Abstract
Duvelisib (IPI-145), an oral, dual inhibitor of phosphoinositide-3-kinase (PI3K)-δ and -γ, was evaluated in a Phase 1 study in advanced hematologic malignancies, which included expansion cohorts in relapsed/refractory (RR) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) and treatment-naïve (TN) CLL. Per protocol, TN patients were at least 65 years old or had a del(17p)/TP53 mutation. Duvelisib was administered twice daily (BID) in 28-day cycles at doses of 8–75 mg in RR patients (n = 55) and 25 mg in TN patients (n = 18.) Diarrhea was the most common nonhematologic AE (TN 78%, RR 47%); transaminase elevations the most frequent lab-abnormality AE (TN 33.3%, RR 30.9%); and neutropenia the most common ≥grade 3 AE (RR 44%, TN 33%). The overall response rates were 56.4% for RR patients (1.8% CR, 54.5% PR) and 83.3% for TN patients (all PRs); median response duration was 21.0 months in RR patients but was not reached for TN patients. Based upon phase 1 efficacy, pharmacodynamics, and safety, duvelisib 25 mg BID was selected for further investigation in a phase 3 study in RR CLL/SLL.
1 |. INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the US, accounting for 30% of all leukemias.1 An estimated 20 110 new CLL cases and 4660 CLL deaths will occur in the US in 2017.2
Novel targeted agents such as anti-CD20 antibodies (ofatumumab and obinutuzumab) in combination with chemotherapy, kinase inhibitors (ibrutinib and idelalisib), and the BCL-2 inhibitor venetoclax have expanded therapeutic options in CLL/SLL.3 Despite these advances and the widespread use of chemoimmunotherapy, many patients with CLL eventually relapse.4 The deletion of chromosome 17p (17p deletion) and/or a mutation in TP53 confer a poorer prognosis, with a predicted survival of only 2–3 years.5 Thus, novel and effective oral treatments with acceptable safety profiles are needed.
Duvelisib (also known as IPI-145)6 is an oral, dual inhibitor of PI3K-δ, and PI3K-γ. PI3K-δ is constitutively expressed in hematologic malignancies, and its inhibition reduces the proliferation of various hematologic tumor cells while allowing normal immune cells to survive.7–12 PI3K-γ plays an important role in the differentiation and migration of myeloid cells and T cells in the tumor microenvironment, while PI3K-δ blocks signals from the microenvironment sustaining leukemia and lymphoma cells in a protective niche.13 Administration of duvelisib to mice engrafted with a peripheral T-cell lymphoma patient-derived xenograft resulted in a shift among tumor-associated macrophages from the immunosuppressive M2-like phenotype to the inflammatory, antitumor M1-like phenotype.14 Thus, there are at least three different mechanisms through which dual inhibition of PI3K-δ and -γ isoforms could be active against lymphoid malignancies, including CLL: blockade of cell autonomous mitogenic and survival signaling, disruption of supportive tumor microenvironment juxtacrine interactions, and activation of anti-lymphoma immune responses. Accordingly, duvelisib may provide greater benefit through its dual inhibition of PI3K-δ and -γ than one PI3K isoform alone.15
Dose escalation, maximum tolerated dose (MTD) determination, and safety for the entire enrolled phase 1 study population are described in a separate publication.16 Here we report on the efficacy, safety, and pharmacodynamics of duvelisib in patients with relapsed/ refractory (RR) CLL/SLL and treatment-naïve (TN) CLL enrolled in expansion cohorts of the first clinical study of duvelisib in hematologic malignancies. These data support the ongoing investigation of duvelisib for CLL/SLL in a phase 3 trial.
1.1 |. Patients and methods
1.1.1 |. Study design and treatment
Study IPI-145–02 was a phase 1, open-label, dose-escalation and cohort expansion study in 210 patients with advanced hematologic malignancies.15 Duvelisib was administered as oral capsules twice daily (BID), continuously in 28-day cycles until disease progression or unacceptable toxicity. Dose interruptions and reductions (up to 2) were allowed. Clinic visits occurred weekly during the first three cycles, biweekly during cycles 4 (C4) and C5, monthly during C6–19, and every three cycles thereafter. Within the broader phase 1 study population, two CLL populations were examined in expansion cohorts: 55 patients with RR CLL/SLL were enrolled during dose escalation or sequentially (nonrandomized) into duvelisib 25 or 75 mg BID expansion cohorts depending upon cohort availability, and 18 patients with TN CLL received 25 mg BID. Peripheral blood and bone marrow samples were submitted at screening for central laboratory biomarker analysis, including del17p, TP53 mutation, and IGHV mutational status.
All patients provided signed informed consent. The protocol was approved by site Institutional Review Boards, and the study was conducted according to local and federal regulations and the Declaration of Helsinki.
1.1.2 |. Key enrollment criteria
Eligible patients were required to have a life expectancy >3 months, an ECOG score of 0–2, adequate hepatic (aspartate aminotransferase [AST] and/or alanine aminotransferase [ALT] ≤ 2.5 × upper limit of normal [ULN]; direct bilirubin ≤1.5 × ULN) and renal function (serum creatinine ≤1.5 × ULN). Baseline cytopenias were permitted, as was prior therapy with PI3K or BTK inhibitors. Key exclusions were HIV infection; history of alcohol abuse, chronic hepatitis, or other chronic liver disease; and pregnancy. In addition, TN patients had to be either ≥65 years old or had a documented TP53 mutation or 17p deletion by local laboratory determination prior to or during screening. The protocol was amended in March 2013 to require concomitant Pneumocystis prophylaxis.
1.1.3 |. Efficacy methods
Response was assessed by the investigator on day 1 of cycles 3, 5, and 7, every third cycle from C10–19, and every sixth cycle thereafter, using computed tomography (CT) scans and other relevant clinical data. The International Workshop on CLL (iwCLL) criteria 200817 were utilized for definitions of complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). PR with lymphocytosis was not defined as a response. Bone marrow biopsy and/or aspiration was performed to confirm a CR.
Efficacy was assessed by overall response rate (ORR; CR + PR), time to response, lesion response (≥50% decrease from baseline in the sum of the product of diameters), duration of response (DOR; months from first response to progressive disease or death), progression-free survival (PFS; months from first dose to either progressive disease or death), and overall survival (OS; months from first dose to death).
1.1.4 |. Safety methods
Adverse events (AEs), blood chemistry, and hematology laboratory parameters were monitored at all clinic visits and summarized for all patients who received at least one dose of duvelisib. AE severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
1.1.5 |. Pharmacodynamic methods
Using serum collected from iNHL or CLL patients, multiplex panels of cytokines, chemokines, and matrix metalloproteinases (74 total analytes) associated with the B-cell malignancy tumor microenvironment were evaluated using Luminex xMAP technology (Luminex, Austin, TX). The PD analysis set consisted of patients with iNHL and CLL with a C1D1 predose sample and at least one other sample (C1D8, C2D1, C3D1, or C5D1).
2 |. RESULTS
2.1 |. Demographic and baseline characteristics, disposition, and treatment
Fifty-five (55) RR patients initiated treatment with duvelisib BID at 8 mg (n = 1), 15 mg (n = 2), 25 mg (n = 28), or 75 mg (n = 24). Eighteen (18) TN patients were treated with duvelisib 25 mg BID. Most TN CLL patients were enrolled in 2013, with the last patient enrolled in January 2014.
The median age of RR patients was 66 years (range 42–82); most were male (76%), white (89%), and had a diagnosis of CLL (91%). Nearly all had an ECOG performance status of 0 (24%) or 1 (71%) (Table 1). Over a third (38%) had a grade 3 or 4 cytopenia at baseline. RR patients had a median of four prior systemic therapies, and 78% had stage ≥3 disease at study entry. Prior anticancer therapies were typical of a relapsed population with CLL. Six patients (11%) had previously received a Bruton’s tyrosine kinase inhibitor (BTKi) and one patient had prior idelalisib plus rituximab therapy. The majority of RR patients had high-risk features, including unmutated IGHV status (86%) and TP53 mutation and/or 17p deletion (56%).
TABLE 1.
Patient demographics and baseline characteristics of patients with CLL
| Relapsed/refractory | Treatment-naïve | |||
|---|---|---|---|---|
| Demographics and baseline characteristics | 25 mg BID n = 28 | 75 mg BID n = 24 | All doses n = 55 | 25 mg BID n = 18 |
| Demographics | ||||
| Age (years), median (range) | 66 (42–82) | 66 (51–79) | 66 (42–82) | 74 (49–83) |
| Race, white, n (%) | 24 (85.7) | 22 (91.7) | 49 (89.1) | 18 (100) |
| Sex, male, n (%) | 25 (89.3) | 15 (62.5) | 42 (76.4) | 13 (72.2) |
| Baseline disease status | ||||
| Diagnosis, n (%) | ||||
| CLL | 24 (85.7) | 23 (95.8) | 50 (90.9) | 18 (100) |
| SLL | 4 (14.3) | 1 (4.2) | 5 (9.1) | NA |
| Years from initial diagnosis, median (range) | 9.25 (1.4–20.9) | 7.15 (0.7–17.8) | 8.50 (0.7–20.9) | 2.90 (0.1–9.4) |
| Baseline disease istage 3, n (%) | 23 (85.2)a | 17 (70.1) | 42 (77.8) | 6 (33) |
| Bulky disease (>5 cm lesion) | 13 (46.4) | 13 (65.0)b | 26 (50.9)b | 5(29) |
| Splenomegaly, n (%) | 6 (21.4) | 6 (25.0) | 14 (25.5) | 8 (44.4) |
| ECOG score, 0/1/2, % | 28.6/64.3/7.1 | 16.7/79.2/4.2 | 23.6/70.9/5.5 | 44.4/55.6/0 |
| Cytopenias at baseline | ||||
| Grade 3, n (%) | 8 (28.6) | 5 (20.8) | 13 (23.6) | 2 (11.1) |
| Grade 4, n (%) | 3 (10.7) | 5 (20.8) | 8 (14.5) | 2 (11.1) |
| Previous anticancer therapies | ||||
| No. prior systemic therapies, median (range) | 5.0(1–11) | 4.0 (1–11) | 4.0 (1–11) | NA |
| <6 mo. from last systemic therapy, n (%) | 16 (57.1) | 19 (79.2) | 35 (63.6) | NA |
| Alkylating agent, n (%) | 27 (96.4) | 23 (95.8) | 53 (96.4) | NA |
| Rituximab, n (%) | 27 (96.4) | 23 (95.8) | 52 (94.5) | NA |
| Purine analog, n (%) | 20 (71.4) | 22 (91.7) | 43 (78.2) | NA |
| Anthracycline, n (%) | 7 (25.0) | 3 (12.5) | 12 (21.8) | NA |
| BTKi, n (%) | 2 (7.1) | 4 (16.7) | 6 (10.9) | NA |
| High-risk mutation status | ||||
| TP53 mutation/17p-deletion, n (%)c | 14 (53.8) | 14 (58.3) | 28 (56.0) | 10 (55.5) |
| Unmutated IGHVd | 21 (84.0) | 14 (87.5) | 38 (86.4) | 14 (82.4) |
| Del11q, n (%)e | 6 (31.6) | 8 (57.14) | 14 (40.0) | 6 (37.5) |
Abbreviations: BID, twice daily; BTKi, Bruton’s tyrosine kinase inhibitor; CLL, chronic lymphocytic leukemia; ECOG, Eastern Cooperative Oncology Group; IGHV, immunoglobulin heavy chain variable; NA, not applicable; RR, relapsed/refractory; SLL, small lymphocytic lymphoma; TN, treatment-naïve.
Baseline disease stage not reported for 1 patient on duvelisib 25 mg BID; the incidence is based upon patients with available data.
Bulky disease information at baseline not reported for 4 patients at 75 mg BID, and 4 patients across all doses for RR; the incidence is based upon patients with available data.
Baseline mutation status not reported for 2 patients at 25 mg BID, 3 patients at 75 mg BID, and 5 patients across all doses for RR. For TN, 1 patient had no mutation status reported at baseline. The incidence is based upon patients with available data.
Baseline IGHV mutation status was not available for 3 patients at 25 mg BID, 8 patients at 75 mg BID, and 11 patients across all doses for RR-CLL/SLL, nor for 1 patient with TN-CLL. The incidence is based upon patients with available data
Baseline mutation status was not available for 9 patients at 25 mg BID, 10 patients at 75 mg BID, and 20 patients across all doses for RR-CLL/SLL, nor for 2 patients with TN-CLL. The incidence is based upon patients with available data.
The median age of TN patients was 74 years (range 49–83); 72% were male, and all 18 were white. At baseline, all TN patients had an ECOG performance status of 0 (44%) or 1 (56%), and four (22%) presented with grade 3/4 cytopenia. Similar to RR CLL, unmutated IGHV status and TP53 mutation and/or 17p deletion was reported in the majority of TN patients (82% and 55%, respectively).
Among RR patients, median duration of duvelisib treatment was 24 weeks (range 3–167) and was similar between the 25-mg (25 weeks) and 75-mg (24 weeks) dose cohorts. As of data cutoff, most of the 49 RR patients who discontinued treatment did so due to an AE (n = 20) or progressive disease (n = 19).
Among TN patients, median duration of treatment was 62.3 weeks (range 3–90). As of data cutoff, 10 TN patients (56%) remained on duvelisib, with AEs (n = 6) the most common reason for discontinuation. Of the 18 TN patients, 11 had received greater than 1 year of duvelisib and 7 received greater than 18 months of duvelisib, all of whom were still on treatment as of the data cutoff.
2.2 |. Safety
2.2.1 |. Relapsed/refractory CLL/SLL
Treatment-emergent AEs and investigation abnormalities were generally similar across doses (Table 2A). Most AEs were grade 1–2. Several common AEs (eg, fatigue, pyrexia, cytopenias, pneumonia) are frequently associated with CLL. The most frequent severe (≥grade 3) Aes were neutropenia (43.6%) and thrombocytopenia (18.1%) and did not result in treatment discontinuation. Diarrhea (47.3%) was the most frequent nonhematologic AE, but resulted in only two treatment discontinuations. Severe diarrhea (9.1%) and colitis (5.5%) were reported less frequently, and some cases overlapped. These events were managed by dose interruptions and standard-of-care interventions including antidiarrheal medications and enteric-acting steroids (ie, budesonide). Dose reduction and budesonide treatment were reported for three patients. Median time to onset of diarrhea was 2.6 months (any grade) and 5 months (grade 3); no grade 4 diarrhea occurred. Transaminase increases were the most frequent nonhematologic laboratory abnormality (30.9%), with a median time to onset of approximately 2 months. All six patients with dose interruptions for grade 3/4 transaminase elevations resumed treatment: two at the same dose and four at a reduced dose. Pneumonitis was reported in 5 (9.1%) patients with no relationship to dose. Otherwise, grade 3/4 nonhematologic and investigation AEs were uncommon.
TABLE 2A.
Incidence of adverse events in patients with relapsed/refractory CLL/SLL (>15% of patients overall)
| Duvelisib 25 mg BID | Duvelisib 75 mg BID | All Duvelisib doses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 28) | (n = 24) | (n = 55) | |||||||
| AE | n (%) | n (%) | n (%) | ||||||
| Gradea, n (%) | Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 |
| Hematologic | |||||||||
| Neutropenia | 19 (67.9) | 5 (17.9) | 10 (35.7) | 10 (41.7) | 2 (8.3) | 5 (20.8) | 31 (56.4) | 7(12.7) | 17 (30.9) |
| Anemia | 8 (28.6) | 5 (17.9) | 1 (3.6) | 10 (41.7) | 5 (20.8) | 0 | 19 (34.5) | 11 (20.0) | 1 (1.8) |
| Thrombocytopenia | 6 (21.4) | 2 (7.1) | 3 (10.7) | 6 (25.0) | 0 | 5 (20.8) | 13 (23.6) | 2 (3.6) | 8 (14.5) |
| Febrile neutropenia | 4 (14.3) | 4 (14.3) | 0 | 4 (16.7) | 5 (16.7) | 0 | 9 (16.4) | 9 (16.4) | 0 |
| Nonhematologic | |||||||||
| Diarrhea | 12 (42.9) | 3 (10.7) | 0 | 13 (54.2) | 1 (4.2) | 0 | 26 (47.3) | 5(9.1) | 0 |
| Fatigue | 12 (42.9) | 3 (10.7) | 0 | 8 (33.3) | 2 (8.3) | 1 (4.2) | 21 (38.2) | 5(9.1) | 1 (1.8) |
| Cough | 8 (28.6) | 0 | 0 | 10 (41.7) | 0 | 0 | 19 (34.5) | 0 | 0 |
| Pyrexia | 4 (14.3) | 0 | 0 | 11 (45.8) | 2 (8.3) | 0 | 17 (30.9) | 2 (3.6) | 0 |
| URTI | 9 (32.1) | 1 (3.6) | 0 | 5 (20.8) | 0 | 0 | 15 (27.3) | 1 (1.8) | 0 |
| Arthralgia | 7 (25.0) | 0 | 0 | 6 (25.0) | 0 | 0 | 14 (25.5) | 0 | 0 |
| Decreased appetite | 8 (28.6) | 1 (3.6) | 0 | 5 (20.8) | 0 | 0 | 14 (25.5) | 1 (1.8) | 0 |
| Nausea | 5 (17.9) | 0 | 0 | 7 (29.2) | 1 (4.2) | 0 | 14 (25.5) | 1 (1.8) | 0 |
| Dyspnea | 6 (21.4) | 1 (3.6) | 0 | 7 (29.2) | 2 (8.3) | 0 | 13 (23.6) | 3 (5.5) | 0 |
| Pneumoniab | 9 (32.1) | 4 (14.2) | 1 (3.6) | 12 (50.0) | 8 (33.3) | 0 | 21 (38.2) | 12 (21.8) | 1 (1.8) |
| Edema (peripheral) | 2 (7.1) | 0 | 0 | 7 (29.2) | 0 | 0 | 10 (18.2) | 0 | 0 |
| Rash (maculopapular) | 4 (14.3) | 0 | 0 | 5 (20.8) | 0 | 0 | 10 (18.2) | 0 | 0 |
| Sinusitis | 5 (17.9) | 0 | 0 | 4 (16.7) | 0 | 0 | 10 (18.2) | 0 | 0 |
| Stomatitis | 5 (17.9) | 2 (7.1) | 0 | 5 (20.8) | 1 (4.2) | 0 | 10 (18.2) | 3(5.5) | 0 |
| Vomiting | 2 (7.1) | 0 | 0 | 7 (29.2) | 1 (4.2) | 0 | 10 (18.2) | 1 (1.8) | 0 |
| Investigations | |||||||||
| AST/ALT increased | 9 (32.1) | 3 (10.7) | 0 | 7 (29.2) | 2 (8.3) | 1 (4.2) | 17 (30.9) | 5(9.1) | 1 (1.8) |
| Hypokalemia | 2 (7.1) | 0 | 0 | 7 (29.2) | 2 (8.3) | 2 (8.3) | 10 (18.2) | 2 (3.6) | 3 (5.5) |
| Hypomagnesaemia | 6 (21.4) | 0 | 0 | 3 (12.5) | 0 | 0 | 9 (16.4) | 0 | 0 |
| Hypophosphatemia | 3 (10.7) | 2 (7.1) | 0 | 6 (25.0) | 3 (12.5) | 1 (4.2) | 9 (16.4) | 5(9.1) | 1 (1.8) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, twice daily; URTI, upper respiratory tract infection. Notes: Patients are counted once within each system organ class and preferred term. Percentages are based on the number of patients in each dose group for the All-Treated Analysis Set. Patients with an event in more than 1 grade are counted only once at the maximum grade within each system organ class, and within each preferred term.
AEs were coded using the Medical Dictionary of Regulatory Activities (MedDRA) version 16.1, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Includes the preferred terns of pneumonia (n = 13), lung infection (n = 2), lung infiltration (n = 1), pneumonia Pseudomonas aeruginosa (n = 1), Bronchopulmonary aspergillosis (n = 1), pneumonia aspiration (n = 1), pneumonia Escherichia (n = 1), and pneumonia respiratory syncytial viral (n = 1).
As typically observed in heavily pretreated CLL, infections of all grades were frequently reported (72.7%), with pneumonias (combined terms) (38.2%) and upper respiratory tract infection (URTI) (27.3%) the most common infectious AEs. One patient who enrolled prior to the protocol amendment requiring Pneumocystis prophylaxis experienced Pneumocystis pneumonia and recovered with antimicrobial therapy. A grade 3 cytomegalovirus infection occurred in one patient and one patient had a grade 1 herpes zoster event (ie, shingles).
Twenty patients discontinued duvelisib due to AEs. Pneumonitis (4 patients), pneumonia, stomatitis, diarrhea, and colitis (all 2 patients) were the only AEs that led to treatment discontinuation in more than one patient. Fatal AEs occurred in nine patients. Four were due to disease progression and three were AEs considered unrelated to study treatment: one each due to pneumonia Pseudomonas aeruginosa sepsis, cardiac arrest/respiratory failure, and sepsis. Two deaths were considered related to treatment by the investigator. One patient receiving 25 mg BID discontinued duvelisib after 158 days due to disease progression and died 21 days later due to respiratory syncytial viral pneumonia. Another patient receiving 75 mg BID died due to metabolic acidosis in the setting of sepsis and renal failure after 70 days of duvelisib.
2.2.2 |. Treatment-naïve CLL
The most frequent treatment-emergent AEs were similar to those observed in RR patients (Table 2B). Neutropenia was the most common ≥ grade 3 AE (6, 33.4%). The incidences of all-grade (14, 77.8%) and severe diarrhea events (4, 22.2%) were higher in TN patients, but manageable with dose modifications and resulted in only one treatment discontinuation. Median times to any-grade or severe diarrhea (grade 3, no grade 4) events were 2.6 and 9.7 months, respectively. AST/ALT elevations occurred in 6 (33.3%) patients, three of whom experienced grade-3 events, resulting in one treatment discontinuation. Similar to the RR population, the most common infectious AEs in TN patients were pneumonia and URTI, in 4 (22.2%) patients each. Other than oral Candida and a Grade 1 herpes zoster event in one subject each, no opportunistic infections were reported. Colitis was reported in three patients, with onset at 7.2, 12.4, and 17 months; two of these patients had grade 3 events and discontinued treatment. Two patients experienced pneumonitis, both grade 3. One patient discontinued treatment and recovered; the other recovered following treatment interruption and was still receiving duvelisib 13 months later at the time of data cutoff. Six TN patients discontinued duvelisib due to AEs, with colitis being the only AE resulting in treatment discontinuation in more than one patient. No TN patients experienced fatal AEs.
TABLE 2B.
Incidence of adverse events in patients with treatment-naïve CLL (>20% of patients)
| Duvelisib 25 mg BID | |||
|---|---|---|---|
| AE | (n = 18) | ||
| Gradea, n (%) | Any | Grade 3 | Grade 4 |
| Hematologic | |||
| Neutropenia | 8 (44.4) | 1 (5.6) | 5 (27.8) |
| Anemia | 5 (27.8) | 1 (5.6) | 0 |
| Thrombocytopenia | 4 (22.2) | 1 (5.6) | 1 (5.6) |
| Nonhematologic | |||
| Diarrhea | 14 (77.8) | 4 (22.2) | 0 |
| Cough | 8 (44.4) | 0 | 0 |
| Peripheral edema | 8 (44.4) | 0 | 0 |
| Fatigue | 7 (38.9) | 1 (5.6) | 0 |
| Nausea | 7 (38.9) | 1 (5.6) | 0 |
| Rash | 7 (38.9) | 1 (5.6) | 0 |
| Pyrexia | 6 (33.3) | 0 | 0 |
| Dizziness | 5 (27.8) | 0 | 0 |
| Abdominal pain | 4 (22.2) | 2(11.1) | 0 |
| Alopecia | 4 (22.2) | 0 | 0 |
| Arthralgia | 4 (22.2) | 1 (5.6) | 0 |
| Constipation | 4 (22.2) | 0 | 0 |
| Contusion | 4 (22.2) | 0 | 0 |
| Decreased appetite | 4 (22.2) | 0 | 0 |
| Headache | 4 (22.2) | 0 | 0 |
| Insomnia | 4 (22.2) | 0 | 0 |
| Pneumonia | 4 (22.2) | 1 (5.6) | 0 |
| Pollakiuria | 4 (22.2) | 0 | 0 |
| URTI | 4 (22.2) | 0 | 0 |
| Investigations | |||
| AST increased | 6 (33.3) | 2(11.1) | 0 |
| ALT increased | 6 (33.3) | 3 (16.7) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, twice daily; URTI, upper respiratory tract infection. Notes: Patients are counted once within each system organ class and preferred term. Percentages are based on the number of patients in each dose group for the All-Treated Analysis Set. Patients with an event in more than 1 grade are counted only once at the maximum grade within each system organ class, and within each preferred term.
AEs were coded using the Medical Dictionary of Regulatory Activities (MedDRA) version 16.1, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
2.3 |. Efficacy
2.3.1 |. Relapsed/refractory CLL/SLL
The ORR for all RR patients was 56.4% (31/55), including 1 (1.8%) CR and 30 (54.5%) PRs and was similar between the 25 and 75-mg cohorts (Table 3). Nodal response (≥50% reduction in adenopathy) occurred in 83.3% (40/48) of patients with radiologically assessed baseline disease across dose groups (Supporting Information Figure 1 A). The median time to response was 1.87 months (range 1.6–16.6). A nondose–dependent lymphocytosis following duvelisib initiation was noted in 75% of patients with absolute lymphocyte counts (ALC) returning to baseline around C5D1 (Supporting Information Figure 2 A). Stable disease (SD) was the best response in 19 (34.5%) patients, 6 of whom (32%) achieved a nodal response and met at least one group B criterion but had persistent treatment-related lymphocytosis; 2 (3.6%) patients did not achieve any response. In patients with TP53 mutation or 17p deletion, the ORR was 46.4% (13/28), including one (3.6%) CR. The ORR in patients with unmutated IGHV was 51.4% (18/35) and included the aforementioned CR. One patient who had received idelalisib and rituximab as a prior therapy achieved PR with duvelisib. Among the six patients with disease progression on prior BTKi therapy, one patient achieved a PR with a response duration of approximately 6.5 months, and another patient maintained SD for approximately 16.5 months. One subject had SD at the time of treatment discontinuation (day 117) due to physician’s decision. The three remaining patients discontinued duvelisib for progressive disease at 2, 3, and 4 months, respectively.
TABLE 3.
Overall response rate in patients with CLL
| Relapsed/refractory | Treatment-naïve | |||||
|---|---|---|---|---|---|---|
| Duvelisib dose | 25 mg BID n = 28 | 75 mg BID n = 24 | All doses n = 55 | TP53 mutation/17p deletion All doses n = 28 | 25 mg BID n =18 | TP53 mutation/17p deletion 25 mg BID n = 10 |
| Overall response rate (ORR) (CR + PR) | ||||||
| ORR, n (%) | 16(57.1) | 13 (54.2) | 31 (56.4) | 13 (46.4) | 15 (83.3) | 8 (80.0) |
| 95% confidence interval | (37.2, 75.5) | (32.8, 74.4) | (42.3, 69.7) | (27.5, 66.1) | (58.6, 96.4) | (44.4, 97.5) |
| Best overall response | ||||||
| Complete response (CR), n (%) | 1 (3.6) | 0 | 1 (1.8) | 1 (3.6) | 0 | 0 |
| Partial response (PR), n (%) | 15 (53.6) | 13 (54.2) | 30 (54.5) | 12 (42.9) | 15 (83.3) | 8 (80.0) |
| Stable diseasea n (%) | 10 (35.7) | 8 (33.3) | 19 (34.5) | 12 (42.9) | 2(11.1) | 1 (10.0) |
| Progressive disease, n (%) | 1 (3.6) | 1 (4.2) | 2 (3.6) | 2 (7.1) | 0 | 0 |
| Unknown, n (%) | 1 (3.6) | 2 (8.3) | 3 (5.5) | 1 (3.6) | 1 (5.6) | 1 (10.0) |
Abbreviations: BID, twice daily; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma. Notes: The Clopper-Pearson confidence interval is presented. Patients with no post-baseline response assessment are represented as having an ‘unknown’ response. Percentages are based on the number of patients in each dose group.
Among RR patients with stable disease, 6/19 (32%) had a lymph node response and persistent lymphocytosis, meeting the criteria for partial response for agents causing increased peripheral blood lymphocytes {Cheson, 2012 #2743}.
The median DOR in responders (n = 31) was 21.0 months. The estimated probability of remaining in response at 6, 12, and 24 months was 73.3%, 62.4%, and 31.2%, respectively. Among patients with TP53 mutation or 17p deletion receiving duvelisib 25 mg BID, median DOR was 23.2 months, with a 71.4% estimated probability of remaining in response at 12 months and 18 months.
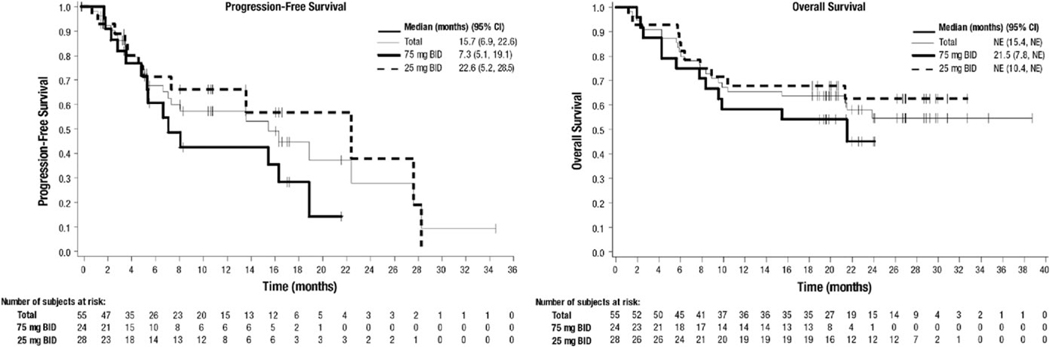
Across all doses, median PFS was 15.7 months (Figure 1). PFS events included 23 disease progressions and three deaths. The estimated probability of being progression-free at 6, 12, and 18 months was 67.7%, 57.1%, and 44.5%, respectively. Among patients with TP53 mutation and/or 17p deletion who received duvelisib 25 mg BID, median PFS was 27.9 months, with an estimated 65.7% and 52.5% probability of being progression free at 12 and 24 months, respectively.
FIGURE 1.
Progression-free survival and overall survival in patients with relapsed/refractory CLL/SLL
The median OS for RR patients was not reached, and the estimated probability of survival at 6, 12, and 18 months was 81.8%, 65.5%, and 63.6%, respectively (Figure 1). The estimated median OS for RR patients with TP53 mutation and/or 17p deletion who received duvelisib 25 mg BID was 21.3 months.
2.3.2 |. Treatment-naïve CLL
The ORR was 83.3% (15/18) including all PRs and was similar among patients with TP53 mutation or 17p deletion (80.0% [8/10]) and unmutated IGHV (85.7% [12/14]) (Table 3). The absence of a postbaseline assessment precluded response determination in one patient, and the remaining two patients (11.1%) had SD as their best response. The median time to response was 3.71 months (range 1.7–8.3). Nodal response (≥50% reduction in adenopathy) occurred in 87.5% (14/16) of patients with radiologically assessed baseline disease (Supporting Information Figure 1B). Similar to RR CLL/SLL, lymphocytosis was observed shortly after duvelisib initiation in 70.6% of TN patients, with return to baseline ALC for the population occurring around C3D1 (Supporting Information Figure 2B).
The median DOR was not reached, with estimated 6- and 12-month probabilities of remaining in response of 100% and 87.5%, respectively. The median PFS and OS were also not reached, with estimated probabilities of remaining progression-free and surviving at 12 months of 94% and 100%, respectively.
2.4 |. Duvelisib pharmacodynamics in CLL
Chemokines, cytokines, and serum factors reflective of CLL tumor cells, the immune system, and the tumor microenvironment were evaluated in an exploratory analysis by collecting blood samples at baseline and at various timepoints during cycles 1–3. Supporting Information Table 1 lists all of the chemokines, cytokines, and serum factors evaluated; 13 of the 74 analytes tested decreased significantly from baseline at cycle 1 Day 8 (C1D8) and displayed ≥50% median change: CCL1, CCL3, CCL4, CCL17, CCL22, CXCL10, CXCL13, IL-6, IL-10, IL-12p40, MMP-9, MMP-12, and TNFα (Supporting Information Figure 3A) with P value <.0018 after Bonferroni correction for the 74 multiple comparisons. All 13 analytes were elevated at baseline in the serum from RR patients compared to healthy donors (n = 33). All but IL-6 were statistically significantly decreased at C2D1 and C3D1 compared to baseline. After 8 days of duvelisib, seven of these analytes were no longer significantly higher than healthy donor samples (data not shown).
Pharmacodynamic data were collected from 17 TN patients (94%); 10 of the 74 serum analytes tested had decreased significantly at C1D8 with ≥50% median change: CCL3, CCL4, CCL17, CCL22, CXCL10, CXCL13, IL-10, IL-12p40, MMP-9, and TNFα (Supporting Information Figure 3B) with P value <.0018 after Bonferroni correction for the 74 multiple comparisons. All analytes except CXCL10 also exhibited significant decreases at C2D1 and C3D1. Only IL-6 changed significantly at C2D1 or C3D1 but not at C1D8; 9 of these 10 analytes (except CXCL10) were elevated at baseline in TN serum compared with healthy donors (n = 33). After 8 days of duvelisib, levels of five of the analytes elevated at baseline were no longer significantly higher than healthy donor samples (data not shown).
3. |. DISCUSSION
Here we present the first demonstration of the anti-leukemic activity and safety of duvelisib in patients with CLL/SLL. Data were collected within a broader phase 1 study in patients with multiple advanced hematologic malignancies, described elsewhere.16
Through dual PI3K-δ and PI3K-γ inhibition, duvelisib targets multiple facets of the pathogenesis and maintenance of CLL, including cell-autonomous survival signaling and growth and migration signaling through supportive cellular interactions and circulating factors provided by the tumor microenvironment.18 Duvelisib demonstrated in vivo pharmacologic activity in CLL cells through rapid, sustained, and maximal inhibition of p-AKT, a downstream marker of PI3K signaling,19 at 25 mg BID. In addition, nearly complete inhibition of CLL cell proliferation, assessed by Ki-67 expression at C2D1, was noted in most RR patients and all TN patients. Neither p-AKT nor Ki-67 inhibition was dose-dependent, with maximal inhibition at 25 mg.16
Significant reductions in chemokines, cytokines, and matrix metalloproteinases expressed by malignant B cells and tumor-supporting myeloid and T cells following duvelisib treatment in RR and TN patients suggest potential CLL tumor microenvironment disruption through dual inhibition of PI3K-δ and PI3K-γ.19–26
The clinical benefit of duvelisib in RR and TN CLL is supported by the induction of durable disease responses. Lymphocytosis was observed in 70–75% of patients shortly after initiating duvelisib and, per recent guidance,27 was not considered progressive disease. In RR CLL patients, ALC returned to baseline levels approximately 4 months into duvelisib treatment, which is shorter than reported for other BCR pathway inhibitors (BCRPIs)28,29 ORRs among all RR patients and the subset with s mutation and/or 17p deletion, 56.4% and 46.4% respectively, were not dose dependent. While PFS appeared to differ between the RR CLL 25 mg BID and 75 mg BID cohorts, interpretation is limited by the phase 1 trial design and conduct, and further accentuated by the small patient numbers within each dose group. Per eligibility criteria, TN CLL patients represented a population for which frontline chemoimmunotherapy would be considered suboptimal. The median age was 74, much older than in most frontline clinical trials of CLL.30 Additionally, the proportion of patients with TP53 mutation and/or 17p deletion (56%) far exceeds the prevalence in the general untreated CLL population and typical frontline CLL therapy trials (5–10%).30 Within this context, the ORR of 83% with 87% of responding patients maintaining response through 12 months is clinically meaningful.
Duvelisib was generally well tolerated in the RR CLL/SLL population, with some patients remaining on treatment for more than 3 years. The safety profile was consistent with the entire phase 1 patient population,16 and characterized by potentially autoimmune toxicities similar to those observed with PI3K-δ inhibitor therapy.31 While there were numerical differences in some individual AE incidences between the 25 mg BID and 75 mg BID dose cohorts, there did not appear to be a definitive dose effect of duvelisib on the overall safety profile in the RR CLL population. Diarrhea (47%) was the most frequently reported nonhematologic event but was managed with dose modifications and supportive-care interventions (eg, antidiarrheals) and rarely resulted in treatment discontinuation. In this report, the incidences of severe diarrhea (9.1%) and colitis (5.5%) are derived from the AE terms reported by investigators and in some cases these events overlapped. Colonoscopy data were not formally collected. Transaminase elevations (25–27%) were mostly low-grade, reversible upon dosing interruptions, and rarely resulted in treatment discontinuation. Hematologic AEs, including grade 3 and 4 events, were also common but expected given baseline cytopenia (grade ≥ 3) in over a third of patients and not preclusive of maintaining therapy.
Infectious complications are a well-recognized major cause of morbidity and mortality in CLL, given the humoral immunodepression inherent to the disease and therapy-induced immunosuppression.32,33 Similar to other BCRPIs, infections, particularly pneumonias and URTIs, were frequently observed and remain an important risk.34,35 Fatal infections were reported in four patients, two of whom previously experienced disease progression. The requirement for prophylaxis added to this study was intended to mitigate the risk for Pneumocystis pneumonia and continues to be implemented in duvelisib clinical trials.
The AE profile in TN was generally similar to RR, although incidences of diarrhea and transaminase elevations were higher. As noted previously, treatment with idelalisib and rituximab resulted in a high incidence of presumed immune-mediated side effects in patients with previously untreated CLL, and was higher in younger patients.36,37 The disruption of Treg signaling via PI3K-δ inhibition and the resultant emergence of a cytotoxic effector T-cell population may account for the autoimmune toxicities with the higher incidence in previously untreated and/or younger patients attributed to a better-preserved population of T cells.37
While differences in study design and treatment exposure preclude direct cross-study comparisons of autoimmune toxicity risk, preclinical data support the rationale that concomitant inhibition of PI3K-γ by duvelisib may mitigate autoimmune complications through maintenance of a safe balance between regulatory and effector T-cell activity. It has been shown that inactivation of PI3K-γ, either through mutations or inhibition by small molecules, impairs the migration of leukocytes from the bloodstream to sites of injury or inflammation; in addition, PI3K-γ is required for optimal T-cell activation and differentiation.38,39 In mouse models of multiple chronic inflammatory diseases, PI3K-γ inhibition or absence abrogated disease activity.38,40–44 While the data from this small cohort suggest a predictable and manageable safety profile in TN patients, additional investigation to elucidate the pharmacologic and clinical sequelae of PI3K-γ inhibition by duvelisib would be informative.
Overall, the clinical and pharmacodynamic activity and manageable safety profile in these heavily pretreated, RR CLL/SLL, and high-risk TN CLL patients highlight the potential of duvelisib as an effective new therapy. These data, together with the data from the overall phase 1 study population, support the selection of 25 mg BID for further clinical development.18 A phase 3 clinical trial (DUO) is ongoing to assess duvelisib as a monotherapy in patients with RR CLL/SLL (NCT02004522).
Supplementary Material
ACKNOWLEDGMENTS
Infinity Pharmaceuticals and Verastem Oncology provided financial support. We would like to thank the investigators, coordinators, nurses, and patients and their families for their contributions. Steven Mousterakis and Justin McLaughlin of Infinity Pharmaceuticals, and Paul Guttry of Acumen Medical Communications, provided graphical and medical writing support.
Funding information
Infinity Pharmaceuticals; Verastem Inc.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
CONFLICT OF INTERESTS
S.O., M.P., B.K., S.H, F.F., P.P., J.J., J.B., N.J., and I.F. declare no competing financial interests. Authors K.A., K.F., M.D., H.M.S., J.S., P.K., and V.K. were employees of Infinity Pharmaceuticals at the time of study conduct.
REFERENCES
- 1.Howlader N, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. [cited April 20, 2014]; http://seer.cancer.gov/csr/1975_2011/ [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results Program, Cancer Stat Facts: Chronic Lymphocytic Leukemia; 2017; https://seer.cancer.gov/statfacts/html/clyl.html
- 3.Patel VM, Balakrishnan K, Douglas M, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199). Leukemia. 2017;31(9):1872–1881. 10.1038/leu.2016.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016; 127(2):208–215. [DOI] [PubMed] [Google Scholar]
- 5.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Am Soci Hematol Educ Book. 2010; 2010(1):481–488. [DOI] [PubMed] [Google Scholar]
- 6.Winkler DG, Faia KL, DiNitto JP, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013;20(11):1364–1374. [DOI] [PubMed] [Google Scholar]
- 7.Billottet C, Banerjee L, Vanhaesebroeck B, Khwaja A. Inhibition of class I phosphoinositide 3-kinase activity impairs proliferation and triggers apoptosis in acute promyelocytic leukemia without affecting ATRA-induced differentiation. Cancer Res. 2009;69(3):1027–1036. [DOI] [PubMed] [Google Scholar]
- 8.Billottet C, Grandage VL, Gale RE, et al. A selective inhibitor of the p110delta isoform of PI 3-kinase inhibits AML cell proliferation and survival and increases the cytotoxic effects of VP16. Oncogene. 2006; 25(50):6648–6659. [DOI] [PubMed] [Google Scholar]
- 9.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman SE, Lapalombella R, Gordon AL, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117(16): 4323–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda H, Hideshima T, Fulciniti M, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116(9): 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meadows SA, Kashishian A, Johnson D, Diehl V, Lannutti B. CAL-101 a potent selective inhibitor of the P1108 isoform of phosphatidylinsitol 3-kinase, attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Poster presented at 52nd Annual Meeting of the American Society of Hematology; 2010 Dec 4; Orlando, FL. [Google Scholar]
- 13.Fruman DA, Rommel C. PI3Kdelta inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 2011;1:562–572. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz SM, Koch R, Porcu P, et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131(8):888–898. 10.1182/blood-2017-08-802470; Epub 2017 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–1822. 10.1038/leu.2015.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887. 10.1182/blood-2017-05-786566 Epub 2017 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. , International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute: Working Group 1996 Guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dil N, Marshall AJ. Role of phosphoinositide 3-kinase p110 delta in TLR4- and TLR9-mediated B cell cytokine production and differentiation. Mol Immunol. 2009;46(10):1970–1978. [DOI] [PubMed] [Google Scholar]
- 19.Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol. 2010;346:31–56. [DOI] [PubMed] [Google Scholar]
- 20.Peluso M, Faia K, Winkler D, et al. Duvelisib (IPI-145) inhibits malignant B-cell proliferation and disrupts signaling from the tumor microenvironment through mechanisms that are dependent on PI3K-δ and PI3K-γ. Blood. 2014;124(21):328.24894774 [Google Scholar]
- 21.Fung-Leung WP. Phosphoinositide 3-kinase delta (PI3Kdelta) in leukocyte signaling and function. Cell Signal. 2011;23(4):603–608. [DOI] [PubMed] [Google Scholar]
- 22.Nashed BF, Zhang T, Al-Alwan M, et al. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol. 2007;37(2):416–424. [DOI] [PubMed] [Google Scholar]
- 23.Soond DR, Bjørgo E, Moltu K, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115(11):2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. [DOI] [PubMed] [Google Scholar]
- 25.Burger JA. Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. Semin Cancer Biol. 2010;20(6):424–430. [DOI] [PubMed] [Google Scholar]
- 26.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30(23):2820–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. 10.1056/NEJMoa1215637; Epub 2013 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. 10.1182/blood-2013-11-535047 Epub 2014 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood.2015; 125(13):2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion, Leuk Lymphoma. 2015;56(10):2779–2786. 10.3109/10428194.2015.1022770 Epub May 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison VA. Infections in patients with leukemia and lymphoma. In: Stosor V, Zembower TR, eds. Infectious Complications in Cancer Patients, Cancer Treatment and Research 161, Switzerland: Springer International Publishing (outside USA); 2014:319–349; 10.1007/978-3-319-04220-6_11 [DOI] [PubMed] [Google Scholar]
- 33.Nosari A. Infectious complications in chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis.2012;4(1):e2012070. 10.4084/MJHID.2012.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imbruvica® [package insert]. Pharmacyclics LLC; 2017. Sunnyvale, CA. https://www.imbruvicahcp.com/now-approved?utm_source=google&utm_medium=cpc&utm_campaign=Imbruvica+HCP+-+Branded+-+2017&utm_content=Package+Insert&utm_term=ibrutinib+package+ insert&gclid=CJrxmfaN7tYCFQWMswodyM4F3Q&gclsrc=ds&utm_source=google&utm_medium=cpc&utm_campaign=Imbruvica+HCP+-+Branded+-+2017&utm_content=Package+Insert&utm_term=ibrutinib+package+insert&gclid=CJrxmfaN7tYCFQWMswodyM4F3Q& gclsrc=ds Accessed October 13, 2017. [Google Scholar]
- 35.Zydelig® [package insert]. Gilead Sciences; 2017. Foster City, CA. https://www.zydelig.com/hcp/about?gclid=CjwKCAjwyIHPBRAIEiwAHPS-GO9hQ_uYlhJPAEVrs5Fc32OmiTII5xp1VpHWcGwkJFDQsQ7Pl3jrFBoC2ZgQAvD_BwE&s-mcid=ps-google-branded-hcp-safety-about&ef_id=V2X4YgAAAXqPdsYg:20171013173203:s Accessed October 13, 2017. [Google Scholar]
- 36.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lampson BL, et al. , editors. Idelalisib given front-line for the treatment of chronic lymphocytic leukemia results in frequent and severe immune-mediated toxicities. Poster and Oral Presentation at the ASH 57th Annual Meeting and Exposition; 2015 Dec 5–8; Orlando, FL. [Google Scholar]
- 38.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25 +Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6598–6602. [DOI] [PubMed] [Google Scholar]
- 39.van Dop WA, Marengo S, te Velde AA, et al. The absence of functional PI3Kgamma prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunol Lett. 2010;131(1):33–39. [DOI] [PubMed] [Google Scholar]
- 40.Ladygina N, Gottipati S, Ngo K, et al. PI3Kgamma kinase activity is required for optimal T-cell activation and differentiation. Eur J Immunol. 2013;43(12):3183–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber DF, Bartolomé A, Hernandez C, et al. Class IB-phosphatidylinositol 3-kinase (PI3K) deficiency ameliorates IA-PI3K-induced systemic lupus but not T cell invasion. J Immunol. 2006;176(1):589–593. [DOI] [PubMed] [Google Scholar]
- 42.Berod L, Heinemann C, Heink S, et al. PI3Kgamma deficiency delays the onset of experimental autoimmune encephalomyelitis and ameliorates its clinical outcome. Eur J Immunol. 2011;41(3):833–844. [DOI] [PubMed] [Google Scholar]
- 43.Camps M, Rückle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11(9):936–943. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Garcia A, Sanchez-Ruiz J, Flores JM, Carrera AC. Phosphatidylinositol 3-kinase gamma inhibition ameliorates inflammation and tumor growth in a model of colitis-associated cancer. Gastroenterology. 2010;138(4):1374–1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.