Abstract
BACKGROUND:
The effects of blood transfusions on oncologic outcomes after surgery remains inconclusive. Thus, we examined the association between receiving a perioperative blood transfusion and oncologic outcomes in patients undergoing curative rectal cancer resection.
OBJECTIVE:
The purpose of this study was to assess the association between receiving a perioperative blood transfusion with disease-free and overall survival in patients undergoing curative resection of clinical stage I-III rectal cancer. We hypothesize that blood transfusion is associated with worse disease-free and overall survival in this patient cohort.
DESIGN:
This was a retrospective cohort study using a propensity score matched analysis.
SETTINGS:
The study involved six tertiary academic medical centers in the United States contributing to the United States Rectal Cancer Consortium.
PATIENTS:
Patients who underwent curative resection for rectal cancer from 2010-2018 were included.
MAIN OUTCOME MEASURES:
The primary outcome was disease-free survival. The secondary outcomes were overall survival, intensive-care unit length of stay, hospital length of stay, surgical site infection, and re-admission.
RESULTS:
Of the 924 patients eligible for matching, 312 patients were matched, including 100 patients who received a transfusion and 212 who did not. In a propensity score matched analysis, receiving a perioperative blood transfusion was not associated with worse 5-year disease-free survival (transfused 78%; not transfused 83%; p = 0.32), but was associated with worse 5-year overall survival (transfused 65% vs not transfused 86%; p < 0.001) and increased hospital length of stay (transfused 9.9 days; not transfused 7.6 days; p=0.001).
LIMITATIONS:
Despite propensity matching, confounding may remain. Propensity matching may limit the power to detect a difference in disease-free survival.
CONCLUSIONS:
Receiving a perioperative blood transfusion is not associated with worse disease-free survival, but is associated with worse overall survival. Such findings are important for clinicians and patients to understand when considering perioperative blood transfusions.
Keywords: Blood transfusions, Outcomes, Perioperative care, Propensity score, Rectal cancer
Abstract
ANTECEDENTES:
El impacto de las transfusiones de sangre en los resultados oncológicos posteriores a la cirugía no son concluyentes. Por lo anterior, estudiamos la asociación entre recibir una transfusión de sangre perioperatoria y los resultados oncológicos en pacientes llevados a resección curativa de cáncer de recto.
OBJETIVO:
El propósito de este estudio fue evaluar la asociación entre recibir una transfusión de sangre perioperatoria con la sobrevida libre de enfermedad y la sobrevida general en pacientes llevados a resección curativa de cáncer de recto en estadio clínico I-III. Nuestra hipótesis es que la transfusión de sangre se asocia con una peor sobrevida global y libre de enfermedad en esta cohorte de pacientes.
DISEÑO:
Es un estudio de cohorte retrospectivo que utilizó un puntaje de propensión por análisis de concordancia.
AMBITO:
El estudio se realizó en seis centros médicos académicos de tercer nivel en los Estados Unidos que contribuían al Consorcio de Cáncer de Recto de los Estados Unidos.
PACIENTES:
Se incluyeron pacientes que fueron llevados a resección curativa por cáncer de recto entre 2010 y 2018.
PRINCIPALES VARIABLES EVALUADAS:
El objeitvo principal fue la sobrevida libre de enfermedad. Los objetivos secundarios fueron la sobrevida global, el tiempo de estancia en la unidad de cuidados intensivos, el tiempo de la estancia hospitalaria, la infección del sitio quirúrgico y el reingreso.
RESULTADOS:
De los 924 pacientes elegibles para el emparejamiento, se emparejaron 312 pacientes, incluidos 100 pacientes que recibieron una transfusión y 212 que no. En el puntaje de propensión por análisis de concordancia, recibir una transfusión de sangre perioperatoria no se asoció con una peor sobrevida libre de enfermedad a 5 años (TRANSFUSIÓN 78%; NO TRANSFUSIÓN 83%; p = 0,32), pero se asoció con una peor sobrevida global a 5 años (TRANSFUSION 65% vs NO TRANSFUSION 86%; p <0,001) y aumento de la estancia hospitalaria (TRANSFUSIÓN 9,9 días; NO TRANSFUSION 7,6 días; p = 0,001).
LIMITACIONES:
A pesar de la concordancia de propensión, pueden existir desviaciones. El emparejamiento de propensión puede limitar el poder para detectar una diferencia en la sobrevida libre de enfermedad.
CONCLUSIONES:
Recibir una transfusión de sangre perioperatoria no se asocia con una peor sobrevida libre de enfermedad, pero sí con una peor sobrevida global. Es importante que los médicos y los pacientes comprendan estos hallazgos al considerar las transfusiones de sangre perioperatorias.
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer death and the fourth most incident cancer in the world, and its incidence is steadily rising worldwide.1 In the United States, the prevalence and incidence of CRC is approximately 1.3 million and 150,000 per year respectively, comprising about 8% of new cancer diagnoses. Among the new diagnoses of CRC, one-third are rectal cancer.2 Surgery remains the mainstay of curative treatment for rectal cancer. The preoperative adjustment of modifiable risk factors has the opportunity to improve postoperative and potentially oncologic outcomes. A significant portion of patients with CRC have preoperative anemia, which can be attributed to tumor bleeding, chronic pro-inflammatory state, malnutrition, or neoadjuvant therapy.3 Anemia has generally been associated with frailty and shortened survival in patients undergoing cancer surgery.4,5 However, the observed association between anemia and oncologic outcomes are likely due to a surrogacy effect of other associated comorbidities that would also impair survival.
While blood transfusion is necessary to correct anemia in many clinical circumstances, it has been thought to induce a proinflammatory state, which is associated with tumorigenesis and tumor progression, particularly in CRC.6-10 Several studies have observed higher morbidity,11-14 higher rates of tumor recurrence,15 and decreased overall survival,16 including a recent propensity weighted analysis of over 4,000 patients undergoing colon cancer resection.17 However, several studies did not demonstrate an association between perioperative blood transfusions and survival.18-21 A meta-analysis involving over 12,000 patients was unable to identify an association due to significant heterogeneity of patients and surgical technique, as well as variability in transfusion threshold.22 Thus, a significant knowledge gap as to the association between blood transfusion and oncologic outcomes remains, especially in rectal cancer.
Determining the relationship between perioperative blood transfusions and oncologic outcomes is of key importance because preoperative measures can be implemented to reduce the need for perioperative blood transfusions. Conversely, if blood transfusions are not associated with worse outcomes, omitting a transfusion for potential oncologic consequence is not clinically justified. We conducted a propensity score matched retrospective analysis to assess the association between receiving a perioperative blood transfusion and 5-year disease-free (DFS) and 5-year overall survival (OS) in a large cohort of patients undergoing curative resection for clinical stage I-III rectal cancer. The authors hypothesize that blood transfusion is associated with decreased disease-free and overall survival in this patient cohort, as well as longer intensive-care unit (ICU) and hospital length of stay (LOS), in addition to higher rates of surgical site infection (SSI) and re-admission.
MATERIALS AND METHODS
Study Design and Patient Population
The present study was approved by Institutional Review Board of Vanderbilt University (IRB # 172094) and utilizes information from the US Rectal Cancer Consortium. This database comprises demographic information, medical history, comorbidities, laboratory values, oncologic data, surgical technique, and post-operative complications for patients who underwent rectal cancer resection at one of 6 tertiary academic medical centers across the United States. Patients with stage I-III rectal cancer who underwent curative-intent primary rectal cancer surgery between January 2010 and December 2018 were identified. Patients with metastatic disease identified before or during surgery were excluded along with patients who underwent surgery for recurrent cancer. Also, patients who underwent an emergent, palliative, or trans-anal procedure were excluded. Finally, patients who did not have documentation of estimated blood loss (EBL), preoperative hemoglobin (Hgb) level, or perioperative transfusion status were excluded from the analysis.
Definitions and Data Collection
We utilized the database to acquire patients’ demographics, comorbidities, American Society of Anesthesiologists (ASA) classification status, cancer stage, tumor data, operative techniques, post-operative morbidity, and oncologic outcomes. The American Joint Commission on Cancer (AJCC) 8th edition was used to determine cancer stage. All operations were performed by a trained colorectal surgeon or a surgical oncologist. Preoperative anemia, as defined by the World Health Organization as a Hgb level <13.0g/dL in men and <12.0g/dL in females, was determined based on the most recent preoperative Hgb level. Surgical site infection was defined as cellulitis requiring antibiotics, infection with purulent drainage from either spontaneous or intentional opening of the wound, or intra-abdominal infection with or without need for drainage or re-operation. Re-admission was considered if the patient developed a condition requiring admission to the hospital within 30 days of discharge from the initial operation. All participating institutions are American College of Surgeons- Commission on Cancer accredited cancer programs with patients undergoing postoperative cancer surveillance as recommended by the National Comprehensive Cancer Network (NCCN).
The cohort of patients available for analysis were divided into two groups: those who received a perioperative blood transfusion and those who did not. A perioperative blood transfusion was defined as receiving at least 1 unit (300 mL) of crossmatched allogenic blood during the time period beginning from the start of the operation to discharge from the post-operative hospital admission. Since there is a growing concern that the immunomodulation and systemic inflammatory response related to receiving a blood transfusion are associated with tumorigenesis and tumor progression, we identified disease-free survival (DFS) as the primary outcome. DFS was defined as the time (months) between initial cancer resection and recurrence. The secondary outcome was overall survival (OS), which was defined as the time (months) between initial cancer resection and death. For those without either event of recurrence or death, their DFS and OS data points were censored at time of last follow-up. Additionally, we examined the association between blood transfusion and other postoperative metrics, including ICU LOS, hospital LOS, rate of SSI, and rate of re-admission.
Statistical Analysis
Prior to analysis of the study endpoints, we constructed a model of the probability of a patient receiving a perioperative blood transfusion, based on patient and disease characteristics potentially related to transfusion. These variables were assessed using a logistic regression model. To allow for nonlinear associations between the predictor variables and the outcomes, continuous variables were included in the model as restricted cubic splines. Categorical variables were regarded as such.
We used the “MatchIt” R package to perform a bipartite propensity score matching of the subjects who did not receive a blood a transfusion to subjects who did. From the regression model, which had a C-index of 0.88, we generated propensity scores on a log relative odds scale. We assessed the degree of covariate overlap by estimating the distance measure using the covariates to predict blood transfusions, which were the following: age, gender, race, BMI, ASA class, diabetes, prior cardiac event, CHF, acute renal failure, chronic renal failure, dialysis, anemia, type of operation, operative time, final stage, grade of tumor differentiation, final resection status of primary rectal tumor only, and EBL. Subjects in one group without a suitable matched in the other group were excluded from the analysis. We used a 3:1 neighbor matching ratio and a caliper of 0.2 of the standard deviation to perform the propensity score matching. We proceeded with the analysis of the subset of patients for which covariate overlap exists between the two groups, thus pruning subjects as needed. We analyzed the primary and secondary endpoints using a covariate-adjusted Cox proportional hazards regression model, excluding all subjects who died within 90 days of surgery. The relative differences in DFS between the two patient groups were estimated as a hazard ratio. We then tested the null hypothesis of no difference in DFS between the two patient groups with the Wald test.
Statistical analyses were performed using the R statistical software (www.r-project.org). A 2-sided P <0.05 was considered statistically significant. All continuous data are expressed as means ± standard deviation and compared using t-tests.
RESULTS
Patient Characteristics and Risk Factors for Blood Transfusion
1881 patients who underwent resection for rectal cancer between January 2010 and December 2018 were identified. After applying our exclusion criteria, a total of 924 patients were eligible for propensity-matched analysis (Figure 1). Of those, 135 patients (14.6%) received a perioperative blood transfusion, whereas 789 patients (85.4%) did not. The median follow-up time was 2.9 years and the 5-year disease-free and overall survival rates for the entire cohort were 84% and 86%, respectively. Patient baseline characteristics for the two patient groups are displayed in Table 1. Oncologic and post-operative outcomes for the unmatched cohort are displayed in Table 2.
Figure 1.
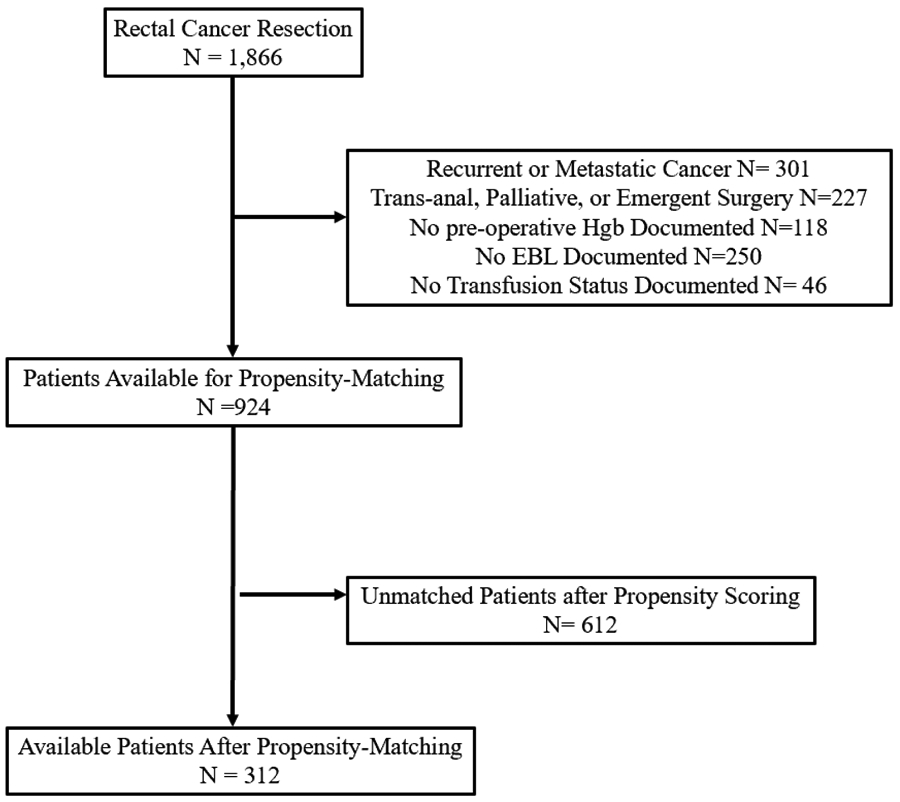
Patient Selection
Table 1:
Patient Baseline Characteristics in Unmatched Cohort
| Patient Characteristics |
Not Transfused N = 789 |
Transfused N = 135 |
Combined N = 924 |
|---|---|---|---|
| Age (years) | 59 ± 12 | 62 ± 14 | 59 ± 12 |
| Gender | |||
| Male | 61% | 56% | 60% |
| Female | 39% | 44% | 40% |
| Race | |||
| White | 89% | 84% | 88% |
| Black | 7% | 15% | 8% |
| Asian | 2% | 0% | 1% |
| Latino | 1% | 0% | 1% |
| Other | 1% | 1% | 2% |
| BMI | 28.9 ± 6.8 | 29.4 ± 9.2 | 29 ± 7.2 |
| ASA Class | |||
| 1 | 0% | 1% | 0% |
| 2 | 47% | 21% | 43% |
| 3 | 51% | 72% | 54% |
| 4 | 2% | 5% | 3% |
| Diabetes | 14% | 24% | 16% |
| CHF | 2% | 3% | 2% |
| Acute Renal Failure | 1% | 1% | 1% |
| Chronic Renal Failure | 2% | 7% | 3% |
| Final Clinical Stage (AJCC 8th edition) | |||
| Stage 0 | 13% | 11% | 13% |
| Stage I | 32% | 30% | 32% |
| Stage IIA | 21% | 23% | 21% |
| Stage IIB | 1% | 1% | 1% |
| Stage IIC | 2% | 7% | 2% |
| Stage IIIA | 8% | 3% | 8% |
| Stage IIIB | 17% | 17% | 17% |
| Stage IIIC | 6% | 8% | 6% |
| Tumor Differentiation | |||
| Well-Differentiated | 12% | 8% | 10% |
| Moderately Differentiated | 67% | 69% | 67% |
| Poorly Differentiated | 13% | 16% | 15% |
| Undifferentiated | 8% | 7% | 8% |
| Mucinous Histology | 5% | 11% | 6% |
| Lymphovascular Invasion | 19% | 19% | 19% |
| Perineural Invasion | 15% | 16% | 15% |
| Lymph Nodes Involved (final pathology) | |||
| 0 | 69% | 67% | 69% |
| 1-3 | 20% | 16% | 18% |
| ≥4 | 11% | 17% | 13% |
| Final Resection Status | |||
| R0 | 93% | 88% | 91% |
| R1 | 8% | 11% | 8% |
| R2 | 1% | 1% | 1% |
| Neoadjuvant Chemotherapy | 21% | 30% | 23% |
| Neoadjuvant Chemoradiation Therapy | 75% | 84% | 76% |
| Total Neoadjuvant Therapy | 24% | 29% | 24% |
| Adjuvant Chemotherapy | 61% | 48% | 59% |
| Pre-operative hemoglobin (g/dl) | 13.1 ± 1.7 | 11.6 ± 1.7 | 12.9 ± 1.8 |
| Anemia | 32% | 73% | 38% |
| Follow-up Time (years) | 2.8 ± 2.1 | 3.1 ± 2.2 | 2.9 ± 2.1 |
Table 2:
Operative Characteristics and Outcomes in Unmatched Cohort
| Operative Characteristics and Outcomes |
Not Transfused N = 789 |
Transfused N = 135 |
Combined N = 924 |
|---|---|---|---|
| Operation Type | |||
| Low Anterior Resection | 71% | 44% | 67% |
| Abdominoperineal Resection | 29% | 56% | 33% |
| Operative Time (minutes) | 259 ± 100 | 317 ± 166 | 267 ± 114 |
| Estimated Blood Loss (milliliters) | 247 ± 238 | 727 ± 811 | 317 ± 415 |
| Intra-operative Blood Transfusion | 0% | 50% | 7% |
| Postoperative Blood Transfusion | 0% | 68% | 10% |
| Postoperative Complication | 49% | 90% | 56% |
| Highest Clavien-Dindo Grade of Complication | |||
| I | 38% | 7% | 30% |
| II | 27% | 55% | 34% |
| III | 31% | 27% | 29% |
| IV | 4% | 12% | 6% |
| V | 1% | 0% | 0% |
| Surgical Site Infection | 17% | 27% | 19% |
| ICU Length of Stay (days) | 2.6± 3.7 | 6.0 ± 6.1 | 4.0 ± 5.1 |
| Hospital Length of Stay (days) | 6.7 ± 5.2 | 11.7 ± 12.4 | 7.5 ± 7 |
| Hospital Re-admission (30 days) | 29% | 38% | 30% |
| 5-year Disease-free survival | 85% | 81% | 84% |
| 5-year Overall survival | 90% | 67% | 86% |
| Time to Death | |||
| 0-30 days | 1% | 2% | 2% |
| 31-60 days | 1% | 7% | 3% |
| 61-90 days | 5% | 0% | 3% |
| > 90 days | 94% | 90% | 92% |
Association of Blood transfusion and Oncologic Outcomes
Before propensity score matched analysis, the 5-year DFS and OS rates were 81% and 66% for patients who received a perioperative blood transfusion as compared to 85% and 90% for patients who did not. In the propensity score matching procedure, 25 patients who did receive a blood transfusion and 549 patients who did not were excluded because these subjects could not be matched to a subject counterpart. Thus, the propensity score-matched analysis is based on 312 patients, 100 of which received a perioperative blood transfusion, and 212 who did not. The propensity score distributions were similar in both groups after matching (Figure 2). The distribution of covariates in the matched cohort are displayed in Supplementary Table 1.
Figure 2: Distribution of Propensity Scores Before and After Matching:
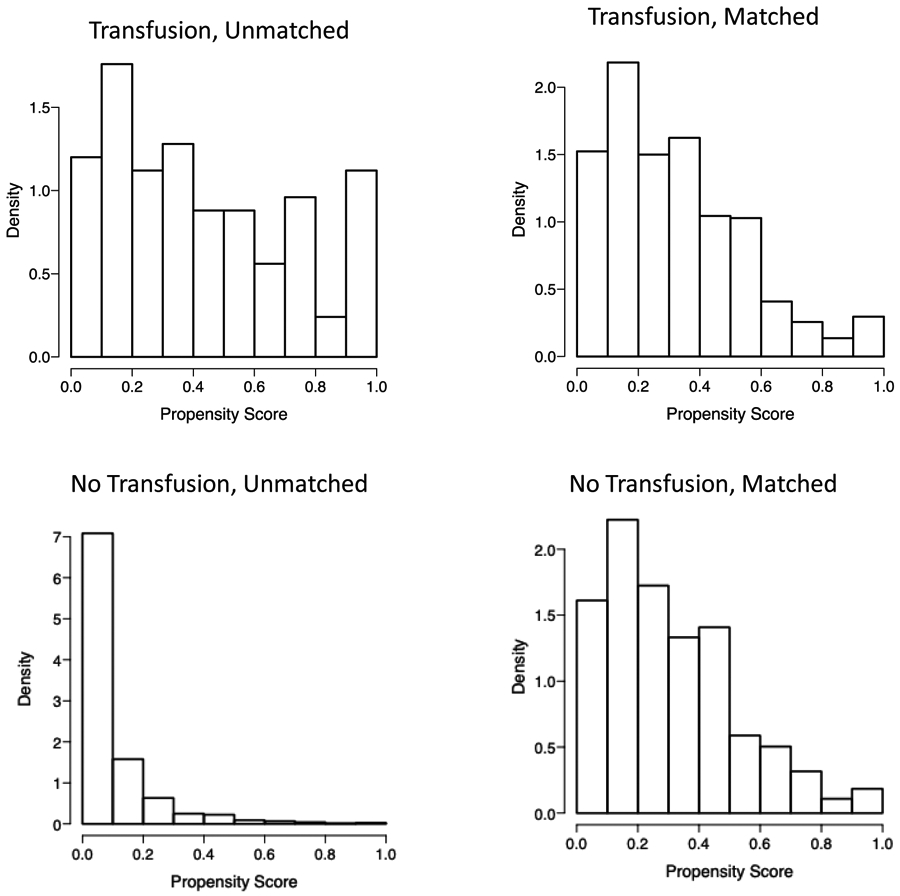
The histograms show the predicted propensity scores by transfusion status. The figures on the left show the propensity scores among all subjects. The figures on the right display the distribution of propensity scores after subjects were matched based on the covariates.
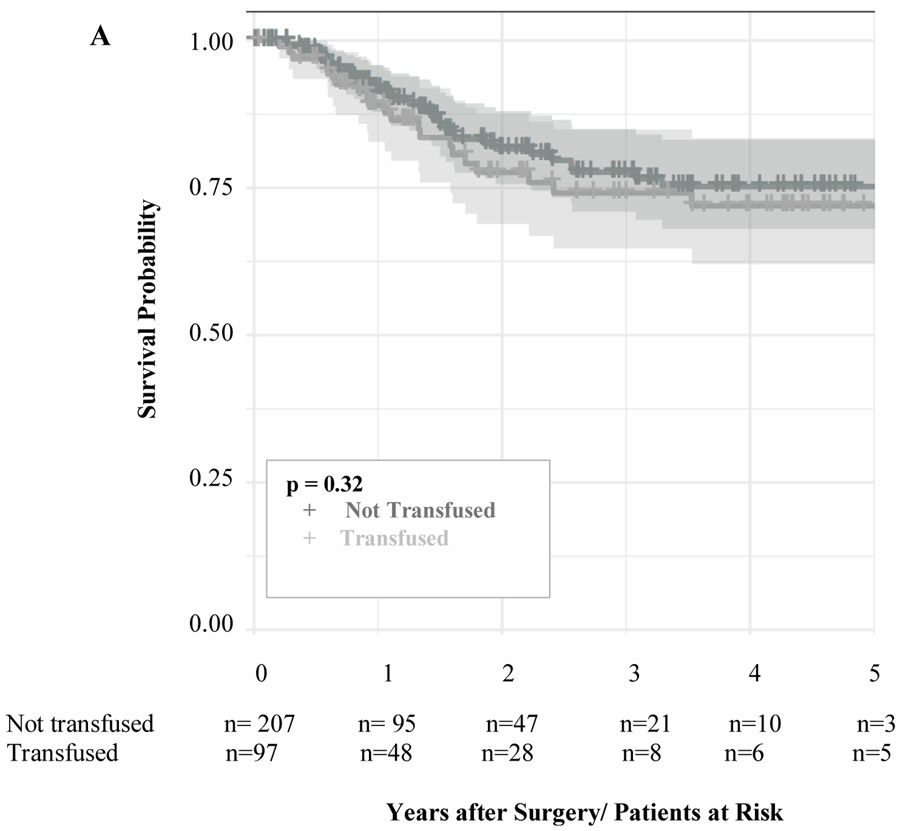
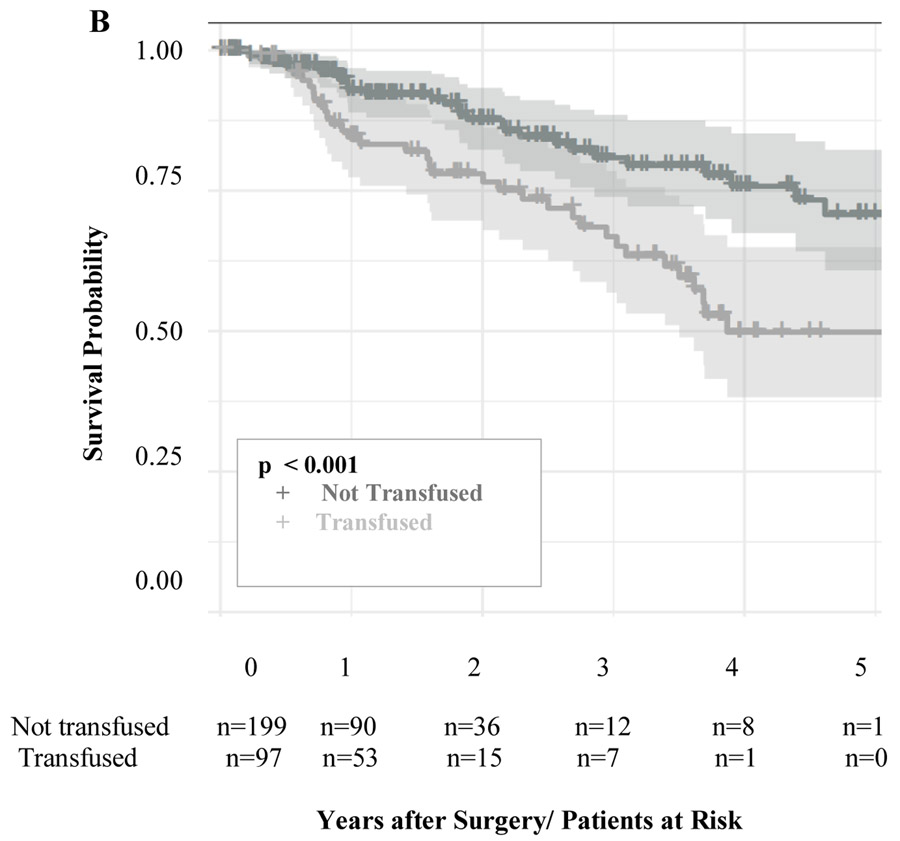
After propensity score matched analysis, the 5-year DFS (TRANSFUSED 78%; NOT TRANSFUSED 83%; p = 0.32, Figure 3a) and time to recurrence (TRANSFUSED 34 months; NOT TRANSFUSED 32 months; p =0.46) were similar among the two groups (Table 3). Also, receiving a perioperative blood transfusion was not associated with increased risk of cancer recurrence (HR 1.08 CI 95% 0.61-1.9; p= 0.8). Additionally, the location of cancer recurrence (distant, locoregional, or both) were similar between the two groups among patients who had cancer recurrence. However, the 5-year OS was significantly different between the two groups (TRANSFUSED 65% vs NOT TRANSFUSED 86%; p < 0.001, Figure 3b) and blood transfusion was associated with decreased OS (HR 2.03 CI 95% 1.21-3.41; p=0.008).
Figure 3:
Adjusted Kaplan-Meier Curves for Disease-free Survival (a) and Overall Survival (b) in a propensity score matched analysis. The number of patients at risk are given below each plot. Survival curves are provided with 95% confidence intervals.
Table 3:
Primary and Secondary Outcomes after Propensity Score Matching
| Outcomes | Not Transfused N = 212 |
Transfused N = 100 |
P - value |
|---|---|---|---|
| 5-year Disease-free Survival | 83% | 78% | 0.32 |
| 5-year Overall Survival | 86% | 65% | <0.001 |
| Recurrence Region | 0.8 | ||
| Locoregional | 26% | 24% | |
| Distant | 59% | 67% | |
| Both | 15% | 10% | |
| Time to Recurrence (months) | 32 ± 27 | 34 ± 27 | 0.46 |
| Hospital Re-admission (30 days) | 30% | 37% | 0.25 |
| ICU Length of Stay | 3.1 ± 4.0 | 4.3 ± 5.7 | 0.091 |
| Hospital Length of Stay | 7.6 ± 7.1 | 9.9 ± 7.3 | 0.001 |
| Surgical Site Infection | 26% | 24% | 0.69 |
| Follow-up time (years) | 2.9 ± 2.3 | 3.2 ± 2.2 | 0.15 |
Association of Blood Transfusion and Postoperative Morbidity
Patients in each group had similar rates of SSI (TRANSFUSED 24%; NOT TRANSFUSED 26%; p=0.69), ICU length of stay (TRANSFUSED 4.3 days; NOT TRANSFUSED 3.1 days; p=0.09), and rate of hospital re-admission within 30 days (TRANSFUSED 37%; NOT TRANSFUSED 30%; p=0.25). However, patients who underwent a perioperative blood transfusion were noted to have a significantly longer overall hospital LOS as compared to their matched subjects who did not undergo a transfusion (TRANSFUSED 9.9 days; NOT TRANSFUSED 7.6 days; p=0.001).
DISCUSSION
We used a propensity score matched analysis to assess the association between perioperative blood transfusions and oncologic outcomes in patients undergoing curative resection for clinical stage I-III rectal cancer. We found that receiving a perioperative blood transfusion was not associated with worse disease-free survival but was associated with worse overall survival. Additionally, blood transfusions were associated with increased hospital length of stay, but not ICU length of stay, surgical site infection, or rate of hospital re-admission.
In this patient cohort, which spanned eight years across six institutions, 14.6% of patients received a blood transfusion, which is slightly less than other similar studies in which the rate of transfusion ranged from 17-62%.18,22 Anemia is common among patients with rectal cancer and previous studies have demonstrated that these patients experience increased post-operative morbidity.3,23,24 However, this association may be due to other variables that are also associated with worse oncologic outcomes, such as age, BMI, ASA class and other medical morbidities. After optimally adjusting for such variables in a propensity score matched analysis, we found that blood transfusion was still associated with worse overall survival, although not disease-free survival. These findings suggest that the immunogenic effects of receiving a blood transfusion may not necessarily be associated with tumor recurrence. However, requiring a perioperative blood transfusion is likely associated with other patient characteristics and clinical factors that cannot be captured in a propensity-score matched model. Thus, the observed difference in overall survival but not disease-free survival can be attributed mostly to the factors contributing to requiring a blood transfusion, rather than the transfusion itself. Overall, the negative associations of receiving a blood transfusion are multifactorial, rather than solely due patient comorbidities or transfusion-associated immunomodulation.
These findings are supported by a series of other studies, including a randomized trial by Busch et al that examined whether cancer prognosis would be different among patients who received an autologous vs an allogeneic blood transfusion. This study as well as other retrospective analyses found no difference between the source of blood transfusion and that transfusions were likely associated with worse oncologic outcomes due to the clinical circumstances that necessitate them.25-30 These authors concluded that the prognostic factors associated with worse DFS are the clinical circumstances leading to blood transfusions, rather than receiving a blood transfusion itself. Further evidence is provided by a recent propensity weighted analysis of a cohort of 400 patients, which failed to demonstrate an association between perioperative blood transfusions and DFS and OS in rectal cancer patients.31 However, this cohort sample size included only patients who underwent an open operation, was small, and over half of these patients received at least one unit of blood, with about 25% of patients receiving 3 or more units of blood.
There are several limitations of this study that must be acknowledged. This is a retrospective cohort study, not a prospective or randomized controlled trial. However, this research question cannot safely be studied in a randomized fashion due to the ethical and clinical implications of withholding a blood transfusion when clinically indicated in the acute setting. While we understand that there are likely several unquantifiable clinical factors that may confound the association between perioperative blood transfusion and oncologic outcomes, the current study design and statistical analysis is the preferred method to answer our research question given its ability to limit the effects of known potential confounding variables. Second, in our efforts to optimally adjust for factors associated with receiving a blood transfusion and worse oncologic outcomes, over 500 patients were excluded from potential analysis. Although this reduction in the number reduces the power of our study, it simultaneously excludes patients from the analysis who are least likely to receive a perioperative blood transfusion. Thus, propensity score matching allowed for a more robust data analysis by restricting the analysis to patients who have similar characteristics and more likely to receive a transfusion. Third, although we adjusted for several factors, there may be other confounders that are unaccounted for. Many covariates were included in this study, including two measures of surgery severity – operative time and estimated blood loss. However, it is possible that confounding may occur from other variables that were not accounted for or are unobtainable. One such set of variables are the clinical factors surrounding administering a blood transfusion, such as intraoperative vital signs, moments of significant blood loss, postoperative urine output, use of postoperative labs, and orthostatic hypotension are likely all related to receiving a blood transfusion to varying degrees. There is no standardized protocol for administering a blood transfusion and is almost solely based on surgeon or anesthesiologist discretion based on clinical circumstance. Despite this, we saw no difference in several secondary outcomes, which indicate a relative balance between the matched groups.
CONCLUSION
In a propensity score matched analysis, receiving a perioperative blood transfusion was not associated with decreased disease-free survival, but was associated with worse overall survival and increased hospital length of stay. These negative associations are likely multifactorial, including a combination of patient characteristics, comorbid diseases, and transfusion-associated immunomodulation. Such findings are important for both the clinical and patient to understand when considering the risks and benefits of perioperative blood transfusions.
Supplementary Material
Supplementary Table 1: Distribution of covariates in matched cohort.
ACKNOWLEDGMENTS
Dr Hawkins’ work on this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number K23DK118192. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project described was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. Additional support was provided by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences.
Funding/Support:
Dr Hawkins work on this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number K23DK118192. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures: None reported.
Presented as a Virtual Plenary Session Podium Presentation in the form of a video abstract for the 2020 ASCRS Annual Scientific Meeting. A 2-minute video abstract is submitted along with this manuscript.
REFERENCES
- 1.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute NC. Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html. Published 2019. Accessed 07/02/2020.
- 3.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116(suppl 7A):11S–26S. [DOI] [PubMed] [Google Scholar]
- 4.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 5.Stapley S, Peters TJ, Sharp D, Hamilton W. The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: a cohort study using medical records. Br J Cancer. 2006;95:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371–379. [PubMed] [Google Scholar]
- 7.Refaai MA, Blumberg N. Transfusion immunomodulation from a clinical perspective: an update. Expert Rev Hematol. 2013;6:653–663. [DOI] [PubMed] [Google Scholar]
- 8.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. [DOI] [PubMed] [Google Scholar]
- 10.McConnell BB, Yang VW. The role of inflammation in the pathogenesis of colorectal cancer. Curr Colorectal Cancer Rep. 2009;5:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995;82:582–587. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi M, Buccianti P, di Sarli M, Galatioto C, Goletti O, Cavina E. Association between perioperative blood transfusion and dehiscence of anastomosis after rectal resection for cancer. Acta Chir Belg. 1996;96:108–111. [PubMed] [Google Scholar]
- 13.Houbiers JG, van de Velde CJ, van de Watering LM, et al. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion. 1997;37:126–134. [DOI] [PubMed] [Google Scholar]
- 14.Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914. [DOI] [PubMed] [Google Scholar]
- 15.Amato A, Pescatori M. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. Br J Surg. 1994;81:313–314. [DOI] [PubMed] [Google Scholar]
- 16.Wolters U, Stützer H, Keller HW, Schröder U, Pichlmaier H. Colorectal cancer--a multivariate analysis of prognostic factors. Eur J Surg Oncol. 1996;22:592–597. [DOI] [PubMed] [Google Scholar]
- 17.Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep. 2018;8:13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–244. [DOI] [PubMed] [Google Scholar]
- 19.Garau I, Benito E, Bosch FX, et al. Blood transfusion has no effect on colorectal cancer survival. A population-based study. Eur J Cancer. 1994;30A:759–764. [DOI] [PubMed] [Google Scholar]
- 20.Steup WH, Hojo K, Moriya Y, et al. An analysis on the effect of blood transfusion on recurrence and survival in patients undergoing extended lymphadenectomy for colorectal cancer. Hepatogastroenterology. 1994;41:253–259. [PubMed] [Google Scholar]
- 21.Jagoditsch M, Pozgainer P, Klingler A, Tschmelitsch J. Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum. 2006;49:1116–1130. [DOI] [PubMed] [Google Scholar]
- 22.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber RS, Jabbour N, Martin RC II. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. 2008;15:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Luo JJ, Pei KY, et al. Joint effect of pre-operative anemia and perioperative blood transfusion on outcomes of colon-cancer patients undergoing colectomy. Gastroenterol Rep (Oxf). 2019;8:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–1376. [DOI] [PubMed] [Google Scholar]
- 26.Busch OR, Hop WC, Marquet RL, Jeekel J. Prognostic impact of blood transfusions on disease-free survival in colorectal carcinoma. Scand J Gastroenterol Suppl. 1993;200:21–23. [DOI] [PubMed] [Google Scholar]
- 27.Busch OR, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer. Evidence of a noncausal relationship. Ann Surg. 1994;220:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busch OR, Marquet RL, Hop WC, Jeekel J. Colorectal cancer recurrence and perioperative blood transfusions: a critical reappraisal. Semin Surg Oncol. 1994;10:195–199. [DOI] [PubMed] [Google Scholar]
- 29.Busch OR, Hop WC, Marquet RL, Jeekel J. Autologous blood transfusions and prognosis in colorectal cancer surgery. J Clin Oncol. 1995;13:1280–1281. [DOI] [PubMed] [Google Scholar]
- 30.Busch OR, Hop WC, Marquet RL, Jeekel J. The effect of blood transfusions on survival after surgery for colorectal cancer. Eur J Cancer. 1995;31A:1226–1228. [DOI] [PubMed] [Google Scholar]
- 31.Warschkow R, Güller U, Köberle D, et al. Perioperative blood transfusions do not impact overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg. 2014;259:131–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Distribution of covariates in matched cohort.