Abstract
The opioid epidemic remains a dire public health crisis with millions of people currently suffering from opioid use disorder (OUD) and tens of thousands dying each year. Synthetic opioids are most responsible for the crisis because of their extreme potency and ease of manufacture. Carfentanil for example has an estimated potency 10,000 times greater than morphine and thus is highly dangerous for human use. Herein, we report two synthetic opioid vaccines that elicited high-affinity antibodies against carfentanil and fentanyl with cross-reactivity to other synthetic opioids in mice and offered protection against opioid-induced respiratory depression, the primary cause of overdose deaths. These vaccines also successfully diminished drug biodistribution to the brain and shielded against opioid analgesic effects. Collectively, these findings provide new insights into the development of immunotherapeutic strategies aimed at opioid abuse and overdose.
Graphical Abstarct

Opioids are a diverse class of drugs, used to relieve pain for more than 200 years. The opioid analgesic morphine, isolated from opium, was heralded as a “miracle drug” in the early 1800s and exploited as a broad-spectrum medication. Decades later, the German pharmaceutical company Bayer marketed the semisynthetic opioid heroin as a nonaddictive morphine-substitute, an over the counter cough suppressant and treatment for common ailments.1 However, the abuse liability of heroin and other opioids soon became increasingly apparent, leading to enactment of the U.S. Harrison Narcotic Act in 1914 and The Heroin Act in 1924, regulating opioid distribution and banning the narcotic heroin outright, respectively.2 The late 1990s marked the beginning of an era plagued by widespread overuse of opioid medications, both from prescriptions and illegal sale, causing an exponential rise in the number of individuals suffering from opioid dependence and addiction, presently referred to as opioid use disorder (OUD).3 From 1999 to 2017, the number of opioid related deaths in the U.S. alarmingly rose from an estimated 800 to more than 47,000.4 In 2017, the U.S. president officially declared the opioid crisis a “public health emergency,” mobilizing a national response to the opioid epidemic.
Currently, synthetic opioids are the main driver of opioid-involved deaths, accounting for approximately 67% of the total recorded in 2018, a 10% increase from 2017, according to the Centers for Disease Control and Prevention (CDC).5 Synthetic opioids readily cross the blood–brain barrier (BBB) stimulating μ-opioid receptors (MORs) in the brain, thereby eliciting analgesic effects.6 Unfortunately, MOR binding also induces on-target side effects including euphoria, sedation, and the development of drug tolerance and dependence, which can lead to OUD. Moreover, excessive MOR activation by opioids causes lethal respiratory depression, the primary cause of drug overdose deaths.7,8
The widespread availability of highly potent synthetic opioids, often nonprescription, on the illegal drug market poses a significant public health threat. Fentanyl, originally developed for cancer pain management, is an estimated 100 times more potent than morphine, and 10 times more powerful than heroin (Figure 1).9 Carfentanil, a structural analog of fentanyl, is the most potent synthetic opioid detected in U.S. forensic toxicology analysis with an estimated potency of 100 times greater than fentanyl.10 In fact, deployment of carfentanil in an aerosolized mixture resulted in the death of 125 people in a 2002 Moscow theater incident, a testament to its extreme potency.11 Carfentanil has utility in veterinary medicine for sedating large animals, such as elephants, but has no approved therapeutic use in humans. Carfentanil and fentanyl, both relatively easy to synthesize, have been added to illicit drugs, such as heroin, cocaine, and methamphetamine, and used in the clandestine manufacturing of counterfeit prescription pills.12 This recent trend is especially dangerous because the illegal drug user is frequently unaware that they are receiving drugs laced with ultrapotent opioids. Although fentanyl has historically been highlighted as the primary adulterant in the illicit drug supply, carfentanil related overdose deaths rose exponentially between 2016 and 2017 among more than 10 states in the U.S., accounting for approximately 11% of the opioid-related deaths during this time.13,14 Fatalities involving carfentanil are not isolated to the U.S., and cases have been documented in countries across Europe and North America.12,15 Unfortunately, the rise in the number of carfentanil and fentanyl related incidents is placing further strain on already overwhelmed public health systems currently battling a pandemic.
Figure 1.

Chemical structure of opioids and conjugate vaccines. Approximate relative potencies of opioids are shown with respect to morphine.
To counteract the harmful and addictive effects of synthetic opioids, our laboratory has implemented an immunotherapeutic strategy centered on the deadliest member, carfentanil. In this approach, inoculation of an opioid conjugate vaccine stimulates the immune system to produce high-affinity, drug-specific antibodies with the ability to alter the drug’s pharmacokinetics. Essentially, the target opioid is sequestered in the periphery through formation of antibody–opioid complexes precluding its dissemination across the BBB. As a result, the potentially rewarding or lethal drug effects are mitigated. Compared to FDA-approved small molecule-based pharmacotherapies (e.g., methadone, buprenorphine, or naltrexone), active vaccination offers many advantages, including protracted protection due to the long half-lives of antibodies and reduced side effects through direct binding to the opioid drug instead of the MOR receptor.16,17 Experimental vaccines against opioids, such as oxycodone, hydrocodone, heroin, and fentanyl, have shown promising results in preclinical animal models.18
Carfentanil belongs to the 4-anilidopiperidine class of synthetic opioids, a class of compounds derived from fentanyl’s core structure.19 Importantly, the approximately 100-fold potency shift observed between fentanyl and carfentanil can be attributed to simple incorporation of the methyl ester at the 4 position of the piperidine ring (Figure 1). Previous disclosures of fentanyl targeted vaccines have focused solely on the fentanyl scaffold.20–23 No vaccine studies reported to date have challenged against carfentanil-dependent respiratory depressive or analgesics effects. Therefore, we undertook the task of designing haptens that consider the complexities of the carfentanil chemical structure, while preserving access to other 4-anilidopiperidine synthetic opioids.
Proper hapten design and linker positioning are crucial for the success of any small molecule conjugate vaccine. In principal, a hapten should closely mirror the native drug structure, maintaining as many of its chemical features as possible. Following this basic strategy, two different approaches were explored: the first exploits the methyl ester functional group of carfentanil as the point of linker attachment (Carfen-ester-TT), whereas the second strategically places the linker at the para position of the phenethyl ring (Carfen-p-phenyl-TT; Figure 1). Both tactics prevent disruption of the 1-phenethyl-4-(N-phenylpropionamido)piperidine-4-carboxylate core, which we hypothesized, would elicit antibody responses capable of recognizing not only carfentanil but also multiple synthetic opioids.
Using this design strategy, haptens Carfen-ester-TT and Carfen-p-phenyl-TT were chemically synthesized, installing a terminal carboxylic acid linker at the appropriate position within each hapten structure (Schemes S1 and S2). The presence of this carboxyl group enabled conjugation to the carrier protein tetanus toxoid (TT) through standard amide coupling. Following bioconjugation, the immunoconjugates were formulated with the two adjuvants, alum and CpG ODN 1826. The vaccine components including the carrier protein (TT) and adjuvant system (alum plus CpG) were chosen because this combination has proven to be highly effective in drugs of abuse vaccines.18 Though recent studies have begun to examine how sex differences may influence vaccine immunogenicity and efficacy, our current study was not designed to investigate these potential variations.24,25
The resulting opioid vaccines were administered to Swiss Webster mice over the course of four intraperitoneal (IP) injections, and the antibody titers were measured by ELISA against the corresponding hapten-BSA conjugate, Carfen-ester-BSA or Carfen-p-phenyl-BSA (Figure 2A, Figure S1). Both vaccines induced very high antibody midpoint titers of similar magnitude, peaking at >200,000. Markedly, a substantial antibody presence persisted for more than 80 days.
Figure 2.
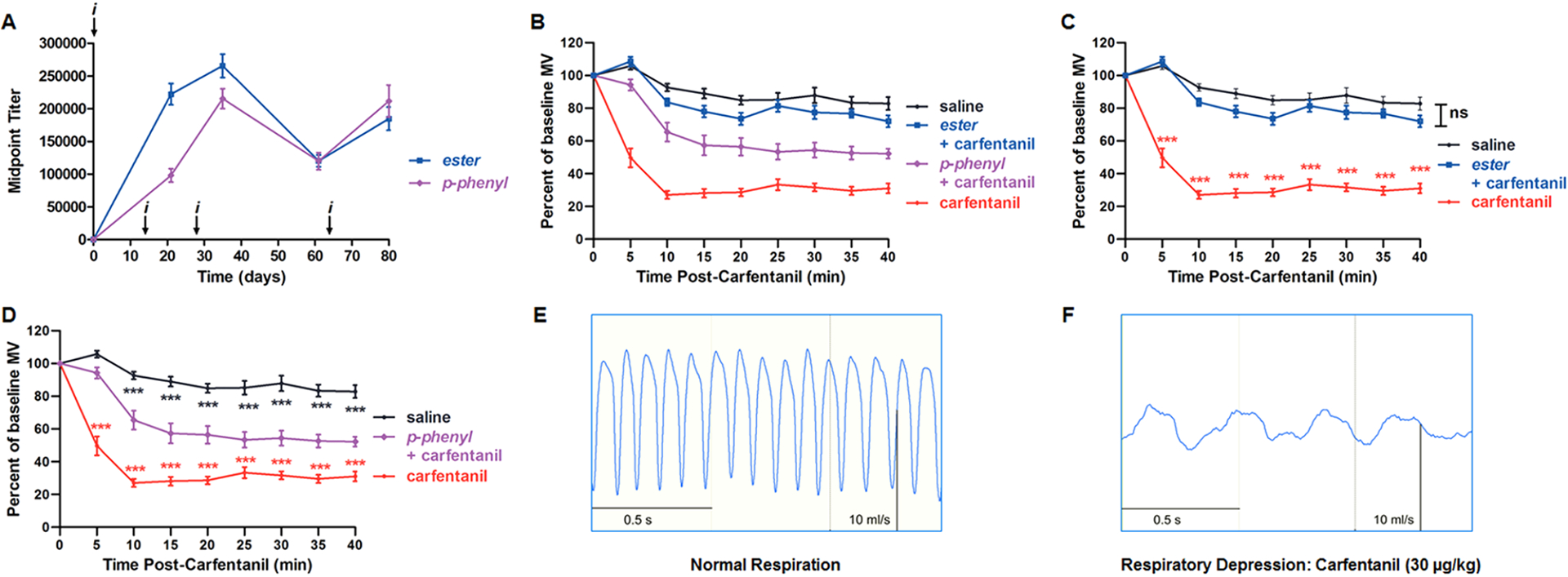
Evaluation of opioid vaccines: antibody titers and respiratory depression results. (A) Antiopioid antibody midpoint titers measured by ELISA using serum from vaccinated mice on days 21, 35, 61, and 80 for each study. i indicates the time of vaccine injections. (B–D) Respiratory depression studies in vaccinated and unvaccinated mice beginning at day 56. Mice received a respiratory depressant dose of carfentanil (30 μg/kg, intraperitoneally), and respiration was measured by whole-body plethysmography (n = 12 per group). Respiratory effects are plotted as percent of baseline minute volume (MV) with respect to the time postdrug injection. A baseline was established for 20 min prior to opioid administration. (B) A total of four groups are presented: three groups receiving carfentanil, two vaccinated (ester + carfentanil and p-phenyl + carfentanil) and one unvaccinated control (carfentanil), and one group receiving saline alone (saline). (C and D) Statistical comparison of carfentanil control and saline groups against either the ester (C) or p-phenyl (D) vaccine group. Data points denote the means ± SEM. Statistical comparison was made by two-way RM ANOVA to confirm a significant effect of treatment conditions [F (3, 44) = 103; P < 0.0001] with Bonferroni’s comparison; *P < 0.05, **P < 0.01, ***P < 0.001. Statistical significance, ***P < 0.001, was observed between carfentanil and saline groups for all time points, except t = 0, but have been omitted for clarity. Respiratory traces recorded in the absence of carfentanil (E) and at maximum respiratory depression by carfentanil (30 μg/kg) at t = 15 min (F).
Carfentanil is an ultrapotent opioid agonist that activates MOR, producing respiratory depression and antinociception that can be reliably measured in rodents. In contrast, antiopioid antibodies from vaccinated animals target the drug and dampen its pharmacological effects, acting as “immunoantagonists.” To assess the ability of our vaccines to block carfentanil-induced respiratory effects, whole-body plethysmography (WBP) was employed, a noninvasive method that measures respiration in freely moving, conscious animals. WBP is a well-established means of measuring opioid-induced respiratory depression, previously validated in mice.26 In the current study, vaccinated and unvaccinated control mice received a 30 μg/kg dose of carfentanil that typically reduces to minute volume (MV) by approximately 70% at maximum respiratory depression (Figure 2B–F). Testing our vaccines in the WBP mouse assay revealed that Carfen-ester-TT completely blocked the respiratory depression induced by carfentanil, with statistical significance between this vaccinated group and the carfentanil control group (Figure 2C); Carfen-p-phenyl-TT provided a partial blockade, lessening the respiratory depressive effects of carfentanil (Figure 2D). As anticipated, the presence of vaccine-generated antibodies does not appear to alter the normal respiration of vaccinated mice in the absence of carfentanil (Figure S2). Overall, both vaccines attenuated the respiratory depressive effects of carfentanil at a dose of 30 μg/kg. Nonetheless, based on the premise that the protective capacity of the vaccine-generated antibodies is dependent upon antibody–drug binding interactions and drug sequestration, the antibody shielding effect could conceivably be overridden at increasing concentrations of carfentanil. More comprehensive dose response studies will be able to uncover such manifestations.
The analgesic potency of opioids in rodents is routinely evaluated through determining response thresholds to thermal stimuli.27 Moreover, opioids increase pain thresholds in a dose-dependent manner, a result that can be quantified by measuring the latency to nociception in hot plate and tail flick tests. As discussed, vide supra, a vaccine’s efficacy can be gauged by its ability to decrease drug potency mediated through antibody–drug binding. Thus, cumulative dose antinociception assays were performed on day 46 where mice were incrementally administered increasing amounts of carfentanil, and latency to nociception was measured 15 min after each dose. In these tests, the carfentanil ED50 values in unvaccinated control mice were determined to be approximately 2.5–3.2 μg/kg (Figure S3). Remarkably, during this initial antinociception study, carfentanil dosing was unable to fully overcome the protective capability of the vaccines. In fact, mice vaccinated with Carfen-ester-TT showed complete neutralization of carfentanil-induced antinociception at a 250 μg/kg cumulative carfentanil dose (approximately 100-fold greater than the ED50 of control mice while mice vaccinated with Carfen-p-phenyl-TT demonstrated a lower but significant attenuation of carfentanil potency (Figure S3)).
To reveal the full potential of our Carfen-ester-TT vaccine and further differentiate the two vaccine candidates, a second carfentanil-based antinociception study was conducted on day 84. In contrast to the previous study wherein mice were injected with small incremental carfentanil amounts over an extended period of time, mice were administered large bolus doses of carfentanil starting at 200 μg/kg. This slightly conservative carfentanil dose was selected with the intent of revealing differences between the two candidates without entirely overwhelming the vaccines and was based on the carfentanil range tested in cumulative studies, vide supra. Thus, following baseline measurements, mice received a single 200 μg/kg bolus dose of carfentanil (t = 0), and latency to nociception was measured 15 min postinjection (Figure 3A and B). As expected, unvaccinated control mice immediately reached 100% of the carfentanil maximal possible effect (MPE), whereas both vaccine groups remained essentially at baseline levels, thus demonstrating a nearly complete vaccine-mediated blockade of carfentanil’s effects. Therefore, an additional 200 μg/kg dose was administered (t = 15), and latency to nociception was monitored for a prolonged period, testing at 15 min intervals. Overall, Carfen-ester-TT out-performed Carfen-p-phenyl-TT, peaking at a combined average of ~38% MPE in both assays compared to ~74% for the latter. Furthermore, the ester vaccine group showed greater recovery from drug antinociception over the course of the experiment.
Figure 3.

Behavioral and pharmacokinetic results in vaccinated and unvaccinated mice administered carfentanil or fentanyl. Baseline nociception was measured, then mice received two consecutive intraperitoneal 200 μg/kg bolus doses of carfentanil, t = 0 and t = 15 min, and latency to nociception was measured at 15 min intervals in the hot plate (A) and tail flick (B) tests. Significance is denoted by an asterisk from a two-way RM ANOVA with Bonferroni’s post hoc test when comparing vaccinated groups to unvaccinated (control), *P < 0.05, **P < 0.01, ***P < 0.001 versus control. Mice were dosed with 20 μg/kg of carfentanil, then trunk blood (C) and the brain (D) were harvested 15 min postinjection and carfentanil was quantified by LCMS analysis. **P < 0.01 and ***P < 0.001 versus control determined by one-way ANOVA. Mice were cumulatively dosed with fentanyl, and latency to nociception was measured by hot plate (E) and tail flick (F) tests. (G) Calculated ED50 values for fentanyl antinociception activity. Statistical comparison was made by one-way ANOVA with a Tukey’s post hoc test, ***P < 0.001 versus control group; #P < 0.05, #P < 0.01 versus other vaccine group. (H) Potency ratios determined for each vaccine group relative to the control group. Dashed line denotes control level. #P < 0.05 two-tailed t-test. Bars denote means ± SEM in all plots; n = 6 per group for all studies.
To corroborate the observed pharmacodynamic effects, carfentanil biodistribution studies were performed on day 91. Vaccinated and unvaccinated control mice received a 20 μg/kg dose of carfentanil and were sacrificed 15 min later, and carfentanil levels in the blood and brain were measured by LCMS. Both Carfen-ester-TT and Carfen-p-phenyl-TT vaccinated groups displayed significantly higher amounts of carfentanil in plasma compared to the control group, 90 and 78 times more, respectively (Figure 3C). Conversely, significantly less carfentanil was observed in the brains of vaccinated mice, 2–4 times less (Figure 3D). These results illustrate the capacity of vaccine-generated serum antibodies to effectively sequester large quantities of free carfentanil in the blood reducing distribution to the brain.
As discussed previously, fentanyl is one of the most common synthetic opioids responsible for opioid-related overdose deaths, therefore we challenged our vaccine candidates against fentanyl as well in cumulative dose antinociception assays. Hence, a cohort of mice on day 46 was evaluated in the hot plate and tail flick tests (Figure 3E–H). Both Carfen-ester-TT and Carfen-p-phenyl-TT vaccinated mice demonstrated significant right shifting of fentanyl dose response curves with respect to unvaccinated control mice resulting in higher ED50 values (Figure 3G). Importantly, potency ratios (vaccine ED50 vs control) of greater than 12 in all assays demonstrate the ability of these vaccines to substantially mitigate the pharmacological effects of fentanyl in addition to carfentanil (Figure 3H).
In support of our in vivo behavioral and pharmacokinetic results, we examined the binding affinities of antibodies raised by Carfen-ester-TT and Carfen-p-phenyl-TT. Competitive surface plasmon resonance (SPR) experiments were performed using the appropriate serum-hapten pair; for example, antiserum from mice immunized with Carfen-ester-TT was tested against the corresponding BSA immunoconjugate immobilized on the BIAcore chip surface in the presence of serial drug dilutions. The resulting competitive IC50 values are representative of the antibody Kd binding constants, a method previously validated in our laboratory.21 Both vaccines produced polyclonal antibody responses with single-digit nanomolar carfentanil affinity, albeit, Carfen-ester-TT displayed ~4-fold higher affinity compared to Carfen-p-phenyl-TT (2.1 versus 9.2 nM, Figure 4). Nanomolar binding was also observed to fentanyl, and again Carfen-ester-TT showed tighter binding, although by a mere 2-fold difference. Generally, the measured binding affinities for carfentanil and fentanyl closely correlate with the behavioral outcomes, a common trend observed in other drugs of abuse vaccines.18
Figure 4.

Synthetic opioids and binding affinities of vaccine-generated antiserum. (A) Chemical structures of opioids recognized by polyclonal antibodies. (B) Antiserum IC50 values were determined by competitive SPR binding assays performed on a BIAcore 3000 instrument. Serial dilutions of compound were incubated with serum, then passed over a sensor chip with the corresponding hapten-BSA conjugate immobilized on the surface. Abbreviations: NLX, naloxone; NLT, naltrexone; MeD, methadone; Oxy, oxycodone; MOP, morphine.
Encouraged by the carfentanil and fentanyl cross-reactivity, we explored the potential of our opioid vaccine antiserum to recognize structurally related fentanyl analogs without binding to approved opioid medications. As illustrated in Figure 4, antibodies from Carfen-ester-TT immunized mice are more tolerant of substitutions at the R1 position, as well as R2 and R3 sites, compared to Carfen-p-phenyl-TT. These binding discrepancies are consistent with the structural elements that are presented to the immune system by each hapten as determined by linker positioning. Neither vaccine antiserum showed detectable affinity for clinically used opioids, such as the pain management medications oxycodone (Oxy) and morphine (MOP), or approved treatments for OUD and opioid overdose including methadone (MeD), naltrexone (NLT), and naloxone (NLX). On the basis of these findings, our active vaccine strategy could potentially be used in combination with current OUD pharmacotherapies and would not interfere with opioid overdose reversal agents.
The ongoing opioid crisis continues to be one of the most serious public health concerns that has been recently exacerbated by the current pandemic. As a consequence, illicit opioid use and opioid overdose deaths have reached unprecedented levels. Interventions for OUD clearly remain in dire need now more than ever. The current study presents two opioid conjugate vaccine candidates with the capacity to mitigate the in vivo pharmacological effects of highly potent opioids, most importantly, neutralizing the often-fatal opioid-induced respiratory depression. In conclusion, our findings are projected to facilitate the advancement of antiopioid immunotherapies toward a clinically viable treatment option for OUD, which may aid in preventing future opioid-related deaths.
METHODS
Details of experimental procedures are provided in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Webb for performing LC-MS/MS analysis of carfentanil samples. Research reported in this manuscript was supported by the National Institute on Drug Abuse under Award No. U01DA046232.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.1c00026.
Experimental procedures, hapten synthesis and bioconjugation chemistry, biochemical and in vivo procedures, and supplemental figures including timeline of experiments, additional respiratory depression measurements, antinociception assays, and biodistribution standard curves (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acschembio.1c00026
The authors declare the following competing financial interest(s): The opioid vaccine technology contained in this manuscript is owned and patented by TSRI, and Cessation Therapeutics has licensed the technology.
REFERENCES
- (1).Sneader W (1998) The discovery of heroin. Lancet 352 (9141), 1697–1699. [DOI] [PubMed] [Google Scholar]
- (2).Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, and Simopoulos TT (2018) A Brief History of the Opioid Epidemic and Strategies for Pain Medicine. Pain Ther 7 (1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Centers for Disease Control and Prevention (2011) Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb. Mortal Wkly Rep 60 (43), 1487–1492. [PubMed] [Google Scholar]
- (4).Seth P, Scholl L, Rudd RA, and Bacon S (2018) Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants - United States, 2015–2016. MMWR Morb Mortal Wkly Rep 67 (12), 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wilson N, Kariisa M, Seth P, Smith H. t., and Davis NL (2020) Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69 (11), 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Stein C (2016) Opioid Receptors. Annu. Rev. Med 67, 433–451. [DOI] [PubMed] [Google Scholar]
- (7).Kieffer BL (1999) Opioids: first lessons from knockout mice. Trends Pharmacol. Sci 20 (1), 19–26. [DOI] [PubMed] [Google Scholar]
- (8).Pattinson KT (2008) Opioids and the control of respiration. Br. J. Anaesth 100 (6), 747–758. [DOI] [PubMed] [Google Scholar]
- (9).Stanley TH (2014) The fentanyl story. J. Pain 15 (12), 1215–1226. [DOI] [PubMed] [Google Scholar]
- (10).Van Bever WF, Niemegeers CJ, Schellekens KH, and Janssen PA (1976) N-4-Substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung 26 (8), 1548–1551. [PubMed] [Google Scholar]
- (11).Riches JR, Read RW, Black RM, Cooper NJ, and Timperley CM (2012) Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J. Anal. Toxicol 36 (9), 647–656. [DOI] [PubMed] [Google Scholar]
- (12).Leen JLS, and Juurlink DN (2019) Carfentanil: a narrative review of its pharmacology and public health concerns. Can. J. Anaesth 66 (4), 414–421. [DOI] [PubMed] [Google Scholar]
- (13).Delcher C, Wang Y, Vega RS, Halpin J, Gladden RM, O’Donnell JK, Hvozdovich JA, and Goldberger BA (2020) Carfentanil Outbreak - Florida, 2016–2017. MMWR Morb Mortal Wkly Rep 69 (5), 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).O’Donnell J, Gladden RM, Mattson CL, and Kariisa M (2018) Notes from the Field: Overdose Deaths with Carfentanil and Other Fentanyl Analogs Detected - 10 States, July 2016-June 2017. MMWR Morb Mortal Wkly Rep 67 (27), 767–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ringuette AE, Spock M, Lindsley CW, and Bender AM (2020) DARK Classics in Chemical Neuroscience: Carfentanil. ACS Chem. Neurosci 11, 3955–3967. [DOI] [PubMed] [Google Scholar]
- (16).Olson ME, Eubanks LM, and Janda KD (2019) Chemical Interventions for the Opioid Crisis: Key Advances and Remaining Challenges. J. Am. Chem. Soc 141 (5), 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Pravetoni M, and Comer SD (2019) Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158, 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bremer PT, and Janda KD (2017) Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev 69 (3), 298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Salle S, Bodeau S, Dhersin A, Ferdonnet M, Goncalves R, Lenski M, Lima B, Martin M, Outreville J, Vaucel J, and Fabresse N (2019) Novel synthetic opioids: A review of the literature. Toxicol Anal Clin 31 (4), 298–316. [Google Scholar]
- (20).Barrientos RC, Bow EW, Whalen C, Torres OB, Sulima A, Beck Z, Jacobson AE, Rice KC, and Matyas GR (2020) Novel Vaccine That Blunts Fentanyl Effects and Sequesters Ultrapotent Fentanyl Analogues. Mol. Pharmaceutics 17 (9), 3447–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, and Janda KD (2016) Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew. Chem., Int. Ed 55 (11), 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Raleigh MD, Baruffaldi F, Peterson SJ, Le Naour M, Harmon TM, Vigliaturo JR, Pentel PR, and Pravetoni M (2019) A Fentanyl Vaccine Alters Fentanyl Distribution and Protects against Fentanyl-Induced Effects in Mice and Rats. J. Pharmacol. Exp. Ther 368 (2), 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Robinson C, Gradinati V, Hamid F, Baehr C, Crouse B, Averick S, Kovaliov M, Harris D, Runyon S, Baruffaldi F, LeSage M, Comer S, and Pravetoni M (2020) Therapeutic and Prophylactic Vaccines to Counteract Fentanyl Use Disorders and Toxicity. J. Med. Chem 63 (23), 14647–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hwang CS, Smith LC, Wenthur CJ, Ellis B, Zhou B, and Janda KD (2019) Heroin vaccine: Using titer, affinity, and antinociception as metrics when examining sex and strain differences. Vaccine 37 (30), 4155–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Townsend EA, Bremer PT, Jacob NT, Negus SS, Janda KD, and Banks ML (2021) A synthetic opioid vaccine attenuates fentanyl-vs-food choice in male and female rhesus monkeys. Drug Alcohol Depend 218, 108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hill R, Santhakumar R, Dewey W, Kelly E, and Henderson G (2020) Fentanyl depression of respiration: Comparison with heroin and morphine. Br. J. Pharmacol 177 (2), 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hargreaves K, Dubner R, Brown F, Flores C, and Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32 (1), 77–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


