Abstract
Biparametric magnetic resonance imaging (bpMRI) of the prostate has emerged as an alternative to multiparametric MRI (mpMRI) for the detection of clinically significant prostate cancer (csPCa). However, while the Prostate Imaging Reporting and Data System (PI-RADS) is widely known for mpMRI, a proper PI-RADS for bpMRI has not yet been adopted. In this review, we report the current status and the future directions of bpMRI, and propose a simplified PI-RADS (S-PI-RADS) that could help radiologists and urologists in the detection and management of PCa.
Keywords: Neoplasms, prostate, biopsy, urogenital system, male urogenital diseases, diagnosis, investigative techniques
Introduction
Prostate mutiparametric magnetic resonance imaging (mpMRI), including T1-weighted (T1W), T2-weighted (T2W), diffusion-weighted imaging (DWI), and dynamic contrast enhancement (DCE) sequences, is proposed by the current Prostate Imaging Reporting and Data System version 2.1 (PI-RADS v2.1) as the guidelines for patients with suspected prostate cancer (PCa).[1] The objective is to promote and improve a standard terminology for both radiologists and urologists to aid prostate mpMRI interpretation for detection of clinically significant prostate cancer (csPca), to help the urologists to make a more accurate diagnosis, determine a treatment plan, and avoid unnecessary needle biopsy.[1]
Despite the improvement of technical parameters and the revision of the interpretation criteria for prostatic mpMRI brought by the PI-RADS v2.1 guidelines to the previous version 2.0, the role of DCE and the management of scoring 3 lesions remain unclear. DCE-MRI has no role in the evaluation of lesions in the transition zone (TZ) and is a tertiary sequence in the peripheral zone (PZ). Furthermore, the potential gadolinium-related risk of adverse events such as nephrogenic systemic fibrosis or gadolinium deposition in the brain, the higher costs, and long examination times of approximately 30–45 minutes[2] should be taken into serious consideration. Finally, reproducibility of PI-RADS v2.1 guidelines among radiologists with different levels of training and experience has not been demonstrated. Although in PI-RADS v2.1, the descriptive terms are defined more precisely, their interpretation is subjective and may be interpreted differently by radiologists.
The rationale to overcome the aforementioned limitations is the simplification and standardization of PI-RADS v2.1.
Biparametric MRI (bpMRI) (including T2W imaging and DWI) represents a potential alternative to mpMRI with similar diagnostic perfomance and reproducibility in detecting PCa. These evidences are demontrated by several studies and meta-analyzes and recently are supported by the PI-RADS committee.[3–11] Finally, the bpMRI can be used in the detection of local recurrence after prostatectomy.[12]
The several systematic reviews and meta-analyzes comparing the diagnostic performance of non-contrast and contrast MRI are affected by heterogeneity of the results and by the lack of a standardized system for bpMRI as well as of a step methodology for images reading.[3, 13–17]
In this review, we report the current status and future directions of bpMRI, and proposes a simplified PI-RADS (S-PI-RADS) system that can help radiologists and urologists in the detection and management of PCa.
Recommendations for acquisition of prostate bpMRI
Patient preparation, technical parameters of acquisition, potential contraindications, and the radiologist’s experience are essential for obtaining a prostate MRI examination of adequate diagnostic quality. The recommendations, in particular for the use of 1.5T MRI with phased-array coil and for performing MRI before biopsy, refer to the European Association of Urology (EAU) prostate guidelines.[18] Knowledge of the pitfalls can help to reduce false positive results using bpMRI.[19] Recommendations for the acquisition of prostate bpMRI are reported in the Figure 1.
Figure 1.
Recommendations for the acquisition of prostate biparametric magnetic resonance imaging.
Diffusion weighted imaging
DWI is a short acquisition time sequence available on most commercial MR scanners. DWI has poor contrast resolution and is used in detecting the signal in tissue where water movement is reduced without exposing patients to radiation. Gleason score (GS) of PCa increases with increasing cell density (becoming more architecturally compact and solid).[20] The signal of DWI derived apparent diffusion coefficient (ADC) map is related to the histopathology of PCa.[21–24] High cell density of clinically significant PCa has a marked reduced diffusion of the water relative to the surrounding tissue and determines a low signal (black) on the DWI derived ADC map [23–25] and a corresponding high signal (white) in DWI at high values of b (≥1500 s/mm2). An inverse relationship is noted between the ADC value and the GS. For example, a decrease in the ADC values (low signal) is significantly correlated with an increase in GS.[26–28]
T2-weighted imaging
T2WI is a sequence that shows zonal anatomy of the prostate which differentiates the high-signal PZ, the heterogeneous mixed-signal TZ, and the low-signal central zone (CZ) and allows to establish the relationship of the prostate and seminal vesicle with the surrounding structures. The role of T2W in S-PI-RADS is to confirm and localize focal lesions (hypointense) detected on DWI/ADC (hyper/hypointense), to evaluate extraprostatic extension, and to guide fusion biopsies. T2W has a low sensitivity to detect PCa in TZ because of low signal intensities of benign prostatic hyperplasia that can mimic PCa.[29]
BpMRI versus mpMRI: diagnostic performance in prostate cancer detection
From a PubMed.gov review using the term “biparametric prostate MRI”, from January 2007 to December 2019, we found 87 articles.
In a meta-analysis by Niu et al.[16], using 33 studies from January 2007 to 2017, the overall sensitivity for general data pooling was 0.81 (95% confidence interval [CI] 0.76–0.85), and overall specificity was 0.77 (95% CI, 0.69–0.84). As for csPCa, bpMRI maintained a high diagnostic value (area under curve 0.85; 95% CI, 0.82–0.88). In the detection of PCa, mpMRI showed significantly higher pooled sensitivity (0.85; 95% CI, 0.78–0.93) than bpMRI (0.80; 95% CI, 0.71–0.90) (p = 0.01); the pooled specificity values were not significantly different (mpMRI, 0.77 [95% CI, 0.58–0.95]; bpMRI, 0.80 [95% CI, 0.64–0.96]; p = 0.82). [16].
In a meta-analysis by Bass et al.[3] including 44 articles, from 01/01/2017 to 06/07/2019, the pooled sensitivity for any cancer detection was 0.84 (95% CI, 0.80–0.88), and the specificity was 0.75 (95% CI, 0.68–0.81) for bpMRI. The pooled sensitivity for csPCa was 0.87 (95% CI, 0.78–0.93) and the specificity was 0.72 (95% CI, 0.56–0.84). Meta-regression analysis revealed no difference in the pooled diagnostic estimates between bpMRI and mpMRI.
In the two meta-analyses by Niu et al.[16] and more recently by Bass et al.[3], bpMRI offers a comparable diagnostic performance to that of mpMRI in the detection of PCa.
From a head-to-head comparison for detection of PCa, mpMRI had a significantly higher pooled sensitivity (0.85; 95% CI, 0.78–0.93) than that of bpMRI (0.80; 95% CI, 0.71–0.90) (p=0.01). However, the pooled specificity values were not significantly different (mpMRI 0.77 [95% CI, 0.58–0.95]; bpMRI 0.80 [95% CI, 0.64–0.96]; p=0.82).[3, 16]
Prostate bpMRI interpretation based on simplified PI-RADS
Before interpreting the images, it is important to assess their quality. The qualitative image analysis is aimed to detect and analyze focal lesions in the PZ and TZ of the prostate gland and seminal vesicles. A suspicious lesion is recognized on the basis of hyper and hypointensities on DWI/ADC maps and hypointensities on T2W images. In our experience, DWI represents the dominant sequence in the detection of lesions, both in PZ and TZ. To detect, localize, and manage a lesion in the prostate, we recommend to adopt a step approach as follows:
After the analysis of axial T1W with fat-saturation enhanced-T1 high-resolution isotropic volume examination (e-THRIVE) to exclude foci of hemorrhage, firstly the ADC map and the corresponding DWI with a high b-value (1500 s/mm2) is analyzed. According to the degree of restriction diffusion, the ADC map distinguishes lesions as homogeneous, moderately hypointense (heterogeneous or homogeneous), markedly hypointense. Then the T2W imaging is analyzed to confirm the lenticular or non-circumscribed, homogeneous hypointense lesion, and to assess the integrity or interruption of the capsule.
Lesion localization according to the 41 sectors/regions prostate map. [30]
Lesion measurement by volume (cc) estimation using the ellipsoidal formula (V=L×H×W×0.52) and 0.5 cc cut-off according to Epstein.[31] DWI with a high b-value (1500 s/mm2) is the preferred sequence for measuring both PZ and TZ lesions.
Index lesion (IL). In multifocal PCa, the index tumor corresponds to the cancer with the highest GS. In a prostate with multiple lesions, bpMRI indicates up to four suspicious areas and probable IL. If the multiple lesions show similar signal intensity on ADC (i.e. moderate or marked hypointensity), the IL can be considered as the one with the greatest volume. Conversely, in prostate with multiple lesions with different signal intensity on ADC map (i.e. moderate and marked hypointensity), the IL can be considered as the smallest markedly hypointense (the most aggressive with higher GS) than the largest but with moderate hypointensity on ADC.[32, 33]
By using this simplified algorithmic approach, readers with varying levels of experience of prostate bpMRI should be able to confidently identify and categorize a focal lesion of the prostate. To simplify the PI-RADS v2.1, on the basis of the aforementioned criteria, we suggested a (S-PI-RADS) (Figure 2) adapted to bpMRI at 3T without endorectal coil.[34–45]
Figure 2.
Simplified prostate imaging reporting and data system version according to biparametric magnetic resonance imaging. (Abbreviations: ADC: Apparent diffusion coefficient; DWI: Diffusion weighted imaging; T2WI: T2-weighted imaging. PSA: Prostate Specific Antigen; bpMRI: biparametric magnetic resonance imaging. Note: * Lesion volume is calculate by ellissoidal formula. ** Accurate evaluation of age and clinical informations are need. *** Category 4 includes lesions with volume < and > 0.5 cc and intra- and extraglandular lesions.
S-PI-RADS assesses four categories and for each, the management is indicated. Lesions with extra-prostatic extension (EPE) and/or invasion of the seminal vesicle are included in category 4 in addition to intraglandular lesions.[19–34]
With T2W spectral presaturation with inversion recovery, the entire pelvis sequences in addition to DWI sequences can help to detect lymph node involvement, bone metastases, and other findings.
Representative cases of S-PI-RADS category 3 and 4 lesions using bpMRI are reported in Figures 3 and 4.
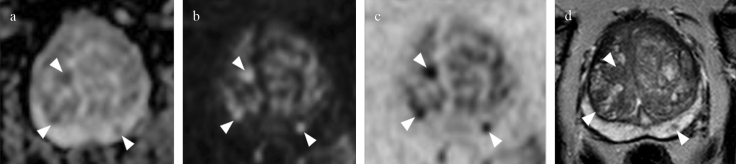
Figure 3. a–d.
Biparametric MRI of the prostate at 3T in 63-year-old, PSA=6.1 ng/mL. Round lesions located in the left posteromedial peripheral zone and in the left anterior and posterior transition zone of the midgland (arrowheads), is assigned to category 3a (volume<0.5 cc) by S-PI-RADS, no biopsy is indicated. (arrowheads in a) Moderately hypointense lesion on axial ADC map, (arrowheads in b) hypointense on axial DWI at high b-value, (arrowheads in c), axial DWI at high b-value inverted, and (arrowheads in d) hypointense on axial T2WI.
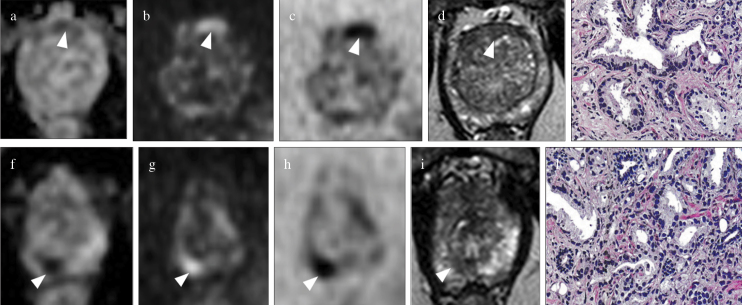
Figure 4. a–l.
Biparametric MRI of the prostate at 3T in a 71-year-old patient with PSA=11 ng/mL with multifocal prostate cancer. Ovalar lesion located in the anterior transition zone at midgland is assigned to S-PI-RADS category 3b lesion (volume>0.5 cc, targeted biopsy is indicated); (arrowhead in a) the lesion is moderately hypointense on axial ADC map, (arrowhead in b) hyperintense on axial DWI at high b-value, (arrowhead in c) hypointense on axial DWI at high b-value inverted, and (arrowhead in d) hypointense on axial T2WI: (e) Gleason score 6 on histology after targeted TRUS/MRI transperineal biopsy. The smallest round (index lesion) in the peripheral posteromedial zone at the apex is assigned to S-PI-RADS category 4 lesion (volume<0.5 cc, targeted biopsy is indicated); (arrowhead in f) the lesion is markedly hypointense on axial ADC map, (arrowhead in g) hyperintense on axial DWI at high b value, (arrowhead in h) hypointense on axial DWI at high b-value inverted, and (arrowhead in i) hypointense on axial T2WI: (l) Gleason score 7 on histology after targeted TRUS/MRI transperineal biopsy.
Discussion
In this review, we aimed to define the current state and the future directions of prostate non-contrast MRI or bpMRI, T2W, and DWI, as well as what radiologists and urologists should know for its appropriate use in the detection and management PCa.
A review of the literature from 2007 to 2019 showed that in addition to the mpMRI based on standardized PI-RADS guidelines, adoption of bpMRI studies in the PCa detection has increased.[3, 16]
From literature, has emerged a minimal role for DCE in the evaluating PCa and a similar diagnostic perfomance and reproducibility for mpMRI and bpMRI without endorectal coil by 3T and 1.5T MRI in detecting PCa is reported. [16–18, 11, 40, 46] Reduction of cost, short examination times, and the non-use of gadolinium work in favor of bpMRI.
In a recent narrative review, the PI-RADS committee supported the progression towards prostate bpMRI acceptance in PI-RADS v2.1 and concluded that bpMRI is a potential solution for meeting the increasing demand for MRI in the PCa diagnostic workup.[9]
Despite the technical improvements and subtle changes of PI-RADS v2.1 in PI-RADS score 3 or “equivocal” clinically significant lesions, their management remains a challenge. A meta-analysis by Niu et al.[16] has confirmed the high diagnostic accuracy of bpMRI in the detection of PCa; however, a proper scoring system is not reported for bpMRI which is therefore penalized in the management of lesions with PI-RADS 3 score when compared to mpMRI. The prevalence of clinically significant PCa for PI-RADS 3 score lesions is 24.9%, [47] but their management is unclear since a robust, clinically useful prediction model for a targeted biopsy decision is not reported.[48] In PI-RADS v2.1, the DCE updates the score of a lesion from 3 to 4 resulting in a reduction in the detection rate for lesions of score 3 and in a potential increase in unnecessary biopsies. In the perspective of a potential validation and standardization of bpMRI, a dedicated system aimed not only at the detection but also at the management of category 3 lesions is needed. This system cannot derive from PI-RADS guidelines or Likert scores by eliminating the DCE. [45]
For proper detection, categorization, and management of score 3 lesions, we adopted a S-PI-RADS using bpMRI by a 3T unit without endorectal coil. Interpreting bpMRI sequences in a standardized stepwise approach (ADC, DWI at high b-values, and T2W imaging) is essential for lesion detection and categorization. The S-PI-RADS assigns a dominant role to DWI in the detection of lesions, both in TZ and PZ. Lesion volume measurement (using a cutoff of 0.5 cc) and lesion categorization on DWI at high b-values and ADC map, respectively, provide a rapid and straightforward detection of category 3 (moderate hypointensity on ADC maps) and category 4 (marked hypointensity on ADC maps). Moreover, S-PI-RADS offers a clinical management option for category 3a lesion (volume<0.5 cc; accurate age assessment, clinical information, and follow-up via PSA and eventually bpMRI within one year is recommended) and category 3b lesions (volume≥0.5 cc; targeted biopsy is recommended).[34] Current PI-RADS v2.1 updated guidelines(1) in multifocal intraglandular PCa indicates IL as the one with the highest PI-RADS v2.1 assessment category. In the presence of extraprostatic extension (EPE), IL is the smaller one, although the larger lesion may have an identical PI-RADS v2.1 score. In a prostate with multiple lesions, according to others[32, 33] using S-PI-RADS, IL (lesion with the higher GS) can be considered the largest (when a lesion with similar moderate or marked hypointensity on ADC coexists) or the smallest markedly hypointense lesion in the ADC, compared to largest but with moderate hypointensity in the ADC.
In conclusion, bpMRI has similar diagnostic efficacy to mpMRI and the potential of replacing it as a simple solution. S-PI-RADS represents a valuable tool for radiologists and urologists in the detection and management of PCa and stimulates further studies for wide clinical use and its potential validation in PI-RADS v2.1.
Main Points.
Non-contrast or biparametric magnetic resonance imaging (MRI) (T2-weighted and diffusion-weighted imaging) shows similar diagnostic performance in detecting prostate cancer compared with multiparametric MRI and can be proposed as a potential alternative.
Diffusion-weighted imaging and apparent diffusion coefficient maps enable the detection and categorization of lesions, both in the transition and the peripheral zone.
Simplified prostate imaging reporting and data system using biparametric MRI is a valuable tool for radiologists and urologists in the detection and management of prostate cancer.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.S., P.S., E.M., A.D., A.D.B.; Design - M.S., P.S., E.M., A.D., A.D.B.; Supervision - M.S., P.S., E.M., A.D.B.; Materials - M.S.; Data Collection and/or Processing - M.S. A.I., M.C.A.; Analysis and/or Interpretation - M.S., A.I., A.D.B., R.T.; Literature Search - M.S., P.S., E.M., A.I., A.D., M.C.A., R.T.; Writing Manuscript - M.S., P.S., E.M.; Critical Review - M.S., P.S., E.M., A.D., A.D.B.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Drost FH, Osses D, Nieboer D, Bangma CH, Steyerberg EW, Roobol MJ, et al. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: a Cochrane systematic review and meta-analysis. Eur Urol. 2020;77:78–94. doi: 10.1016/j.eururo.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Bass EJ, Pantovic A, Connor M, Gabe R, Padhani AR, Rockall A, et al. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2020. Nov 20, [DOI] [PubMed]
- 4.Bjurlin MA, Carroll PR, Eggener S, Fulgham PF, Margolis DJ, Pinto PA, et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol. 2020;203:706–12. doi: 10.1097/JU.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202:343–51. doi: 10.2214/AJR.13.11046. [DOI] [PubMed] [Google Scholar]
- 6.Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2- weighted imaging. J Magn Reson Imaging. 2007;25:146–52. doi: 10.1002/jmri.20793. [DOI] [PubMed] [Google Scholar]
- 7.Delongchamps NB, Beuvon F, Eiss D, Flam T, Muradyan M, Zerbib M, et al. Multiparametric MRI is helpful to predict tumor focality, stage, and size in patients diagnosed with unilateral low-risk prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:232–7. doi: 10.1038/pcan.2011.9. [DOI] [PubMed] [Google Scholar]
- 8.Vilanova JC, Barceló-Vidal C, Comet J, Boada M, Barcelo J, Ferrer J, et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-total prostate-specific antigen ratio in the detection of prostate cancer. AJR Am J Roentgenol. 2011;196:W715–22. doi: 10.2214/AJR.10.5700. [DOI] [PubMed] [Google Scholar]
- 9.Schoots IG, Barentsz JO, Bittencourt LK, Haider MA, Macura KJ, Margolis DJA, et al. PI-RADS committee position on MRI without contrast medium in biopsy-naive men with suspected prostate cancer: narrative review. AJR Am J Roentgenol. 2021;216:3–19. doi: 10.2214/AJR.20.24268. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl CK, Bruhn R, Kramer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology. 2017;285:493–505. doi: 10.1148/radiol.2017170129. [DOI] [PubMed] [Google Scholar]
- 11.Tamada T, Kido A, Yamamoto A, Takeuchi M, Miyaji Y, Moriya T, et al. Comparison of biparametric and multiparametric MRI for clinically significant prostate cancer detection with PI-RADS version 2.1. J Magn Reson Imaging. 2021;53:283–91. doi: 10.1002/jmri.27283. [DOI] [PubMed] [Google Scholar]
- 12.Aisa MC, Piscioli I, Di Blasi A, Scialpi M. PSA/biparametric MRI: an accurate potential diagnostic approach for detection and management of local recurrence after radical prostatectomy. Turk J Urol. 2020;46:87–88. doi: 10.5152/tud.2019.19242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo S, Suh CH, Kim SY, Cho JY, Kim SH, Moon MH. Head-to-head comparison between biparametric and multiparametric MRI for the diagnosis of prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2018;211:W226–41. doi: 10.2214/AJR.18.19880. [DOI] [PubMed] [Google Scholar]
- 14.Alabousi M, Salameh JP, Gusenbauer K, Samoilov L, Jafri A, Yu H, et al. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis. BJU Int. 2019;124:209–20. doi: 10.1111/bju.14759. [DOI] [PubMed] [Google Scholar]
- 15.Liang Z, Hu R, Yang Y, An N, Duo X, Liu Z, et al. Is dynamic contrast enhancement still necessary in multiparametric magnetic resonance for diagnosis of prostate cancer: a systematic review and meta-analysis. Transl Androl Urol. 2020;9:553–73. doi: 10.21037/tau.2020.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu XK, Chen XH, Chen ZF, Chen L, Li J, Peng T. Diagnostic performance of biparametric MRI for detection of prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2018;211:369–78. doi: 10.2214/AJR.17.18946. [DOI] [PubMed] [Google Scholar]
- 17.Kang Z, Min X, Weinreb J, Li Q, Feng Z, Wang L. Abbreviated biparametric versus standard multiparametric MRI for diagnosis of prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;212:357–65. doi: 10.2214/AJR.18.20103. [DOI] [PubMed] [Google Scholar]
- 18.European Association of Urology EAU guidelines on prostate cancer. 2019. https://uroweb.org/guideline/prostate-cancer/
- 19.Scialpi M, D’Andrea A, Martorana E, Malaspina CM, Aisa MC, Napoletano M, et al. Biparametric MRI of the prostate. Turk J Urol. 2017;43:401–9. doi: 10.5152/tud.2017.06978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleason DF. Histologic grading of prostate cancer: a perspective. Hum Pathol. 1992;23:273–9. doi: 10.1016/0046-8177(92)90108-F. [DOI] [PubMed] [Google Scholar]
- 21.Ren J, Huan Y, Wang H, Zhao H, Ge Y, Chang Y, et al. Diffusion-weighted imaging in normal prostate and differential diagnosis of prostate diseases. Abdom Imaging. 2008;33:724–8. doi: 10.1007/s00261-008-9361-2. [DOI] [PubMed] [Google Scholar]
- 22.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–61. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 23.Downes MR, Gibson E, Sykes J, Haider M, van der Kwast TH, Ward A. Determination of the association between T2-weighted MRI and Gleason sub-pattern: a proof of principle study. Acad Radiol. 2016;23:1412–21. doi: 10.1016/j.acra.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 24.De Visschere PJ, Vral A, Perletti G, Pattyn E, Praet M, Magri V, et al. Multiparametric magnetic resonance imaging characteristics of normal, benign and malignant conditions in the prostate. Eur Radiol. 2017;27:2095–109. doi: 10.1007/s00330-016-4479-z. [DOI] [PubMed] [Google Scholar]
- 25.Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797–810. doi: 10.1148/rg.296095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boesen L, Chabanova E, Logager V, Balslev I, Thomsen HS. Apparent diffusion coefficient ratio correlates significantly with prostate cancer Gleason score at final pathology. J Magn Reson Imaging. 2015;42:446–53. doi: 10.1002/jmri.24801. [DOI] [PubMed] [Google Scholar]
- 28.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Fütterer J, Bouwense S, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–84. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 29.Engels RRM, Israël B, Padhani AR, Barentsz JO. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 1: acquisition. European Urology. 2020;77:457–68. doi: 10.1016/j.eururo.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Scialpi M, Scialpi P, Pusiol T, D’andrea A. Biparametric MRI and 41 sector map for MRI/Transrectal ultrasound fusion biopsy to increase diagnostic accuracy of prostate cancer. Turk J Urol. 2018;44:453–4. doi: 10.5152/tud.2018.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271:368–74. doi: 10.1001/jama.1994.03510290050036. [DOI] [PubMed] [Google Scholar]
- 32.Rosenkrantz AB, Deng FM, Kim S, Lim RP, Hindman N, Mussi TC, et al. Prostate cancer: multiparametric MRI for index lesion localization--a multiple-reader study. AJR Am J Roentgenol. 2012;199:830–7. doi: 10.2214/AJR.11.8446. [DOI] [PubMed] [Google Scholar]
- 33.Scialpi M, Scialpi P, Martorana E. Prostate cancer index lesion detection and volume estimation: is dynamic contrast-enhanced MRI really reliable. AJR Am J Roentgenol. 2019;213:W289. doi: 10.2214/AJR.19.21764. [DOI] [PubMed] [Google Scholar]
- 34.Scialpi M, Aisa MC, D’Andrea A, Martorana E. Simplified prostate imaging reporting and data system for biparametric prostate MRI: a proposal. AJR Am J Roentgenol. 2018;211:79–382. doi: 10.2214/AJR.17.19014. [DOI] [PubMed] [Google Scholar]
- 35.Scialpi M, Scialpi P, Aisa MC, Martorana E, D’Andrea A. Simplified PI-RADS with biparametric MRI: a practical approach to improve management of PI-RADS version 2 category 3 lesions. Radiology. 2018;289:882–3. doi: 10.1148/radiol.2018182092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scialpi M, Falcone G, Scialpi P, D’Andrea A. Biparametric MRI: a further improvement of PIRADS 2.0? Diagn Interv Radiol. 2016;22:297–8. doi: 10.5152/dir.2016.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scialpi M, Martorana E, D’Andrea A. Standardizing biparametric MRI to simplify and improve prostate imaging reporting and data system, version 2, in prostate cancer management. AJR Am J Roentgenol. 2016;207:W74–5. doi: 10.2214/AJR.16.16519. [DOI] [PubMed] [Google Scholar]
- 38.Scialpi M, Martorana E, Aisa MC, Rondoni V, D’Andrea A, Brunese L. Abbreviated biparametric prostate MR imaging: is it really an alternative to multiparametric MR imaging? Radiology. 2018;286:360. doi: 10.1148/radiol.2017171883. [DOI] [PubMed] [Google Scholar]
- 39.Scialpi M, Rondoni V, Aisa MC, Martorana E, D’Andrea A, Malaspina CM, et al. Is contrast enhancement needed for diagnostic prostate MRI? Transl Androl Urol. 2017;6:499–509. doi: 10.21037/tau.2017.05.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scialpi M, Prosperi E, D’Andrea A, Martorana E, Malaspina C, Palumbo B, et al. Biparametric versus multiparametric MRI with non-endorectal coil at 3T in the detection and localization of prostate cancer. Anticancer Res. 2017;37:1263–71. doi: 10.21873/anticanres.11443. [DOI] [PubMed] [Google Scholar]
- 41.Scialpi M, Martorana E, D’Andrea A. Standardizing biparametric MRI to simplify and improve prostate imaging reporting and data system, version 2, in prostate cancer management. AJR Am J Roentgenol. 2016;207:W74–5. doi: 10.2214/AJR.16.16519. [DOI] [PubMed] [Google Scholar]
- 42.Scialpi M, Martorana E, Scialpi P, D’Andrea A, Torre R, Di Blasi A, et al. Round table: arguments in supporting abbreviated or biparametric MRI of the prostate protocol. Abdom Radiol (NY) 2020;45:3974–81. doi: 10.1007/s00261-020-02510-w. [DOI] [PubMed] [Google Scholar]
- 43.Scialpi M, Di Blasi A, Scialpi P, Martorana E. In defense to arguments against using an abbreviated or biparametric prostate MRI protocol. Abdom Radiol (NY) 2020;45:4271–2. doi: 10.1007/s00261-020-02537-z. [DOI] [PubMed] [Google Scholar]
- 44.Martorana E, Scialpi P, Grisanti R, Scialpi M. Risk stratification system for biparametric prostate magnetic resonance imaging. Transl Androl Urol. 2019;8:S482–3. doi: 10.21037/tau.2019.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scialpi M, Martorana E, Aisa MC, Rondoni V, D’Andrea A, Bianchi G. Score 3 prostate lesions: a gray zone for PI-RADS v2. Turk J Urol. 2017;43:237–40. doi: 10.5152/tud.2017.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwazawa J, Mitani T, Sassa S, Ohue S. Prostate cancer detection with MRI: is dynamic contrast-enhanced imaging necessary in addition to diffusion-weighted imaging? Diagn Interv Radiol. 2011;17:243–8. doi: 10.4261/1305-3825.DIR.3605-10.1. [DOI] [PubMed] [Google Scholar]
- 47.Wadera A, Alabousi M, Pozdnyakov A, Kashif Al-Ghita M, Jafri A, McInnes MD, et al. Impact of PI-RADS category 3 lesions on the diagnostic accuracy of MRI for detecting prostate cancer and the prevalence of prostate cancer within each PI-RADS category: A systematic review and meta-analysis. Br J Radiol. 2021;94:20191050. doi: 10.1259/bjr.20191050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osses DF, Arsov C, Schimmöller L, Schoots IG, van Leenders GJLH, Esposito I, et al. Equivocal PI-RADS three lesions on prostate magnetic resonance imaging: risk stratification strategies to avoid MRI-targeted biopsies. J Pers Med. 2020;10:270. doi: 10.3390/jpm10040270. [DOI] [PMC free article] [PubMed] [Google Scholar]