Abstract
Sensing of heat, high light (HL), or mechanical injury by a single leaf of a plant results in the activation of different systemic signals that reach systemic tissues within minutes and trigger systemic acquired acclimation (SAA) or systemic wound responses (SWRs), resulting in a heightened state of stress readiness of the entire plant. Among the different signals associated with rapid systemic responses to stress in plants are electric, calcium, and reactive oxygen species (ROS) waves. These signals propagate from the stressed or injured leaf to the rest of the plant through the plant vascular bundles, and trigger SWRs and SAA in systemic tissues. However, whether they can propagate through other cell types, and whether or not they are interlinked, remain open questions. Here we report that in response to wounding or heat stress (HS), but not HL stress, the ROS wave can propagate through mesophyll cells of Arabidopsis (Arabidopsis thaliana). Moreover, we show that ROS production by mesophyll cells during these stresses is sufficient to restore SWR and SAA transcript accumulation in systemic leaves, as well as SAA to HS (but not HL). We further show that propagation of the ROS wave through mesophyll cells could contribute to systemic signal integration during HL and HS stress combination. Our findings reveal that the ROS wave can propagate through tissues other than the vascular bundles of plants, and that different stresses can trigger different types of systemic signals that propagate through different cell layers and induce stress-specific systemic responses.
In addition to vascular bundles, mesophyll cells can mediate the reactive oxygen species wave during systemic responses to wounding or heat stress in Arabidopsis.
Introduction
In response to different abiotic stresses, plants mount an acclimation response that counters the adverse effects of stress on plant metabolism, reproduction, and overall survival (Zhu, 2016; Kollist et al., 2019; Cheung et al., 2020). This response is triggered upon perception of stress at the tissues immediately subjected to stress (termed local tissues), as well as in other tissues of the plant that have not yet experienced the stress (termed as systemic tissues). The perception of stress at the local tissues activates therefore a signal transduction process that links the different tissues (local to all systemic tissues) over long distances, sometime spanning the entire length of the plant (e.g. Miller et al., 2009; Szechyńska-Hebda et al., 2010; Christmann et al., 2013; Choi et al., 2014, 2017; Gilroy et al., 2016; Guo et al., 2016; Choudhury et al., 2018; Devireddy et al., 2018; Takahashi et al., 2018; Toyota et al., 2018; Fichman et al., 2019; Wang et al., 2019; Zandalinas et al., 2019; Devireddy et al., 2020a, 2020b; Farmer et al., 2020; Fichman and Mittler, 2020). This process is termed systemic signaling, and the acclimation of systemic tissues to stress, upon perception of the systemic signal, is called systemic acquired acclimation (SAA; Karpinski et al., 1999). A similar systemic signaling process occurs in plants upon wounding of a local leaf, and this process is termed systemic wound response (SWR; Walker-Simmons et al., 1984). During SAA or SWR, many different abiotic stress- or wound-response transcripts and hormones that rapidly accumulate in the local leaf upon stress or wounding also accumulate within minutes in the systemic tissues, and these transcripts and hormones are thought to mediate SAA or SWR at the systemic tissues (e.g. Galvez-Valdivieso et al., 2009; Miller et al., 2009; Suzuki et al., 2013; Zandalinas et al., 2019; Fichman et al., 2020b). Although the process of SAA or SWR can be easily traced back to some of the regulatory transcripts and hormones that accumulate in systemic tissues during stress, how the systemic signal initiating at the local leaf and reaching the systemic tissues is propagated, and what is its nature, are still ongoing subjects of active research (e.g. Fichman et al., 2020a; Fichman and Mittler, 2020). Among the main candidates for systemic signals mediating SAA or SWRs are electric, calcium, reactive oxygen species (ROS), and hydraulic pressure waves (Miller et al., 2009; Christmann et al., 2013; Mousavi et al., 2013; Choi et al., 2014; Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020).
Because plants lack a true nervous system that connects different tissues, systemic signals that travel from the local tissue, initially subjected to stress, to the entire plant are transmitted by cell-to-cell signaling events that involve changes in calcium, membrane potential and ROS (Fichman and Mittler, 2020). It is thought that during this process the different cells along the path of the cell-to-cell signaling chain are being activated one-by-one (similar to a domino effect) starting at the initial (local) tissue and ending at the systemic tissue, and that this activation process propagates and maintains the different systemic signals. This concept was initially proposed as a way to transmit ROS signals over long distances in plants (Miller et al., 2009, Mittler et al., 2011), and was later adopted for explaining calcium and other systemic signals (Choi et al., 2014, 2017). According to this model, each cell along the cell-to-cell path that transmits the signal starts to actively generate ROS upon sensing that the cell preceding it in the chain is producing ROS. It was found that in Arabidopsis (Arabidopsis thaliana) the ROS produced by each cell during this process is generated by the RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) protein and that this process is controlled by calcium-dependent activation of RBOHD (Fichman and Mittler, 2020; Fichman et al., 2021). The ROS being used as a systemic signal, most likely H2O2 (Miller et al., 2009), is therefore actively generated by each cell along the path of the signal, as opposed to being made in the local tissue and somehow transported over long distances (Mittler et al., 2011).
Recently, wound-induced systemic cell-to-cell electric and calcium signals were shown to be dependent on the function of GLUTAMATE RECEPTOR-LIKE (GLR) calcium channels expressed at the vascular bundles of Arabidopsis, and a double mutant for glr3.3;glr3.6 was shown to be deficient in wound-induced systemic signaling (Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020). In contrast, systemic cell-to-cell signaling, and SAA to high light (HL) or heat stress (HS) were found to be dependent on ROS produced in each cell along the path of the signal by RBOHD and/or RBOHF (Miller et al., 2009; Fichman et al., 2019; Zandalinas et al., 2020b). At least in response to HL stress, this process was also found to occur at the vascular bundles of Arabidopsis (Zandalinas et al., 2020b). A new study has now revealed that GLR3.3 and/or GLR3.6 are not absolutely required for HL-induced systemic ROS signaling, and that the systemic signal mediating SAA to HL stress in Arabidopsis requires a coordinated function of plasmodesmata (PD) proteins (i.e. PLASMODESMATA-LOCALIZED PROTEINS 1 and 5; PDLP1 and PDLP5) and RBOHD (Fichman et al., 2021). It was further found that RBOHD-produced ROS opens PD pores between cells and facilitates cell-to-cell transport of carboxyfluorescein during this process, suggesting that enhancing transport through PDs is one possible role for ROS during systemic cell-to-cell signaling in plants (Fichman et al., 2021). In addition, aquaporins such as PLASMA MEMBRANE INTRINSIC PROTEIN 2;1 (PIP2;1) and calcium-permeable channels, such as CYCLIC NUCLEOTIDE-GATED CALCIUM CHANNEL 2 (CNGC2), and MECHANOSENSITIVE SMALL CONDUCTANCE-LIKE (MSL) channels 2 and 3 were found to be involved in this process (Fichman et al., 2021). Moreover, in response to wounding the systemic ROS signal was shown to induce a systemic redox signal (wave of change in the redox state of the glutathione pool) that propagated throughout the plant within minutes (Fichman and Mittler, 2021).
A recent study has also revealed that in contrast to the local application of HL or HS to a single leaf of Arabidopsis, or the co-application of HL and HS to the same leaf (HL+HS), the co-application of HL and HS to two different leaves of the same plant (HL&HS) resulted in a stronger ROS wave response (Zandalinas et al., 2020a). It was further found that the plant hormone jasmonic acid (JA) suppresses the activation of the ROS wave in local leaves simultaneously subjected to a combination of HL and HS (HL+HS; Zandalinas et al., 2020a). Although the ROS wave was found to propagate through the vascular bundles of Arabidopsis during systemic responses to HL stress (Zandalinas et al., 2020b), it is unknown at present whether it propagates through the same plant tissues during other stresses, such as HS or wounding. Finding, for example, that the ROS wave propagates through other plant tissues during HS, could provide a potential explanation to the stronger ROS wave signal observed under conditions of HL&HS (Zandalinas et al., 2020a). In addition, it could provide initial evidence for the propagation of rapid systemic signals outside the vascular bundles of plants.
To identify the plant tissues that mediate RBOHD-dependent systemic ROS signal propagation during responses to HS or wounding, we used the rbohD transgenic lines we previously developed to study the propagation of the ROS wave during HL stress (Zandalinas et al., 2020b). Our findings reveal that in contrast to RBOHD-dependent systemic responses to HL stress, that were exclusively mediated through the vascular bundles of Arabidopsis (Zandalinas et al., 2020b), RBOHD-dependent systemic signaling during HS (Zandalinas et al., 2020a), or wounding (Miller et al., 2009; Fichman et al., 2019; Fichman and Mittler, 2021), are mediated through the vascular bundles and/or mesophyll cells. We further show that propagation of the ROS wave through mesophyll cells could contribute to the stronger systemic ROS signal observed in plants subjected to HL and HS simultaneously applied to two different leaves (HL&HS; Zandalinas et al., 2020a). Our findings demonstrate that ROS production in mesophyll cells is required for the propagation of rapid systemic ROS signals during responses to HS or wounding.
Results
Vascular bundles or mesophyll cells can mediate the ROS wave during the systemic response of Arabidopsis to wounding
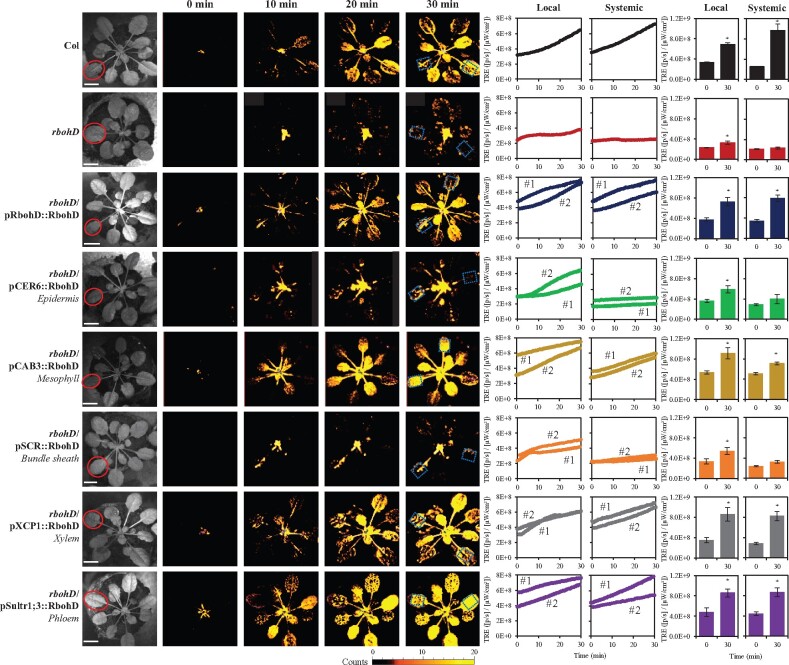
To identify the plant tissues that transmit RBOHD-dependent systemic signals (i.e. the ROS wave; Miller et al., 2009) in response to a local application of wounding, we used the different transgenic lines we previously developed of rbohD, in which RBOHD was expressed under its native promoter or different tissue-specific promoters (Zandalinas et al., 2020b). These lines were previously characterized for their ROS wave propagation, SAA and Zat12 expression in response to a local application of HL stress, and the localization and stable expression of the RBOHD protein in their different tissues was confirmed using GFP-RBOHD fusions driven by the tissue-specific promoters (Zandalinas et al., 2020b). In our analysis we included wild-type, rbohD null mutants, and rbohD mutants in which RBOHD was expressed under its native promoter, or epidermis-, mesophyll-, xylem parenchyma-, phloem-, or bundles sheath-specific promoters (Zandalinas et al., 2020b; Supplemental Figure S1). All plants were wounded on a single local leaf and local and systemic ROS levels were imaged in whole-plants grown in soil using the new live-imaging method we developed to image ROS (Fichman et al., 2019; Zandalinas et al., 2020a, 2020b). As shown in Figure 1 and Supplemental Figure S2, wound-induced systemic ROS accumulation was suppressed in the rbohD mutant and this suppression was complemented to wild-type levels by expression of RBOHD in the rbohD mutant using its native promoter. Expressing the RBOHD protein in rbohD plants using the mesophyll-, xylem parenchyma-, or phloem-specific promoters also complemented the systemic accumulation of ROS to wild-type levels in the rbohD mutant in response to wounding. In contrast, as shown in Supplemental Figures S2 and S3, as well as previously reported (Zandalinas et al., 2020b), in response to a local application of HL stress, expression of the RBOHD protein in rbohD plants using the native promoter of RBOHD, or using the xylem parenchyma- or phloem-specific promoters (but not the mesophyll-specific promoter), complemented the systemic accumulation of ROS in rbohD mutants to wild-type levels in response to HL. These findings reveal that in response to a local wounding treatment, the ROS wave can propagate through the vascular bundles, or mesophyll cells of Arabidopsis.
Figure 1.
Complementation of wound-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by different tissue-specific promoters. Representative time-lapse images of whole-plant ROS levels in wild-type, rbohD and the different rbohD-complemented A. thaliana plants subjected to a local wound treatment (red circles), are shown on left; representative line graphs showing continuous measurements of ROS levels in local and systemic leaves of wild-type, rbohD, and two independent homozygous complemented lines (#1 and #2), over the entire course of the experiment (0–30 min) are shown in the middle (ROIs for some of them are indicated with blue boxes); and statistical analysis of ROS levels in local and systemic leaves at 0 and 30 min is shown on right (Student’s t test, sd, N = 10, *P < 0.05). All experiments were repeated at least three times with similar results. Scale bar indicates 1 cm. CER, eceriferum; CAB, chlorophyll A/B binding protein; SCR, scarecrow; XCP, xylem cysteine peptidase; ROI, region of interest; Sultr, sulfate transporter; TRE, total radiant efficiency.
Vascular bundles or mesophyll cells can mediate the ROS wave during the systemic response of Arabidopsis to HS
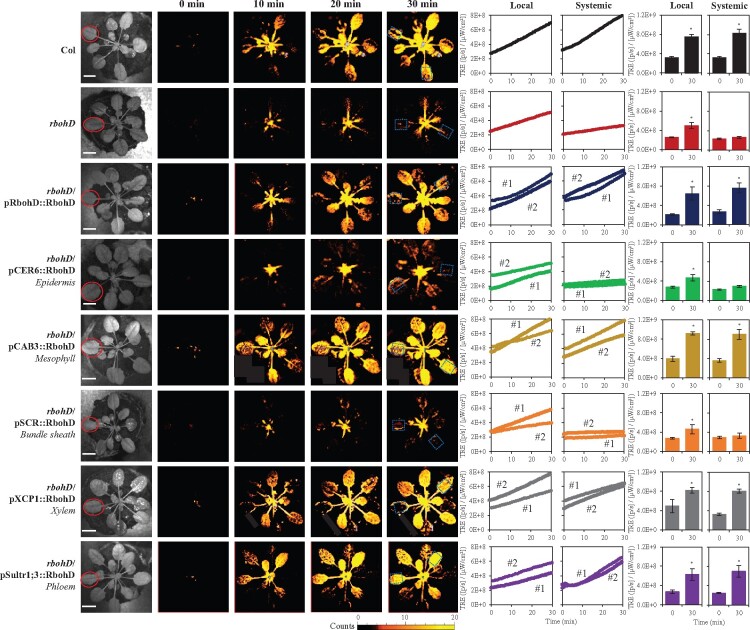
As shown in Figure 2 and Supplemental Figure S2, a similar result to that shown in Figure 1 was obtained when a local Arabidopsis leaf was subjected to HS. Thus, similar to the local application of wounding (Figure 1), but different from the local application of HL (Supplemental Figures S2 and S3; Zandalinas et al., 2020b), expression of the RBOHD protein in rbohD plants using its native promoter, or using the mesophyll-, xylem parenchyma-, or phloem-specific promoters, complemented the systemic accumulation of ROS in rbohD mutants to wild-type levels in response to a local application of HS. The findings shown in Figures 1 and 2, and Supplemental Figure S2, reveal therefore that unlike rapid systemic ROS responses to HL, that could only be complemented to wild-type levels in the rbohD mutant by expressing the RBOHD protein in xylem parenchyma or phloem cells (Supplemental Figures S2 and S3; Zandalinas et al., 2020b), tissues limited in their localization to the vascular bundles, systemic ROS signals (i.e. the ROS wave) to wounding or HS can be mediated by RBOHD protein found in mesophyll cells, that are primarily localized outside the vascular bundles of plants.
Figure 2.
Complementation of HS-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by different tissue-specific promoters. Representative time-lapse images of whole-plant ROS levels in wild-type, rbohD and the different rbohD-complemented A. thaliana plants subjected to a local HS treatment (red circles), are shown on left; representative line graphs showing continuous measurements of ROS levels in local and systemic leaves of wild-type, rbohD, and two independent homozygous complemented lines (#1 and #2), over the entire course of the experiment (0–30 min) are shown in the middle (ROIs for some of them are indicated with blue boxes); and statistical analysis of ROS levels in local and systemic leaves at 0 and 30 min is shown on right (Student’s t test, sd, N = 10, *P < 0.05). All experiments were repeated at least three times with similar results. Scale bar indicates 1 cm. CER, eceriferum; CAB, chlorophyll A/B binding protein; SCR, scarecrow; XCP, xylem cysteine peptidase; ROI, region of interest; Sultr, sulfate transporter; TRE, total radiant efficiency.
Complementing the ROS wave by expression of RBOHD in mesophyll cells restores SAA- and SWR-associated transcript expression in systemic leaves in response to a local HS or wounding treatment
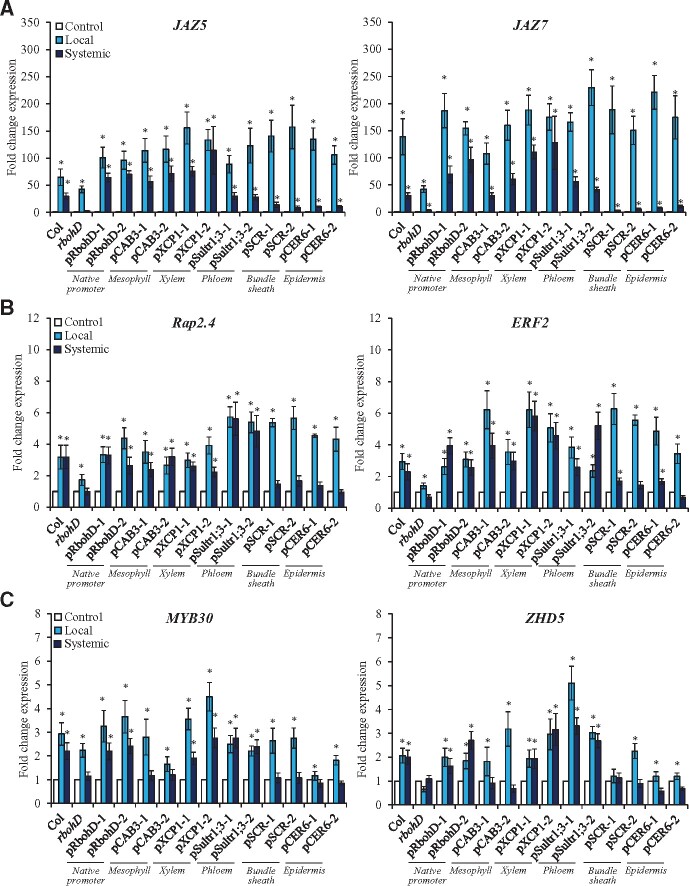
Complementing the ROS wave by expression of RBOHD in mesophyll cells (Figures 1 and 2, and Supplemental Figures S1 and S2) might or might not complement the expression of systemic transcripts previously associated with SAA or SWR in response to a local application of HS or wounding, respectively. Complementation of RBOHD expression in the rbohD mutant using the xylem parenchyma- or phloem- (but not mesophyll-) specific promoters restored the expression of the Zat12 SAA and SWR gene in response to local application of HL stress (measured using Zat12:luciferase;rbohD double mutants complemented with the different tissue-specific RBOHD transformation vectors; Zandalinas et al., 2020b). Because Zat12 reporter plants might not be a good experimental tool to study stress-specific responses to HS, HL or wounding (Zat12 is expressed in response to HL or wounding; Miller et al., 2009), we elected to study the expression of different wounding-, HS-, or HL-specific transcripts in the different lines shown in Figures 1 and 2 in response to a local application or HS, wounding, or HL using reverse transcription quantitative polymerase chain reaction (RT-qPCR). We chose the transcripts and timing for this analysis based on our previous RNA-Seq studies of systemic signaling in response to HL and/or HS (Suzuki et al., 2013; Zandalinas et al., 2019, 2020a; Fichman et al., 2020b), as well as based on studies of systemic wound responses using transcriptomics and RT-qPCR analyses (Suzuki et al., 2013; Toyota et al., 2018). As shown in Figure 3A, expression of the wound-response transcripts JAZ5 and JAZ7 was enhanced in local and systemic leaves of wild-type plants upon local wounding. In contrast, in response to the same treatment, the expression of these transcripts was suppressed in systemic (but not local) leaves of the rbohD mutant. Complementation of RBOHD expression with the RBOHD native promoter, or the mesophyll-, xylem parenchyma-, or phloem-specific promoters restored the systemic expression of JAZ5 and JAZ7 in response to a local wounding treatment. In contrast, complementation of RBOHD expression with the bundle sheath- or epidermis-specific promoters failed to restore the systemic expression of JAZ5 and JAZ7 to wild-type levels in response to the local wounding treatment. These findings reveal that complementing the ROS wave by expression of RBOHD in mesophyll, xylem parenchyma or phloem cells of the rbohD mutant was sufficient to restore some SWR-specific transcript expression in response to a local wounding treatment.
Figure 3.
Local- and systemic stress-induced transcript expression in wild-type, rbohD, and the rbohD mutant complemented with RBOHD driven by different tissue-specific promoters. A, Local and systemic steady-state levels of JAZ5 (AT1G17380) and JAZ7 (AT2G34600) transcripts in wild-type, rbohD, and the different rbohD-complemented A. thaliana plants subjected to a local wound treatment. B, Local and systemic steady-state levels of Rap2.4 (AT1G78080) and ERF2 (AT5G47220) transcripts in wild-type, rbohD, and the different rbohD-complemented Arabidopsis plants subjected to a local HS treatment. C, Local and systemic steady-state levels of MYB30 (AT3G28910) and ZHD5 (AT1G75240) transcripts in wild-type, rbohD, and the different rbohD-complemented Arabidopsis plants subjected to a local HL stress treatment. Student’s t test, sd, N = 3, *P < 0.05 compared to Control. CER, eceriferum; CAB, chlorophyll A/B binding protein; SCR, scarecrow; XCP, xylem cysteine peptidase; Sultr, sulfate transporter; JAZ, jasmonate-zim-domain protein; ERF, ethylene response factor; ZHD, zinc-finger homeodomain.
To test the effect of restoring RBOHD expression in the different tissues on SAA responses to HS, we studied the expression of Rap2.4 and ERF2, two transcripts previously associated with SAA to HS (Suzuki et al., 2013; Zandalinas et al., 2020a), in local and systemic leaves of the different wild-type, rbohD and rbohD-complemented lines, in response to a local HS treatment. As shown in Figure 3B, expression of the HS-response transcripts Rap2.4 and ERF2 was enhanced in local and systemic leaves of wild-type plants upon a local HS treatment. In contrast, in response to the same treatment, the enhanced expression of these transcripts was blocked in systemic and suppressed in local leaves or the rbohD mutant. Complementation of RBOHD expression with the RBOHD native promoter, or the mesophyll-, xylem parenchyma-, or phloem-specific promoters restored the systemic expression of Rap2.4 and ERF2 in response to a local HS treatment. In contrast, complementation of RBOHD expression with the bundle sheath- or epidermis-specific promoters did not restore the systemic expression of Rap2.4 and ERF2 to wild-type levels in response to a local HS treatment. These findings reveal that similar to the response of JAZ5 and JAZ7 to wounding (Figure 3A), restoring the ROS wave by expression of RBOHD in mesophyll, xylem parenchyma or phloem cells was sufficient to restore some SAA transcript expression in systemic leaves in response to a local HS treatment.
To study whether a similar effect would occur in complemented rbohD plants subjected to a local treatment of HL, we studied the expression of MYB30 and ZHD5, two transcripts associated with the SAA response of Arabidopsis to HL stress (Zandalinas et al., 2019, 2020a; Fichman et al., 2020b). As shown in Figure 3C, similar to Zat12 expression in the different rbohD-complemented lines (Zandalinas et al., 2020b), complementation of RBOHD expression in xylem parenchyma or phloem (but not mesophyll) cells of the rbohD mutant supported the systemic expression of MYB30 and ZHD5 in response to a local treatment of HL stress. Complementation of RBOHD expression in mesophyll cells of the rbohD mutant did however result in enhanced local (but not systemic) expression of MYB30 and ZHD5 (Figure 3C), demonstrating that local leaves of these plants were able to sense the HL stress but were unable to initiate the systemic ROS signal in response to it. Taken together, the results presented in Figures 1–3;Supplemental Figures S1–S3 reveal that complementing the expression of RBOHD in the mesophyll, xylem parenchyma or phloem cells of the rbohD mutant restores not only the ROS wave, but also the expression of certain systemic transcripts specific to wounding or HS. In contrast, complementing the expression of RBOHD in mesophyll cells of the rbohD mutant did not complement the ROS wave or systemic HL-specific SAA transcripts in response to a local application of HL stress (Figure 3;Supplemental Figure S3).
Complementing the ROS wave by expression of RBOHD in mesophyll cells restores local HS-induced SAA
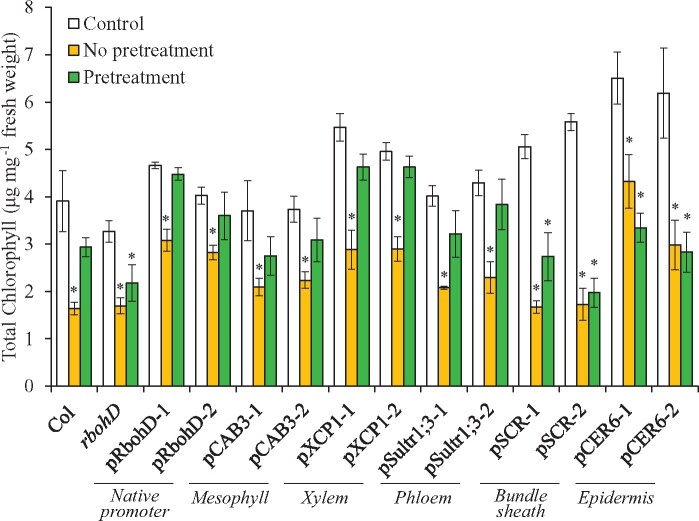
Complementing the expression of RBOHD in the xylem parenchyma or phloem cells of the rbohD mutant restored SAA to HL (Supplemental Figure S4; Zandalinas et al., 2020b). Although we do not have a biological assay for SAA during SWR, aside from measuring systemic wound-induced transcript expression as shown in Figure 3, an assay for SAA to HS was previously reported (Suzuki et al., 2013; Zandalinas et al., 2020a). We therefore used this assay to study whether restoring RBOHD expression in mesophyll cells could restore SAA to HS of the rbohD mutant. As shown in Figure 4, complementing the expression of RBOHD in the rbohD mutant using its native promoter, or the mesophyll-, xylem parenchyma-, or phloem-specific promoters restored SAA to HS. In contrast, complementing the expression of RBOHD in the rbohD mutant using the mesophyll-specific promoter failed to restore SAA to HL (Supplemental Figure S4; Zandalinas et al., 2020b). The findings presented in Figures 1–4;Supplemental Figures S1–S4 reveal therefore that expression of RBOHD in mesophyll cells can restore the ROS wave, systemic transcript expression, and SAA to HS (but not HL stress) in the rbohD mutant.
Figure 4.
Complementation of HS-induced SAA in the rbohD mutant with RBOHD driven by different tissue-specific promoters. HS-induced changes in systemic leaf chlorophyll content of wild-type, rbohD and the different rbohD-complemented A. thaliana plants are shown. Systemic leaves were obtained from plants that were either untreated and unstressed (Control), untreated at their local leaves and subjected to a systemic HS treatment (No pretreatment), or subjected to a local HS pre-treatment before being subjected to a systemic HS treatment (Pretreatment). SAA is evident when the systemic leaf of a pre-treated plant does not show a loss of chlorophyll content following a systemic HS treatment. Ten different plants each from two independent complemented lines for each construct were subjected to the SAA HS assay and chlorophyll content was measured in systemic leaves. Student’s t test, sd, N = 10, *P < 0.05 compared to Control. CER, eceriferum; CAB, chlorophyll A/B binding protein; SCR, scarecrow; XCP, xylem cysteine peptidase; Sultr, sulfate transporter.
Could expression of RBOHD in mesophyll cells contribute to the stronger systemic ROS signal observed in plants subjected to HL&HS?
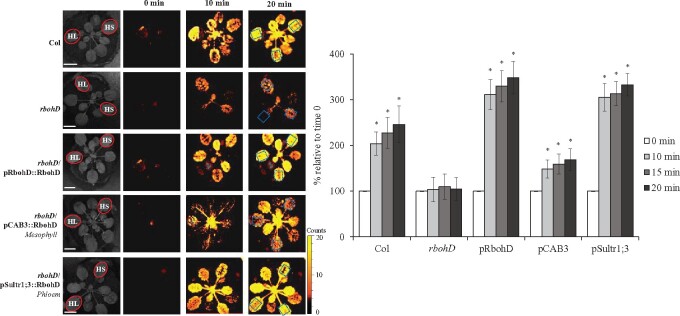
We previously reported that HS and HL, when applied to two different leaves of the same Arabidopsis plant (HL&HS), result in a stronger ROS wave response compared to HS or HL applied to a single leaf, or to the same leaf (HL+HS; Zandalinas et al., 2020a). Our current findings that in response to HS the ROS wave could be mediated through mesophyll, xylem parenchyma, and/or phloem cells (Figures 2–4), but in response to HL it could only be mediated through xylem parenchyma and/or phloem cells (Supplemental Figures S2–S4; Zandalinas et al., 2020b), might provide a potential explanation to this phenomena. In response to HL and HS applied to two different leaves (HL&HS), the systemic ROS wave might be stronger because it would propagate through an additional cell layer (mesophyll, contributed by the HS treatment). This could not occur of course when the two stresses are applied to the same leaf because under these conditions the ROS wave induced by HL+HS applied to the same leaf is suppressed by JA (Zandalinas et al., 2020a). To test whether the ROS wave could propagate through mesophyll cell layers during HL&HS combination, we compared the intensity of the ROS wave between wild-type, rbohD, and rbohD in which RBOHD expression was complemented at the mesophyll or phloem cells, subjected to a HL&HS treatment (Figure 5;Supplemental Figure S5). As shown in Figure 5, compared to wild-type plants, the ROS wave was suppressed in rbohD plants subjected to the HL&HS treatment. Complementation of RBOHD expression with RBOHD expressed under the control of its native promoter, or a phloem-specific promoter (that could restore HS- or HL-response ROS wave functions from the two different leaves; Figures 2–4 and Supplemental Figures S2, S3, and S5; Zandalinas et al., 2020b) restored the ROS wave to its high level of expression. In contrast, complementation of the rbohD mutant with RBOHD expressed under the mesophyll-specific promoter (that could only restore HS-, but not HL-response ROS wave functions from the HL-treated leaf; Figures 2–4;Supplemental Figures S2, S3, and S5; Zandalinas et al., 2020b), could not restore the ROS wave to its maximal intensity. These finding demonstrate that under conditions of HL&HS at least part of the ROS wave that spreads throughout the plant (originating from the HS-treated leaf) could be mediated through mesophyll cells. Complementation of RBOHD expression with RBOHD expressed under the control of the phloem-specific promoter was nonetheless sufficient to restore the ROS wave to wild-type or rbohD mutant complemented with RBOHD under its native promoter levels (Figure 5), suggesting that in wild-type plants transmission of the ROS wave signal through phloem cells is sufficient to cause a higher signal during HL&HS combination.
Figure 5.
Complementation of light- and heat-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by the phloem- or mesophyll tissue-specific promoters, during stress combination. Representative time-lapse images of whole-plant ROS levels of wild-type, rbohD, and rbohD-complemented A. thaliana plants subjected to a local light (HL) and HS treatments, simultaneously applied to two leaves of the same plant (red circles; HL&HS; Zandalinas et al., 2020a) are shown on left (ROIs for some of them are indicated with blue boxes), and statistical analysis of ROS levels in systemic leaves of treated plants at 0, 10, 15, and 20 min is shown on right (Student’s t test, sd, N = 10, *P < 0.05 compared to 0 min). All experiments were repeated at least three times with similar results. Scale bar indicates 1 cm. CAB, chlorophyll A/B binding protein; Sultr, sulfate transporter.
Discussion
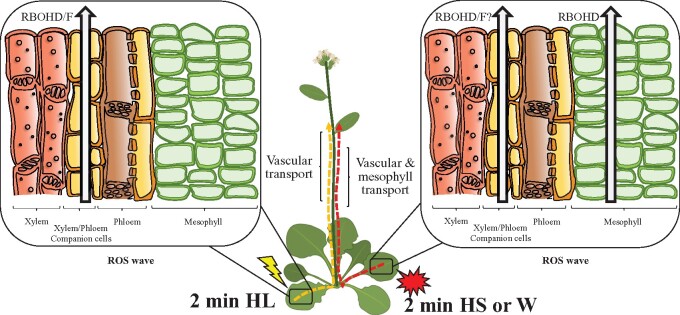
Abiotic, mechanical injury, and biotic stresses trigger a rapid systemic signal transduction process that activates different acclimation and defense mechanisms in systemic tissues within minutes of stress sensing at the local tissues (Fichman et al., 2019; Kollist et al., 2019; Fichman and Mittler, 2020). Up until now, the systemic electric, calcium, and ROS waves, triggered by wounding or HL stress, were shown to be mediated through the vascular bundles of plants (Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Farmer et al., 2020; Shao et al., 2020; Zandalinas et al., 2020b). Here, we present evidence that in addition to vascular bundles, mesophyll cells can also mediate the systemic ROS wave in response to a local treatment of wounding or HS (Figures 1–6). Mesophyll cells are not typically considered part of the vascular bundles of plants and are found within leaves and stems as cell layers that connect the vascular tissues to the epidermis, stomata and/or other leaf/stem structures and cell types. Because the ROS wave propagates from cell-to-cell via mechanisms that require apoplastic and symplastic connectivity between cells (Miller et al., 2009; Fichman et al., 2021), and mesophyll cells are connected with each other via PD and/or their shared apoplastic microenvironment, as well as express RBOHD under controlled growth conditions (Supplemental Figure S1; Zandalinas et al., 2020b), the basic mechanisms that allow the ROS wave to propagate from cell-to-cell through mesophyll cell layers appear to be present. In contrast, GLR3.3 and/or GLR3.6 that are required for rapid wound-response systemic signaling are not thought to be localized to mesophyll cells (they are thought to be exclusively localized to the xylem parenchyma and phloem cells; Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020). A recent study has shown that GLR3.3 and/or GLR3.6 are not absolutely required for the ROS wave to propagate in response to a local treatment of HL stress (Fichman et al., 2021). Taking this study into consideration, it is plausible that the ROS wave will propagate through tissues that do not express GLR3.3 and/or GLR3.6, possibly using other calcium-permeable channels such as CNGCs or MSLs (Fichman et al., 2021). Having many of the required proteins and physical connections/proximity required for ROS cell-to-cell signals to function, support the possibility that the ROS wave can propagate through layers of mesophyll cells that are outside the vascular bundles (Figure 6).
Figure 6.
A model showing that when light stress is applied to a local leaf, the ROS wave is mediated through vascular bundles. In contrast, when heat stress or wounding are applied to a local leaf, both vascular and mesophyll cells can mediate the ROS wave W, wounding.
Considering the extensive literature and established role of chloroplasts in the perception of light stress, as well as ROS production (Karpinski et al., 1999; Mittler, 2002), it is somewhat surprising that perception of light stress in rbohD mutants expressing RBOHD in mesophyll cells does not trigger the systemic ROS wave (Supplemental Figure S3; Zandalinas et al., 2020b). Although local leaves of rbohD/pCAB3::GFP-RbohD plants express MYB30 and ZHD5 in response to HL stress, showing that they can perceive the stress, they are nevertheless unable to trigger the systemic ROS signal and cause accumulation of these transcripts in systemic leaves (Figure 3;Supplemental Figure S3). One possible explanation to this finding stem from recent studies showing that HL-induced ROS in Arabidopsis leaves and bundle sheath cells of rice requires RBOH proteins (Devireddy et al., 2020b; Xiong et al., 2021). It is therefore possible that triggering of the systemic ROS signal during light stress requires RBOH present in vascular cells and that this process is independent of ROS accumulation in chloroplasts of mesophyll cells. In addition, it is possible that under longer and more pervasive HL treatments, additional ROS signaling mechanisms may be involved, and these could be mediated through additional cell layers. Further studies are required to address this intriguing possibility.
In addition to showing that the ROS wave can propagate outside the vascular bundles of Arabidopsis (Figures 1 and 2;Supplemental Figure S2), supporting systemic wound- and HS-induced transcript expression in systemic leaves (Figure 3) and mediating SAA to HS (Figure 4), our findings further highlight the interesting possibility that different stresses, e.g. HS, HL, and wounding, trigger different types of systemic waves that propagate through different tissues, and could even be spatially separated from each other. For example, complementing RBOHD expression in mesophyll cells of the rbohD mutant can complement systemic responses to wounding (Figure 3). Under these conditions, the electric and calcium waves could propagate through the vascular bundles (supported by GLR3.3;GLR3.6; Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020), while the ROS wave could propagate through mesophyll cells (supported by RBOHD; Figures 1 and 3; Zandalinas et al., 2020b). This possibility suggests that the ROS wave can be spatially separated from the calcium and electric waves. Different stresses could therefore trigger different combinations of waves that could travel through different tissues and cell layers of the plant. During systemic responses to HL stress, however, the separation of systemic signals cannot occur (for reasons unknown at present), and the ROS wave must propagate together with the electric and calcium waves through the vascular bundles. Further studies are of course needed to address these intriguing possibilities.
Under all stresses studied here (HL, HS, wounding), RBOHD appeared to be required for systemic transcript accumulation (Figure 3), suggesting that even though GLRs were present and most likely functional in the rbohD mutant, they could not mediate their function to drive the expression of systemic transcript accumulation in the absence of the ROS wave. The ROS wave, even occurring at tissues other than the vascular bundles (i.e. mesophyll cell layers; Figures 1 and 2) could therefore be required to support other systemic signal propagation (such as electric and calcium waves) occurring at the vascular bundles during HS or wound responses. Although it is unknown at present how changes in ROS at the mesophyll cell layers impact electric and calcium signaling at the vascular bundles, one intriguing possibility is that different metabolites, ions, ROS, hormones, and/or pH changes, occurring at the mesophyll cell layers are diffused/transported to the vascular bundles, and these are needed to link the different waves (Fichman et al., 2020a; Fichman and Mittler, 2020). In this respect, it should be mentioned that changes in localized pH levels were recently linked to the triggering and propagation of electric and calcium waves in Arabidopsis (Shao et al., 2020). An alternative explanation could of course be that RBOHF at the vascular tissues replaces the function of RBOHD in linking the different waves during all stresses studied, and that the levels of RBOHF-produced ROS in the vascular bundles of rbohD plants complemented by RBOHD expressed under the control of a mesophyll-specific promoter are too low to be detected by our assay. Further studies, for example, transformation of the rbohD;rbohF double mutant with the pCAB3::RbohD construct, are of course needed to address these possibilities, as well as to resolve the different spatial and temporal relationships that could potentially exist between the different waves, signals and hormones involved in systemic signaling (e.g. Kangasjärvi et al., 2009; Miller et al., 2009, 2011; Dubiella et al., 2013; Gilroy et al., 2014, 2016; Evans et al., 2016; Choi et al., 2017; Fichman et al., 2020a; Fichman and Mittler, 2020).
Our findings that the ROS wave can propagate through multiple cell layers in response to different stresses could also partially explain how the integration of different systemic signals during a combination of HL and HS results in a stronger ROS wave signal (Zandalinas et al., 2020a). It is possible that during a combination of HL and HS applied to two different leaves of the same plant (HL&HS; Zandalinas et al., 2020a), the ROS wave initiated from the two different leaves propagates through all three cell types of the plant (mesophyll, xylem parenchyma, and phloem, initiated by the local HS treatment, and xylem parenchyma and phloem, initiated by the local HL treatment). In contrast, during a combination of HL+HS applied to the same leaf, JA suppresses the ROS wave and the signal is lower (Zandalinas et al., 2020a). Our findings that restoring RBOHD expression in mesophyll cells did not result in a stronger systemic ROS signal during a HL&HS treatment (Figure 5), reveals that during HL&HS combination in Arabidopsis the ROS wave could indeed propagate through mesophyll cells (Figures 5 and 6;Supplemental Figure S5). The ROS wave triggered by the HL treatment (propagating through xylem parenchyma and/or phloem cells) could therefore merge with the ROS wave triggered by the HS treatment (propagating through mesophyll and xylem parenchyma and/or phloem cells) to generate a stronger systemic ROS signal during HL&HS combination that is mediated through multiple cell layers (Figures 5 and 6;Supplemental Figure S5). Because complementing RBOHD expression in the rbohD mutant using RBOHD expressed under the phloem-specific promoter was sufficient to restore the strong signal observed during HL&HS combination (Zandalinas et al., 2020a; Figure 5;Supplemental Figure S5), it is also possible that the stronger signal observed during HL&HS combination is simply the result of two different ROS wave signals merging together, regardless of the type of tissue supporting their transmission. Further studies are of course needed to dissect the mode of systemic signal integration through the different cell layers during stress combination.
Materials and methods
Plant material, growth conditions and stress treatments
Arabidopsis (A. thaliana) Col-0 (cv Columbia-0), rbohD plants (Fichman et al., 2019) and two independent lines each of the different rbohD complemented plants (Zandalinas et al., 2020b) were grown in peat pellets (Jiffy-7, Jiffy, http://www.jiffygroup.com/) at 23°C under short day growth conditions (10-h light/14-h dark, 50 µmol m−2 s−1). Wounding was achieved by puncturing a single leaf with 18 dressmaker pins (Singer, Murfreesboro, TN, USA) as described in Fichman et al. (2019). HS was induced by placing a heat block 2 cm underneath the treated leaf for 2 min, increasing the leaf temperature to 31–33°C (Zandalinas et al., 2020a).
HL stress was applied by subjecting a single leaf to a light intensity of 1700 µmol m−2 s−1 for 2 min using a ColdVision fiber optic LED light source (Schott A20980, Southbridge, MA, USA) as described in Devireddy et al. (2018) and Zandalinas et al. (2019, 2020a, 2020b). The spectrum of this light stress treatment was shown in previous studies to contain all components required for triggering the systemic ROS signal through phytochrome B-mediated signaling (Devireddy et al., 2020b), as well as, when applied for more than 45 min, cause photosynthetic inhibition and light-induced cell death (Balfagón et al., 2019; Zandalinas et al., 2019, 2020a, 2020b). However, when applied for 2 min, this light stress treatment did not increase leaf temperature (Supplemental Table S1; Zandalinas et al., 2020a). Local and systemic leaf temperatures were measured under all conditions and treatment using an infrared camera (C2; FLIR Systems; Zandalinas et al., 2020a).
Measurements of ROS accumulation
To image whole-plant ROS levels, plants were fumigated with 50-μM H2DCFDA (excitation/emission 495 nm/517 nm; Millipore-Sigma, St Louis, MO, USA) in 50-mM phosphate buffer (pH 7.4) containing 0.01% (v/v) Silwet L-77 (LEHLE seeds, Round Rock, TX, USA), using a portable mini nebulizer (Punasi Direct, Hong Kong, China) for 30 min as described previously (Fichman et al., 2019; Zandalinas et al., 2020a, 2020b). Following H2DCFDA application, local leaves were exposed to wounding, HL stress, HS, or HL and HS applied to two different leaves located at opposite sides of the plant as described by Zandalinas et al. (2020a). Imaging of ROS accumulation in response to a local stress treatment was conducted with an IVIS Lumina S5 platform using Living Image 4.7.2 software (PerkinElmer) as described in Fichman et al. (2019) and Zandalinas et al. (2020a, 2020b). All experiments were repeated at least three times each with 10 wild-type, rbohD and the different complemented plants.
RT-qPCR analysis
To analyze transcript expression by RT-qPCR, plants were subjected to a local treatment of wounding, 8-min HL or 8-min HS as described above. Local and systemic leaves were collected and immediately frozen in liquid nitrogen following the 8-min HL or HS treatments, or 30 min following wounding. Relative expression analysis by RT-qPCR was performed according to Balfagón et al. (2019) by using the CFX Connect Real-Time PCR Detection System (Bio-Rad) and gene-specific primers (Supplemental Table S2; Primer efficiency range of 0.99–1.04). All experiments were repeated at least three times each with at least five wild-type, rbohD and the different rbohD complemented plants.
HS acclimation assay
For HS acclimation, a single leaf was pre-treated for 15 min at 31–33°C by placing a heat block 2 cm underneath the treated leaf (Zandalinas et al., 2020a). Plants were then incubated for 45 min under controlled conditions. Following the recovery period, a systemic leaf of pre-treated and untreated plants was dipped in a 42°C (or 23°C as control) water bath for 60 min and allowed to recover under controlled growth conditions. Systemic leaves were sampled 6 d after the water bath HS treatment for chlorophyll measurements, as previously described (Zandalinas et al., 2020a, 2020c). For HL-induced SAA, a single leaf was pre-treated for 15 min with a light intensity of 1,700 µmol m−2 s−1 using a ColdVision fiber optic LED light source (Schott A20980, Southbridge, MA, USA). Plants were then incubated for 45 min under controlled conditions. Following the recovery period, a systemic leaf was exposed to a light intensity of 1,700 µmol m−2 s−1 for 45 min. Control systemic leaves (untreated) and systemic leaves of plants that were pretreated with HL stress, as described above (SAA), were then analyzed for electrolyte leakage as previously described (Zandalinas et al., 2019, 2020a, 2020b). Acclimation assays were repeated at least three times with 10 plants per repeat.
GFP imaging
Localization of RBOHD-GFP in leaves of mature (4- to 5-week-old) rbohD plants complemented with the RBOHD-GFP protein driven by its native or CAB3 promoter was performed using a TCS SP8 (Leica) multiphoton confocal microscope (Buffalo Grove, IL, USA) as described in Zandalinas et al. (2020b), as follows: ×20 and ×40 magnification, 10% laser intensity and excitation/emission of 488 nm/510 nm.
Statistical analysis
Results are presented as the mean ± sd. At least three different biological repeats were performed. Statistical analyses were performed by a two-tailed Student’s t test (asterisks denote statistical significance at P < 0.05 with respect to controls).
Accession numbers
CAB3 (AT1G29910); CER6 (AT1G68530); ERF2 (AT5G47220); GLR3.3 (AT1G42540); GLR3.6 (AT3G51480); JAZ5 (AT1G17380); JAZ7 (AT2G34600); MYB30 (AT3G28910); Rap2.4 (AT1G78080); RBOHD (AT5G47910); RBOHF (AT1G64060); SCR (AT3G54220); Sultr1;3 (AT1G22150); XCP1 (AT4G35350); ZHD5 (AT1G75240).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Representative confocal images of RBOHD-GFP fusion protein expression in mature leaves of transgenic rbohD mutants.
Supplemental Figure S2. Linear regression analysis conducted using scatter plots of continuous ROS measurements in local and systemic leaves of wild-type, rbohD and the different complemented lines over the entire course of each experiment (0–30 min).
Supplemental Figure S3. Complementation of light stress (HL)-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by different tissue-specific promoters.
Supplemental Figure S4. Complementation of light stress (HL)-induced SAA in the rbohD mutant with RBOHD driven by different tissue-specific promoters.
Supplemental Figure S5. Complementation of light (HL)- and heat (HS)-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by the phloem- or mesophyll tissue-specific promoters, during stress combination.
Supplemental Table S1. FLIR camera measurements showing the surface temperature of treated (local) and systemic leaves for each stress treatment (C2, FLIR systems AB).
Supplemental Table S2. Transcript-specific primers used for relative expression analysis by RT-qPCR.
Supplementary Material
Acknowledgments
Confocal images were acquired and analyzed at the University of Missouri Molecular Cytology Core facility.
Funding
This work was supported by funding from the National Science Foundation (IOS-1353886, MCB-1936590, IOS-1932639), the Interdisciplinary Plant Group, and the University of Missouri.
Conflict of interest statement. The authors declair no conflict of interest.
References
- Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad R, Mittler R, Zandalinas SI (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181: 1668–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Qu LJ, Russinova E, Zhao Y, Zipfel C (2020) Update on receptors and signaling. Plant Physiol 182: 1527–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Miller G, Wallace I, Harper J, Mittler R, Gilroy S (2017) Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J 90: 698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim S-H, Hilleary R, Gilroy S (2014) Salt stress-induced Ca 2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury FK, Devireddy AR, Azad RK, Shulaev V, Mittler R (2018) Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol 178: 1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Arbogast J, Mittler R (2020a) Coordinated and rapid whole‐plant systemic stomatal responses. New Phytol 225: 21–25 [DOI] [PubMed] [Google Scholar]
- Devireddy AR, Liscum E, Mittler R (2020b) Phytochrome B is required for systemic stomatal responses and ROS signaling during light stress. Plant Physiol 184: 1563–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Zandalinas SI, Gómez-Cadenas A, Blumwald E, Mittler R (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte C-P, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Choi W-G, Gilroy S, Morris RJ (2016) A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol 171: 1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Gao YQ, Lenzoni G, Wolfender JL, Wu Q (2020) Wound- and mechanostimulated electrical signals control hormone responses. New Phytol 227: 1037–1050 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Miller G, Mittler R (2019) Whole-plant live imaging of reactive oxygen species. Mol Plant 12: 1203–1210 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2020) Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. DOI: 10.1111/tpj.14685 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2021) A systemic whole-plant change in redox levels accompanies the rapid systemic response to wounding. Plant Physiol (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y, Myers RJ, Grant DG, Mittler R (2021) Plasmodesmata-localized proteins and reactive oxygen species orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci Signal. 14: eabf0322. [DOI] [PubMed] [Google Scholar]
- Fichman Y, Zandalinas SI, Mittler R (2020a) Untangling the ties that bind different systemic signals in plants. Sci Signal 13: eabb9505. [DOI] [PubMed] [Google Scholar]
- Fichman Y, Zandalinas SI, Sengupta S, Burks D, Myers RJ, Azad RK, Mittler R (2020b) MYB30 orchestrates systemic reactive oxygen signaling and plant acclimation. Plant Physiol 184: 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, Asami T, Davies WJ, Jones AM, Baker NR, et al. (2009) The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21: 2143–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171: 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Suzuki N, Miller G, Choi W-G, Toyota M, Devireddy AR, Mittler R (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19: 623–630 [DOI] [PubMed] [Google Scholar]
- Guo Z, Wang F, Xiang X, Ahammed GJ, Wang M, Onac E, Zhou J, Xia X, Shi K, Yin X, et al. (2016) Systemic induction of photosynthesis via illumination of the shoot apex is mediated sequentially by phytochrome b, auxin and hydrogen peroxide in tomato. Plant Physiol 172: 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi S, Nurmi M, Tikkanen M, Aro EME, Kangasjarvi S, Nurmi M, Tikkanen M, Aro EME (2009) Cell-specific mechanisms and systemic signalling as emerging themes in light acclimation of C3 plants. Plant Cell Environ 32: 1230–1240 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Kollist H, Zandalinas SI, Sengupta S, Nuhkat M, Kangasjärvi J, Mittler R (2019) Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci 24: 25–37 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16: 300–309 [DOI] [PubMed] [Google Scholar]
- Mousavi SARR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE (2018) Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Natl Acad Sci USA 115: 10178–10183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Gao Q, Lhamo D, Zhang H, Luan S (2020) Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci Signal 13: eaba1453. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, et al. (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S (2010) Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K (2018) A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556: 235–238 [DOI] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai-Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Xiong H, Hua L, Reyna-Llorens I, Shi Y, Chen K-M, Smirnoff N, Kromdijk J, Hibberd JM (2021) The rice bundle sheath produces reactive oxygen species during high light stress via NADPH oxidase. bioRxiv 2020.07.06.189381. doi: https://doi.org/10.1101/2020.07.06. 189381 [DOI] [PMC free article] [PubMed]
- Walker-Simmons M, Hollander-Czytko H, Andersen JK, Ryan CA (1984) Wound signals in plants: a systemic plant wound signal alters plasma membrane integrity. Proc Natl Acad Sci USA 81: 3737–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Hu C, Zhou J, Liu Y, Cai J, Pan C, Wang Y, Wu X, Shi K, Xia X, et al. (2019) Systemic root-shoot signaling drives jasmonate-based root defense against nematodes. Curr Biol 29: 3430–3438.e4 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Devireddy AR, Sengupta S, Azad RK, Mittler R (2020a) Systemic signaling during abiotic stress combination in plants. Proc Natl Acad Sci USA 117: 13810–13820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Mittler R (2020b) Vascular bundles mediate systemic reactive oxygen signaling during light stress in Arabidopsis. Plant Cell 32: 3425–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Sengupta S, Burks D, Azad RK, Mittler R (2019) Identification and characterization of a core set of ROS wave‐associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J 98: 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Song L, Sengupta S, McInturf SA, Grant DG, Marjault H, Castro‐Guerrero NA, Burks D, Azad RK, Mendoza‐Cozatl DG, et al. (2020c) Expression of a dominant‐negative AtNEET‐H89C protein disrupts iron–sulfur metabolism and iron homeostasis in Arabidopsis. Plant J 101: 1152–1169 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.