Abstract
Aim:
To evaluate the preliminary effectiveness of the BRief Evaluation of Asthma THerapy intervention, a 7-min primary care provider-delivered shared decision-making protocol that uses motivational interviewing to address erroneous asthma disease and medication beliefs.
Design:
A multi-centre masked two-arm group-randomized clinical trial.
Methods:
This 2-year pilot study is funded (September 2016) by the National Institute of Nursing Research. Eight providers will be randomized to one of two arms: the active intervention (N = 4) or a dose-matched attention control (N = 4). Providers will deliver the intervention to which they were randomized to 10 Black adult patients with uncontrolled asthma (N = 80). Patients will be followed three months postintervention to test the preliminary intervention effects on asthma control (primary outcome) and on medication adherence, lung function, and asthma-related quality of life (secondary outcomes).
Discussion:
This study will evaluate the preliminary impact of a novel shared decision-making intervention delivered in a real world setting to address erroneous disease and medication beliefs as a means of improving asthma control in Black adults. Results will inform a future, large-scale randomized trial with sufficient power to test the intervention’s effectiveness.
Impact:
Shared decision-making is an evidence-based intervention with proven effectiveness when implemented in the context of labour- and time-intensive research protocols. Medication adherence is linked with the marked disparities evident in poor and minority adults with asthma. Addressing this requires a novel multifactorial approach as we have proposed. To ensure sustainability, shared decision-making interventions must be adapted to and integrated into real-world settings. Trial registration: Registered at clincialtrials.gov as NCT03036267 and NCT03300752.
Keywords: asthma, community–based participatory research, health beliefs, minority, mixed methods, motivational interviewing, nursing, qualitative research, shared decision-making
1 |. INTRODUCTION
More than 339 million people worldwide have asthma, representing a 3.6% increase since 2006 (The Global Asthma Network, 2018). In the USA, prevalence is relatively high among non-Hispanic Blacks (9.1%) and persons living below 100% of the poverty level (11.1%) (Centers for Disease Control and Prevention [CDC], 2016). As many as 50% of adults with asthma fail to achieve disease control (CDC, 2014) with Black adults disproportionately representing that population (Akinbami, Moorman, & Liu, 2011). Additionally, adults die at a rate more than four times that of children (Moorman et al., 2012) and Blacks at nearly three times the rate of Whites (Moorman et al., 2012). Most asthma-related hospitalizations and deaths could be prevented with appropriate self-management that achieves and maintains disease control (Expert Panel Report-3 [EPR-3], 2007). Inhaled corticosteroids (ICS) are a safe first-line treatment for uncontrolled asthma, but ICS non-adherence is common. ICS adherence being higher in whites, the primary cause of uncontrolled and/or fatal asthma (EPR-3, 2007), has been found to be consistently higher among White adults than Blacks: 74% versus 52% (Apter et al., 2003); 55% versus 35% (Krishnan et al., 2001); and 51% versus 29% (Le et al., 2008).
1.1 |. Background
ICS non-adherence among Black patients is due, in part, to a greater preference for non-prescription treatments (e.g., “natural therapies”) (George, Birck, Hufford, Jemmott, & Weaver, 2006; George, Campbell, & Rand, 2009; George et al., 2014) and erroneous medication beliefs (e.g., ICS is addicting) (Apter et al., 2003; George, Freedman, Norfleet, Feldman, & Apter, 2003; George et al., 2006; Le et al., 2008). In prior research we have identified high rates of erroneous asthma beliefs (93%) and negative ICS beliefs (68%) among urban Black adults and demonstrated that erroneous health beliefs were associated with uncontrolled asthma (George et al., 2014) and ICS non-adherence (George et al., 2006).
Federally qualified health centers (FQHC) are a type of primary care setting designated to receive enhanced Medicaid reimbursement because their patients are underserved, underinsured, and uninsured individuals who tend to receive episodic care (Chang, Lewis, Meara, Lurie, & Bynum, 2016). Asthma has a disproportionate impact on FQHC patients, with prevalence as high as 18% and asthma being a top cause of hospitalization (Drake, Lim, Mallya, & Robbins, 2009). FQHCs have unique challenges to achieving asthma control among patients including limited time for primary care providers (PCPs) to evaluate asthma control, to assess ICS non-adherence and to identify erroneous beliefs (US Department of Health & Human Services, 2008). Compared with specialists, PCPs’ patients more commonly have uncontrolled asthma, which is significant given that PCPs deliver most asthma care (Murphy et al., 2012). This speaks to the pressing need for brief interventions that facilitate asthma management in FQHCs.
Good patient-provider communication is universally accepted as foundational for achieving optimum asthma outcomes (Global Initiative for Asthma, 2018). One approach, Shared Decision-Making (SDM), is an effective communication strategy that improve health behaviours (Stiggelbout, Pieterse, & De Haes, 2015) by facilitating discussion of the risks and merits associated with specific options in the context of patients’ goals and preferences in a manner that activates patients to engage in disease self-management. Patients and PCPs jointly consider options, reconciling differences and reaching agreed on higher-quality decisions that more closely align patients’ needs with evidence-based care (Street, Makoul, Arora, & Epstein, 2009).
To date, SDM interventions have focused on improving ICS adherence, quality of life, and disease control among children and White privately insured adults, using highly trained interventionists who intercede over multiple lengthy sessions (Kew, Malik, Aniruddhan, & Normansell, 2017). This approach, while effective, is difficult to translate into primary care where interventions must be brief and are sustainable only if they can be integrated into routine visits.
Motivational interviewing (MI) is a patient-centred counselling approach that elicits behavioural change by helping patients explore and resolve ambivalence towards change by engaging them in collaborative partnerships, focusing on connecting behaviour to outcomes, evoking the individual’s internal motivation to change and planning a course of action (Miller & Rollnick, 2007). While the effectiveness of brief SDM interventions using MI has been established in acute care settings (D’Onofrio, Pantalon, Degutis, Fiellin, & O’Connor, 2005; D’Onofrio et al., 2012; Pantalon et al., 2013), its translation to FQHCs has not been tested.
2 |. THE STUDY
2.1 |. Aims
To address this significant gap, we developed the BRief Evaluation of Asthma THErapy (BREATHE) intervention, a single session, brief (7-min) PCP-led SDM intervention that uses MI to address erroneous asthma beliefs and negative ICS beliefs associated with ICS non-adherence. This study will (a) evaluate the feasibility and acceptability of intervention procedures and (b) assess the preliminary evidence of intervention effects on asthma control (primary outcome), ICS adherence, lung functioning, and asthma-related quality of life (secondary outcomes) over a 3-month follow-up period. To accomplish this, we will conduct a pilot clinical trial with 80 Black adult patients with uncontrolled asthma from two FQHCs who are treated by eight PCPs (10 patients per PCP). We hypothesize that BREATHE will be feasible and acceptable as evidenced by: (a) high rates of PCP fidelity to the intervention protocol; (b) high satisfaction ratings by patients, patients’ loved ones, and PCPs; and (c) improvements over three months in outcomes in patients receiving the BREATHE intervention relative to controls.
2.2 |. Design
2.2.1 |. Conceptual framework
An adaptation of Satterfield et al. (2009) Model of Transdisciplinary Evidence-Based Practice (Figure 1), guides this study. In this model, SDM is defined as the nexus of patient preferences, research evidence, and clinician expertise. We operationally define research evidence as the patient-reported outcomes of asthma control and asthma quality of life and by lung functioning, objectively measured. Patient preference is defined as ICS adherence, beliefs (disease and medications). Furthermore, clinician expertise is operationalized as the delivery of BREATHE using SDM and MI techniques. These evidence-based practices are considered in the context of the FQHC.
FIGURE 1.
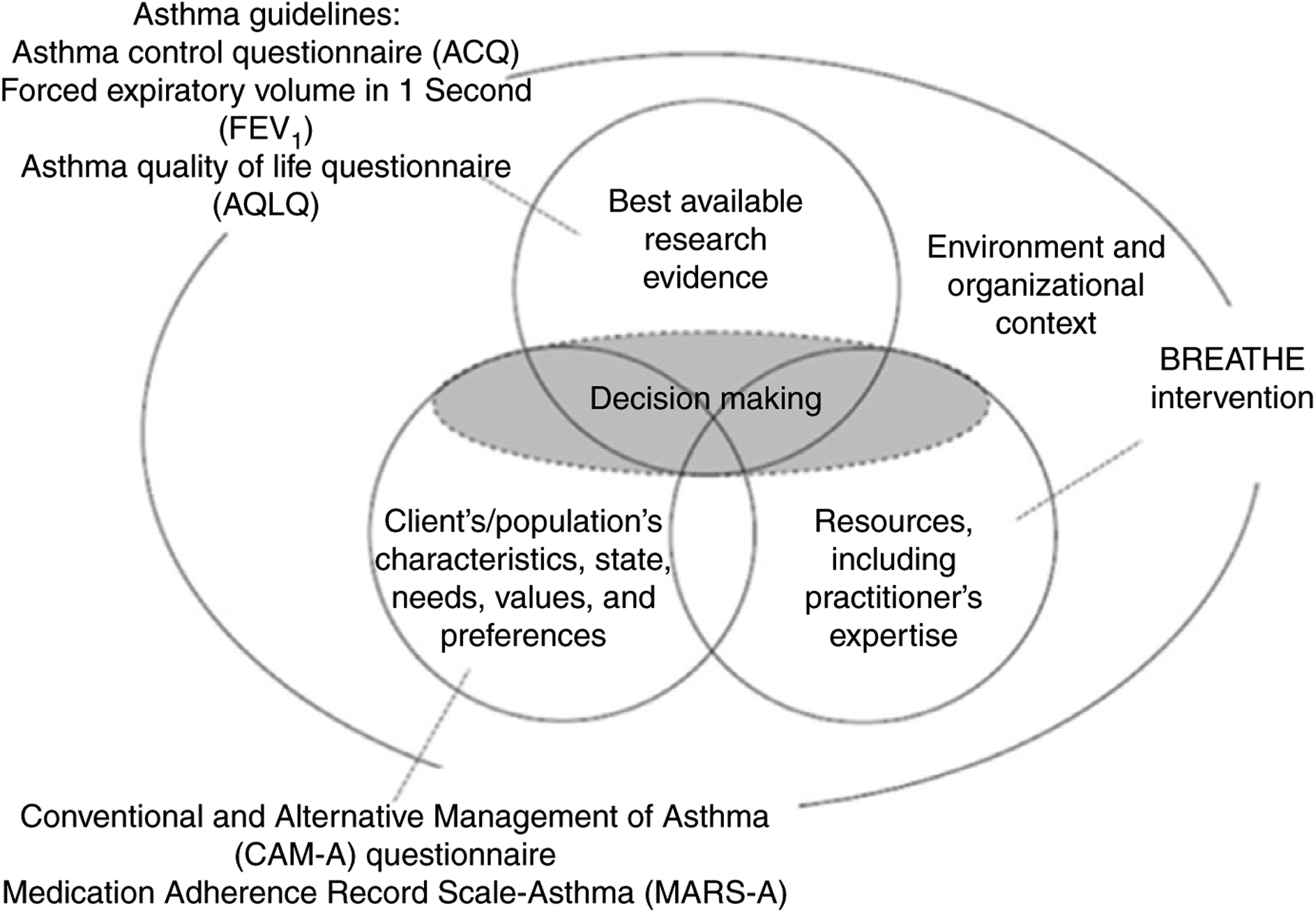
Satterfield et al. (2009) revised model of transdisciplinary evidence-based practice for the BREATHE intervention
2.2.2 |. Study design overview
We will conduct a pilot clinical trial with eight PCPs from two urban FQHCs. Using a group-randomization method, we will randomize PCPs to one of two interventions: the BREATHE intervention or a dose-matched attention control condition. We will enroll 10 Black adult patients with uncontrolled persistent asthma per PCP (N = 80, 40 per intervention arm); patients will complete assessments monthly for three months postintervention. Data are analysed at the patient level. To evaluate intervention satisfaction, we will conduct postintervention interviews with patients, their loved ones, and PCPs. This clinical trial will allow for estimation of parameters crucial for a larger randomized control trial (RCT) including final content specification, patient and PCP recruitment rates, and effect sizes.
2.3 |. Setting
This study will be conducted in two unrelated FQHCs in Philadelphia, each serving the same zip codes with the highest asthma burden in the city. Patients are largely uninsured, receiving Medicaid, or dually eligible (Medicare/Medicaid) with ~95% self-reporting race as non-Hispanic Black.
2.4 |. PCP sample
All PCPs with responsibility for the medical management of adult asthma patients will be eligible for randomization. At one site four physicians and two nurse physicians (NPs) were eligible to be randomized and at the second site three physicians and three NPs were eligible.
2.5 |. Patient sample
We will enroll 80 FQHC patients (10/PCP), aged 18+ who self-identify as Black with: (a) persistent asthma defined as PCP-diagnosed asthma requiring ICS; (b) uncontrolled asthma assessed by the Asthma Control Questionnaire (ACQ) (Juniper, O’Byrne, Guyatt, Ferrie, & King, 1999); and (c) erroneous asthma beliefs and/or ICS beliefs measured by the Conventional and Alternative Management for Asthma (CAM-A) instrument (George et al., 2014) (see Outcomes section for details on these instruments). Exclusion criteria include patients who are non-English speakers; have serious mental health conditions (e.g., psychosis) that preclude completing study procedures; and participated in focus groups that informed the intervention.
2.6 |. Patient recruitment
We will recruit patients using methods we have used successfully in our prior work (i.e., flyers and referrals by the FQHC staff members) (George et al., 2014), and electronic health record searches. For the latter, both sites will generate a list of potentially eligible patients using a combination of ICD-10 (Asthma 493) queries filtered for provider, age, race, and ICS prescription. We will mail these patients opt-out letters indicating three options: call the study team if interested; call the FQHC administrator to indicate that they are not to be contacted; or do nothing. As specified in the letter, if patients choose to do nothing study personnel will call them two weeks later to ascertain interest in the study and screen for eligibility.
2.7 |. Two-step screening for eligibility
Using an institutional review board (IRB)-approved telephone consent script, the study team will screen interested patients for uncontrolled asthma using the ACQ (Juniper et al., 1999). Those with uncontrolled asthma will be scheduled for an office visit to complete the CAM-A. Those who endorse at least one erroneous asthma beliefs will be eligible for enrollment at which time written consent will be obtained.
2.8 |. Sample size
We performed a power analysis to estimate the sample size needed to detect significant differences between BREATHE and control patient participants on the primary outcome, asthma control. Power calculations were based on: (a) four repeated observations/patient over the three-month follow-up with repeated measures correlation of ρ = 0.8; (b) intra-cluster correlation among PCPs = 0.2 to account for clustering of patients from the same PCP; and (c) linear mixed models to estimate mean score differences. On the basis of our prior studies, we estimated 10% attrition over the 3-month postintervention period. With a sample size of 10 patients per PCP and four PCPs in the intervention group and four in the control group (total N = 10 × 4 × 2 = 80) and 10% attrition, we calculated the reliability of estimated mean score differences between the two groups. For the ACQ, the half-width of 95% CI for the estimated mean score difference between the two groups is 0.32 (assuming SD 0.76). This is equivalent to having 91% power to detect a medium effect size (Cohen’s d = 0.5) on the ACQ which is clinically meaningful.
2.9 |. Procedures
2.9.1 |. Randomization
We will randomize in equal numbers eight PCPs (four/FQHC site) into BREATHE or the dose-matched attention control condition stratified for provider type (physician vs. NPs). Remaining PCPs will be placed on a standby list that will be used to replace PCPs who decline to enroll or who drop-out. Our biostatistician will create computer-generated randomization lists in advance.
2.9.2 |. Masking
Patients, data collectors, data managers, and the statistician will be blinded to assignment.
2.10 |. Intervention conditions
2.10.1 |. BREATHE intervention
BREATHE is a one-time, brief (7-min), tailored SDM intervention session that uses MI techniques delivered by PCPs at the time of a medical visit to address erroneous asthma and medication beliefs underlying ICS non-adherence. To develop BREATHE, we adapted an evidence-based acute care setting SDM intervention (D’Onofrio et al., 2005, 2012; Pantalon et al., 2013). We first identified intervention components to retain for tailoring to asthma in the FQHC setting population using six focus groups at the two FQHCs (three/FQHC) that included 32 Black adult patients with persistent asthma and 14 patients’ loved ones using conventional community-based participatory research approaches (Minkler, 2005). Last, four expert reviewers (2 NPs, a respiratory therapist, and our patient co-investigator) provided feedback on the intervention components identified for adaptation by the focus groups.
PCPs deliver BREATHE during a medical visit using four steps: raise the subject (role of ICS non-adherence in uncontrolled asthma); provide feedback; enhance motivation using MI techniques and assess readiness to change (Figure 2); and advise and negotiate treatment options with patients (SDM). To increase fidelity to the intervention protocol, PCPs are guided by a script on a tablet that automatically populates the script with patients’ ACQ scores and CAM-A responses allowing PCPs to tailor the session to each patient. The tablet also houses optional data including statistics on the association of ICS non-adherence with hospitalizations and/or death. The PCP also has access to the results of office spirometry performed that day. In case of tablet failure, or if the PCP prefers, the BREATHE steps are also summarized on a single-page laminated pocket guide. Table 1 provides a condensed version of the script.
FIGURE 2.
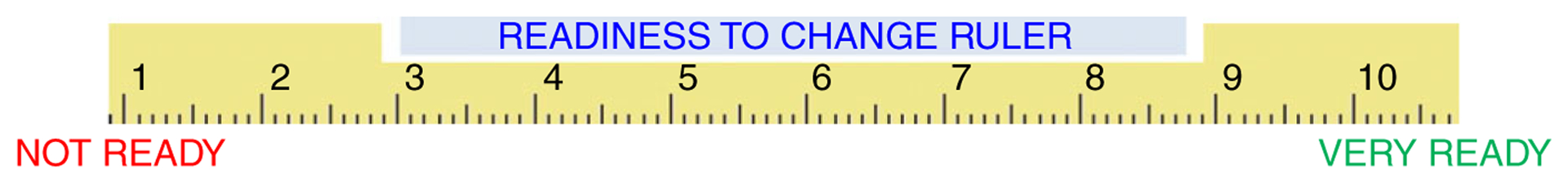
Readiness ruler
TABLE 1.
Abbreviated script of the BREATHE intervention
| Step | Objectives | Script and actions | Time (min) |
|---|---|---|---|
| 1. Raise the subject | PCP establishes rapport by asking the patient’s permission to discuss their asthma control and health beliefs | “Would you mind taking a few minutes to talk with me about your asthma control and your use of asthma controller medicine? (insert BRAND NAME ICS)” | 1.5 |
| 2. Provide feedback | PCP provides feedback to the patient based on assessments of the patient’s uncontrolled asthma and erroneous health/ICS beliefs, drawing a connection between current symptoms and ICS non-adherence. The PCP also asks the patient to make their own connections between non-adherence and current and future symptoms/outcomes. Medically accurate connections are reinforced via reflective listening by the PCP | “From what I see here your asthma is not in control.” (REVIEW ACQ SCORE) “What connection (if any) do you see between how you use your asthma controller medicine (insert BRAND NAME ICS) and having asthma that is not in control?” Show measures of asthma status again and discuss relationship between suboptimal ICS, asthma control, and risk for hospitalization/death |
1.5 |
| 3. Enhance motivation | The PCP attempts to enhance the patient’s motivation to increase ICS adherence using MI techniques such as assessing his/her level of readiness to change and reasons/motives for any readiness, as well as empathy, concern, and acceptance of ambivalence about increased ICS adherence. In this step, the PCP may elicit the patient’s beliefs regarding the benefits, and negative sequelae, of their current self-management approach (pros/cons) | (Show Readiness Ruler [Figure 2]) “Based on what we just discussed, how ready might you be to increase your (insert BRAND NAME ICS) use, on a scale from 1–10, where 1 means not at all ready and 10 means totally ready?” If patient says: ≥2, ask “Why did you choose that number and not a lower one?” (Be ready to explain why you’re asking this.) If patient says: <1 or unwilling, ask- What would it take for you to become a “2”? PROBES: What would make this a problem for you? How important would it be for you to prevent that from happening? Have you not been able to do something you wanted to because of your asthma? Reflect/reiterate patients reasons for making a change |
2 |
| 4. Negotiate and advise (shared decision-making) | The PCP and patient jointly consider treatment options. The PCP will actively attempt to build consensus around ICS adherence, reconciling conflicts to better align health beliefs with evidence-based guidelines. For example, if the patient uses ICS intermittently rather than twice-daily because of a fear that tolerance will develop, then the PCP will attempt to counter that belief using responses gleaned from the focus groups, as well as from national guidelines. This may include encouraging ICS use once a day as an initial short-term plan to be followed by a return visit and re-evaluation. If the patient declines to engage in SDM or declines attempts at negotiating ICS use, then the PCP and the patient agree to disagree | “What might be your next step, if any?” “If you can take more (25%; 50%) of your (insert BRAND NAME ICS) dose you will be less likely to have a serious asthma attack.” “This is what I’ve heard you say, you have agreed to…. (state actual amounts of ICS use) because you want/it will lead to…” (summarize their most motivational reasons for change). “I also suggest that you remind yourself every day of these reasons and even add new ones to the list. This is an agreement between you and yourself because we know that only you can decide it is important enough to you to make these changes.” Suggest follow-up to discuss asthma control and ICS use. Ask the patient if they have any questions and when questions have been addressed, thank patient for his/her time |
2 |
PCP: primary care provider; ICS: inhaled corticosteroid; ACQ: asthma control questionnaire.
2.10.2 |. Attention control condition
The control condition is a 7-min unscripted healthy lifestyle (e.g., diet, exercise) discussion. Control PCPs use the tablets to time the discussion. Additionally, they have access to the same patient data that the BREATHE providers have: spirometry, ACQ, and CAM-A scores, as well as the optional statistics. While control PCPs are trained on where to locate this data, they do not receive training on how to use it or on any BREATHE intervention components.
2.10.3 |. Intervention fidelity
We will follow the NIH Treatment Fidelity Workgroup recommendations for training and implementation standardizations and plan to assess consistency in dose in the active and control conditions (Bellg et al., 2004). We will ensure intervention fidelity using several methods. In addition to BREATHE being a manualized intervention, we will ensure consistency across PCPs by implementing manualized training procedures and having a team member review intervention visits audiotapes; these steps are detailed below.
2.10.4 |. PCP training
We will train PCPs randomized to BREATHE using a structured training curriculum adapted from our prior research (D’Onofrio et al., 2005, 2012; Pantalon et al., 2013). The core training will consist of one 2-hr session: 30 min of didactic instruction addressing intervention delivery; 60 min of role-playing clinical scenario; 50 min of SDM skills training (e.g., creating a trusting environment, relinquishing sole decision-making, motivating, and empowering patients to engage in self-management decisions); and a 10-min question and answer period.
Prior to delivering BREATHE in the trial, PCPs will have to demonstrate proficiency in a standardized patient scenario to evaluate if she/he can accurately deliver the intervention components in 7 min or less. A team member (AAN) will assess the PCP using the Brief Negotiated Interview Adherence (BNI) Scale, a validated scale; a score >75% indicates proficiency. If proficiency is not achieved, PCPs will receive additional instruction and be re-evaluated by the trainer. Prior studies have shown that such re-training results in 97% proficiency (Pantalon et al., 2012).
Because we are randomizing PCPs in each FQHC, there is the chance of contamination. We will minimize the risk of contamination by: (a) training only BREATHE PCPs to deliver the active intervention; and (b) encouraging PCP confidentiality regarding training and intervention content.
2.10.5 |. Delivery consistency of BREATHE
To assess PCPs adherence to the intervention protocol, we will audio record visits and then review recording using the BNI Scale. Written feedback, free of patient identifiers, will be returned to PCPs by email within 24 hr of each visit. If there is fidelity drift (BNI Scale score <75%), additional training will be conducted prior to the next study-related medical visit.
2.10.6 |. Delivery consistency of the control condition
The study team will audio record control visits and review them to determine the content and length of the healthy lifestyle discussions (e.g., diet, exercise) and if contamination occurred (i.e., BREATHE components used). Written feedback, free of patient identifiers, will be returned to PCPs by email within 24 hr of each visit.
2.11 |. Data collection
Trained study staff members, masked to study condition, will collect assessments at baseline and 1, 2, and 3 months postintervention. All data were collected at the FQHCs. Baseline data are collected on the same day as the medical visit where the patient receives the BREATHE intervention or control condition. Because we anticipate that patients will have low health literacy, study staff members will read all survey questions and answers to the patient, requiring about 30 min to complete. To address potential assessment reactivity, both groups will receive an equal number of assessments.
2.12 |. Outcomes, variables, and measurement instruments
Measures will be collected as noted in Table 2. The primary outcome is asthma control and the secondary outcomes are medication adherence, lung function, and asthma quality of life. All primary and secondary measures have published evidence of reliability and validity in adult asthma populations. Permission has been obtained from all survey copyright holders.
TABLE 2.
Timeline of study measurement
| Phone screening | In-person screening | Baseline | 1-month | 2-month | 3-month | |
|---|---|---|---|---|---|---|
| Patient measures | ||||||
| ACQ (Juniper et al., 1999) | X | X | X | X | ||
| CAM-A (George et al., 2014) | X | X | ||||
| AQLQ (Juniper et al., 1993) | X | X | X | X | ||
| MARS-A (Mora et al., 2011) | X | X | X | X | ||
| FEV1 (clinical data) | X | X | X | X | ||
| NVS (Weiss et al., 2005) | X | |||||
| SDMQ-9 (Kriston et al., 2010) | X | X | ||||
| PROMIS-29 (“Validation, nD) | X | |||||
| Asthma diary | Dispensed | X | X | X | ||
| Doser™ | Dispensed | X | X | X | ||
| Demographics and asthma history | X | |||||
| Patient debriefing and masking assessment | X | |||||
| CSQ-8 (Larsen et al., 1979) | X | |||||
| PCP measures | ||||||
| PCMI (Norful et al., 2018) | X | |||||
| PCP debriefing | X | |||||
| Demographics and work history | X |
Note. ACQ: asthma control questionnaire; CAM-A: conventional and alternative management for asthma; AQLQ: asthma quality of life questionnaire; MARS-A: medication adherence record scale—asthma; FEV1: forced expiratory volume in one second; NVS: newest vital sign; SDMQ-9: shared decision-making questionnaire-9; PROMIS-29: patient-reported outcome measurement information system-29; CSQ-8: Client satisfaction questionnaire-8; PCMI: patient co-management index.
2.13 |. Patient measures—primary outcome
2.13.1 |. ACQ
The ACQ is a 6-item (nighttime and daytime symptoms, breathlessness, wheeze, activity limitations, rescue medication use) 7-point Likert scale survey reported as a mean (range 0–6); lower scores indicate better control (Juniper et al., 1999). The ACQ has a positive predictive value of 88% in identifying uncontrolled asthma in clinical trials (Juniper, Bousquet, Abetz, & Bateman, 2006).
2.14 |. Patient measures—secondary outcomes
2.14.1 |. Asthma quality of life questionnaire (AQLQ)
The AQLQ, a 32-item 7-point Likert scale survey, represents four domains: symptoms (11 items); emotions (5 items); environment (4 items); and activities (12 items). Total mean score and domain means (range 1–7) are reported; higher scores represent less impairment (Juniper, Guyatt, Ferrie, & Griffith, 1993).
2.14.2 |. Medication Adherence Record Scale-Asthma (MARS-A)
The MARS-A is a 10-item 5-point Likert scale survey of ICS adherence reported as a total mean score (range 10–50) with higher scores indicating higher adherence (Mora et al., 2011). The MARS-A correlates with objective ICS adherence as measured by electronic monitoring (Clatworthy, Price, Ryan, Haughney, & Horne, 2009) and with pharmacy refills (Cohen et al., 2009).
2.14.3 |. Forced expiratory volume in 1 s (FEV1)
The FEV1, an objective measurement of lung function, is the volume of air blown out in the first second of maximum exhalation. A FEV1 < 80% may indicate airway obstruction (Miller et al., 2005).
2.15 |. Other patient measurements
2.15.1 |. CAM-A
The CAM-A is a 17-item 7-point Likert scale survey comprised of two subscales: erroneous asthma beliefs and negative ICS beliefs. A summary score is calculated for each subscale (range 0–9 for negative ICS beliefs and 0–6 for inaccurate asthma beliefs; 2 items not scored) with higher scores indicating more erroneous asthma beliefs and/or more negative ICS beliefs. Higher scores are associated with uncontrolled asthma (George et al., 2014).
2.15.2 |. Newest vital sign (NVS)
The NVS, a 6-item health literacy/numeracy test, evaluates the ability to read and apply information from an ice cream nutrition label (Weiss et al., 2005).
2.15.3 |. SDM
The SDM Questionnaire-9 (SDMQ-9) (Kriston et al., 2010) is a 9- item 6-point Likert scale instrument to evaluate patients’ perspective of the degree to which a provider uses SDM during medical visits. Higher scores indicate greater SDM.
2.15.4 |. Patient-reported outcomes measurement information system-29 (PROMIS-29)
The PROMIS-29 assesses anxiety, depression, fatigue, pain interference and intensity, physical function, sleep disturbance, and social roles and activities (Health Measures, n.d.). It will be used to capture co-morbid conditions in this trial.
2.15.5 |. Asthma dairy
An investigator-developed daily asthma diary, written at a 4th grade reading level, will capture asthma symptoms and self-reported ICS use.
2.16 |. Doser™
The Doser™, an electronic monitor capable of recording number of puffs/day, will be attached to compatible ICS MDIs. Due to unique delivery systems for ICS, there is no single electronic device to monitor ICS use from dry powder inhalers. Therefore, we will only objectively measure ICS adherence in those prescribed an ICS MDI.
2.17 |. Demographics and asthma history
Demographics include age, sex, educational level, and employment. Asthma history includes age of diagnosis, history of life-threatening asthma, and current asthma medication use.
2.18 |. Patient debriefing and masking assessment
All patients regardless of treatment condition will complete an open-ended survey to capture perceptions of the PCP visit, immediately following the visit. Patients will also be asked to guess if they were assigned to the active or control condition.
2.19 |. Provider measures
2.19.1 |. Provider co-management
The Provider Co-Management Index (PCMI) (Norful, Ye, Shaffer, & Poghosyan, 2018) captures how well PCPs co-manage the same patient. It is a 20-item survey with three subscales measuring effective communication, mutual respect and trust, and shared philosophy of care. Each item is scored on a scale from 1 (Strongly Disagree) to 4 (Strongly Agree). Higher total scores indicate greater co-management.
2.19.2 |. PCP debriefing
PCPs will complete an open-ended survey to capture their perception of the visit regardless of whether they are delivering the active or control condition.
2.19.3 |. Demographics and work history
Demographics include age, sex, race/ethnicity. Work history includes years in practice and number of asthma patients seen weekly.
2.20 |. Study process outcomes—patients
2.20.1 |. Recruitment and retention
Our success in recruiting and retaining patient participants will be documented, including time to achieve recruitment goals, number of missed visits, and attrition.
2.20.2 |. Patient satisfaction
Patient satisfaction will be measured with the Client Satisfaction Questionnaire-8 (CSQ-8) (Larsen, Attkisson, Hargreaves, & Nguyen, 1979), an 8-item measurement of patients’ perspectives of the value of treatment received. Scores range from 8 to 32, with higher values indicating higher satisfaction.
2.20.3 |. Patient feedback (acceptability and satisfaction with trial components)
Consistent with Qualitative Descriptive methodology, we will use maximum variation sampling (Suri, 2011) to purposively select participants to interview who have a range of patient experiences: five patients who completed the trial and five who did not. The PI will conduct the interviews.
2.20.4 |. Study process outcomes—satisfaction of patients’ loved ones
We will purposively select five loved ones of patients who completed the trial and five loved ones of patients who did not. The PI will interview them to assess perceptions of the effects of study participation on their loved ones’ asthma management and barriers to participation.
2.21 |. Study process outcomes—providers
2.21.1 |. Recruitment and retention
Our success in recruiting and retaining PCPs will be documented, including their accepting assignment (active and control) and completing the study.
2.21.2 |. Satisfaction
Five PCPs who completed the trial and up to five who declined to enroll, or who drop out, will be selected for in-depth interviews using methods above.
2.21.3 |. Quantitative data management
Double-data entry will be made into a secured electronic database, REDCap (Harris et al., 2009).
2.21.4 |. Qualitative data management
All interviews will be audio recorded; deidentified verbatim transcripts will be stored in a qualitative data management program, Atlas.ti (n.d.).
3 |. ANALYTIC METHODS
3.1 |. Quantitative
Data analysis in the context of a feasibility trial will balance two primary goals: (a) estimation of effect size and (b) statistical significance testing. Our primary goal is to examine intervention feasibility, which will guide modifications of design elements for a future RCT, if warranted. We will first examine the data distribution and check for outliers using descriptive statistics. Next, we will proceed with hypothesis testing.
We will document BREATHE’s penetration using PCP recruitment and retention data. The process evaluation will include descriptive statistics on PCPs’ intervention proficiency and fidelity (e.g., content and length) and will assess site and discipline (e.g., NP vs. physician) differences. If we find variability, we will explore potential causes (e.g., FQHC size).
Intervention effects will be assessed using linear regression models. All hypothesis tests will be two-sided at level α = 0.05. Outcomes will be examined to inform the future RCT. Separate models will be fitted for different outcomes. If yijt is the outcome for patient j of provider i at time t, without loss generality then the following linear mixed model will be used: yijt = β0 + β1 Group + β3Xij + v1i + v2ij + ɛijt; where Xi and Xij are vectors of possible PCP- and patient-level confounders, respectively. The random term is a PCP-specific random effect and is a patient-specific random effect; is the model random error. We assume that v1i, v2ij and ɛijt are mutually independent. To test for a time and group interaction, we will add the time*group term to the model.
Linear mixed models will be used to adjust for the heterogeneity of the sites and participants by including PCP- and patient-specific random effects adjusting for clustering of data due to the repeated measurement of the same patient and to associations in a PCP with both PCP-level and patient-level random effects. We will examine patterns of missing data; linear mixed models provide unbiased estimates for data with missing values. Intention-to-treat analyses will be conducted.
3.2 |. Qualitative
Using multiple coders working independently, we will analyse transcripts using conventional inductive line-by-line content analysis, consistent with Qualitative Descriptive methodologies, to identify code/categories associated with trial satisfaction/dissatisfaction. Coders will meet weekly to develop the codebook and to resolve coding conflicts. While we do not expect to reach data saturation, posttrial interviews will provide adequate data to refine the intervention.
3.3 |. Ethical consideration
The Institutional Review Boards of Columbia University and the University of Pennsylvania approved this protocol. Written consent will be obtained from PCPs and patient subjects.
3.4 |. Remuneration
PCPs will receive $400 in graduated installments ($50, $50, $100, $200) received after their first, second, sixth, and tenth interventions. Patients will receive graduated payments at each of the four visits ($20, $30, $40, and $60 [total $150]). PCPs, patients and patients’ loved ones will receive additional payment if selected for posttrial interviews ($100, $50 and $50, respectively).
3.5 |. Safety monitoring committee (SMC) and adverse events (AEs)
While the behavioural intervention itself is low-risk, participants must have uncontrolled asthma to be eligible for enrollment. Because of this, we appointed a SMC to establish a threshold for AEs (defined below) with input from the principal investigator. The SMC is comprised of a chair with expertise in biostatistics, a pulmonologist, and a behavioural scientist.
The SMC defined AEs as Emergency Department visits or hospitalizations, regardless of its cause, as well as the use of oral corticosteroids. Study participants will be instructed to notify the study team within 48 hr of any AE. We will also ask them to report these at monthly data collection points in an attempt to capture any AEs that might otherwise go unreported. The SMC will hold regular meetings, create/review study reports, monitor AEs to determine if they are study-related, as they occur and implement stop rules.
3.6 |. Validity and reliability/rigour
The clinical trial offers a rigorous and unbiased plan as it relates to pre-experimental power calculation, masking of study staff members and subjects, use of validated instruments and plan for intervention fidelity (training and implementation). To ensure trustworthiness (rigour) of the qualitative data, we will incorporate Guba’s (1981) recommendations: (a) triangulating data (using more than one source) and performing member checks (returning codes to interviewees for confirmation) to enhance credibility; (b) providing thick descriptive data about participants to enhance transferability (generalizability to other populations and settings); (c) maintaining audit trails (documentation of all study-related decisions) to enhance dependability (reliability); and (d) creating reflexive audit trails to document biases and researchers’ effect on the data (confirmability or objectivity).
4 |. DISCUSSION
Low rates of ICS adherence are linked with the marked disparities evident in poor and minority adults with asthma. ICS non-adherence due to erroneous asthma beliefs or negative ICS beliefs is more common in poor and minority adults. These beliefs have an impact on self-management choices and pose some of the most daunting challenges to improving care in these communities. Addressing this requires a novel multifactorial approach as we have proposed. To ensure their sustainability, evidence-based interventions must be adapted to and integrated into real-world settings. A 7-min SDM intervention that uses MI techniques can focus on suboptimal asthma control while being delivered by PCPs during the 10–15 min typically allocated for an appointment. This feasibility trial proposes to ascertain our ability to recruit and to train providers to deliver a brief intervention in the FQHC setting and to test whether minority adult patients with uncontrolled asthma receive benefit from this intervention by comparing the active condition to a dose-matched control condition. Furthermore, satisfaction with the intervention will be examined in providers, patients, and patients’ loved ones. We anticipate that this trial will provide data that will inform a future full-scale RCT.
This study’s innovations are numerous. Although this proposed study uses a relatively well-established intervention paradigm—SDM coupled with MI—its extension to Black adults with uncontrolled asthma who receive care in FQHCs is novel. Few adult asthma interventions are conducted in primary care and even fewer in FQHCs; none have used PCPs to deliver the intervention (Kew et al., 2017). Additionally, PCPs success in delivering SDM interventions for asthma has not been evaluated. Another innovation is that by drawing on existing resources (i.e., PCPs integrated into the intervention setting and have a relationship with patients), we maximize the likelihood of sustaining the intervention and building capacity in underserved communities. Using PCPs as interventionists offer several advantages for patient care, including keeping PCPs more informed regarding their patients’ status and health needs. The potential for scalability is exemplified by our plan for a one-time brief intervention delivered during routine primary care visits. Our intervention is also innovative because, to our knowledge, we are among the first to address ICS non-adherence by concurrently targeting both erroneous health beliefs and ICS beliefs, which has the potential to have a synergistic effect; targeting multiple factors may amplify intervention benefits compared with interventions that target just one of these important contributors to ICS non-adherence.
4.1 |. Limitations
This feasibility trial is not without important limitations. First, we will use two sites with very similar patient demographics which will limit the generalizability of our findings. Second, we will randomize at the level of the PCP and not the clinics, which may allow for the introduction of contamination of the active and control conditions.
5 |. CONCLUSIONS
Poorly controlled asthma among low-income, Black adults is a significant problem that is due, in part, to ICS non-adherence. A brief single session SDM clinic-based intervention delivered by PCPs that addresses ICS adherence by focusing on erroneous beliefs about asthma and negative beliefs about ICS has the potential to greatly improve asthma control in this high risk group. This has important implication for nursing in that it will provide a novel, low-risk tool to improve care. Because erroneous beliefs about asthma and ICS transcends cultures, the BREATHE intervention has the potential to be applied to other patient populations both in the USA and beyond.
Funding information
This study was supported by the National Institute of Nursing Research, National Institutes of Health (R21 NR016507). Dr. Norful’s efforts were supported, in part, by the National Center for Advancing Translational Sciences,National Institutes of Health (TL1 TR001875)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Akinbami LJ, Moorman JE, & Liu X (2011). Asthma prevalence, health care use and mortality: United States, 2005–2009. National Health Statistics Report, 12(32), 1–14. [PubMed] [Google Scholar]
- Apter AJ, Boston RC, George M, Norfleet AL, Tenhave T, Coyne JC, … Feldman HI (2003). Modifiable barriers to adherence to inhaled steroids among adults with asthma: It’s not just black and white. Journal of Allergy and Clinical Immunology, 111(6), 1219–1226. 10.1067/mai.2003.1479 [DOI] [PubMed] [Google Scholar]
- Atlas.ti. (n.d.). Retrieved from https://atlasti.com/
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, … Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2014). Uncontrolled asthma among persons with current asthma. Retrieved from https://www.cdc.gov/asthma/asthma_stats/uncontrolled_asthma.htm
- Centers for Disease Control and Prevention (CDC). (2016). Most recent asthma data. Retrieved from https://www.cdc.gov/asthma/most_recent_data.htm
- Chang CH, Lewis VA, Meara E, Lurie JD, & Bynum JP (2016). Characteristics and service use of medicare beneficiaries using federally qualified health centers. Medical Care, 54(8), 804–809. 10.1097/MLR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J, Price D, Ryan D, Haughney J, & Horne R (2009). The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Primary Care Respiratory Journal, 18, 300–305. 10.4104/pcrj.2009.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JL, Mannm DM, Wisnivesky JP, Home R, Leventhal H, Musumeci-Szabo TJ, & Halm EA (2009). Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: The Medication Adherence Report Scale for Asthma. Annals of Allergy Asthma and Immunology, 103, 325–331. 10.1016/S1081-1206(10)60532-7 [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, … O’Connor PG (2012). A brief intervention reduces hazardous and harmful drinking in emergency department patients. Annals of Emergency Medicine, 60(2), 181–192. 10.1016/j.annemergmed.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, Pantalon MV, Degutis LC, Fiellin DA, & O’Connor PG (2005). Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Academic Emergency Medicine, 12(3), 249–256. 10.1197/j.aem.2004.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R, Lim S, Mallya G, & Robbins JH; Philadelphia Department of Public Health. (2009). Health center service areas: Examining population health. Retrieved from https://www.phila.gov/health/pdfs/2009_Health_Center_Service_Area_Report.pdf
- Expert Panel Report 3 (EPR-3). (2007). National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma.NIH pub no 07–4051 Bethesda, MD: National Heart, Lung and Blood Institute, National Institutes of Health. Retrieved from https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma. [Google Scholar]
- George M, Birck K, Hufford D, Jemmott LS, & Weaver TE (2006). Beliefs about asthma and complementary and alternative medicine (CAM) in low-income inner city African American adults. Journal of General Internal Medicine, 21, 1317–1324. 10.1111/j.1525-1497.2006.00624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Campbell J, & Rand C (2009). Self-management of acute asthma among low-income urban adults. Journal of Asthma, 46, 618–624. 10.1080/02770900903029788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Freedman TG, Norfleet AL, Feldman HI, & Apter AJ (2003). Qualitative research enhanced understanding of patients’ beliefs: Results of focus groups with low-income urban African American adults with asthma. Journal of Allergy and Clinical Immunology, 111, 967–973. 10.1067/mai.2003.1459 [DOI] [PubMed] [Google Scholar]
- George M, Topaz M, Rand C, Sommers MS, Glanz K, Pantalon MV, … Shea J (2014). Inhaled corticosteroid beliefs, complementary and alternative medicine and uncontrolled asthma in urban minority adults. Journal of Allergy and Clinical Immunology, 134, 1252–1259. 10.1067/j.jaci.2014.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Asthma. (2018). Global strategy for asthma management and prevention (2018 update). Retrieved from https://ginasthma.org/
- Guba E (1981). Criteria for assessing the trustworthiness of naturalistic inquiries. Educational Communication and Technology Journal, 29(2), 75–91. [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Measures. (n.d.). Patient Reported Outcomes Measurement Information System Measures. Retrieved from http://www.healthmeasures.net/explore-measurement-systems/promis/measure-development-research/validation.
- Juniper EF, Bousquet J, Abetz L, & Bateman ED (2006). Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respiratory Medicine, 100(4), 616–621. 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Ferrie PJ, & Griffith LE (1993). Measuring quality of life in asthma. American Review of Respiratory Disease, 147(4), 832–838. 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, & King DR (1999). Development and validation of a questionnaire to measure asthma control. European Respiratory Journal, 14(4), 902–907. 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- Kew KM, Malik P, Aniruddhan K, & Normansell R (2017). Shared decision-making for people with asthma. Cochrane Database of Systematic Review, 10, CD012330. 10.1002/14651858.cd012330.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, & Wu AW (2001). Race and sex differences in consist.ncy of care with national asthma guidelines in managed care organizations. Archives of Internal Medicine, 161(13), 1660–1668. 10.1001/archinte.161.13.1660 [DOI] [PubMed] [Google Scholar]
- Kriston L, Scholl I, Hölzel L, Simon D, Loh A, & Härter M (2010). The 9-item shared decision making questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Education and Counseling, 80(1), 94–99. 10.1016/j.pec.2009.09.034 [DOI] [PubMed] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, & Nguyen TD (1979). Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning, 2(3), 197–207. 10.1016/0149-7189(79)90094-6 [DOI] [PubMed] [Google Scholar]
- Le TT, Bilderback A, Bender B, Wamboldt FS, Turner CF, Rand CS, & Bartlett SJ (2008). Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? Journal of Asthma, 45(1), 33–37. 10.1080/02770900701815552 [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, … Wanger J (2005). Standardisation of spirometry. European Respiratory Journal, 26, 319–338. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2007). Ten things that motivational interviewing is not. Behavioural and Cognitive Psychotherapy, 37(2), 129–140. 10.1017/S1352465809005128 [DOI] [PubMed] [Google Scholar]
- Minkler M (2005). Community-based research partnerships: Challenges and opportunities. Journal of Urban Health, 82(2), 3–12. 10.1093/jurban/jti0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, & Liu X (2012). National surveillance of asthma: United States, 2001–2010. Vital Health Statisitcs, 3(35), 1–58. [PubMed] [Google Scholar]
- Mora PA, Berkowitz A, Contrada RJ, Wisnivesky J, Horne R, Leventhal H, & Halm EA (2011). Factor structure and longitudinal invariance of the Medical Adherence Report Scale-Asthma. Psychology and Health, 26(6), 713–727. 10.1080/08870446.2010.490585 [DOI] [PubMed] [Google Scholar]
- Murphy KR, Meltzer EO, Blaiss MS, Nathan RA, Stoloff SW, & Doherty DE (2012). Asthma management and control in the United States: Results of the 2009 asthma insight and management survey. Allergy and Asthma Proceedings, 33(1), 54–64. 10.2500/aap.2011.32.3518 [DOI] [PubMed] [Google Scholar]
- Norful AA, Ye S, Shaffer J, & Poghosyan L (2018). Development and psychometric testing of the provider co-management index (PCMI): Measuring nurse practitioner-physician co-management. Journal of Nursing Measurement, 26(3), E127–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalon MV, Martino S, Dziura J, Li FY, Owens PH, Fiellin DA, … D’Onofrio G (2012). Development of a scale to measure practitioner adherence to a brief intervention in the emergency department. Journal of Substance Abuse and Treatment, 43(4), 382–388. 10.1016/j.jsat.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalon MV, Sledge WH, Bauer SF, Brodsky B, Giannandrea S, Kay J, … Rockland LH (2013). Important medical decisions: Using brief motivational interviewing to enhance patients’ autonomous decision-making. Journal of Psychiatric Practice, 19(2), 98–108. 10.1097/01.pra.0000428556.48588.22 [DOI] [PubMed] [Google Scholar]
- Satterfield JM, Spring B, Brownson RC, Mullen EJ, Newhouse RP, Walker BB, & Whitlock EP (2009). Toward a transdisciplinary model of evidence-based practice. Milbank Quarterly, 87(2), 368–390. 10.1111/j.1468-0009.2009.00561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiggelbout AM, Pieterse AH, & De Haes JC (2015). Shared decision making: Concepts, evidence and practice. Patient Education and Counseling, 98(10), 1172–1179. 10.1016/j.pec.2015.06.022 [DOI] [PubMed] [Google Scholar]
- Street RL, Makoul G, Arora NK, & Epstein RM (2009). How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Education and Counseling, 74(3), 295–301. 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Suri H (2011). Purposeful sampling in qualitative research synthesis. Qualitative Research Journal, 11, 63–75. 10.3316/QRJ1102063 [DOI] [Google Scholar]
- The Global Asthma Network. (2018). The Global Asthma Report 2018. Retrieved from http://globalasthmareport.org/Global%20Asthma%20Report%202018.pdf
- U.S. Department of Health & Human Services, Health Resources and Services Administration, Bureau of Health Professions, The Physician Workforce. (2008). Projections and research into the current issues affecting supply and demand. Retrieved from http://bhpr.hrsa.gov/healthworkforce/reports/physwfissues.pdf
- Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, … Hale FA (2005). Quick assessment of literacy in primary care: The newest vital sign. Annals of Family Medicine, 3(6), 514–522. 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]


