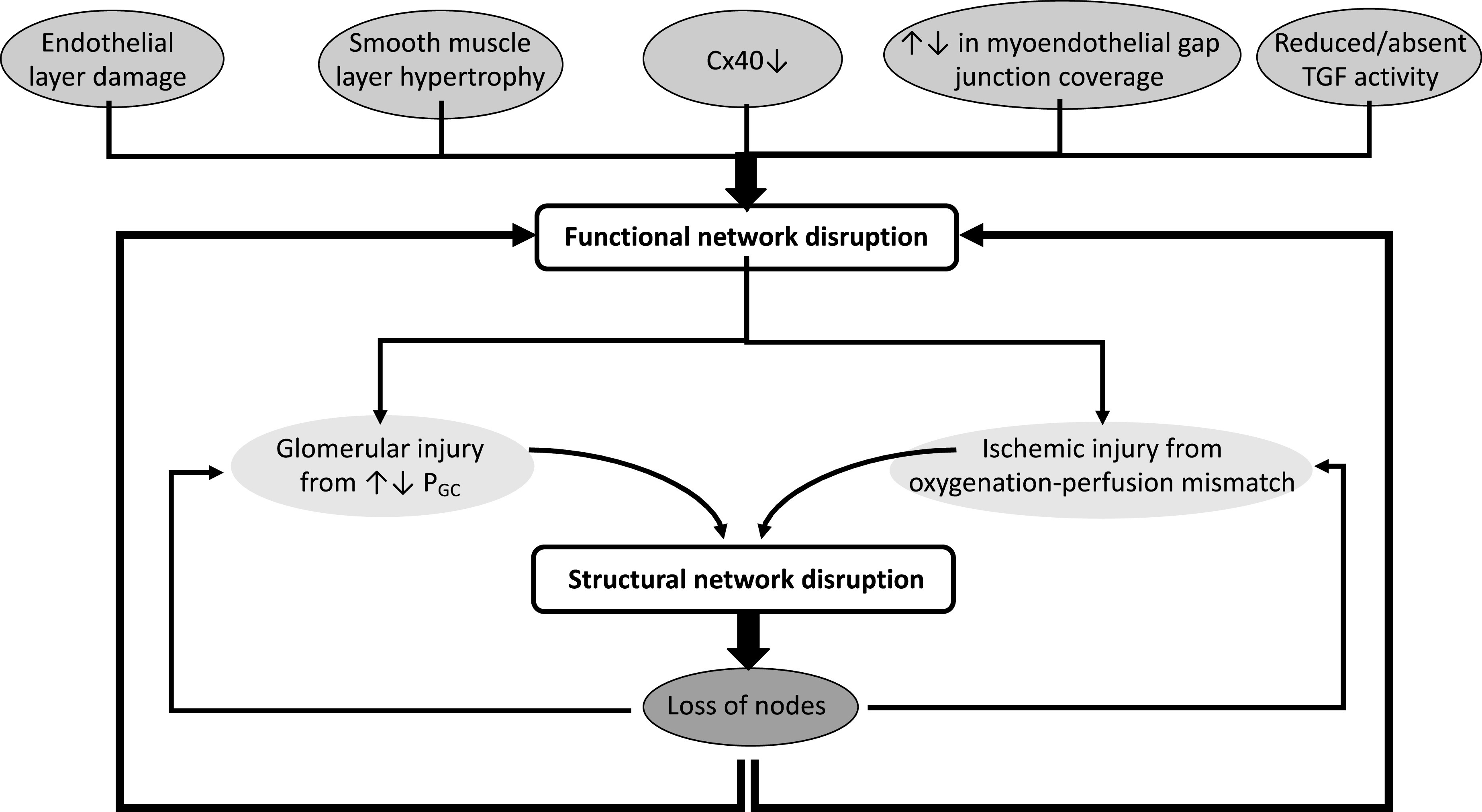
Various pathophysiologic changes in the nephrovascular network cause functional disruption to the nephrovascular network, resulting in structural damage to the network that propagates the initial insult. Functional disturbances to the nephrovascular network contribute to failing or loss of nodes from the network. These derailments include microvascular alteration or damage, decreased Cx40 expression, changes in MEGJs, and reduced or absent TGF signals; they increase susceptibility to glomerular injury from unregulated PGC and ischemic injury from oxygenation-perfusion mismatches. As the network starts to fall apart and lose elements, the likelihood of glomerular and ischemic injury increases and functional network disruptions worsens, exacerbating structural disruption.
Figure 4.
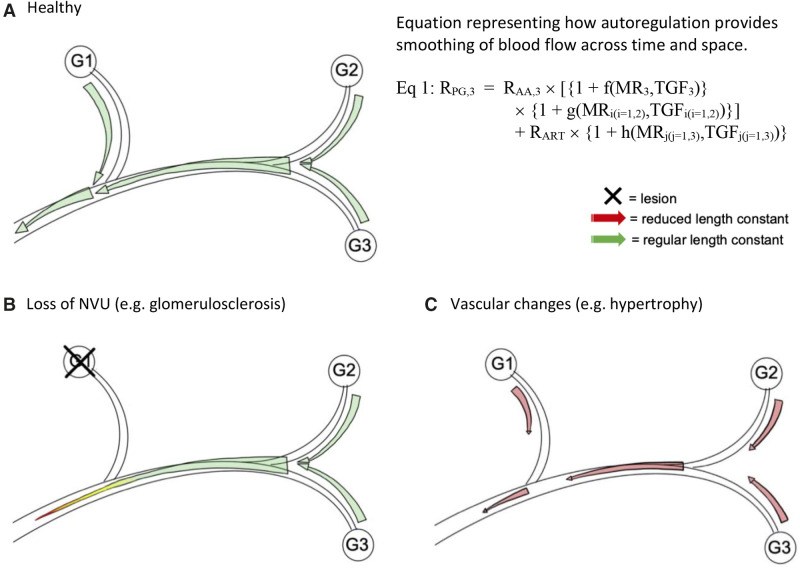
Ascending TGF signals from NVUs interact along an artery, and this interaction has a shorter radius when NVUs are lost from the network and when vascular hypertrophy reduces the distance of TGF signal transmission. (A) Shown is the upstream transmission of the TGF signal from each glomerulus (G1-3), where it can interact with other TGF signals and affect autoregulation in other NVUs as they continue upstream. When MR and TGF signals transmit far enough to create interactions between NVUs, the autoregulatory mechanisms can recruit more resistance to increase autoregulatory effectiveness. Equation (Eq) 1 shows the preglomerular resistance (RPG) of NVU 3 is determined by its own TGF and MR acting on its afferent arteriole (AA) plus MR and TGF contributions form NVUs 1 and 2. Note all three NVUs contribute to upstream resistance (RART). The distributed RPG creates smoothing of resistance in space and time. (B) In situations of glomerular or vascular lesions, the NVU completely “drops out” of the nephrovascular network. The TGF signal from G2 and G3 will have a shorter interaction radius without the addition of the signal from G1. (C) Changes to the vessel, such as hypertrophy of the smooth muscle layer in hypertension, also shorten the interaction radius and result in reduced length constant, meaning the signal does not transmit as far. Thickness of the arrows represents the magnitude of the TGF signal. Not shown here for the sake of simplicity is that TGF signals also travel upstream to neighboring AAs, where the signals can also interact. Equation 1 reprinted from ref. 66, with permission.
Structural damage will eventually lead to the loss of NVUs, increase the interglomerular distance, and reduce the total amount of TGF signal that is generated along with its propagation. Depending on how extensive the loss is, the outcome of the loss of NVUs on total TGF-signaling effectiveness is difficult to predict. Similarly, cortical hypertrophy increases the distance between successive nodes (vascular branch sites) at which TGF information is shared, even if afferent arterioles contribute to TGF signal amplification. The increased distance of travel until a signal amplifier is reached may reduce the strength (amount) of TGF information reaching successive nodes.
At the level of the vascular wall, endothelial damage is predicted not only to reduce signal transmission generally, but also to disorganize the communication pathway. Hypertrophy of arteriolar/arterial VSMCs (i.e., increased numbers of VSMCs) will increase the drain of current from the endothelium, thus reducing the length of transmission and making communication much more local. Increased fractional salt reabsorption from proximal tubule, as in diabetes mellitus, reduces salt concentration at the macula densa; shifting TGF signaling toward a more dilated, preglomerular vasculature; and reducing the negative feedback regulation of the arterial microcirculation. Interventions that could strengthen TGF signaling include the carbonic anhydrase inhibitor acetazolamide and sodium-glucose cotransporter-2 (SGLT2) inhibitors, which increase NaCl delivery to the macula densa, thereby reducing GFR.95 Thomson et al. 100 administered SGLT2 inhibitors (acutely and chronically) to early diabetic rats and demonstrated that they had a continuous operation of TGF along the same curve, but that increased capacity for Na+ reabsorption in the ascending limb of the Loop of Henle contributed to the GT balance resetting to an operating point with maximum gain. The result of interventions that improve the effectiveness of TGF would be to enhance synchronization, thus strengthening operation of the vascular network, which might be able to prevent progression of diseases such as AKI, CKD, and diabetic nephropathy.
Conclusions
In this review, we have examined the emerging concept of distributed autoregulation from anatomic and physiologic standpoints. Autonomous oscillations of TGF facilitate the entrainment and synchronization of multiple oscillating NVUs. Gap junction–mediated communication provides the highway on which the TGF-initiated VCRs travel. Electrical communication (i.e., VCRs) results in information processing at every arterial branch point within the radius of TGF depolarization. This optimizes partly shared resistance upstream for a cluster of glomeruli, resulting in smoothing of the resistance differences in all of the NVUs within that cluster, so the differences in glomerular blood flow and PGC are minimized. Oxygenation-perfusion matching is an inevitable consequence of glomerular blood-flow optimization.
We have discussed that, instead of autoregulation being the averaged response of single nephrons, it may actually be the coordinated behavior of many nephrons working together to optimize perfusion. This view of synchronized network behavior represents a paradigm shift that has a large effect on the understanding of disease, as discussed. A comprehensive understanding of how pathophysiologic conditions—such as endothelial dysfunction, hypertension, diabetes, and kidney disease—affects the behavior of the nephrovascular network and synchronization warrants further study.
Perspectives
The importance of autoregulation is that, of the many control systems which regulate renal function, it is the only one that stabilizes RBF when BP fluctuates and/or increases. All other vasoconstrictor systems tend to increase renal vascular resistance as BP is reduced.
Many inputs into RBF are unrelated to BP. Although efferent renal sympathetic nerve activity is heavily coded by the baroreflex, this information is rarely transduced into RBF.102 Renal sympathetic nerve activity also carries behavioral information that is transduced into RBF.103–105 Renal denervation and NE infusions have little, if any, effect on steady-state and dynamic autoregulation, suggesting neural and autoregulatory RBF regulation are independent.106 Similarly, circadian changes of RBF, being partly behavioral, are neurally coded, and can be larger—in relative terms—than circadian changes of BP.104 Although most studies exploring autoregulation are performed during the light period in anesthetized rodents that are nocturnal, some studies have been performed during the light period in conscious dogs and rabbits. The results of these studies21,107 were comparable to similar rat studies.108,109
This leads one to the issue of timescales: hours, weeks, and years. Where timescales become important, and where they become very difficult to address, is when we get into pathologic situations, such as the transition from AKI to CKD, hypertension, and even more so in the very prolonged pathogenesis of diabetes. At this time, there are few relevant data to guide our thinking, but clearly there is considerable potential for various disease states to interfere with the structural substrate and/or operation of TGF synchronization.
The health burden of AKI, CKD, and diabetic nephropathy mandates the need to better understand their dysregulation and prevent progression to the point of RRTs. Strengthening network interactions among NVUs, by means of increasing TGF activity or by increasing the responsiveness of the TGF system, could mitigate further renal injury in these situations. For instance, SGLT2 inhibitors are widely used for treatment of diabetes, and are well known for improving cardiovascular and renal outcomes. These drugs may enhance TGF activation in NVUs which, in turn, is expected to enhance synchronization. The effect of such a strategy is to improve coordinated behavior of NVUs, thereby augmenting network autoregulation, the inevitable consequence of which is oxygenation-perfusion matching in the renal parenchyma. This concept has the potential to inform strategies used to treat renal disease. Using this new lens to view disease progression and improving synchronization to improve oxygenation-perfusion matching has a vast potential to inform the management of kidney disease.
Disclosures
B. Braam reports being on the editorial board for the American Journal of Physiology-Renal Physiology; receiving research funding from Amgen (a quality-improvement grant) and Fresenius Canada (an equipment grant); and receiving honoraria from AstraZeneca (for serving on the advisory board; CA$1000 in the year 2020) and Otsuka (for serving on the advisory board; CA$1000 in the year 2020). All remaining authors have nothing to disclose.
Funding
This work was supported by the Division of Nephrology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta Kidney Health Translational Research Chair grant/award. This work was also supported by the Canadian Institutes of Health Research grant MOP-102694.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. Beeuwkes R 3rd: Vascular-tubular relationships in the human kidney. In: Renal Pathophysiology: Recent Advances, edited by Leaf A, Giebisch G, Bolis L, Gorini S, New York, Raven Press, 1980, pp 155–163 [Google Scholar]
- 2. Beeuwkes R 3rd, Bonventre JV: Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol 229: 695–713, 1975. [DOI] [PubMed] [Google Scholar]
- 3. Marsh DJ, Postnov DD, Rowland DJ, Wexler AS, Sosnovtseva OV, Holstein-Rathlou NH: Architecture of the rat nephron-arterial network: Analysis with micro-computed tomography. Am J Physiol Renal Physiol 313: F351–F360, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Postnov DD, Marsh DJ, Postnov DE, Braunstein TH, Holstein-Rathlou NH, Martens EA, et al.: Modeling of kidney hemodynamics: Probability-based topology of an arterial network. PLOS Comput Biol 12: e1004922, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tran CHT, Vigmond EJ, Goldman D, Plane F, Welsh DG: Electrical communication in branching arterial networks. Am J Physiol Heart Circ Physiol 303: H680–H692, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zechariah A, Tran CHT, Hald BO, Sandow SL, Sancho M, Kim MSM, et al.: Intercellular conduction optimizes arterial network function and conserves blood flow homeostasis during cerebrovascular challenges. Arterioscler Thromb Vasc Biol 40: 733–750, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright FS: Flow-dependent transport processes: Filtration, absorption, secretion. Am J Physiol 243: F1–F11, 1982. [DOI] [PubMed] [Google Scholar]
- 8. Casellas D, Moore LC: Autoregulation of intravascular pressure in preglomerular juxtamedullary vessels. Am J Physiol 264: F315–F321, 1993. [DOI] [PubMed] [Google Scholar]
- 9. Braus H: Anatomie des Menschen: Zweiter Band Eingeweide, Berlin, Springer, 1924. [Google Scholar]
- 10. Casellas D, Dupont M, Bouriquet N, Moore LC, Artuso A, Mimran A: Anatomic pairing of afferent arterioles and renin cell distribution in rat kidneys. Am J Physiol 267: F931–F936, 1994. [DOI] [PubMed] [Google Scholar]
- 11. Casellas D, Bouriquet N, Moore LC: Branching patterns and autoregulatory responses of juxtamedullary afferent arterioles. Am J Physiol 272: F416–F421, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Marsh DJ, Sosnovtseva OV, Mosekilde E, Holstein-Rathlou NH: Vascular coupling induces synchronization, quasiperiodicity, and chaos in a nephron tree. Chaos 17: 015114, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Beeuwkes R 3rd: Efferent vascular patterns and early vascular-tubular relations in the dog kidney. Am J Physiol 221: 1361–1374, 1971. [DOI] [PubMed] [Google Scholar]
- 14. Burger CH, Cross RB: Aspects of renal vascular organization and early vascular tubular relations of the marsupial Isoodon obesulus . Anat Rec 203: 47–54, 1982. [DOI] [PubMed] [Google Scholar]
- 15. Yip KP, Holstein-Rathlou NH, Marsh DJ: Dynamics of TGF-initiated nephron-nephron interactions in normotensive rats and SHR. Am J Physiol 262: F980–F988, 1992. [DOI] [PubMed] [Google Scholar]
- 16. Holstein-Rathlou NH: Synchronization of proximal intratubular pressure oscillations: Evidence for interaction between nephrons. Pflugers Arch 408: 438–443, 1987. [DOI] [PubMed] [Google Scholar]
- 17. Leyssac PP, Baumbach L: An oscillating intratubular pressure response to alterations in Henle loop flow in the rat kidney. Acta Physiol Scand 117: 415–419, 1983. [DOI] [PubMed] [Google Scholar]
- 18. Holstein-Rathlou NH, Marsh DJ: Oscillations of tubular pressure, flow, and distal chloride concentration in rats. Am J Physiol 256: F1007–F1014, 1989. [DOI] [PubMed] [Google Scholar]
- 19. Holstein-Rathlou NH, Marsh DJ: A dynamic model of renal blood flow autoregulation. Bull Math Biol 56: 411–429, 1994. [DOI] [PubMed] [Google Scholar]
- 20. Daniels FH, Arendshorst WJ, Roberds RG: Tubuloglomerular feedback and autoregulation in spontaneously hypertensive rats. Am J Physiol 258: F1479–F1489, 1990. [DOI] [PubMed] [Google Scholar]
- 21. Just A, Wittmann U, Ehmke H, Kirchheim HR: Autoregulation of renal blood flow in the conscious dog and the contribution of the tubuloglomerular feedback. J Physiol 506: 275–290, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsh DJ, Sosnovtseva OV, Chon KH, Holstein-Rathlou NH: Nonlinear interactions in renal blood flow regulation. Am J Physiol Regul Integr Comp Physiol 288: R1143–R1159, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Pikovsky A, Rosenblum M, Kurths J, Hilborn RC: Synchronization: A Universal Concept in Nonlinear Science, Cambridge, United Kingdom, Cambridge University Press, 2003. [Google Scholar]
- 24. Daniels FH, Arendshorst WJ: Tubuloglomerular feedback kinetics in spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol 259: F529–F534, 1990. [DOI] [PubMed] [Google Scholar]
- 25. Holstein-Rathlou NH, Wagner AJ, Marsh DJ: Tubuloglomerular feedback dynamics and renal blood flow autoregulation in rats. Am J Physiol 260: F53–F68, 1991. [DOI] [PubMed] [Google Scholar]
- 26. Marsh DJ, Sosnovtseva OV, Pavlov AN, Yip KP, Holstein-Rathlou NH: Frequency encoding in renal blood flow regulation. Am J Physiol Regul Integr Comp Physiol 288: R1160–R1167, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Sosnovtseva OV, Pavlov AN, Mosekilde E, Yip KP, Holstein-Rathlou NH, Marsh DJ: Synchronization among mechanisms of renal autoregulation is reduced in hypertensive rats. Am J Physiol Renal Physiol 293: F1545–F1555, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Sosnovtseva OV, Pavlov AN, Mosekilde E, Holstein-Rathlou NH, Marsh DJ: Double-wavelet approach to study frequency and amplitude modulation in renal autoregulation. Phys Rev E Stat Nonlin Soft Matter Phys 70: 031915, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Yip KP, Holstein-Rathlou NH, Marsh DJ: Mechanisms of temporal variation in single-nephron blood flow in rats. Am J Physiol 264: F427–F434, 1993. [DOI] [PubMed] [Google Scholar]
- 30. Chon KH, Raghavan R, Chen Y-M, Marsh DJ, Yip K-P: Interactions of TGF-dependent and myogenic oscillations in tubular pressure. Am J Physiol Renal Physiol 288: F298–F307, 2005. [DOI] [PubMed] [Google Scholar]
- 31. Bell PD, Komlosi P, Zhang ZR: ATP as a mediator of macula densa cell signalling. Purinergic Signal 5: 461–471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen YM, Yip KP, Marsh DJ, Holstein-Rathlou NH: Magnitude of TGF-initiated nephron-nephron interactions is increased in SHR. Am J Physiol 269: F198–F204, 1995. [DOI] [PubMed] [Google Scholar]
- 33. Wagner AJ, Holstein-Rathlou NH, Marsh DJ: Internephron coupling by conducted vasomotor responses in normotensive and spontaneously hypertensive rats. Am J Physiol 272: F372–F379, 1997. [DOI] [PubMed] [Google Scholar]
- 34. Källskog O, Marsh DJ: TGF-initiated vascular interactions between adjacent nephrons in the rat kidney. Am J Physiol 259: F60–F64, 1990. [DOI] [PubMed] [Google Scholar]
- 35. Arensbak B, Mikkelsen HB, Gustafsson F, Christensen T, Holstein-Rathlou NH: Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol 115: 479–487, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Krüger O, et al.: Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 295: R371–R380, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taugner R, Schiller A, Kaissling B, Kriz W: Gap junctional coupling between the JGA and the glomerular tuft. Cell Tissue Res 186: 279–285, 1978. [DOI] [PubMed] [Google Scholar]
- 38. Pricam C, Humbert F, Perrelet A, Orci L: Gap junctions in mesangial and lacis cells. J Cell Biol 63: 349–354, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forssmann WG, Taugner R: Studies on the juxtaglomerular apparatus. V. The juxtaglomerular apparatus in Tupaia with special reference to intercellular contacts. Cell Tissue Res 177: 291–305, 1977. [DOI] [PubMed] [Google Scholar]
- 40. Taugner R, Kirchheim H, Forssmann WG: Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri . Cell Tissue Res 235: 319–325, 1984. [DOI] [PubMed] [Google Scholar]
- 41. Marsh DJ, Toma I, Sosnovtseva OV, Peti-Peterdi J, Holstein-Rathlou NH: Electrotonic vascular signal conduction and nephron synchronization. Am J Physiol Renal Physiol 296: F751–F761, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peti-Peterdi J: Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006. [DOI] [PubMed] [Google Scholar]
- 43. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J: Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sorensen CM, Holstein-Rathlou NH: Cell-cell communication in the kidney microcirculation. Microcirculation 19: 451–460, 2012. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Hill CE: Differential connexin expression in preglomerular and postglomerular vasculature: Accentuation during diabetes. Kidney Int 68: 1171–1185, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Abed AB, Kavvadas P, Chadjichristos CE: Functional roles of connexins and pannexins in the kidney. Cell Mol Life Sci 72: 2869–2877, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haas TL, Duling BR: Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res 53: 113–120, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Pohl U Connexins: Key players in the control of vascular plasticity and function. Physiol Rev 100:525–572, 2020. [DOI] [PubMed] [Google Scholar]
- 49. Just A, Kurtz L, de Wit C, Wagner C, Kurtz A, Arendshorst WJ: Connexin 40 mediates the tubuloglomerular feedback contribution to renal blood flow autoregulation. J Am Soc Nephrol 20: 1577–1585, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sorensen CM, Giese I, Braunstein TH, Brasen JC, Salomonsson M, Holstein-Rathlou NH: Role of connexin40 in the autoregulatory response of the afferent arteriole. Am J Physiol Renal Physiol 303: F855–F863, 2012. [DOI] [PubMed] [Google Scholar]
- 51. Oppermann M, Carota I, Schiessl I, Eisner C, Castrop H, Schnermann J: Direct assessment of tubuloglomerular feedback responsiveness in connexin 40-deficient mice. Am J Physiol Renal Physiol 304: F1181–F1186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Wit C, Roos F, Bolz S-S, Pohl U: Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13: 169–177, 2003. [DOI] [PubMed] [Google Scholar]
- 53. Fang JS, Angelov SN, Simon AM, Burt JM: Compromised regulation of tissue perfusion and arteriogenesis limit, in an AT1R-independent fashion, recovery of ischemic tissue in Cx40(-/-) mice. Am J Physiol Heart Circ Physiol 304: H816–H827, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Wit C, Roos F, Bolz SS, Kirchhoff S, Krüger O, Willecke K, et al.: Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86: 649–655, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Møller S, Jacobsen JCB, Braunstein TH, Holstein-Rathlou NH, Sorensen CM: Influence of connexin45 on renal autoregulation. Am J Physiol Renal Physiol 318: F732–F740, 2020. [DOI] [PubMed] [Google Scholar]
- 56. Møller S, Jacobsen JCB, Holstein-Rathlou NH, Sorensen CM: Lack of connexins 40 and 45 reduces local and conducted vasoconstrictor responses in the murine afferent arterioles. Front Physiol 11: 961, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heberlein KR, Straub AC, Isakson BE: The myoendothelial junction: Breaking through the matrix? Microcirculation 16: 307–322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sandow SL, Hill CE: Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000. [DOI] [PubMed] [Google Scholar]
- 59. Gustafsson F, Holstein-Rathlou N: Conducted vasomotor responses in arterioles: Characteristics, mechanisms and physiological significance. Acta Physiol Scand 167: 11–21, 1999. [DOI] [PubMed] [Google Scholar]
- 60. Pogoda K, Kameritsch P: Molecular regulation of myoendothelial gap junctions. Curr Opin Pharmacol 45: 16–22, 2019. [DOI] [PubMed] [Google Scholar]
- 61. Pogoda K, Mannell H, Blodow S, Schneider H, Schubert KM, Qiu J, et al.: NO augments endothelial reactivity by reducing myoendothelial calcium signal spreading: A novel role for Cx37 (connexin 37) and the protein tyrosine phosphatase SHP-2. Arterioscler Thromb Vasc Biol 37: 2280–2290, 2017. [DOI] [PubMed] [Google Scholar]
- 62. Steinhausen M, Endlich K, Nobiling R, Parekh N, Schütt F: Electrically induced vasomotor responses and their propagation in rat renal vessels in vivo . J Physiol 505: 493–501, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Welsh DG, Tran CHT, Hald BO, Sancho M: The conducted vasomotor response: Function, biophysical basis, and pharmacological control. Annu Rev Pharmacol Toxicol 58: 391–410, 2018. [DOI] [PubMed] [Google Scholar]
- 64. Little TL, Xia J, Duling BR: Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res 76: 498–504, 1995. [DOI] [PubMed] [Google Scholar]
- 65. Hald BO, Jacobsen JCB, Sandow SL, Holstein-Rathlou NH, Welsh DG: Less is more: Minimal expression of myoendothelial gap junctions optimizes cell-cell communication in virtual arterioles. J Physiol 592: 3243–3255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sorensen CM, Cupples WA: Myoendothelial communication in the renal vasculature and the impact of drugs used clinically to treat hypertension. Curr Opin Pharmacol 45: 49–56, 2019. [DOI] [PubMed] [Google Scholar]
- 67. Rhodin JA: The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res 18: 181–223, 1967. [DOI] [PubMed] [Google Scholar]
- 68. Behringer EJ, Segal SS: Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels. Circ Res 110: 1311–1321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hald BO, Welsh DG, Holstein-Rathlou NH, Jacobsen JCB: Gap junctions suppress electrical but not [Ca(2+)] heterogeneity in resistance arteries. Biophys J 107: 2467–2476, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Diep HK, Vigmond EJ, Segal SS, Welsh DG: Defining electrical communication in skeletal muscle resistance arteries: A computational approach. J Physiol 568: 267–281, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hosoyamada Y, Ichimura K, Sakai T: Organ specificity and functional relevance of the arterial structure: A comparative study in the kidney and the skeletal muscle with electron microscopy. J Vasc Res 52: 265–272, 2015. [DOI] [PubMed] [Google Scholar]
- 72. Ushiyama A, Kataoka H, Iijima T: Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care 4: 59, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sgouralis I, Layton AT: Autoregulation and conduction of vasomotor responses in a mathematical model of the rat afferent arteriole. Am J Physiol Renal Physiol 303: F229–F239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Q, Cao C, Mangano M, Zhang Z, Silldorff EP, Lee-Kwon W, et al.: Descending vasa recta endothelium is an electrical syncytium. Am J Physiol Regul Integr Comp Physiol 291: R1688–R1699, 2006. [DOI] [PubMed] [Google Scholar]
- 75. Zhang Z, Payne K, Pallone TL: Syncytial communication in descending vasa recta includes myoendothelial coupling. Am J Physiol Renal Physiol 307: F41–F52, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Z, Lin H, Cao C, Payne K, Pallone TL: Descending vasa recta endothelial cells and pericytes form mural syncytia. Am J Physiol Renal Physiol 306: F751–F763, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Z, Payne K, Pallone TL: Descending vasa recta endothelial membrane potential response requires pericyte communication. PLoS One 11: e0154948, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pallone TL: Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol 266: F850–F857, 1994. [DOI] [PubMed] [Google Scholar]
- 79. Kennedy-Lydon TM, Crawford C, Wildman SSP, Peppiatt-Wildman CM: Renal pericytes: Regulators of medullary blood flow. Acta Physiol (Oxf) 207: 212–225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kennedy-Lydon T, Crawford C, Wildman SSP, Peppiatt-Wildman CM: Nonsteroidal anti-inflammatory drugs alter vasa recta diameter via pericytes. Am J Physiol Renal Physiol 309: F648–F657, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vaz PG, Humeau-Heurtier A, Figueiras E, Correia C, Cardoso J: Laser speckle imaging to monitor microvascular blood flow: A review. IEEE Rev Biomed Eng 9: 106–120, 2016. [DOI] [PubMed] [Google Scholar]
- 82. Allen J, Howell K: Microvascular imaging: Techniques and opportunities for clinical physiological measurements. Physiol Meas 35: R91–R141, 2014. [DOI] [PubMed] [Google Scholar]
- 83. Postnov DD, Tang J, Erdener SE, Kılıç K, Boas DA: Dynamic light scattering imaging. Sci Adv 6: eabc4628, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holstein-Rathlou NH, Sosnovtseva OV, Pavlov AN, Cupples WA, Sorensen CM, Marsh DJ: Nephron blood flow dynamics measured by laser speckle contrast imaging. Am J Physiol Renal Physiol 300: F319–F329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mitrou N, Scully CG, Braam B, Chon KH, Cupples WA: Laser speckle contrast imaging reveals large-scale synchronization of cortical autoregulation dynamics influenced by nitric oxide. Am J Physiol Renal Physiol 308: F661–F670, 2015. [DOI] [PubMed] [Google Scholar]
- 86. Mitrou N, Braam B, Cupples WA: A gap junction inhibitor, carbenoxolone, induces spatiotemporal dispersion of renal cortical perfusion and impairs autoregulation. Am J Physiol Heart Circ Physiol 311: H582–H591, 2016. [DOI] [PubMed] [Google Scholar]
- 87. Kapela A, Behringer EJ, Segal SS, Tsoukias NM: Biophysical properties of microvascular endothelium: Requirements for initiating and conducting electrical signals. Microcirculation 25: e12429, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cole WC, Welsh DG: Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 510: 160–173, 2011. [DOI] [PubMed] [Google Scholar]
- 89. Hald BO, Welsh DG: Conceptualizing conduction as a pliant electrical response: Impact of gap junctions and ion channels. Am J Physiol Heart Circ Physiol 319: H1276–H1289, 2020. [DOI] [PubMed] [Google Scholar]
- 90. Hald BO, Welsh DG: Conceptualizing conduction as a pliant vasomotor response: Impact of Ca2+ fluxes and Ca2+ sensitization [published online ahead of print September 18, 2020]. Am J Physiol Heart Circ Physiol 10.1152/ajpheart.00286.2020 [DOI] [PubMed] [Google Scholar]
- 91. Scully CG, Mitrou N, Braam B, Cupples WA, Chon KH: Detecting interactions between the renal autoregulation mechanisms in time and space. IEEE Trans Biomed Eng 64: 690–698, 2017. [DOI] [PubMed] [Google Scholar]
- 92. Müller-Suur R, Ulfendahl HR, Persson AEG: Evidence for tubuloglomerular feedback in juxtamedullary nephrons of young rats. Am J Physiol 244: F425–F431, 1983. [DOI] [PubMed] [Google Scholar]
- 93. Müller-Suur R, Persson AEG, Ulfendahl HR: Tubuloglomerular feedback in juxtamedullary nephrons. Kidney Int Suppl 12: S104–S108, 1982. [PubMed] [Google Scholar]
- 94. Cupples WA, Wexler AS, Marsh DJ: Model of TGF-proximal tubule interactions in renal autoregulation. Am J Physiol 259: F715–F726, 1990. [DOI] [PubMed] [Google Scholar]
- 95. Christensen EI, Grann B, Kristoffersen IB, Skriver E, Thomsen JS, Andreasen A: Three-dimensional reconstruction of the rat nephron. Am J Physiol Renal Physiol 306: F664–F671, 2014. [DOI] [PubMed] [Google Scholar]
- 96. Marsh DJ, Postnov DD, Sosnovtseva OV, Holstein-Rathlou NH: The nephron-arterial network and its interactions. Am J Physiol Renal Physiol 316: F769–F784, 2019. [DOI] [PubMed] [Google Scholar]
- 97. Scully CG, Mitrou N, Braam B, Cupples WA, Chon KH: Segmentation of renal perfusion signals from laser speckle imaging into clusters with phase synchronized dynamics. IEEE Trans Biomed Eng 61: 1989–1997, 2014. [DOI] [PubMed] [Google Scholar]
- 98. Legrand M, Bezemer R, Kandil A, Demirci C, Payen D, Ince C: The role of renal hypoperfusion in development of renal microcirculatory dysfunction in endotoxemic rats. Intensive Care Med 37: 1534–1542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Konukoglu D, Uzun H: Endothelial dysfunction and hypertension. Adv Exp Med Biol 956: 511–540, 2017. [DOI] [PubMed] [Google Scholar]
- 100. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al.: Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thomson SC, Vallon V: Renal effects of sodium-glucose Co-transporter inhibitors. Am J Cardiol 124[Suppl 1]: S28–S35, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. DiBona GF, Sawin LL: Renal hemodynamic effects of activation of specific renal sympathetic nerve fiber groups. Am J Physiol 276: R539–R549, 1999. [DOI] [PubMed] [Google Scholar]
- 103. Grady HC, Bullivant EMA: Renal blood flow varies during normal activity in conscious unrestrained rats. Am J Physiol 262: R926–R932, 1992. [DOI] [PubMed] [Google Scholar]
- 104. Barrett CJ, Navakatikyan MA, Malpas SC: Long-term control of renal blood flow: What is the role of the renal nerves? Am J Physiol Regul Integr Comp Physiol 280: R1534–R1545, 2001. [DOI] [PubMed] [Google Scholar]
- 105. Johns EJ, Kopp UC, DiBona GF: Neural control of renal function. Compr Physiol 1: 731–767, 2011. [DOI] [PubMed] [Google Scholar]
- 106. Cupples WA, Braam B: Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007. [DOI] [PubMed] [Google Scholar]
- 107. Malpas SC, Evans RG, Head GA, Lukoshkova EV: Contribution of renal nerves to renal blood flow variability during hemorrhage. Am J Physiol 274: R1283–R1294, 1998. [DOI] [PubMed] [Google Scholar]
- 108. Lau C, Sudbury I, Thomson M, Howard PL, Magil AB, Cupples WA: Salt-resistant blood pressure and salt-sensitive renal autoregulation in chronic streptozotocin diabetes. Am J Physiol Regul Integr Comp Physiol 296: R1761–R1770, 2009. [DOI] [PubMed] [Google Scholar]
- 109. Griffin KA, Abu-Naser M, Abu-Amarah I, Picken M, Williamson GA, Bidani AK: Dynamic blood pressure load and nephropathy in the ZSF1 (fa/fa cp) model of type 2 diabetes. Am J Physiol Renal Physiol 293: F1605–F1613, 2007. [DOI] [PubMed] [Google Scholar]