Abstract
Objective:
Turner Syndrome women (monosomy X) have high risk of aortopathies consistent with a role for sex chromosomes in disease development. We demonstrated that sex chromosomes influence regional development of angiotensin II (AngII)-induced aortopathies in mice. In this study, we determined if the number of X chromosomes regulates regional development of AngII-induced aortopathies.
Approach and Results:
We used females with varying numbers of X chromosomes (XXF or XOF) on an C57BL/6J (ascending aortopathies) or Ldlr−/− background (descending and abdominal aortopathies) compared to XY males (XYM). To induce aortopathies, mice were infused with AngII. XOF (C57BL/6J) exhibited larger percent increases in ascending aortic lumen diameters than AngII-infused XXF or XYM. AngII-infused XOF (Ldlr−/−) exhibited similar incidences of thoracic (XOF, 50%; XYM, 71%) and abdominal aortopathies (XOF, 83%; XYM, 71%) as XYM, which were greater than XXF (XXF, 0%). Abdominal aortic lumen diameters and maximal external diameters were similar between XOF and XYM but greater than XXF, and these effects persisted with extended AngII infusions. Larger aortic lumen diameters, abdominal aortopathy incidence (XXF, 20%; XOF, 75%), and maximal aneurysm diameters (XXF, 1.02 ± 0.17; XOF, 1.96 ± 0.32 mm; P=0.027) persisted in ovariectomized AngII-infused XOF mice. Data from RNA-seq demonstrated that X chromosome genes that escape X-inactivation (histone lysine demethylases Kdm5c and Kdm6a) exhibited lower mRNA abundance in aortas of XOF than XXF (P=0.033 and 0.024 respectively). Conversely, DNA methylation was higher in aortas of XOF than XXF (P=0.038).
Conclusion:
The absence of a second X chromosome promotes diffuse AngII-induced aortopathies in females.
Keywords: Monosomy X, Turner Syndrome, angiotensin, aneurysm, sex chromosome, vascular disease, women, vascular biology
Introduction
Women with Turner Syndrome, or monosomy X, have an increased risk of aortic dissection1 that is associated with stiffer aortas2 which exhibit lower distensibility3. A higher frequency of aortic dissection in Turner Syndrome females (≈40 per 100,000 compared to 6 per 100,000 in the general population) occurs at small ascending aortic diameters4–6 and is often fatal. Thus, the absence of a second X chromosome in women has a striking effect to increase susceptibility to aortopathies, but the mechanisms for the enhancements are unclear.
Aortopathies, including ascending, descending thoracic (DTA), abdominal aortic aneurysms (AAAs) and aortic dissection exhibit sexual dimorphism.7–10 We demonstrated that male mice, similar to humans, exhibit a 4-fold higher incidence of angiotensin II (AngII)-induced AAAs than females.11, 12 Infusion of AngII also promoted aortopathies in the ascending aorta to a greater extent in male than female mice.13, 14 Results from previous studies demonstrated that sex hormones contribute to sex differences in experimental AngII-induced aortopathies, with testosterone identified as a primary facilitator of higher AngII-induced AAAs in male mice.11, 12, 15 Recent studies indicated that both determinants of biologic sex, namely sex hormones and sex chromosome complement (XX versus XY), influenced the development and regional location of AngII-induced aortopathies.13, 16 Specifically, XY male mice (XYM) exhibited diffuse aortopathies extending from the ascending to abdominal aorta when infused with AngII, while XX females (XXF), typically resistant to aortopathy development, developed focal AAAs when susceptibility was increased by exposures to testosterone.13 Moreover, when ovary-intact female mice with XX versus XY sex chromosomes were compared, infusion of AngII caused more diffuse and severe aortopathies throughout the aorta of XY females, with increased incidence of aortic rupture.16 Conversely, when comparing testes-intact XY and XX males, AngII-infusion resulted in more focal aortopathy development within the suprarenal aorta of XX relative to XY males.13 These results suggest a role for sex chromosome genes to regulate sex differences in regional aortopathy development; however, the relative contribution of two X chromosomes in females, versus the presence of the Y chromosome in males to these findings is unclear.
In this study, we defined the effect of one (analogous to Turner Syndrome) versus two X chromosomes in female mice on the regional development of AngII-induced aortopathies. We hypothesized that two X chromosomes protect females in an aortic region-specific manner from AngII-induced aortopathies.
Materials and Methods.
Data that support the findings reported in this manuscript are available from the corresponding author upon reasonable request.
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Kentucky. We used XY* male mice17–19 with an aberrant Y chromosome (recombines abnormally with the X chromosome) on a C57BL/6J background to develop mice with selected numbers of X chromosomes. XY* male mice bred to XXF produce genotypes including XY*X (XO) or XXF. Here we bred XY* male mice (C57BL/6J or on an low density lipoprotein receptor deficient (Ldlr−/−) background) to wild type female mice (C57BL/6J, stock#000664) or to female Ldlr−/− mice (Stock#002207, The Jackson Laboratory) to obtain female mice with two (XX) or one (XO) X chromosome. In some studies, we compared XXF and XOF to wild type AngII-infused Ldlr−/− XYM mice. Mice with these sex chromosome genotypes were developed on an Ldlr−/− background to increase susceptibility to AngII-induced descending and abdominal aortopathies19, or maintained on a C57BL/6J background to study ascending aortopathies. Ldlr−/− mice were fed a Western-diet (TD88137, Envigo) starting one week before implantation of AngII containing osmotic minipumps while C57BL/6J mice were fed normal rodent laboratory diet for the duration of the experiment. Mice were infused subcutaneously with AngII (1,000 ng/kg/min, Bachem, lot#1010697) by osmotic micropumps (Alzet model 1004, Durect Co) for specified intervals. At the onset of AngII infusion mice were between 14-18 weeks of age (Figure 1–3 and 5) or 20-24 weeks of age (Figure 4). At study endpoint, mice were euthanized under anesthesia (ketamine/xylazine 100:10 mg/kg, i.p.) for exsanguination by cardiac puncture.
Figure 1.
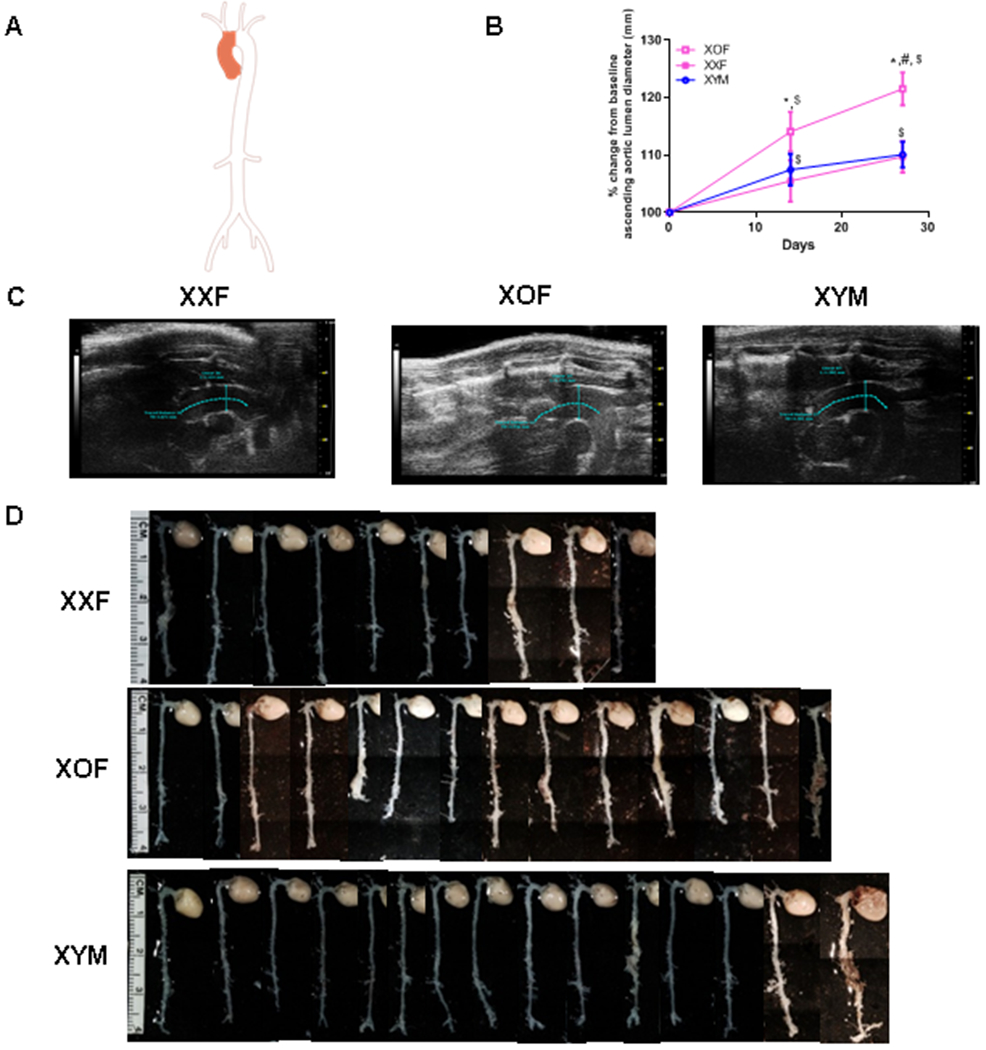
XOF C57BL/6 mice have dilated ascending aortas when infused with AngII. A, Aorta illustration with shaded area depicting ascending aorta. B, Percent change from baseline of ascending aortic lumen diameters of XXF, XOF or XYM mice during AngII infusions. Data are mean ± SEM from FXX=14, FXO=14, and MXY=15. C, Representative ultrasound images of ascending aorta, blue lines delineate areas of measurement. D, Representative aortas from mice of each group, the last aortas on the right within XOF and XYM groups were classified as ruptures. *, P<0.05 FXO compared to XXF. #, P<0.05 XOF compared to XYM. $, p<0.05 compared to day 0 within genotype.
Figure 3.
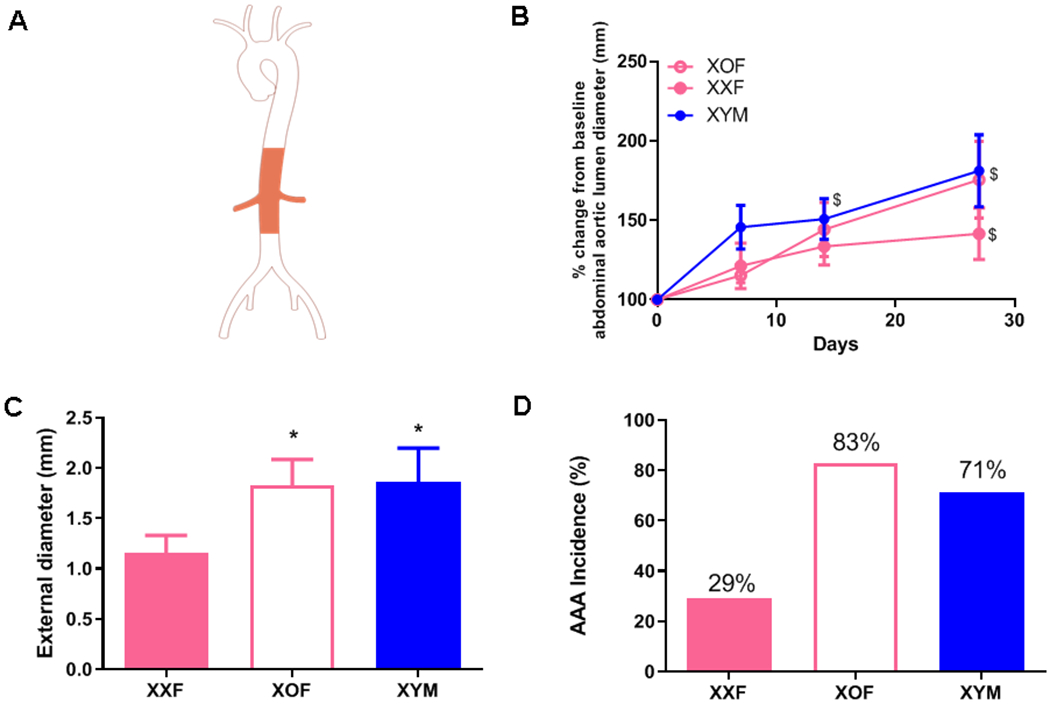
XOF Ldlr−/− mice develop severe abdominal aortic aneurysms (AAAs) to the level of XY males (M) when infused with AngII. A, Aorta illustration with shaded area depicting abdominal aorta. B, Abdominal aorta lumen diameters of FXO, FXX and MXY mice during AngII infusions. C, Maximal external diameters of abdominal aortas from mice of each group. D, AAA incidence from mice of each group, AAA incidence percentage illustrated within each bar. Data are mean ± SEM from n = 6-7/group. *, P<0.05 compared to FXX.
Figure 5.
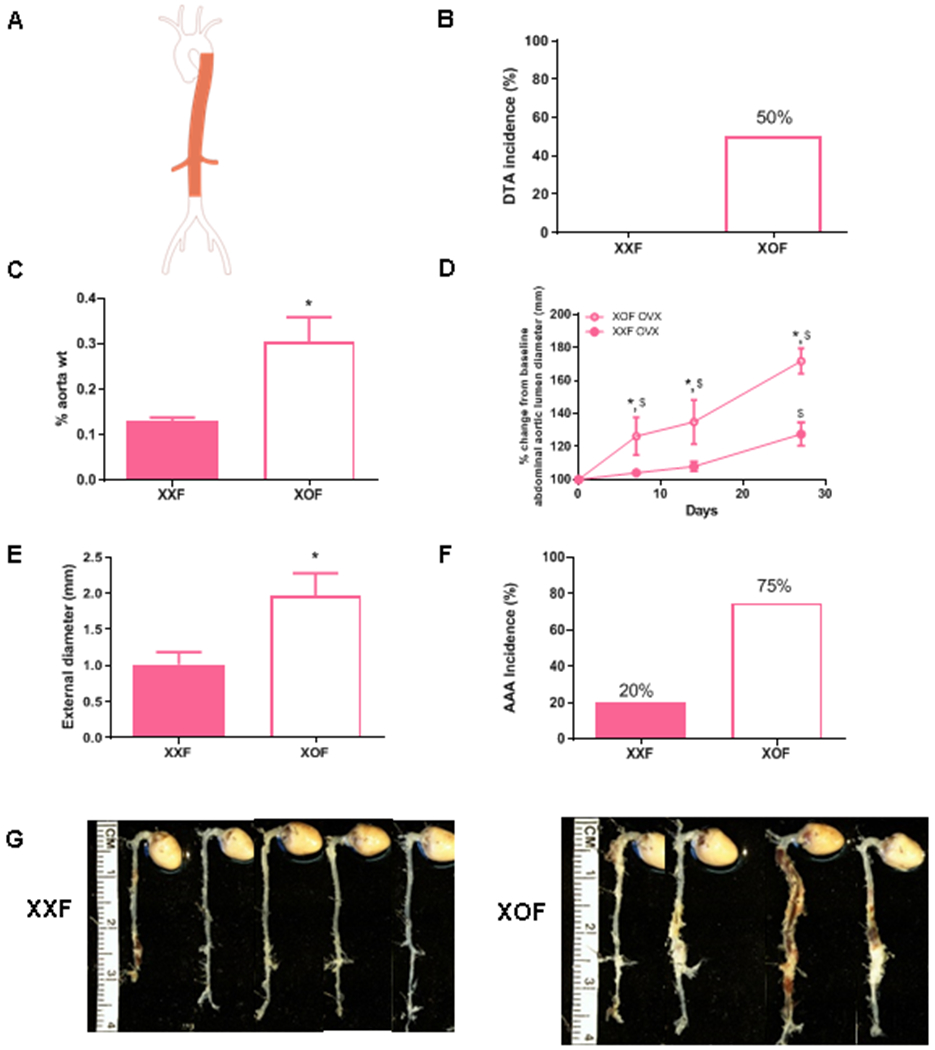
Ovariectomy does not influence augmented descending thoracic (DTA) and abdominal (AAA) aortopathies of XOF Ldlr−/− mice. A, Aorta illustration with shaded areas depicting thoracic and abdominal aortas. B, DTA incidence (%) of XXF and XOF mice. C, Aortic weights as a percentage of body weight in female ovariectomized (OVX) mice of each genotype. D, % change in abdominal aortic lumen diameters from baseline in OVX female mice of each genotype during AngII infusions. E, Maximal external diameters of abdominal aortas of OVX female mice of each genotype. F, AAA incidence (%) of OVX female mice of each genotype. G, Aortas from mice of each group. Data are mean ± SEM from XXF (n=5) and XOF (n=4) . *, P<0.05 compared to XXF. $, p<0.05 compared to day 0 within genotype.
Figure 4.
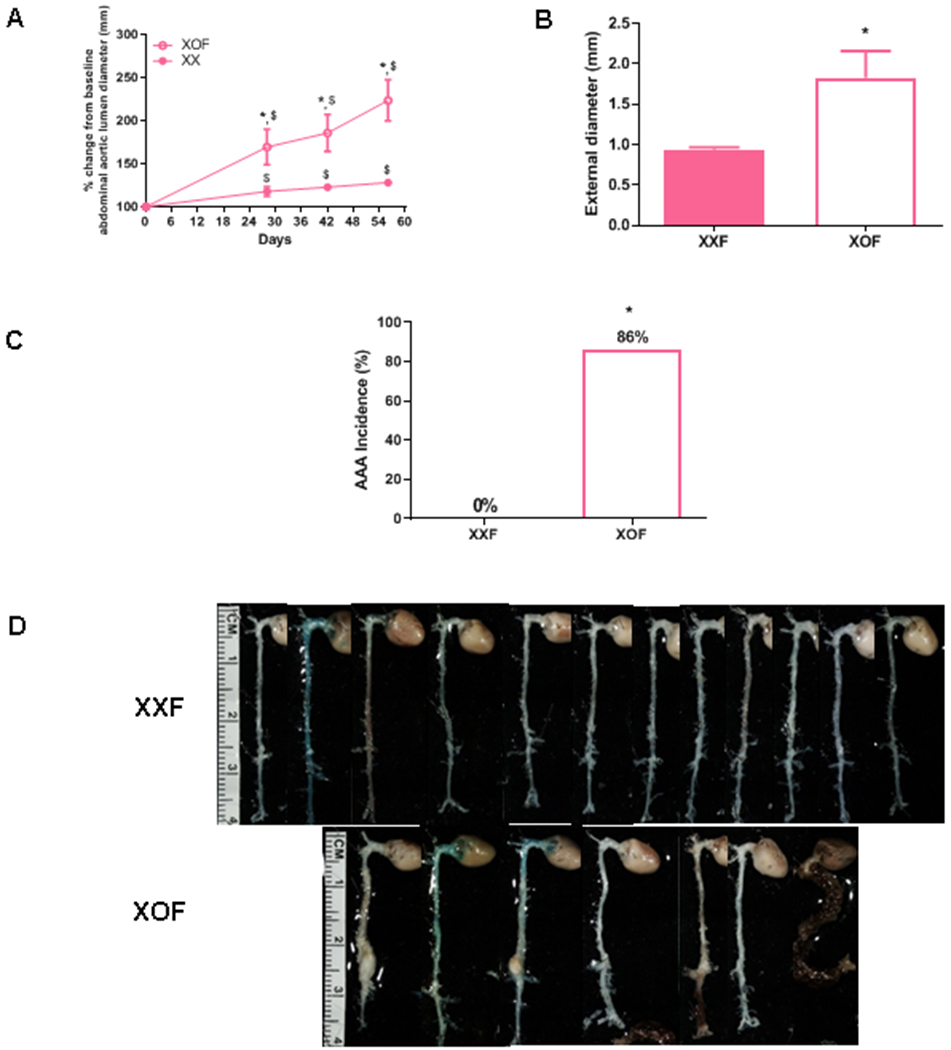
Prolonged AngII infusion results in pronounced abdominal aortic lumen dilation and increased AAA size in XOF compared to XXF. A, % change in abdominal aortic lumen diameters from baseline at various time points during prolonged infusions of AngII to XXF and XOF mice. B, Maximal AAA external diameters of XXF and XOF mice. C, AAA incidence of XXF and XOF mice at study endpoint. AAA incidence percentage illustrated within each bar. D, Aortas from mice of each group, the last aorta on the right within the XOF group was classified as ruptured. Data are mean ± SEM from XXF (n=12), XOF (n=7). *, P<0.05 compared to XXF. $, p<0.05 compared to day 0 within genotype.
Ovariectomy
To remove female gonadal hormones, Ldlr−/− XXF and XOF mice (14 weeks of age; n = 5 mice/genotype) were ovariectomized as described previously.11, 20 In brief, in anesthetized (isoflurane, to effect) females, an incision (1 cm) was made at each flank, fallopian tubes were located and the blood supply to ovaries was interrupted via a hemostat. Ovaries were excised, the peritoneum was sutured (5–0 black monofilament nylon suture, Ethilon 1668G), and the skin was closed (staples). Mice were allowed to recover on a heating pad and monitored for 7 days. Studies were initiated 12 days following surgery.
Ascending Aortic Aneurysm Quantification
Ultrasound (Vevo 2100 high-resolution imaging system, 55 MHz probe, VisualSonics, Inc) was performed on anesthetized (2-3% isoflurane) C57BL/6J mice to quantify ascending aorta lumen diameters (scans of aortic root through the ascending aorta) on day 0, 14, and 27 of AngII infusions.13, 16 Ascending aortic lumen diameters were quantified during systole. At study endpoint, maximal ex vivo diameters of the excised, cleaned ascending aorta were quantified from images recorded with a Nikon SMZ800 dissecting microscope, and image analyses were performed using Nikon Elements version 3.2.
Descending Thoracic Aortic Aneurysm Quantification
The percent incidence of DTA was quantified based on visual appearance of pathology in the descending thoracic aortic region of Ldlr−/− mice by two observers blinded to the experimental groups. We also used the aortic weight (ascending aorta to diaphragm), expressed as a percentage of the body weight, as a mode of quantifying DTA.
AAA Quantification
Ultrasound (Vevo 2100 high-resolution imaging system, 55 MHz probe, VisualSonics, Inc) was performed on anesthetized (2-3% vol/vol isoflurane) Ldlr−/− mice to quantify aortic lumen diameters of the suprarenal abdominal aorta on days 0, 7, 14, and 27 of AngII infusion. Abdominal hair was removed by shaving and applying a depilatory cream to anesthetized mice. Maximal ex vivo abdominal (AAA) diameters were quantified on cleaned, excised abdominal aortas at study endpoint using images obtained with a Nikon SMZ800 dissecting microscope. Image analysis was performed using Nikon Elements Version 3.2 software. AAA incidence was defined by visual inspection of cleaned aortas by two observers blinded to the experimental groups. Mice that died of a confirmed rupture in the abdominal region were included in the calculation of AAA incidence.
Serum and Plasma Components
Sera total cholesterol concentrations were quantified from Ldlr−/− mice using an enzymatic assay kit (Total cholesterol; FUJIFILM Wako Diagnostics USA). Plasma renin concentrations were quantified by assaying angiotensin I concentrations (IBL, CAT#IB59131) generated in the absence or presence of an excess of exogenous rat angiotensinogen as described previously.21
Blood Pressure
Systolic blood pressure was quantified on conscious mice using a Visitech system (BP-2000, Apex, NC) as described previously.13, 16 Blood pressure of each mouse was quantified before pump implantation and during the 3rd week of AngII infusion. Measurements were recorded for 4 days at the same time of day. The first day of measurement was used to acclimate mice to the system, and measurements from each mouse were averaged over the other 3 days of recording.
RNA sequencing (RNA seq)
Transcriptional profiling:
Harvested thoracic and abdominal aorta RNA samples from GDX female XX and XO FCG Ldlr−/− mice (n = 5 mice/group) were collected. Samples were provided to the University of Kentucky Genomics Core for sample preparation and sequencing. RNA Integrity Number [RIN]: 8.97 ± 0.04 were of sufficient quality. Poly-A enriched mRNA underwent whole transcriptome sequencing (TruSeq library kit in an Illumina HiSeq 2500 High Output, single-end 100 bp reads). Pre-alignment quality control showed an average read depth of 37.4 ± 2.2 million reads per sample at a read length of 99.5 ± 0.1/ 100 base pairs, with a mean read quality of 36.21 ± 0.01 phred (between 99.9 and 99.99% base identification accuracy). Reads were aligned to Genome Reference Consortium Mouse Build 38 (mm10) using the Spliced Transcripts Alignment to a Reference [STAR] (2.6.1) sequence aligning algorithm.22 Post-alignment assembled results (Partek Flow, v 8.0) retained 30.9 ± 1.9 million unique reads per sample with a read quality of 36.30 ± 0.01 phred. The resulting counts were normalized to abundance measures with the trimmed-mean of M values (TMM) algorithm 23 in WebMev 24. 19,296 rows of data were filtered to retain protein encoding genes for which at least 2 samples had TMM signal ≥ 1.5, resulting in 13,270 rows of prestatistically filtered data. The filtered data set underwent statistical analysis using LIMMA 25. The fdr procedure 26, as modified by Storey27 was used to estimate the error of multiple testing. Functional categorization was determined with the prestatistically filtered gene list as a background using DAVID bioinformatic tools’ functional overrepresentation clustering algorithm output 28 on the Gene Ontology annotation set67,29.
Data Availability:
Quality control data (disambiguated per sample), STAR alignment settings, and significant results are provided as supplementary material. Complete FASTQ and TMM normalized RNA-seq data are available through the Gene Expression Omnibus (GSE154036, www.ncbi.nlm.nih.gov/geo/).
RT-PCR Quantification of mRNA Abundance
RNA was extracted from the thoracic aorta (defined as the segment extending from the aortic root to the diaphragm) and abdominal aorta (defined as the segment extending from the diaphragm to the iliac bifurcation) from XXF and XOF Ldlr−/− mice fed a Western diet for 1 week. RNA was extracted using a Maxwell Rapid Sample Concentrator (RSC) simply RNA Tissue kit (REF#AS1340, Promega, Madison, WI). RNA (226 ng) was reverse transcribed to cDNA using Supermix (cat#95048-500, Quanta Biosciences, Gaithersburg, MD). cDNA was diluted (1:10) and mRNA abundance of Kdm5c or Kdm6a was quantified by RT-PCR using SYBER Green FastMix (cat#95071-012, Gaithersburg, MD) on a BioRad quantitative real-time PCR thermocycler (CF96 Real-Time system, BioRad, Hercules, CA). mRNA abundance was determined using the ΔΔCt method and normalized to β-actin as a housekeeping gene.
Primer sequences for Kdm5c were: Forward 5’-ACCCACCTGGCAAAAACATTGG-3’; reverse 5’-ACTGTCGAAGGGGGATGCTGTG-3’. Primers sequences for β-actin were: 5’-GCTCTGGCTCCTAGCACCAT-3’; reverse 5’-GCCACCGATCCACACAGAGT-3’. Primer sequences for Kdm6a were: Forward 5’-CCAATCCCCGCAGAGCTTACCT-3’; reverse 5’-TTGCTCGGAGCTGTTCCAAGTG-3’.
Global DNA Methylation Quantification
Female Ldlr−/− (20 weeks of age) XX and XO mice (n = 5-8 mice/genotype) were fed a Western diet for 1 week before infusing them with AngII (1,000 ng/kg/min) for 3 days by osmotic pump. Mice were anesthetized (ketamine/xylaxine 100:10 mg/kg, i.p.) for exsanguination, and aortas were cleaned from extraneous tissues under a dissecting microscope. Aortas were separated into thoracic (root to diaphragm) and abdominal regions (diaphragm to iliac bifurcation) and processed for DNA extraction using Maxwell RSC Tissue DNA kit (CAT#AS1610, Promega, Madison, WI). The percentage of 5-methyl cytosine (5-mC) in DNA extracts was quantified using a commercial Zymo Research kit (CAT#D5325) for quantification of global DNA methylation.
Aorta Sectioning
We chose aortas from studies of AngII-infused C57BL/6J mice (ascending aortopathy) or Ldlr−/− mice (descending thoracic or abdominal aortopathies)(n = 1/sex chromosome genotype) that had a primary endpoint measure (internal or external aortic diameters) close to the mean for that group. A pair of 5 μm paraffin embedded aortic sections was placed on each of 5 slides. Three more sets of 5 pairs were placed such that each slide had 4 pairs of sections, each pair from a location on the artery 50 μm distant from the adjacent pairs, with 100 μm discarded between each set of 5 slides from thoracic and abdominal segments, and 60 μm discarded between each set from ascending aortas. A slide from each level was selected and stained with Van Gieson (Sigma HT25A-1KT).
Statistical Analysis
For illustration, data are presented as mean ± standard error (SEM). Data sets were tested for normality (Shapiro-Wilk) and equal variance (Brown-Forsythe). If data did not pass normality, then data were statistically transformed to natural log. To define differences between groups (normally distributed data), data were analyzed using unpaired Student t-test for two sample comparisons, by repeated measures two-way ANOVA (ultrasound), or by one-way ANOVA for multiple group comparisons using Holm-Sidak posthoc tests. For categorical data (aneurysm incidence), data were analyzed using Fisher’s Exact test. Statistical significance was defined as P<0.05. Statistical analyses were performed using either SigmaPlot software (Version 13) or GraphPad Prism 5.
Results
Absence of a second X chromosome in females promoted diffuse AngII-induced aortopathies to the incidence and severity of XYM
To define the role of one versus two X chromosomes on AngII-induced aortopathies, we used XY* mice with an aberrant pseudoautosomal region (PAR) that recombines variably with the X PAR during meiosis to generate XXF and XOF.17–19 For studies quantifying ascending aortopathies (Figure 1A), gonad-intact XXF and XOF C57BL/6J and XYM mice were infused with AngII for 28 days. Body weights, plasma renin concentrations, and sex organ weights were not significantly different between XXF and XOF mice (Table 1). However, XYM mice had greater body weights than females of either genotype. Systolic blood pressures were significantly lower in XYM AngII-infused mice compared to females of either genotype (Table 1; P<0.05). At baseline, absolute ascending aortic diameters were significantly larger in XYM than XXF or XOF mice (XYM: 1.03 ± 0.23; XXF: 0.90 ± 0.17; XOF: 0.95 ± 0.03 mm; P<0.05). Because of baseline differences in aortic lumen dimensions between sexes, we calculated the percent change from baseline of ascending lumen diameters following AngII infusions within each sex and genotype (Figure 1B). Following AngII infusion, the percent change in ascending aortic lumen diameters was similar between XXF and XYM mice (Figure 1B). However, the percent change in ascending aortic lumen diameters was significantly larger following AngII infusion in XOF compared to XXF (days 14 and 27) or XYM mice (day 27) (Figure 1B; P<0.05). At study endpoint, we quantified external diameters within the descending and abdominal aortas of AngII-infused C57BL/6J XXF, XOF and XYM mice. External diameters of DTA were significantly larger in XYM than XXF mice (Supplemental Figure IA; P<0.05). However, there was no significant difference between sexes or genotypes of AAA external diameters (Supplemental Figure IB; P>0.05). Moreover, the number of mice that died of aortic rupture in each group was not significantly different between sexes (male versus female) or sex chromosome genotype (Table 1; P> 0.05). Representative images of ascending aortic lumens are illustrated for each genotype in Figure 1C, and representative aortas from mice of each genotype are illustrated in Figure 1D. Representative tissue sections from ascending aortas were Van Gieson stained to assess morphology and illustrate, in general, that adventitial expansion occurred in ascending aortas from XOF and XYM, but not XXF mice (Supplemental Figure I).
Table 1.
Characteristics of AngII-infused mice.
| Parameter | XXF | XOF | XYM |
|---|---|---|---|
| C57BL/6J ascending aortopathy formation | |||
| Body weight (g) | 21.93 ± 0.33 | 20.64 ± 0.45 | 25.33 ± 0.64$ |
| Blood Pressure (mmHg) | 150 ± 3.2 | 150 ± 2.3 | 141 ± 2.1$ |
| Plasma renin (ng/ml/30 min) | 3.61 ± 0.19 | 3.36 ± 0.09 | 3.56 ± 0.11 |
| Sex organs weight/ body weight% | 0.48 ± 0.04 | 0.53 ± 0.06 | 0.68 ± 0.04 |
| No. of ruptured mice/ total number of mice | 0/14 | 1/14 | 1/15 |
| Intact Ldlr−/− DTA and AAA formation | |||
| Body weight (g) | 23.01 ± 0.7 | 20.10 ± 1.2* | 26.8 ± 0.6$ |
| Blood pressure (mmHg) | 140 ± 6 | 122 ± 4.8* | 139 ± 6.3$ |
| Plasma renin (ng/ml/30 min) | 3.25 ± 0.19 | 2.34 ± 0.21* | 3.1 ± 0.31 |
| Sex organs weight/ body weight% | 0.37 ± 0.06 | 0.40 ± 0.04 | 0.58 ± 0.03 |
| No. of ruptured mice/ total number of mice | 0/7 | 1/6 | 1/7 |
| Females Intact Ldlr−/− AAA progression | |||
| Body weight (g) | 26.08 ± 0.7 | 23.45 ± 0.5* | |
| Plasma renin (ng/ml/30 min) | 2.8 ± 0.17 | 2.8 ± 0.12 | |
| Sex organs weight/ body weight% | 0.46 ± 0.04 | 0.38 ± 0.04 | |
| No. of ruptured mice/ total number of mice | 0//12 | 1/7 | |
| Females Ovariectomized Ldlr−/− DTA and AAA formation | |||
| Body weight (g) | 25.70 ± 0.8 | 21.95 ± 0.94* | |
| Blood Pressure (mmHg) | 146 ± 5 | 153 ± 4.8 | |
| Plasma renin (ng/ml/30 min) | 4.4 ± 1.0 | 3.63 ± 0.2 | |
| Sex organs weight/ body weight% | 0.06 ± 0.004 | 0.05 ± 0.01 | |
| No. of ruptured mice/ total number of mice | 0/5 | 0/4 | |
P<0.05 XXF vs. XOF
P<0.05 XYM vs. XXF or XOF
For studies focused on sex chromosome influences on DTA or AAA, we used XXF, XOF and XYM mice on an Ldlr−/− background and fed a Western diet, which heightens the sensitivity to these AngII-induced aortopathies.30–32 After one week of the diet, all mice were infused with AngII for 28 days. Body weights were significantly lower in XOF compared to AngII-infused XXF mice (Table 1; P<0.05). Moreover, body weights of XOF were significantly lower than those of XYM mice (Table 1; P<0.05). Systolic blood pressures of XXF and XYM mice infused with AngII were not significantly different (Table 1). However, systolic blood pressures of AngII-infused XOF mice were significantly lower than those of XXF or XYM mice (Table 1; P<0.05). Similarly, plasma renin concentrations of XXF and AngII-infused XYM mice were similar, but plasma renin concentrations of XXF were higher than those of XOF mice (Table 1; P<0.05). Weights of sex organs were not significantly different between XXF and XOF mice (Table 1). The incidence of DTAs increased from 0 (XXF) to 50% (XOF; Figure 2B; P<0.05), to an incidence that was not significantly different from XYM mice (71%; Figure 2B). The diffuse nature of aortopathies could be quantified by larger aortic weights in XOF than XXF, resulting in aortic weights similar to those of AngII-infused XYM mice (Figure 2C; P<0.05). The diffuse nature of aortopathies of XOF mice is also evident in excised aortas at study endpoint (Figure 2D). Representative tissue sections from descending thoracic aortas were Van Gieson stained to assess morphology and illustrate, in general, that adventitial expansion occurred in descending thoracic aortas from XOF and XYM, but not XXF mice (Supplemental Figure III).
Figure 2.
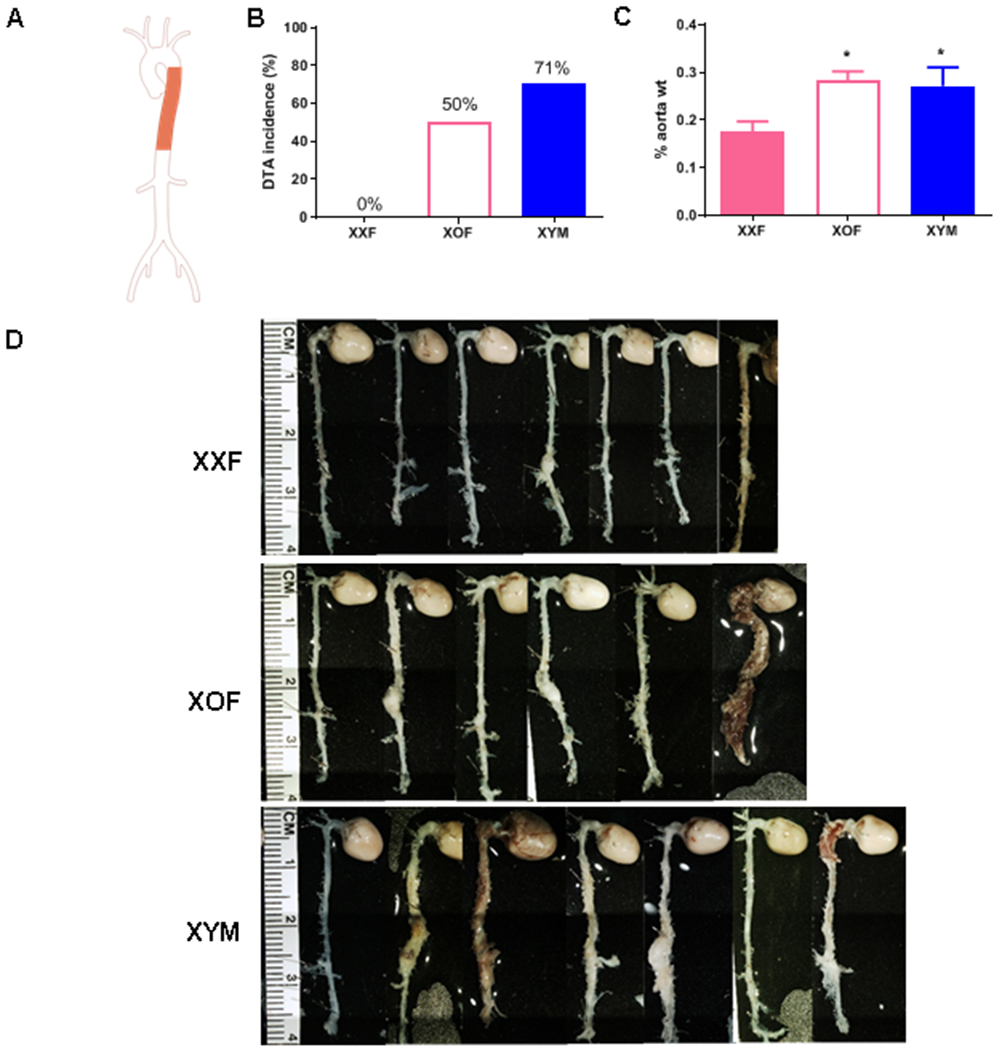
XOF Ldlr−/− mice develop descending thoracic aortic aneurysms (DTA) to the level of XYM following AngII infusions. A, Aorta illustration with shaded area depicting descending thoracic aorta. B, Incidence of DTA in female XXF, XO or XYM mice infused with AngII. C, Aortic weights, expressed as a percentage of body weight, from mice of each genotype. Data are mean ± SEM from (XXF=7, XOF=6, and XYM=7). *, P<0.05 compared to XXF. D, Aortas from mice of each group, the last aorta on the right within XOF and XYM groups was classified as aortic rupture.
By day 27 of AngII infusion, XOF Ldlr−/− mice had larger percent abdominal aortic lumen dilation over baseline than XXF (there was no difference in baseline abdominal aortic lumen diameters between groups), with aortic dilation similar to that of AngII-infused XYM mice (Figure 3A,B). Maximal AAA external diameters (Figure 3C; P<0.05) and AAA incidence (Figure 3D; P<0.05) were similar between XOF and XYM mice, and significantly greater than XXF mice. Representative tissue sections from abdominal aortas were Van Gieson stained to assess morphology and illustrate, in general, that adventitial expansion occurred in abdominal aortas from XOF and XYM, but not XXF mice (Supplemental Figure IV).
To determine the role of the number of X chromosomes on the progression of aortic pathology in females, we extended the duration of AngII infusions to two months in XXF and Ldlr−/− XOF mice. XOF mice exhibited more progressive dilation of abdominal aortic lumen diameters as illustrated by percent change from baseline following prolonged AngII infusion (Figure 4A; P<0.05) and exhibited larger maximal external AAA diameters (Figure 4B; P<0.05) than XXF mice. The incidence of AngII-induced AAAs increased from 0% (XXF) to 86% in XOF mice with prolonged AngII infusion (Figure 4C), and aortas from XOF mice exhibited diffuse and severe aortopathies (Figure 4D). However, the number of mice that died from aortic rupture was not significantly different between sexes or genotypes (Table 1; P>0.05).
Ovariectomized Ldlr−/− XOF females exhibited diffuse AngII-induced aortopathies
Humans with Turner Syndrome exhibit reduced concentrations of ovarian sex hormones.33 However, it is unclear if XOF mice exhibit similar differences in circulating concentrations of ovarian sex hormones that could influence aortopathy development. Since ovariectomy augmented progression of AngII-induced AAAs in female Ldlr−/− mice34, we repeated studies examining AngII-induced aortopathies (Figure 5A) in ovariectomized XXF and XOF mice. Body weights were significantly lower in AngII-infused XOF compared to XXF mice (Table 1; P<0.05). However, systolic blood pressures and plasma renin concentrations were not significantly different between genotypes (Table 1). Moreover, sex organ weights, while much lower than observed in intact females of each genotype, were not significantly different between genotypes (Table 1). Similar to findings from non-ovariectomized females, removal of ovarian sex hormones did not influence higher DTA incidences (Figure 5B), larger aortic weights (Figure 5C; P<0.05), more dilated abdominal aortic lumen diameters (percent change from baseline; Figure 5D; P<0.05; there was no difference in baseline abdominal aortic lumen diameters between groups) and larger maximal AAA external diameters (Figure 5E; P<0.05) of XOF than XXF mice. Moreover, AAA incidence was 20% in AngII-infused XXF mice compared to 75% in ovariectomized XOF mice, where aortic disease was diffuse and severe (Figure 5F). However, there were no aortic ruptures in either genotype of ovariectomized female mice (Table 1).
X-chromosome lysine histone demethylases, Kdm5c and Kdm6a, exhibited higher mRNA abundance in aortas from XXF than XOF mice, and were associated with reduced aortic global DNA methylation
Gene dosage effects occur for X-chromosome genes that escape X-inactivation, which could have ramifications for aortopathy development in XOF mice.35 To define genes influenced by the number of X chromosomes in an aortic region-specific manner, we performed RNA-seq on thoracic and abdominal aortas from XXF and XOF Ldlr−/− mice fed a Western diet for one week (but not infused with AngII). Global unbiased analysis of mRNA transcript levels detected 8,214 differentially expressed genes (DEGs) (q≤0.01) between thoracic and abdominal aortas of XOF mice (Figure 6A). Using the same criteria, 4,950 DEGs were found between thoracic and abdominal aortas of XXF mice. Of these, 94.6% (4,682/4,950) segment-based DEGs in XXF mice were similar to those in XOF mice. This was further evidenced by graphing the log 2-fold changes for the 4,682 genes that differed between thoracic and abdominal aortas within both genotypes, with 100% directional agreement and a high magnitude of change correlation (Pearson’s R=0.98) between genotypes (Figure 6A, inset). Transcriptome-wide analysis between genotypes within an aortic region, after correction using a False Discovery Rate q-value > 1, did not reveal differences between thoracic or abdominal aortas of XXF compared to XOF mice, as anticipated based on the small number of genes that escape X-inactivation in mice.36
Figure 6.
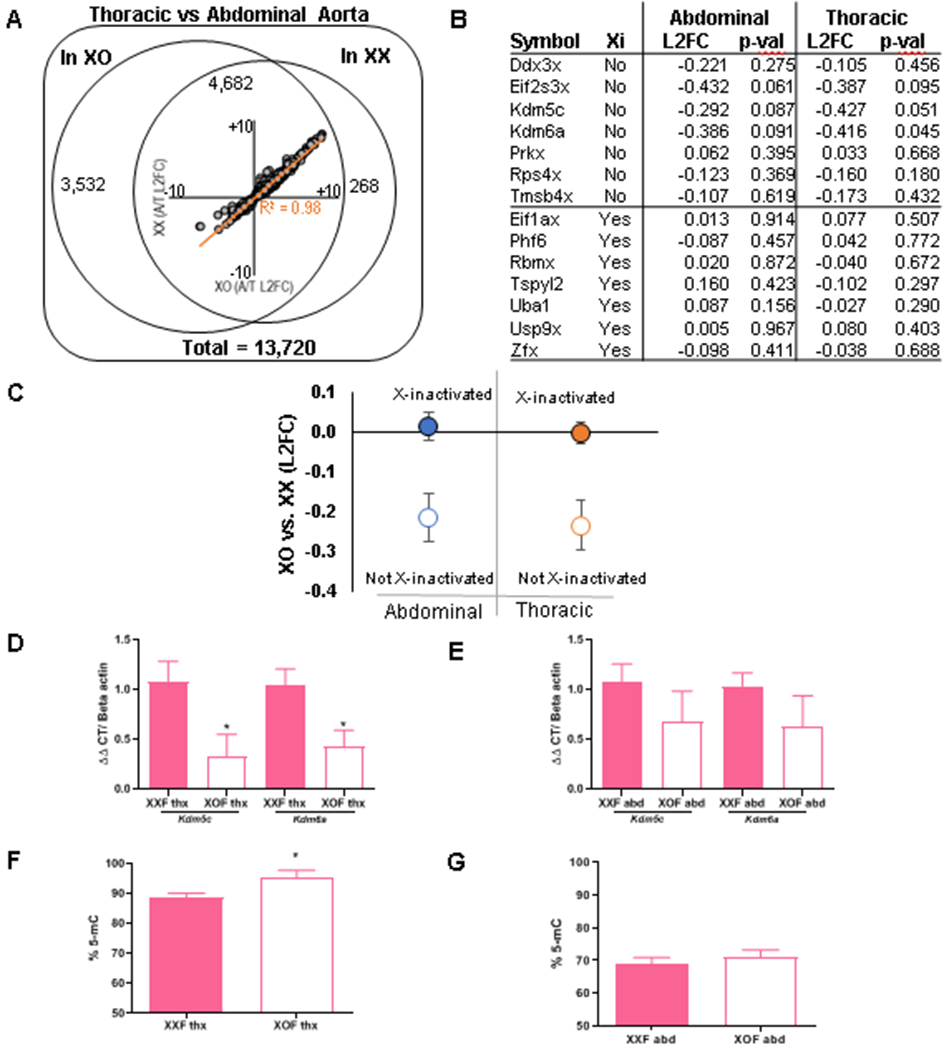
Genes escaping X-inactivation and percent DNA methylation within aortic regions of XXF and XOF Ldlr−/− mice. A-E, Mice were fed a Western diet for one week prior to RNA seq on thoracic and abdominal aortas. A) A total of 13,270 mRNA species annotated to protein-coding genes, of which 8,214 gene transcripts varied between thoracic and abdominal aortas of both sex chromosome genotypes. Of these, 4,950 genes differed between abdominal and thoracic aortas of XXF, and 4,682 of these genes also differed between aortic regions of XOF mice, resulting in large directional agreement and a high correlation between transcripts varying across aortic regions of both genotypes (inset). B) We analyzed 7 genes that either escape or do not escape X-inactivation in aortic regions from each genotype. C, In aggregate, X-inactivated genes (filled circles) show no detectable difference in expression, while genes escaping X inactivation (hollow circles) show significantly decreased expression in abdominal (p = 0.024) and thoracic (p = 0.016) aortas of XOF than XXF mice. D,E) mRNA abundance of Kdm5c and Kdm6a in thoracic and abdominal aortas, respectively. F,G) Percent DNA methylation (% 5-mC) of thoracic and abdominal aortas, respectively, of mice infused with AngII for 3 days. Data are mean ± SEM from n = 5-8 mice/genotype. *, P<0.05 compared to XXF.
Genes on the X chromosome that escape X-inactivation may exhibit gene dosage influences on AngII-induced aortopathies. Thus, we focused on regional aortic transcript expression levels of seven X chromosome genes that do not escape X-inactivation versus seven genes on the X chromosome that have been shown to escape X-inactivation in mice (Figure 6B,C).18, 36 In aggregate, genes on the X chromosome that do not escape X-inactivation were not different between thoracic or abdominal aortas from XXF and XOF mice (Figure 6C; P>0.05). However, the seven examined genes on the X chromosome reported to escape X-inactivation had higher transcript levels (log 2-fold change) in both thoracic and abdominal aortas of XXF than XOF mice (Figure 6C; P<0.05). We confirmed mRNA abundance of two X chromosome genes reported to escape X-inactivation (Kdm5c, Kdm6a) and with log 2-fold changes in RNA-seq analysis that were significant in thoracic aorta with trends in abdominal aortas (Thoracic: Kdm5c p = 0.0505; Kdm6a p = 0.0446; Abdominal: Kdm5c p = 0.0865; Kdm6a p = 0.0907; Figure 6B), and found higher mRNA abundance of both genes in thoracic and trends for higher abundance in abdominal aortas of XXF than XOF mice (Figure 6D,E). Both Kdm5c and Kdm6a are lysine histone demethlyases that remove methyl groups from DNA. Thus, we quantified the percent global methylation of DNA in thoracic and abdominal aortas from XXF and XOF mice. In thoracic aortas the percentage of global DNA methylation was significantly greater in XOF than XXF mice, with differences in global DNA methylation between genotypes inversely related to the mRNA abundance of these X chromosome genes (Figure 6F,G; P<0.05).
Discussion
Major findings of this study are: (1) XXF mice are protected from AngII-induced aortopathy development in the ascending, descending thoracic and abdominal aorta due to the presence of two X chromosomes; (2) the number of X chromosomes influences the regional development of AngII-induced aortopathies in females, with an absence of a second X chromosome resulting in diffuse and severe aortopathies to the level of XYM mice; (3) alterations in ovarian sex hormones in XOF mice are not responsible for augmented aortopathy development; (4) gene dosage effects of X chromosome genes escaping X-inactivation may contribute to augmented aortopathy development of XOF; and (5) mRNA abundance of two X chromosome histone lysine demethylase genes, Kdm5c and Kdm6a, are lower in thoracic aortas from XOF than XXF and are inversely related to the percent of aortic global DNA methylation. These results suggest that gene dosage effects from X chromosome genes, including Kdm5c and Kdm6a, that escape X-inactivation and that exert epigenetic influences on DNA transcription may contribute to augmented aortopathies of XOF mice.
Mice have not been widely embraced for study of Turner Syndrome, because XO mice do not share some overt syndromic features of the disease such as sterility and embryonic lethality.37 Other differences that may impact the use of XO mice as a model of Turner Syndrome include differences in pseudoautosomal region (PAR) genes (humans have at least 25 genes, mice have two38, 39), differences in X-Y gene pairs (more ancestral X-Y gene pairs survive in humans than mice40), and differences in the percentage of genes escaping X-inactivation between species (up to 25% of X genes in humans escape X-inactivation, 3% of X genes in mice36, 41–45). Nevertheless, the comparison of XO and XX mice gives information regarding the characteristics of X genes that are expected to account for the syndromic features of Turner Syndrome.20 We used Ldlr−/− mice fed a Western diet for studies focused on aortopathy development in the descending and abdominal aorta, as hypercholesterolemia has been demonstrated to promote AngII-induced aneurysms in these regions.30–32 While our focus was on aortopathies that are prevalent in Turners Syndrome subjects, this population also exhibits several cardiometabolic features including hypertension2, 46, 47, coronary calcification48 and atherogenic lipid profiles49–51, several of which are recapitulated in AngII-infused hypercholesterolemic mice52, 53. Our results demonstrate that in contrast to other phenotypes where XO mice may not parallel the human condition of Turner Syndrome, XOF mice exhibit diffuse and severe aortopathies similar to those experienced by humans. Moreover, other common pathologies of Turner Syndrome, such as hypertension and atherosclerosis, which were influenced by the lack of a second X chromosome, may be of interest in future studies using AngII-infused XOF Ldlr−/− mice. Notably, lower blood pressures of AngII-infused XOF compared to XXF mice suggest that blood pressure differences, while intriguing, are likely not a contributor to higher aortopathies of XOF mice.
Recent results from our laboratory demonstrated that XYF Ldlr−/− mice with ovaries, from the Four Core Genotypes model18, 54, exhibited a high level of susceptibility to diffuse AngII-induced aortopathies, most especially AAAs, relative to XXF.13, 16 Adult XY females did not have appreciable concentrations of circulating testosterone, which has been demonstrated to markedly augment susceptibility to AngII-induced AAAs in both XYM and XXF mice.12 These results suggest that in female mice, addition of the Y chromosome, through use of the Four Core Genotypes model, or the loss of the second X chromosome contribute to increased susceptibility to AngII-induced aortopathy.16 In this study, we tested these possibilities by contrasting the regional location and severity of AngII-induced aortopathies in XXF and XOF in comparison to XYM mice. We used XY* mice that have an abnormal pseudoautosomal region (PAR) which recombines variably with the PAR of the X chromosome during meiosis.17–19 This mouse model generates female mice that have one or two copies of X chromosomes (XX vs XO), while having ovaries. These experimental genotypes allow for determination of the role of the second X chromosome in disease development and progression in females.
Our results demonstrate that an XX sex chromosome genotype in females is important for the protection against AngII-induced aortopathies. These effects were observed in different aortic regions of C57BL/6J (ascending aorta) and Ldlr−/− (descending and abdominal aorta) female mice, under different experimental paradigms (short and long-term AngII infusions), and were independent of ovarian sex hormones because they persisted in ovariectomized female mice. Moreover, since XOF exhibited diffuse and severe AngII-induced aortopathies with a similar morphology as observed in XYM mice, these results indicate that the second X chromosome is the mechanism for protection of females against aortic disease development and progression (Figure 7). In contrast, our results do not support an effect of the Y chromosome on AngII-induced aortopathies, since XOF, XY females16 and XYM mice exhibit similar levels of aortopathy susceptibility. Moreover, these results suggest that the previously observed high susceptibility of XYM mice to AngII-induced aortopathies11 likely resulted from the absence of a second X chromosome, as well as the presence of testosterone, rather than the presence of the Y chromosome.
Figure 7.
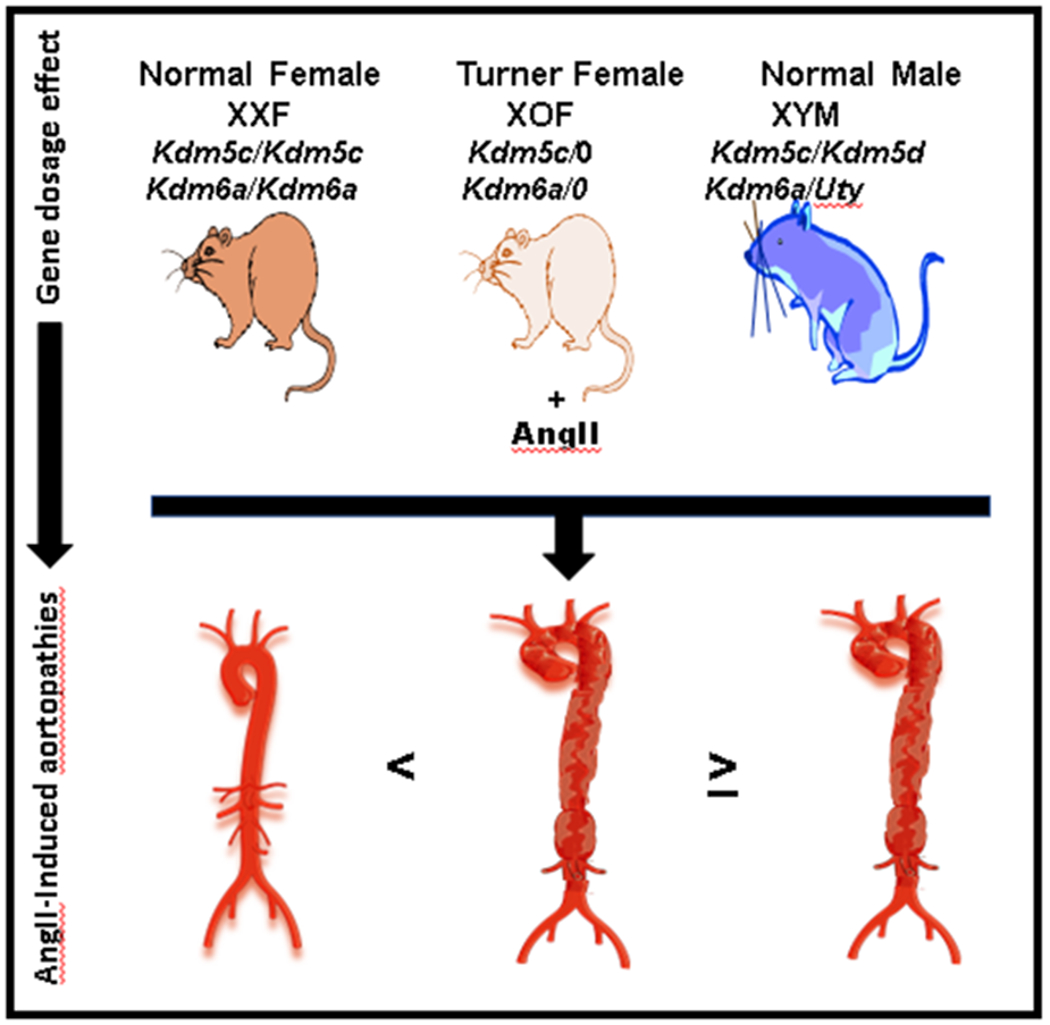
Graphic illustrating how the number of X chromosomes results in an aortic gene dosage effect for pivotal genes that protect XX females, and are lower in XO females and XY males, against AngII-induced aortopathies.
Multiple studies have shown that ovarian sex hormones, specifically estradiol, have a protective role against AAA development and progression in female and male mice and rats.34, 55–57 It is well established that Turner Syndrome females have lower plasma concentrations of estrogen.58 Since ovariectomy of XOF mice had no effect on AngII-induced aortopathies, these results do not support a role for differences in circulating concentrations of ovarian hormones as mechanisms for augmented susceptibility of XOF mice to AngII-induced aortopathies. In all, the present study and previous studies suggest that sex differences in aortopathies are the combined result of harmful effects of testosterone in males, and the beneficial effects of a second X chromosome in XXF mice.
Previous studies in our laboratory demonstrated that in Four Core Genotypes mice, allowing for production of XX and XY mice with the same type of gonads, XY mice had diffuse aortic disease while XX mice had focal AAAs.13 In this study, we found that XOF mice exhibit aortopathies in the ascending, descending, and abdominal aorta, similar to that observed in XYM and XY female mice.13, 16 Similar to previous findings13, aortopathies in each region of the aorta from XOF mice exhibited adventitial expansion that was similar to that observed in XYM mice. These results demonstrate that the absence of a second X chromosome, rather than the presence of the Y chromosome, results in diffuse aortopathies along the length of the aorta. While ascending and descending thoracic aortopathies of XOF in the present study are similar to the human Turner Syndrome phenotype, it is of interest that AAA development in mice was also augmented by an XO genotype. It is unclear if these findings have relevance to humans with Turner Syndrome, but our results suggest additional studies on abdominal aortopathies in this population.
To define mechanisms for effects of an XO sex chromosome genotype to promote diffuse AngII-induced aortopathies, we analyzed regional differences in gene transcript levels in thoracic and abdominal aortas from XXF and XOF mice. We found a large number of genes that differed in transcript expression levels between thoracic and abdominal aortas of both genotypes. However, as might be expected given that a small number of X chromosome genes in mice escape X-inactivation36, we did not find global differences between genotypes. Therefore, we focused on X chromosome genes that have been reported to escape X-inactivation in mice, and demonstrate that two X chromosome lysine demethylases had higher transcript levels in aortas from XXF than XOF mice. As a lysine-specific histone demethylase that reduces histone methylation of H3K459, Kdm5c is considered a dosage-sensitive gene60, meaning that differences in expression levels would be predicted to result in a pronounced phenotype.61 Similarly, Kdm6a is a highly specific lysine histone demethylase removing methyl groups from H3K27, allowing transcription to proceed.62, 63 Each of these X chromosome genes has a Y chromosome homolog (Kdm5c: Kdm5d; Kdm6a: Uty). However, it is unclear if Y chromosome homologs of Kdm5c or Kdm6a in males have sufficient activity levels or whether other differences (e.g., cell expression) influence whether they compensate for the X chromosome allele.64, 65 In general, our results confirm higher expression levels of these X chromosome genes in aortas of XXF than XOF mice. These gene dosage effects were significant in thoracic aortas, with trends for higher expression levels in abdominal aortas of XXF than XOF mice, and were inversely related to DNA methylation differences between genotypes. Since higher DNA methylation in aortas of XOF could result in more tightly packed chromatin that is not accessible to transcription factors, these results suggest that XOF mice may exhibit reduced abundance of genes that protect against AngII-induced aortopathies. Rare human mutations of Kdm5c or Kdm6a have not been reported to be associated with aortopathies, but future studies should investigate locus-specific changes in DNA methylation of target genes that influence aortic disease development, as a recent retrospective review of five biobliographic databases from GWAS data identified Kdm6a as an epigenetic modifier related to sex differences in cardiometabolic risk.66 Moreover, replication of these findings in a different experimental model of aortopathies in future studies will augment translational relevance of these findings.
In summary, XOF mice, independent of ovarian sex hormones, exhibit diffuse AngII-induced aortopathies as evidenced by pronounced ascending and abdominal aortic dilation and higher DTA and AAA incidences compared to XX females. Moreover, diffuse and severe AngII-induced aortopathies of XOF were similar to those observed in XY male mice. Genes that escape X-inactivation such as Kdm5c and Kdm6a may afford protection against AngII-induced aortopathies in XXF mice. Finding and exploring autosomal genes that are affected or controlled by Kdm5c or Kdm6a and other X-inactivation escapees could identify potential therapeutic targets for females with Turner Syndrome.
Supplementary Material
Highlights.
A second X chromosome protects female mice from angiotensin II (AngII)-induced aortopathies in the ascending, descending thoracic and abdominal aorta.
Findings of augmented AngII-induced aortopathies in XO females may have relevance to aortic disease development in women with Turner Syndrome.
X-chromosome genes that escape X-inactivation, such as the lysine-specific demethylases Kdm5c or Kdm6a, may regulate aortic genes that are protective against aortopathies in XX females.
Acknowledgments:
We acknowledge the technical assistance of Ms. Victoria English, Dr. Mark Ensor and Ms. Heba Ali for technical contributions to the manuscript, and Medical Servier (www.smart.servier.com) for art illustration used in figures.
Source of Funding:
LAC, HA, SL, and AD are partially supported by 18SFRN3390001 from the American Heart Association. LAC is partially supported by R01HL107326 from the National Institutes of Health. SL’s work is supported in part by the Jimmy and Roberta Howell Professorship in Cardiovascular Surgery at Baylor College of Medicine.
Abbreviations
- DTA
descending thoracic aneurysm
- AAA
abdominal aortic aneurysm
- AngII
angiotensin II
- XYM
XY male mice
- XXF
XX female mice
- XOF
XO female mice
- Ldlr−/−
low density lipoprotein receptor deficient
- PAR
pseudoautosomal region
- DEGs
differentially expressed genes
Footnotes
Disclosure: None.
Reference List
- 1.Bondy CA. Aortic dissection in Turner syndrome. Curr Opin Cardiol. 2008;23:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox DA, Kang KT, Potts JE, Bradley TJ, Stewart LL, Dionne JM and Sandor GGS. Non-invasive assessment of aortic stiffness and blood pressure in young Turner syndrome patients. J Pediatr Endocrinol Metab. 2019;32:489–498. [DOI] [PubMed] [Google Scholar]
- 3.Wen J, Trolle C, Viuff MH, Ringgaard S, Laugesen E, Gutmark EJ, Subramaniam DR, Backeljauw P, Gutmark-Little I, Andersen NH et al. Impaired aortic distensibility and elevated central blood pressure in Turner Syndrome: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2018;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson AP, Yonas HM and Turner PT. Disorders of tumoral calcification of the spine: illustrative case study and review of the literature. J Spinal Disord Tech. 2007;20:97–103. [DOI] [PubMed] [Google Scholar]
- 5.Carlson M, Airhart N, Lopez L and Silberbach M. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation. 2012;126:2220–2226. [DOI] [PubMed] [Google Scholar]
- 6.Matura LA, Ho VB, Rosing DR and Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663–1670. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AP, Cassis LA, Eghbali M, Reue K and Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2017;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannawa KK, Eliason JL and Upchurch GR Jr. Gender differences in abdominal aortic aneurysms. Vascular. 2009;17 Suppl 1:S30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roman MJ, Devereux RB, Preiss LR, Asch FM, Eagle KA, Holmes KW, LeMaire SA, Maslen CL, Milewicz DM, Morris SA et al. Associations of age and sex with marfan phenotype: The National Heart, Lung, and Blood Institute GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) Registry. Circ Cardiovasc Genet. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gawinecka J, Schonrath F and von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly. 2017;147:w14489. [DOI] [PubMed] [Google Scholar]
- 11.Henriques TA, Huang J, D’Souza SS, Daugherty A and Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–3872. [DOI] [PubMed] [Google Scholar]
- 12.Henriques T, Zhang X, Yiannikouris FB, Daugherty A and Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsiraj Y, Thatcher SE, Blalock E, Fleenor B, Daugherty A and Cassis LA. Sex chromosome complement defines diffuse versus focal angiotensin II-induced aortic pathology. Arterioscler Thromb Vasc Biol. 2018;38:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rateri DL, Davis FM, Balakrishnan A, Howatt DA, Moorleghen JJ, O’Connor WN, Charnigo R, Cassis LA and Daugherty A. Angiotensin II induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am J Pathol. 2014;184:2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A and Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circ Res. 2012;110:e73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsiraj Y, Thatcher SE, Charnigo R, Chen K, Blalock E, Daugherty A and Cassis LA. Female mice with an XY sex chromosome complement develop severe angiotensin II-induced abdominal aortic aneurysms. Circulation. 2017;135:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT and Washburn LL. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. [DOI] [PubMed] [Google Scholar]
- 18.Burgoyne PS and Arnold AP. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol Sex Differ. 2016;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ and Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40. [DOI] [PubMed] [Google Scholar]
- 20.AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, Katz W, Ali HM, Petriello M, Deng P, Morris AJ, Wang X, Lusis AJ, Arnold AP, Reue K, Thompson K, Tso P and Cassis LA. XX sex chromosome complement promotes atherosclerosis in mice. Nat Commun. 2019;10:2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty A, Rateri DL, Lu H, Inagami T and Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. [DOI] [PubMed] [Google Scholar]
- 22.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson MD and Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YE, Kutnetsov L, Partensky A, Farid J and Quackenbush J. WebMeV: A cloud platform for analyzing and visualizing cancer genomic data. Cancer Res. 2017;77:e11–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W and Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochberg Y and Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. [DOI] [PubMed] [Google Scholar]
- 27.Clement K, Viguerie N, Diehn M, Alizadeh A, Barbe P, Thalamas C, Storey JD, Brown PO, Barsh GS and Langin D. In vivo regulation of human skeletal muscle gene expression by thyroid hormone. Genome Res. 2002;12:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang da W, Sherman BT and Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM and Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Lu H, Howatt DA, Balakrishnan A, Moorleghen JJ, Sorci-Thomas M, Cassis LA and Daugherty A. Associations of ApoAI and ApoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2015;35:1826–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H, Howatt DA, Balakrishnan A, Graham MJ, Mullick AE and Daugherty A. Hypercholesterolemia Induced by a PCSK9 Gain-of-Function Mutation Augments Angiotensin II-Induced Abdominal Aortic Aneurysms in C57BL/6 Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:1753–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Sawada H, Howatt DA, Moorleghen JJ, Vsevolozhskaya O, Daugherty A and Lu HS. Hypercholesterolemia Accelerates Both the Initiation and Progression of Angiotensin II-induced Abdominal Aortic Aneurysms. Ann Vasc Med Res. 2020;6. [PMC free article] [PubMed] [Google Scholar]
- 33.Viuff MH and Gravholt CH. Care of adult women with Turner syndrome: the state of affairs in Germany. Endocr Connect. 2019;8:C1–C4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thatcher SE, Zhang X, Woody S, Wang Y, Alsiraj Y, Charnigo R, Daugherty A and Cassis LA. Exogenous 17-beta estradiol administration blunts progression of established angiotensin II-induced abdominal aortic aneurysms in female ovariectomized mice. Biol Sex Differ. 2015;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peeters SB, Korecki AJ, Simpson EM and Brown CJ. Human cis-acting elements regulating escape from X-chromosome inactivation function in mouse. Hum Mol Genet. 2018;27:1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berletch JB, Ma W, Yang F, Shendure J, Noble WS, Disteche CM and Deng X. Escape from X inactivation varies in mouse tissues. PLoS Genet. 2015;11:e1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urbach A and Benvenisty N. Studying early lethality of 45,XO (Turner’s syndrome) embryos using human embryonic stem cells. PLoS One. 2009;4:e4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helena Mangs A and Morris BJ. The Human Pseudoautosomal Region (PAR): Origin, Function and Future. Curr Genomics. 2007;8:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raudsepp T and Chowdhary BP. The Eutherian Pseudoautosomal Region. Cytogenet Genome Res. 2015;147:81–94. [DOI] [PubMed] [Google Scholar]
- 40.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S et al. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014;508:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaton BP, Dixon-McDougall T, Peeters SB and Brown CJ. The eXceptional nature of the X chromosome. Hum Mol Genet. 2018;27:R242–R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrel L and Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. [DOI] [PubMed] [Google Scholar]
- 43.Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berletch JB, Yang F and Disteche CM. Escape from X inactivation in mice and humans. Genome Biol. 2010;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopes AM, Burgoyne PS, Ojarikre A, Bauer J, Sargent CA, Amorim A and Affara NA. Transcriptional changes in response to X chromosome dosage in the mouse: implications for X inactivation and the molecular basis of Turner Syndrome. BMC Genomics. 2010;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YJ, Kim SM, Lee YA, Kim GB, Shin CH and Yang SW. Relationship between systolic hypertension assessed by 24-hour ambulatory blood pressure monitoring and aortic diameters in young women with Turner syndrome. Clin Endocrinol (Oxf). 2019;91:156–162. [DOI] [PubMed] [Google Scholar]
- 47.Strader WJ 3rd, Wachtel HL and Lundberg GD Jr. Hypertension and aortic rupture in gonadal dysgenesis. J Pediatr. 1971;79:473–475. [DOI] [PubMed] [Google Scholar]
- 48.Schoepp M, Hannah-Shmouni F, Matta J, Ghanem AM, Hanover JA, Abd-Elmoniem KZ and Gharib AM. Coronary calcification in adults with Turner syndrome. Genet Med. 2018;20:664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pirgon O, Atabek ME, Oran B and Guclu R. Atherogenic lipid profile and systolic blood pressure are associated with carotid artery intima-media thickness in children with Turner syndrome. J Clin Res Pediatr Endocrinol. 2008;1:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozlowska-Wojciechowska M, Jez W, Zdrojewski T and Chwojnicki K. Are young women with Turner syndrome at greater risk of coronary artery disease? Eur J Cardiovasc Prev Rehabil. 2006;13:467–469. [DOI] [PubMed] [Google Scholar]
- 51.Van PL, Bakalov VK and Bondy CA. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab. 2006;91:2867–2870. [DOI] [PubMed] [Google Scholar]
- 52.Daugherty A, Lu H, Rateri DL and Cassis LA. Augmentation of the renin-angiotensin system by hypercholesterolemia promotes vascular diseases. Future Lipidol. 2008;3:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daugherty A, Manning MW and Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS and Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin-McNulty B, Tham DM, da Cunha V, Ho JJ, Wilson DW, Rutledge JC, Deng GG, Vergona R, Sullivan ME and Wang YX. 17 Beta-estradiol attenuates development of angiotensin II-induced aortic abdominal aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1627–1632. [DOI] [PubMed] [Google Scholar]
- 56.Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS, Henke PK, Stanley JC, Eagleton MJ and Upchurch GR. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41:108–114. [DOI] [PubMed] [Google Scholar]
- 57.Wu XF, Zhang J, Paskauskas S, Xin SJ and Duan ZQ. The role of estrogen in the formation of experimental abdominal aortic aneurysm. Am J Surg. 2009;197:49–54. [DOI] [PubMed] [Google Scholar]
- 58.Turner JW. Brown-Sequard Syndrome at C 2 Level due to Vascular Lesion of Cord. Proc R Soc Med. 1938;31:296–297. [PMC free article] [PubMed] [Google Scholar]
- 59.Grafodatskaya D, Chung BH, Butcher DT, Turinsky AL, Goodman SJ, Choufani S, Chen YA, Lou Y, Zhao C, Rajendram R, Abidi FE, Skinner C, Stavropoulos J, Bondy CA, Hamilton J, Wodak S, Scherer SW, Schwartz CE and Weksberg R. Multilocus loss of DNA methylation in individuals with mutations in the histone H3 lysine 4 demethylase KDM5C. BMC Med Genomics. 2013;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naqvi S, Bellott DW, Lin KS and Page DC. Conserved microRNA targeting reveals preexisting gene dosage sensitivities that shaped amniote sex chromosome evolution. Genome Res. 2018;28:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ZX and Oliver B. X Chromosome and autosome dosage responses in drosophila melanogaster heads. G3 (Bethesda). 2015;5:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greer EL and Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. [DOI] [PubMed] [Google Scholar]
- 64.Iwase S, Brookes E, Agarwal S, Badeaux AI, Ito H, Vallianatos CN, Tomassy GS, Kasza T, Lin G, Thompson A, Gu L, Kwan KY, Chen C, Sartor MA, Egan B, Xu J and Shi Y. A mouse model of X-linked intellectual disability associated with impaired removal of histone methylation. Cell Rep. 2016;14:1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gazova I, Lengeling A and Summers KM. Lysine demethylases KDM6A and UTY: The X and Y of histone demethylation. Mol Genet Metab. 2019;127:31–44. [DOI] [PubMed] [Google Scholar]
- 66.Asllanaj E, Zhang X, Ochoa Rosales C, Nano J, Bramer WM, Portilla-Fernandez E, Braun KVE, Gonzalez-Jaramillo V, Ahrens W, Ikram A, Ghanbari M, Voortman T, Franco OH, Muka T and Glisic M. Sexually dimorphic DNA-methylation in cardiometabolic health: A systematic review. Maturitas. 2020;135:6–26. [DOI] [PubMed] [Google Scholar]
- 67.The Gene Ontology C The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Quality control data (disambiguated per sample), STAR alignment settings, and significant results are provided as supplementary material. Complete FASTQ and TMM normalized RNA-seq data are available through the Gene Expression Omnibus (GSE154036, www.ncbi.nlm.nih.gov/geo/).


