Significance Statement
In patients receiving maintenance dialysis, strategies for managing hyperphosphatemia are only moderately effective. In this 4-week randomized trial involving 236 patients experiencing hyperphosphatemia despite phosphate binder use, tenapanor (a small molecule that inhibits paracellular phosphate absorption) significantly reduced serum phosphorus concentration from baseline, compared with placebo, when used with phosphate binders as a dual-mechanism treatment. A significantly larger proportion of patients randomized to tenapanor plus binder achieved a serum phosphorus concentration <5.5 mg/dl at all time points compared with placebo plus binder. Few patients discontinued tenapanor because of diarrhea (the most frequently reported adverse event) or other adverse events. These findings indicate that evaluation of long-term safety and efficacy of this dual-mechanism treatment of hyperphosphatemia is merited.
Keywords: tenapanor, hyperphosphatemia, NHE3 dialysis, phosphorus, phosphate binders, phosphate uptake, FGF23
Abstract
Background
Hyperphosphatemia is associated with cardiovascular morbidity and mortality in patients receiving maintenance dialysis. It is unknown whether combining two therapies with different mechanisms of action—tenapanor, an inhibitor of paracellular phosphate absorption, and phosphate binders—is safe and effective for the management of hyperphosphatemia in patients receiving maintenance dialysis.
Methods
This double-blind phase 3 trial enrolled 236 patients undergoing maintenance dialysis with hyperphosphatemia (defined in this trial as serum phosphorus 5.5–10 mg/dl inclusive) despite receiving phosphate binder therapy (sevelamer, nonsevelamer, sevelamer plus nonsevelamer, or multiple nonsevelamer binders). These participants were randomly assigned to receive oral tenapanor 30 mg twice daily or placebo for 4 weeks. The primary efficacy end point was the change in serum phosphorus concentration from baseline to week 4.
Results
Of the 236 randomized patients, 235 (99.6%) were included in the full analysis set; this included 116 in the tenapanor plus binder group and 119 in the placebo plus binder group. A total of 228 patients (96.6%) completed the 4-week treatment period. In the full analysis set (mean age 54.5 years, 40.9% women), patients treated with tenapanor plus binder achieved a larger mean change in serum phosphorus concentration from baseline to week 4 compared with placebo plus binder (−0.84 versus −0.19 mg/dl, P<0.001). Diarrhea was the most commonly reported adverse event, resulting in study drug discontinuation in four of 119 (3.4%) and two of 116 (1.7%) patients receiving tenapanor plus binder or placebo plus binder, respectively.
Conclusions
A dual-mechanism treatment using both tenapanor and phosphate binders improved control of hyperphosphatemia in patients undergoing maintenance dialysis compared with phosphate binders alone.
Clinical Trial registry name and registration number:
AMPLIFY, NCT03824587
Patients receiving dialysis for the management of CKD are highly likely to be hyperphosphatemic.1 Hyperphosphatemia plays a key role in the calcification of soft tissues, such as blood vessels, heart valves, and myocardium, and has been shown to be an independent risk factor for cardiovascular mortality in these patients.2–6 Current strategies for the management of hyperphosphatemia, including more frequent hemodialysis, dietary phosphate restriction, and phosphate binder (hereafter referred to as “binder”) therapy, are only moderately effective and difficult to implement.7–10
Tenapanor hydrochloride (hereafter referred to as “tenapanor”), an inhibitor of gastrointestinal sodium/hydrogen exchanger isoform 3 (NHE3), has been shown to reduce paracellular phosphate transport and facilitate management of hyperphosphatemia.11 As monotherapy, tenapanor significantly decreased serum phosphorus concentration in patients receiving maintenance dialysis (CKD stage 5D).12,13 Acting via a unique, nonphosphate binding mechanism of action, tenapanor offers a potential management option for reducing serum phosphorus concentration as part of a dual treatment approach with binders. Herein, we report on the safety and efficacy of tenapanor 30 mg twice daily versus placebo in combination with stabilized doses of binder for the management of hyperphosphatemia in patients receiving maintenance dialysis.
Methods
Study Design
AMPLIFY was a multicenter, phase 3, randomized, double-blind, placebo-controlled trial enrolling patients from 46 centers in the United States between February 2019 and July 2019 (Figure 1). After a 2- to 4-week run-in period, during which their existing binder dose was maintained, we randomized eligible patients to receive oral tenapanor 30 mg or placebo twice daily for the 4-week treatment period while they continued to receive their dose of binder. We used a stratified randomization scheme, with type of binder (sevelamer [alone or in combination with nonsevelamer] or nonsevelamer agents [alone or in combination]) and serum phosphorus concentration at the end of the run-in period (<7.5 or ≥7.5 mg/dl) as two stratification factors. We used an interactive response technology (IRT) to implement randomization; the IRT provided the patient randomization number and the appropriate bottle identification for the treatment allocated.
Figure 1.
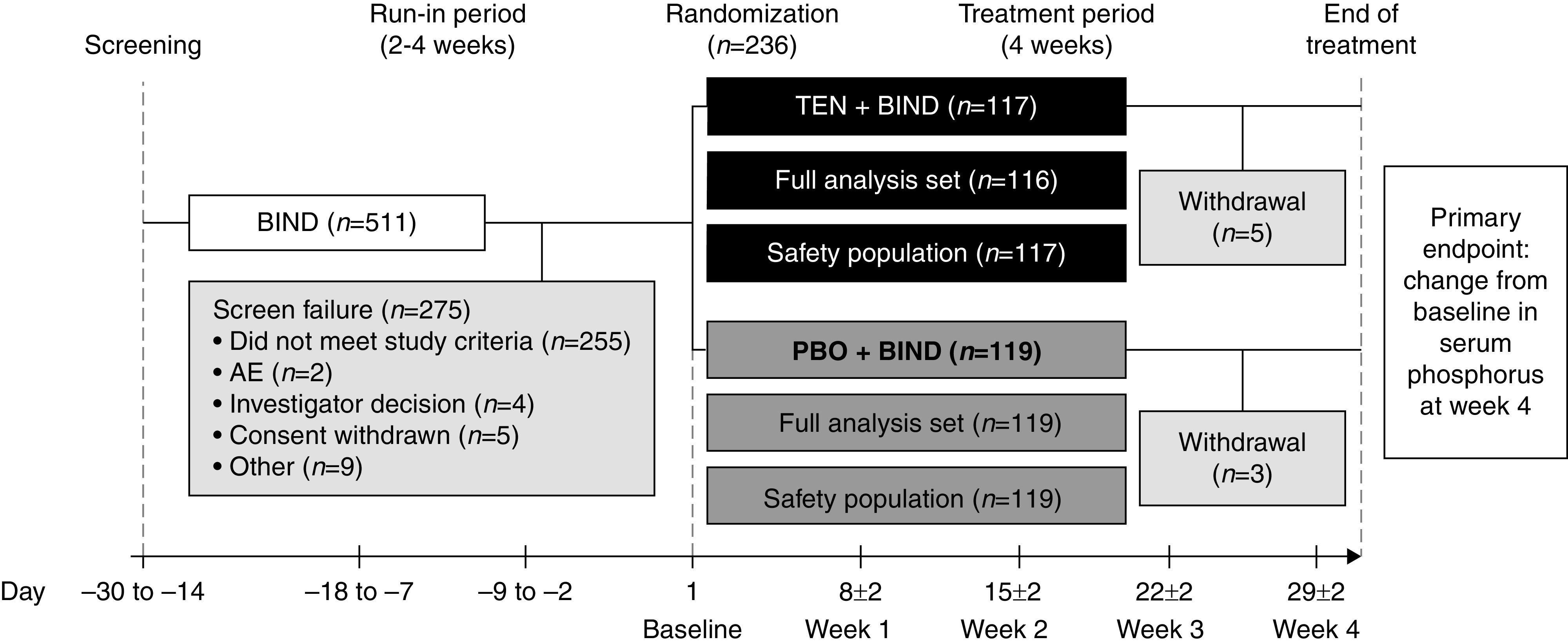
Overview of patient flow through the trial. The safety population set included all randomized patients who received at least one dose of treatment. The FAS included all randomized patients who had at least one postbaseline serum phosphorus measurement during the trial. Baseline was defined as the measurement collected at day 1 (randomization). If this value was missing, the last measurement before the first dose of study drug was used. BIND, binder; PBO, placebo; TEN, tenapanor.
Each patient’s binder dose remained stable during the run-in and treatment periods. During the first 2 weeks of treatment, investigators were permitted to titrate the study drug dose on the basis of serum phosphorus concentrations and gastrointestinal tolerability. Investigators could down titrate tenapanor or placebo from 30 to 20 mg and from 20 to 10 mg twice daily in response to adverse events (AEs) and could also up titrate from 10 to 20 mg and from 20 to 30 mg twice daily if tolerated. We selected these doses on the basis of results from phase 2 clinical trials of tenapanor.12 Patients did not receive specific dietary recommendations prior to or during their participation in the trial.
We conducted the trial in accordance with the Declaration of Helsinki. All patients provided written informed consent before trial entry. All participating sites obtained independent ethics committee/institutional review board approval.
Patients
Full inclusion and exclusion criteria used in this trial are listed in Supplemental Material. Briefly, men and women aged 18–80 years at screening (week −4 to −3) were eligible for randomization if they had received maintenance hemodialysis thrice weekly for at least 3 months or maintenance peritoneal dialysis for at least 6 months; had a per-session Kt/Vurea of 1.2 or more at the most recent assessment before screening; had been prescribed and were taking one or more binders at least thrice daily, with an unchanged dose during the 4 weeks before screening; and had a serum phosphorus concentration of 5.5–10 mg/dl (inclusive) both at screening and at the end of the run-in period. Patients who did not meet the criterion for serum phosphorus concentration at the end of the run-in period (“screen failure”) could be rescreened if they had met the serum phosphorus criterion at screening, but they were required to wait a minimum of 1 week after screen failure for rescreening. Key exclusion criteria were serum plasma parathyroid hormone concentration exceeding 1200 pg/ml, clinical signs of hypovolemia at screening, and a history of inflammatory bowel disease/irritable bowel syndrome with diarrhea.
Prior/Concomitant Medications
Unless indicated for the treatment of an AE, concomitant medications that we permitted during the treatment period were those recorded at screening and used during the run-in period. All patients were to remain on a stable binder dose for the duration of the trial. Patients receiving active vitamin D or calcimimetics were required to have maintained a stable dose during the 4 weeks before screening.
Efficacy End Points and Assessments
The primary efficacy end point was the change in serum phosphorus concentration from baseline to week 4; the efficacy of tenapanor + binder was evaluated by the difference between the tenapanor + binder and placebo + binder groups in mean change from baseline in serum phosphorus concentration at week 4. Serum phosphorus concentration was measured at each scheduled visit; baseline was defined as the last measurement prior to the first dose of study drug, and it was typically the measurement collected at day 1 of the treatment period (randomization).
Key secondary efficacy end points included the proportion of patients achieving serum phosphorus below 5.5 mg/dl at week 4 and the relative change from baseline in intact fibroblast growth factor 23 (iFGF23) and C-terminal fibroblast growth factor 23 (cFGF23) concentrations at week 4 (derived from the fibroblast growth factor 23 [FGF23] value at week 4 divided by the baseline value –1). Measurement of FGF23 was scheduled at baseline and week 4 (or at early termination). Other secondary end points included the change from baseline in serum phosphorus concentration and the proportions of patients achieving serum phosphorus below 5.5 mg/dl at weeks 1, 2, and 3.
We recorded study drug adherence at each scheduled visit after baseline. We calculated adherence rate by dividing the number of tablets taken by the patient since the preceding visit by the number of tablets expected to be taken since the preceding visit.
Safety Outcomes and Assessments
Safety assessments were on the basis of AEs, clinical laboratory tests, vital signs, electrocardiograms, and physical examinations. We assessed BP and pulse at screening, baseline, and week 4 (or early termination). We measured predialysis body weight and performed predialysis clinical laboratory tests (serum electrolytes, hematology, and urinalysis), 12-lead electrocardiogram, and physical examinations at screening and week 4 (or early termination).
Statistical Methods
We included all randomized patients in the intent to treat (ITT) analysis set. The full analysis set (FAS), used for the evaluation of efficacy end points, included all ITT patients with at least one postbaseline measurement of serum phosphorus. The safety population, used for the analysis of safety data, consisted of all ITT patients who received at least one dose of study drug (tenapanor or placebo) in combination with binder.
A sample size of 107 in each treatment group was expected to achieve 95% power to detect a difference of −0.5 mg/dl in the serum phosphorus concentration (primary efficacy end point) between the tenapanor + binder and placebo + binder groups at the 0.050 significance level. Statistical analyses were performed at the two-sided significance levels of 0.010 and 0.050.
All measures were summarized by randomized group. We calculated geometric mean and geometric coefficient of variation for iFGF23 and cFGF23, which we expressed as percentage change. We performed all data manipulation, descriptive statistics, and statistical hypothesis testing using SAS version 9.3 or later.
We followed a sequential testing procedure to control the overall type I error rate associated with multiple comparisons on the primary efficacy end point and the three key secondary efficacy end points at the 0.05 level. For the analysis of the primary efficacy end point, we performed the treatment comparison using a mixed effects model for repeated measures on observed cases of the FAS. The mixed effects model for repeated measures included the IRT-recorded binder type (sevelamer or nonsevelamer), serum phosphorus concentration at week −1 (<7.5 or ≥7.5 mg/dl), treatment, visit (week 1 to week 4), and treatment by visit interaction as fixed effects. We included the baseline serum phosphorus concentration and baseline by visit interaction as covariates and patient as a random effect. We calculated the proportion of patients achieving serum phosphorus below 5.5 mg/dl at week 4 and analyzed the relative change from baseline in iFGF23 and cFGF23 at week 4 using an ANOVA model on the log-transformed relative value at week 4.
We summarized and analyzed the primary and key secondary end point data by age group (<45 or ≥45 years and <65 or ≥65 years), sex (man or woman), race (White or Black), pooled site (West, Central, or East), type of binder (sevelamer or nonsevelamer), baseline serum phosphorus concentration (<7.5 or ≥7.5 mg/dl), and type of dialysis (hemodialysis or peritoneal dialysis).
Results
Patients
Of 511 patients who were screened for enrollment, 236 (46.2%) were randomly assigned to treatment with tenapanor (117 patients) or placebo (119 patients) for 4 weeks in combination with prior binder therapy (Figure 1). All 119 patients treated with placebo + binder and 116 of 117 patients (99.1%) receiving tenapanor + binder were included in the FAS.
One hundred and twelve of 117 patients (95.7%) assigned to tenapanor + binder and 116 of 119 patients (98.3%) assigned to placebo + binder completed the treatment period. Eight patients (3.4%) prematurely discontinued from the trial overall; one (0.8%) patient in the placebo group and four (3.4%) patients in the tenapanor group withdrew owing to an AE, one (0.8%) patient receiving placebo and one (0.9%) patient receiving tenapanor withdrew owing to a kidney transplant, and one (0.8%) patient in the placebo group opted to withdraw from the trial.
Treatment groups were well balanced with respect to patient demographics, baseline disease characteristics (Table 1), and mean daily dose of binder (Supplemental Table 1).
Table 1.
Patient demographics and baseline characteristics (FAS)
| Demographic or Characteristic | TEN + BIND, n=116 | PBO + BIND, n=119 | Overall, n=235 |
|---|---|---|---|
| Age, yr | 55.1 (12.4) | 53.9 (12.7) | 54.5 (12.5) |
| Men, n (%) | 65 (56.0) | 74 (62.2) | 139 (59.1) |
| Race, n (%) | |||
| White | 57 (49.1) | 60 (50.4) | 117 (49.8) |
| Black | 51 (44.0) | 50 (42.0) | 101 (43.0) |
| Other a | 8 (6.9) | 9 (7.6) | 17 (7.2) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 37 (31.9) | 30 (25.2) | 67 (28.5) |
| Not Hispanic or Latino | 78 (67.2) | 88 (73.9) | 166 (70.6) |
| Not reported/unknown | 1 (0.9) | 1 (0.8) | 2 (0.9) |
| Body mass index, kg/m2 | 33.4 (7.7) | 30.8 (8.2) | 32.1 (8.0) |
| Time since first dialysis, mo b | 42.6 (11.4, 111.1) | 35.7 (8.1, 104.0) | 51.4 (45.3) |
| Type of dialysis, n (%) | |||
| Hemodialysis | 104 (89.7) | 107 (89.9) | 211 (89.8) |
| Peritoneal dialysis | 12 (10.3) | 12 (10.1) | 24 (10.2) |
| Most recent Kt/Vurea before screening | |||
| Hemodialysis | 1.5 (0.3) | 1.6 (0.4) | 1.6 (0.4) |
| Peritoneal dialysis | 2.1 (0.5) | 2.1 (0.3) | 2.1 (0.4) |
| Baseline serum phosphorus concentration, mg/dl | 6.7 (1.3) | 6.9 (1.4) | 6.8 (1.4) |
| Baseline iFGF23 concentration, pg/ml b | 8426.9 (1094.0, 36,690.0) | 10,472.0 (1239.9, 40,275.0) | 9435.8 (1189.7, 36,690.0) |
| Baseline cFGF23 concentration, RU/ml b | 13,508.0 (2766.3, 57,567.0) | 16,100.0 (3450.1, 50,234.0) | 14,573.0 (3266.4, 53,570.0) |
| Baseline PTH concentration, pg/ml b | 308.5 (112.0, 692.0) | 316.0 (147.0, 681.0) | 314.0 (123.0, 681.0) |
| Concomitant binder, n (%) | |||
| Sevelamer only | 56 (48.3) | 58 (48.7) | 114 (48.5) |
| Nonsevelamer | 60 (51.7) | 61 (51.3) | 121 (51.5) |
| Concomitant calcimimetic, n (%) | 47 (40.5) | 46 (38.7) | 93 (39.6) |
Baseline was defined as the measurement collected at day 1. If this value was missing, the last measurement before the first dose of study drug was used. Race was self-reported by the patient at trial enrollment. Data are mean (SD) unless otherwise specified. TEN, tenapanor; BIND, binder; PBO, placebo; RU, relative unit; PTH, parathyroid hormone.
Includes Asian, Native American or Alaskan, Native Hawaiian or Pacific Islander, and other.
Data are median (10th percentile, 90th percentile).
Study Assessments
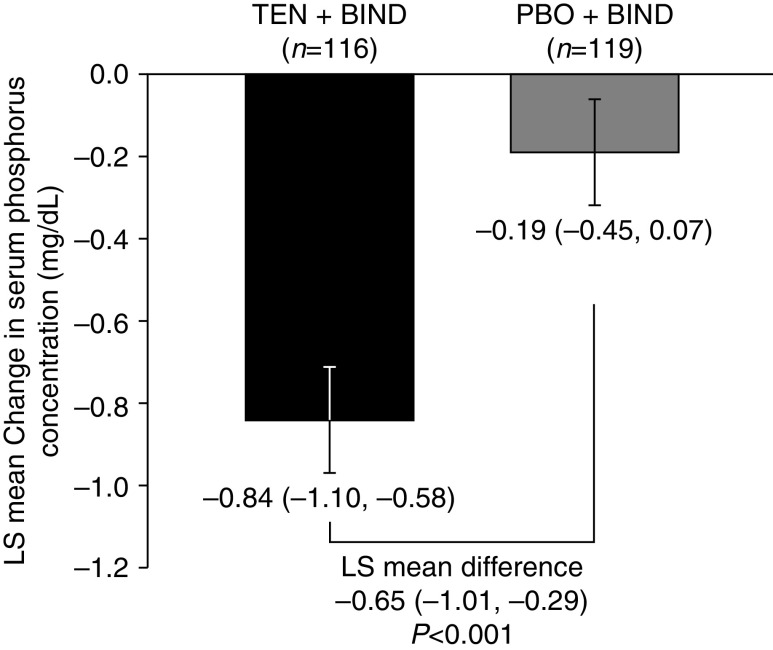
Patients treated with tenapanor + binder had a significantly larger least squares (LS) mean change in serum phosphorus concentration from baseline to week 4 compared with placebo + binder (−0.84 versus −0.19 mg/dl, P<0.001) (Figure 2). Subgroup analyses performed on the primary end point confirmed the efficacy of tenapanor + binder in reducing serum phosphorus; at week 4, patients treated with tenapanor + binder achieved a larger LS mean reduction from baseline than placebo + binder in all 16 subgroups analyzed despite the smaller sample size compared with the FAS (Supplemental Figure 1).
Figure 2.
LS mean change in serum phosphorus concentration from baseline at week 4 (primary end point, FAS). Mean baseline serum phosphorus was 6.9 mg/dl for the PBO + BIND group and 6.7 mg/dl for the TEN + BIND group. Baseline was defined as the measurement collected at day 1. If this value was missing, the last measurement before the first dose of study drug was used. Data are LS mean change (95% confidence interval) in serum phosphorus concentration, P value, and SEM from a mixed effects model for repeated measures. BIND, binder; PBO, placebo; TEN, tenapanor.
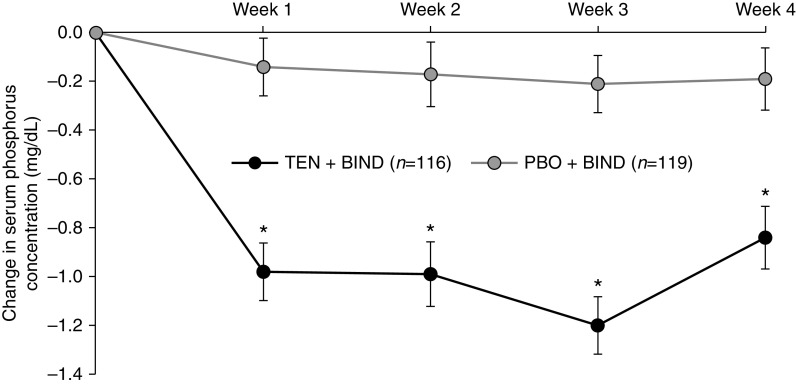
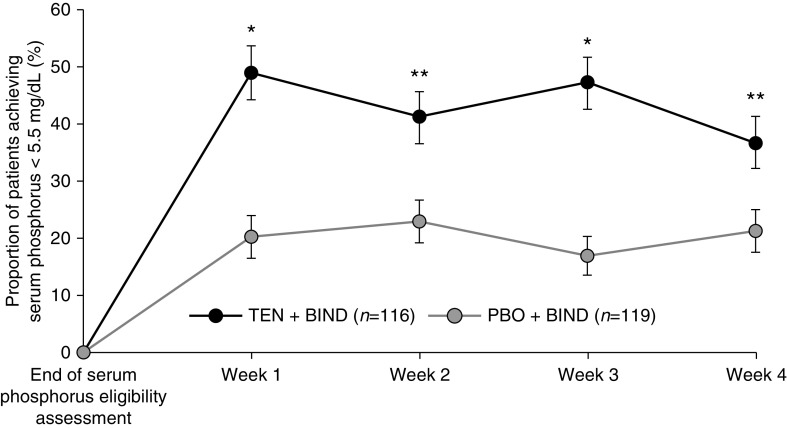
Figure 3 shows the effect of tenapanor versus placebo, in combination with binder, on serum phosphorus concentration over the treatment period. Treatment with tenapanor + binder resulted in significantly more pronounced LS mean reductions from baseline at all time points compared with placebo + binder (0.84–1.21 versus 0.14–0.21 mg/dl, P<0.001). Additionally, a significantly larger proportion of patients receiving tenapanor + binder achieved a serum phosphorus concentration below 5.5 mg/dl at week 1 (49.1% versus 21.0%, P<0.001), week 2 (41.4% versus 23.5%, P=0.003), week 3 (47.4% versus 17.6%, P<0.001), and week 4 (37.1% versus 21.8%, P=0.01) compared with placebo + binder (Figure 4).
Figure 3.
Change in serum phosphorus concentration from baseline over time to week 4 (FAS). Mean baseline serum phosphorus was 6.9 mg/dl for the PBO + BIND group and 6.7 mg/dl for the TEN + BIND group. Baseline was defined as the measurement collected at day 1. If this value was missing, the last measurement before the first dose of study drug was used. Data are LS mean change in serum phosphorus concentration, P value, and SEM from a mixed effects model for repeated measures. BIND, binder; PBO, placebo; TEN, tenapanor. *P<0.001 versus placebo.
Figure 4.
Proportion of patients achieving serum phosphorus below 5.5 mg/dl at weeks 1–4 (± SEM; FAS). The P value was obtained from the Cochran–Mantel–Haenszel test adjusting for type of binder and serum phosphorus concentration before randomization (<7.5 or ≥7.5 mg/dl). BIND, binder; PBO, placebo; TEN, tenapanor. *P<0.001 versus placebo; **P<0.01 versus placebo.
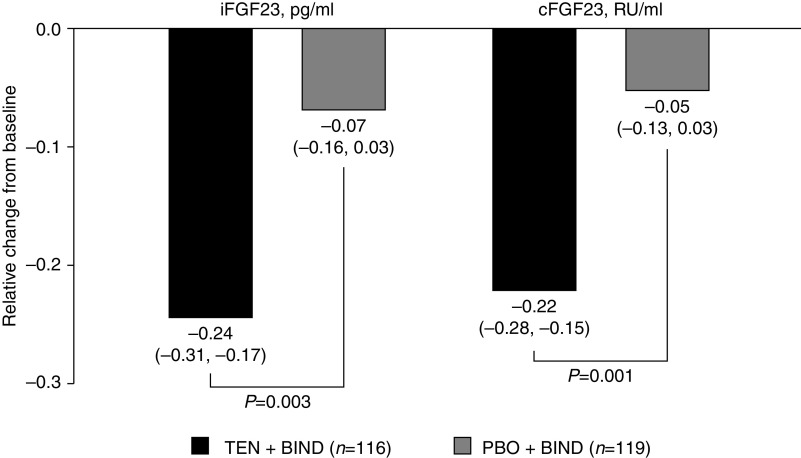
Figure 5 shows the relative change from baseline in concentrations of iFGF23 and cFGF23 with tenapanor and placebo, in combination with binder. At week 4, patients treated with tenapanor + binder achieved significantly more pronounced relative reductions in concentrations of iFGF23 (24.4% versus 6.9%, P=0.003) and cFGF23 (22.1% versus 5.3%, P=0.001). Statistical significance was achieved for the primary efficacy end point and the three key secondary efficacy end points after multiplicity adjustment.
Figure 5.
Relative change from baseline at week 4 in concentrations of iFGF23 and cFGF23 (FAS). Mean baseline iFGF23 (SD) was 15,436.9 (16,804.2) pg/ml for the PBO + BIND group and 14,032.4 (15,729.8) pg/ml for the TEN + BIND group. Mean baseline cFGF23 (SD) was 24,108.0 (26,238.8) RU/ml for the PBO + BIND group and 22,203.7 (25,068.6) RU/ml for the TEN + BIND group. Relative change from baseline at week 4 was derived as “week 4 value/baseline value –1.” Baseline was defined as the measurement collected at day 1. If this value was missing, the last measurement before the first dose of study drug was used. The P values come from an analysis of covariates model with the natural logarithm of “week 4 value/baseline value” as the dependent variable and serum phosphorus level before randomization (<7.5 or ≥7.5 mg/dl) and treatment as factors. BIND, binder; PBO, placebo; RU, relative unit; TEN, tenapanor.
Study drug adherence was generally similar between the tenapanor + binder and placebo + binder groups, and the overall mean adherence rates (since the preceding scheduled visit) were 85.1%, 90.9%, 91.0%, and 87.2% at treatment weeks 1, 2, 3, and 4, respectively. For those patients randomly assigned to receive tenapanor, the final twice-daily dose was 30 mg for 65 patients (55.6%), 20 mg for 35 patients (29.9%), and 10 mg for 17 patients (14.5%); the mean (± SD) final dose of tenapanor was 24.1 (±7.3) mg twice daily. For those randomly assigned to receive placebo, the final twice-daily dose was 30 mg for 106 patients (89.1%), 20 mg for 12 patients (10.1%), and 10 mg for one patient (0.8%).
Safety Assessments
Table 2 provides an overview of the AEs that occurred during the treatment period. Three patients in the tenapanor + binder group and five patients in the placebo + binder group experienced serious AEs, most commonly respiratory, thoracic, and mediastinal disorders. Of patients receiving placebo + binder, one patient (0.8%) experienced acute pulmonary edema, acute respiratory failure, and a pneumothorax, and one patient (0.8%) experienced dyspnea. Within the tenapanor + binder group, one patient (0.9%) experienced acute pulmonary edema, and one patient (0.9%) experienced pulmonary edema. None of the serious AEs were judged to be related to treatment. No deaths occurred over the course of the trial.
Table 2.
Overview of AEs (safety population)
| AEs | TEN + BIND, n=117 | PBO + BIND, n=119 | Overall, n=236 |
|---|---|---|---|
| Any AE | 60 (51.3) | 33 (27.7) | 93 (39.4) |
| Treatment-related AEs | 51 (43.6) | 15 (12.6) | 66 (28.0) |
| Any SAE | 3 (2.6) | 5 (4.2) | 8 (3.4) |
| Treatment-related SAEs | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AEs leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AEs leading to study drug discontinuation | 5 (4.3) | 2 (1.7) | 7 (3.0) |
| Treatment-related AEs leading to study drug discontinuation | 5 (4.3) | 2 (1.7) | 7 (3.0) |
| AEs by preferred term a | |||
| Diarrhea | 50 (42.7) | 8 (6.7) | 58 (24.6) |
| Nausea | 6 (5.1) | 3 (2.5) | 9 (3.8) |
| Treatment-related AEs by preferred term a | |||
| Diarrhea | 47 (40.2) | 8 (6.7) | 55 (23.3) |
| Nausea | 6 (5.1) | 3 (2.5) | 9 (3.8) |
Data are presented as n (%). Treatment-related AEs were those considered to be related or possibly related to study drug by the investigator. There are no MedDRA terms available to indicate 'softening of stool.' Therefore, any such change in stool consistency was designated "diarrhea." TEN, tenapanor; BIND, binder; PBO, placebo; SAE, serious adverse event.
Occurring in at least 3.0% of patients in the TEN group and at a higher incidence than in the PBO group.
Diarrhea was the most common AE with tenapanor + binder treatment, reported at a placebo-adjusted rate of 36.0%, and it was the only AE by preferred term that occurred at an adjusted rate >3.0%. Diarrhea was typically transient (≤1 week in duration) and mild to moderate in severity. Approximately two-thirds of cases occurred within 1 week of commencing treatment with tenapanor + binder (Supplemental Figure 2). Treatment-related AEs were reported for 51 patients (43.6%) receiving tenapanor + binder and 15 patients (12.6%) receiving placebo + binder during the 4-week treatment period. By system organ class, most treatment-related AEs were gastrointestinal in nature, with diarrhea and nausea the only treatment-related AEs by preferred term reported in at least 2.0% of patients receiving tenapanor + binder. Diarrhea was judged to be treatment-related for 47 patients (40.2%) in the tenapanor + binder group and eight patients (6.7%) in the placebo + binder group; study drug dose was reduced for 37 patients (31.6%) in the tenapanor + binder group and five patients (4.2%) in the placebo + binder group. Diarrhea resolved following dose titration in almost all cases. Most patients who received dose adjustment were being treated concomitantly with one or more nonsevelamer binders. Two patients (1.7%) in the placebo + binder group and five patients (4.3%) in the tenapanor + binder group discontinued the study drug owing to an AE. Diarrhea was the cause for discontinuation of the study drug for the two patients who discontinued placebo + binder treatment and for four of five patients who discontinued tenapanor + binder treatment.
We observed no clinically significant differences between groups in terms of laboratory parameters, electrocardiographic parameters, vital signs, and physical examinations during the trial.
Discussion
In this 4-week, randomized, phase 3 trial of patients receiving maintenance dialysis with hyperphosphatemia despite the use of binders, a dual-mechanism treatment with tenapanor and stable doses of phosphate binders significantly reduced serum phosphorus concentrations from baseline (mean reductions of 0.84–1.21 mg/dl) compared with placebo + binder (0.14–0.21 mg/dl) across 4 weeks of treatment. The effect of tenapanor in combination with binder was consistent with findings from studies of tenapanor as monotherapy; compared with baseline, reductions of 0.48–1.98 mg/dl were achieved with tenapanor monotherapy in a 4-week phase 2 dose-finding trial,12 reductions of 1.00–1.19 mg/dl were achieved in an 8-week phase 3 trial,13 and a reduction of 1.27 mg/dl was achieved in a 26-week phase 3 trial.
In patients receiving maintenance dialysis, hyperphosphatemia is associated with all-cause and cardiovascular mortality; clinical practice guidelines for management recommend reductions toward normal serum phosphorus concentrations.8,14 Despite efforts to manage hyperphosphatemia, approximately 70% of patients on dialysis continue to have elevated serum phosphorus concentrations.7 In our trial, a significantly larger proportion of patients receiving tenapanor + binder achieved a serum phosphorus concentration below 5.5 mg/dl (37.1%–49.1%) compared with placebo + binder (17.6%–23.5%) across the 4 weeks of treatment. Additionally, treatment with tenapanor + binder resulted in significantly more pronounced relative reductions in iFGF23 and cFGF23 concentrations at week 4 compared with placebo + binder, consistent with previous studies of tenapanor.12,13,15 Elevated FGF23 concentrations16–18 have been shown to be an independent risk factor for cardiovascular and noncardiovascular death.19 Further research is necessary to evaluate the effect of reducing FGF23 concentration on health outcomes, but initial analyses indicate a potential therapeutic benefit.20
There is an unmet need for safe and effective phosphorus-lowering medications with novel mechanisms of action, simple dosing schedules, and acceptable tolerability profiles. Recent evidence has highlighted the substantial contribution of passive paracellular phosphate transport to overall intestinal absorption and serum phosphorus concentration, which is not adequately addressed by current treatment measures.11,21,22 Tenapanor is a minimally absorbed small molecule inhibitor of NHE3 that acts within the gastrointestinal tract to inhibit sodium absorption.11 The effect of tenapanor on phosphate absorption is a result of NHE3 inhibition, which transiently increases the intracellular proton concentration of cells lining the gastrointestinal lumen and induces a conformational change in tight junction proteins that reduces permeability specific to paracellular phosphate transport; effects on the paracellular transport of other ions or molecules have not been observed.11 The use of a dual-mechanism treatment with tenapanor and phosphate binders may result in enhanced control of serum phosphorus concentration compared with monotherapy with either agent, which may be particularly relevant for the management of patients with persistent hyperphosphatemia. The use of tenapanor may also allow for a reduction in phosphate binder dose and pill burden, increasing the overall tolerability of hyperphosphatemia treatment and thereby, improving patient adherence.23–25
The combination of tenapanor and binder was generally well tolerated; the most common AE recorded was diarrhea, which was typically transient or resolved with dose titration. Diarrhea was reported if the patient described an increase in the frequency of bowel movements or a change in stool form, consistent with a softer stool, compared with their typical bowel movements; the change from baseline was not necessarily considered bothersome by the patient. A randomized, controlled, phase 3 study that evaluated the gastrointestinal effects of tenapanor found that the average effect of tenapanor on stool form and frequency was a detectable softening (an increase of 0.8, as per Bristol Stool Form Scale criteria) and a modest increase in bowel movement frequency (equivalent to one additional bowel movement every 2.5 days).13 Real-world experience with tenapanor, as monotherapy or as part of a dual-mechanism treatment with binders, may potentially help to define the optimal management of hyperphosphatemia by facilitating tailored therapy on the basis of individual patient characteristics and comorbid (including gastrointestinal) conditions to allow for more patients to achieve target serum phosphorus concentrations.
Potential limitations of the trial should be acknowledged. The study was designed to be of short-term duration; for patients who did not achieve serum phosphorus <5.5 mg/dl with tenapanor + binder, additional time to optimize doses may be beneficial. Evaluation of the long-term safety and efficacy of tenapanor for patients with hyperphosphatemia is ongoing; an 18-month open-label trial (NORMALIZE; ClinicalTrials.gov identifier: NCT03988920) will evaluate the capacity of tenapanor, when used as monotherapy or as part of a dual-mechanism treatment with sevelamer, to achieve “normal” (within the population reference range) serum phosphorus concentrations (>2.5 and ≤4.5 mg/dl) in patients with CKD stage 5D and serum phosphorus concentrations over 4.5 mg/dl.
In summary, the AMPLIFY trial demonstrates that a dual-mechanism treatment using tenapanor and phosphate binders facilitates control of hyperphosphatemia in patients receiving maintenance dialysis.
Disclosures
G.M. Chertow reports consultancy agreements with Akebia, Amgen, Ardelyx, Inc., AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, and Vertex; ownership interest in Ardelyx, Inc., CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, and PuraCath; research funding from the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); scientific advisor or membership as serving on the board of directors for Satellite Healthcare and as coeditor for Brenner & Rector’s The Kidney (Elsevier); and other interests/relationships with Angion, Bayer, NIDDK, and ReCor. P.E. Pergola is a consultant for Akebia Therapeutics, Ardelyx, Inc., AstraZeneca, Bayer, Corvidia Therapeutics, Gilead Sciences, Otsuka, Reata Pharmaceuticals, Tricida, and Unicycive; reports ownership interest in Unicycive Therapeutics; reports research funding as a principal or subinvestigator on multiple clinical trials (the contracts are with the practice, not the individual); reports scientific advisor or membership with Ardelyx, Inc. and Unicycive; and reports serving on the speakers bureau from AstraZeneca. D.P. Rosenbaum is an employee of, and has ownership interest in, Ardelyx, Inc. Y. Yang is employed by Ardelyx, Inc.
Funding
None.
Data Sharing Statement
Individual deidentified participant data and additional study-related documents will not be available.
Supplementary Material
Acknowledgments
The authors thank all of the patients and investigators involved. Medical writing support was provided by Svetha Sankar, Doctor of Veterinary Medicine, of Oxford PharmaGenesis and was funded by Ardelyx, Inc.
Ardelyx, Inc. was the sponsor of the trial.
Dr. Pablo E. Pergola, Dr. David P. Rosenbaum, Dr. Yang Yang, and Dr. Glenn M. Chertow contributed to the planning of the trial, interpretation of the data, and critical revision of the manuscript for important intellectual content; Dr. Yang Yang reviewed the manuscript critically for statistical content; and Dr. Glenn M. Chertow, Dr. Pablo E. Pergola, Dr. David P. Rosenbaum, and Dr. Yang Yang approved the final version of the manuscript for submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020101398/-/DCSupplemental.
Supplemental Material. Selection of trial population.
Supplemental Figure 1. Least squares mean difference in change in serum phosphorus (milligrams per deciliter) from baseline to week 4 (subgroup analysis of full analysis set).
Supplemental Figure 2. Reports of any diarrhea event after randomization.
Supplemental Table 1. Summary of type of phosphate binder and mean daily dose in use at baseline (intent to treat set).
References
- 1. Rastogi A, Bhatt N, Rossetti S, Beto J: Management of hyperphosphatemia in end-stage renal disease: A new paradigm. J Ren Nutr 31: 21–34, 2021. [DOI] [PubMed] [Google Scholar]
- 2. Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Cano-Megías M, Guisado-Vasco P, Bouarich H, de Arriba-de la Fuente G, de Sequera-Ortiz P, Álvarez-Sanz C, et al.: Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: A prospective long-term follow-up study. BMC Nephrol 20: 188, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cozzolino M, Ciceri P, Galassi A, Mangano M, Carugo S, Capelli I, et al.: The key role of phosphate on vascular calcification. Toxins (Basel) 11: 213, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al.; ARO Investigators: Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26: 1948–1955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The DOPPS Practice Monitor: Serum phosphorus (most recent): National sample, 2020. Available at: http://www.dopps.org/DPM. Accessed August 27, 2020
- 8. Barreto FC, Barreto DV, Massy ZA, Drüeke TB: Strategies for phosphate control in patients with CKD. Kidney Int Rep 4: 1043–1056, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umeukeje EM, Mixon AS, Cavanaugh KL: Phosphate-control adherence in hemodialysis patients: Current perspectives. Patient Prefer Adherence 12: 1175–1191, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, et al.: Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev 8: CD006023, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, et al.: Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 10: eaam6474, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Block GA, Rosenbaum DP, Leonsson-Zachrisson M, Åstrand M, Johansson S, Knutsson M, et al.: Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 28: 1933–1942, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Block GA, Rosenbaum DP, Yan A, Chertow GM: Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: A randomized phase 3 trial. J Am Soc Nephrol 30: 641–652, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lertdumrongluk P, Rhee CM, Park J, Lau WL, Moradi H, Jing J, et al.: Association of serum phosphorus concentration with mortality in elderly and nonelderly hemodialysis patients. J Ren Nutr 23: 411–421, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Block GA, Rosenbaum DP, Yan A, Greasley PJ, Chertow GM, Wolf M: The effects of tenapanor on serum fibroblast growth factor 23 in patients receiving hemodialysis with hyperphosphatemia. Nephrol Dial Transplant 34: 339–346, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, et al.: Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390: 325–331, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ix JH, Shlipak MG, Wassel CL, Whooley MA: Fibroblast growth factor-23 and early decrements in kidney function: The Heart and Soul Study. Nephrol Dial Transplant 25: 993–997, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waziri B, Duarte R, Naicker S: Chronic kidney disease-mineral and bone disorder (CKD-MBD): Current perspectives. Int J Nephrol Renovasc Dis 12: 263–276, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, et al.: Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: A meta-analysis. J Am Soc Nephrol 29: 2015–2027, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, et al.; Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators*: Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: The evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 132: 27–39, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Fouque D, Vervloet M, Ketteler M: Targeting gastrointestinal transport proteins to control hyperphosphatemia in chronic kidney disease. Drugs 78: 1171–1186, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saurette M, Alexander RT: Intestinal phosphate absorption: The paracellular pathway predominates? Exp Biol Med (Maywood) 244: 646–654, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fissell RB, Karaboyas A, Bieber BA, Sen A, Li Y, Lopes AA, et al.: Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial Int 20: 38–49, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gray K, Ficociello LH, Hunt AE, Mullon C, Brunelli SM: Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis 12: 1–8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA: Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant 29: 2092–2099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.