Abstract
Characterizing molecular aspects of haustorium development by parasitic plants in the Orobanchaceae family has identified hormone signaling/transport and specific genes as major players.
Nutrient availability is one of the critical factors for plant survival and is mediated mainly by the root system. In addition to the roots, some species have evolved novel organs specialized for the acquisition of organic and inorganic nutrients. For example, most legume species form root nodules that accommodate nitrogen-fixing bacteria to acquire fixed nitrogen, whereas carnivorous plants have developed digestive leaves that trap and kill prey to absorb additional nutrients (Markmann and Parniske, 2009; Thorogood et al., 2018). Parasitic plants form a multicellular organ, the haustorium, emanating from either their shoots or roots, to parasitize other plants in order to acquire water and nutrients (Yoshida et al., 2016). Plant parasitism has independently evolved at least 12 times among angiosperms (Westwood et al., 2010). The Orobanchaceae family includes the largest number of parasitic species and all, except the genus Lindenbergia, Rehmannia, and Triaenophora, are root parasitic plants (Angiosperm Phylogeny Group, 2016; Li et al., 2019). Haustorium development proceeds in stages, including initiation, host penetration, and the formation of vascular connections. Recent progress in molecular and cellular biology has revealed that each phase is modulated by specific plant hormones, cellular programs, and signal exchanges between the host and parasite. In this review, we discuss recent updates on the molecular details of haustorium organogenesis in the Orobanchaceae family by focusing on genetic components and signaling molecules that function at each stage of haustorium development.
Advances
Molecular actors functioning at different stages of haustorium development have been identified, including quinone receptors, ethylene signaling components, and auxin transporters.
Haustorial cells change their identities throughout multiple developmental stages of the haustorium in response to host signals.
Genes involved in normal plant growth and development were co-opted for haustorium organogenesis by parasitic plants during their evolution.
Haustorium development in Orobanchaceae parasitic plants
The Orobanchaceae, with approximately 90 genera and 2,000 species, is the largest family of parasitic angiosperms and exhibits the entire spectrum of parasitism, from facultative (host-independent) to obligate (host-dependent), and from hemiparasitic (photosynthetic) to holoparasitic (nonphotosynthetic). Members of the Orobanchaceae family include notorious parasitic weeds, including Striga spp., Orobanche spp., Phelipanche spp., Rhamphicarpa fistulosa (Rodenburg et al., 2016), and Pedicularis kansuenses (Xiang et al., 2018), that pose great threats to the agriculture and ecology in Africa, Europe, and Asia.
There are two classes of haustoria, depending on the formation site: lateral haustoria and terminal haustoria. Terminal haustoria develop at root tips by terminating the meristematic activity of radical tips, followed by cell expansion (Olivier et al., 1991; Masumoto et al., 2020). Terminal haustoria result, therefore, from root tip transformation into a new organ. In contrast, lateral haustoria are initiated on the side of parasitic roots near the transition zone without permanently terminating meristematic activity at the root tip (Tomilov et al., 2005; Masumoto et al., 2020). Consequently, a single root can produce multiple lateral haustoria. Facultative parasitic plants produce only lateral haustoria throughout their life-cycle, whereas obligate parasites form a single terminal haustorium after germination and multiple lateral haustoria on later-emerging adventitious roots (Mutuku et al., 2020). As obligate parasites rely on host nutrients for postseedling growth, the formation of terminal haustoria is essential for survival.
Regardless of the morphological differences between lateral and terminal haustoria, both types have similar developmental processes. Haustorial development can be separated into three steps: haustorium initiation, host penetration, and haustorium maturation. Haustorium initiation requires host-derived haustorium-inducing factors (HIFs) in most species (Figure 1A; Xiang et al., 2018; Goyet et al., 2019). Upon HIF perception, the root cells start to expand and divide until the root assumes a dome-shaped swollen structure. In facultative parasites, cellar vacuolation and radial enlargement become apparent approximately 6 h after HIF treatment, and in parallel, cell division occurs within each cell layer of the root, including the epidermis, cortex, and stele (Baird and Riopel, 1984; Matvienko et al., 2001; Ishida et al., 2016; Wakatake et al., 2018; Cui et al., 2016; Wang et al. 2019). In terminal haustoria of the obligate parasite Striga asiatica, the first morphological changes, including termination of radicle elongation and subsequent radial expansion of the meristematic region, are apparent within 5–8 h after HIF treatment (Smith et al., 1990). Epidermal cells of the haustorium initiation site form protrusions, called haustorial hairs in hemiparasites and papillae in holoparasites (Cui et al., 2016, 2018a; Fernandez-Aparicio et al., 2016; Goyet et al., 2017, 2019). Once the parasite tissue reaches the host roots, host penetration begins; the epidermal cells at the host contact site differentiate into intrusive cells, slender-shaped cells capable of penetrating the host, and grow toward the host’s vascular center. After reaching the host’s vascular center, some of the intrusive cells differentiate into tracheary elements, eventually connecting the host’s xylem with the parasite’s xylem via newly established xylem bridges to form a mature haustorium.
Haustorium or prehaustorium?
The term haustorium is defined as “the special organ of parasitic plants, which invades host tissues and serves as the structural and physiological bridge that allows the parasites to withdraw water and nutrients from the conductive systems of living host plants” (Joel, 2013). In all cases, the hemispherical structures induced either by HIFs or host exudates/extracts lack internal cell components for nutrient absorption and host invasion, such as xylem bridges and intrusive cells, and therefore are distinguished from “haustoria” in the context of the tissue structure. In this regard, these early structures were referred to as an “early haustorial structure” (Goyet et al., 2019), “proto-haustorium” (Spallek et al., 2017), “pre-attachment haustorium” (Zhang et al., 2015), or “prehaustorium” (Ranjan et al., 2014; Goyet et al., 2019). Nevertheless, a majority of studies to date have not specified the term “haustorium,” sometimes leading to confusion, especially in nonparasitic plant spheres. In the stem parasite Cuscuta spp., the term “prehaustorium” was proposed by Peirce (1893) to describe the central cells behind meristematic tissue, and a similar structure was observed in Striga (Okwonkwo, 1967; Kuijt, 1969, 1977). Currently, the term “prehaustorium” in Cuscuta spp. is widely used to distinguish mechanically induced (immature) haustoria from host-infected (mature) haustoria (Ranjan et al., 2014; Costea and Tardif, 2006). In this review, we use “prehaustorium” to describe a haustorium induced in vitro or at the stage before host attachment, “invading haustorium” for that at the penetration stage bearing intrusive cells, and “haustorium” for that with a successfully established vascular connection to the host characterized by the presence of a xylem bridge (Figure 1A).
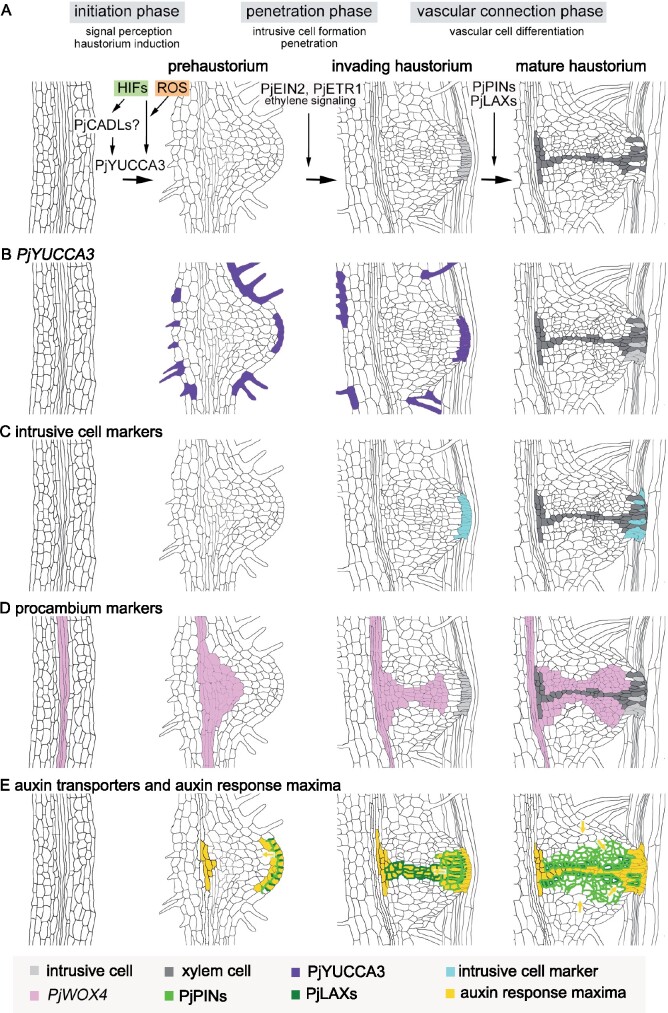
Figure 1.
Developmental stages of a facultative parasite haustorium. A, A schematic diagram of the three phases of haustorium formation. Signals and genes involved in each developmental phase are indicated in the figure. Quinone-type HIFs may be perceived by the orthologs of Arabidopsis CARD1 (CANNOT RESPOND TO DMBQ 1), named CARD-like proteins (CADLs), which drive expression of the auxin biosynthesis gene YUCCA3 (YUCCA-flavin monooxygenase 3) at the tip of the prehaustorium. During the host invasion stage, ethylene signaling driven by ETR1 and EIN2 is required. Directional localization of the auxin efflux transporter PIN (PIN-FORMED auxin transporter family proteins) and the auxin influx transporter LAX (LIKE-AUX1 transporter family proteins) define auxin flow at the center of haustoria where xylem bridges and plate xylem develop. B–E, Schematic diagrams of the expression patterns of select genes during haustorium development. B, Expression patterns (purple) of the auxin biosynthesis gene PjYUCCA3. C, Expression patterns (aqua) of intrusive cell marker genes. D, Expression patterns (pink) of procambium marker genes. E, Expression patterns of auxin transporters and local auxin maxima. Areas highlighted in yellow indicate the sites of localized auxin responses, and yellow arrows indicate direction of auxin flow. Dark and light green lines indicate the localization sites for the auxin transporters PIN and LAX, respectively
Initiation phase: converting a root into a prehaustorium
Haustorium-inducing factors
Prehaustorium induction in a majority of Orobanchaceae members requires HIFs, host-derived signaling molecules. Various chemicals, mainly phenolic compounds, and quinones, have been reported to be capable of inducing prehaustorium development in vitro (Albrecht et al., 1999; Goyet et al., 2019). Among them, 2,6-dimethoxybenzoquinone (DMBQ), a quinone isolated from sorghum root extracts (Chang and Lynn, 1986; Lynn and Chang, 1990) has the widest range of activity against numerous parasitic species, including the facultative parasites Agalinis purpurea, Tryphisaria spp., and Phtheirospermum japonicum, and the obligate hemiparasite Striga spp., but not for holoparasites such as Phelipanche or Orobanche spp. In Phelipanche ramosa, cytokinin plant hormones serve as HIFs; analysis of root exudates of the host plant Brassica napus identified cytokinin-like compounds responsible for prehaustorium induction (Goyet et al., 2017). Cytokinins were also reported to induce prehaustoria in Striga asiatica (Keyes et al., 2000). Sphaeropsidones, the phytotoxic cyclohexene oxides isolated from fungi, can induce prehaustoria in Orobanche crenata and O. cumana as well as in S. hermonthica (Fernandez-Aparicio et al., 2016).
Although DMBQ was identified from abraded sorghum roots, the origin of HIFs in host exudates remains obscure. The lack of any host mutant impaired in prehaustorium induction also hampers a complete understanding of the HIF generation pathway. A recent study demonstrates that HIFs are partially derived from the lignin biosynthetic pathway (Cui et al., 2018b). Several lignin monomers can induce prehaustoria in P. japonicum and S. hermonthica. Arabidopsis and rice mutants with altered lignin compositions are partially impeded in their abilities to induce prehaustoria formation in these parasites (Cui et al., 2018b). Chemical reactions during lignin degradation or polymerization, processes that require oxidative enzymes such as peroxidases and/or laccases, can produce DMBQ (Kamaya et al., 1981; Umezawa et al., 1982). Therefore, lignin, a highly conserved polymer in land plants, seems to be an important player in HIFs production. Notably, some parasites, including P. japonicum, S. hermonthica, and T. versicolor, do not induce prehaustoria against their own species or closely related species (Yoder, 1997; Yoshida and Shirasu, 2009). A recent report showed that peroxidase or laccase treatments on T. versicolor roots can produce HIFs, implying that parasitic plant roots may contain the substrates for HIFs (Wang et al., 2020). Interestingly, however, DMBQ was not detected in such oxidative enzyme-treated root extracts obtained from T. versicolor. Together with the fact that the DMBQ content in the host root exudates was three orders of magnitude lower than the concentration required to produce prehaustoria, there may be additional HIFs or HIF enhancers present in host plant root exudates (Wang et al., 2020). Deciphering the mechanisms for parasites to avoid self-haustorium development requires a systematic comparison of root exudates between hosts and parasites and will open innovative methods for generating crops resistant to parasitic weeds.
HIF perception
Functional genes directly regulating haustorium initiation have not been identified. Recently, a plasma membrane-localized leucine-rich-repeat receptor kinase (LRR-RK), named CANNOT RESPOND TO DMBQ 1 (CARD1), was identified as a molecule mediating quinone perception in Arabidopsis (Laohavisit et al., 2020). Wild-type Arabidopsis responds to DMBQ with an elevated cytosolic Ca2+ level and MAPK activation as outputs, whereas the card1 mutants are impaired in these responses. With the identification of CARD1, the study further uncovered the positive role of DMBQ signaling in the control of stomatal closure and plant immunity (Figure 2). Introduction of the P. japonicum and S. asiatica orthologs of CARD1 (CADLs) into the Arabidopsis card1 mutant restored DMBQ responses, indicating the functional conservation of CARD1 in quinone perception in these parasites. Importantly, DMBQ treatment induces the elevation of cytosolic Ca2+ only in the root elongation zone in P. japonicum where prehaustoria emerge, suggesting that the functional site of PjCADLs when the root is exposed to DMBQ is at the root tip. In P. japonicum, PjCADL1 is consistently expressed throughout the root, whereas PjCADL2 and PjCADL3 are expressed within the root epidermis; however, knockdown of individual PjCADL genes results in normal prehaustorium induction. Either these PjCADLs function redundantly or other genes are responsible. Note that because no direct interaction was observed between CARD1 and DMBQ, quinone signaling mediated by CARD1 may require genuine quinone receptor(s) and/or an additional co-receptor(s). A pair of cysteine residues in the extracellular domain of CARD1 was shown to be crucial for the signaling output (Laohavisit et al., 2020). The sulfur-containing amino acids cysteines are often the oxidation target of reactive oxygen species (ROS) that modulate protein conformation and/or activity via disulfide bond formation. It is conceivable that CARD1 may sense DMBQ in the form of extracellular redox changes, provided that quinones are redox-active molecules. Interestingly, CARD1 was also reported to be an H2O2 sensor in Arabidopsis (also designated as HYDROGEN-PEROXIDE-INDUCED Ca2+ INCREASE 1 (HPCA1); Wu et al., 2020). Similar to quinone sensing, pairs of extracellular cysteine residues in CARD1/HPCA1 are crucial for H2O2 sensing; however, the positions of crucial residues are different for H2O2 and quinone sensing (Laohavisit et al., 2020, Wu et al., 2020). This may suggest that CARD1/HPCA1 has dual function for perceiving extracellular H2O2 and quinones, although both signals are likely to be perceived via oxidation of the extracellular domain.
Figure 2.
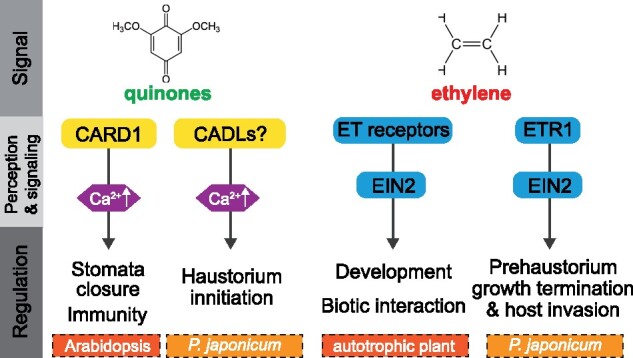
Functional divergence of plant regulatory genes in haustorium formation and function. Quinone molecules released from plants are perceived by CARD1 to regulate stomatal closure and plant immunity through cellular Ca2+ elevation in Arabidopsis. CARD1 orthologs (CADLs) are potentially involved in prehaustorium initiation through Ca2+ elevation. Ethylene (ET) signaling mediated by ethylene receptors, including ETR1, and ethylene signaling component EIN2, mediate organ development and biotic interactions in autotrophic plants. Both ETR1 and EIN2 are employed by P. japonicum to terminate prehaustorium growth in the absence of a host and to promote tissue penetration upon host attachment
Earlier studies have shown that redox potentials are associated with prehaustorium induction activity, and redox modifying chemicals inhibit prehaustorium induction in T. versicolor (Smith et al., 1996; Wang et al., 2019). Moreover, quinone oxidoreductases that are involved in the redox cycling of quinones are required in DMBQ-treated prehaustorium formation in T. versicolor and P. japonicum (Bandaranayake et al., 2010; Ishida et al., 2017). These results further suggest the involvement of redox signaling in the process from HIF perception to prehaustorium morphogenesis. ROS are also important in prehaustorium induction (Wada et al., 2019). Inhibitors of NADPH oxidases, superoxide dismutases, and peroxidases involved in ROS production or regulation block prehaustorium formation upon DMBQ treatment in S. hermonthica, suggesting that ROS are required for transducing the DMBQ signal (Figure 1A). To date, whether quinones and phenolics induce ROS in parasitic plants has not been clearly shown. In S. hermonthica, DMBQ treatment induces H2O2 production in prehaustoria at approximately 7-h post treatment, but not at the radical tip during the earlier initiation stage (Wada et al., 2019). Furthermore, DMBQ does not induce ROS generation in Arabidopsis seedlings (Laohavisit et al., 2020). The functional relationship between HIFs and ROS in prehaustorium formation remains to be solved.
Auxin-biosynthesis directs prehaustorium initiation
Auxin is known to have a central role in promoting cell division and, thus, meristematic activities in organ development. Several studies have documented the importance of the phytohormone auxin in haustorium initiation. Microarray analysis in DMBQ-treated P. japonicum revealed the upregulation of auxin-responsive genes (Ishida et al., 2016). PjYUC3, which encodes a catalytic enzyme for auxin biosynthesis, and the auxin-responsive DR5 marker are specifically expressed in the epidermis and outer cortical cells of the haustorium initiation site (Figure 1B; Ishida et al., 2016). Knockdown of PjYUC3 resulted in reduced haustorium formation levels, whereas ectopic expression of PjYUC3 in root epidermal cells induced the formation of haustorium-like structures (Ishida et al., 2016). Thus, auxin biosynthesis within epidermal cells is required and sufficient for prehaustorium formation.
In T. versicolor, an auxin-responsive marker and an ethylene-responsive marker are expressed in the root tips upon DMBQ treatment (Tomilov et al., 2005). Excess auxin application substantially increased the frequency of haustorium formation in T. versicolor, whereas disturbed auxin fluxes, either by application of an auxin efflux or auxin activity inhibitor, reduced the number of haustoria (Tomilov et al., 2005). Transcriptome analysis of Santalum album, a facultative root parasitic plant in the Santalaceae family, showed that auxin-related genes are upregulated in prehaustoria (preattached haustoria) and downregulated in postattached haustoria (Zhang et al., 2015).
Penetration phase
Firm attachment to the host by haustorial hairs
Haustorial hairs, also recognized as papillae in holoparasites, are tubular extensions from the haustorial surface and function in providing tight adhesion with the host surface by secreting mucilage (Baird and Riopel, 1983; Joel and Losner-Goshen, 1994; Heide-Jorgensen and Kuijt, 1995). Immunolabeling of cell-wall components indicates the presence of low-esterified homogalacturonan and arabinogalactan proteins at the epidermal surface of the holdfast that may act as adhesive compounds between the host and parasite cells in the stem parasite Cuscuta (Striberny and Krause, 2015; Hozumi et al., 2017). Phtheirospermum japonicum haustorial hair defective mutants (hhd) form fewer haustoria compared with the wild-type when infecting hosts; this impairment is restored by providing external forces that allow the host and parasite to grow side-by-side, implying that haustorial hairs support parasitism by attaching to the host root (Cui et al., 2016). Developmental programs for haustorial hairs are shared with those of root hairs since Pjhhd mutants are also defective in root hair formation. The auxin biosynthesis enzyme encoded by PjYUC3 and the auxin signaling marker DR5 are expressed during the development of haustorial hairs, suggesting that auxin may also be involved in haustorial hair formation, as in the root hairs (Ishida et al., 2016).
Intrusive cell differentiation
Soon after prehaustoria reach the host tissue surface, the prehaustorium begins penetrating the host (Figure 1A). The most prominent feature during penetration is the formation of intrusion-specific cells, called “intrusive cells” in Orobanchaceae parasitic plants and “searching hyphae” in dodders. These cells have a distinct slender shape and are laterally aligned to each other at the host interface. A recent forward genetic study in P. japonicum identified two mutants defective in intrusive cell formation upon host attachment and failure in host invasion; the responsible genes encode key ethylene signaling genes ETHYLENE INSENSITIVE 2 (EIN2) and ETHYLENE RESPONSE 1 (ETR1), respectively (Figure 1A; Cui et al., 2020). Both mutants are insensitive to ethylene, and the exogenous application of the ethylene signaling inhibitor AgNO3 during host infection completely blocked wild-type P. japonicum invasion. Pjetr1 and Pjein2 also exhibit prolonged cell division within the prehaustorium apex, giving rise to an elongated prehaustorium. These findings imply that ethylene signaling in parasitic plants plays a key role in host invasion by regulating cell division and differentiation within the haustorial apical region. Regulation of prehaustorium apex cell division may function to keep the prehaustorium elongated until it reaches the host root surface where invasion occurs. Interestingly, prehaustoria induced by root exudates or extracts, but not by DMBQ or syringic acid, occasionally showed prolonged cell division resulting in elongated prehaustoria (Cui et al., 2020). Importantly, these elongated prehaustoria can invade host tissues and establish xylem connections, suggesting that prehaustoria can sense the presence of the host and regulate apical cell division to reach the host’s location.
Host ethylene also contributes to parasite invasion. Arabidopsis mutants impaired in ethylene signaling (etr1, ein2) or biosynthesis (heptuple; defective in eight ACS enzymes) were less frequently invaded by wild-type P. japonicum. Overexpression of Ethylene Response Factor 2 (ERF2) in Arabidopsis reduced haustorium attachment in Phelipanche aegyptiaca at an early infection stage, possibly by affecting intrusive cell formation (Clarke et al., 2020). These findings indicate that parasitic plants adopted host recognition mechanisms by utilizing ethylene, a ubiquitous gaseous hormone widely implicated in plant developmental processes and biotic interactions (Figure 2).
Laser microdissection-based transcriptome analysis identified several intrusive cell-specific genes in P. japonicum, including a homolog of Haesa-like1 (HSL1) encoding LRR-RLK, Germin-Like Protein 1 (GLP1), Constitutive Disease Resistance 1 (CDR1), and four subtilases (SBTs; Figure 1C; Ogawa et al., 2020). Inhibition of subtilase activity by ectopic production of extracellular proteinase inhibitor 10 (Epi10) protein reduced expression of an intrusive cell-specific marker gene and caused defects in subsequent xylem bridge formation. These observations suggest that subtilase activity is important for intrusive cell formation and later haustorium development. In S. hermonthica, in-situ hybridization revealed a peroxidase specifically expressed in intrusive cells during host invasion (Yoshida et al., 2019).
Cell-wall modification enzymes during penetration
Immediately after entering the cortex layers, intrusive cells grow toward the host’s vascular center by pushing away and compressing host cells (Neumann et al., 1999), a process driven by not only mechanical force but also by modification of host cell walls. Numerous transcriptome analyses have confirmed the upregulation of cell-wall modifying enzymes in haustoria during host infection (Yang et al., 2015; Yoshida et al., 2019). Specifically, pectate lyases are commonly enriched in T. versicolor, S. hermonthica, and O. aegypitiaca (Yang et al., 2015). Also, genes encoding pectin methyl esterases (PMEs) and polygalacturonases that are involved in primary cell-wall modification, and Carbohydrate-Active enzymes (CAzyme) that are involved in cell-wall loosening, lignification, and suberization, are highly upregulated in S. hermonthica during rice infection (Yoshida et al., 2019; Mutuku et al., 2019). High pectinolytic activity and accumulation of pectate lyase were also confirmed in Cuscuta haustoria in their susceptible host interaction (Johnsen et al., 2015). Interestingly, in the interaction between nitrogen-fixing rhizobial bacteria and the legume Lotus japonicus, mutations in a pectate lyase gene in the host plant (LjNPL) arrested rhizobium infection and reduced the number of mature nodules (Xie et al., 2012), indicating a common requirement for pectin degradation in bacteria and parasitic plants in the context of tissue invasion.
Grafting and plant parasitism share many common cellular events including wounding, tissue adhesion, and forming a vascular connection (Melnyk, 2017). β-1,4-Glucanase (GH9B3), a secreted type of primary cell-wall modifying enzyme targeting cellulose, was recently shown to function in both interfamily grafting and haustorium maturation (Kurotani et al., 2020; Notaguchi et al., 2020). Nicotiana benthamiana GH9B3 and PjGH9B3 are induced in the grafting junction during compatible grafting and at the interface with the host in haustoria during the invasion stage, respectively. The genetic disruption of the corresponding genes impaired tobacco intergrafting to Arabidopsis and haustorium maturation in P. japonicum. These results suggest that extracellular GH9B3 facilitates tissue adhesion by targeting cellulose in the apoplast; however, many details about how GH9B3 modulates the cell wall to successfully attain grafting and parasitism remain to be investigated.
The question remains open as to how parasites degrade host cell walls, while simultaneously maintaining the integrity of their own cell wall. This is a fascinating topic as bacteria, fungi, and nematodes also digest cell walls in interactions with their hosts; however, among these organisms, only parasitic plants possess cell walls that are structurally similar to those of their hosts. Tissue invasion by parasitic plants, therefore, needs to target cell wall composition or modifications specific to the hosts but not their own, or spatiotemporally produce unique cell walls that are resistant to their own cell-wall degrading enzymes at the interface, or both. Molecular evidence for either of these proposed strategies is scarce as the biochemical activity of cell-wall modifying enzymes and the cell wall modification by parasitic plants have been poorly characterized. There is an interesting link to haustorium initiation: because the HIFs are also derived from cell walls and parasitic plants can avoid self-haustorium formation, parasitic plants may be able to distinguish hosts’ cell walls from their own with yet undiscovered mechanisms.
Vascular connection phase
Establishing cells with procambium-like identity in central haustoria
For absorbing water and nutrients, parasitic plants need to connect their vasculatures to those of the hosts. When the intrusive cells reach the host vasculature, some intrusive cells differentiate into tracheary elements. At the same time, some cells near the parasite’s root vasculature also differentiate into tracheary elements, thereby forming a mass of tracheary elements known as plate xylem (Dobbins and Kuijt, 1973; Mutuku et al., 2020). These tracheary elements are connected in the middle of haustoria and form a xylem bridge, establishing a xylem connection between the parasite and host (Ishida et al., 2016; Wakatake et al., 2018).
After host invasion but before xylem bridge formation, cells in the central part of the haustorium acquire a procambium cell-like identity (Figure 1D). Procambium cells act as vascular meristems and proliferate and differentiate into xylem, phloem, and cambium during plant vascular development upon receiving appropriate positional and molecular cues (Furuta et al., 2014). ARABIDOPSIS HOMEOBOX PROTEIN 15 (HB15) and WUSCHEL-RELATED HOMEOBOX 4 (WOX4) are known to regulate the formation and maintenance of procambial/cambial tissues in Arabidopsis (Prigge et al., 2005; Carlsbecker et al., 2010; Hirakawa et al., 2010; Etchells et al., 2013). Phtheirospermum japonicum orthologs of HB15 (PjHB15a and b) and WOX4 (PjWOX4) are expressed in the central region of haustoria before xylem bridge formation (Wakatake et al., 2018), suggesting a procambium-like identity of these cells. The sequential expression of PjHB15 and an ortholog of the xylem-differentiating marker CELLULOSE SYNTHASE CATALYTIC SUBUNIT 7/IRREGULAR XYLEM 3 (PjCESA7/IRX3) resembles the xylem differentiation processes in roots (Wakatake et al., 2018). The expression of these procambial markers is retained in actively dividing cells surrounding the xylem bridge, indicating that these cells maintain meristemic activity for tracheary element differentiation. These activities lead to more xylem bridge formation, while simultaneously proliferating cells to expand haustorium size comparable to the lateral growth of the roots and shoots (Sanchez et al., 2012; Miyashima et al., 2019).
Xylem bridge formation defined by auxin flow
Xylem continuity with host plants is a prominent feature observed in most parasitic plants (Kuijt 1969). In the Orobanchaceae, the formation of xylem bridges is mediated by coordinated expression of auxin transporter and auxin biosynthesis genes. An auxin biosynthesis gene, PjYUC3, is expressed primarily in the prehaustorial epidermis and neighboring cortex cells; expression of this gene is also maintained at the haustorial apex during the early phase of host invasion (Figure 1B; Ishida et al., 2016; Wakatake et al., 2020). After host invasion, the expression of DR5, an auxin-responsive marker, indicates the presence of auxin-response maxima in intrusive cells, the plate xylem-forming region, and the middle of haustoria (Figure 1E). The temporal and spatial expression of DR5 coincides with the expression of PjCESA7/IRX3, a xylem cell differentiation marker, at the site for xylem bridge formation (Wakatake et al., 2020). High levels of cellular auxin signaling are a key determinant for xylem cell identity in Arabidopsis roots (Smetana et al., 2019). Therefore, the function of auxin in the control of cell pattern formation is likely conserved in haustorial vessel differentiation.
The creation of auxin-response maxima in undifferentiated cells at the xylem bridge-forming site is seemingly regulated by auxin transporters, PINs and LAXs. The auxin efflux carriers, PjPIN1and PjPIN9, and the auxin influx carriers, PjLAX1, PjLAX2, and PjLAX5, are expressed in substantially different regions before and during haustorium formation (Wakatake et al., 2020). Application of inhibitors of auxin-efflux transport blocks xylem bridge formation, and knockdown of PjPIN1 or PjPIN9 by RNAi disrupts tracheary element differentiation at the host interface or plate xylem region, respectively, suggesting different roles for each PIN transporter in xylem bridge formation (Wakatake et al., 2020). Treatment with inhibitors of auxin-influx transport did not block xylem bridge formation but resulted in distorted xylem bridges, indicating that the LAX family of transporters shapes auxin gradients to form simple xylem paths between the host and parasite.
The processes of xylem bridge formation, including the expression of procambium markers and auxin transporters, direct the formation of local auxin maxima followed by xylem cell differentiation. These steps are similar to those for vascular differentiation in Arabidopsis roots (Smetana et al., 2019). In Arabidopsis, AtHB8 encodes an HD-ZIP III whose ectopic expression induces xylem vessel formation. In P. japonicum, expression of PjHB8, the ortholog of AtHB8, is observed in the central region of haustoria (Figure 1D; Wakatake et al., 2018). This finding suggests that procambium-like cells in central haustoria have xylem procambium identity rather than vascular stem cell identity, a proposal supported by the observation that only xylem cells, and not phloem cells, are present in the haustoria of Orobanchaceae hemiparasites.
Phloem development in haustoria
Phloem is a nutrient-conductive tissue in plant vascular systems. Some holoparasitic species, including Orobanche crenata (Dörr and Kollmann, 1995), Orobanche cumana (Krupp et al., 2019), and Cuscuta (Forste et al., 2020), directly connect their phloem sieve elements with those of host plants in the haustorium. These phloem–phloem connections have not been observed in hemiparasites regardless of whether they are obligate or facultative parasites (Dörr and Kollmann, 1977; Dörr et al., 1979; Dörr, 1990, Masumoto et al., 2020). Consistently, host-driven mobile green fluorescent protein (GFP) is transported to parasitic plants through haustoria in holoparasitic Phelipanche ramosa and P. aegyptiaca (Ackroyd and Graves, 1997; Peron et al., 2016), but not in the hemiparasitic P. japonicum (Spallek et al., 2017). Furthermore, the phloem differentiation marker, Arabidopsis ALTERED PHLOEM DEVELOPMENT (APL; Bonke et al., 2003) was not expressed within haustoria except in the basal region of P. japonicum (Masumoto et al., 2020), suggesting that haustoria lack characteristic phloem cells. These observations suggest that phloem development in haustoria may be associated with the loss of photosynthetic ability. Interestingly, although host-derived GFP is transported to haustoria in holoparasitic P. aegyptiaca, phloem sieve elements with typical characteristics of callose accumulation and enucleation are not observed in haustoria (Ekawa and Aoki, 2017); however, NAC45, NEN1, and NEN4, putative orthologs of key regulatory genes for enucleation in Arabidopsis (Furuta et al., 2014), are expressed in haustoria (Ekawa and Aoki, 2017). These observations suggest that the GFP-transporting cells in P. aegyptiaca haustoria may have partially acquired features of phloem sieve elements despite lacking the typical morphological characteristics of well-studied Arabidopsis phloem sieve elements.
Material transfer
The fundamental function of haustoria is to absorb water and nutrients from their hosts. Nutrient flow from host to parasite suggests that haustoria act as sink organs, whereas the host plants become sources; however, how and whether haustoria create sink strength remain obscure. Orobanche spp. accumulate large amounts of mannitol and may use this sugar-alcohol to generate an osmotic gradient for material flow from the host toward the parasite (Aly et al., 2009). Potential regulatory mechanisms for xylem flow from the host to the parasites were recently revealed at the molecular level in S. hermonthica (Fujioka et al., 2019). The study demonstrated that S. hermonthica bears mutations in a gene encoding protein phosphatase 2C (PP2C), a key regulator of abscisic acid (ABA) signaling, and is incapable of closing stomata due to its insensitivity to ABA. This insensitivity leads to a higher transpiration rate in Striga than in its host, generating a water potential gradient toward the parasite (Ackroyd and Graves, 1997).
Hyaline bodies, specialized parenchyma cells in the center of haustoria with especially dense cytoplasm, extracellular deposits, and high metabolic activity (Visser et al., 1984; Riopel and Timko, 1995; Pielach et al., 2014), may function as storage organs that can generate sink pressure. Consistent with this hypothesis, the formation of hyaline bodies corresponds well with host compatibility (Gurney et al., 2003; Pielach et al., 2014); however, some parasites including T. versicolor and P. japonicum do not develop hyaline bodies but can increase their fitness upon host infection (Heide-Jorgensen and Kuijt, 1995; Spallek et al., 2017; Honaas et al., 2019; Irving et al., 2019; Masumoto et al., 2020). These observations suggest that strategies for nutrient processing or transport may vary depending on the species.
Recent findings in Cuscuta have remarkably extended the scope of macromolecules that are transported between parasitic plants and their hosts, including mRNAs, miRNAs, artificial siRNAs, and proteins (Alakonya et al., 2012; Kim and Westwood, 2015; Shahid et al., 2018; Liu et al., 2019). Some macromolecules are functional upon delivery, for example, Cuscuta australis whose genome lacks the FLOWERING LOCUS T (FT) gene obtains the FT from the host to regulate its own flowering (Shen et al., 2020). In root parasites, P. japonicum produces cytokinin plant hormones and transfers them to the host to induce host cell proliferation and vascular cell differentiation. These activities result in the thickening of host roots, a phenomenon called hypertrophy, that is thought to be beneficial for the parasite’s postattachment growth (Spallek et al., 2017). Together, these observations indicate that haustoria actuate selective transport; however, the regulatory mechanisms for such directional transport remain an open question. Analyses of the expression and localization of transporter proteins may give us a clue to resolve these questions. Recent transcriptome analysis indicates that sets of sugar transporters are expressed during haustorium formation in Orobanchaceae parasitic plants (Misra et al., 2019). Although the localization and transport direction of these transporters are unknown, they may participate in effective nutrient transport.
Concluding remarks and future perspectives
Recent advances in the molecular and cellular biology of parasitic plants have begun to reveal the genetic programs for haustorium organogenesis (Ichihashi et al., 2020). A haustorium in the Orobanchaceae develops through reprogramming of root tissues, and the cellular functions and identities change depending on the developmental stage and the relationship with the host. Host signals, such as HIFs, ethylene, and as yet unknown factors for intrusive cell differentiation and vascular differentiation, provoke changes in haustorial cell identity. Concomitantly, parasitic plants avoid self-recognition during haustorium initiation and self-digestion during penetration. The plant hormone auxin functions across all stages of haustorium development. Auxin accumulation at the organ initiation site and xylem pattern formation are also manifested in other plant tissues, suggesting that basic programs for haustorium organogenesis are co-opted from the developmental programs of other plant tissues. Gene expression similarity between lateral root development and haustorium formation has been proposed (Yoshida et al., 2019); however, there are still many gaps remaining to completely understand the regulatory mechanisms controlling haustorium organogenesis (see Outstanding questions). Notably, how interspecies communication regulates organ development remains unknown. Although identifying quinone receptors could provide clues for elucidating the entire signaling pathway for haustorium organogenesis, how the known and unknown HIFs execute a developmental program for haustorium initiation, including the activation of YUCCA3-mediated auxin signaling, remain unknown. Furthermore, the signals for intrusive cell differentiation and how intrusive cells penetrate host tissues without damaging their own cell walls are also unknown. Importantly, the substance used as a host signal for haustorial xylem induction and the subsequent signaling events, such as perception and the downstream targets, are still undetermined. Identifying the signaling compounds and the transduction pathway components will provide a more complete picture of haustorium formation. Advances in these areas will contribute to understanding how interspecies communication affects organ development during plant evolution.
Outstanding questions
What are the identities of host signals that trigger cell fate changes at each step of haustorium development, e.g., key HIF(s), differentiation of intrusive cells, and induction of xylem bridges?
Are all HIFs perceived by CARD1-like receptors?
What are the signaling cascades that link HIF perception to the induction of YUCCA3 and other downstream genes to effect haustorium initiation?
How do parasitic plants avoid self-recognition during haustorium initiation?
How do parasitic cell-wall modifying enzymes act specifically on host cell walls?
How are organic materials transported from the host to parasitic plants, and how is the direction of transport defined?
Funding
This work was partly supported by KAKENHI [Grant number 17K15139 to K.M.F., 19K16169 to SC, 18H02464, 18H04838, and 20H05909 to S.Y.], JST PRESTO [Grant number JPMJPR194D], and the International Atomic Energy Agency Research [Contract number 20645 to S.Y.]. K.M.F. was supported by JSPS Restart Postdoctoral Fellowship program (RPD).
Conflict of interest statement. Authors declare that they have no conflicts of interests.
K.M.F., L.X., S.C., and S.Y. conceived and wrote the manuscript. K.M.F. and S.C. drew the figures. S.C. and S.Y. finalized the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Satoko Yoshida (satokoy@bs.naist.jp).
References
- Ackroyd RD, Graves JD (1997) The regulation of the water potential gradient in the host and parasite relationship between Sorghum bicolor and Striga hermonthica. Ann Bot 80: 649–656 [Google Scholar]
- Alakonya A, Kumar R, Koenig D, Kimura S, Townsley B, Runo S, Garces HM, Kang J, Yanez A, David-Schwartz R, et al. (2012) Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts Cuscuta pentagona plant parasitism. Plant Cell 24: 3153–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht H, Yoder JI, Phillips DA (1999) Flavonoids promote haustoria formation in the root parasite Triphysaria versicolor. Plant Physiol 119: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R, Cholakh H, Joel DM, Leibman D, Steinitz B, Zelcer A, Naglis A, Yarden O, Gal-On A (2009) Gene silencing of mannose 6-phosphate reductase in the parasitic weed Orobanche aegyptiaca through the production of homologous dsRNA sequences in the host plant. Plant Biotechnol J 7: 487–498 [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181: 1–20 [Google Scholar]
- Baird WM V, Riopel JL (1983) Experimental studies of the attachment of the parasitic angiosperm Agalinis purpurea to a host. Protoplasma 218: 206–218 [Google Scholar]
- Baird WM V, Riopel JL (1984) Experimental studies of haustorium initiation and early development in Agalinis purpurea (L.) RAF. (Scrophulariaceae). Am J Bot 71: 803–814 [Google Scholar]
- Bandaranayake PCG, Filappova T, Tomilov A, Tomilova NB, Jamison-McClung D, Ngo Q, Inoue K, Yoder JI (2010) A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. Plant Cell 22: 1404–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mahonen AP, Hauser MT, Helariutta Y (2003) APL regulates vascular tissue identity in Arabidopsis. Nature 426: 181–186 [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vaten A, Thitamadee S, et al. (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Lynn DG (1986) The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol 12: 561–579 [DOI] [PubMed] [Google Scholar]
- Clarke CR, Park SY, Tuosto R, Jia X, Yoder A, Van Mullekom J, Westwood J (2020) Multiple immunity-related genes control susceptibility of Arabidopsis thaliana to the parasitic weed Phelipanche aegyptiaca. PeerJ 8: e9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea M, Tardif FJ (2006) The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Can J Plant Sci 86: 293–316 [Google Scholar]
- Cui S, Kubota T, Nishiyama T, IJ K., Shigenobu S, Shibata TF, Toyoda A, Hasebe M, Shirasu K, Yoshida S (2020) Ethylene signaling mediates host invasion by parasitic plants. Sci Adv 6: eabc2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Suzaki T, Tominaga-Wada R, Yoshida S (2018a) Regulation and functional diversification of root hairs. Semin Cell Dev Biol 83: 115–122 [DOI] [PubMed] [Google Scholar]
- Cui S, Wada S, Tobimatsu Y, Takeda Y, Saucet SB, Takano T, Umezawa T, Shirasu K, Yoshida S (2018b) Host lignin composition affects haustorium induction in the parasitic plants Phtheirospermum japonicum and Striga hermonthica. New Phytol 218: 710–723 [DOI] [PubMed] [Google Scholar]
- Cui S, Wakatake T, Hashimoto K, Saucet SB, Toyooka K, Yoshida S, Shirasu K (2016) Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant Phtheirospermum japonicum. Plant Physiol 170: 1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins DR, Kuijt J (1973) Studies on the haustorium of Castilleja (Scrophulariaceae). II. The endophyte. Can J Botany 51: 923–931 [Google Scholar]
- Dörr I (1990) Sieve elements in haustoria of parasitic angiosperms. InBehnke HD, Sjolund RD, eds, Sieve Elements. Springer, New York, pp 239–256 [Google Scholar]
- Dörr I, Kollmann R (1977) Strukturelle grundlagen des parasitismus bei Orobanche. II. Die differenzierung der assimilat-leitungsbahn im haustorialgewebe. Protoplasma 83: 185–199 [Google Scholar]
- Dörr I, Kollmann R (1995) Symplasmic sieve element continuity between Orobanche and its host. Botanica Acta 108: 47–55 [Google Scholar]
- Dörr I, Visser JH, Kollmann R (1979) On the parasitism of Alectra vogelii Benth. (Scrophulariaceae) III. The occurrence of phloem between host and parasite. Z Pflanzenphysiol 84: 213–222 [Google Scholar]
- Ekawa M, Aoki K (2017) Phloem-conducting cells in haustoria of the root-parasitic plant Phelipanche aegyptiaca retain nuclei and are not mature sieve elements. Plants 6: 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Mishra L, Turner SR (2013) WOX4 and WOX14 act downstream of the PXY receptor kinase to regulate plant vascular proliferation independently of any role in vascular organisation. Development 140: 2224–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Aparicio M, Masi M, Maddau L, Cimmino A, Evidente M, Rubiales D, Evidente A (2016) Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J Agric Food Chem 64: 5188–5196 [DOI] [PubMed] [Google Scholar]
- Forste F, Mantouvalou I, Kanngiesser B, Stosnach H, Lachner LA, Fischer K, Krause K (2020) Selective mineral transport barriers at Cuscuta-host infection sites. Physiol Plant 168: 934–947 [DOI] [PubMed] [Google Scholar]
- Fujioka H, Samejima H, Suzuki H, Mizutani M, Okamoto M, Sugimoto Y (2019) Aberrant protein phosphatase 2C leads to abscisic acid insensitivity and high transpiration in parasitic Striga. Nat Plants 5: 258–262 [DOI] [PubMed] [Google Scholar]
- Furuta KM, Yadav SR, Lehesranta S, Belevich I, Miyashima S, Heo JO, Vaten A, Lindgren O, De Rybel B, Van Isterdael G, et al. (2014) Plant development. Arabidopsis NAC45/86 direct sieve element morphogenesis culminating in enucleation. Science 345: 933–937 [DOI] [PubMed] [Google Scholar]
- Goyet V, Billard E, Pouvreau J-B, Lechat M-M, Pelletier S, Bahut M, Monteau F, Spíchal L, Delavault P, Montiel G, et al. (2017) Haustorium initiation in the obligate parasitic plant Phelipanche ramosa involves a host-exudated cytokinin signal. J Exp Bot 68: 5539–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyet V, Wada S, Cui S, Wakatake T, Shirasu K, Montiel G, Simier P, Yoshida S (2019) Haustorium inducing factors for parasitic Orobanchaceae. Front Plant Sci 10: 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney AL, Grimanelli D, Kanampiu F, Hoisington D, Scholes JD, Press MC (2003) Novel sources of resistance to Striga hermonthica in Tripsacum dactyloides, a wild relative of maize. New Phytol 160: 557–568 [DOI] [PubMed] [Google Scholar]
- Heide-Jorgensen HS, Kuijt J (1995) The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastrucutre. Am J Bot 82: 782–797 [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22: 2618–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaas LA, Jones S, Farrell N, Kamerow W, Zhang H, Vescio K, Altman NS, Yoder JI, dePamphilis CW (2019) Risk versus reward: host dependent parasite mortality rates and phenotypes in the facultative generalist Triphysaria versicolor. BMC Plant Biol 19: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi A, Bera S, Fujiwara D, Obayashi T, Yokoyama R, Nishitani K, Aoki K (2017) Arabinogalactan proteins accumulate in the cell walls of searching hyphae of the stem parasitic plants, Cuscuta campestris and Cuscuta japonica. Plant Cell Physiol 58: 1868–1877 [DOI] [PubMed] [Google Scholar]
- Ichihashi Y, Hakoyama T, Iwase A, Shirasu K, Sugimoto K, Hayashi M (2020) Common mechanisms of developmental rerogramming in plants—lessons from regeneration, symbiosis, and parasitism. Front Plant Sci 11: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida JK, Wakatake T, Yoshida S, Takebayashi Y, Kasahara H, Wafula E, dePamphilis CW, Namba S, Shirasu K (2016) Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 28: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida JK, Yoshida S, Shirasu K (2017) Quinone oxidoreductase 2 is involved in haustorium development of the parasitic plant Phtheirospermum japonicum. Plant Signal Behav 12: e1319029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving LJ, Kim D, Schwier N, Vaughan JKE, Ong G, Hama T (2019) Host nutrient supply affects the interaction between the hemiparasite Phtheirospermum japonicum and its host Medicago sativa. Environ Exp Bot 162: 125–132 [Google Scholar]
- Joel DM (2013) The haustorium and the life cycles of parasitic Orobanchaceae. InJoel DM, Gressel J, Musselman LJ, eds, Parasitic Orobanchaceae. Springer, New York, pp 21–24 [Google Scholar]
- Joel DM, Losner-Goshen D (1994) The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can J Bot 72: 564–574 [Google Scholar]
- Johnsen HR, Striberny B, Olsen S, Vidal-melgosa S, Fangel JU, Willats WGT, Rose JKC, Krause K (2015) Cell wall composition profiling of parasitic giant dodder (Cuscuta reflexa) and its hosts: a priori differences and induced changes. New Phytol 207: 805–881 [DOI] [PubMed] [Google Scholar]
- Kamaya Y, Nakatsubo F, Higuchi T, Iwahara S (1981) Degradation of d,l-syringaresinol, a β-β′ linked lignin model compound, by Fusarium solani M-13-1. Arch Microbiol 129: 305–309 [Google Scholar]
- Keyes WJ, O’Malley RC, Kim D, Lynn DG (2000) Signaling organogenesis in parasitic angiosperms: xenognosin generation, perception, and response. J Plant Growth Regul 19: 217–231 [DOI] [PubMed] [Google Scholar]
- Kim G, Westwood JH (2015) Macromolecule exchange in Cuscuta-host plant interactions. Curr Opin Plant Biol 26: 20–25 [DOI] [PubMed] [Google Scholar]
- Krupp A, Heller A, Spring O (2019) Development of phloem connection between the parasitic plant Orobanche cumana and its host sunflower. Protoplasma 256: 1385–1397 [DOI] [PubMed] [Google Scholar]
- Kuijt J (1969) The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA [Google Scholar]
- Kuijt J (1977) Haustoria of phanerogamic parasites. Annu Rev Phytopath 15: 91–118 [Google Scholar]
- Kurotani K-I, Wakatake T, Ichihashi Y, Okayasu K, Sawai Y, Ogawa S, Cui S, Suzuki T, Shirasu K, Notaguchi M (2020) Host-parasite tissue adhesion by a secreted type of β-1,4-glucanase in the parasitic plant Phtheirospermum japonicum. Commun Biol 3: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Wakatake T, Ishihama N, Mulvey H, Takizawa K, Suzuki T, Shirasu K (2020) Quinone perception in plants via leucine-rich-repeat receptor-like kinases. Nature 587: 92–97 [DOI] [PubMed] [Google Scholar]
- Li X, Feng T, Randle C, Schneeweiss GM (2019) Phylogenetic relationships in Orobanchaceae inferred from low-copy nuclear genes: consolidation of major clades and identification of a novel position of the non-photosynthetic Orobanche clade sister to all other parasitic Orobanchaceae. Front Plant Sci 10: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Shen G, Xu Y, Liu H, Zhang J, Li S, Li J, Zhang C, Qi J, Wang L, Wu J (2019) Extensive inter-plant protein transfer between Cuscuta parasites and their host plants. Mol Plant 13: 573–585 [DOI] [PubMed] [Google Scholar]
- Lynn DG, Chang M (1990) Phenolic signals in cohabitation - Implications for plant development. Annu Rev Plant Physiol Plant Mol Biol 41: 497–526 [Google Scholar]
- Markmann K, Parniske M (2009) Evolution of root endosymbiosis with bacteria: how novel are nodules? Trends Plant Sci 14: 77–86 [DOI] [PubMed] [Google Scholar]
- Masumoto N, Suzuki Y, Cui S, Wakazaki M, Sato M, Shibata A, Furuta KM, Ichihashi Y, Shirasu K, Toyooka K, et al. (2021) Three-dimensional reconstructions of haustoria in two parasitic plant species in the Orobanchaceae. Plant Physiol 185: 1429–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matvienko M, Torres MJ, Yoder JI (2001) Transcriptional responses in the hemiparasitic plant Triphysaria versicolor to host plant signals. Plant Physiol 127: 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk CW (2017) Plant grafting: insights into tissue regeneration. Regeneration (Oxf) 4: 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra VA, Wafula EK, Wang Y, dePamphilis CW, Timko MP (2019) Genome-wide identification of MST, SUT and SWEET family sugar transporters in root parasitic angiosperms and analysis of their expression during host parasitism. BMC Plant Biol 19: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Roszak P, Sevilem I, Toyokura K, Blob B, Heo JO, Mellor N, Help-Rinta-Rahko H, Otero S, Smet W, et al. (2019) Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 565: 490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku JM, Cui S, Hori C, Takeda Y, Tobimatsu Y, Nakabayashi R, Mori T, Saito K, Demura T, Umezawa T, et al. (2019) The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiol 179: 1796–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku JM, Cui S, Yoshida S, Shirasu K (2020) Orobanchaceae parasite-host interactions. New Phytol 230: 46–59 [DOI] [PubMed] [Google Scholar]
- Neumann U, Vian B, Weber HC, Salle G (1999) Interface between haustoria of parasitic members of the Scrophulariaceae and their hosts: a histochemical and immunocytochemical approach. Protoplasma 207: 84–97 [Google Scholar]
- Notaguchi M, Kurotani KI, Sato Y, Tabata R, Kawakatsu Y, Okayasu K, Sawai Y, Okada R, Asahina M, Ichihashi Y, et al. (2020) Cell-cell adhesion in plant grafting is facilitated by beta-1,4-glucanases. Science 369: 698–702 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Wakatake T, Spallek T, Ishida JK, Sano R, Kurata T, Demura T, Yoshida S, Ichihashi Y, Schaller A, et al. (2020) Subtilase activity in the intrusive cells mediates haustorium maturation in parasitic plants. Plant Physiol 185: 1381–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwonkwo SNC (1967) Studies on Striga senegalensis Benth. I. Mode of host-parasite union and haustorial structure. Phytomorph 16: 453–463 [Google Scholar]
- Olivier A, Benhamou N, Leroux GD (1991) Cell surface interactions between sorghum roots and the parasitic weed Striga hermonthica: cytochemical aspects of cellulose distribution in resistant and susceptible host tissues. Can J Bot 69: 1679–1690 [Google Scholar]
- Peirce GJ (1893) On the structure of the haustoria of some phanerogamic parasites. Ann Bot os7: 291–324 [Google Scholar]
- Peron T, Candat A, Montiel G, Veronesi C, Macherel D, Delavault P, Simier P (2016) New insights into phloem unloading and expression of sucrose transporters in vegetative sinks of the parasitic plant Phelipanche ramosa L. (Pomel). Front Plant Sci 7: 2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielach A, Leroux O, Domozych DS, Knox JP, Popper Z a (2014) Arabinogalactan protein-rich cell walls, paramural deposits and ergastic globules define the hyaline bodies of rhinanthoid Orobanchaceae haustoria. Ann Bot 114: 1359–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, Ichihashi Y, Farhi M, Zumstein K, Townsley B, David-Schwartz R, Sinha NR (2014) De novo assembly and characterization of the transcriptome of the parasitic weed Cuscuta pentagona identifies genes associated with plant parasitism. Plant Physiol 166: 1186–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopel JL, Timko MP (1995) Haustorial initiation and differentiation. InPress MC, Graves JD, eds, Parasitic Plants. Chapman & Hall, New York, pp 39–79 [Google Scholar]
- Rodenburg J, Demont M, Zwart SJ, Bastiaans L (2016) Parasitic weed incidence and related economic losses in rice in Africa. Agric Ecosyst Environ 235: 306–317 [Google Scholar]
- Sanchez P, Nehlin L, Greb T (2012) From thin to thick: major transitions during stem development. Trends Plant Sci 17: 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S, Kim G, Johnson NR, Wafula E, Wang F, Coruh C, Bernal-Galeano V, Phifer T, dePamphilis CW, Westwood JH, et al. (2018) MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553: 82–85 [DOI] [PubMed] [Google Scholar]
- Shen G, Liu N, Zhang J, Xu Y, Baldwin IT, Wu J (2020) Cuscuta australis (dodder) parasite eavesdrops on the host plants' FT signals to flower. Proc Natl Acad Sci USA 117: 23125–23130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana O, Makila R, Lyu M, Amiryousefi A, Sanchez Rodriguez F, Wu MF, Sole-Gil A, Leal Gavarron M, Siligato R, Miyashima S, et al. (2019) High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 565: 485–489 [DOI] [PubMed] [Google Scholar]
- Smith CE, Dudley MW, Lynn DG (1990) Vegetative/parasitic transition: control and plasticity in Striga development. Plant Physiol 93: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Ruttledge T, Zeng Z, O'Malley RC, Lynn DG (1996) A mechanism for inducing plant development: the genesis of a specific inhibitor. Proc Natl Acad Sci USA 93: 6986–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallek T, Melnyk CW, Wakatake T, Zhang J, Sakamoto Y, Kiba T, Yoshida S, Matsunaga S, Sakakibara H, Shirasu K (2017) Interspecies hormonal control of host root morphology by parasitic plants. Proc Natl Acad Sci USA 114: 5283–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striberny B, Krause K (2015) Cell wall glycoproteins at interaction sites between parasitic giant dodder (Cuscuta reflexa) and its host Pelargonium zonale. Plant Signal Behav 10: e1086858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood CJ, Bauer U, Hiscock SJ (2018) Convergent and divergent evolution in carnivorous pitcher plant traps. New Phytol 217: 1035–1041 [DOI] [PubMed] [Google Scholar]
- Tomilov AA, Tomilova NB, Abdallah I, Yoder JI (2005) Localized hormone fluxes and early haustorium development in the hemiparasitic plant Triphysaria versicolor. Plant Physiol 138: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Nakatsubo F, Higuchi T (1982) Lignin degradation by Phanerochaete chrysosporium: metabolism of a phenolic phenylcoumaran substructure model compound. Arch Microbiol 131: 124–128 [Google Scholar]
- Visser JH, Inge D, Kollmann R (1984) The “hyaline body” of the root parasite Alectra orobanchoides benth. (Scrophulariaceae)—its anatomy, ultrastructure and histochemistry. Protoplasma 121: 146–156 [Google Scholar]
- Wada S, Cui S, Yoshida S (2019) Reactive oxygen species (ROS) generation is indispensable for haustorium formation of the root parasitic plant Striga hermonthica. Front Plant Sci 10: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatake T, Ogawa S, Yoshida S, Shirasu K (2020) An auxin transport network underlies xylem bridge formation between the hemi-parasitic plant Phtheirospermum japonicum and host Arabidopsis. Development 147: dev187781 [DOI] [PubMed] [Google Scholar]
- Wakatake T, Yoshida S, Shirasu K (2018) Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants. Development 145: dev164848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Murdock M, Lai SWT, Steele DB, Yoder JI (2020) Kin recognition in the parasitic plant Triphysaria versicolor is mediated through root exudates. Front Plant Sci 11: 560682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Steele D, Murdock M, Lai S, Yoder J (2019) Small-molecule screens reveal novel haustorium inhibitors in the root parasitic plant Triphysaria versicolor. Phytopathology 109: 1878–1887 [DOI] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW (2010) The evolution of parasitism in plants. Trends Plant Sci 15: 227–235 [DOI] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, Xu Y, Xie L, Huang F, Wan D, Ni J, Yuan F, Wu X, et al. (2020) Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578: 577–581 [DOI] [PubMed] [Google Scholar]
- Xiang L, Li Y, Sui X, Li A (2018) Fast and abundant in vitro spontaneous haustorium formation in root hemiparasitic plant Pedicularis kansuensis Maxim. (Orobanchaceae) . Plant Divers 40: 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Murray JD, Kim J, Heckmann AB, Edwards A, Oldroyd GED, Downie JA (2012) Legume pectate lyase required for root infection by rhizobia. Proc Natl Acad Sci USA 109: 633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Wafula EK, Honaas LA, Zhang HT, Das M, Fernandez-Aparicio M, Huang K, Bandaranayake PCG, Wu B, Der JP, et al. (2015) Comparative transcriptome analyses reveal core parasitism genes and suggest gene duplication and repurposing as sources of structural novelty. Mol Biol Evol 32: 767–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JI (1997) A species-specific recognition system directs haustorium development in the parasitic plant Triphysaria (Scrophulariaceae). Planta 202: 407–413 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Cui S, Ichihashi Y, Shirasu K (2016) The haustorium, a specialized invasive organ in parasitic plants. Annu Rev Plant Biol 67: 643–667 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kim S, Wafula EK, Tanskanen J, Kim YM, Honaas L, Yang Z, Spallek T, Conn CE, Ichihashi Y, et al. (2019) Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr Biol 29: 3041–3052 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K (2009) Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytol 183: 180–189 [DOI] [PubMed] [Google Scholar]
- Zhang X, Berkowitz O, Teixeira da Silva JA, Zhang M, Ma G, Whelan J, Duan J (2015) RNA-Seq analysis identifies key genes associated with haustorial development in the root hemiparasite Santalum album. Front Plant Sci 6: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]