Abstract
Introduction:
There are increasing reports of posterior reversible encephalopathy syndrome (PRES) associated with the use of chemotherapeutic agents. Recognition of PRES is crucial given its reversibility with appropriate supportive management. We report a patient presenting with PRES after treatment with Rituximab, Cyclophosphamide, Hydroxydaunorubicin/Adriamycin, Oncovin/Vincristine, Prednisone (R-CHOP) and intrathecal methotrexate. We also perform a systematic review of the literature on chemotherapy-associated PRES.
Case Report:
A 72-year-old man with recently diagnosed diffuse large B-cell lymphoma became unresponsive 4 days after initiation of R-CHOP and intrathecal methotrexate. Brain magnetic resonance imaging showed interval development of occipital and temporal fluid attenuation inversion recovery hyperintensities consistent with PRES. The patient’s blood pressure was aggressively controlled and he received 5 days of high-dose methylprednisone. He subsequently regained consciousness and his mental status gradually improved. Repeat magnetic resonance imaging showed interval resolution of the bilateral fluid attenuation inversion recovery hyperintensities.
Review Summary:
We performed a systematic review of the literature and included a total of 70 unique cases involving chemotherapy-associated PRES. Platinum-containing drugs, Cyclophosphamide, Hydroxydaunorubicin/Adriamycin, Oncovin/Vincristine, Prednisone/ R-CHOP regimens, and gemcitabine were the agents most commonly used in patients who developed suspected chemo-associated PRES. Median onset of symptoms occurred 8 days after chemotherapy. Hypertension was the most commonly reported risk factor associated with the development of chemotherapy-associated PRES. In most cases, PRES improved with supportive management alone within 2 weeks.
Conclusions:
Chemotherapy-associated PRES is an increasingly encountered syndrome. Both neurologists and non-neurologists should be familiar with the most commonly implicated agents, symptoms, risk factors, and clinical course of chemotherapy-associated PRES, given its favorable prognosis with appropriate management.
Keywords: chemotherapy, posterior reversible encephalopathy syndrome, reversible posterior leukoencephalopathy
Posterior reversible encephalopathy syndrome(PRES), also known as reversible posterior leukoencephalopathy, is a clinical syndrome characterized by headache, seizures, altered mental status, and visual disturbances. It is often associated with radiographic findings of posterior cerebral white matter edema. First described in 1996 by Hinchey et al,1 PRES is most commonly associated with hypertensive encephalopathy, eclampsia or preeclampsia, and immunosuppressive therapies such as cyclosporine and tacrolimus. Recently there have been increased reports of PRES associated with chemotherapy. The mechanism of PRES is believed to be related to endothelial dysfunction and failure of cerebral autoregulation, leading to breakdown of the blood-brain barrier (BBB) and resulting cerebral edema.1,2 In chemotherapy-associated PRES, the cytotoxic effects of treatment may cause direct vascular damage to the BBB. If clinical risk factors for PRES such as acute hypertension, renal dysfunction, or electrolyte imbalances are combined with chemotherapy-related cytotoxic effects, patients may be at higher risk of developing PRES.3,4
As use of chemotherapeutic agents increases, the diagnosis of PRES is likely to become more frequent. Prompt recognition of this syndrome is crucial, as PRES is usually reversible with appropriate supportive management.1 The purpose of this article is to familiarize the physician with the clinical course, outcomes, and risk factors seen with chemotherapy-associated PRES. We report a patient who developed PRES shortly after administration of combination chemotherapy for diffuse large B-cell lymphoma (DLBCL). We then perform a systematic review on chemotherapy-associated PRES in the literature.
CASE REPORT
A 72-year-old man with recently diagnosed stage IV DLBCL presented to the hospital with 4 weeks of increasing confusion. The family reported the patient was not responding appropriately to questions and had stopped eating and drinking. The patient denied any headaches, visual problems, or seizure-like activity. His past medical history included hypertension, type 2 diabetes mellitus, asthma, and osteoarthritis. His family history was negative for known cancers or neurological disease. He was a former cigarette smoker with a 7 pack year history. He had no history of alcohol or drug abuse. Neurological examination demonstrated no focal deficits. However, the patient was only intermittently oriented to self, time and place, and displayed difficulties with naming, repetition, and following commands. A magnetic resonance imaging (MRI) of the brain showed hyperintensity of the medial temporal lobe and a lumbar puncture demonstrated elevated protein with otherwise negative cytology and no evidence of infection. The patient’s confusion persisted despite medical optimization and a negative infectious disease work-up. There was concern for central involvement of his DLBCL and chemotherapy was initiated.
The patient received one cycle of Rituximab, Cyclophosphamide, Hydroxydaunorubicin/Adriamycin, Oncovin/Vincristine, Prednisone (R-CHOP) and one dose of intrathecal methotrexate. Three days later he received a second dose of intrathecal methotrexate. The next day the patient was found to be unresponsive, with an episode of right gaze deviation and right upper extremity shaking for 1 to 2 minutes. Repeat neurological examination now showed the patient to be nonverbal and withdrawing only to painful stimuli. His blood pressure was elevated to 200/101. Computed tomography of the head showed no acute intracranial abnormalities accounting for his sudden change in consciousness. His labs and examination were notable for an elevated creatinine to 1.53 (baseline unknown) and significant fluid overload. He was transferred to the intensive care unit where his blood pressure was aggressively controlled. An electroencephalogram showed no evidence of seizure activity, but he was treated with levetiracetam for seizure prophylaxis. A subsequent MRI showed interval development of Fluid attenuation inversion recovery (FLAIR) hyperintensities involving the bilateral occipital and temporal lobes, consistent with PRES (Fig. 1).
FIGURE 1.
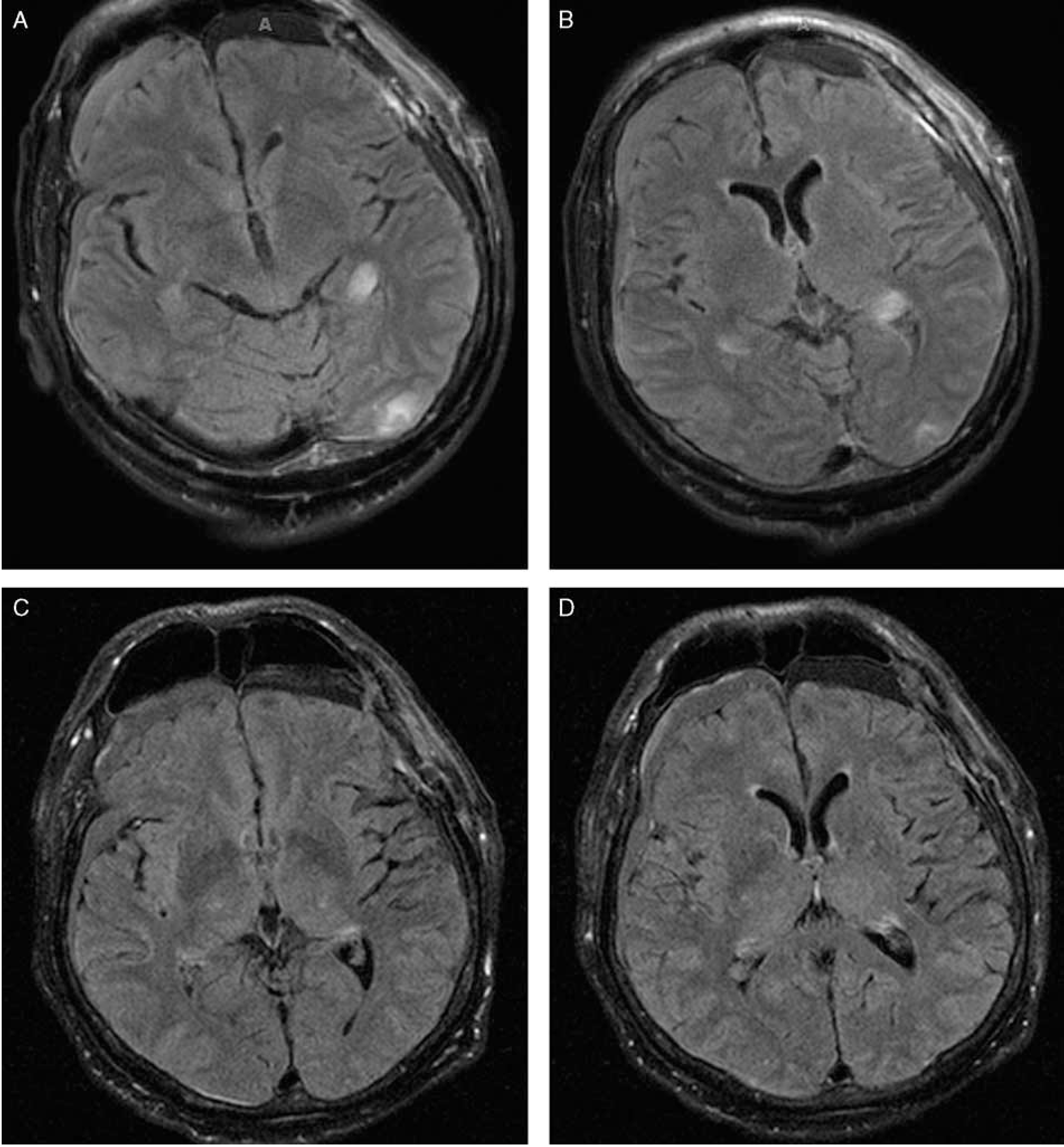
A and B, Magnetic resonance imaging of the brain without contrast demonstrating bilateral occipital FLAIR hyperintensities consistent with posterior reversible encephalopathy syndrome. C and D, Interval resolution of bilateral FLAIR hyperintensities after 11 days.
The patient remained unresponsive for 1 week despite adequate blood pressure control. The patient then received empirical treatment with a 5-day course of high-dose steroids (methylprednisone 1 g/d). On the last day of treatment a repeat MRI showed interval resolution of the bilateral occipital FLAIR hyperintensities (Figs. 1C, D). The following day the patient regained consciousness and his mental status gradually improved. One month later, the patient was oriented to self and able to answer simple questions and follow commands. He was discharged home.
DISCUSSION AND RESULTS
We performed a systematic review of the literature to better characterize the clinical course, treatment, and risk factors associated with chemotherapy-associated PRES. The use of targeted therapies, including the VEGF inhibitor bevacizumab, was excluded as this topic has been reviewed elsewhere.5,6 Search keywords included posterior leukoencephalopathy syndrome and chemotherapy treatments, and were executed in Ovid Medline 1946-, Embase 1947-, and Scopus 1823-. All searches were completed in December 2015 and limited to the English language using database supplied limits. The results were exported to EndNote. A total of 98 unique citations were identified. Additional case reports were identified through article references. Case reports that lacked radiographic data or cited a targeted therapy as a likely inciting cause of PRES were excluded. A total of 70 cases were ultimately included in the review.4,7–71 To our knowledge, this is the largest systematic review of chemotherapy-associated PRES in the literature.
Table 1 details the clinical characteristics of chemotherapy-associated PRES. Women were more commonly affected than men, accounting for 77% of the cases. Symptoms and radiographic findings were largely consistent with PRES due to other causes.1,72 Seizures were the most frequent presenting symptom, followed by altered mental status (most commonly manifesting as decreased consciousness and lethargy), visual changes, headache, and focal neurological deficits. Comatose states, as seen in our patient, were seen in 10 cases (14%), but were generally reversible. Radiographic findings also tended to occur in the posterior distribution, with the occipital lobe implicated in 93% of cases. However, involvement of the frontal lobes, thalamus, cerebellum, and basal ganglia structures were also seen (Table 1). Median blood pressure was 170/92.5, with a median mean arterial pressure (MAP) of 118 mm Hg. This was similar to the median MAP (117 mm Hg) found in a large, retrospective study of 96 patients with all-cause PRES.72 However, 5 case reports noted a “normal” blood pressure in their patients without providing actual measurements, and were excluded from analysis. Thus, our median MAP was likely an overestimation of systemic blood pressures. Indeed, Liman et al72 noted a significantly lower MAP in patients with chemotherapy-associated PRES (110 mm Hg) compared with PRES due to other causes (122 mm Hg), which may argue for endothelial dysfunction in precipitating cerebral edema. In our patient and this review, systemic hypertension was an important risk factor associated with the development of PRES, given the elevated median MAP. However, chemotherapy-associated PRES can still develop in the presence of relatively normal blood pressures. Some authors have concluded that a 25% increase of blood pressures from the baseline values will put a patient at risk for chemotherapy-associated PRES.4
TABLE 1.
Clinical and Radiographic Characteristics of Patients With Chemotherapy-associated PRES
| Age (N = 70) | 18–79, median: 55 |
| Sex (%) | |
| Female | 77 |
| Male | 23 |
| Blood pressure (N = 63)* | 106–240/95–124, median: 170/92.5 |
| Days until onset (N = 63)† | 1–60, median: 8 |
| Duration(N = 36)‡ | 1–14, median: 4 |
| Fatal (%) | 7 |
| Symptoms (%) | |
| Seizure | 64 |
| Altered mental status | 47 |
| Visual changes | 43 |
| Headache | 40 |
| Other neurological deficit | 19 |
| Coma | 14 |
| MRI findings (%) | |
| Occipital | 93 |
| Parietal | 57 |
| Frontal | 26 |
| Temporal | 17 |
Only case reports that included numeric measurements of presenting blood pressures were included.
Number of days between last administration of implicated chemotherapy and onset of posterior reversible encephalopathy syndrome symptoms.
Duration of symptoms was defined as number of days to significant clinical improvement.
MRI indicates magnetic resonance imaging.
Table 2 lists the most frequently implicated chemotherapeutic agents. Our patient received R-CHOP and IT-methotrexate, both of which have been implicated in PRES. Interestingly, in our review, the majority (74%) of PRES cases were associated with combination chemotherapy, of which CHOP/R-CHOP was the most frequent. IT-methotrexate was implicated in 5 of the 70 cases (7%). Although IT-methotrexate neurotoxicity has been described in the pediatric population, PRES is relatively rare, and is even less frequently encountered in the adult population.9 In our review, gemcitabine was the most frequently encountered single chemotherapeutic agent. However, platinum-based drugs were the most commonly encountered agents when accounting for chemotherapy regimens. Given the retrospective nature of this study, it is difficult to ascertain whether the increased frequency of platins reported in PRES is confounded by reporting bias, oncologist practice, or type of cancer being treated. However, platins are known to have neurotoxic side effects, although usually these side effects are restricted to the peripheral nervous system.73
TABLE 2.
Implicated Chemotherapeutic Agents in the Development of Posterior Reversible Encephalopathy Syndrome
| Chemotherapeutic Agent | N |
|---|---|
| Cisplatin, carboplatin, oxaliplatin* | 30 |
| CHOP/R-CHOP | 14 |
| Daunorubicin | 24 |
| Vincristine, vinorelbine, vinflunine, vinblastine, vindesine | 21 |
| Cyclophosphamide | 16 |
| Gemcitabine | 14 |
| Capecitabine, 5-fluorouracil* | 13 |
| Cytarabine | 9 |
| IT cytarabine | 3 |
| Etoposide | 8 |
| Methotrexate | 7 |
| IT methotrexate | 5 |
| Taxane | 5 |
| Ifosfamide | 3 |
| Bleomycin | 2 |
| Lomustin | 1 |
| Dacarbazine | 1 |
| Irinotecan | 1 |
FOLFOX: 7.
CHOP indicates Cyclophosphamide, Hydroxydaunorubicin/Adriamycin, Oncovin/Vincristine, Prednisone; R-CHOP, Rituximab, Cyclophosphamide, Hydroxydaunorubicin/Adriamycin, Oncovin/Vincristine, Prednisone.
The mechanism for chemotherapy-induced PRES is unclear, but it may largely be related to toxic effects of chemotherapeutic agents on endothelial cells, resulting in BBB dysfunction and cerebral vasogenic edema.74 These toxic effects are likely more potent when combined with systemic risk factors associated with cancer. A study on rats demonstrated that intracarotid injection of cisplatin, the agent most frequently implicated in cases of chemotherapy-induced PRES, increases BBB permeability.75 Bleomycin has also been shown to have dose-dependent cytotoxicity on endothelial cells in vitro.76 Chemotherapeutic agents may also lead to immune reactions that increase BBB permeability. Chemotherapies may increase tumor cell recognition by the body’s innate immune system and trigger a complex cascade of endothelial cell activation and cytokine production, ultimately resulting in vascular instability and BBB dysfunction.77–79 Therefore, both indirect and indirect effects of chemotherapeutic agents may cause BBB dysfunction and result in PRES.
Our patient suffered an acute change in mental status four days after administration of his first dose of R-CHOP and IT methotrexate. In our review, median onset of symptoms occurred 8 days after chemotherapy administration, although often PRES occurred in the second or third cycle of chemotherapy. Two cases reported an onset of PRES 60 days after their last chemotherapy dose.35,54 Chemotherapy-associated PRES was also largely reversible, and in all nonlethal cases patients showed symptom resolution within two weeks. However, a significant proportion (7% in our study) had fatal outcomes due to neurological and clinical deterioration.7,10,12,19,21,51,55 Treatment of PRES consists largely of blood pressure control and withdrawal of the inciting factor; however, in some cases, hemorrhagic conversion and infarction can occur.8,26,51,56,60 Interestingly, the use of high-dose steroids was reported in 9 cases (13%), although outcomes and duration of symptoms were similar to patients treated without high-dose steroids.8,14,24,27,31,46,47,56,69 In our patient, abrupt neurologic recovery was seen 1 day after pulse steroid conclusion. It is unclear whether or not high-dose steroids have a real impact on the clinical course, given that in most cases the syndrome will resolve on its own with appropriate management.1 However, there is a theoretical benefit of reducing inflammation and resulting edema, although there are also reported cases of precipitating PRES with initiation of high-dose steroids.80 The use of steroids in PRES should therefore be exercised with caution, although in the above 9 case reports and our patient, symptoms were not worsened with steroid administration.
Besides hypertension, reported risk factors for PRES include renal dysfunction, fluid overload, and electrolyte imbalances such as hypomagnesemia.4,81,82 Our patient exhibited an acute kidney injury and marked fluid overload at the time of symptom development. In this review, 8 cases (11%) reported an elevated creatinine, and 6 (9%) reported a below normal magnesium. It is difficult to estimate the true contribution of acute kidney injury and hypomagnesemia in the development of PRES. Therefore, physicians should be vigilant to the possibility of PRES in the presence of these risk factors, and work to correct renal dysfunction and electrolyte imbalances as part of supportive management.
The differential for neurological symptoms in a cancer patient after administration of chemotherapy is broad. However, all physicians should maintain a high clinical suspicion of chemotherapy-associated PRES, as most patients will have a favorable prognosis. Chemotherapy-associated PRES is an increasingly common syndrome during oncologic treatment. Platinum-containing drugs, CHOP/R-CHOP regimens, and gemcitabine are the most frequently implicated agents, although nearly every drug class has been implicated. For the most part, the symptoms, radiographic findings, duration, and outcomes are similar to PRES precipitated by other causes. The onset of symptoms occurs a median of 8 days after chemotherapy administration, but has been reported up to 2 months later. Blood pressures are generally elevated and should be aggressively controlled, although hypertension may be absent in some cases of chemotherapy-associated PRES. High-dose steroids have not been shown to improve outcomes, however they have been used empirically in the management of PRES. Patients typically improve within 2 weeks of symptom onset. Prompt recognition of the syndrome is critical given its reversibility with appropriate management.
Acknowledgments
S.E.S is supported by UL1 TR00448, Sub-Award KL2 TR000450.
Footnotes
The remaining authors declare no conflict of interest.
REFERENCES
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RB. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:1743. Author reply 6. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10:86–91. [DOI] [PubMed] [Google Scholar]
- 4.Tam CS, Galanos J, Seymour JF, et al. Reversible posterior leukoencephalopathy syndrome complicating cytotoxic chemotherapy for hematologic malignancies. Am J Hematol. 2004;77: 72–76. [DOI] [PubMed] [Google Scholar]
- 5.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354: 980–982; discussion-2. [DOI] [PubMed] [Google Scholar]
- 6.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354: 980–982; discussion-2. [PubMed] [Google Scholar]
- 7.Abali H, Eren OO, Dizdar O, et al. Posterior leukoencephalopathy after combination chemotherapy in a patient with lymphoma. Leuk Lymphoma. 2005;46:1825–1828. [DOI] [PubMed] [Google Scholar]
- 8.Akins PT, Axelrod Y, Silverthorn JW, et al. Management and outcomes of malignant posterior reversible encephalopathy syndrome. Clin Neurol Neurosurg. 2014;125:52–57. [DOI] [PubMed] [Google Scholar]
- 9.Aradillas E, Arora R, Gasperino J. Methotrexate-induced posterior reversible encephalopathy syndrome. J Clin Pharm Ther. 2011;36: 529–536. [DOI] [PubMed] [Google Scholar]
- 10.Battipaglia G, Avilia S, Morelli E, et al. Posterior reversible encephalopathy syndrome (PRES) during induction chemotherapy for acute myeloblastic leukemia (AML). Ann Hematol. 2012;91: 1327–1328. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt A, Farooq MU, Majid A, et al. Chemotherapy-related posterior reversible leukoencephalopathy syndrome. Nat Clin Pract Neurol. 2009;5:163–169. [DOI] [PubMed] [Google Scholar]
- 12.Cain MS, Burton GV, Holcombe RF. Fatal leukoencephalopathy in a patient with non-Hodgkin’s lymphoma treated with CHOP chemotherapy and high-dose steroids. Am J Med Sci. 1998;315: 202–207. [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, Huang CH. Reversible posterior leukoencephalopathy syndrome induced by vinorelbine. Clin Breast Cancer. 2012;12: 222–225. [DOI] [PubMed] [Google Scholar]
- 14.Chue AL, Fernando IN, Hussain SA, et al. Chemotherapy related encephalopathy in a patient with Stage IV cervical carcinoma treated with cisplatin and 5-fluorouracil: a case report. Cases J. 2009;2:8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cioffi P, Laudadio L, Nuzzo A, et al. Gemcitabine-induced posterior reversible encephalopathy syndrome: a case report. J Oncol Pharm Pract. 2012;18:299–302. [DOI] [PubMed] [Google Scholar]
- 16.Connolly RM, Doherty CP, Beddy P, et al. Chemotherapy induced reversible posterior leukoencephalopathy syndrome. Lung cancer. 2007;56:459–463. [DOI] [PubMed] [Google Scholar]
- 17.Edwards MJ, Walker R, Vinnicombe S, et al. Reversible posterior leukoencephalopathy syndrome following CHOP chemotherapy for diffuse large B-cell lymphoma. Ann Oncol. 2001;12: 1327–1329. [DOI] [PubMed] [Google Scholar]
- 18.Eichler FS, Wang P, Wityk RJ, et al. Diffuse metabolic abnormalities in reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 2002;23:833–837. [PMC free article] [PubMed] [Google Scholar]
- 19.Femia G, Hardy TA, Spies JM, et al. Posterior reversible encephalopathy syndrome following chemotherapy with oxaliplatin and a fluoropyrimidine: a case report and literature review. Asia Pac J Clin Oncol. 2012;8:115–122. [DOI] [PubMed] [Google Scholar]
- 20.Foreid H, Pires C, Albuquerque L, et al. Posterior reversible encephalopathy manifested by refractory status epilepticus in two patients under chemotherapy. BMJ Case Rep. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood MJ, Dodds AJ, Garricik R, et al. Posterior leukoencephalopathy in association with the tumour lysis syndrome in acute lymphoblastic leukaemia—a case with clinicopathological correlation. Leuk Lymphoma. 2003;44:719–721. [DOI] [PubMed] [Google Scholar]
- 22.Haefner MD, Siciliano RD, Widmer LA, et al. Reversible posterior leukoencephalopathy syndrome after treatment of diffuse large B-cell lymphoma. Onkologie. 2007;30:138–140. [DOI] [PubMed] [Google Scholar]
- 23.Han CH, Findlay MP. Chemotherapy-induced reversible posterior leucoencephalopathy syndrome. Intern Med J. 2010;40:153–159. [DOI] [PubMed] [Google Scholar]
- 24.Helissey C, Chargari C, Lahutte M, et al. First case of posterior reversible encephalopathy syndrome associated with vinflunine. Invest New Drugs. 2012;30:2032–2034. [DOI] [PubMed] [Google Scholar]
- 25.Hemmaway C, Mian A, Nagy Z. Images in haematology. Irreversible blindness secondary to posterior reversible encephalopathy syndrome following CHOP combination chemotherapy. Br J Haematol. 2010;150:129. [DOI] [PubMed] [Google Scholar]
- 26.Henderson RD, Rajah T, Nicol AJ, et al. Posterior leukoencephalopathy following intrathecal chemotherapy with MRA-documented vasospasm. Neurology. 2003;60:326–328. [DOI] [PubMed] [Google Scholar]
- 27.Imai H, Okuno N, Ishihara S, et al. Reversible posterior leukoencephalopathy syndrome after carboplatin and paclitaxel regimen for lung cancer. Intern Med. 2012;51:911–915. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Arahata Y, Goto Y, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998;19:415–417. [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal A, Sabnani I, Baran DA, et al. A unique case of rituximab-related posterior reversible encephalopathy syndrome in a heart transplant recipient with posttransplant lymphoproliferative disorder. Am J Transplant. 2015;15:823–826. [DOI] [PubMed] [Google Scholar]
- 30.Kaneda H, Okamoto I, Satoh T, et al. Reversible posterior leukoencephalopathy syndrome and trastuzumab. Invest New Drugs. 2012;30:1766–1767. [DOI] [PubMed] [Google Scholar]
- 31.Kim CH, Kim CH, Chung CK, et al. Unexpected seizure attack in a patient with spinal metastasis diagnosed as posterior reversible encephalopathy syndrome. J Korean Neurosurg Soc. 2011;50: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kistler CA, McCall JC, Ghumman SS, et al. Posterior reversible leukoencephalopathy syndrome secondary to hepatic transarterial chemoembolization with doxorubicin drug eluting beads. J Gastrointest Oncol. 2014;5:E43–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon EJ, Kim SW, Kim KK, et al. A case of gemcitabine and cisplatin associated posterior reversible encephalopathy syndrome. Cancer Res Treat. 2009;41:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladwa R, Peters G, Bigby K, et al. Posterior reversible encephalopathy syndrome in early-stage breast cancer. Breast J. 2015;21:674–677. [DOI] [PubMed] [Google Scholar]
- 35.Loo SW, Ford H, Lu SJ. Blindness, confusion and seizures in a cancer patient. Neth J Med. 2010;68:95–96. [PubMed] [Google Scholar]
- 36.Maeda T, Kikuchi E, Matsumoto K, et al. Gemcitabine and cisplatin chemotherapy induced reversible posterior leukoencephalopathy syndrome in a bladder cancer patient. Int J Clin Oncol. 2010;15:508–511. [DOI] [PubMed] [Google Scholar]
- 37.Marrone LC, Marrone BF, de la Puerta Raya J, et al. Gemcitabine monotherapy associated with posterior reversible encephalopathy syndrome. Case Rep Oncol. 2011;4:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porcello Marrone LC, Marrone BF, Pascoal TA, et al. Posterior reversible encephalopathy syndrome associated with FOLFOX chemotherapy. Case Rep Oncol Med. 2013;23:535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsunaga M, Miwa K, Araki K, et al. A case of reversible posterior leukoencephalopathy syndrome (RPLS) induced by modified FOLFOX6. Gan To Kagaku Ryoho. 2012;39: 1283–1286. [PubMed] [Google Scholar]
- 40.Modi N, Monga D. PRES—a potential side effect of gemcitabine. Communy Oncol. 2012;9:27–28. [Google Scholar]
- 41.Moris G, Ribacoba R, Gonzalez C. Delayed posterior encephalopathy syndrome following chemotherapy with oxaliplatin and gemcitabine. J Neurol. 2007;254:534–535. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen MT, Virk IY, Chew L, et al. Extended use dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. J Clin Neurosci. 2009;16:1688–1690. [DOI] [PubMed] [Google Scholar]
- 43.Norden AD, Batchelor TT. Reversible posterior leukoencephalopathy syndrome. Onkologie. 2007;30:90–91. [DOI] [PubMed] [Google Scholar]
- 44.Ocvirk J, Boc M, Rebersek M, et al. Cisplatin-induced non-convulsive posterior reversible encephalopathy syndrome in a 41-year-old woman with metastatic malignant melanoma. Radiol Oncol. 2009;43:120–125. [Google Scholar]
- 45.Onujiogu N, Lengyel E, Yamada SD. Reversible posterior leukoencephalopathy syndrome following intravenous paclitaxel and intraperitoneal cisplatin chemotherapy for fallopian tube cancer. Gynecol Oncol. 2008;111:537–539. [DOI] [PubMed] [Google Scholar]
- 46.Ozkan A, Hakyemez B, Ozkalemkas F, et al. Tumor lysis syndrome as a contributory factor to the development of reversible posterior leukoencephalopathy. Neuroradiology. 2006; 48: 887–892. [DOI] [PubMed] [Google Scholar]
- 47.Papayannidis C, Volpato F, Iacobucci I, et al. Posterior reversible encephalopathy syndrome in a B-cell acute lymphoblastic leukemia young adult patient treated with a pediatric-like chemotherapeutic schedule. Hematol Rep. 2014;6:5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel A, Ayto R, MacDonald DH. Posterior reversible encephalopathy after intrathecal methotrexate therapy in diffuse large B-cell lymphoma. Br J Haematol. 2013;161:607. [DOI] [PubMed] [Google Scholar]
- 49.Pawar PS, Noviawaty I, Zaidat OO. Unusual case of intra-arterial doxorubicin chemoembolization-associated posterior reversible encephalopathy syndrome. Neurologist. 2012;18:49–50. [DOI] [PubMed] [Google Scholar]
- 50.Piccin A, Dossi RC, Cassibba V, et al. Anti-thrombin-III reduction and posterior reversible encephalopathy syndrome (PRES) in acute lymphoblastic leukaemia (ALL). New insight into PRES pathophysiology. Ann Hematol. 2012;91:1153–1155. [DOI] [PubMed] [Google Scholar]
- 51.Dedic Plavetic N, Rakusic Z, Ozretic D, et al. Fatal outcome of posterior “reversible” encephalopathy syndrome in metastatic colorectal carcinoma after irinotecan and fluoropyrimidine chemotherapy regimen. World J Surg Oncol. 2014;12:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajasekhar A, George TJ Jr. Gemcitabine-induced reversible posterior leukoencephalopathy syndrome: a case report and review of the literature. Oncologist. 2007;12:1332–1335. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandran A, Klein R. Posterior reversible encephalopathy syndrome (PRES) after combination chemotherapy for lymphoma. Am J Ther. 2014;21:e137–e142. [DOI] [PubMed] [Google Scholar]
- 54.Rangi PS, Partridge WJ, Newlands ES, et al. Posterior reversible encephalopathy syndrome: a possible late interaction between cytotoxic agents and general anaesthesia. Neuroradiology. 2005;47:586–590. [DOI] [PubMed] [Google Scholar]
- 55.Roy S, Gandhi AK, Jana M, et al. Recurrent posterior reversible encephalopathy syndrome after chemotherapy in hematologic malignancy-posterior reversible encephalopathy syndrome can strike twice!!!. J Cancer Res Ther. 2014;10:393–396. [DOI] [PubMed] [Google Scholar]
- 56.Russell MT, Nassif AS, Cacayorin ED, et al. Gemcitabine-associated posterior reversible encephalopathy syndrome: MR imaging and MR spectroscopy findings. Magn Reson Imaging. 2001;19:129–132. [DOI] [PubMed] [Google Scholar]
- 57.Ryan SA, Maceneaney P, O’Reilly SP, et al. Reversible posterior leukoencephalopathy induced by carboplatin and etoposide. Med Oncol. 2012;29:1287–1291. [DOI] [PubMed] [Google Scholar]
- 58.Saito B, Nakamaki T, Nakashima H, et al. Reversible posterior leukoencephalopathy syndrome after repeat intermediate-dose cytarabine chemotherapy in a patient with acute myeloid leukemia. Am J Hematol. 2007;82:304–306. [DOI] [PubMed] [Google Scholar]
- 59.Sengupta S, Benkers T, Blitstein M, et al. Posterior reversible encephalopathy syndrome (PRES) complicating newly-diagnosed diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2014;14:e111–e113. [DOI] [PubMed] [Google Scholar]
- 60.Sharief U, Perry DJ. Delayed reversible posterior encephalopathy syndrome following chemotherapy with oxaliplatin. Clin Colorectal Cancer. 2009;8:163–165. [DOI] [PubMed] [Google Scholar]
- 61.Siddiqi AI. Rituximab as a possible cause of posterior reversible encephalopathy syndrome. Australas Med J. 2011;4:513–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simkens GA, Hanse MC, de Hingh IH. Acute neurological disorders following intraperitoneal administration of cisplatin. Int J Gynaecol Obstet. 2013;120:291. [DOI] [PubMed] [Google Scholar]
- 63.Skelton MR, Goldberg RM, O’Neil BH. A case of oxaliplatin-related posterior reversible encephalopathy syndrome. Clin Colorectal Cancer. 2007;6:386–388. [DOI] [PubMed] [Google Scholar]
- 64.Sueblinvong T, Noophun P, Pataradool K, et al. Posterior leukoencephalopathy following cisplatin, bleomycin and vinblastine therapy for germ cell tumor of the ovary. J Obstet Gynaecol Res. 2002;28:99–103. [DOI] [PubMed] [Google Scholar]
- 65.Truong QV, Abraham J, Nagaiah G, et al. Gemcitabine associated with posterior reversible encephalopathy syndrome (PRES): a case report and review of the literature. Clin Adv Hematol Oncol. 2012;10:611–613. [PubMed] [Google Scholar]
- 66.Truman N, Nethercott D. Posterior reversible encephalopathy syndrome (PRES) after treatment with oxaliplatin and 5-fluorouracil. Clin Colorectal Cancer. 2013;12:70–72. [DOI] [PubMed] [Google Scholar]
- 67.Tsukamoto S, Takeuchi M, Kawajiri C, et al. Posterior reversible encephalopathy syndrome in an adult patient with acute lymphoblastic leukemia after remission induction chemotherapy. Int J Hematol. 2012;95:204–208. [DOI] [PubMed] [Google Scholar]
- 68.Vaughn DJ, Jarvik JG, Hackney D, et al. High-dose cytarabine neurotoxicity: MR findings during the acute phase. AJNR Am J Neuroradiol. 1993;14:1014–1016. [PMC free article] [PubMed] [Google Scholar]
- 69.Vieillot S, Pouessel D, De Champfleur N, et al. Reversible posterior leukoencephalopathy syndrome after carboplatin therapy. Ann Oncol. 2007;18:608–609. [DOI] [PubMed] [Google Scholar]
- 70.Wright KL, Polito MH, French AE. Posterior reversible encephalopathy syndrome: a case study. Am J Nurs. 2012;112:36–40. quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 71.Zahir MN, Masood N, Shabbir-Moosajee M. Cisplatin-induced posterior reversible encephalopathy syndrome and successful re-treatment in a patient with non-seminomatous germ cell tumor: a case report. J Med Case Reports. 2012; 6:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liman TG, Bohner G, Heuschmann PU, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2012;259:155–164. [DOI] [PubMed] [Google Scholar]
- 73.Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol. 1992;10:795–803. [DOI] [PubMed] [Google Scholar]
- 74.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugimoto S, Yamamoto YL, Nagahiro S, et al. Permeability change and brain tissue damage after intracarotid administration of cisplatin studied by double-tracer autoradiography in rats. J Neurooncol. 1995;24:229–240. [DOI] [PubMed] [Google Scholar]
- 76.Dirix LY, Libura M, Libura J, et al. In vitro toxicity studies with mitomycins and bleomycin on endothelial cells. Anticancer Drugs. 1997;8:859–868. [DOI] [PubMed] [Google Scholar]
- 77.Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baethmann A, Maier-Hauff K, Kempski O, et al. Mediators of brain edema and secondary brain damage. Crit Care Med. 1988;16:972–978. [DOI] [PubMed] [Google Scholar]
- 79.Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irvin W, MacDonald G, Smith JK, et al. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25:2484–2486. [DOI] [PubMed] [Google Scholar]
- 81.Gorman DJ, Kefford R, Stuart-Harris R. Focal encephalopathy after cisplatin therapy. Med J Aust. 1989;150:399–401. [DOI] [PubMed] [Google Scholar]
- 82.Boulos MI, Shoamanesh A, Aviv RI, et al. Severe hypomagnesemia associated with reversible subacute ataxia and cerebellar hyperintensities on MRI. Neurologist. 2012;18:223–225. [DOI] [PubMed] [Google Scholar]


