Abstract
What are emotions and how should we study them? These questions give rise to ongoing controversy amongst scientists in the fields of neuroscience, psychology and philosophy, and have resulted in different views on emotions [1, 2, 3, 4, 5, 6]. In this review, we define emotions as functional states that bear essential roles in promoting survival and thus have emerged through evolution. Emotions trigger behavioral, somatic, hormonal, and neurochemical reactions, referred to as expressions of emotion. We discuss recent studies on emotion expression across species and highlight emerging common principles. We argue that detailed and multidimensional analyses of emotion expressions are key to develop biology-based definitions of emotions and to reveal their neuronal underpinnings.
Current Opinion in Neurobiology 2021, 68:57–66
This review comes from a themed issue on The social brain
Edited by Michael Brecht and Hailan Hu
For a complete overview see the Issue and the Editorial
Available online 3rd February 2021
https://doi.org/10.1016/j.conb.2021.01.003
0959-4388/© 2021 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Emotions are one of the most fascinating yet mysterious products of brain function. Sometimes defined from the perspective of their conscious experience in humans, here we adopt the definition of emotions as a class of internal states that are expressed by specific behaviors as well as somatic responses and exist across the animal kingdom [1,5,9] (see Box 1 ‘A functional definition of emotions’). In line with this definition, there is ample evidence that basic ‘emotion states’, such as defensive, aggressive or hedonic states, exist across species and elicit observable behavioral and physiological adaptations. These expressions of emotion affect the entire organism and involve motor, somatic and biochemical (e.g. hormonal, neurochemical) reactions. Intriguingly, modes of emotion expression, as well as the underlying brain mechanisms, show great similarities across highly distinct species, suggesting that emotions are central brain functions, which rely on universal principles of brain computation. By presenting recent evidence from studies across diverse species, we attempt to answer questions on how, when, and why are emotions expressed, and where in the brain is emotion expression triggered. We mostly focus on model organisms commonly used in neuroscience laboratories, but also elaborate on the value of broadening the scope of observations to non-typical model organisms. Finally, we briefly discuss the recent technological advancements in the field and the challenges future studies on the expression of emotions across species face.
Box 1. A functional definition of emotions.
What is an emotion? There are still vigorous debates about how to define emotions and as a consequence how to approach their investigation [2, 3, 4, 5, 6, 7, 8, 9]. One of the most divisive aspects of this debate is whether consciousness is regarded as an indispensable, core feature of ‘emotion’ or not. Indeed, leading scientists in the field have argued that emotions are cognitively assembled and by definition conscious concepts of one’s own (human) internal state [4,10,11]. These views have led to recommendations to refrain from using words referring to human emotions when describing animal research. Other influential scientists have promoted a different view on emotions, which does not imply consciousness as a prerequisite but rather as a feature that has evolved in higher species and was added on top of otherwise conserved primitive emotion processes [1,9,12, 13, 14]. Maybe the most important consequence of these views is whether or not animal research can, and if so to what extent, teach us about emotion processes in humans. A further important implication of some theories, such as the ‘theory of constructed emotion’ developed by Lisa Feldman Barrett [11] is that emotions have, according to this view, no specific neuronal substrates in the brain but are solely inferred from circumstances (environmental and internal).
Here, we adopt a definition of emotions adhering to the framework proposed by Anderson & Adolphs [1,5,15]. This framework defines emotions as evolutionary conserved functional and central brain states that drive organismal responses (expressions of emotions) to stimuli. Emotion states have thus specific and evolutionary conserved brain correlates and expression patterns. We support this definition for several reasons. Most importantly, behavioral regularities, or patterns of multimodal adaptive changes, are observed across animal species, when confronted with circumstances that influence the individual’s prospect of survival or procreation [5,16]. If one accepts this functionality of emotions, it is likely that emotions have emerged from evolutionary processes and thus primitive forms and conserved features must exist across phylogeny as already suggested by Darwin [16].
Another reason to regard consciousness as a feature rather than a prerequisite for emotion is the finding that humans can exhibit measureable behavioral adaptations even when they are unaware of changes in their emotion state [17]. Thus, humans can express emotions unconsciously and conscious awareness of an emotion is not necessary for the underlying emotion state to affect behavior. Analogously, many core functions of the brain, such as vision, memory or decision-making regularly involve consciousness in humans yet do not rely on it. There are multiple examples of blind sight, non-declarative memory, and unconscious decision-making in humans. It thus seems that several core functions of the brain can be executed in absence of conscious appraisal.
A further argument for an evolutionary origin of emotion states, is strong evidence that the brain circuits and regions regulating similar types of emotion expressions are conserved across species [18••]. However, even if we agree that primitive forms of emotion may exist in animals, and we believe that we can use insights gained from studying brain mechanisms of emotion in animal models, identifying and defining emotion states and their expressions in distant species is not trivial.
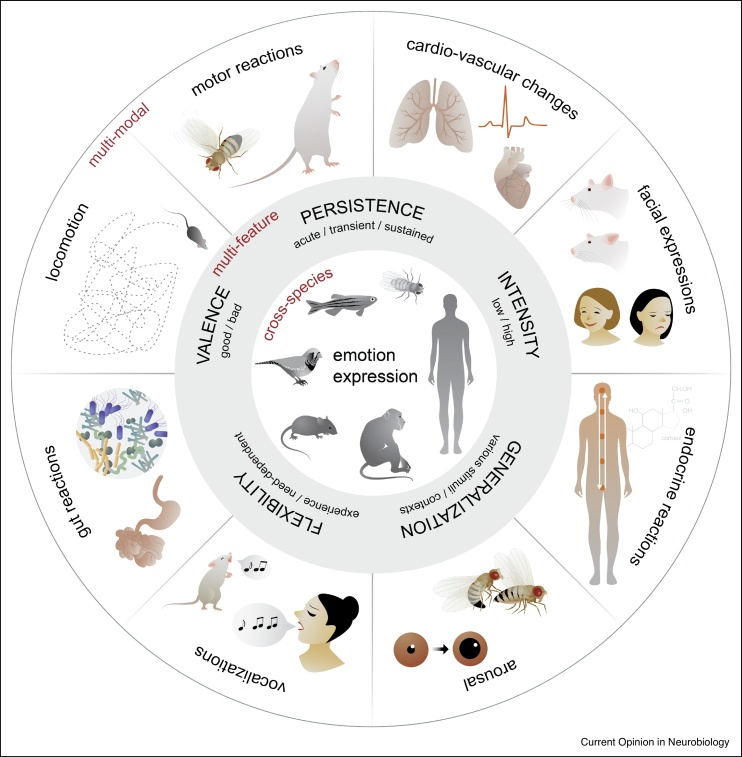
Towards a cross-species approach of emotion studies, Anderson and Adolphs therefore proposed that emotions and their expressions are characterized by general features, also called ‘emotion primitives’ [5]. Emotion expressions are commonly more complex than reflexes, such that they are highly regulated by prior experience, physiological state and context. On the other hand, emotion expressions are dissimilar from planned, learned or volitional behaviors, but rather constitute recurring, regular action patterns, that span multiple modalities. Therefore, the basic emotion features proposed by Anderson and Adolphs include valence (emotions are positive or negative), persistence (emotions tend to outlast their trigger), intensity (emotions can be weak or strong) and generalization (the same emotion can occur in different contexts or be triggered by different stimulus conditions). Behaviors in humans down to the most primitive model organism should adhere to these emotion features to qualify as an expression of emotion (see also Figure 1).
Alt-text: Box 1
How are emotions expressed?
Multiple modalities of emotion expression
Emotion states trigger ‘action patterns’ in multiple modalities, such as behavioral, physiological, and biochemical [16,19,20] (Figure 1). For example, the emotion state of fear may prompt widening of the eyes, heavier breathing, shifts in attention, redistribution of blood, release of hormones, motor responses such as freezing or flight, and sustained avoidance. Despite the fact that various modalities of emotion expression commonly occur simultaneously, we first consider them separately, giving examples from diverse species to illustrate evolutionarily conserved principles.
Figure 1.
Modes of emotion expression.
Emotions are expressed through several modalities summarised in this schematic. Across species (inner circle) organismal response to a relevant stimulus can evoke changes in locomotion, motor behaviour, facial expressions, vocalisations, cardiovascular, gut and endocrine reactions and arousal (outer circle). Emotions, together with their expressions, have been proposed to encompass different features (middle circle) which are explained in further detail in Box 1.
Locomotion and motor behaviors
Changes in locomotion and acute motor reactions accompany many, if not all, emotion states. Particularly obvious motor reactions are observed in multiple species when they are in a state of fear. Vertebrates, including fish and mice, as well as invertebrates, such as flies, exhibit strikingly similar defensive motor responses including freezing and escape in response to threat, such as a visually approaching object [21, 22, 23, 24]. The reliability of these responses and the accessibility of model organisms for neuroscientific investigations have enabled scientists to map the mechanisms underlying the detection of threat and the expression of fear onto specialized circuits in the brain [24,25,26•,27,28•]. Indeed, the ease to trigger and recognize behavioral expressions of fear and anxiety in laboratory animals, in particular rodents, has led to a great abundance of neuroscientific studies. Consequently, to date, we have detailed insights into the neuronal mechanisms underlying the expression, regulation and learning of fear and anxiety [29,30] that exceed our knowledge about many other emotion states by far. These mechanistic insights are particularly remarkable in the context of studies providing evidence that fear expression and the underlying neuronal mechanisms are to some extent conserved across species [31]. For instance, Terburg et al. recently demonstrated that the same neuronal circuit mechanisms control defensive behaviors, such as freezing and escape, in rodents and humans [18••].
It is important to note that emotions are often expressed in a non-linear fashion. The seemingly same emotion state can elicit diverse reactions dependent on the intensity of the underlying state, the context, or the features of the trigger event. Fear, for example, can trigger various, even opposing, motor reactions, such as freezing, escape and approach. This observation has led to the formulation of the ‘threat imminence theory’ which postulates that these diverse behaviors are a direct consequence of the distance to the threat, such that imminent danger will result in escape, while more distant harm will lead to freezing [32]. Other emotions are also expressed non-linearly. Aggressive behaviors, for example, comprise multiple diverse actions, which largely depend on the context and trigger in multiple species including flies, birds, mice and primates [33]. Interestingly, aggression in flies and mice has been shown to be the behavioral expression of a persistent internal state, that can be triggered by the activation of analogous brain regions [34,35], and is thus reminiscent of ‘rage’ or ‘anger’ states in higher species.
Somatic expressions and endocrine responses
In parallel to changes in motor behaviors, emotions trigger somatic reactions, which are so noticeable that in the nineteenth century James and Lange proposed that bodily changes in themselves cause emotions, rather than being part of their expression [36,37]. The James-Lange theory was later challenged on several grounds [38]. For instance, somatic expressions may be too slow, or not differentiated enough to account for diverse and distinct emotion states. However, there is still evidence that somatic signals exert strong influence on emotions and their expression in humans and other species [39, 40, 41]. Indeed, recent studies in rodents have dissected precise neuronal connections between the peripheral organs and the brain which monitor bodily parameters and provide reward-associated or negatively valenced teaching signals to guide behavior [42••,43••]. These may well be examples of how bodily inputs can trigger, or at the very least, regulate emotions. The intimate relationship between somatic reactions and physiological feedback on emotion processes are further illustrated by the fact that many, if not all, emotion-relevant brain regions that have been reported to evoke somatic changes are also sensitive to visceral input [44,45]. For example, a recent study in mice showed that the visceroceptive (posterior) insular cortex gives rise to changes in emotion expression, such as persistent changes in anxiety-related behaviors, and at the same time triggers bodily reactions, such as increases in breathing rate [46••]. It is thus likely that most emotion-relevant brain regions engage in tight loops between emotion expression and physiological feedback.
Vocalizations and facial expressions
It seems intuitive that humans communicate emotions vocally. Indeed, both prosody [47] and vocal bursts, such as shrieks, laughter or crying [48] are used and recognized as indicators of distinct emotion states across human cultures. However, also many other animals, including rodents and monkeys use vocal abilities to express and communicate both positive as well as negative emotion states [49, 50, 51]. For instance, multi-parametric analyses of squirrel monkey vocalizations revealed that specific acoustic parameters of their calls were correlated to the degree of aversion displayed by the monkeys [49].
Apart from vocalizations, one of the historically best-recognized indicators of emotions are facial expressions. Most famously, Paul Ekman studied human facial expressions cross-culturally and proposed the existence of six universal basic emotions expressed on the human face [52]. Together with his colleague Wallace Friesen, he also developed the ‘facial action coding system’ (FACS), an anatomy-based system for classifying emotions into categories based on changes in facial expressions [53]. More recently, the FACS was successfully applied to seven other species, including chimpanzees [54] and macaques [55]. Strong indications that also rodents express affective value via orofacial expressions already existed since the 1970s when Grill and Norgren demonstrated in a series of elegant studies that rodents exhibit ‘liking’ versus ‘disgust’ in response to tastants of different intrinsic or learned values [56,57]. Further evidence for rodent affective facial expressions came from a study introducing a mouse grimace scale as a measure of pain [58], that was later also translated to rats [59]. Recently, Dolensek et al. employed machine vision and machine learning tools to classify and quantitatively describe facial expressions of several distinct emotion states in mice [60••]. Importantly, the authors were able to show that mouse facial expressions reveal basic emotion features, such as intensity, persistence, generalization and flexibility. Taken together, facial expressions appear to be evolutionarily conserved across mammalian species and in combination with modern analysis methods hold great promise as universal tools for classifying emotions in rodent species and beyond.
When are emotions expressed?
Emotions are expressed at very different time points and scales. Some expressions, such as freezing, escape or vocalizations, occur immediately and can be very short-lived, commonly lasting only a few seconds. Other expressions, such as sustained states of avoidance or aggression have been shown to persist for minutes to hours across species including flies, mice and humans [11,21,22,46••,61].
Emotion states do not only change behaviors but also have lasting consequences on cognitive abilities. While in humans, cognition and emotion are strongly intertwined [63,64], intriguingly, even honeybees exposed to threat (e.g. vigorous shaking) showed subsequent pessimistic cognitive bias towards an ambiguous stimulus [65], and bumblebees when offered sucrose to elicit pleasure, exhibited an opposite response [66••]. These findings clearly suggest that even invertebrates exhibit primitive forms of cognitive changes as expression of emotion state.
While the persistence of emotion expressions may on the one hand arise from prolonged effects of released hormones or neuromodulators, recent evidence also highlights the involvement of neuronal activity mechanisms. For instance, Kennedy et al. showed that in the mouse hypothalamus, sustained states characterized by prolonged occurrence of defensive actions depend on stimulus-specific and slow neural dynamics [62••]. Taken together, a characteristic feature of diverse emotion states is that a single state is commonly expressed and represented in the brain at diverse timescales.
Why are emotions expressed?
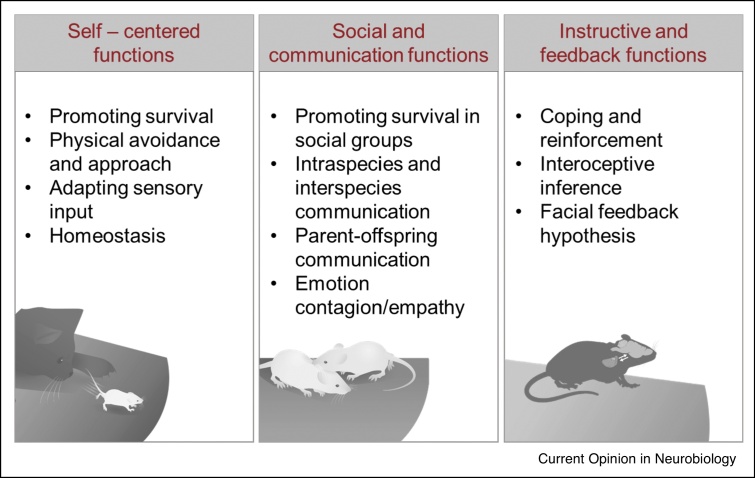
Charles Darwin proposed already in the 19th century that emotion expression might serve diverse functions: preparing the organism adaptively to environmental stimuli, communicating critical information, and possibly contributing to the emergence or maintenance of the emotion state itself [16] (Figure 2).
Figure 2.
Functional roles of emotion expression.
Expression of emotion across species has been shown to support: self-centered, social and communication as well as instructive and feedback functions.
Self-centered functions
Maybe the most ancient function of emotion expression is that of self-preservation. Escape or freezing reactions in case of fear can prevent being detected or caught by predators. The accompanying bodily changes, such as changes in blood flow and breathing, famously support fight or flight reactions. Shifts in attention and sensory processing facilitate detection and identification of threat. Following a similar logic, reactions to pleasurable or disgusting stimuli evoke patterns of rejection or intake across rodents, monkeys and human babies [67] and suggest a role for facial expressions in sensory regulation. Indeed, facial expressions, which we intuitively relate to communicative functions in humans, were shown to bear such sensory functions. In a study by Susskind et al. human facial expressions of fear were shown to enhance sensory acquisition, while expressions of disgust diminished sensory exposure [68]. Therefore, in many instances expression of emotions provide very clear functional advantages and promote the survival of the individual.
Social and communication functions
While emotion expressions thus certainly have self-preservation functions, they undoubtedly also gained communicative value [20,69]. In a two-stage model, emotion expressions initially evolved as self-centered, adaptive functions. Soon after, both the exaggerated display of emotion state, as well as the interpretation of emotional display, became adaptive [69].
Especially in social animals, emotions are recognized across individuals of the same and occasionally different species [70]. The recognition and interpretation, as well as sharing a similar emotion state, have long been regarded as a human-specific capacity. However, to date, we have ample evidence that emotion contagion and even empathy-like behaviors, as well as some suggested neuronal correlates thereof, for instance in the form of mirror neurons, exist across species [70,71,72••,73]. Interestingly, several factors influence the capacity of emotion recognition. For example, communication of emotion state is usually facilitated by familiarity, such as belonging to the same social group [74, 75, 76]. Also, experience with a given emotion state may enhance or be required for emotion recognition. This has been shown both in observational fear [74] but also empathy [75,77] in mice and monkeys. Further factors that can influence emotion communication and recognition within a species are sex, competition and rank [70]. Especially in social animals, emotion communication is thus tightly regulated and of outstanding functional importance.
Instructive and feedback functions
Emotion expressions powerfully change the behavioral and bodily states. The somatic expression of emotions has influenced many emotion theories [36,37,78,79] and today most experts in affective neuroscience would agree that physiological and behavioral feedback modulate emotional behavior and experience.
Indeed, emotion expressions, whether in the form of freezing or heartbeat acceleration, provide feedback signals into emotion circuits at all levels and may even be sufficient to reinforce or trigger certain emotions states [78,80]. Freezing responsive neurons, for instance, have been described in the amygdala, insular cortex, and the prefrontal cortex [46••,81••,82,83•] and a recent study highlights how freezing evoked changes in breathing may provide an important feedback signal for fear maintenance [84]. In humans, the ‘facial feedback hypothesis’ suggests that facial expressions provide feedback that can in itself influence the emotion state of an individual [85,86]. Furthermore, the theory of ‘interoceptive inference’ postulates that the brain compares predictions of internal state with feedback from the body that are then used to infer emotion states [87,88]. While these are all good indications for important roles of emotion expressions in feedback or instructive functions, much remains to be learned about the influence of body and behavior on emotion processes.
Where in the brain are emotion expressions triggered?
Emotions may be best regarded as distributed brain states since evidence from diverse species suggests that emotion states engage networks across the entire brain and across time [6,13,46••,62••,64,83•,89,90]. Interestingly, a recurrent set of brain regions participates in these ‘emotion networks’ and contributes to emotion states as diverse as ‘fear’, ‘disgust’, ‘reward’ or ‘social emotions’. Amongst those common ‘emotion’ brain regions are the prefrontal cortex, including orbitofrontal, insula, and anterior cingulate cortices, and subcortical structures such as the nucleus accumbens, the ventral pallidum, the amygdala, the periaqueductal grey and the hypothalamus [29,60••,67,89,91,92].
Indications that certain basic emotion states and their expressions engage homologues brain regions across-species come from studies on social emotions, such as aggression and mating, in flies and mice [93], or expressions of ‘pleasure’ in humans, monkeys and rodents [67].
A recurrent motif found within these brain regions is that opposite emotion expressions, such as related to ‘aggression’ versus ‘mating’, ‘fear’ versus ‘desire’, or ‘pleasure’ versus ‘disgust’, are mapped onto neighboring, intricately interwoven and antagonistic neuronal populations and circuits. This is true at all levels of the neuraxis, such as in the orbitofrontal or insular cortices, the central and basolateral amygdala, the nucleus accumbens, or the hypothalamus [13,30,60••,67,93,94•,95•,96, 97, 98, 99, 100, 101, 102]. Importantly, studies across many species suggest that this organization may be a functional principle of emotion processing rather than a particularity of one type of brain organization. Strikingly, antagonist sets of emotional behaviors had already been proposed by Darwin in his ‘Principle of Antithesis’ [16].
It is further important to note that emotion expression can be triggered at various sites along this ‘emotion network’, suggesting a nested representation with feedback loops between all levels [30]. Fear reactions, for instance, can be triggered by activity manipulations in the prefrontal cortex [103], the insular cortex [46••], the amygdala [97] and related midbrain structures, such as the periaqueductal grey, which further project to pre-motor targets in the medulla [104]. However, the course of activation is not unidirectional and functional feedback loops exist amongst all levels [30].
Thus, across emotions, interconnected circuits control and select appropriate expression patterns. However, how this coordination amongst different components along the neuraxis occurs and which parts of an emotion can be dissociated without losing the core of the emotion state is still poorly understood. While different aspects of emotion processes, such as detection of trigger events, emotion expression or regulation, may require the contribution of specialized brain regions and neuronal circuits, it has been suggested that the contributions of distinct parts within this network may not be separable but rather intimately linked [64]. While we have learned a lot about different aspects of certain emotion states, one of the most prominent outstanding questions may therefore be how the brain links the diverse processes that constitute an emotion state into a cohesive internal state.
Future directions and concluding remarks
We have argued here that emotion expressions are windows into the internal affective state of an individual across species, from insects to humans. Historically, research into the neuronal underpinnings of emotion has leveraged this proximity of internal state and consequent behavioral adaptation. Indeed, we have gained detailed mechanistic knowledge about certain emotion processes, especially for those emotion states where behavioral readouts have been well established, such as fear [29,30] or, to some lesser extent, aggression [33]. In these instances, quantitative assessments of motor behavior were matched with targeted activity measurements and manipulations. The tractability of certain animal models to neuroscientific investigation and established behavioral readouts within these species, have allowed the identification of detailed mechanisms underlying emotions, including specialized brain circuits and genetically defined neuronal populations [29,30,33]. In the case of fear, we now have in-depth accounts for neuronal correlates of fear learning, expression, and regulation that have started to translate into generalized principles of emotion encoding across species [18••,29, 30, 31]. The importance of behavior for our understanding of brain mechanisms of emotion becomes obvious when comparing the abundance of knowledge about the neuronal underpinnings of ‘fear’ to the lack of knowledge about other emotion states, where expressions may be less recognizable and/or ecologically relevant triggers are more difficult to identify. Indeed, we may not even be able to list many relevant emotion states in distant species, because we oftentimes lack crucial evidence, which emotion states exist, and how they are defined. However, the recent increase in methodological developments to measure and quantify naturalistic behaviors in unsupervised manner provides an unmatched opportunity for advancing our understanding of emotions. Machine vision and machine learning approaches are blossoming [105••,106,107,108], and methods for comprehensive quantifications of precise movements, behavioral syllables, sequences and patterns have been established [109,110••]. This precision and ability to assess hidden or highly dimensional parameters of motor behavior have started to yield unprecedented details about behavioral alterations. Amongst those are accounts of the behavioral consequences of treatments with psychoactive drugs [110••], or the identification and quantitative assessment of previously poorly established emotion states in widely used model organisms [60••,106].
However, the assessment of motor behavior alone may oftentimes lead to ambiguous results. As an example, approach behavior is displayed in states of fear, aggression and hedonia. Combining further analytic parameters with the classification of motor behavior, such as by adding measures of heart rate, breathing, arousal, body temperature, release of neuromodulators, or facial expressions, may greatly enhance the resolution and reliability of the analysis of emotion states. We therefore suggest that a key to a biology-based definition of emotions, and a more precise means to address the fundamental principles of their brain basis, is to leverage the high dimensionality of emotion expression and combine assessments in multiple dimensions, such as motor, somatic and chemical. While to date most studies have focused on a single or very few parameters of expression, future studies may greatly benefit from quantitative assessment of emotion expression across multiple modalities simultaneously, attempting to create multidimensional representations and classifications. Multidimensional analyses achieved through computational approaches may be crucial to address whether emotion states are truly discrete categories or to what extent they are confluent. Furthermore, by providing more graded emotion readouts, computational analysis methods may allow to address brain correlates of diverse emotion features, such as the processing of valence, intensity or persistence across different emotion states.
Despite the great promises of computational techniques to analyze highly dimensional data, important and difficult challenges remain. One particular challenge may relate to identifying ecologically relevant emotion triggers and exploring behavior in naturalistic settings. Another challenge may be the brain-wide encoding of emotions. Our current knowledge strongly suggests that emotion states engage networks spanning the entire brain. Many currently employed neuroscience techniques are thus likely to miss crucial dynamics of the entire brain for a function, which depends on activity in networks rather than single brain regions. Recent developments, such as functional ultrasound imaging, a technique able to measure the brain-wide activity and functional connectivity in real-time in awake behaving animals, may therefore hold great promise for the investigation of emotions in mammals [113,114]. Future challenges may also lie in ideally matching high-dimensional behavioral and neuronal data, distinguishing emotion-relevant from irrelevant behavior or neuronal activity, and identifying triggers and conditions that can be employed to evoke and study emotion across species [9,111,112]. Since emotions are evolutionarily evolved products of brain function, it may reveal crucial to investigate how emotion states are implemented across diverse species, wherever possible including classical and non-typical model organisms [115]. Only this approach may allow us to extract universal principles of emotions and their neuronal basis and distinguish them from mechanistic details in specific brains.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors would like to thank the members of the Gogolla lab for comments on the manuscript, and Julia Kuhl for figure illustrations. We would also like to thank colleagues in the fields of affective and systems neurosciences, psychology, and ethology for inspiration. The scope of this opinion article does unfortunately not allow to comprehensively cite the rich literature on emotions and their expression that inspired this work. Our lab is supported by the Max Planck Society and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC2017-STG, grant agreement no. 758448 to N.G.).
References
- 1.Adolphs R. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc Cogn Affect Neurosci. 2017;12:24–31. doi: 10.1093/scan/nsw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adolphs R., Mlodinow L., Barrett L.F. What is an emotion? Curr Biol. 2019;29:R1060–R1064. doi: 10.1016/j.cub.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett L.F. Functionalism cannot save the classical view of emotion. Soc Cogn Affect Neurosci. 2017;12:34–36. doi: 10.1093/scan/nsw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson D.J., Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shackman A.J., Wager T.D. The emotional brain: fundamental questions and strategies for future research. Neurosci Lett. 2019;693:68–74. doi: 10.1016/j.neulet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mobbs D., Adolphs R., Fanselow M.S., Barrett L.F., LeDoux J.E., Ressler K., Tye K.M. Viewpoints: approaches to defining and investigating fear. Nat Neurosci. 2019;22:1205–1216. doi: 10.1038/s41593-019-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panksepp J., Lane R.D., Solms M., Smith R. Reconciling cognitive and affective neuroscience perspectives on the brain basis of emotional experience. Neurosci Biobehav Rev. 2017;76:187–215. doi: 10.1016/j.neubiorev.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Panksepp J. Toward a general psychobiological theory of emotions. Behav Brain Sci. 1982;5:407–422. [Google Scholar]
- 10.Barrett L.F., Adolphs R., Marsella S., Martinez A.M., Pollak S.D. Emotional expressions reconsidered: challenges to inferring emotion from human facial movements. Psychol Sci Public Interes. 2019;20:1–68. doi: 10.1177/1529100619832930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett L.F. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci. 2017;12:1–23. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanselow M.S., Pennington Z.T. A return to the psychiatric dark ages with a two-system framework for fear. Behav Res Ther. 2018;100:24–29. doi: 10.1016/j.brat.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chikazoe J., Lee D.H., Kriegeskorte N., Anderson A.K. Population coding of affect across stimuli, modalities and individuals. Nat Neurosci. 2014;17:1114–1122. doi: 10.1038/nn.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adolphs R. Emotion. Curr Biol. 2010;20:R549–R552. doi: 10.1016/j.cub.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Adolphs Ralph, Anderson David J. Princeton University Press; 2018. The Neuroscience of Emotion a New Synthesis. [Google Scholar]
- 16.Darwin C. 1872. The Expression of the Emotions in Man and Animals. London, Murray. [Google Scholar]
- 17.Winkielman P., Berridge K.C., Wilbarger J.L. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personal Soc Psychol Bull. 2005;31:121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- 18••.Terburg D., Scheggia D., Triana del Rio R., Klumpers F., Ciobanu A.C., Morgan B., Montoya E.R., Bos P.A., Giobellina G., van den Burg E.H. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell. 2018;175:723–735.e16. doi: 10.1016/j.cell.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a translational approach from rodents to humans, authors show that the basolateral amygdala controls via the central amygdala active behavioral responses to threat. The paper provides evidence for an evolutionarily conserved fear response mechanism.
- 19.Damasio A., Carvalho G.B. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 20.Keltner D., Sauter D., Tracy J., Cowen A. Emotional expression: advances in basic emotion theory. J Nonverbal Behav. 2019;43:133–160. doi: 10.1007/s10919-019-00293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zacarias R., Namiki S., Card G.M., Vasconcelos M.L., Moita M.A. Speed dependent descending control of freezing behavior in Drosophila melanogaster. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-05875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temizer I., Donovan J.C., Baier H., Semmelhack J.L. A visual pathway for looming-evoked escape in larval zebrafish. Curr Biol. 2015;25:1823–1834. doi: 10.1016/j.cub.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka R., Clark D.A. Object-displacement-sensitive visual neurons drive freezing in drosophila. Curr Biol. 2020;30:2532–2550.e8. doi: 10.1016/j.cub.2020.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz M., Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23:2011–2015. doi: 10.1016/j.cub.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn T.W., Gebhardt C., Naumann E.A., Riegler C., Ahrens M.B., Engert F., Del Bene F. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron. 2016;89:613–628. doi: 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Gibson W.T., Gonzalez C.R., Fernandez C., Ramasamy L., Tabachnik T., Du R.R., Felsen P.D., Maire M.R., Perona P., Anderson D.J. Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in drosophila. Curr Biol. 2015;25:1401–1415. doi: 10.1016/j.cub.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; Implementing a novel behavioral paradigm in flies, which relies on exposure to repetitive visual threat stimuli, the authors show that even invertebrates express persistent emotion states.
- 27.Shang C., Chen Z., Liu A., Li Y., Zhang J., Qu B., Yan F., Zhang Y., Liu W., Liu Z. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat Commun. 2018;9:1–17. doi: 10.1038/s41467-018-03580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Kunwar P.S., Zelikowsky M., Remedios R., Cai H., Yilmaz M., Meister M., Anderson D.J. Ventromedial hypothalamic neurons control a defensive emotion state. eLife. 2015;4 doi: 10.7554/eLife.06633. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors provide evidence that the hypothalamus mediates innate defensive responses and supports emotional learning in mice. Specifically, the authors show that a hypothalamus-controlled defensive state displays core features of emotion, including scalability, valence, generalization and persistence.
- 29.Calhoon G.G., Tye K.M. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tovote P., Fadok J.P., Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 31.Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Perusini J.N., Fanselow M.S. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem. 2015;22:417–425. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lischinsky J.E., Lin D. Neural mechanisms of aggression across species. Nat Neurosci. 2020;23:1317–1328. doi: 10.1038/s41593-020-00715-2. [DOI] [PubMed] [Google Scholar]
- 34.Hoopfer E.D., Jung Y., Inagaki H.K., Rubin G.M., Anderson D.J. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife. 2015;4 doi: 10.7554/eLife.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashikawa Y., Hashikawa K., Falkner A.L., Lin D. Ventromedial hypothalamus and the generation of aggression. Front Syst Neurosci. 2017;11:94. doi: 10.3389/fnsys.2017.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James W. What is an emotion? Mind. 2007;9:188–205. [Google Scholar]
- 37.Lange C.G. Williams & Wilkins; 1885. The Emotions. [Google Scholar]
- 38.Cannon W.B. The James-Lange Theory of emotions: a critical examination and an alternative theory. Am J Psychol. 1927;39:106. [PubMed] [Google Scholar]
- 39.Vianna E.P.M., Weinstock J., Elliott D., Summers R., Tranel D. Increased feelings with increased body signals. Soc Cogn Affect Neurosci. 2006;1:37–48. doi: 10.1093/scan/nsl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Betley J.N., Xu S., Cao Z.F.H., Gong R., Magnus C.J., Yu Y., Sternson S.M. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that hypothalamic populations of starvation-sensitive and thirst-promoting neurons also show properties associated with a negative-valence teaching signals. These findings provide evidence for a close link between metabolic need and emotion state.
- 43••.Han W., Tellez L.A., Perkins M.H., Perez I.O., Qu T., Ferreira J., Ferreira T.L., Quinn D., Liu Z.W., Gao X.B. A neural circuit for gut-induced reward. Cell. 2018;175:665–678.e23. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence for the involvement of the gut in motivation and reward. Specifically, optogenetic activation of vagal sensory neurons innervating the gut is shown to induce reward, mimicking the effects of stimulation of reward-neurons.
- 44.Sternson S.M. Exploring internal state-coding across the rodent brain. Curr Opin Neurobiol. 2020;65:20–26. doi: 10.1016/j.conb.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 46••.Gehrlach D.A., Dolensek N., Klein A.S., Roy Chowdhury R., Matthys A., Junghänel M., Gaitanos T.N., Podgornik A., Black T.D., Reddy Vaka N. Aversive state processing in the posterior insular cortex. Nat Neurosci. 2019;22:1424–1437. doi: 10.1038/s41593-019-0469-1. [DOI] [PubMed] [Google Scholar]; The authors reveal that the posterior insular cortex processes and modulates diverse internally and externally generated aversive states and dissect the functional contribution of segregated subcortical projections mediating inhibition of ongoing behaviors.
- 47.Laukka P., Thingujam N.S., Iraki F.K., Elfenbein H.A., Rockstuhl T., Chui W., Althoff J. The expression and recognition of emotions in the voice across five nations: a lens model analysis based on acoustic features. J Pers Soc Psychol. 2016;111:686–705. doi: 10.1037/pspi0000066. [DOI] [PubMed] [Google Scholar]
- 48.Laukka P., Elfenbein H.A., Söder N., Nordström H., Althoff J., Chui W., Iraki F.K., Rockstuhl T., Thingujam N.S. Cross-cultural decoding of positive and negative non-linguistic emotion vocalizations. Front Psychol. 2013;4:353. doi: 10.3389/fpsyg.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fichtel C., Hammerschmidt K., Jürgens U. On the vocal expression of emotion. A multi-parametric analysis of different states of aversion in the squirrel monkey. Behaviour. 2001;138:97–116. [Google Scholar]
- 50.Portfors C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 51.Seyfarth R.M., Cheney D.L. Annals of the New York Academy of Sciences. New York Academy of Sciences; 2003. Meaning and emotion in animal vocalizations; pp. 32–55. [DOI] [PubMed] [Google Scholar]
- 52.Ekman P. An argument for basic emotions. Cogn Emot. 1992;6:169–200. [Google Scholar]
- 53.Ekman P., Friesen W. Facial action coding system: a technique for the measurement of facial movement. Paola Alto Consult Psychol Press. 1978;3:203–221. [Google Scholar]
- 54.Parr L.A., Waller B.M., Vick S.J., Bard K.A. Classifying chimpanzee facial expressions using muscle action. Emotion. 2007;7:172–181. doi: 10.1037/1528-3542.7.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parr L.A., Waller B.M., Burrows A.M., Gothard K.M., Vick S.J. Brief communication: MaqFACS: a muscle-based facial movement coding system for the rhesus macaque. Am J Phys Anthropol. 2010;143:625–630. doi: 10.1002/ajpa.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grill H.J., Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 57.Grill H.J., Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 58.Langford D.J., Bailey A.L., Chanda M.L., Clarke S.E., Drummond T.E., Echols S., Glick S., Ingrao J., Klassen-Ross T., Lacroix-Fralish M.L. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 59.Sotocinal S.G., Sorge R.E., Zaloum A., Tuttle A.H., Martin L.J., Wieskopf J.S., Mapplebeck J.C.S., Wei P., Zhan S., Zhang S. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Dolensek N., Gehrlach D.A., Klein A.S., Gogolla N. Facial expressions of emotion states and their neuronal correlates in mice. Science. 2020;368:89–94. doi: 10.1126/science.aaz9468. [DOI] [PubMed] [Google Scholar]; The authors identify mouse facial expressions as sensitive indicators of the internal emotion state in mice. Facial expression can be quantified at millisecond time scales and classified into emotion-like categories. Combining facial expression analysis with two-photon calcium imaging identified single neurons whose activity closely correlates with specific facial expressions in the insular cortex.
- 61.Lin D., Boyle M.P., Dollar P., Lee H., Lein E.S., Perona P., Anderson D.J. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–227. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Kennedy A., Kunwar P.S., Li L., Stagkourakis S., Wagenaar D.A., Anderson D.J. Stimulus-specific hypothalamic encoding of a persistent defensive state. Nature. 2020;586:730–734. doi: 10.1038/s41586-020-2728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study identifies the neural activity in ventromedial hypothalamus as a novel mechanism involved in the persistence of the internal emotion state of fear.
- 63.Phelps E.A. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 64.Salzman C.D., Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu Rev Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bateson M., Desire S., Gartside S.E., Wright G.A. Agitated honeybees exhibit pessimistic cognitive biases. Curr Biol. 2011;21:1070–1073. doi: 10.1016/j.cub.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Perry C.J., Baciadonna L., Chittka L. Unexpected rewards induce dopamine-dependent positive emotion-like state changes in bumblebees. Science. 2016;353:1529–1531. doi: 10.1126/science.aaf4454. [DOI] [PubMed] [Google Scholar]; The authors report the existence of positive-valenced emotion states in invertebrates. Importantly, these states affect decision-making pointing to an influence of emotion on cognitive functions even in insects.
- 67.Berridge K.C., Kringelbach M.L. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Susskind J.M., Lee D.H., Cusi A., Feiman R., Grabski W., Anderson A.K. Expressing fear enhances sensory acquisition. Nat Neurosci. 2008;11:843–850. doi: 10.1038/nn.2138. [DOI] [PubMed] [Google Scholar]
- 69.Shariff A.F., Tracy J.L. What are emotion expressions for? Curr Dir Psychol Sci. 2011;20:395–399. [Google Scholar]
- 70.Ferretti V., Papaleo F. Understanding others: emotion recognition in humans and other animals. Genes Brain Behav. 2019;18 doi: 10.1111/gbb.12544. [DOI] [PubMed] [Google Scholar]
- 71.Bartal I.B.A., Decety J., Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72••.Carrillo M., Han Y., Migliorati F., Liu M., Gazzola V., Keysers C. Emotional mirror neurons in the rat’s anterior cingulate cortex. Curr Biol. 2019;29:1301–1312.e6. doi: 10.1016/j.cub.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper shows the existence of mirror neurons in rat’s anterior cingulate cortex, which activate during pain (but not fear) experience of both self and others. It confirms the existence of neuronal correlate of empathy in a rodent brain.
- 73.Scheggia D., Managò F., Maltese F., Bruni S., Nigro M., Dautan D., Latuske P., Contarini G., Gomez-Gonzalo M., Requie L.M. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat Neurosci. 2020;23:47–60. doi: 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- 74.Allsop S.A., Wichmann R., Mills F., Burgos-Robles A., Chang C.J., Felix-Ortiz A.C., Vienne A., Beyeler A., Izadmehr E.M., Glober G. Corticoamygdala transfer of socially derived information gates observational learning. Cell. 2018;173:1329–1342.e18. doi: 10.1016/j.cell.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell M.W., de Waal F.B.M. Chimpanzees empathize with group mates and humans, but not with baboons or unfamiliar chimpanzees. Proc R Soc B Biol Sci. 2014;281 doi: 10.1098/rspb.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Engen H.G., Singer T. Empathy circuits. Curr Opin Neurobiol. 2013;23:275–282. doi: 10.1016/j.conb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Ben-Ami Bartal I., Shan H., Molasky N.M.R., Murray T.M., Williams J.Z., Decety J., Mason P. Anxiolytic treatment impairs helping behavior in rats. Front Psychol. 2016;7:850. doi: 10.3389/fpsyg.2016.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Critchley H.D., Garfinkel S.N. Interoception and emotion. Curr Opin Psychol. 2017;17:7–14. doi: 10.1016/j.copsyc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Seth A.K., Friston K.J. Active interoceptive inference and the emotional brain. Philos Trans R Soc B Biol Sci. 2016;371 doi: 10.1098/rstb.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Critchley H.D., Eccles J., Garfinkel S.N. Elsevier B.V.; 2013. Interaction Between Cognition, Emotion, and the Autonomic Nervous System. [DOI] [PubMed] [Google Scholar]
- 81••.Karalis N., Dejean C., Chaudun F., Khoder S., Rozeske R.R., Wurtz H., Bagur S., Benchenane K., Sirota A., Courtin J. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat Neurosci. 2016;19:605–612. doi: 10.1038/nn.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors present 4 Hz oscillations as a novel mechanisms to synchronize prefrontal-amygdala circuits during freezing behavior. The results highlight the implication of brain-wide networks in emotion expression.
- 82.Courtin J., Chaudun F., Rozeske R.R., Karalis N., Gonzalez-Campo C., Wurtz H., Abdi A., Baufreton J., Bienvenu T.C.M., Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- 83•.Gründemann J., Bitterman Y., Lu T., Krabbe S., Grewe B.F., Schnitzer M.J., Lüthi A. Amygdala ensembles encode behavioral states. Science. 2019;364 doi: 10.1126/science.aav8736. [DOI] [PubMed] [Google Scholar]; The authors identify opposing activity dynamics in two major ensembles in the basolateral amygdala that predict transitions between exploratory and non-exploratory states.
- 84.Bagur S., Lefort J., Lacroix M., de Lavilléon G., Herry C., Billand C., Geoffroy H., Benchenane K. Dissociation of fear initiation and maintenance by breathing-driven prefrontal oscillations. bioRxiv. 2018 doi: 10.1101/468264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIntosh D.N. Facial feedback hypotheses: evidence, implications, and directions. Motiv Emot. 1996;20:121–147. [Google Scholar]
- 86.Davis J.I., Senghas A., Brandt F., Ochsner K.N. The effects of BOTOX injections on emotional experience. Emotion. 2010;10:433–440. doi: 10.1037/a0018690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allen M. Unravelling the neurobiology of interoceptive inference. Trends Cogn Sci. 2020;24:265–266. doi: 10.1016/j.tics.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Seth A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 89.Saarimäki H., Gotsopoulos A., Jääskeläinen I.P., Lampinen J., Vuilleumier P., Hari R., Sams M., Nummenmaa L. Discrete neural signatures of basic emotions. Cereb Cortex. 2016;26:2563–2573. doi: 10.1093/cercor/bhv086. [DOI] [PubMed] [Google Scholar]
- 90.Nummenmaa L., Saarimäki H. Emotions as discrete patterns of systemic activity. Neurosci Lett. 2019;693:3–8. doi: 10.1016/j.neulet.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 91.Kragel P.A., LaBar K.S. Decoding the nature of emotion in the brain. Trends Cogn Sci. 2016;20:444–455. doi: 10.1016/j.tics.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saarimäki H., Ejtehadian L.F., Glerean E., Jääskeläinen I.P., Vuilleumier P., Sams M., Nummenmaa L. Distributed affective space represents multiple emotion categories across the human brain. Soc Cogn Affect Neurosci. 2018;13:471–482. doi: 10.1093/scan/nsy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson D.J. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci. 2016;17:692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- 94•.Namburi P., Al-Hasani R., Calhoon G.G., Bruchas M.R., Tye K.M. Architectural representation of valence in the limbic system. Neuropsychopharmacology. 2015;41:1697–1715. doi: 10.1038/npp.2015.358. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors provide an insightful and comprehensive review on valence coding in the brain, providing an unified model of the neuronal substrates of ‘good’ and ‘bad’.
- 95•.Douglass A.M., Kucukdereli H., Ponserre M., Markovic M., Gründemann J., Strobel C., Alcala Morales P.L., Conzelmann K.K., Lüthi A., Klein R. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci. 2017;20:1384–1394. doi: 10.1038/nn.4623. [DOI] [PubMed] [Google Scholar]; The authors identify a new population in the central amygdala neurons expressing serotonin receptor Htr2a which promote feeding behavior and positive reinforcement.
- 96.Herry C., Ciocchi S., Senn V., Demmou L., Muller C., Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 97.Fadok J.P., Krabbe S., Markovic M., Courtin J., Xu C., Massi L., Botta P., Bylund K., Müller C., Kovacevic A. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–99. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 98.Beyeler A., Namburi P., Glober G.F., Luck R., Wildes C.P., Tye K.M., Calhoon G.G. Divergent routing of positive and negative information from the amygdala during memory article divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron. 2016;90:348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L., Gillis-Smith S., Peng Y., Zhang J., Chen X., Salzman C.D., Ryba N.J.P., Zuker C.S. The coding of valence and identity in the mammalian taste system. Nature. 2018;558:127–131. doi: 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faure A., Richard J.M., Berridge K.C. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berridge K.C., Kringelbach M.L. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim J., Pignatelli M., Xu S., Itohara S., Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dejean C., Courtin J., Karalis N., Chaudun F., Wurtz H., Bienvenu T.C.M., Herry C. Prefrontal neuronal assemblies temporally control fear behaviour. Nature. 2016;535:420–424. doi: 10.1038/nature18630. [DOI] [PubMed] [Google Scholar]
- 104.Tovote P., Esposito M.S., Botta P., Chaudun F., Fadok J.P., Markovic M., Wolff S.B.E., Ramakrishnan C., Fenno L., Deisseroth K. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- 105••.Mathis A., Mamidanna P., Cury K.M., Abe T., Murthy V.N., Mathis M.W., Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 2018;21:1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]; The authors introduce DeepLabCut, a video-based software package based on deep-learning, which enables easy and rapid motion tracking in any species.
- 106.Goodwin N.L., Nilsson S.R.O., Golden S.A. Rage against the machine: advancing the study of aggression ethology via machine learning. Psychopharmacology. 2020;237:2569–2588. doi: 10.1007/s00213-020-05577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pereira T.D., Shaevitz J.W., Murthy M. Quantifying behavior to understand the brain. Nat Neurosci. 2020;23:1537–1579. doi: 10.1038/s41593-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mathis A., Schneider S., Lauer J., Mathis M.W. A primer on motion capture with deep learning: principles, pitfalls, and perspectives. Neuron. 2020;108:44–65. doi: 10.1016/j.neuron.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Wiltschko A.B., Johnson M.J., Iurilli G., Peterson R.E., Katon J.M., Pashkovski S.L., Abraira V.E., Adams R.P., Datta S.R. Mapping sub-second structure in mouse behavior. Neuron. 2015;88:1121–1135. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110••.Wiltschko A.B., Tsukahara T., Zeine A., Anyoha R., Gillis W.F., Markowitz J.E., Peterson R.E., Katon J., Johnson M.J., Datta S.R. Revealing the structure of pharmacobehavioral space through motion sequencing. Nat Neurosci. 2020;23:1433–1443. doi: 10.1038/s41593-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors implement MoSeq behavioral analysis method to classify behavioral changes triggered by psychoactive drugs. The study provides a powerful demonstration on how behavioral analyses can be used as a window into diverse neural and psychological processes of interest, which has the potential to greatly benefit the neuroscientific investigation of complex brain functions.
- 111.Krakauer J.W., Ghazanfar A.A., Gomez-Marin A., MacIver M.A., Poeppel D. Neuroscience needs behavior: correcting a reductionist bias. Neuron. 2017;93:480–490. doi: 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 112.Datta S.R., Anderson D.J., Branson K., Perona P., Leifer A. Computational neuroethology: a call to action. Neuron. 2019;104:11–24. doi: 10.1016/j.neuron.2019.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macé É., Montaldo G., Trenholm S., Cowan C., Brignall A., Urban A., Roska B. Whole-brain functional ultrasound imaging reveals brain modules for visuomotor integration. Neuron. 2018;100:1241–1251.e7. doi: 10.1016/j.neuron.2018.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brunner C., Grillet M., Sans-Dublanc A., Farrow K., Lambert T., Macé E., Montaldo G., Urban A. A platform for brain-wide volumetric functional ultrasound imaging and analysis of circuit dynamics in awake mice. Neuron. 2020;108:861–875. doi: 10.1016/j.neuron.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 115.Laurent G. On the value of model diversity in neuroscience. Nat Rev Neurosci. 2020;21:395–396. doi: 10.1038/s41583-020-0323-1. [DOI] [PubMed] [Google Scholar]