1. Introduction
Viruses are the ideal vehicles to deliver therapeutic genes to target tissues. Viral vectors used for gene therapy differ from their natural parent viruses in that they are non-replicative, non-pathogenic, and are delivered as a high-titer bolus at an unnatural site.1 However, similarities between the natural virus and viral delivery vector, such as capsid proteins, may result in host immune responses that may limit gene transfer or transgene expression. Additionally, the transgene product may also be targeted by the immune system resulting in ocular inflammation and removal of transduced cells. Finally, treatment emergent adverse events (TEAEs) may be associated with technical challenges of delivering viral vectors to the eye. Here, we review host immune responses to viral-mediated gene transfer, the role of ocular immune privilege, and factors that determine immunogenicity of retinal gene therapies. We discuss ocular inflammation and TEAEs from preclinical and clinical studies and explore strategies to mitigate these responses.
1.1. Viral Vectors for Retinal Gene Therapy
The effectiveness of a viral vector system for gene delivery depends on the target tissue, cellular tropism, packaging capacity, genome integration, and immunogenicity.1 Three viral vectors that have been widely used in ocular gene therapy include adenovirus (Ad), lentivirus (LV), and adeno-associated virus (AAV). Although gene transfer can be accomplished ex vivo using an integrating virus such as lentivirus to introduce transgenes into stem cells, for example, for transplantation, this review will focus on in vivo gene transfer into postmitotic cells using viral vectors that enable either integrating or episomal DNA for long term expression.2
The Ad vector contains ~36 kilobases (kb) double-stranded DNA (dsDNA) in a viral capsid. First evaluated in respiratory conditions such as cystic fibrosis3, its high packaging capacity and high transduction efficiency was thought to make it an ideal viral vector. However, its durability is limited by severe innate and adaptive host immune responses. A sobering reminder of this limitation is when an 18-year-old with partial ornithine deficiency who 18 hours after hepatic gene transfer went into multisystem organ failure due to disseminated intravascular coagulation.3 When the viral capsid becomes tagged by coagulation Factor X, toll-like receptor (TLR) 4 on splenic macrophages are activated and set off a cascade of immune responses to create an anti-viral environment.4 Ad also binds to complement component C3 and IgM antibodies resulting in neutrophil activation, while Ad-antibody complexes attract macrophages. An adaptive immune response also occurs with activation of CD8+ T cells and development of neutralizing antibodies (NAbs) which can interfere with viral vector re-administration.4 This overwhelming immune response triggered by Ad rendered its application in in vivo gene transfer ineffective and unsafe. Alternatively, its short-term gene expression and ability to induce CD8+ T cells makes it ideal for use in vaccines and anticancer therapy, for which Ad could deliver cytotoxic genes to tumor cells and also induce a strong immune response against them.4 Use of Ad allowed the scientific community to understand the intricacies of the immune response to viral vectors and laid down the foundation for exploring other viral gene therapies.
Lentivirus (LV), such as the human immunodeficiency virus (HIV), is an enveloped virus with single-stranded RNA which upon infection is reverse transcribed in the host cell’s cytoplasm. It has a high packaging capacity of ~8 kb and can transduce multiple cell types, making it suitable for inherited retinal diseases (IRDs) such as Stargardt disease.5 However, it primarily integrates into the host’s DNA and can lead to insertional mutagenesis. 4 Although preexisting immunity to LV is low, high levels of T1 interferon (IFN) mediated by TLRs limits its efficacy for gene transduction in vivo. Furthermore, efficient transduction of macrophages and dendritic cells can trigger a strong immune response to the transgene product. Nevertheless, its stable integration allows for effective long-term gene expression for ex vivo gene transfer.
AAV is a small non-enveloped parvovirus with a single-stranded DNA genome of ~4.8 kb. AAV is not naturally pathogenic and does not replicate autonomously, relying on a helper virus such as adenovirus for replication. The removal of viral-coding sequences except for the inverted terminal repeats maximizes the packaging capacity of recombinant AAVs (rAAV) and contributes to their low immunogenicity and cytotoxicity in vivo.6 Its low immunogenicity may be due in part to low antigen presenting cell transduction.7 AAV also exists largely as extra-genomic episomal concatemers and seldom integrates into the host genome, thus reducing the risk of insertional mutagenesis.8 Neuronal cells are also ideal targets for AAV vectors because episomal persistence is enhanced in nondividing cells. For these reasons, rAAV has emerged as the leading viral vector for in vivo ocular gene therapy.
1.2. Applications of Retinal Gene Therapy
rAAV has been evaluated for retinal gene therapy for various IRDs.8 In 2017, the Food and Drug Administration (FDA) approved the first retinal gene therapy application with subretinal delivery of a rAAV vector carrying the RPE65 transgene (voretigene neparvovec-rzyl, Luxturna®, Spark Therapeutics, Philadelphia, PA) for the treatment of biallelic RPE65mutation–associated retinal dystrophy.9 Among 37 subjects who received 1.5×1011 vector genomes (vg) in at least 1 eye in the phase 1 and 3 studies, performance on multi-luminance mobility testing and mean white light full-field light sensitivity threshold, which measures the lowest illumination perceived over the entire visual field, was stable out to 4 years.9 All patients who received the intervention received perioperative systemic steroids and the safety profile was consistent with the vitrectomy and subretinal injection procedure. Although one patient developed endophthalmitis, no other adverse immune responses were noted. These studies suggest that the effect of subretinal AAV-mediated gene therapy can be durable for at least 4 years without a major immune response.
Although the defective RPE65 gene in type 2 Leber Congenital Amaurosis (LCA) is naturally expressed primarily by retinal pigment epithelial (RPE) cells, other IRDs may result from genetic defects in other cell types such as photoreceptors, bipolar cells, Muller glia, or choroidal cells. Other retinal gene therapies currently under evaluation include transgene delivery of the rab-escort protein REP-1 for choroideremia,10-13 rod photoreceptor ATP-binding cassette transporter ABCA4 for Stargardt disease,14 cone photoreceptor cyclic nucleotide-gated channel subunits CNGA315 and CNGB316 for achromatopsia, retinoschisin RS1 for X-linked retinoschisis,17 myosin MYO7A for Usher syndrome,18 and the GTPase regulator RPGR for X-linked retinitis pigmentosa (XLRP).8 Gene therapies can also be applied as a biofactory approach for treating neovascular and degenerative conditions such as age-related macular degeneration (AMD) by employing rAAV to enable sustained production of antibodies or inhibitory proteins targeting vascular endothelial growth factor (VEGF).19-22
2. Host Immune Responses
While host immune responses are typically directed against microbial pathogens such as AAV, they can also recognize transgene products as foreign in patients with inborn genetic mutations, as evident in AAV-based gene therapy trials for Duchenne’s muscular dystrophy, where patients developed an adaptive immune response against the dystrophin transgene product.23 Additionally, immune responses can be triggered not only by viral capsids and/or transgene proteins, but also by the viral genome or transgene itself. Although the eye is considered to be a site of immune privilege, the immunogenicity of ocular gene therapies using AAV varies with the viral capsid, vector dose, route of administration, biodistribution, as well as the specific promoter and transgene. Thus, while the impact of host immune responses on the efficacy, safety, and durability of viral gene transfer remain unclear, and likely varies with different approaches, they are critical to understanding ocular inflammation and TEAEs in retinal gene therapy.
2.1. Innate Immune Response
The innate immune response is the first line of host defense against viral infections. Pattern recognition receptors (PRRs) on immune cells such as macrophages, monocytes, granulocytes, natural killer (NK) cells and dendritic cells (DC) recognize conserved pathogen associated molecular patterns (PAMPs) on viruses. Critical PRRs include toll-like receptors, a family of transmembrane (TLR 1, 2,4, 5, 6, 10) and intracellular (TLR 3, 7, 8, 9) PRRs,24 which upon recognizing PAMPs can trigger a signaling cascade to activate innate immune responses.25 Surface membrane-bound TLR2 can sense viral capsid proteins,26,27 while endosomal TLR9 binds to specific unmethylated DNA sequences such as cytosine-phosphate-guanine (CpG) motifs in viral genomes.28 Transgenes with a significant number of CpG dinucleotides can also activate innate responses through TLRs as well.29
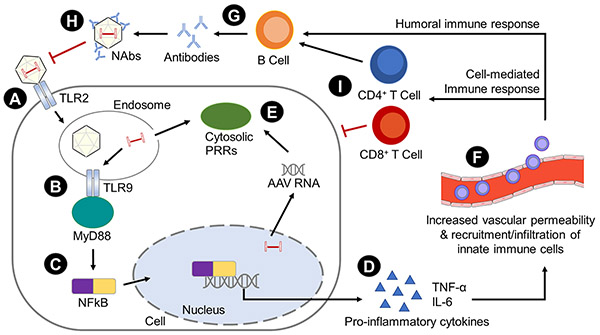
TLRs mediate a downstream signaling pathway via adaptor myeloid differentiation primary response gene 88 (MyD88) which leads to the release of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), an interferon regulatory factor, which activates transcription of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (see Figure 1).24 Hepatic injections of AAV2 in mice triggers the TLR-MyD88 pathway in DCs and NF-kB-dependent release of cytokines, resulting in activation of CD8+ T-cell responses to both AAV capsid and transgene product30 and eventual loss of transgene expression.31 MyD88 signaling also takes place in B cells and regulates the production of Th1-dependent antibodies to AAV.32
Figure 1. Cellular and molecular mechanisms of host immune responses to viral vectors.
(A) TLRs can recognize PAMPs on viruses and trigger a signaling cascade to activate innate immune responses. Surface-bound TLR2 can sense viral capsid proteins.
(B) Endosomal TLR9 binds to specific unmethylated DNA sequences like CpG motifs on viral genomes.
(C) TLRs connects with signaling adaptor myeloid differentiation primary response gene 88 (MyD88) and leads to release of nuclear factor NF-kB.
(D) This then activates transcription of pro-inflammatory cytokines like TNF-α and IL-6,
(E) Cytosolic PRRs respond to PAMPs in the cytoplasm, including NLRs, ALRs, and RLRs.
(F) The pro-inflammatory cytokines can also promote vascular permeability and disruption of the blood-retinal barrier.
(G) CD4+ helper T cells interact with B cells to generate humoral responses. B cells produce antibodies that interact with Fc receptors on immune cells, complement-activating antibodies, and NAbs.
(H) Pre-existing NAbs to certain AAV serotypes can limit viral infectivity by interfering with receptor interactions between the virus and the host cells, thereby inhibiting transgene expression.
(I) Cellular adaptive immune responses are mediated by T cells, which are triggered by AAV or transgene antigens. The presentation of proteasomally-degraded antigens on class I MHC molecules induces CD8+ cytotoxic T cells, while presentation of endosomally-processed antigens on class II MHC molecules induces CD4+ helper T cells. Interactions of APCs with CD4+ T cells induces antigen-specific B cell responses, while APCs stimulate CD8+ T cells to directly destroy transduced cells.
While TLRs are activated by extracellular and endolysosomal PAMPs, cytosolic PRRs respond to PAMPs in the cytoplasm. Examples of cytosolic PRRs include nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs), retinoic acid-inducible gene-I-like (RIG-I-like) receptors (RLRs), and intracellular sensors of DNA like cyclic GMP-AMP synthase (cGAS).28 NLRs and ALRs can induce inflammasome formation which triggers the release of IL-1β and IL-18.33 RLRs contribute to a late innate immune response to double-stranded RNA, an AAV-derived replication intermediate, after long-term AAV transduction.34 cGAS signaling can reduce AAV transduction in mouse embryonic fibroblasts, and may impact retinal gene therapy as it is upregulated after subretinal AAV delivery.28
Viral proteins can also interact with the complement system. Immunoprecipitation studies demonstrated binding of iC3b complement protein to AAV2 capsids in human serum, and both complement receptor 1/2- and C3-deficient mice display impaired humoral immunity against AAV2 vectors.35 Although AAV2 capsids do not activate the alternate complement pathway, they bind to complement regulator protein H, which may protect the virus from the complement system.35
Together, the innate immune responses to AAV vectors lead to activation and release of multiple pro-inflammatory cytokines and chemokines, which then promotes vascular permeability and compromises the blood-retinal barrier (BRB). This results in recruitment and infiltration of innate immune cells including antigen presenting cells (APCs) to shape the adaptive immune response.36
2.2. Adaptive Immune Response
In contrast to the nonspecific nature of innate immunity, the adaptive host immune response is generated in response to specific pathogens and contributes to immunological memory. APCs first interact with naïve T cells in secondary lymphoid tissues, which then differentiate into antigen-specific T cells, including CD4+ helper T cells, which then interact with B cells to generate humoral responses and CD8+ cytotoxic T cells that mediate cellular immune responses. Because NAbs produced by B cells can inhibit vector transduction, while cytokine-secreting T cells can directly destroy infected cells, both humoral and cellular immune responses can contribute to limiting the effectiveness of AAV-mediated retinal gene therapy by eliminating vectors and transduced cells.
2.2.1. Humoral Immune Response
Humoral immunity to viral vectors is mediated by antibodies that interact with Fc receptors on immune cells, complement-activating antibodies, and NAbs.37 While the first two types of antibodies can trigger death of the infected cells, NAbs limit viral infectivity by interfering with receptor interactions between the virus and host cells, and may also promote clearance of viral particles by sequestering them to the spleen.38
Because humans are natural hosts for wild-type AAV, pre-existing NAbs to certain AAV serotypes can be highly prevalent among individuals.39 Serum NAb titers as low as 1:5 can inhibit transgene expression in mice40,41 and nonhuman primates (NHPs).42 Furthermore, many patients demonstrate NAbs to multiple AAV serotypes, indicating high co-prevalence and cross-reactivity which likely results from the highly-conserved amino acid sequence of the viral capsid. Although NAb titers are correlated with IgG rather than IgM levels, no correlation was found between serum NAbs and AAV-specific T cells, perhaps because T cells are tissue-resident and circulate at low levels. Beside NAbs, non-neutralizing binding antibodies (BAbs) may also be present, although their role is unclear. Some studies suggest that BAbs may enhance clearance of AAV vectors through opsonization.38 However, another study showed that hepatic transduction with AAV8 was increased in mice that were passively immunized with BAbs compared to those immunized with NAbs.43
The clinical impact of NAbs on gene therapy remains an area of controversy. In clinical trials involving hepatic AAV gene transfer for hemophilia B patients, one subject with a pretreatment anti-AAV2 NAb titer of 1:17 experienced a reduction of factor IX levels to baseline after 4 weeks, while another subject with a NAb titer of 1:2 retained higher factor IX levels beyond 10 weeks following vector administration.44 The immune response was found to be related to the AAV capsid rather than the factor IX transgene. Similarly, viral transduction by direct intraparenchymal injection of AAV2 and AAV5 vectors into mouse brains, an immune-privileged site, was also limited by circulating NAbs.45 By contrast, circulating anti-AAV9 NAbs up to 1:20-1:40 did not compromise intracranial AAV9 gene delivery.46 For retinal applications, Heier et al. showed that exudative AMD patients with higher baseline NAbs receiving intravitreal AAV2 to express soluble Flt1 to neutralize the VEGF receptor demonstrated reduced transgene expression.20 However, NAbs may not have significant clinical impact for subretinal vector delivery, as patients who received subretinal rAAV2 carrying the RPE65 gene did not demonstrate significant elevation in anti-AAV2 NAbs. These patients therapeutically benefited from vector readministration to the second eye without clinically significant adverse effects.47
2.2.2. Cell-mediated Immune Response
While humoral responses originate from B cells, cellular adaptive immune responses are mediated by T cells, which are triggered by AAV or transgene antigens that have been either passively transduced or actively phagocytosed by APCs. The presentation of proteasomally-degraded cytosolic antigens on class I major histocompatibility complex (MHC) molecules by most nucleated cells induces CD8+ cytotoxic T cells, while presentation of endosomally-processed antigens on class II MHC molecules on immune cells, such as dendritic cells, macrophages, monocytes, as well as RPE cells, trigger CD4+ T cells. The ability of APCs to cross present viral or transgene antigens to either MHC class I or II drives the T cell response, as interaction of APCs with CD4+ T cells can induce antigen-specific B cell responses, while also stimulating the CD8+ T cell response that directly destroy transduced cells.48
Unlike humoral immunity, the prevalence of pre-existing T cells directed against AAV is less common. This due to the fact that capsid-reactive T cells are more often localized to the spleen than the peripheral blood,49 and because methods to detect T cell responses are not as sensitive as NAbs, which accounts for the discrepancy between prevalence of AAV-specific NAbs and circulating CD8+ T cells.39 Similar to the cross-reactivity of NAbs to different AAV serotypes, CD8+ T cells can respond to multiple AAVs due the shared processing and presentation of capsid epitopes.
The impact of AAV-specific T cell responses was noted in AAV-mediated gene therapy trials for Hemophilia B, where similar to the effect of NAbs, increases in AAV2-specific CD8+ T cell responses were associated with elevated transaminases and reduced gene expression after 4-6 weeks.44 In another study using AAV1-mediated gene transfer to muscle, capsid-specific CD8+ T-cells resulted in a decrease of transgene expression and a rise in creatine phosphokinase, an indicator of muscle cell damage.50 While stronger adaptive immune responses have been noted in hepatic and muscle gene transfer, gene transfer to immune-privileged sites such as the brain and eye have exhibited little to no detectable immune response to capsid or transgene in peripheral blood.2
3. Ocular Immune Privilege
Like other parts of the central nervous system, the eye benefits from ocular immune privilege due to 1) the presence of physical blood-ocular barriers, 2) an immunosuppressive molecular microenvironment, and 3) deviant immune responses mediated by immunosuppressive regulatory T cells (Tregs) known as anterior chamber associated immune deviation (ACAID).
3.1. Blood-Ocular Barriers
Blood-ocular barriers consists of tight junctions between RPE cells, and between endothelial cells of retinal and corneal capillaries. Along with the lack of direct lymphatic drainage, these barriers sequester ocular antigens from the developing immune system and limits immune cell entry into the eye. Importantly, the blood-retinal barrier separates different compartments of the eye with unique immunological properties, including the vitreous, subretinal, and suprachoroidal compartments to which viral vectors for gene therapy can be delivered.
3.2. Immunosuppressive Ocular Environment
In addition to physical barriers, the eye expresses high levels of soluble immunomodulatory factors including α-melanocyte stimulating hormone (α-MSH),51 transforming growth factor-β (TGF-β),52 soluble Fas ligand,53 and macrophage migration inhibitory factor (MIF),54 which are potent inhibitors of neutrophils, macrophages, and NK cells. The eye also expresses high levels of immunomodulatory cell-surface molecules such as membrane-bound Fas ligand53 and complement regulatory proteins55 among others, which together creates an immunosuppressive microenvironment to limit the inflammatory responses in the eye.
3.3. Anterior Chamber Associated Immune Deviation (ACAID)
Much our initial understanding of ocular immune privilege came from introducing antigens into the anterior chamber (AC), as in corneal transplantation, which results in an acquired immune response known as anterior chamber-associated immune deviation (ACAID). This is a process by which tolerance-inducing F4/80+ APCs bearing the immunogens migrate through the trabecular meshwork to the spleen, where instead of triggering a typical adaptive immune response, they induce systemic immune tolerance to the antigen by stimulating splenic CD1-reactive natural killer T (NKT) cells.56 These cells then produce immunosuppressive factors such as IL-10 and TGF-β and generating antigen-specific Tregs cells.57-59
A similar mechanism of immune deviation occurs in the vitreous and subretinal compartments.60-62 Unlike antigens in the anterior and vitreous chambers which drain into systemic circulation, those in the subretinal space are thought to exit through the choroid and travel through lymphatics to cervical lymph nodes.62 While F4/80+ APCs are key mediators in ACAID, DCs in the choroid and microglia in the neurosensory retina may play a similar role for immune deviation in the subretinal space. In particular, the RPE may serve an important role in subretinal space immune deviation. The RPE secretes immunosuppressive factors such as TGF-β, somatostatin, thrombospondin and pigment epithelial derived factor (PEDF)63 and constitutively expresses membrane-bound Fas ligand64 which induces apoptosis in inflammatory cells.65 RPE disruption with intravenous sodium iodate results in loss of immune deviation in the subretinal and vitreous compartments, but not the AC, 62 highlighting the importance of the RPE barrier in subretinal gene therapy. However, blood-retinal barriers such as the RPE may be compromised by the underlying retinal pathology in patients with IRDs or surgical maneuvers associated with delivering the viral vector. Thus, the risk for ocular inflammation and host immune responses to retinal gene therapy depends not only on the viral vector, but also on the route of delivery to different ocular compartments, especially with respect to blood-ocular barriers, as well as the therapeutic transgene and underlying retinal condition.
4. Factors Affecting Host Immune Responses
Factors that affect host immune responses to viral-mediated gene therapy include 1) the viral vector and dose, 2) the mode of delivery and vector biodistribution, 3) promoter and transgene, and 4) the disease to be treated.
4.1. Type of Viral Vector
The choice of viral vectors used for retinal gene therapy has largely been guided by host immune responses and safety considerations. Intravenous Ad triggered greater chemokine and cytokine expression with widespread liver inflammation compared with AAV, which only induced a transient response, likely due to differences in surface receptor usage, virus internalization, or intracellular trafficking.66 Furthermore, the dsDNA in Ad is a stronger agonist for TLR9 and thus may stimulate a greater innate immune response. 67
Among AAV vectors, different serotypes not only exhibit distinct tropism for different types of retinal cells but are also impacted by different prevalence of pre-existing NAbs and degrees of immunogenicity. NAbs against AAV1 and AAV2 have the highest prevalence, with reported rates of 30-70%, while NAbs against AAV5, AAV7, AAV8 and AAV9 are lower at 15-30%.39,68 The impact of pre-existing NAbs on retinal gene delivery is controversial, however, and likely depends on the degree of immune privilege of the ocular compartment to which the virus is delivered. Structural differences between AAV8 and AAVrh32.33 viral capsids also produce different adaptive immune responses to the capsid and transgene product,69 while AAV1 transduces DCs more efficiently than AAV8 and triggers a stronger immune response.70 Strategies to reduce immunogenicity include the use of proteosome inhibitors to reduce AAV2-capsid presentation MHC I molecules, 71 and amino acid alterations in the AAV2 capsid to evade ubiquitination and cell death.72 Interestingly, the use of self-complimentary (sc) viral genomes with dsDNA rather than the native single-stranded DNA to improve transduction efficacy actually led to more severe adaptive immune responses, independent of capsid serotype.73 Other novel variants such as AAV7m8 and AAV8BP2 also appear to exhibit different levels of immunogenicity, with studies in nonhuman primates (NHPs) suggesting that AAV8BP2 may have a better safety profile compared to AAV7m8.74
4.2. Route of Administration & Biodistribution
The route of viral vector delivery into the eye has significant impact on the immunogenicity of the ocular gene therapy. Different ocular compartments not only preferentially expose the viral particles to different retinal cell types, but also exhibits different local and systemic biodistribution, as well as exposure to host immune surveillance. Intravitreal injections can be easily performed in an outpatient setting, and the technique is familiar to most retinal specialists. Viral vectors delivered into the vitreous cavity could theoretically transduce broad areas of the retina, but are inhibited from reaching the photoreceptors and RPE by the internal limiting membrane (ILM) barrier. By contrast, subretinal injections must be performed in an operating room and requires the use of a subretinal cannula, which necessitates vitreoretinal surgical maneuvers. The therapeutic effect of the viral vector is also limited to the area of the subretinal bleb that is created, though the viral particles can readily access and transduce photoreceptors and RPE.
Another important distinction between intravitreal and subretinal AAV delivery is the difference in vector outflow and systemic biodistribution. Seitz et al. compared the biodistribution of subretinal and intravitreal AAV8 in NHPs and found that intravitreal delivery showed higher levels of virus in aqueous humor, while subretinal delivery resulted in higher levels in the retina and downstream tissues such as the optic nerve and chiasm.75 Interestingly, compared to subretinal injections, the intravitreal AAV8 group showed a 464-fold higher virus load in the bloodstream, as well as higher levels in the spleen and draining lymph nodes.75 These findings suggest that intravitreal delivery or leakage of vector into the vitreous cavity results in viral vector egress through Schlemms canal into systemic circulation and lymphatic tissues, where it could activate effector immune cells to trigger the host immune response. Indeed, Reichel et al. showed that intravitreal AAV8 produced higher antibody titers as compared to subretinal delivery at same or lower doses.76 This strong humoral response appears to be independent of viral serotype, transgene, vector dose, or number of eyes injected, and increases after a second AAV injection.77 In mice, the antibody response to intravitreal AAV can inhibit vector expression upon subsequent administration via the same route to the fellow eye, but had no effect on subretinal vector readministration. 78 In contrast to intravitreal delivery, delivery of rAAV2 vector into subretinal space did not generate any humoral response against the viral capsid.78 In fact, sequential bilateral subretinal injections of AAV2 carrying RPE65 in dogs and NHPs demonstrated an elevation of NAb after the first treatment, and additional increase after the second eye was treated, but showed minimal inflammatory changes or other adverse effects.79 Subretinal injections of the same AAV2 vector into the second eye of human patients also appear to be safe and effective, with no evidence of functional deficit.47 Thus, the ocular immune privilege and decreased outflow to systemic circulation from the subretinal compartment may be less impacted by either pre-existing immunity or adaptive responses to a therapeutic vector than the vitreous cavity, and better enable sequential bilateral AAV treatments.
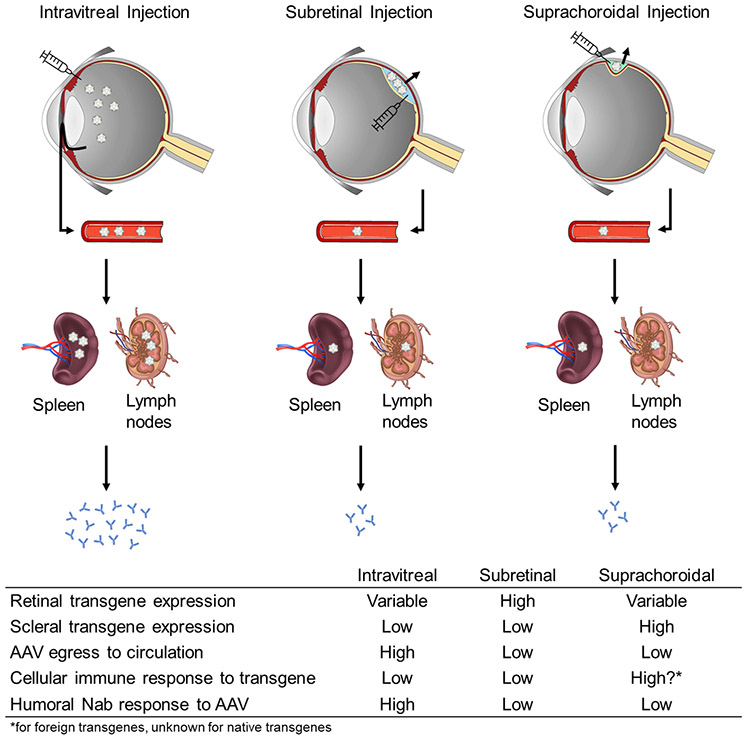
A novel mode of vector delivery involves injection into the suprachoroidal space.80,81 The suprachoroid is a potential space outside the blood-retinal barrier82 that is located between the choroidal vasculature and scleral wall of the eye. This space could be accessed using different methods, including transscleral microneedles that has been successfully employed to deliver triamcinolone for the management of macular edema.83,84 Ding et al evaluated suprachoroidal delivery of AAV8 using conventional 30-gauge needles, and demonstrated widespread green fluorescent protein (GFP) expression across various cell types in rats, pigs, and NHPs.85 No major adverse inflammatory reactions were noted, although all three NHPs showed elevated NAbs at 21 days after injections despite being seronegative at baseline.85 Yiu et al. employed transscleral microneedles similar to those in human studies, and compared suprachoroidal, subretinal and intravitreal delivery of AAV8 expressing GFP in NHPs with longitudinal imaging, and found that suprachoroidal delivery produced mostly peripheral transgene expression in RPE that was highest after 1 month but subsequently declined at month 3.86 The loss in transgene expression was attributed to chorioretinal infiltration of inflammatory cells which was accompanied by greater humoral and cellular responses to the GFP transgene, whereas intravitreal injection of the same vector and dose produced minimal transgene expression but greater sequestration to the spleen and stronger antibody responses to the AAV8 capsid.87 Interestingly, GFP expression after suprachoroidal vector delivery appears to persist in scleral fibroblasts at month 3. Together, these studies suggest that the greater trabecular outflow of viral vectors from the vitreous cavity to the bloodstream and spleen can trigger stronger host immune responses to the AAV capsid, compared with the slower uveoscleral outflow of virus from the suprachoroidal space. However, the greater transgene expression in scleral tissues outside the blood-retinal barrier after suprachoroidal delivery could also elicit a greater immune response to the transgene (see Figure 2).
Figure 2. Routes of viral vector delivery.
Effects of different routes of viral vector delivery on transgene expression, egress to circulation and immune response.
4.3. Transgene and Promoter
Beside the type of viral vector and route of delivery, the specific transgene and promoter88 may also contribute to ocular inflammatory and immune responses. For example, GFP is a foreign protein to the host, and is intrinsically linked to immunogenicity and cytotoxicity.89 Although fluorescent proteins enable in vivo visualization of transgene expression, they are prone to generating oxidative damage when expressed at high levels. 89 For example, comparison studies have shown that retinal expression of GFP is more toxic that retinoschisin, a mammalian protein.90
Interestingly, different promoters can also impact retinal toxicity after viral transduction. Xiong et al. found that AAV vectors with broadly-active promoters such as cytomegalovirus immediate-early promoter (CMV), human ubiquitin C promoter (UbiC), chicken beta actin promoter (CAG), and RPE-specific Bestrophin promoter (Best1 or VMD2), resulted in dose-dependent retinal inflammation, toxicity, and function loss, whereas vectors with photoreceptor-specific promoters, including human red opsin (RedO), human rhodopsin (Rho), human rhodopsin kinase (RK), and mouse cone arrestin (CAR) were not toxic.88 This pattern of toxicity was found across various capsid types including AAV8, AAV5 and engineered capsids, and did not depend on the transgene, as AAV-CMV-null displayed similar toxicity as AAV8-CMV-GFP.88 The authors hypothesized that RPE and/or microglia are the primary activators of this immune-mediated retinal toxicity, although the precise mechanism remains elusive.88
Despite these concerns, voretigene neparvovec-rzyl (Luxturna) has been FDA-approved with use of the CAG promoter, with no clear evidence of toxicity reported in clinical trials.91 Similarly, phase 1 studies using an AAV2 with VMD2 promoter for MERTK mutation-related retinitis pigmentosa (RP) have not shown major adverse effects.92,93 Local immune dysregulation or impaired RPE function may account for this relative sparing, but additional studies are needed to investigate the relative safety of different promoter and transgenes for retinal gene therapy.
5. Ocular Inflammation in Retinal Gene Therapies
5.1. Preclinical Studies
Pre-clinical and clinical studies demonstrate a viral dose-dependent relationship with host immune responses and intraocular inflammation, depending on the route of administration (see Table 1). Intravitreal injection of AAV2 expressing sFlt1 in NHPs demonstrated mild to moderate, though self-resolving, vitreous inflammation in the high dose group (2.4 x 1010 vg) but not in the low dose group (2.4 x 109 vg).94 Intravitreal delivery of a tyrosine-mutant variant of AAV2 to express human retinoschisin 1 (rAAV2tYF-CB-hRS1) in macaques also showed anterior and posterior inflammatory responses at the 4 x 1010 and 4 x 1011 vg doses with development of serum anti-AAV antibodies and evidence of mononuclear infiltrates on histological examination, but no adverse effects on electroretinography or visual evoked potentials.95
Table 1.
AAV vector, promoter and doses used in preclinical and clinical studies.
| AAV vector |
Promoter | Gene | Dose-range without inflammation (vg/eye) |
Dose-range with inflammation (vg/eye) |
Reference | |
|---|---|---|---|---|---|---|
| Intravitreal studies | ||||||
| NHP | AAV2 | Chicken β-actin | sFLT01 | 2.4 x 109 | MacLachlan et al 2011 | |
| AAV8 | Arrestin 3 | CNGA3 | 1 x 1012 | -- | Reichel et al 2017 | |
| AAV8 | CMV | GFP | 7 x 1012 | -- | Chung et al 2020 | |
| Human | AAV2 | CMV | ND4 | 1 x 1010 - 2 x 1010 | -- | Wan et al 2016 |
| AAV2 | Chicken β-actin | sFLT01 | 2 x 108 - 6 x 109 | 2 x 1010 | Heier et al 2017 | |
| AAV8 | RS1 | RS1 | 1 x 109 | 1 x 1010 - 1 x 1011 | Cukras et al 2018 | |
| AAV2 | CMV | ND4 | -- | 9 x 109 - 1.8 x 1011 | Bouquet et al 2019 | |
| AAV.7m8 | CMV | anti-VEGF | -- | 2 x 1011 - 6 x 1011 | Khanani et al 2020 | |
| Subretinal studies | ||||||
| Rat | AAV8 | CB7 | GFP | 1.2 x 107 | -- | Ding et al 2019 |
| Dog | AAV5 | PR0.5 | CNGB3 | 1.53 x 1014 | -- | Komáromy et al 2010 |
| AAV5 | 3LCR-PR0.5 | CNGB3 | 1.73 x 1012 - 7.48 x 1012 | -- | Komáromy et al 2010 | |
| AAV5 | PR2.1 | CNGB3 | 8.47 x 1010 - 9.39 x 1012 | 9.43 x 1012 - 1.65 x 1013 | Komáromy et al 2010 | |
| AAV2/5 | mOP500 | GFP | 3.27 x 109 - 3.27 x 1010; 3.27 x 1012 | 3.27 x 1011 | Beltran et al 2010 | |
| AAV2/5 | hGRK1 | GFP | 1.51 x 109 - 1.51 x 1010 | 1.51 x 1011 - 1.51 x 1012 | Beltran et al 2010 | |
| AAV2/5 | CBA | GFP | 4.79 x 109 - 4.79 x 1011 | 4.79 x 1012 | Beltran et al 2010 | |
| AAV5 | PR2.1 | CNGB3 | 7.5 x 109 - 1 x 1011 | 5 x 1011 - 1.1 x 1013 | Ye et al 2017 | |
| AAV2tYF | GRK1 | RPGR | 8.4 x 109 - 4.2 x 1010 | 2.1 x 1011 | Dufour et al 2020 | |
| NHP | AAV2 | CMV | GFP | -- | 1 x 1010 - 1 x 1011 | Vandenberghe et al 2011 |
| AAV8 | CMV | GFP | 1 x 108 - 1 x 1010 | 1 x 1011 | Vandenberghe et al 2011 | |
| AAV2 | VMD2 | MERTK | 1 x 1010 - 5 x 1010 | -- | Ghazi et al 2016 | |
| AAV2tYF | PR1.7 | CNGB3 | -- | 1.2 x 1011 - 1.2 x 1012 | Ye et al 2016 | |
| AAV8 | Arrestin 3 | CNGA3 | -- | 1 x 1011 - 1 x 1012 | Reichel et al 2017 | |
| AAV8 | CPK850 | RLBP1 | 3.3 x 106 - 3.3 x 107 | 3.3 x 108 - 3.3 x 109 | MacLachlan et al 2017 | |
| AAV8 | CMV | GFP | -- | 7 x 1012 | Chung et al 2020 | |
| Human | AAV2 | CBA | RPE65 | 5.96 x 1010 - 17.88 x 1010 | -- | Jacobson et al 2012 |
| AAV2/2 | RPE65 | RPE65 | 1 x 1010 | 1 x 1011 | Bainbridge et al 2015 | |
| AAV | CMV | sFLT01 | 1 x 1010 - 1 x 1011 | -- | Rakoczy et al 2015 | |
| AAV2 | VMD2 | MERTK | 5.96 x 1010 - 17.88 x 1010 | -- | Ghazi et al 2016 | |
| AAV | CMV | sFLT01 | 1 x 1011 | -- | Constable et al 2016 | |
| AAV2/4 | RPE65 | RPE65 | 1.22 x 109 - 4.8 x 109 | -- | Le Meur et al 2018 | |
| AAV2 | CAG | REP1 | 1 x 1010 | 1 x 1011 | Xue et al 2018 | |
| AAV2 | Chicken β-actin | REP1 | -- | 1 x 1011 | Dimopoulos et al 2018 | |
| AAV2 | Chicken β-actin | REP1 | 1 x 1011 | -- | Lam et al 2019 | |
| AAV2 | Chicken β-actin | RPE65 | 1.5 x 1011 | -- | Maguire et al 2019 | |
| AAV2 | Chicken β-actin | REP1 | 1 x 1011 | -- | Fischer et al 2019 | |
| Suprachoroidal Studies | ||||||
| Rat | AAV8 | CB7 | GFP | 1.2 x 107; 2.85 x 109 | -- | Ding et al 2019 |
| Pig | AAV8 | CB7 | GFP | 4.75 x 1010 | -- | Ding et al 2019 |
| NHP | AAV8 | CB7 | GFP | 4.75 x 1010 | -- | Ding et al 2019 |
| NHP | AAV8 | CMV | GFP | -- | 7 x 1011 - 7 x 1012 | Chung et al 2020 |
Like intravitreal injections, subretinal delivery of AAV vectors also exhibit dose-dependent inflammation, but generally shows less immunogenicity at similar vector doses. In NHPs, Vandenberghe’s team compared subretinal injection of AAV2 and AAV8 to express GFP with dose-ranging studies from 1 x 108 to 1 x 1011 vg. They found that serum NAbs were correlated with degree of GFP expression, and were mostly detected at viral titers of 1010 vg or greater.96 NAbs were also detected in the AC, but only in animals receiving the highest dose (1 x 1011 vg) of either serotype.96 Similar doses of subretinal AAV2 (1 x 1010 - 5 x 1010 vg)91 did not elicit ocular inflammation in preclinical NHP studies for rAAV2-VMD2-hMERTK for treatment of MERTK-associated RP, but subretinal delivery of AAV8 for CNGA3-based achromatopsia (1 x 1011 – 1 x 1012 vg)97 and AAV2tYF expressing human CNGB3 (1.2 × 1011 – 1.2 × 1012 vg)95 were associated with posterior segment inflammation in NHPs. AAV also triggered intraocular inflammation in dogs, with subretinal AAV5 expressing CNGB3 showing multifocal retinitis from 5 × 1011 – 1.65 x 1013 vg,16,98 AAV2 expressing RPGR showing inflammation at 2.1 x 1011 vg,99 and a comparison of AAV2/5 expressing GFP under 3 different promoters (mOP, hGRK1, and CBA) all showing ocular inflammation and retinal toxicity at the highest doses (1.51 x 1011 – 4.79 x 1012 vg).100 Interestingly, in one study by MacLachlan et al., subretinal AAV8 carrying the human RLBP1 gene triggered ocular inflammation in NHPs at doses as low 3.3 x 108 vg, with photoreceptor thinning and reduced ERG signal,101 highlighting the variability of immune responses between these preclinical studies.
Because the suprachoroidal space is outside the blood-retinal barrier, this ocular compartment may not afford the same degree of immune privilege as the subretinal or even vitreous space. In NHPs, suprachoroidal AAV8 expressing GFP triggered AC cells, vitritis, and peripheral chorioretinitis that resolved after a 2-week oral course of steroids, while intravitreal injections of the same vector and dose showed no notable intraocular inflammation.87 An important drawback of animal studies is that expression of human transgenes or proteins such as GFP are foreign to the host and could contribute to ocular inflammation regardless of the viral vector. Moreover, the host immune responses in these species may differ from humans. For example, while prevalence of NAbs against AAV2 are higher than AAV7, AAV8, or AAV9 in humans,102,103 the prevalence NAbs against AAV7, AAV8, and AAV9 are much higher in NHPs.104 Thus, evaluating inflammatory and immune responses in human studies, using vectors that express therapeutic human proteins, may provide greater insight on the safety of these treatments.
5.2. Human clinical trials
In an early phase 1 dose-escalation study evaluating the safety of intravitreal AAV2-sFlt01 for neovascular AMD (2 x 108 - 2 x 1010 vg), two patients who received the highest dose developed intraocular inflammation that resolved with topical steroids, and anti-AAV2 antibodies developed in most of the higher-dose cohorts.20 A more recent phase 1 trial using the novel AAV.7m8 vector to express the anti-VEGF agent aflibercept (2 x 1011 – 6 x 1011 vg) also showed ocular inflammation across all cohorts, despite the use of perioperative steroids.105
In human trials with X-linked retinoschisis (XLRS) patients, intravitreal AAV2-RS1 demonstrated dose-dependent intraocular inflammation which responded to topical and oral steroids. An increase in serum NAbs noted in all groups except for the lowest dose (1 x 109 vg), and substantial titers against AAV8 (1:320–1:2,560) noted in the highest dose (1 x 1011 vg), despite being screened for the absence pre-existing NAbs.17 Patients with Leber’s Hereditary Optic Neuropathy (LHON) who received 1.0 – 2.0 x 1010 vg intravitreal AAV2 expressing the NADH dehydrogenase subunit 4 (ND4) gene demonstrated no NAbs after 6 months.106 A later phase 1/2 dose-escalation clinical study with the same vector up to 1.8 x 1011 vg demonstrated vitritis in all patients. No dose response relationship was noted between viral dose or NAb levels and the degree of inflammation.107 Although the threshold for host immune responses to intravitreal AAV delivery varies between vector, disease, and study, less inflammation is generally noted at <1x1010 vg.
The majority of past and ongoing gene therapy clinical trials employ subretinal injections, which generally exhibit less inflammatory and immune responses. In the phase I study of 15 patients with the RPE65 form of LCA who received subretinal AAV2 therapy up to 17.78 x 1010 vg by Jacobson and colleagues, all patients demonstrated anti-AAV2 antibodies at baseline, but a majority showed no increase in antibody titers greater than two-fold up to 3 years after treatment.108 One patient experienced three-fold increase at day 14 which returned to baseline at day 90, but spiked again at year 2, presumably due to re-exposure to wild type AAV.108 Although some patients had pre-existing T cell memory, no increase in response was noted in any patient.108 In another phase I/II trial of 12 patients with RPE65 mutations in the United Kingdom, Bainbridge et al. noted inflammatory or immune responses in 5 out of 8 patients who received the higher dose (1 x 1011vg), while none were noted in the 4 who received the lower dose (1 x 1010vg) after subretinal injections.109 One patient developed mild anterior uveitis and focal pigmentary changes in the macula, with sustained visual acuity reduction of 15 letters, and was found to exhibit AAV2 NAbs and some AAV2-specific T cell response at 4 weeks, although the other participants with intraocular inflammation were largely asymptomatic and showed no sustained functional impact.109 In a later study by Le Meur et al. which evaluated both subretinal AAV2 and AAV4 for RPE65-related LCA in 9 patients, no intraocular inflammation was noted up to 4.8 x 109 vg.110 Long-term safety data from phase 3 studies of the FDA-approved voretigene neparvovec-rzyl by Maguire et al. showed no adverse immune or inflammatory responses out to 4 years.9
A series of clinical trials have also been conducted employing subretinal AAV2 delivery of REP-1 for choroidermia. In Xue et al., 1 in 14 patients who received the higher 1 x 1011vg dose developed significant retinal inflammation that was attributed to vector reflux into the vitreous cavity.10 In the Alberta study by Dimopoulos and colleagues, 1 in 6 subjects receiving the same dose showed localized intraretinal inflammation, loss of photoreceptor layer, and significant visual decline, which upon review of the surgical video, was noted to have developed intraretinal and subretinal hemorrhage during the injection procedure, as well as introduction of subretinal air bubbles.11 A later study using microscope-integrated optical coherence tomography (OCT) in 6 patients using the same vector and dose showed improved safety profile, with only one patient showing mild vitreous cell and one showing mild AC cell, both of which resolved within 1 week of treatment.12 The most recent phase 2 study of 6 patients did not report any notable adverse effects.13
Other recent human trials using subretinal AAV injections include the use of rAAV2-VMD2-hMERTK for MERTK-associated RP which showed no inflammation or toxicity up to 2 years despite some patients developing a rise in anti-AAV2 antibodies,92 as well as rAAV.sFLT-1 for neovascular AMD, in which patients did not exhibit significant intraocular inflammation, and only 3 of 9 seronegative patients developed NAbs that did not appear to impact protein production.21,22 Together, these studies confirm that vector delivery to the subretinal space may be less likely to trigger ocular inflammation or a pronounced host immune response.
6. Other Treatment Emergent Adverse Effects
Beside intraocular inflammation, TEAEs of retinal gene therapy may also be related to the injection procedure itself. Intravitreal injections are commonly performed by retinal specialists and are generally well tolerated. The risk of endophthalmitis after intravitreal injections are generally low with proper antiseptic techniques.111 Patients with XLRS who received intravitreal injections of rAAV8 noted minimal pain and subconjunctival hemorrhage associated with the procedure.17
Unlike intravitreal injections,111 subretinal viral vector delivery typically requires pars plana vitrectomy, where risks include elevated intraocular pressure (IOP), glaucoma, hypotony, endophthalmitis, hemorrhage, retinal detachment, cataract progression, epiretinal membrane formation, and macular hole formation. Although most gene therapy clinical trials used 23-gauge vitrectomy systems, smaller 25- or 27-gauge vitrectomy instruments may provide lower rates of surgical complications.5
Delivery of the viral vectors requires the insertion of a 39- to 41-gauge cannula through the retina to access the subretinal space. This can be accomplished in 2 steps with a “pre-bleb” of balanced salt solution, or by direct subretinal injection. A saline pre-bleb theoretically minimizes inadvertent vector injection into the vitreous or choroid, but passage of a second cannula may stretch the retinotomy causing vector reflux into the vitreous also. Use of intraoperative OCT and automated injectors may enhance the accuracy and precision of surgical technique and prevent complications.12 A novel subretinal injection device that is deployed from a flexible cannula passed through the suprachoroidal space may overcome the risk of vitreous spillage altogether.112,113
In the clinical trials leading to the approval of voretigene-neparvovec-rzyl, 68% of patients experienced ocular TEAEs, most of which were related to the surgery itself.9 One patient had loss of foveal function due to the procedure, and one had elevated IOP with optic atrophy in setting of endophthalmitis. Seven (18%) patients developed cataracts, 7 (18%) had elevated IOP, 3 (8%) experienced ocular inflammation, of which one was endophthalmitis, 2 (5%) with epiretinal membrane, 1 (3%) with choroidal hemorrhage, and other patients reported eye pain, irritation and pruritus.9 A meta-analysis of 164 eyes of 82 patients with RPE-associated LCA across 6 prospective clinical trials revealed central retinal thinning of 19.2 μm in treated eyes at 2-3 years post-treatment as compared to controls, which may be attributed to the temporary retinal edema or detachment caused by subretinal injection.114
In the choroideremia trials, TEAEs besides ocular inflammation include retinal stretch during surgery and subsequent retinal thinning in 1 patient,9 and intravitreal, intraretinal and subretinal hemorrhage that accompanied the introduction of subretinal air bubbles.10 The use of intraoperative OCT in a later trial helped investigators identify preexisting retinal thinning and avoid excess foveal stretching and macular hole formation.12
In other gene therapy trials for IRDs, 1 in 6 patients who received rAAV2-VMD2-hMERTK developed a shallow foveal detachment without macular hole formation that persisted until 6 weeks.92 Filamentary keratitis, oscillopsia and cataract progression were also reported in the other patients. In a phase I/II study of 18 X-linked RP patients, no severe ocular TEAEs were reported, though mild corticosteroid-responsive inflammation related to viral vector, hemorrhage, ocular hypertension, pain, corneal abrasion and suture granuloma were noted. Similar minor procedure related adverse outcomes were noted in 9 patients with CNGA3 mutation-related achromatopsia.115
7. Mitigation Strategies
7. 1. Mitigation of Humoral Immune Responses
One approach employed in clinical trials to avoid complications of pre-existing immunity is to screen for and exclude patients with pre-existing NAbs. However, due to the endemic nature of AAV and high seroprevalence of NAbs, this strategy may exclude a large portion of patients.68,116 AAV vectors isolated from other species such as the AAV hybrid rh32.33 isolated from rhesus macaques exhibit low prevalence of NAbs among human subjects.116 Methods such as directed evolution may also be used to engineer AAV capsids to evade antibody neutralization,117 although such strategies may result in altered tropism and still be affected by antibody cross-reactivity.
Another approach to evade the humoral response is to co-administer empty AAV capsids to competitively inhibit NAbs. Mingozzi et al. showed that inclusion of empty capsids reduces neutralizing activity of NAbs and increases genetic transduction in a dose-dependent manner.118 To address the higher antigen load and presentation on MHC class I molecules, they mutagenized the AAV capsid so that it could not enter the cell, but noted that other modes of engulfment of capsid such as pinocytosis may result in eventual antigen presentation and immunogenicity.118
Use of immunosuppressive agents to target B cells and plasma cells has also been used to limit humoral responses.119 Anti-CD20 treatment was shown to be partially effective in reducing AAV NAbs in some rheumatoid arthritis patients,120 but the use of immunosuppression must be weighed against potential risks and side effects. Plasmapheresis may also help reduce serum NAb levels,121 although this type of therapy does not completely eliminate T cell responses unless combined by T cell-directed immunosuppressive agents.
7.2. Mitigation of Cell-Mediated Immune Responses
Few clinical trials exclude patients for pre-existing T cell immunity due to the limited sensitivity of methods for detection. Assays to identify antigen-specific T cells from peripheral blood mononuclear cells (PBMCs) may not detect T cells that circulate at very low frequencies.122 An approach used in hepatic gene transfer involves the engineering of a hyperactive variant of the transgene, which would allow the vector dose to be reduced and thus less likely to stimulate a T cell response.123 This approach may not be feasible for some forms of retinal gene therapy, however, where the transgene may not encode a catalytic protein.
Immunosuppressive agents such as proteasome inhibitors can decrease T cell activation and proliferation, decrease antigen presentation and enhance AAV transduction.72 Although immunosuppression can reduce cytotoxic responses, it can also downregulate anti-inflammatory regulatory T cell responses.124 Novel AAV capsids could be engineered to minimize antigen presentation but may still be limited by immune cross reactivity.49
8. Conclusion
Gene therapy using viral vectors such as AAV has been shown to be effective for many IRDs and some acquired conditions such as AMD. However, host immune responses, ocular inflammation, and other TEAEs continue to pose barriers to widespread adoption. A variety of factors contribute to the safety of each therapeutic platform. Different viral capsids determine cellular tropism, but also immunogenicity. The route of administration to different ocular compartments determines local and systemic biodistribution and exposure of viral or transgene product to immune surveillance. Degenerative retinal conditions may compromise blood-ocular barriers depending on the severity of the condition, while certain promoters and transgenes may contribute to immunogenicity and retinal toxicity. Variations in study design, patient population, surgical techniques, and detection assays for immune responses contribute to the difficulty of cross-study comparisons.125 Nevertheless, our mounting experience across past and ongoing clinical trials is providing greater insight into host immunity, ocular inflammation, and technical challenges of gene delivery. Innovations in engineering novel vectors, surgical instrumentation, and mitigation strategies will help provide a path to broaden the application of viral gene therapy to treating retinal diseases.
References
- 1.Shirley JL, de Jong YP, Terhorst C, Herzog RW. Immune Responses to Viral Gene Therapy Vectors. Mol Ther. 2020. March 4;28(3):709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003. Sep-Oct;80(1-2):148–58. [DOI] [PubMed] [Google Scholar]
- 4.Crystal RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. 2014. January;25(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuzbrokh Y, Kassotis AS, Ragi SD, Jauregui R, Tsang SH. Treatment-Emergent Adverse Events in Gene Therapy Trials for Inherited Retinal Diseases: A Narrative Review. Ophthalmol Ther. 2020. December;9(4):709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mays LE, Wang L, Lin J, Bell P, Crawford A, Wherry EJ, Wilson JM. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol Ther. 2014. January;22(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramlogan-Steel CA, Murali A, Andrzejewski S, Dhungel B, Steel JC, Layton CJ. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: Trials, future directions and safety considerations. Clin Exp Ophthalmol. 2019. May;47(4):521–536. [DOI] [PubMed] [Google Scholar]
- 9.Maguire AM, Russell S, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, Marshall KA, McCague S, Reichert H, Davis M, Simonelli F, Leroy BP, Wright JF, High KA, Bennett J. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology. 2019. September;126(9):1273–1285. [DOI] [PubMed] [Google Scholar]
- 10.Xue K, Jolly JK, Barnard AR, Rudenko A, Salvetti AP, Patrício MI, Edwards TL, Groppe M, Orlans HO, Tolmachova T, Black GC, Webster AR, Lotery AJ, Holder GE, Downes SM, Seabra MC, MacLaren RE. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med. 2018. October;24(10):1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulos IS, Hoang SC, Radziwon A, Binczyk NM, Seabra MC, MacLaren RE, Somani R, Tennant MTS, MacDonald IM. Two-Year Results After AAV2-Mediated Gene Therapy for Choroideremia: The Alberta Experience. Am J Ophthalmol. 2018. September;193:130–142. [DOI] [PubMed] [Google Scholar]
- 12.Lam BL, Davis JL, Gregori NZ, MacLaren RE, Girach A, Verriotto JD, Rodriguez B, Rosa PR, Zhang X, Feuer WJ. Choroideremia Gene Therapy Phase 2 Clinical Trial: 24-Month Results. Am J Ophthalmol. 2019. January;197:65–73. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MD, Ochakovski GA, Beier B, Seitz IP, Vaheb Y, Kortuem C, Reichel FFL, Kuehlewein L, Kahle NA, Peters T, Girach A, Zrenner E, Ueffing M, MacLaren RE, Bartz-Schmidt KU, Wilhelm B. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients With Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019. August 29;137(11):1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker MA, Choi D, Erker LR, Pennesi ME, Yang P, Chegarnov EN, Steinkamp PN, Schlechter CL, Dhaenens CM, Mohand-Said S, Audo I, Sahel J, Weleber RG, Wilson DJ. Test-Retest Variability of Functional and Structural Parameters in Patients with Stargardt Disease Participating in the SAR422459 Gene Therapy Trial. Transl Vis Sci Technol. 2016. October 1;5(5):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banin E, Gootwine E, Obolensky A, Ezra-Elia R, Ejzenberg A, Zelinger L, Honig H, Rosov A, Yamin E, Sharon D, Averbukh E, Hauswirth WW, Ofri R. Gene Augmentation Therapy Restores Retinal Function and Visual Behavior in a Sheep Model of CNGA3 Achromatopsia. Mol Ther. 2015. September;23(9):1423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komáromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, Tanaka JC, Acland GM, Hauswirth WW, Aguirre GD. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010. July 1;19(13):2581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, Vijayasarathy C, Marangoni D, Ziccardi L, Kjellstrom S, Park TK, Hiriyanna S, Wright JF, Colosi P, Wu Z, Bush RA, Wei LL, Sieving PA. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol Ther. 2018. September 5;26(9):2282–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyka FM, Boye SL, Chiodo VA, Hauswirth WW, Boye SE. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum Gene Ther Methods. 2014. April;25(2):166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SH, Frick SL, Yiu G. Targeting vascular endothelial growth factor using retinal gene therapy. Annals of Translational Medicine (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heier JS, Kherani S, Desai S, Dugel P, Kaushal S, Cheng SH, Delacono C, Purvis A, Richards S, Le-Halpere A, Connelly J, Wadsworth SC, Varona R, Buggage R, Scaria A, Campochiaro PA. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017. July 1;390(10089):50–61. [DOI] [PubMed] [Google Scholar]
- 21.Constable IJ, Pierce CM, Lai CM, Magno AL, Degli-Esposti MA, French MA, McAllister IL, Butler S, Barone SB, Schwartz SD, Blumenkranz MS, Rakoczy EP. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine. 2016. December;14:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakoczy EP, Lai CM, Magno AL, Wikstrom ME, French MA, Pierce CM, Schwartz SD, Blumenkranz MS, Chalberg TW, Degli-Esposti MA, Constable IJ. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015. December 12;386(10011):2395–403. [DOI] [PubMed] [Google Scholar]
- 23.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010. October 7;363(15):1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011. September 19;2:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014. September 25;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hösel M, Broxtermann M, Janicki H, Esser K, Arzberger S, Hartmann P, Gillen S, Kleeff J, Stabenow D, Odenthal M, Knolle P, Hallek M, Protzer U, Büning H. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012. January;55(1):287–97. [DOI] [PubMed] [Google Scholar]
- 27.312. Innate Immune Response towards Adeno-Associated Viral (AAV) Vectors in Human Liver Non-Parenchymal Cells. Molecular therapy. 2010;18:S120–S120. [Google Scholar]
- 28.Bucher K, Rodríguez-Bocanegra E, Dauletbekov D, Fischer MD. Immune responses to retinal gene therapy using adeno-associated viral vectors - Implications for treatment success and safety. Prog Retin Eye Res. 2020. October 15:100915. [DOI] [PubMed] [Google Scholar]
- 29.Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, Wilson JM. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013. July;123(7):2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayandharan GR, Aslanidi G, Martino AT, et al. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy [retracted in: Proc Natl Acad Sci U S A. 2017 Dec 26;:]. Proc Natl Acad Sci U S A. 2011;108(9):3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119(8):2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudres M, Ciré S, Vasseur V, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther. 2012;20(8):1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lugrin J, Martinon F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol Rev. 2018. January;281(1):99–114. [DOI] [PubMed] [Google Scholar]
- 34.Shao W, Earley LF, Chai Z, Chen X, Sun J, He T, Deng M, Hirsch ML, Ting J, Samulski RJ, Li C. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight. 2018. June 21;3(12):e120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM, Bartlett JS, Muruve DA. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol. 2008. March;82(6):2727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers GL, Shirley JL, Zolotukhin I, Kumar SRP, Sherman A, Perrin GQ, Hoffman BE, Srivastava A, Basner-Tschakarjan E, Wallet MA, Terhorst C, Biswas M, Herzog RW. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017. June 15;129(24):3184–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forthal D 2015. Functions of Antibodies, p 25–48. In Crowe J, Boraschi D, Rappuoli R (ed), Antibodies for Infectious Diseases. ASM Press, Washington, DC. [Google Scholar]
- 38.Wang L, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandamme C, Adjali O, Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum Gene Ther. 2017. November;28(11):1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy SL, Li H, Zhou S, et al. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver. Mol Ther 2008;16:138–145 [DOI] [PubMed] [Google Scholar]
- 41.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006;108:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzpatrick Z, Leborgne C, Barbon E, Masat E, Ronzitti G, van Wittenberghe L, Vignaud A, Collaud F, Charles S, Simon Sola M, Jouen F, Boyer O, Mingozzi F. Influence of Pre-existing Anti-capsid Neutralizing and Binding Antibodies on AAV Vector Transduction. Mol Ther Methods Clin Dev. 2018. February 13;9:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006. March;12(3):342–7. [DOI] [PubMed] [Google Scholar]
- 45.Sanftner LM, Suzuki BM, Doroudchi MM, Feng L, McClelland A, Forsayeth JR, Cunningham J. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004. March;9(3):403–9. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Zhong L, Li M, Li J, Tran K, Ren L, He R, Xie J, Moser RP, Fraser C, Kuchel T, Sena-Esteves M, Flotte TR, Aronin N, Gao G. Adeno-Associated Virus Neutralizing Antibodies in Large Animals and Their Impact on Brain Intraparenchymal Gene Transfer. Mol Ther Methods Clin Dev. 2018. October 4;11:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012. February 8;4(120):120ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nidetz NF, McGee MC, Tse LV, Li C, Cong L, Li Y, Huang W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol Ther. 2020. March;207:107453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007. April;13(4):419–22. [DOI] [PubMed] [Google Scholar]
- 50.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, Hutnick NA, Betts MR, Kastelein JJ, Stroes ES, High KA. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009. September 3;114(10):2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992. December;11(12):1199–206. [DOI] [PubMed] [Google Scholar]
- 52.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992. January;22(1):165–73. [DOI] [PubMed] [Google Scholar]
- 53.Sugita S, Taguchi C, Takase H, Sagawa K, Sueda J, Fukushi K, Hikita N, Watanabe T, Itoh K, Mochizuki M. Soluble Fas ligand and soluble Fas in ocular fluid of patients with uveitis. Br J Ophthalmol. 2000. October;84(10):1130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998. June 15;160(12):5693–6. PMID: 9637476. [PubMed] [Google Scholar]
- 55.Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993. December;34(13):3579–84. [PubMed] [Google Scholar]
- 56.Wilbanks GA, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1. Evidence that an antigen-specific, ACAID-inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991. April 15;146(8):2610–7 [PubMed] [Google Scholar]
- 57.Kosiewicz MM, Alard P, Streilein JW. Alterations in cytokine production following intraocular injection of soluble protein antigen: impairment in IFN-gamma and induction of TGF-beta and IL-4 production. J Immunol. 1998. November 15;161(10):5382–90. [PubMed] [Google Scholar]
- 58.Jiang LQ, Streilein JW. Immune privilege extended to allogeneic tumor cells in the vitreous cavity. Invest Ophthalmol Vis Sci. 1991. January;32(1):224–8. [PubMed] [Google Scholar]
- 59.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999. November 1;190(9):1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang LQ, Jorquera M, Streilein JW. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Invest Ophthalmol Vis Sci. 1993. November;34(12):3347–54. [PubMed] [Google Scholar]
- 61.Sonoda KH, Sakamoto T, Qiao H, Hisatomi T, Oshima T, Tsutsumi-Miyahara C, Exley M, Balk SP, Taniguchi M, Ishibashi T. The analysis of systemic tolerance elicited by antigen inoculation into the vitreous cavity: vitreous cavity-associated immune deviation. Immunology. 2005. November;116(3):390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998. September;39(10):1823–34. [PubMed] [Google Scholar]
- 63.Zamiri P, Sugita S, Streilein JW. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem Immunol Allergy. 2007;92:86–93. [DOI] [PubMed] [Google Scholar]
- 64.Wenkel H, Streilein JW. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest Ophthalmol Vis Sci. 2000. October;41(11):3467–73. [PubMed] [Google Scholar]
- 65.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, Hauswirth WW. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008. September 24;14:1760–9. [PMC free article] [PubMed] [Google Scholar]
- 66.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76(9):4580–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martino AT, Markusic DM. Immune Response Mechanisms against AAV Vectors in Animal Models. Mol Ther Methods Clin Dev. 2019;17:198–208. Published 2019 December 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruzik A, Fetahagic D, Hartlieb B, Dorn S, Koppensteiner H, Horling FM, Scheiflinger F, Reipert BM, de la Rosa M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol Ther Methods Clin Dev. 2019. June 7;14:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M, Wilson JM. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol. 2009. May 15;182(10):6051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu Y, Song S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc Natl Acad Sci U S A. 2009. October 6;106(40):17158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F, Zhou S, High KA. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010. January;18(1):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gabriel N, Hareendran S, Sen D, Gadkari RA, Sudha G, Selot R, Hussain M, Dhaksnamoorthy R, Samuel R, Srinivasan N, Srivastava A, Jayandharan GR. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Methods. 2013. April;24(2):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, Ertl HC, Muruve DA, Lee B, Herzog RW. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011. June 16;117(24):6459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramachandran PS, Lee V, Wei Z, Song JY, Casal G, Cronin T, Willett K, Huckfeldt R, Morgan JI, Aleman TS, Maguire AM, Bennett J. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum Gene Ther. 2017. February;28(2):154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seitz IP, Michalakis S, Wilhelm B, Reichel FF, Ochakovski GA, Zrenner E, Ueffing M, Biel M, Wissinger B, Bartz-Schmidt KU, Peters T, Fischer MD; RD-CURE Consortium. Superior Retinal Gene Transfer and Biodistribution Profile of Subretinal Versus Intravitreal Delivery of AAV8 in Nonhuman Primates. Invest Ophthalmol Vis Sci. 2017. November 1;58(13):5792–5801. [DOI] [PubMed] [Google Scholar]
- 76.Reichel FF, Peters T, Wilhelm B, Biel M, Ueffing M, Wissinger B, Bartz-Schmidt KU, Klein R, Michalakis S, Fischer MD; RD-CURE Consortium. Humoral Immune Response After Intravitreal But Not After Subretinal AAV8 in Primates and Patients. Invest Ophthalmol Vis Sci. 2018. April 1;59(5):1910–1915. [DOI] [PubMed] [Google Scholar]
- 77.Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015. February;22(2):116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, Hauswirth WW. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008. September 24;14:1760–9. [PMC free article] [PubMed] [Google Scholar]
- 79.Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, Bote E, Grant RL, Golden JA, Narfstrom K, Syed NA, Orlin SE, High KA, Maguire AM, Bennett J. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010. March 3;2(21):21ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016. January 25;10:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emami-Naeini P, Yiu G. Medical and Surgical Applications for the Suprachoroidal Space. Int Ophthalmol Clin. 2019. Winter;59(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yiu G, Pecen P, Sarin N, Chiu SJ, Farsiu S, Mruthyunjaya P, Toth CA. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol. 2014. February;132(2):174–81. [DOI] [PubMed] [Google Scholar]
- 83.Yeh S, Khurana RN, Shah M, Henry CR, Wang RC, Kissner JM, Ciulla TA, Noronha G; PEACHTREE Study Investigators. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology. 2020. July;127(7):948–955. [DOI] [PubMed] [Google Scholar]
- 84.Willoughby AS, Vuong VS, Cunefare D, Farsiu S, Noronha G, Danis RP, Yiu G. Choroidal Changes After Suprachoroidal Injection of Triamcinolone Acetonide in Eyes With Macular Edema Secondary to Retinal Vein Occlusion. Am J Ophthalmol. 2018. February;186:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding K, Shen J, Hafiz Z, Hackett SF, Silva RLE, Khan M, Lorenc VE, Chen D, Chadha R, Zhang M, Van Everen S, Buss N, Fiscella M, Danos O, Campochiaro PA. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest. 2019. August 13;129(11):4901–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yiu G, Chung SH, Mollhoff IN, Nguyen UT, Thomasy SM, Yoo J, Taraborelli D, Noronha G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol Ther Methods Clin Dev. 2020. January 21;16:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung SH, Mollhoff IN, Mishra A, Sin TN, Ngo T, Ciulla T, Sieving PA, Thomasy S, Yiu G. Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes. Hum Gene Ther. 2021. January 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong W, Wu DM, Xue Y, Wang SK, Chung MJ, Ji X, Rana P, Zhao SR, Mai S, Cepko CL. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc Natl Acad Sci U S A. 2019. March 19;116(12):5785–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, Harmon JW, Sun Z. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev Rep. 2016. October;12(5):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khabou H, Cordeau C, Pacot L, Fisson S, Dalkara D. Dosage Thresholds and Influence of Transgene Cassette in Adeno-Associated Virus-Related Toxicity. Hum Gene Ther. 2018. November;29(11):1235–1241. [DOI] [PubMed] [Google Scholar]
- 91.Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008. May 22;358(21):2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, Hou R, Deng WT, Boye SL, Almaghamsi A, Al Saikhan F, Al-Dhibi H, Birch D, Chung C, Colak D, LaVail MM, Vollrath D, Erger K, Wang W, Conlon T, Zhang K, Hauswirth W, Alkuraya FS. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016. March;135(3):327–43. [DOI] [PubMed] [Google Scholar]
- 93.Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, Clément N, Cleaver B, McDoom I, Boye SE, Peden MC, Sherwood MB, Abernathy CR, Alkuraya FS, Boye SL, Hauswirth WW. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013. March;24(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, Malatos S, Dufresne E, Morris J, Calcedo R, Veres G, Scaria A, Andrews L, Wadsworth S. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011. February;19(2):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye GJ, Budzynski E, Sonnentag P, Nork TM, Miller PE, Sharma AK, Ver Hoeve JN, Smith LM, Arndt T, Calcedo R, Gaskin C, Robinson PM, Knop DR, Hauswirth WW, Chulay JD. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-PR1.7-hCNGB3, a Recombinant AAV Vector for Treatment of Achromatopsia. Hum Gene Ther Clin Dev. 2016. March;27(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, Wang L, Castle MJ, Maguire AC, Grant R, Wolfe JH, Wilson JM, Bennett J. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011. June 22;3(88):88ra54. doi: 10.1126/scitranslmed.3002103. Erratum in: Sci Transl Med. 2011 Dec 7;3(112):112er9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reichel FF, Dauletbekov DL, Klein R, Peters T, Ochakovski GA, Seitz IP, Wilhelm B, Ueffing M, Biel M, Wissinger B, Michalakis S, Bartz-Schmidt KU, Fischer MD; RD-CURE Consortium. AAV8 Can Induce Innate and Adaptive Immune Response in the Primate Eye. Mol Ther. 2017. December 6;25(12):2648–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye GJ, Komáromy AM, Zeiss C, Calcedo R, Harman CD, Koehl KL, Stewart GA, Iwabe S, Chiodo VA, Hauswirth WW, Aguirre GD, Chulay JD. Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs. Hum Gene Ther Clin Dev. 2017. December;28(4):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dufour VL, Cideciyan AV, Ye GJ, Song C, Timmers A, Habecker PL, Pan W, Weinstein NM, Swider M, Durham AC, Ying GS, Robinson PM, Jacobson SG, Knop DR, Chulay JD, Shearman MS, Aguirre GD, Beltran WA. Toxicity and Efficacy Evaluation of an Adeno-Associated Virus Vector Expressing Codon-Optimized RPGR Delivered by Subretinal Injection in a Canine Model of X-linked Retinitis Pigmentosa. Hum Gene Ther. 2020. February;31(3-4):253–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beltran WA, Boye SL, Boye SE, Chiodo VA, Lewin AS, Hauswirth WW, Aguirre GD. rAAV2/5 gene-targeting to rods:dose-dependent efficiency and complications associated with different promoters. Gene Ther. 2010. September;17(9):1162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacLachlan TK, Milton MN, Turner O, Tukov F, Choi VW, Penraat J, Delmotte MH, Michaut L, Jaffee BD, Bigelow CE. Nonclinical Safety Evaluation of scAAV8-RLBP1 for Treatment of RLBP1 Retinitis Pigmentosa. Mol Ther Methods Clin Dev. 2017. December 22;8:105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Calcedo R, Wilson JM. Humoral Immune Response to AAV. Front Immunol. 2013. October 18;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010. June;21(6):704–12. [DOI] [PubMed] [Google Scholar]
- 104.Gao G, Alvira MR, Somanathan S, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A. 2003;100(10):6081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khanani Arshad M., Kiss Szilard, Turpcu Adam, Hoang Carol, Osborne Aaron; Phase 1 Study of Intravitreal Gene Therapy ADVM-022 for neovascular AMD (OPTIC Trial). Invest. Ophthalmol. Vis. Sci 2020;61(7):1154. [Google Scholar]
- 106.Wan X, Pei H, Zhao MJ, Yang S, Hu WK, He H, Ma SQ, Zhang G, Dong XY, Chen C, Wang DW, Li B. Efficacy and Safety of rAAV2-ND4 Treatment for Leber's Hereditary Optic Neuropathy. Sci Rep. 2016. February 19;6:21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouquet C, Vignal Clermont C, Galy A, Fitoussi S, Blouin L, Munk MR, Valero S, Meunier S, Katz B, Sahel JA, Thomasson N. Immune Response and Intraocular Inflammation in Patients With Leber Hereditary Optic Neuropathy Treated With Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 2019. April 1;137(4):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012. January;130(1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015. May 14;372(20):1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Meur G, Lebranchu P, Billaud F, Adjali O, Schmitt S, Bézieau S, Péréon Y, Valabregue R, Ivan C, Darmon C, Moullier P, Rolling F, Weber M. Safety and Long-Term Efficacy of AAV4 Gene Therapy in Patients with RPE65 Leber Congenital Amaurosis. Mol Ther. 2018. January 3;26(1):256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stem MS, Rao P, Lee IJ, Woodward MA, Faia LJ, Wolfe JD, Capone A Jr, Covert D, Dass AB, Drenser KA, Garretson BR, Hassan TS, Margherio A, Oh KT, Raephaelian PV, Randhawa S, Sneed S, Trese MT, Yedavally S, Williams GA, Ruby AJ. Predictors of Endophthalmitis after Intravitreal Injection: A Multivariable Analysis Based on Injection Protocol and Povidone Iodine Strength. Ophthalmol Retina. 2019. January;3(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Smet MD, Lynch JL, Dejneka NS, Keane M, Khan IJ. A Subretinal Cell Delivery Method via Suprachoroidal Access in Minipigs: Safety and Surgical Outcomes. Invest Ophthalmol Vis Sci. 2018. January 1;59(1):311–320. [DOI] [PubMed] [Google Scholar]
- 113.Heier JS, Ho AC, Samuel MA, Chang T, Riemann CD, Kitchens JW, Slakter JS, Leiderman YI, Spencer R, Williams GA, Hickson-Curran SB, Keane M, JS Baldassarre; Prelude Study Group. Safety and Efficacy of Subretinally Administered Palucorcel for Geographic Atrophy of Age-Related Macular Degeneration: Phase 2b Study. Ophthalmol Retina. 2020. April;4(4):384–393. [DOI] [PubMed] [Google Scholar]
- 114.Wang X, Yu C, Tzekov RT, Zhu Y, Li W. The effect of human gene therapy for RPE65-associated Leber's congenital amaurosis on visual function: a systematic review and meta-analysis. Orphanet J Rare Dis. 2020. February 14;15(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fischer MD, Michalakis S, Wilhelm B, Zobor D, Muehlfriedel R, Kohl S, Weisschuh N, Ochakovski GA, Klein R, Schoen C, Sothilingam V, Garcia-Garrido M, Kuehlewein L, Kahle N, Werner A, Dauletbekov D, Paquet-Durand F, Tsang S, Martus P, Peters T, Seeliger M, Bartz-Schmidt KU, Ueffing M, Zrenner E, Biel M, Wissinger B. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020. June 1;138(6):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009. February 1;199(3):381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bartel MA, Hwang B, Stone D, Koerber JT, Cuoto L, Mingozzi F, High KA, Schaffer DV. 358. Directed Evolution of AAV for Enhanced Evasion of Human Neutralizing Antibodies. Molecular Therapy. 2012. May 1;20(1): S140. [Google Scholar]
- 118.Mingozzi F, Anguela XM, Pavani G, Chen Y, Davidson RJ, Hui DJ, Yazicioglu M, Elkouby L, Hinderer CJ, Faella A, Howard C, Tai A, Podsakoff GM, Zhou S, Basner-Tschakarjan E, Wright JF, High KA. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013. July 17;5(194):194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miao CH. Advances in Overcoming Immune Responses following Hemophilia Gene Therapy. J Genet Syndr Gene Ther. 2011. December 23;S1:007. [PMC free article] [PubMed] [Google Scholar]
- 120.Mingozzi F, Chen Y, Edmonson SC, Zhou S, Thurlings RM, Tak PP, High KA, Vervoordeldonk MJ. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013. April;20(4):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, Moullier P, Benveniste O, Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011. November;19(11):2084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hui DJ, Edmonson SC, Podsakoff GM, Pien GC, Ivanciu L, Camire RM, Ertl H, Mingozzi F, High KA, Basner-Tschakarjan E. AAV capsid CD8+ T-cell epitopes are highly conserved across AAV serotypes. Mol Ther Methods Clin Dev. 2015. September 30;2:15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cantore A, Nair N, Della Valle P, Di Matteo M, Màtrai J, Sanvito F, Brombin C, Di Serio C, D'Angelo A, Chuah M, Naldini L, Vandendriessche T. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012. November 29;120(23):4517–20. [DOI] [PubMed] [Google Scholar]
- 124.Gernoux G, Wilson JM, Mueller C. Regulatory and Exhausted T Cell Responses to AAV Capsid. Hum Gene Ther. 2017. April;28(4):338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Buck TM, Wijnholds J. Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays. Int J Mol Sci. 2020. June 12;21(12):4197. [DOI] [PMC free article] [PubMed] [Google Scholar]