Abstract
The hypothalamic paraventricular nucleus (PVN) controls neuroendocrine axes and the autonomic nervous system to mount responses that cope with the energetic burdens of psychological or physiological stress. Neurons in the PVN that express the angiotensin Type 1a receptor (PVNAgtr1a) are implicated in neuroendocrine and autonomic stress responses; however, the mechanism by which these neurons coordinate activation of neuroendocrine axes with sympathetic outflow remains unknown. Here, we use a multidisciplinary approach to investigate intra-PVN signaling mechanisms that couple the activity of neurons synthesizing corticotropin-releasing-hormone (CRH) to blood pressure. We used the Cre-Lox system in male mice with in vivo optogenetics and cardiovascular recordings to demonstrate that excitation of PVNAgtr1a promotes elevated blood pressure that is dependent on the sympathetic nervous system. Next, neuroanatomical experiments found that PVNAgtr1a synthesize CRH, and intriguingly, fibers originating from PVNAgtr1a make appositions onto neighboring neurons that send projections to the rostral ventrolateral medulla and express CRH type 1 receptor (CRHR1) mRNA. We then used an ex vivo preparation that combined optogenetics, patch-clamp electrophysiology, and Ca2+ imaging to discover that excitation of PVNAgtr1a drives the local, intra-PVN release of CRH, which activates rostral ventrolateral medulla-projecting neurons via stimulation of CRHR1(s). Finally, we returned to our in vivo preparation and found that CRH receptor antagonism specifically within the PVN lowered blood pressure basally and during optogenetic activation of PVNAgtr1a. Collectively, these results demonstrate that angiotensin II acts on PVNAgtr1a to conjoin hypothalamic-pituitary-adrenal axis activity with sympathetically mediated vasoconstriction in male mice.
SIGNIFICANCE STATEMENT The survival of an organism is dependent on meeting the energetic demands imposed by stressors. This critical function is accomplished by the CNS's ability to orchestrate simultaneous activities of neurosecretory and autonomic axes. Here, we unveil a novel signaling mechanism within the paraventricular nucleus of the hypothalamus that links excitation of neurons producing corticotropin-releasing-hormone with excitation of neurons controlling sympathetic nervous system activity and blood pressure. The implication is that chronic stress exposure may promote cardiometabolic disease by dysregulating the interneuronal cross-talk revealed by our experiments.
Keywords: autonomic, cardiovascular, glucocorticoids, HPA axis, hypertension, stress
Introduction
Survival of an organism is dependent on meeting the energetic demands imposed by stressors, defined broadly, as real or perceived threats to homeostasis. The CNS coordinates the activity of neuroendocrine axes and the autonomic nervous system to initiate cardiometabolic responses that anticipate the energetic burdens associated with psychological or physiological threats. While the secretagogues, nuclei, and ganglia controlling the neuroendocrine and autonomic nodes of the stress response are well established (Ulrich-Lai and Herman, 2009), the signaling mechanism(s) governing their integration and coordination remains to be elucidated.
The paraventricular nucleus of the hypothalamus (PVN) is recognized as a key integrative center that moderates cardiometabolic function to cope with homeostatic threats (Sladek et al., 2015). As such, the PVN contains anatomically and functionally distinct neuronal phenotypes that release neuroendocrine factors into the systemic circulation or regulate sympathetic outflow to cardiovascular tissues (Swanson and Sawchenko, 1980). The firing activity of these neuronal phenotypes controls the neurosecretory and sympathetic outflows from the PVN (Cazalis et al., 1985; Chen and Toney, 2010; Holbein et al., 2018) and is fine-tuned by the combined action of intrinsic properties (e.g., ionic conductance) or extrinsic properties (e.g., synaptic connectivity) (Bourque et al., 1993; Brown et al., 2013; Colmers and Bains, 2018). While the intrinsic and extrinsic mechanisms governing the firing and output of each independent neuronal phenotype are generally well understood, the local signals that coordinate their activities to enable multimodal neuroendocrine and autonomic responses to homeostatic threats remain largely unknown. In this regard, we discovered that within the PVN, the firing of vasopressin neurons is accompanied by dendritic release of vasopressin that creates a paracrine signal which couples the secretion of vasopressin into the systemic circulating with augmented sympathetic outflow to the cardiovascular system (Son et al., 2013). Still, whether the coordinated activities of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic outflow typically observed in the context of the stress response also involves a local interpopulation signaling mechanism has not been discerned.
Within the PVN, the neuropeptide, angiotensin-II, and its angiotensin Type 1a receptors (Agtr1a) have long been implicated in orchestrating neuroendocrine and autonomic responses to stress (Jezova et al., 1998; Saavedra et al., 2004). Recent studies from our laboratories using genetically modified mice have found that the activity of neurons in the PVN that synthesize Agtr1a(s) is intimately coupled to the neuroendocrine and cardiovascular nodes of the stress response (de Kloet et al., 2017). Specifically, selective optogenetic excitation of Agrt1a-expressing neurons in the PVN (PVNAgtr1a) promotes activation of the HPA and hypothalamic-pituitary-thyroid (HPT) axes, whereas optogenetic inhibition of these neurons elicits the opposite effect (de Kloet et al., 2017). Interestingly, optogenetic excitation was also found to increase systolic blood pressure (SBP) (de Kloet et al., 2017); however, selective deletion of Agtr1a(s) from the PVN blunts cardiovascular reactivity and indices of sympathetic nervous system activity (SNA) during psychogenic stress (L. Wang et al., 2016). Collectively, these results suggest that, during stress, PVNAgtr1a couple activation of neuroendocrine axes mediating energy utilization with the increased sympathetic outflow and cardiovascular reactivity that is a hallmark of the fight-or-flight response. However, the precise signaling mechanisms that couple the activity of PVNAgtr1a to alterations in autonomic outflow that affect cardiovascular function remain to be identified.
To address this gap, we implement a multidisciplinary approach based on the complementary use of (1) in vivo optogenetics and telemetric cardiovascular recordings, (2) genetic reporting, fluorescence ISH, and neuronal tract-tracing, and (3) ex vivo optogenetics, patch-clamp electrophysiology, and Ca2+ imaging. We reveal a novel signaling mechanism that couples the activity of neurons regulating the HPA axis and sympathetically mediated vasoconstriction. We propose that this novel interneuronal cross-talk has important implications for mounting responses that cope with acute threats to homeostasis, but also contribute to the neuroendocrine and autonomic dysregulation that occurs with cardiometabolic diseases that follow chronic stress exposure.
Materials and Methods
Animals
Studies were conducted in male mice on a C57BL/6J background or in Wistar male rats (n = 4, purchased from Harlan, 4-5 weeks old). Mice were 8-10 weeks old at the initiation of the studies. All animals were maintained in temperature and humidity-controlled rooms on 12:12 h light-dark cycles. In all cases, food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committees at the University of Florida or Georgia State University and were conducted in accordance with the National Institutes of Health's Guide for the care and use of laboratory animals.
The majority of studies were conducted using an Agtr1a-Cre knock-in mouse line. These mice were generated by Biocytogen and the University of Florida, were extensively validated (de Kloet et al., 2017), and have been made available at The Jackson Laboratory (stock #030553). In one study, mice homozygous for this Agtr1a-Cre knock-in gene were bred with mice heterozygous for the Ai32 stop-flox-ChR2-eYFP gene (The Jackson Laboratory, stock #024109). This leads to two genotypes of offspring: (1) mice that express the light-sensitive channel-2 rhodopsin (ChR2) and enhanced yellow fluorescent protein (eYFP) in all cells that express the Agtr1a (referred to as Agtr1a-ChR2 mice) and (2) their littermates that express only the Agtr1a-Cre gene that serve as controls for the cardiovascular studies depicted in Figure 1.
Figure 1.
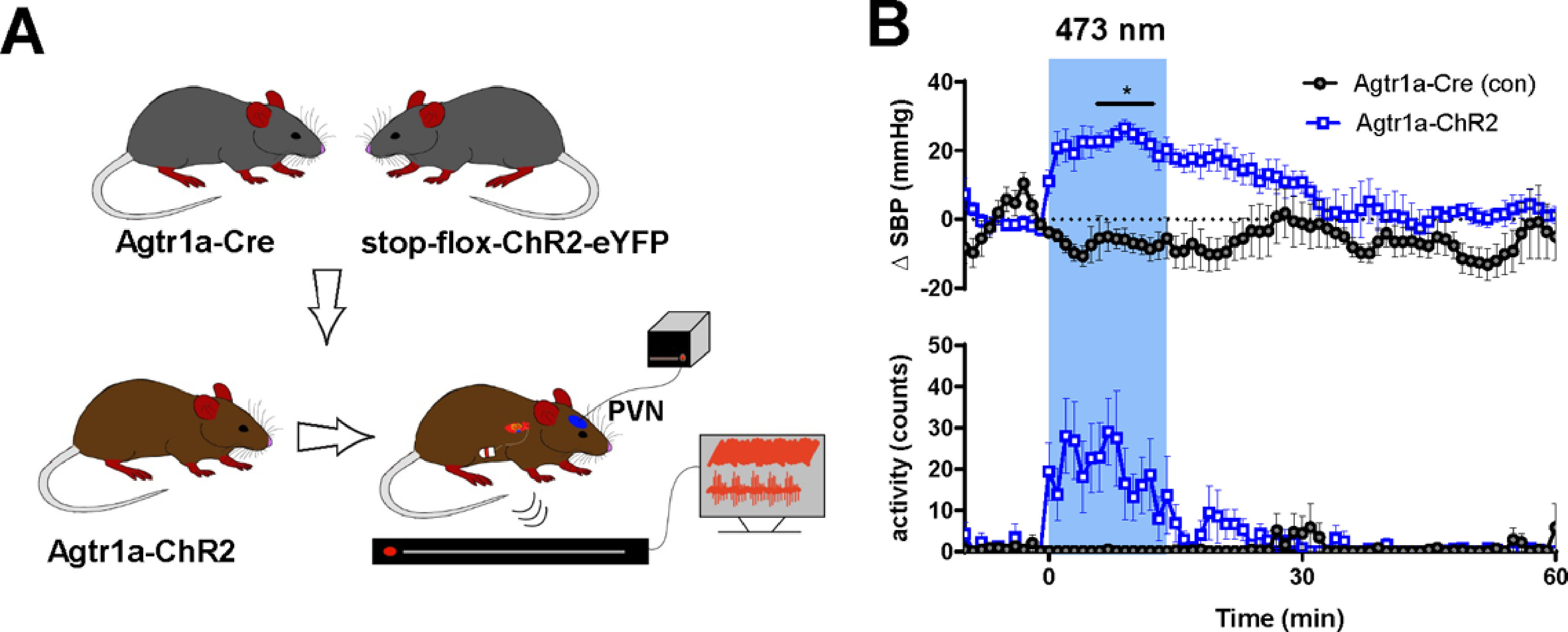
SBP and locomotor activity are robustly elevated when the PVN is optogenetically stimulated in mice with ChR2 expression directed to the Agtr1a gene. A, Schematic depicting the mouse line and experimental protocol. In this experiment, Agtr1a-ChR2 mice and littermate controls undergo optogenetic stimulation (10 mW; 15 Hz; 5 s on/off; 15 min) of Agtr1a neurons and afferents localized to the PVN. B, Change in SBP and locomotor activity of mice before, during, and after optogenetic stimulation. Both SBP and locomotor activity were measured using the radiotelemetry transmitters. Error bars indicate SEM. Blue box represents period of optogenetic stimulation. p < 0.05. n = 5 per group.
Initial neuroanatomical studies used mice that have the red fluorescent protein variant, tdTomato, directed to either corticotrophin-releasing hormone (CRH)-containing cells or Agtr1a-containing cells. These lines are referred to as CRH-tdTomato or Agtr1a-tdTomato mice, respectively. To generate CRH-tdTomato mice, CRH-Cre knock-in mice (The Jackson Laboratory, stock #012704) were crossed with stop-flox-tdTomato mice (The Jackson Laboratory, stock #007914). The fidelity of this approach to label CRH neurons residing in the PVN was previously validated (Smith et al., 2014). The same approach was used to generate Agtr1a-tdTomato mice, which we have previously found exhibit 97% overlap between tdTomato and Agtr1a-mRNA within the PVN (de Kloet et al., 2017).
Viral constructs
Adeno-associated viral vectors (AAVs) that allow for the Cre-inducible expression of fluorophores (eYFP or mCherry) and/or light sensitive ion channels were obtained from the viral vector core at the University of North Carolina. For expression of eYFP and ChR2, pAAV2-EF1a-DIO-hChR2(H134R)-eYFP-WPRE (referred to as AAV-ChR2-eYFP) was used; for the expression of only eYFP, pAAV-EF1a-DIO-eYFP (referred to as AAV-eYFP) was used; and for the expression of mCherry and the red-shifted ChR2: rAAV2/Ef1a-DIO-C1V1-(E122t/E162T)-TS-mCherry (referred to as AAV-rsChR2-mCherry) was used. In order to retrogradely transfect neurons, we used AAV vectors (of the AAVrg serotype) that were obtained from Addgene. These AAVs lead to expression of the calcium indicator, GCaMP7s, under control of the synapsin promoter (i.e., pGP-AAVrg-syn-JGCaMP7s-WPRE; catalog #104488) or the tdTomato fluorophore under control of the CAG promoter (i.e., AAVrg-CAG-tdTomato; catalog #59462).
Radiotelemetry
Radiotelemeters (PAC-10, DSI) were used to continuously monitor cardiovascular parameters and locomotor activity during optogenetic stimulation in awake, freely-moving mice. Agtr1a-ChR2 mice and their littermate Agtr1a-Cre controls were anesthetized using isoflurane and administered analgesic (Buprenex; 0.1 mg kg−1, s.c.). An incision (∼1 cm) was made on the midline of the ventral neck to expose the left carotid artery. Subsequently, a 1-cm-long segment of the carotid artery was separated from the vagus nerve using two silk sutures (size: 5-0); the cranial end of the segment was permanently ligated and the caudal end of the segment was temporally occluded. Next, the carotid artery was punctured and the catheter was slid into the artery lumen such that the tip extended into the aortic arch. The catheter was then secured in place using suture and the telemetry device was positioned subcutaneously, in the left flank region. Skin was closed with 5-0 monofilament suture, and mice were allowed to recover for at least 7 d before undergoing stereotaxic surgery. During the study, cardiovascular parameters were continuously recorded and analyzed using Ponemah software (version 6.42, DSI).
Stereotaxic surgery
Stereotaxic surgery was performed to deliver AAVs or the retrograde neuronal tract tracer fluorogold (FG) into specific brain nuclei. We also used it to implant fiber optics targeting the PVN as previously described (de Kloet et al., 2017). Fiber optic implants allowed for optogenetic stimulation during cardiovascular recordings obtained in conscious freely moving mice or under general anesthesia. In preparation for aseptic stereotaxic surgery, mice were anesthetized using isoflurane and administered analgesic (Buprenex; 0.1 mg kg−1, s.c.). To retrogradely label presympathetic neurons of the PVN, Agtr1a-tdTomato mice received bilateral stereotaxic microiontophoretic injections of FG (6 μA 7 s pulses, 8 min) into the rostral ventrolateral medulla (RVLM) using the following coordinates from λ: 1.57 mm posterior, ±1.3 mm lateral, and 5.07 mm ventral from the surface of the brain. These same coordinates were used to deliver AAVrg-syn-JGCaMP7s-WPRE or AAVrg-CAG-tdTomato bilaterally into the RVLM using a picospritzer III (Parker). For AAV-mediated gene transfer to the PVN, Cre-inducible AAVs described above were injected bilaterally into the PVN using the following coordinates from bregma: AP: ±0 mm, ML: ±0.25 mm, DV: – 4.75 mm. For each AAV injection, the pipette was left in the ROI for 5 min to allow for diffusion of the AAVs (100-150 nl) into the brain. Mice administered FG were killed 7 d after the microiontophoretic injections to collect brains for neuroanatomical studies. Mice receiving AAV injections were allowed to recover for at least 3 weeks before subsequent procedures.
To retrogradely label PVN presympathetic neurons of male rats, rhodamine-labeled microspheres (Lumaflor) were unilaterally injected into the RVLM as previously described (Sonner et al., 2011). A stereotaxic apparatus was used to pressure inject 200 nl of the tracer into the RVLM (starting from bregma: AP: −12 mm, ML: ±2 mm, DV: −8 mm). In general, RVLM injection sites were contained within the caudal pole of the facial nucleus to ∼1 mm more caudal and were ventrally located with respect to the nucleus ambiguus. The location of the tracer was verified histologically (Sonner et al., 2011). Rats were used 3-5 d after surgery.
RNAscope ISH and immunohistochemistry
In order to collect tissue for neuroanatomical studies, mice were anesthetized with pentobarbital (50 mg kg−1, i.p.) and perfused transcardially with RNase free-isotonic saline followed by 4% PFA. Brains were then postfixed for ∼4 h, after which they were stored in RNase-free 30% sucrose for up to 1 week before further processing. For RNAscope ISH, the PVN (from bregma; −0.22 mm to −1.22 mm) was sectioned at 20 µm into 6 serial sections using a Leica CM3050 S cryostat (Leica Microsystems). Sections were immediately mounted onto Fisherbrand SuperFrost Plus Gold Microscope Slides (Thermo Fisher Scientific). After air-drying at room temperature for 20-30 min, slides were dipped in EtOH, again allowed to dry for 10-15 min and then stored at −80°C until further processing. For immunohistochemical labeling of FG, mouse brains were sectioned at 30 µm into four serial sections and stored in cryoprotective solution at −20°C, until further processing.
RNAscope ISH was performed using the RNAscope V2 Multiplex Fluorescent Reagent Kit (Advanced Cell Diagnostics) as per the manufacturer's instructions with slight modification to the pretreatment procedure that allows for preservation of the reporter genes (i.e., tdTomato and/or eYFP), while still providing optimal mRNA signal. The probes used for these studies were as follows: Agtr1a (Mm-Agtr1a-O1; catalog #481161), Crhr1 (Mm-Crhr1; catalog #418011), DapB (Negative control probe-DapB; catalog #310043); Ubc (Mm-Ubc; catalog #310771). Upon completion of the ISH for Agtr1a that is depicted in Figure 1, slides were immediately coverslipped using Prolong Gold Antifade Mountant (Thermo Fisher Scientific). For labeling that is depicted in Figure 2, sections underwent immunohistochemical for GFP (used to amplify the eYFP signal) immediately following the ISH protocol.
Figure 2.
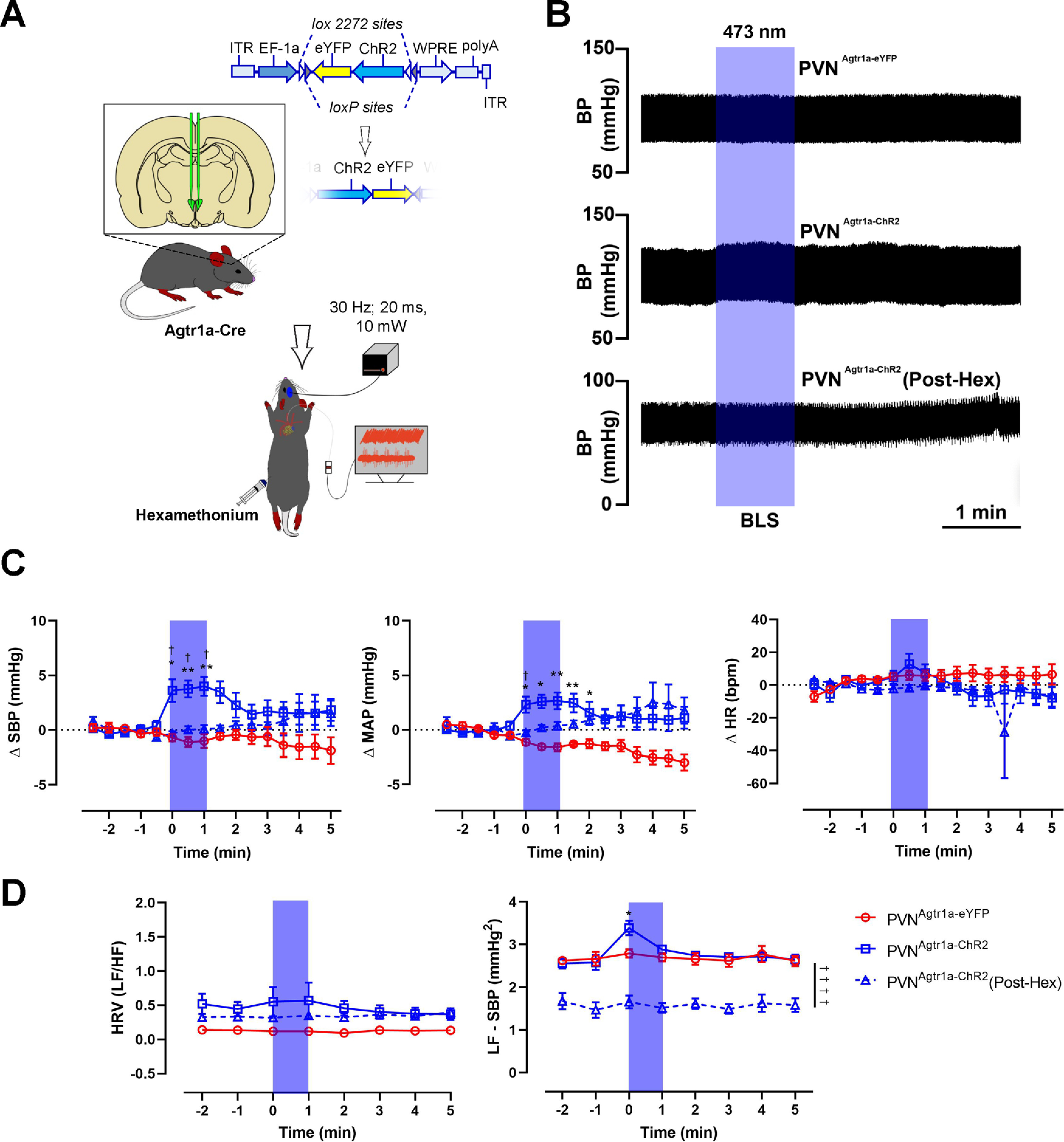
Optogenetic stimulation of PVNAgtr1a elicits sympathetically mediated increases in blood pressure. A, Schematic depicting the mouse line and experimental protocol. In this experiment, Agtr1a-Cre mice received a Cre-inducible AAV-ChR2-eYFP into the PVN. Following recovery, PVNAgtr1a were optogenetically stimulated (10 mW; 20 ms pulse width; 30 Hz; 1 min) while cardiovascular parameters were recorded via a Millar catheter inserted into the left carotid artery. B, Representative pulsatile arterial blood pressure traces following blue laser stimulation (BLS) in PVNAgtr1a-eYFP mice, PVNAgtr1a-ChR2 mice, or PVNAgtr1a-ChR2 mice following the systemic administration of hexamethonium (30 mg/kg, i.p.). C, Group means demonstrating the change in SBP (left), mean arterial pressure (MAP; middle), and heart rate (HR; right). D, Group means demonstrating the change in heart rate variability (left; HRV – ratio of the LF domain over the HF domain) and the LF domain of SBP variability (right; LF – SBP). Error bars indicate SEM. p < 0.05; p < 0.05; two-way ANOVA followed by Tukey's post for PVNAgtr1a-eYFP versus PVNAgtr1a-ChR2. †p < 0.05; two-way ANOVA followed by Tukey's post for PVNAgtr1a-ChR2 versus PVNAgtr1a-ChR2 (Post-Hex), n = 6: PVNAgtr1a-eYFP, n = 11: PVNAgtr1a-ChR2, n = 10: PVNAgtr1a-ChR2 (Post-Hex).
Standard immunohistochemistry protocols were used to amplify FG or eYFP signal (de Kloet et al., 2017). Primary antibodies and dilutions used are as follows: GFP (Invitrogen [A10262]; 1:1000) and FG (Millipore [AB153]; 1:1000). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, raised in donkey, and used at a 1:500 dilution. Briefly, brain sections were rinsed then incubated first in blocking solution (2% normal donkey serum and 0.2% Triton X in 50 mm KPBS) for 2 h at 25°C and then in the primary antibody (diluted in blocking solution) for 18 h at 4°C. Sections were again rinsed 5 × 5 min (50 mm KPBS) before incubation in the secondary antibody in blocking solution for 2 h at 25°C. After a final series of rinses, slides were allowed to air dry and then coverslipped using Prolong Gold Antifade Mountant.
Image capture and processing
Images were captured and processed using Axiovision 4.8.2 software and a Zeiss AxioImager fluorescent Apotome microscope. Using the Apotome, z stacks of the proteins and mRNAs of interest were captured at 20 × magnification throughout the PVN using neuroanatomical landmarks (Franklin and Paxinos, 2008). An average of 10 optical sections were collected per z stack (1 µm between z steps), and these were used to generate projection images depicted in Figure 3. For ISH experiments, sections hybridized with the probes of interest (Agtr1a or Crhr1) were used to determine the exposure time and image processing required to provide optimal visualization of RNA signal. As described in detail by de Kloet et al. (2016), these same parameters were then used to assess background fluorescence in sections hybridized with the negative control probe (DapB). Importantly, using these exposure times and image processing parameters, there was minimal or no fluorescence in sections hybridized with the negative control probe. All final figures were then prepared using Adobe Photoshop 2020, and the brightness and contrast were adjusted to provide optimal visualization.
Figure 3.
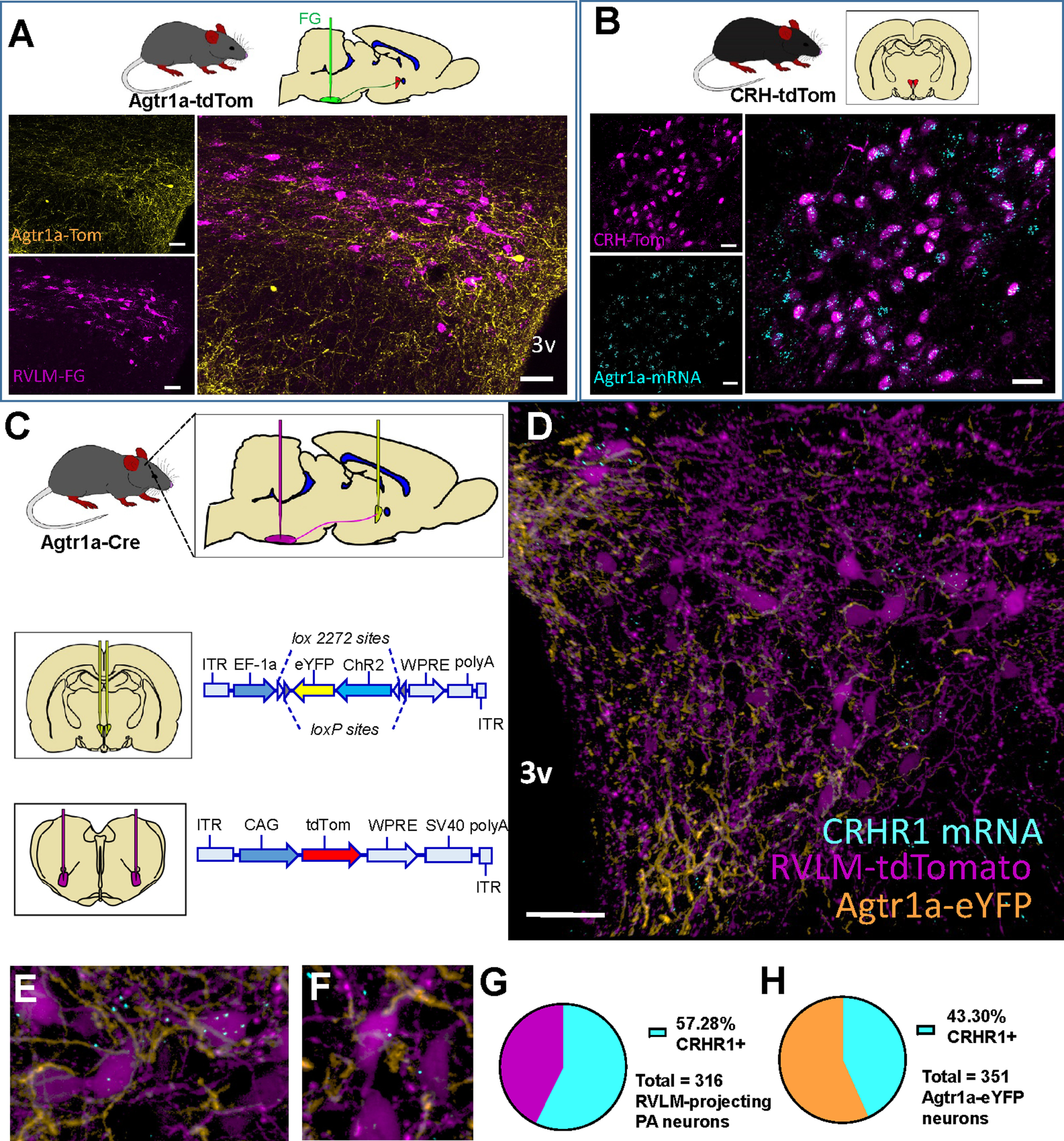
Presympathetic neurons of the PVN express CRHR1 mRNA and are juxtaposed to processes arising from PVNAgtr1a. A, Top, Agtr1a-tdTomato mice received FG injections into the RVLM and were perfused 7 d later. Left, In the PVN, tdTomato (yellow) depicts Agtr1a neurons and FG labeling (magenta) depicts RVLM-projecting neurons. Right, Merged image showing that Agtr1a neurons and RVLM-projecting neurons are separate populations. 3v, Third cerebral ventricle. Scale bar, 50 µm. B, Top, Sections through the PVN from CRH-tdTomato mice were processed for ISH for Agtr1a mRNA. Left, Projection images through the PVN of a CRH-tdTomato reporter mouse depicting CRH-Tomato (magenta) and Agtr1a mRNA (cyan). Right, Merged image showing that CRH neurons in the PVN robustly express Agtr1a mRNA. Scale bar, 20 µm. C, Top, Schematic of the AAV injections used to label (middle) PVNAgtr1a neurons with eYFP and (bottom) retrogradely label presympathetic neurons that project to the RVLM with tdTomato. D, 20× image of a coronal section through the PVN depicting CRH1R mRNA (cyan), presympathetic neurons that project to the RVLM (magenta), and neurons that express Agtr1a (yellow). E, F, 40× projection images highlighting that presympathetic neurons in the PVN express CRHR1 mRNA and are in close proximity to processes arising from PVNAgtr1a. G, Percentage of RVLM-projecting or (H) PVNAgtr1a that express CRHR1 mRNA. n = 4 mice.
Slice preparation
Experimental Agtr1a-Cre mice for electrophysiological and in vitro Ca2+ imaging studies underwent stereotactic surgery (described above) at the University of Florida to deliver the AAVs described above bilaterally into the RVLM and PVN. After surgical recovery, they were transported via courier service (Optimize Courier) to Georgia State University for use in electrophysiological and/or in vitro Ca2+ imaging studies that were conducted ∼8-12 weeks after stereotactic surgery.
On the day of the experiment, mice and rats were anesthetized with pentobarbital (50 mg kg−1, i.p.) and then perfused transcardially with 30 ml of ice-cold aCSF solution with NaCl replaced by equal-osmol sucrose. This sucrose aCSF solution contained (in mm): 200 sucrose, 2.5 KCl, 1 MgSO4, 26 NaHCO3, 1,25 NaH2PO4, 20 D-glucose, 0.4 ascorbic acid, and 2.0 CaCl2, pH 7.2, 300-305 mOsmol l−1. The animal was then rapidly decapitated, and the brain was subsequently removed, mounted in the chamber of a vibrotome with superglue (Leica VT1200s, Leica Microsystems), and submerged in the same sucrose solution and bubbled constantly with 95% O2/5% CO2. Slices were cut at 240 µm thickness and placed in a holding chamber containing aCSF bubbled with 95% O2/5% CO2. The aCSF is identical in composition to the sucrose solution, but with 200 mm sucrose replaced by 119 mm NaCl. The slice chamber was warmed using a water bath at 32°C for 20 min before placement at room temperature.
Simultaneous imaging and electrophysiology
Coronal hypothalamic slices were placed into a specimen chamber on the stage of a Nikon Eclipse FN1 microscope and perfused constantly (∼3 ml/min) with aCSF bubbled continuously with 95% O2/5% CO2 and warmed to 32°C. Neurons expressing GCaMP7s and mCherry were visualized using the Dragonfly 200 laser spinning disk confocal imaging system and an iXon 888 EMCCD camera (Andor Technology). ChR2 or rsChR2 was stimulated using an LED excitation wavelength of 460 or 525 nm, respectively, emitted from a pE-300 Coolpparatus (Andor Technology). The LED stimulus timing interval was controlled by an Andor Mosaic. ChR2 was stimulated using 30 pulses with a 100 ms pulse width and 50 ms interval. Images were acquired using Andor Fusion Software and analyzed using ImageJ.
Whole-cell current-clamp recordings were obtained from fluorescently labeled PVN neurons using pipettes (2.5-4 mΩ) pulled from borosilicate glass (O.D. 1.5 mm) using a P-97 flaming/brown horizontal micropipette puller (Sutter Instruments). The pipette internal solution consisted of the following (in mm): 135 KMeSO4, 8 NaCl, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, 6 phosphocreatine, 0.2 EGTA with pH 7.2-7.3 and 285-295 mOsmol (kg H2O)−1. The liquid junction potential for the KMeSO4 internal was ∼−10 mV and was not corrected. For current-clamp recordings, traces were obtained with an Axopatch 200B amplifier (Molecular Devices) and digitized using an Axon 1440B Digitizer (Molecular Devices) at 10 kHz on a Dell desktop computer running Clampex 10 software (Molecular Devices). Data were discarded if series resistance exceeded a 20% change over the course of the recording.
Anesthetized assessment of blood pressure in response to optogenetic manipulations
Agtr1a-Cre male mice received stereotactic injections of the Cre-inducible AAV-ChR2-eYFP or AAV-eYFP into the PVN as described above. Three to 4 weeks later, mice were anesthetized using isoflurane and a Millar catheter (model SPR1000, Millar) was implanted into the aortic arch by way of the carotid artery using a similar procedure as described above for the implantation of telemetry devices. After catheterization, mice underwent stereotactic surgery to lower a fiber optic into the PVN for optical stimulation using the following coordinates from bregma: AP: ±0 mm, ML ±0.25 mm, DV −3.8 mm.
In order to collect cardiovascular data, the Millar catheter was connected to a PowerLab signal transduction unit (AD Instruments). Blood pressure and heart rate data were sampled/recorded at 1 kHz and analyzed using Labchart8 software (AD Instruments). To test the effect of optical stimulation of Agtr1a neurons of the PVN on blood pressure, mice were optically stimulated with 473 nm blue light for 60 s (10 mW output; 20 ms pulse width; 30 Hz). Mice then received injections of hexamethonium (30 mg/kg, i.p.) or saline vehicle. Once blood pressure again stabilized (∼15-20 min after the injections), mice were again subjected to optical stimulation using the same parameters. Blood pressure data were condensed into 30 s bins to perform statistical analyses and to generate the figures.
Anesthetized assessment of blood pressure in response to pharmacological injections
Age-matched C57BL/6J male mice were anesthetized using isoflurane and a Millar catheter was implanted into the aortic arch as described above. Following catheterization, a glass micropipette (borosilicate glass with filament, O.D. 1.5 mm, I.D. 0.86 mm, Sutter Instrument) was lowered into the PVN using a stereotaxic frame with the following coordinates from bregma: AP: ±0 mm, ML ± 0.25 mm, DV −4.75 mm. The PVN was then microinjected with either Astressin at 50, 100, 200 μm (Astressin, Tocris Bioscience), or vehicle (10% acetic acid in H2O, pH 7.4). Microinjections were conducted at different orders in all mice, alternating between the right and the left PVN with a 30 min interval between each microinjection. The volume of all injections was ∼100 nl. At the end of all microinjections, mice received hexamethonium (30 mg/kg, i.p.) and were then subjected to a PVN microinjection of Astressin (200 μm/100 nl). Blood pressure was sampled at 30 s every 60 s for 2 min and 10 min before and following microinjections, respectively.
In a different set of experiments, Agtr1a-Cre mice received stereotactic injections of the Cre-inducible AAV-ChR2-eYFP into the PVN, and following 3 weeks of recovery mice were anesthetized and a Millar catheter (model SPR1000, Millar) was implanted into the aortic arch as described above. Subsequently, a dual optical injector cannula (Optical Injector, Doric) was lowered above the PVN, allowing microinjections and optogenetic stimulation within the same site while blood pressure was simultaneously recorded. Either vehicle (10% acetic acid, pH 7.4) or Astressin (200 μm) was microinjected, and pulses of 473 nm laser light (10 mW output; 20 ms pulse width; 30 Hz; 1 min) were delivered 5 min following microinjections. Blood pressure was sampled at 30 s every 60 s for 2 min and 5 min before and following optical stimulation, respectively.
Spectral analysis of heart rate and SBP variability
Heart rate variability (HRV) was analyzed using a Heart Rate Variability Analysis Software provided in Labchart8 (AD Instruments), whereas SBP variability was analyzed using a fast Fourier transform spectral variation in SBP using Labchart 8. Briefly, HRV and SBP variability were sampled at 1 min bins before and following optogenetic stimulation, and the frequency bands were as follows: very low frequency (LF), LF, and high frequency (HF) at 0-0.15 Hz, 0.15-1.5 Hz, and 1.5-5 Hz, respectively. HRV was expressed as an LF/HF ratio, which is a surrogate clinical marker of sympatho-vagal balance to the heart (Tasić et al., 2017). The LF domain of SBP variability indicates the level of sympathetic vasoconstrictor activity (deBoer et al., 1987; Madwed et al., 1989).
Statistics
All electrophysiological traces were analyzed in ClampFit 10.7 (Molecular Devices) and Igor Pro (Wavemetrics). Calcium data were analyzed in ImageJ and Igor Pro. Statistics were performed with Prism GraphPad. The following statistical tests were used: paired-samples t test (see Figs. 4C, 6Civ, 9E), and two-way ANOVA with Tukey's post hoc test (see Figs. 5E, 7F,G, 8B). All reported data are represented as mean ± SEM.
Figure 4.
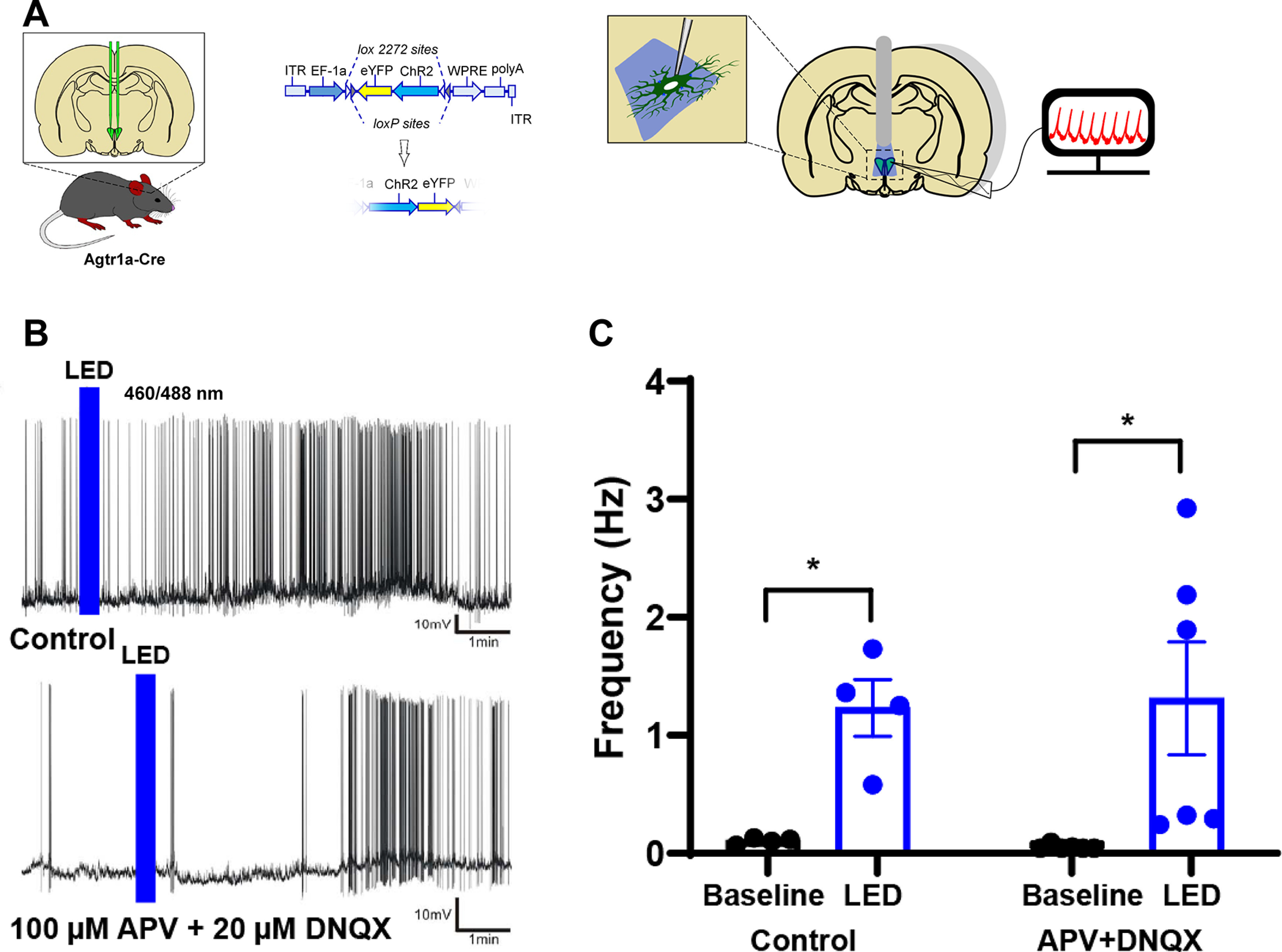
Selective optogenetic excitation of PVNAgtr1a evokes a delayed firing response from putative presympathetic neurons. A, Schematic of the AAV-mediated gene transfer approach used to direct expression of eYFP and ChR2 to PVNAgtr1a. B, Current-clamp recordings of putative presympathetic neurons. LED stimulation evokes increased firing frequency under control conditions (top) and in the presence of DNQX/APV (bottom). C, Summary data showing the increased firing of putative presympathetic neurons before and after LED-induced excitation of PVNAgtr1a (p < 0.05, n = 4 cells from 2 animals). The LED-evoked firing increase persisted in the presence of DNQX/APV (p < 0.05, n = 6 cells from 3 animals)). p < 0.05. Error bars indicate SEM.
Figure 6.
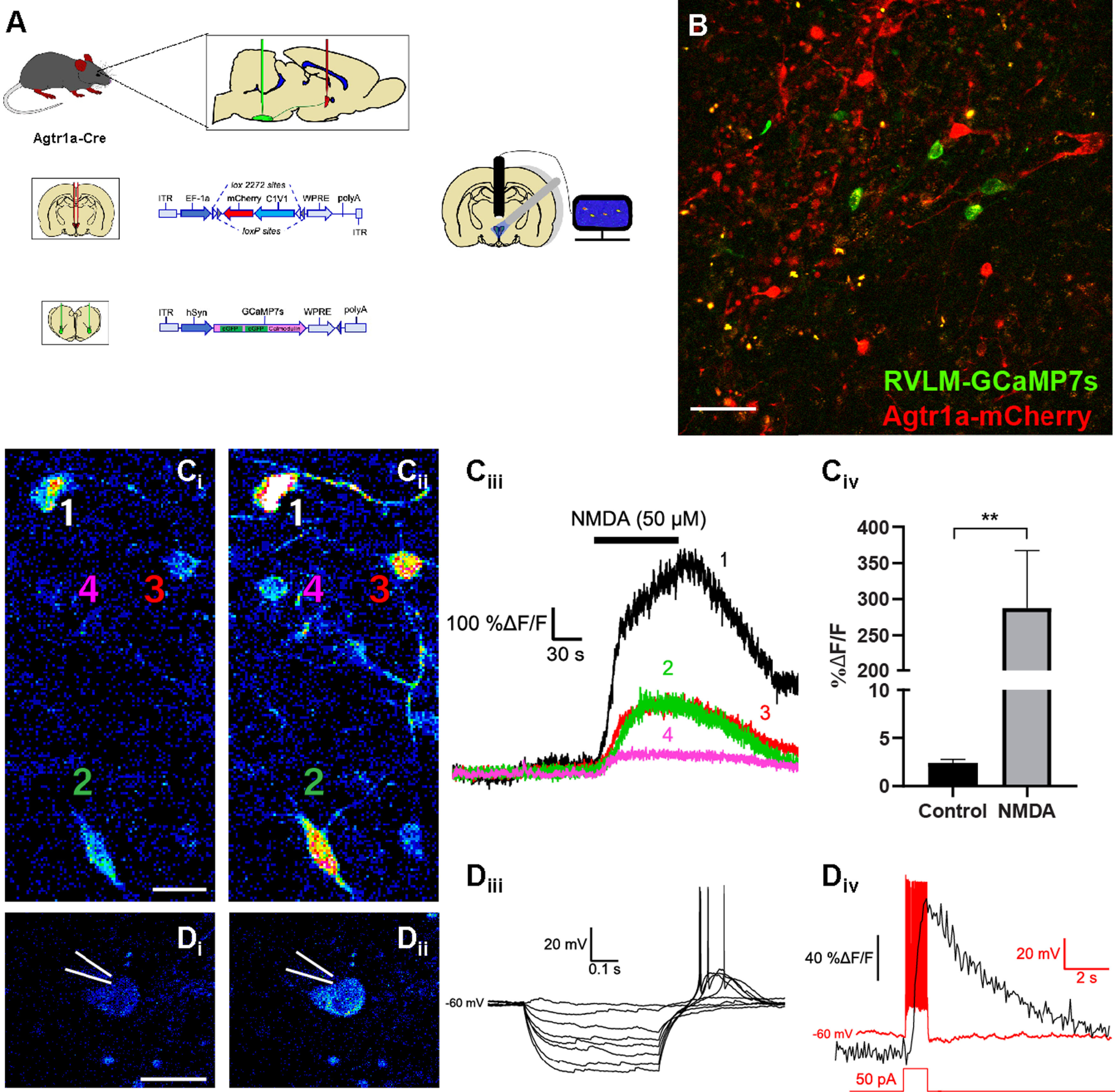
Characterization of GCaMP7s expression in PVN neurons that project to the RVLM. A, Schematic of the AAV injections used to direct the expression of rsChR2-mCherry to PVNAgtr1a while also driving retrograde expression of GCaMP7s in PVN neurons projecting to the RVLM. Fluorescence intensity is used as a proxy for neuronal firing and can be simultaneously recorded from multiple neurons in a slice through the PVN. B, Two-photon microscopy depicting a coronal section through the PVN with GCaMP7s (green) expression within RVLM-projecting neurons and rsChR2-mCherry (red) expression within PVNAgtr1a. Scale bar, 50 µm. C, Two-photon microscopy showing the fluorescence intensity of GCaMP7s within RVLM-projecting (Ci) before and (Cii) during NMDA (50 μm) application. Scale bar, 25 µm. Ciii, Corresponding measurements of %ΔF/F changes in neurons from Ci-ii in response to a 50 μm application of NMDA. Civ, Summary data of average peak %ΔF/F changes in response to NMDA (p = 0.006, paired t test, n = 10 cells from 2 animals). D, Individual GCaMP7s expressing neurons are patch-clamped and fluorescence is recorded (Di) before and (Dii) during the peak increase after an injection of current. Scale bar, 25 µm. n = 2 cells from 1 animals. Diii, I–V relationship in current-clamp mode of the neuron from Di-ii. This cell exhibited the low-threshold depolarization that is indicative of PVN presympathetic neurons. Div, Current-clamp trace (red) and the corresponding change in GCaMP7s fluorescence (black) when current (1 s, 50 pA) is injected into the neuron in Di-ii. Error bars indicate SEM. P < 0.01.
Figure 9.
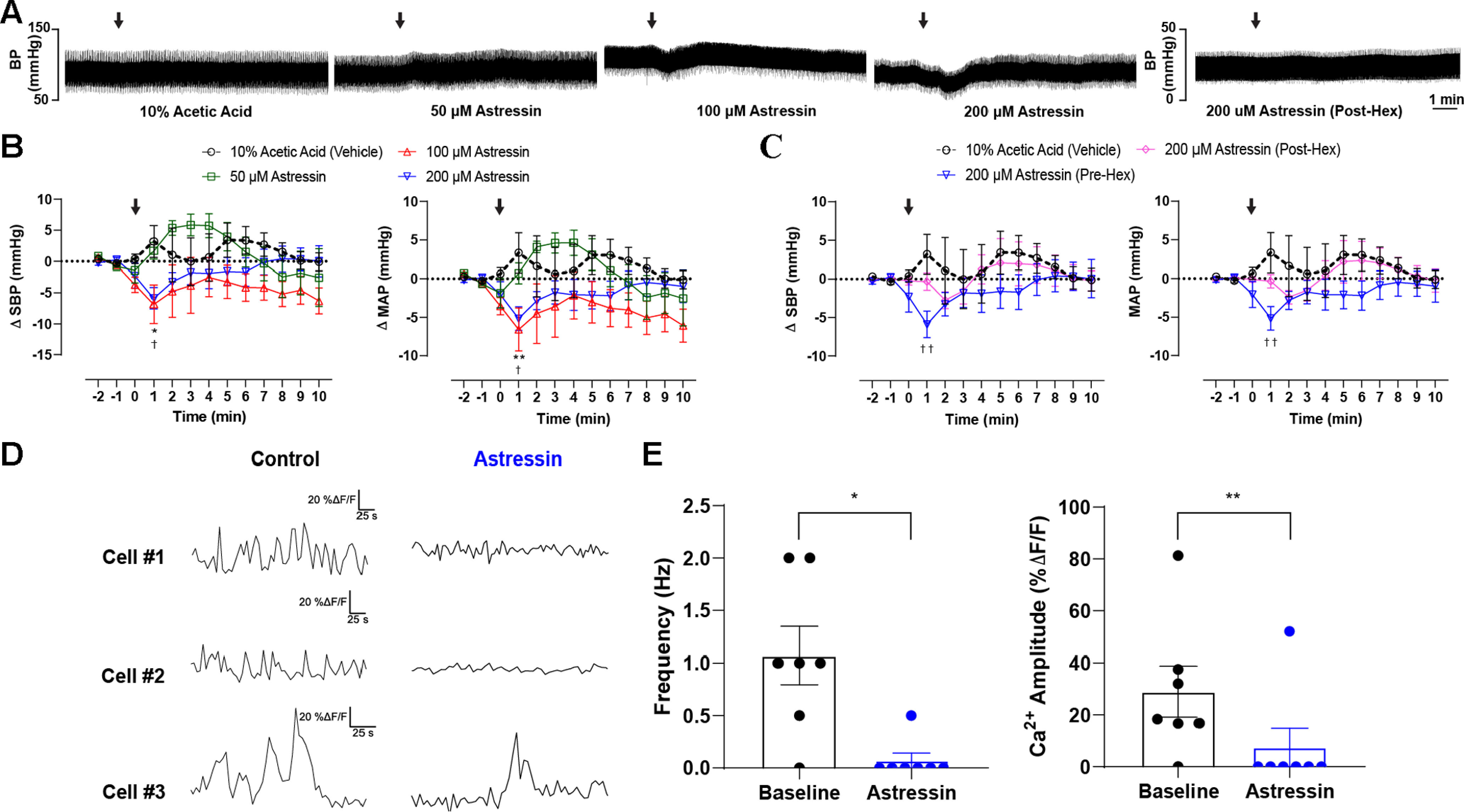
Blockade CRH1R(s) in the PVN lowers basal blood pressure and diminishes the firing activity of presympathetic neurons. A, Representative pulsatile arterial blood pressure traces following PVN microinjection (100 nl) with vehicle, different doses of Astressin (50, 100, 200 μm), and Astressin following systemic administration of hexamethonium. B, Grouped data demonstrating (left) the change in SBP and (right) mean arterial pressure (MAP) following the administration of vehicle and the different doses of Astressin into the PVN (n = 6 per group). C, PVN administration of Astressin (200 μm) before (n = 6) or after (n = 4) the systemic administration of hexamethonium (30 mg/kg, i.p.). p < 0.05; p < 0.01; two-way ANOVA followed by Tukey's post hoc test for vehicle versus Astressin (100 μm). †p < 0.05; ††p < 0.05; two-way ANOVA followed by Tukey's post hoc test for vehicle versus Astressin (200 μm). Arrows indicate time of injection. D, Representative traces of basal GCaMP7s activity in three independent recordings obtained from RVLM-projecting neurons (left) before and (right) subsequent to delivery of Astressin (0.5 μm). E, Summary data showing mean changes in GCaMP7s transients (left) frequency and (right) amplitude evoked by Astressin administration (n = 7 cells from 3 animals). Error bars indicate SEM.
Figure 5.
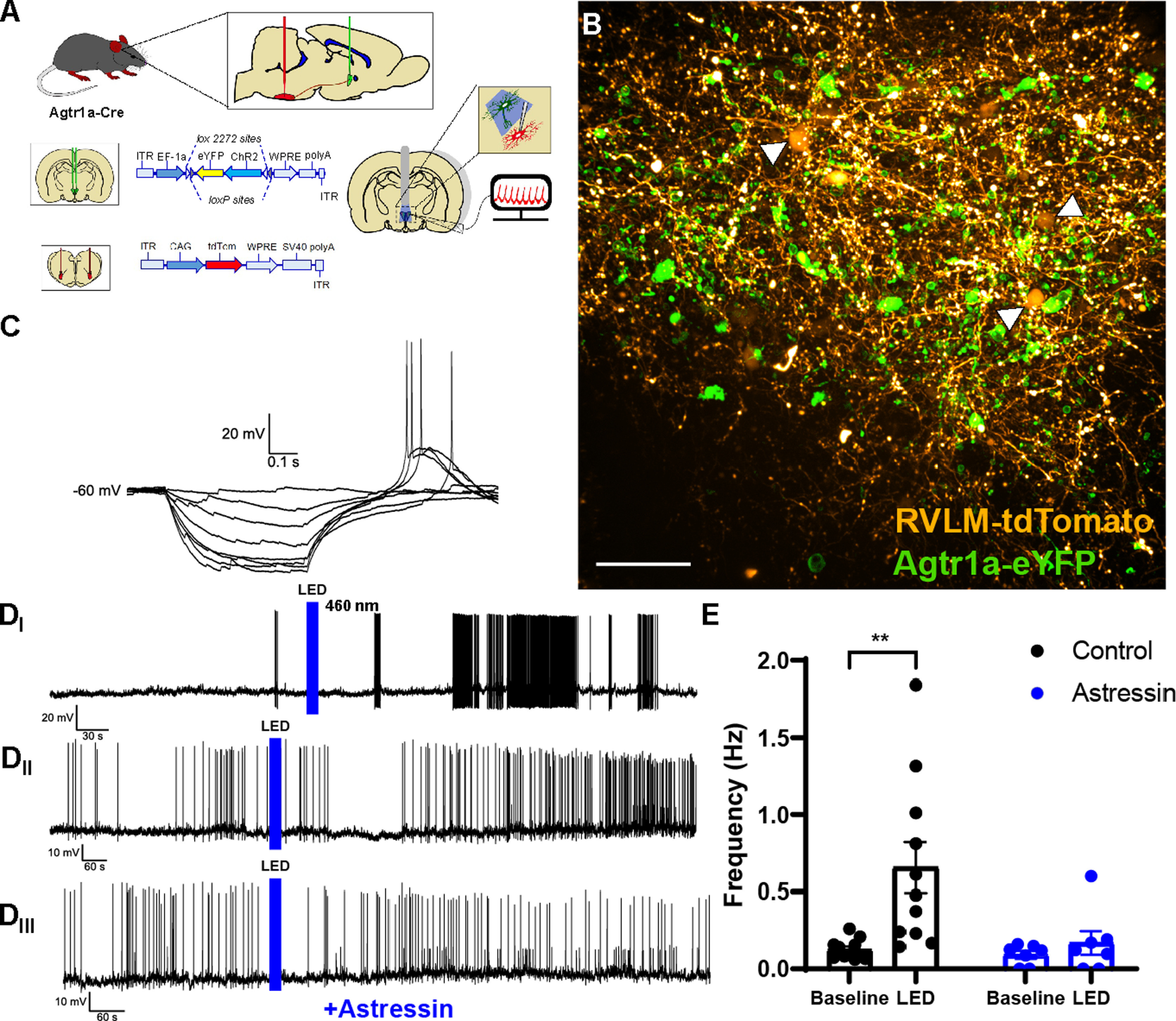
LED stimulation of PVNAgtr1a increases firing frequency of PVN neurons retrogradely labeled from the RVLM, which can be abrogated by Astressin. A, Schematic of the AAV injections used to direct the expression of eYFP-ChR2 to PVNAgtr1a while also driving retrograde expression of tdTomato in PVN neurons projecting to the RVLM. B, Confocal image of coronal slice through the PVN depicting RVLM-projecting neurons labeled with tdTomato (yellow) and PVNAgtr1a expressing eYFP and ChR2 (green). Scale bar, 50 µm. C, Current-clamp recording of the I–V relationship of a RVLM-projecting neuron. The presence of a low-threshold transient depolarization confirms that the cell is consistent with electrophysiological properties attributed to presympathetic neurons of the PVN. Di, Current-clamp recording of a patch-clamped RVLM-projecting neuron. LED stimulation of PVNAgtr1a causes a delayed upregulation in firing of RVLM-projecting neurons. Dii, Current-clamp recording from another RVLM-projecting neuron that also exhibits a delayed firing response to LED stimulation of PVNAgtr1a that is suppressed in the presence of Astressin (Diii, 0.5 μm). E, Summary data of firing frequency from recordings of RVLM-projecting neurons before and after the LED stimulation. LED stimulation of PVNAgtr1a significantly increased the firing frequency of RVLM-projecting neurons (p < 0.01, two-way ANOVA followed by Tukey's post hoc test, n = 11 cells from 5 animals). The LED stimulation of PVNAgtr1a did not significantly increase the firing frequency of RVLM-projecting neurons in the presence of Astressin (p > 0.05, two-way ANOVA followed by Tukey's post hoc test n = 7 cells from 3 animals). Error bars indicate SEM.
Figure 7.
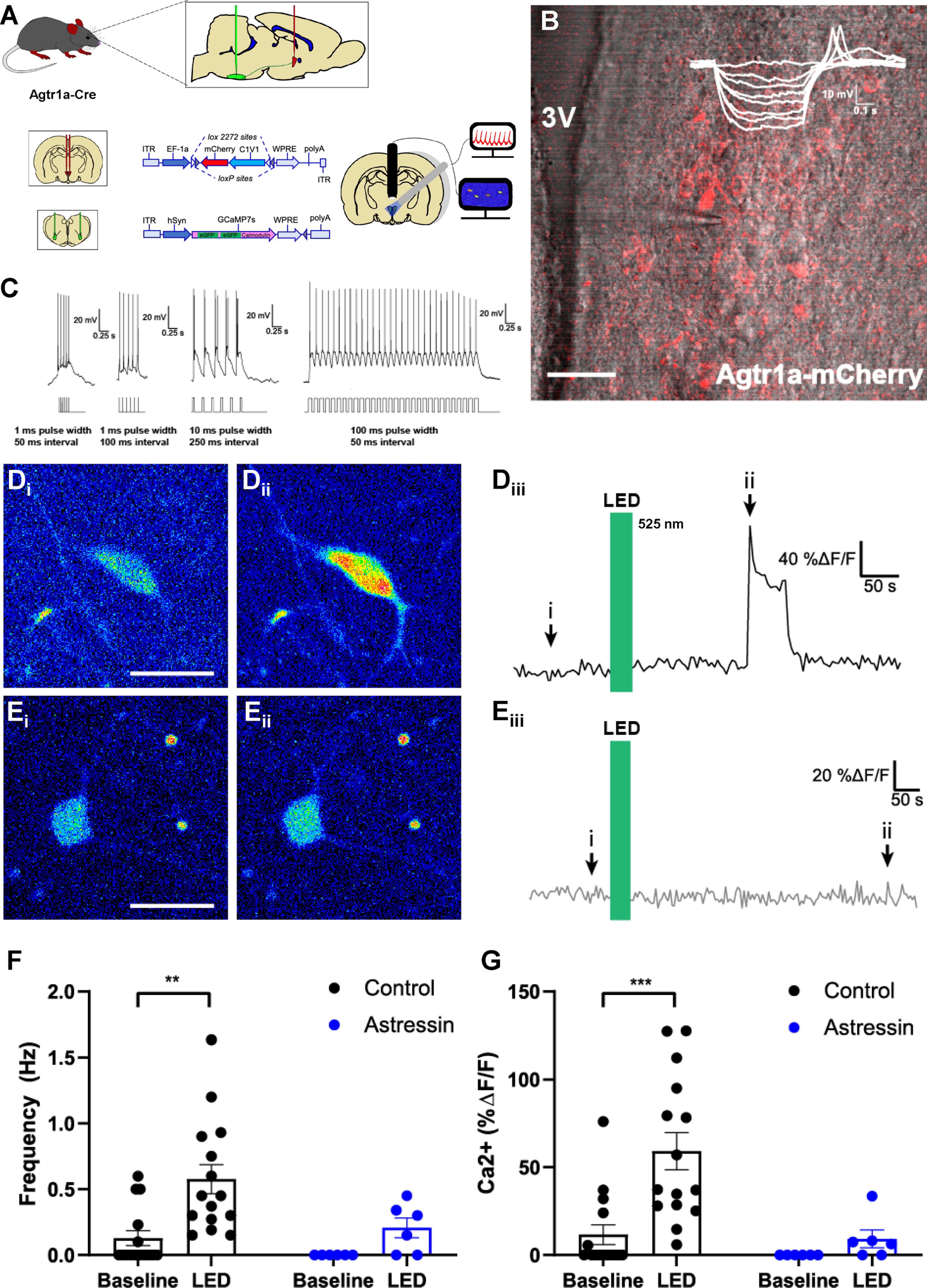
Optogenetic excitation of PVNAgtr1a augments GCaMP7s fluorescence within PVN neurons that project to the RVLM. A, Schematic of the AAV injections used to direct the expression of rsChR2-mCherry to PVNAgtr1a while also driving retrograde expression of GCaMP7s in PVN neurons projecting to the RVLM. Fluorescence intensity is used as a proxy for neuronal firing and can be simultaneously recorded from multiple neurons in a slice through the PVN. B, Two-photon microscopy depicting PVNAgtr1a expressing mCherry (red) and rsChR2 within coronal slice through the PVN captured using Dodt gradient contrast imaging. Inset, I–V curve with a low threshold depolarization, indicating the patched neurons exhibit electrophysiological characteristics of a parvocellular cell. 3V, Third ventricle. Scale bar, 50 µm. C, Further characterization of a patched PVNAgtr1a neuron with different optogenetic stimulation protocols. Stimulus waveforms appear below the neuron's response. When pulse interval becomes too short, the spikes occur on the same depolarization, but spike count corresponds with LED pulses. A protocol consisting of 30 pulses with a 100 ms width and a 50 ms interval is the standard protocol used in these experiments unless otherwise noted. D, GCaMP7s fluorescence within RVLM-projecting neurons (Di) under basal conditions and (Dii) after LED stimulation of PVNAgtr1a. Scale bar, 25 µm. Diii, ROI trace from neuron shown in Di,ii representing the time course of the increased GCamP7s fluorescence that was observed before, during, and after LED stimulation of PVNAgtr1a. E, GCaMP7s fluorescence within RVLM-projecting neuron (Ei) under basal conditions and (Eii) after LED stimulation of PVNAgtr1a in the presence of Astressin (0.5 μm). Scale bar, 25 µm. Eiii, ROI trace from neuron shown in Ei,ii representing the time course of GCamP7s fluorescence that was observed before, during, and after LED stimulation of PVNAgtr1a when Astressin (0.5 μm) is present. F, Left, Ca2+ frequency (Hz), as indicated by GCaMP7s fluorescence within RVLM-projecting neurons, increases after LED stimulation of PVNAgtr1a (p = 0.001, two-way ANOVA followed by Tukey's post hoc test, n = 15 cells from 5 animals). Right, Ca2+ frequency (Hz) does not significantly increase after LED stimulation of PVNAgtr1a in the presence of Astressin (p = 0.64, two-way ANOVA followed by Tukey's post hoc test, n = 6 cells from 3 animals). G, Left, Ca2+ amplitude (%ΔF/F), as indicated by GCaMP7s fluorescence within RVLM-projecting neurons, increases after LED stimulation (p = 0.0003, two-way ANOVA followed by Tukey's post hoc test, n = 15 cells from 5 animals). Right, Ca2+ amplitude (%ΔF/F) does not significantly increase in the presence of Astressin (p = 0.9, two-way ANOVA followed by Tukey's post hoc test, n = 6 cells from 3 animals).
Figure 8.
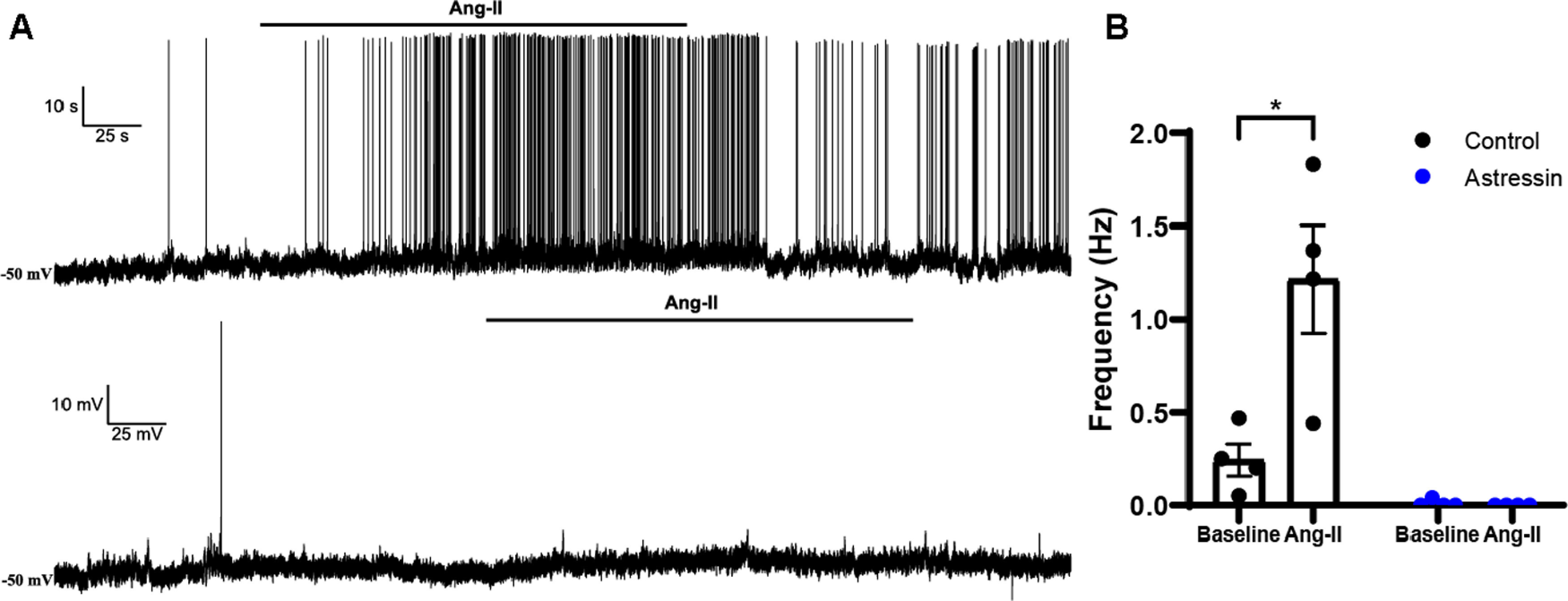
Blockade of CRH1R(s) prevents the firing of PVN presympathetic neurons that follows administration of Ang-II. A, Representative electrophysiological recordings obtained from presympathetic neurons showing an Ang-II mediated increase in firing rate (top), an effect that was absent when slices were preincubated in Astressin (0.5 μm) (bottom). B, Left, Summary plots showing a significant increase in firing frequency evoked by Ang-II under control conditions (p = 0.003, two-way ANOVA followed by Tukey's post hoc test, n = 2 of 4); this effect that was blunted by Astressin (p = 0.99, two-way ANOVA followed by Tukey's post hoc test, n = 4 cells from 2 animals). P < 0.05. Error bars indicate SEM.
Telemetric recordings of blood pressure and activity in awake freely-moving mice were performed and analyzed using Ponemah software (DSI), while cardiovascular parameters obtained from anesthetized mice were analyzed in Labchart 8 (AD Instruments). For all cardiovascular parameters, two-way ANOVAs with Tukey's multiple comparison tests were performed using GraphPad (see Figs. 1B, 2C,D, 9B,C, 10C). Data are expressed as mean ± SEM.
Figure 10.
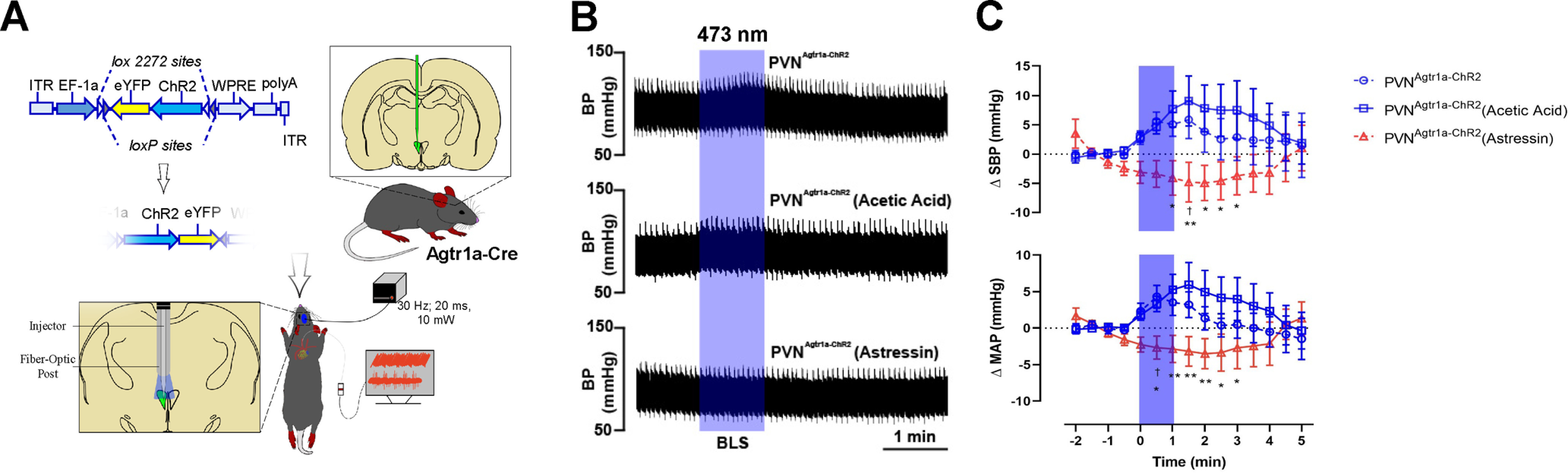
Blockade of CRH1R(s) within the PVN abolishes the increased blood pressure evoked by the optogenetic stimulation of PVNAgtr1a. A, Schematic depicting the mouse line and experimental protocol. In this experiment, Agtr1a-Cre mice received a Cre-inducible AAV-ChR2-eYFP into the PVN. Following recovery, optogenetic stimulation (10 mW; 20 ms pulse width; 30 Hz; 1 min) of PVNAgtr1a was performed after PVN microinjections using a dual optical injector cannula. B, Representative pulsatile arterial blood pressure traces following blue laser stimulation (BLS) in PVNAgtr1a-ChR2, and BLS 5 min following the injection of vehicle (10% acetic acid, pH 7.4 – PVNAgtr1a-ChR2 (acetic acid)) or Astressin (200 μm; PVNAgtr1a-ChR2 (Astressin)) into the PVN. C, Grouped data demonstrating the change in SBP and mean arterial pressure (MAP). *p < 0.05; **p < 0.05; two-way ANOVA followed by Tukey's post for PVNAgtr1a-ChR2 (Astressin) versus PVNAgtr1a-ChR2. †p < 0.05; two-way ANOVA followed by Tukey's post for PVNAgtr1a-ChR2 (Astressin) versus PVNAgtr1a-ChR2 (acetic acid); n = 6 per group. Error bars indicate SEM.
Results
In vivo optogenetic stimulation of the PVN elicits robust elevations in blood pressure when ChR2 expression is directed to the Agtr1a gene
The PVN coordinates the release of neuroendocrine factors with alterations in autonomic outflow to maintain blood pressure at levels optimal for survival (Sladek et al., 2015). Prior research heavily implicates Agtr1a(s) in the regulation of these processes. In particular, Ang-II induced activation of Agtr1a(s) evokes changes in neuronal activity associated with activation of the HPA and HPT axes, augmented sympathetic outflow, vasopressin release, and elevated blood pressure (Bains et al., 1992; Jezova et al., 1998; Q. Zhu et al., 2005; Y. F. Li et al., 2006; de Kloet et al., 2017). To begin to elucidate the precise signaling mechanisms associating Agtr1a(s), neuronal activity, and cardiovascular function within the PVN, we bred mice homozygous for the Agtr1a-Cre knock-in gene to mice heterozygous for the knock-in of the stop-flox-ChR2-eYFP gene at the ubiquitous ROSA26 locus. This breeding scheme resulted in offspring with expression of the light-sensitive cation ion channel, ChR2, directed to the Agtr1a gene or littermate controls harboring only the Agtr1a-Cre gene (Fig. 1A). These two groups of male mice were implanted with radiotelemetry devices and chronic dwelling fiber optics targeting the PVN, thereby allowing for continuous monitoring of cardiovascular parameters in conscious freely moving subjects during optogenetic activation of PVNAgtr1a. After surgical recovery and habituation to the tethering procedures, fiber optics were connected to a 473 nm laser light source and mice underwent optical stimulation of the PVN (10 mW; 15 Hz, 5 s on/off; 15 min). In the absence of optical stimulation, control mice and Agtr1a-ChR2 mice exhibited similar SBPs (Agtr1a-Cre = 107.2 ± 2.5 mmHg; Agtr1a-ChR2 = 101 ± 2.5 mmHg; t(8) = 1.643; p = 0.1389) and locomotor activity (Agtr1a-Cre = 0.70 ± 0.49 counts/min; Agtr1a-ChR2 = 1.16 ± 0.93 counts/min; t(8) = 0.44; p = 0.67). As illustrated in Figure 1B, relative to controls harboring only the Agtr1a-Cre gene, optical stimulation of the PVN in Agtr1a-ChR2 mice elicited pronounced elevations in SBP (main effects of genotype [F(1,8) = 12.3; p = 0.008] and time [F(4.834,38.67) = 2.92; p = 0.026] and a time × genotype interaction [F(70,560) = 4.32; p < 0.0001]) and locomotor activity (main effects of genotype [F(1,8) = 9.815; p = 0.014] and time [F(3.5,28) = 3.5; p = 0.023] and a time × genotype interaction [F(70,560) = 4.18; p < 0.0001]) that persisted throughout the period of optical stimulation and, in the case of blood pressure, extended several minutes thereafter.
Specific in vivo optogenetic activation of PVNAgtr1a increases blood pressure, an effect that is abolished by ganglionic blockade
The previous experiment established that Cre-Lox-mediated direction of ChR2 to the Agtr1a gene results in altered cardiovascular function when blue light is pulsed into the PVN; however, there are caveats to consider. In particular, Cre recombination that occurs with our breeding scheme results in production of ChR2 in all cells that express the Agtr1a gene throughout the course of development, which includes neurons residing outside the PVN. In this regard, the subfornical organ and the median preoptic nucleus are brain regions that express Agtr1a(s) that send axonal projections into the PVN (Bains et al., 1992; Z. Li and Ferguson, 1993; Krause et al., 2011; Leib et al., 2017; Frazier et al., 2021). Thus, the optogenetic approach implemented in the prior experiment likely excites these axonal projections, as well as the soma of PVNAgtr1a. To circumvent this caveat and further delineate the specific role that PVNAgtr1a play in cardiovascular function, we stereotaxically administered Cre-inducible AAVs (AAV2-eYFP or AAV2-ChR2-eYFP) into the PVN of Agtr1a-Cre male mice (Fig. 2A). This produced mice with ChR2 expression specifically within PVNAgtr1a or controls expressing only the eYFP fluorophore. After 3-4 weeks of recovery, mice were anesthetized and a Millar catheter (model SPR1000) was inserted into the carotid artery to record cardiovascular parameters. After the catheter was secured, mice were placed into a stereotaxic apparatus and a microcraniotomy was performed dorsal to the PVN. Subsequently, a fiber optic connected to a laser light source was slowly lowered into the PVN, and cardiovascular responses to pulses of 473 nm light were recorded (10 mW output; 20 ms pulse width; 30 Hz; 1 min). As shown in Figure 2B, C, optical stimulation had no effect on cardiovascular parameters when given to Agtr1a-Cre mice administered the control virus producing only eYFP. In contrast, pulsing 473 nm light into the PVN of Agtr1a-Cre mice given AAV-ChR2-eYFP reliably produced a significant increase in blood pressure that was sustained until the cessation of illumination (Fig. 2B,C; PVNAgtr1a-eYFP; −1.0 ± 0.6 mmHg, PVNAgtr1a-ChR2; 4.0 ± 0.9 mmHg at 1 min, main effects of genotype [F(15,240) = 2.2; p = 0.0064] and time [F(1,240) = 58.15; p < 0.0001], n = 6-11). However, no effects were observed on heart rate (Fig. 2C; PVNAgtr1a-eYFP; 5.7 ± 3.4 bpm, PVNAgtr1a-ChR2; 7.9 ± 5.2 bpm at 1 min [F(15,240) = 0.81; p = 0.6693], n = 6-11). While anesthesia may have blunted the cardiovascular response to optogenetic stimulation, these results indicate that the elevations in the blood pressure observed in the prior experiment cannot be solely attributed to concurrent increases in locomotor activity. As mentioned, the PVN contains neurosecretory and presympathetic neurons that influence cardiovascular function by controlling the release of endocrine factors into the systemic circulation or sympathetic nervous activity, respectively. To gain insight into mechanisms coupling excitation of PVNAgtr1a to altered cardiovascular function, we combined systemic administration of the nicotinic receptor antagonist, hexamethonium (30 mg/kg, i.p.), with in vivo optogenetics to determine whether altered autonomic function contributes to the increase in blood pressure that accompanies the excitation of PVNAgtr1a. Figure 2B, C shows that the cardiovascular responses previously observed with optogenetic excitation of PVNAgtr1a were abolished in the presence of hexamethonium (Fig. 2C; PVNAgtr1a-ChR2; 4.0 ± 0.9 mmHg, PVNAgtr1a-ChR2 (Post-Hex); 0.1 ± 0.4 mmHg at 1 min, main effects of treatment [F(15,302) = 1.94; p = 0.0197] and time [F(1,302) = 18.26; p < 0.0001], n = 10 or 11). To further establish that the increases in blood pressure are sympathetically mediated, we conducted heart rate variability and SBP variability analyses (Fig. 2D). Following blue light stimulation, no changes were observed in heart rate variability, a surrogate indicator of sympatho-vagal balance to the heart (Fig. 2D; PVNAgtr1a-eYFP; 0.1 ± 0.1, PVNAgtr1a-ChR2; 0.6 ± 0.3 at 1 min [F(7,120) = 0.15; p = 0.9931], n = 6–11). This suggests that the selective excitation of PVNAgtr1a does not affect heart rate nor the autonomic drive to the heart. Intriguingly, optical stimulation of PVNAgtr1a produced a significant increase in the LF domain of SBP variability, an indicator of sympathetic vasoconstrictor activity (Fig. 2D; PVNAgtr1a-eYFP; 2.8 ± 0.1 mmHg2, PVNAgtr1a-ChR2; 3.4 ± 0.2 mmHg2 [F(7,120) = 2.76; p = 0.0107], n = 6-11). Changes in the LF domain of SBP variability were attenuated by the systemic administration of hexamethonium (Fig. 2D; PVNAgtr1a-ChR2; 3.4 ± 0.2 mmHg2, PVNAgtr1a-ChR2 (Post-Hex); 1.7 ± 0.2 mmHg2 [F(7,152) = 2.31; p = 0.0287], n = 10 or 11). Furthermore, the administration of hexamethonium decreased the baseline of the LF domain of SBP variability, suggesting that hexamethonium does indeed block autonomic outflow to the vasculature (Fig. 2D). Together, these results suggest that the increased blood pressure that follows selective excitation of PVNAgtr1a is dependent on increased sympathetic outflow to the vasculature.
Fibers arising from PVNAgtr1a make appositions onto neighboring neurons that express mRNA for the CRH1 receptor and send direct projections to the RVLM
Follow-up experiments probed the neuroanatomical basis underlying the cardiovascular effects observed with optogenetic activation of PVNAgtr1a. A recent study determined that optogenetic excitation of neurons in the PVN that have direct axonal projections to the RVLM increases sympathetic nervous system activity and blood pressure (Koba et al., 2018). This prior study, in conjunction with our present results, suggests that optogenetic activation of PVNAgtr1a may increase blood pressure by exciting RVLM-projecting neurons within the PVN. In particular, it is possible that RVLM-projecting neurons synthesize Agtr1a(s) and the increased blood pressure that is observed with optogenetic stimulation is the result of their direct excitation. To confirm or refute this possibility, mice with the red fluorescent protein variant, tdTomato, directed to the Agtr1a gene were iontophoretically administered the retrograde neuronal tract-tracer, FG, bilaterally into the RVLM (Fig. 3A). Seven days later, Agtr1a-tdTomato mice were perfused and brains were processed for FG immunohistochemistry to evaluate whether PVN neurons with identified projections to the RVLM synthesize Agtr1a(s) as indicated by colocalization with tdTomato. As shown in Figure 3A, neurons with direct projections to the RVLM, here, deemed presympathetic, mostly populated the caudal (≈−1.06 mm from bregma) region of the PVN (Franklin and Paxinos, 2008). As can be seen, somatic expression of tdTomato in this region was scant and did not colocalize with FG, suggesting that Agtr1a(s) are not present on presympathetic neurons in the PVN.
In contrast to the caudal region, the medial region (≈−0.82 mm from bregma) of the PVN is densely populated with Agtr1a-tdTomato labeling (de Kloet et al., 2017). The medial PVN contains neurosecretory parvocellular neurons that synthesize CRH and initiate activation of the HPA axis. Subsequent experiments evaluated whether such neurons express Agtr1a(s). Toward this end, mice with the tdTomato expression directed to the CRH gene (CRH-tdTomato) were perfused and coronal sections through the PVN were processed for RNAscope ISH for Agtr1a mRNA. Figure 3B depicts tdTomato and blue puncta, indicative of labeling for CRH-synthesizing neurons and mRNA coding for Agtr1a(s), respectively. Quantitative analysis revealed that the majority of tdTomato-labeled neurons colocalized with Agtr1a mRNA (176 of 215 CRH-tdTomato cells express Agtr1a mRNA; n = 3 mice), thereby demonstrating extensive expression of Agtr1a(s) on CRH neurons of the PVN. Together, these results suggest that PVNAgtr1a are not presympathetic neurons that project to the RVLM but, rather, are CRH-synthesizing neurons that coupled their excitation to changes in autonomic outflow that elevate blood pressure.
Given that prior studies revealed a local intra-PVN cross-talk among peptidergic neurosecretory and presympathetic neurons (Son et al., 2013; Jiang et al., 2018), we next sought to determine whether PVNAgtr1a, which synthesize CRH, have processes that come into close proximity with presympathetic neurons that express CRHR1(s). To visualize soma and efferents arising from PVNAgtr1a, we delivered a Cre-inducible anterograde viral tracer (AAV-ChR2-eYFP) into the PVN of Agtr1a-Cre male mice. The same mice were delivered a pan-neuronal retrograde virus (AAVrg-CAG-tdTomato) into the RVLM to label presympathetic neurons with tdTomato (Fig. 3C). After 3-4 weeks of recovery, mice were perfused and brains were processed for immunohistochemistry and RNAscope ISH for eYFP and CRH 1 receptor (CRH1R) mRNA, respectively. As shown in Figure 3D-F, PVNAgtr1a send a dense plexus of fibers (yellow) to the caudal PVN (≈−1.06 mm from bregma). These fibers are in close proximity to neurons retrogradely labeled with tdTomato (magenta). Efforts to identify neurons colabeled with eYFP and tdTomato were unsuccessful, consistent with our prior results demonstrating that presympathetic neurons in the PVN are devoid of the Agtr1a. A subset of these RVLM-projecting neurons exhibited colocalization of tdTomato and CRHR1 mRNA (magenta soma with blue puncta; Fig. 3E,F). Quantitative analysis revealed that 57% (181 of 316 neurons; n = 4 mice) of RVLM-projecting neurons expressed mRNA for the CRHR1. Interestingly, a subset (43% or 152 of 351 neurons; n = 4 mice) of PVNAgtr1a were also found to synthesize CRHR1 mRNA. Collectively, these results demonstrate that, within the PVN, the Agtr1a is robustly expressed on CRH neurons, but presympathetic neurons do not produce this receptor but, rather, synthesize CRHR1(s). The implication is that excitation of PVNAgtr1a may trigger the release of CRH, which stimulates CRHR1(s) on neighboring presympathetic neurons to alter sympathetic outflow and cardiovascular function.
Ex vivo optogenetic stimulation of PVNAgtr1a evokes excitation of presympathetic neurons that is mediated by CRHR1(s)
To probe for functional connectivity between PVNAgtr1a and presympathetic neurons, we used an ex vivo approach that combined optogenetics, patch-clamp electrophysiology, and Ca2+ imaging. As a first strategy, we delivered a Cre-inducible AAV that expresses ChR2 and eYFP (AAV-ChR2-eYFP) into the PVN of Agtr1a-Cre mice (Fig. 4A). This allowed selective excitation of PVNAgtr1a with ex vivo optogenetics. To determine whether optogenetic excitation of PVNAgtr1a affects the activity of presympathetic neurons, we obtained patch-clamp recordings from parvocellular neurons that were in the vicinity of ChR2-eYFP-expressing fibers originating from PVNAgtr1a, but that were themselves devoid of fluorescent protein expression. The majority of these neurons (n = 8 cells from 7 animals) exhibited a low-threshold spike and lacked a transient outward rectification, electrophysiological characteristics often exhibited by presympathetic neurons (Luther and Tasker, 2000; Stern, 2001). As shown in Figure 4B, optogenetic stimulation of PVNAgtr1a (LED 460/488 nm; 20-30 pulse train, 50 ms pulse, 100 ms interval) consistently evoked a delayed (195.0 ± 19.4 s) excitatory response in putative presympathetic PVN neurons, which resulted in a significant increase in firing discharge under control conditions (Fig. 4C; t(3) = 4.73, p = 0.018, paired t test).
These results suggest that excitation of PVNAgtr1a spurs neighboring neurons in the PVN to fire action potentials and subsequent experiments investigated the basis for this coupling. We previously determined that PVNAgtr1a neurons express GLUT2 mRNA, a marker for glutamate, the major excitatory neurotransmitter in the hypothalamus (van den Pol, 1982; de Kloet et al., 2017). Accordingly, we repeated these experiments in the presence of the NMDA and AMPA glutamate receptor antagonists, APV (100 μm) and DNQX (20 μm), respectively, to evaluate whether the concomitant excitation of putative presympathetic neurons was mediated by glutamate. As shown in Figure 4C, application of APV and DNQX had no effect on the increased action potentials exhibited by parvocellular neurons subsequent to optogenetic excitation of PVNAgtr1a. That is, similar to what we observed in the previous experiment, optogenetic stimulation consistently evoked a delayed (mean delay: 145.0 ± 41 s) excitatory response in putative presympathetic neurons in the presence of the synaptic blockers (Fig. 4C; t(5) = 2.64, p = 0.046, paired t test). Together, these results indicate that activation PVNAgtr1a evokes an excitatory response in putative presympathetic neurons, and that this interpopulation cross-talk displays a slow time course, and is not mediated by glutamate, a fast-acting excitatory neurotransmitter.
Based on these initial studies and our neuroanatomical results showing the robust expression of Agtr1a(s) on CRH neurons and the intriguing presence of CRHR1(s) on presympathetic neurons, we set up to directly test the hypothesis that presympathetic neurons are responsive to excitation of PVNAgtr1a, and that this cross-talk is mediated by a slow acting neuropeptide, namely, CRH. Toward this end, we again delivered a Cre-inducible AAV-ChR2-eYFP into the PVN of Agtr1a-Cre mice, but this time, we also bilaterally administered into the RVLM a pan neuronal viral construct (AAVrg-CAG-tdTomato) that promotes retrograde expression of tdTomato (Fig. 5A). This approach allows for simultaneous optogenetic excitation of PVNAgtr1a with patch-clamp recordings from known presympathetic neurons identified with the presence of tdTomato. First, we obtained patch-clamp recordings from neurons retrogradely labeled with tdTomato in slices through the PVN that also contained soma and fibers arising from PVNAgtr1a (Fig. 5B). As expected, we found that eYFP and tdTomato labeled separate populations of neurons in the PVN and tdTomato-expressing neurons exhibited electrophysiological characteristics indicative of having direct projections to the RVLM (Fig. 5C) (Stern, 2001). Optogenetic excitation of PVNAgtr1a (LED 460 nm; 30 pulse train, 50 ms pulse, 100 ms interval) consistently evoked a delayed (157.4 ± 16.1 s) excitatory response in identified presympathetic neurons, that resulted in a significant increase in firing discharge (∼430% change; Fig. 5CDi,E; [F(1,32) = 5.70, p = 0.003], two-way ANOVA followed by Tukey's post hoc test, n = 11 cells from 5 animals). This effect was not dependent on the baseline degree of activity of presympathetic neurons (Pearson r2 = 0.08, p = 0.4). Importantly, the increased activity of presympathetic neurons that was evoked by optogenetic excitation of PVNAgtr1a was abrogated when slices were preincubated with the CRH receptor antagonist, Astressin (0.5 μm; Fig. 5CDii,iii,E; [F(1,32) = 5.70, p = 0.96], two-way ANOVA followed by Tukey's post hoc test, n = 7 cells from 3 animals).
Prior work from our group determined that within the PVN, dendritically released arginine vasopressin (AVP) evokes excitatory responses in presympathetic neurons by stimulating V1a receptors (Son et al., 2013). Therefore, it is possible that V1a receptors act as an intermediary between the activation of PVNAgtr1a and concomitant excitation of presympathetic neurons. To rule out this possibility, we repeated our ex vivo experiments in the presence of a V1a receptor antagonist, [β-mercapto-β,β-cyclopentamethylenepropionyl1, O-me-Tyr2, Arg8]-vasopressin (V1aR-A; 1 μm). We found that the increase in presympathetic neuronal firing activity following optogenetic excitation of PVNAgtr1a persisted in the presence of the V1aR-A (baseline: 0.14 ± 0.1 Hz; LED 460: 0.77 ± 0.2 Hz, p < 0.05, n = 3). Together, these results support the overall notion that activity-dependent release of CRH from PVNAgtr1a evokes a CRHR1-mediated excitatory response in presympathetic neurons.
Ex vivo optogenetic stimulation of PVNAgtr1a evokes a CRHR1-mediated increase in intracellular Ca2+ levels in presympathetic neurons
Follow-up experiments combined ex vivo optogenetics and ex vivo calcium imaging as a complementary strategy to further support the functional cross-talk between PVNAgtr1a and presympathetic neurons. Accordingly, we delivered an AAV (AAV-rsChR2-mCherry) producing red-shifted ChR2 (C1V1) and red fluorescent protein (mCherry) into the PVN of Agtr1a-Cre mice (Fig. 6A). These male mice were also bilaterally administered into the RVLM a viral construct that allows retrograde expression of the ultrasensitive fluorescent protein calcium sensor, GCaMP7s, under the control of the synapsin promoter (Fig. 6A). Eight to 10 weeks later, coronal slices through the PVN revealed efficacious transfection of GCaMP7s (range = 1-5 neurons/slice), particularly in the caudal region of the nucleus (≈−1.06 mm from bregma; Fig. 6B). To first validate this approach, we bath-applied NMDA (50 μm) and observed a robust and synchronous increase in intracellular calcium ([Ca2+]ic) in several presympathetic neurons within the slice (Fig. 6Ci-iv; t(7) = 4.82, p = 0.006, paired t test n = 10 cells from 2 animals). Additionally, in a few cases, we patched presympathetic neurons expressing GCaMP7s and found clear increases in [Ca2+]ic following bursts of action potentials evoked by current injection through the patch pipette (Fig. 6Di-iv). These results support efficient retrograde expression and sensitivity of the genetically encoded Ca2+ sensor in presympathetic neurons. Next, we used the same viral and Ca2+ imaging approaches to evaluate whether excitation of PVNAgtr1a affected [Ca2+]ic of presympathetic neurons in the PVN (Fig. 7A). First, we validated that PVNAgtr1a transfected with rsChR2 responded to pulses of 525 nm of light by patch clamping PVN neurons labeled with mCherry (Fig. 7B). These neurons lacked both a low threshold spike and a delayed outward rectification (Fig. 7B, inset) (Luther and Tasker, 2000). Stimulation of PVNAgtr1a with varying protocols using the 525 nm LED excitation reliably and consistently evoked firing activity in the patched neurons (Fig. 7C). These results demonstrate that PVNAgtr1a exhibit electrophysiological characteristics of neurosecretory parvocellular neurons and validate our ability to optogenetically excite PVNAgtr1a using a red-shifted opsin. Optogenetic excitation of PVNAgtr1a (LED 525 nm; 30 pulse train, 50 ms pulse, 100 ms interval) consistently evoked a delayed (157.9 ± 17.2 s) increase in the frequency (Fig. 7F; [F(1,38) = 10.1, p = 0.001], two-way ANOVA followed by Tukey's post hoc test, n = 15 cells from 5 animals) and amplitude (Fig. 7G; [F(1,38) =8.4, p = 0.0003], two-way ANOVA followed by Tukey's post hoc test, n = 5 of 15) of [Ca2+]ic in presympathetic neurons (Fig. 7Di–iii). The delay in the [Ca2+]ic response was not different from that observed with spiking (see Fig. 5D, left, E). The responses were almost completely abolished when slices were preincubated with Astressin (0.5 μm and no change was observed for either frequency; Fig. 7F; [F(1,38) = 10.13, p = 0.64], two-way ANOVA followed by Tukey's post hoc test, n = 6 cells from 3 animals) or amplitude (Fig. 7G; [F(1,38) = 8.4, p = 0.9], two-way ANOVA followed by Tukey's post hoc test, n = 6 cells from 3 animals).
These results indicate that the activity of PVNAgtr1a is coupled to increased firing of presympathetic neurons via activation of CRHR1(s); however, whether stimulation of Agtr1a(s) per se also elicits this interpopulation cross-talk was not evaluated. Consequently, follow-up experiments examined whether application of Ang-II, the endogenous ligand for Agtr1a(s), evoked a similar CRH-mediated increased firing of PVN presympathetic neurons. In order to visualize presympathetic neurons in the PVN, male rats were administered RetroBeads into the RVLM. Three to five days later, coronal sections were made through the PVN and patch-clamp recordings were obtained from identified presympathetic neurons before, during, and after bath application of Ang-II (Fig. 8A). Bath application of Ang-II (0.5 μm, 1 min) significantly increased the firing frequency of presympathetic PVN neurons (Fig. 8B; [F(1,12) = 10.15, p = 0.003], two-way ANOVA followed by Tukey's post hoc test, n = 4 cells from 2 animals). Importantly, this increase was completely abolished when slices were preincubated in Astressin (0.5 μm, Fig. 8B; [F(1,12) = 10.15, p = 0.99], two-way ANOVA followed by Tukey's post hoc test, n = 4 cells from 2 animals). Collectively, results from our ex vivo experiments support the notion that within the PVN, Ang-II activates Agtr1a(s) expressed on CRH neurons, which evokes a delayed and indirect excitation of presympathetic neurons that is dependent on stimulation of CRH1R(s).
In vivo blockade of CRH receptors in the PVN alters cardiovascular function under basal conditions and during optogenetic excitation of PVNAgtr1a
The results from our anatomic and electrophysiological studies predict that excitation of PVNAgtr1a promotes the release of CRH, which engages CRHR1(s) expressed on presympathetic neurons to augment sympathetic outflow and elevate blood pressure. Here, we conducted complementary in vivo and ex vivo studies to test the validity of this prediction. Initial experiments used WT male C57BL/6J mice to evaluate how administering varying doses of Astressin affects blood pressure. Toward this end, mice were anesthetized and a Millar catheter was implanted into the left common carotid artery to record cardiovascular parameters. Subsequently, a microcraniotomy was performed dorsal to the PVN, using a stereotaxic frame, and a glass micropipette was lowered into the PVN to perform unilateral microinjections (100 nl) of either the vehicle (10% acetic acid, pH 7.4) or Astressin (50, 100, 200 μm). Before microinjections, there were no differences in baseline blood pressure among the groups (vehicle; 106.5 ± 9.2 mmHg, Astressin; 110.2 ± 4.7 mmHg, 110.3 ± 8.5 mmHg, 99.2 ± 10.2 mmHg for 50, 100, and 200 μm, respectively [F(3,20) = 0.3775; p = 0.7702], n = 6 per group). However, relative to microinjection of vehicle, Astressin induced a dose-dependent and transient depressor response in blood pressure (Fig. 9A). Statistical analysis revealed significant depressor responses induced by Astressin 60 s following microinjection (Fig. 9B, Vehicle; 3.2 ± 2.6 mmHg, Astressin; 1.7 ± 1.3 mmHg, −6.9 ± 3.1 mmHg, −5.9 ± 1.7 mmHg for 50, 100, and 200 μm, respectively [F(3,260) = 13.66; p < 0.0001], n = 6 per group). No effects were observed on heart rate (vehicle; 1.5 ± 6.5 bpm, Astressin; −4.1 ± 5.5 bpm, −0.2.3 ± 4.5 bpm, −4.2 ± 6.0 bpm for 50, 100, and 200 μm, respectively [F(36,260) = 0.6888; p = 0.9110], n = 6 per group). These results suggest that under basal conditions, CRHR1(s) in the PVN contribute to the tonic maintenance of blood pressure. To evaluate a role for presympathetic neurons in this effect, we combined ganglionic blockade via systemic administration of hexamethonium with microinjections of Astressin (200 μm) into the PVN. As expected, microinjection of Astressin (200 μm) significantly lowered blood pressure relative to administration of the vehicle; however, this effect was eliminated in the presence of hexamonethonium (30 mg/kg, i.p.; Fig. 9C, −5.9 ± 1.7 vs −0.3 ± 1.1 mmHg for Astressin pre (n = 6) and post (n = 4) hexamethonium, respectively [F(1,98) = 4.25; p = 0.0420).
These results demonstrate that the decreases in blood pressure that follow CRH1R antagonism in the PVN are dependent on the autonomic nervous system and suggest that CRH exerts an endogenous excitatory tone on presympathetic neurons. To investigate this possibility, we performed ex vivo experiments directly assessing the effects of Astressin on basal Ca2+ activity in retrogradely labeled presympathetic neurons expressing GCaMP7s. As shown in Figure 9D, we found that Astressin per se significantly blunted ongoing basal Ca2+ activity in these neurons, drastically decreasing both Ca2+ transient frequency (Fig. 9E; t(6) = 3.46, p = 0.01, paired t test, n = 7 cells from 3 animals) and amplitude (Fig. 9E; t(6) = 4.54, p = 0.004, paired t test, n = 7 cells from 3 animals). These results further support the notion that endogenous CRH acts on CRHR1(s) expressed on presympathetic neurons to promote tonic excitation that contributes to the maintenance of blood pressure in male mice.
Final experiments tested the hypothesis that the elevations in blood pressure that follow in vivo optogenetic excitation of PVNAgtr1a results from the interneuronal cross-talk revealed by our anatomic and electrophysiological studies. Male Agtr1a-Cre mice were again delivered Cre-inducible AAV-ChR2-eYFP into the PVN (Fig. 10A). After 3-4 weeks of recovery, mice were anesthetized, and a Millar catheter was implanted into the left common carotid artery. Subsequently, mice were placed into a stereotaxic apparatus, and a microcraniotomy was performed dorsal to the PVN, where a dual optrode/microinjection cannula was lowered above the PVN. This allowed microinjections and optogenetic stimulation within the same site while blood pressure was simultaneously recorded. Either vehicle (10% acetic acid, pH 7.4) or Astressin (200 μm) was microinjected into the PVN; and 5 min later, pulses of 473 nm laser light (10 mW output; 20 ms pulse width; 30 Hz; 1 min) were delivered above the site of injection. As shown in Figure 10B, C, optogenetic excitation of PVNAgtr1a reliably produced a significant increase in blood pressure that was sustained until the cessation of illumination. This response was replicated when optical stimulation was performed 5 min following the administration of vehicle. Remarkably, the pressor response induced by optogenetic excitation of PVNAgtr1a was abolished by pretreatment with Astressin (200 μm) (Fig. 10B,C; PVNAgtr1a-ChR2; 5.1 ± 2.0 mmHg, PVNAgtr1a-ChR2 (acetic acid); 7.7 ± 3.1 mmHg, PVNAgtr1a-ChR2 (Astressin); −4.1 ± 3.0 mmHg, [F(2,223) = 17.58; p < 0.0001], n = 6 per group). Together, these results support the overall notion that activity-dependent release of CRH from PVNAgtr1a evokes a CRHR1-mediated excitatory response in presympathetic neurons that ultimately increases blood pressure in male mice.
Discussion
Stressors are met with coordinated neuroendocrine and autonomic responses to cope with the energetic demands of physiological and psychological threats. The present study unveils a paracrine signaling mechanism within the PVN that couples the activity of neurosecretory and presympathetic neurons mediating these responses. In this regard, in vivo optogenetic excitation of PVNAgtr1a was found to significantly increase blood pressure, an effect that was dependent on recruitment of the autonomic nervous system. Puzzlingly, PVNAgtr1a did not exhibit characteristics of presympathetic neurons involved in the regulation of blood pressure but, rather, were CRH-synthesizing neurons that initiate activation of the HPA axis. Intriguingly, fibers originating from PVNAgtr1a made appositions onto presympathetic neurons that expressed mRNA coding for CRHR1(s), thereby providing anatomic and molecular evidence supporting a local cross-talk between these neuronal phenotypes. Indeed, ex vivo optogenetic excitation of PVNAgtr1a elicited increased firing of presympathetic neurons that persisted in the presence of glutamate receptor antagonists but was completely abolished by Astressin, a CRHR1 antagonist. Delivery of Astressin into the PVN significantly decreased blood pressure in vivo and suppressed basal Ca2+ activity within presympathetic neurons ex vivo, indicating that CRHR1(s) exert an excitatory tone on presympathetic neurons that contributes to the maintenance of blood pressure. Additionally, pretreatment with Astressin eliminated elevations in blood pressure that followed optogenetic excitation of PVNAgtr1a, demonstrating that CRH and its receptors couple the activity of neurosecretory and presympathetic neurons in the PVN. Collectively, these results reveal a novel mechanism underlying interneuronal cross-talk within the PVN that coordinates neuroendocrine release with autonomic outflow in male mice. Cross-talk among PVNAgtr1a and presympathetic neurons is likely engaged under conditions of increased RAS activity, such as hyponatremia, hypotension, or psychogenic stress, that elicit homeostatic multimodal responses involving coordinated HPA axis, autonomic and behavioral limbs orchestrated by the PVN. The implication is that chronic stress may promote cardiometabolic disease through dysregulation of the cross-talk revealed by our experiments.
Early studies using rats determined that electrical stimulation of the parvocellular subdivision of the PVN increases blood pressure and SNA (Porter and Brody, 1986; Kannan et al., 1989). Subsequently, it was reported that microinjections of Ang-II into the PVN also increase blood pressure and SNA, but this effect could be abrogated by Agtr1a antagonists (Bains et al., 1992; Jensen et al., 1992; G. Q. Zhu et al., 2002; Y. F. Li et al., 2006). Consistent with these prior studies, our results demonstrate that optogenetic excitation of PVNAgtr1a elicits elevations in blood pressure that are dependent on engagement of the autonomic nervous system. These results suggest that, within the PVN, Ang-II acts on Agtr1a(s) to increase firing of neurons that control autonomic outflow to cardiovascular tissues. Indeed, prior electrophysiological studies found that Ang-II depolarizes neurons in the PVN with identified projections to the hindbrain and spinal cord (Ferguson, 1988; D. P. Li et al., 2003; Cato and Toney, 2005), and our results showing that Ang-II increases the firing of presympathetic neurons are consistent with these earlier findings. A parsimonious explanation for these effects is that Ang-II binds to Agtr1a(s) expressed on presympathetic neurons to evoke excitation; however, presympathetic neurons do not appear to express Agtr1a(s); and consistent with prior reports, we found that PVNAgtr1a are parvocellular neurosecretory neurons that synthesize CRH (Oldfield et al., 2001; L. A. Wang et al., 2019). A recent study by Jiang et al. (2018) used genetically modified mice, in vitro patch-clamp electrophysiology, and viral tract tracing to convincingly demonstrate that functional CRH1R(s) are present on PVN neurons that project to the hindbrain. Here, we replicate these results by showing that PVN neurons that project to the RVLM express CRH1R mRNA. This, in conjunction with the observation that PVNAgtr1a produce CRH and make appositions onto presympathetic neurons, predicts cross-talk among these neuronal phenotypes that is mediated by CRH1R(s).
While each neuronal phenotype within the PVN can be linked to a distinct function, there is growing evidence that a local, interpopulation cross-talk among the neurons influences physiology and behavior (Brown et al., 2013; Son et al., 2013; Pati et al., 2020). Here, we used in vitro optogenetics with whole-cell patch-clamp electrophysiology and Ca2+ imaging to reveal that selective activation of PVNAgtr1a is followed by a significant increase in firing discharge of presympathetic neurons. The activation of the latter was slow and delayed in nature (tens of seconds following PVNAgtr1a activation) and was not mediated by the fast-acting excitatory transmitter glutamate. Indeed, our results showing that the PVNAgtr1a to presympathetic neuronal cross-talk was blunted by Astressin support CRH as the key underlying signaling mediator. The slow temporal dynamics of this cross-talk is very similar to the cross-talk between AVP neurosecretory and presympathetic neurons we recently reported in the PVN (Son et al., 2013). We showed that the AVP to presympathetic cross-talk was mediated by dendritically released AVP and its diffusion into the extracellular space (Son et al., 2013). Still, whether the PVNAgtr1a to presympathetic neuronal cross-talk involves dendritic and/or axonal release of CRH remains to be determined. The fact that presympathetic neurons were activated following stimulation of PVNAgtr1a neurons either by direct optical stimulation or by Ang-II, the endogenous ligand of Agtr1a, suggests that this cross-talk is not an artifactual response associated with optogenetic manipulations. Finally, indices of cross-talk among PVNAgtr1a and presympathetic neurons were observed in male mice as well as male rats, suggesting that this unique signaling mechanism coupling neuroendocrine secretion and autonomic outflow may be conserved across species.
Our present results, along with previous reports (Brown et al., 2013; Son et al., 2013; Pati et al., 2020), support the growing notion that neuropeptides are efficient signaling molecules mediating interneuronal communication within the PVN, contributing to the ability of this nucleus to generate complex, multimodal homeostatic responses. In this regard, administration of Astressin (200 μm) into the PVN produced a transient, but significant, decrease in blood pressure that was eliminated by systemic hexamethonium, suggesting that CRH receptors in the PVN contribute to the maintenance of basal blood pressure by influencing autonomic outflow. In support of this interpretation, we found that ex vivo application of Astressin silenced Ca2+ activity within presympathetic neurons of the PVN, thereby revealing that CRH receptors exert a basal excitatory tone on presympathetic neurons that affects blood pressure. To the best of our knowledge, this is the first report of CRH receptor blockade within the PVN affecting basal blood pressure. Previous studies found that intracerebroventricular delivery of CRH increases blood pressure (Tarjan et al., 1995; Yosten and Samson, 2014) and selective delivery of CRH into the RVLM also increases blood pressure and SNA, effects that are blocked by pretreatment with Astressin (Bardgett et al., 2014). Our final experiments tested the hypothesis that elevations in blood pressure that followed optogenetic excitation of PVNAgtr1a were dependent on stimulation of CRH receptors. Consistent with this hypothesis, microinjection of Astressin into the PVN prevented increased blood pressure during optogenetic activation of PVNAgtr1a. These results are consistent with a recent report by L. A. Wang et al. (2019), showing that optogenetic excitation of CRH neurons in the PVN increases blood pressure in mice by stimulating CRH receptors; however, the same study determined that optogenetic excitation of nerve terminals in the nucleus of the solitary tract that originated from CRH neurons in the PVN also increase blood pressure (L. A. Wang et al., 2019). While it is established that CRH neurons in the PVN project to hindbrain structures (e.g., nucleus of the solitary tract, RVLM) (Sawchenko, 1987; Milner et al., 1993), this pattern of innervation may not reflect that of PVNAgtr1a, which represents a subset (≈80%) of CRH neurons. The vast majority of PVNAgtr1a project to the median eminence and control the activity of the HPA and HPT axes (de Kloet et al., 2017). Here, we propose that Agtr1a(s) within the PVN couple the activity of these neuroendocrine axes to sympathetic outflow and blood pressure to coordinate metabolic and cardiovascular responses to stress in male mice.
Cross-talk among neurosecretory and presympathetic neurons of the PVN has important implications for cardiometabolic diseases. Stress is an established risk factor for cardiometabolic disease (Yusuf et al., 2004; Penninx, 2017), and impaired HPA axis activity and autonomic dysfunction are commonly observed in hypertension, myocardial infarction, obesity, and Type 2 diabetes (Lemche et al., 2016). Conversely, overactivation of the RAS is associated with stress-related pathologies, such as affective disorders (Baghai et al., 2006; Reinecke et al., 2018), which are often comorbid with diabetes, hypertension, and cardiovascular disease (Penninx, 2017; Wallace et al., 2018). Intriguingly, within the PVN, the expression of Agtr1a(s) and CRH is upregulated subsequent to chronic stress (Aguilera et al., 1995; Herman et al., 1995; Leong et al., 2002), suggesting enhanced cross-talk among PVNAgtr1a and presympathetic neurons that couples heightened HPA axis activity to augmented sympathetic drive. Chronic engagement of neuroendocrine and autonomic limbs of the stress response contributes to the glucose intolerance and hypertension that is a hallmark of the metabolic syndrome. Thus, dysregulation of the cross-talk revealed by our experiments could be a contributing mechanism linking comorbidity between chronic stress and cardiometabolic disease, thereby representing a novel therapeutic target.
Footnotes
This work was supported by American Heart Association (AHA) Grant 17GRNT33660969; and National Institutes of Health Grants HL125805 and HL145028 to A.D.d.K., HL136595, HL096830, and HL122494 to E.G.K., and HL090948 and NS094640 to J.E.S.
The authors declare no competing financial interests.
References
- Aguilera G, Young WS, Kiss A, Bathia A (1995) Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin-II. Neuroendocrinology 61:437–444. 10.1159/000126866 [DOI] [PubMed] [Google Scholar]
- Baghai TC, Binder EB, Schule C, Salyakina D, Eser D, Lucae S, Zwanzger P, Haberger C, Zill P, Ising M, Deiml T, Uhr M, Illig T, Wichmann HE, Modell S, Nothdurfter C, Holsboer F, Müller-Myhsok B, Möller HJ, Rupprecht R, et al. (2006) Polymorphisms in the angiotensin-converting enzyme gene are associated with unipolar depression, ACE activity and hypercortisolism. Mol Psychiatry 11:1003–1015. 10.1038/sj.mp.4001884 [DOI] [PubMed] [Google Scholar]
- Bains JS, Potyok A, Ferguson AV (1992) Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599:223–229. 10.1016/0006-8993(92)90395-P [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Sharpe AL, Toney GM (2014) Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexcitation. Am J Physiol Endocrinol Metab 307:E944–E953. 10.1152/ajpendo.00291.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Oliet SH, Kirkpatrick K, Richard D, Fisher TE (1993) Extrinsic and intrinsic modulatory mechanisms involved in regulating the electrical activity of supraoptic neurons. Ann NY Acad Sci 689:512–519. 10.1111/j.1749-6632.1993.tb55581.x [DOI] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M, Stern JE (2013) Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25:678–710. 10.1111/jne.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MJ, Toney GM (2005) Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93:403–413. 10.1152/jn.01055.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ (1985) The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol 369:45–60. 10.1113/jphysiol.1985.sp015887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Toney GM (2010) In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103:4–15. 10.1152/jn.00094.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers PL, Bains JS (2018) Balancing tonic and phasic inhibition in hypothalamic corticotropin-releasing hormone neurons. J Physiol 596:1919–1929. 10.1113/JP275588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J (1987) Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol 253:H680–H689. 10.1152/ajpheart.1987.253.3.H680 [DOI] [PubMed] [Google Scholar]
- de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG (2016) Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct 221:891–912. 10.1007/s00429-014-0943-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, Nguyen D, Cahill KM, Sumners C, Stern JE, Krause EG (2017) A unique 'angiotensin-sensitive' neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci 37:3478–3490. 10.1523/JNEUROSCI.3674-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV (1988) Paraventricular nucleus neurons projecting to the dorsomedial medulla are influenced by systemic angiotensin. Brain Res Bull 20:197–201. 10.1016/0361-9230(88)90179-7 [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G (2008) The mouse brain in stereotaxic coordinates, Ed 3. Amsterdam: Elsevier. [Google Scholar]
- Frazier CJ, Harden SW, Alleyne AR, Mohammed M, Sheng W, Smith JA, Elsaafien K, Spector EA, Johnson DN, Scott KA, Krause EG, de Kloet AD (2021) An angiotensin-responsive connection from the lamina terminalis to the paraventricular nucleus of the hypothalamus evokes vasopressin secretion to increase blood pressure in mice. J Neurosci 41:1429–1442. 10.1523/JNEUROSCI.1600-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C (1995) Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180–190. 10.1159/000126839 [DOI] [PubMed] [Google Scholar]
- Holbein WW, Blackburn MB, Andrade MA, Toney GM (2018) Burst patterning of hypothalamic paraventricular nucleus-driven sympathetic nerve activity in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol 314:H530–H541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LL, Harding JW, Wright JW (1992) Role of paraventricular nucleus in control of blood pressure and drinking in rats. Am J Physiol 262:F1068–F1075. 10.1152/ajprenal.1992.262.6.F1068 [DOI] [PubMed] [Google Scholar]
- Jezova D, Ochedalski T, Kiss A, Aguilera G (1998) Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol 10:67–72. 10.1046/j.1365-2826.1998.00182.x [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rajamanickam S, Justice NJ (2018) Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J Neurosci 38:1874–1890. 10.1523/JNEUROSCI.1492-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H (1989) Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol 256:R1325–R1330. 10.1152/ajpregu.1989.256.6.R1325 [DOI] [PubMed] [Google Scholar]
- Koba S, Hanai E, Kumada N, Kataoka N, Nakamura K, Watanabe T (2018) Sympathoexcitation by hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla. J Physiol 596:4581–4595. 10.1113/JP276223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR (2011) Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 31:15009–15015. 10.1523/JNEUROSCI.0892-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DE, Zimmerman CA, Poormoghaddam A, Huey EL, Ahn JS, Lin YC, Tan CL, Chen Y, Knight ZA (2017) The forebrain thirst circuit drives drinking through negative reinforcement. Neuron 96:1272–1281.e1274. 10.1016/j.neuron.2017.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemche E, Chaban OS, Lemche AV (2016) Neuroendocrine and epigenetic mechanisms subserving autonomic imbalance and HPA dysfunction in the metabolic syndrome. Front Neurosci 10:142. 10.3389/fnins.2016.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong DS, Terron JA, Falcon-Neri A, Armando I, Ito T, Johren O, Tonelli LH, Hoe KL, Saavedra JM (2002) Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B) and AT(2) receptors. Neuroendocrinology 75:227–240. 10.1159/000054714 [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL (2003) Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23:5041–5049. 10.1523/JNEUROSCI.23-12-05041.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ferguson AV (1993) Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol 265:R302–R309. 10.1152/ajpregu.1993.265.2.R302 [DOI] [PubMed] [Google Scholar]
- Li YF, Wang W, Mayhan WG, Patel KP (2006) Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290:R1035–R1043. 10.1152/ajpregu.00338.2004 [DOI] [PubMed] [Google Scholar]
- Luther J, Tasker JG (2000) Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523:193–209. 10.1111/j.1469-7793.2000.t01-1-00193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madwed JB, Albrecht P, Mark RG, Cohen RJ (1989) Low-frequency oscillations in arterial pressure and heart rate: a simple computer model. Am J Physiol 256:H1573–H1579. 10.1152/ajpheart.1989.256.6.H1573 [DOI] [PubMed] [Google Scholar]
- Milner TA, Reis DJ, Pickel VM, Aicher SA, Giuliano R (1993) Ultrastructural localization and afferent sources of corticotropin-releasing factor in the rat rostral ventrolateral medulla: implications for central cardiovascular regulation. J Comp Neurol 333:151–167. 10.1002/cne.903330203 [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ (2001) Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13:139–146. 10.1111/j.1365-2826.2001.00597.x [DOI] [PubMed] [Google Scholar]
- Pati D, Harden SW, Sheng W, Kelly KB, de Kloet AD, Krause EG, Frazier CJ (2020) Endogenous oxytocin inhibits hypothalamic corticotrophin-releasing hormone neurones following acute hypernatraemia. J Neuroendocrinol 32:e12839. 10.1111/jne.12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW (2017) Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev 74:277–286. 10.1016/j.neubiorev.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Porter JP, Brody MJ (1986) A comparison of the hemodynamic effects produced by electrical stimulation of subnuclei of the paraventricular nucleus. Brain Res 375:20–29. 10.1016/0006-8993(86)90954-6 [DOI] [PubMed] [Google Scholar]
- Reinecke A, Browning M, Klein Breteler J, Kappelmann N, Ressler KJ, Harmer CJ, Craske MG (2018) Angiotensin regulation of amygdala response to threat in high-trait-anxiety individuals. Biol Psychiatry Cogn Neurosci Neuroimaging 3:826–835. 10.1016/j.bpsc.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Jezova M, Zhou J (2004) Brain angiotensin II, an important stress hormone: regulatory sites and therapeutic opportunities. Ann NY Acad Sci 1018:76–84. 10.1196/annals.1296.009 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE (1987) Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res 437:253–263. 10.1016/0006-8993(87)91641-6 [DOI] [PubMed] [Google Scholar]
- Sladek CD, Michelini LC, Stachenfeld NS, Stern JE, Urban JH (2015) Endocrine-autonomic linkages. Compr Physiol 5:1281–1323. 10.1002/cphy.c140028 [DOI] [PubMed] [Google Scholar]
- Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG (2014) Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol Behav 136:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE (2013) Dendritic peptide release mediates interpopulation cross-talk between neurosecretory and preautonomic networks. Neuron 78:1036–1049. 10.1016/j.neuron.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Lee S, Ryu PD, Lee SY, Stern JE (2011) Imbalanced K+ and Ca2+ subthreshold interactions contribute to increased hypothalamic presympathetic neuronal excitability in hypertensive rats. J Physiol 589:667–683. 10.1113/jphysiol.2010.198556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE (2001) Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537:161–177. 10.1111/j.1469-7793.2001.0161k.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31:410–417. 10.1159/000123111 [DOI] [PubMed] [Google Scholar]
- Tarjan E, Ferraro T, May CN (1995) Effect of ICV infusion of CRF on blood pressure and adrenal steroids in rabbits. Neuropeptides 28:325–331. 10.1016/0143-4179(95)90097-7 [DOI] [PubMed] [Google Scholar]
- Tasić T, Djordjević DM, De Luka SR, Trbovich AM, Japundžić-Žigon N (2017) Static magnetic field reduces blood pressure short-term variability and enhances baro-receptor reflex sensitivity in spontaneously hypertensive rats. Int J Radiat Biol 93:527–534. 10.1080/09553002.2017.1276307 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN (1982) The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol 206:317–345. 10.1002/cne.902060402 [DOI] [PubMed] [Google Scholar]
- Wallace K, Zhao X, Misra R, Sambamoorthi U (2018) The humanistic and economic burden associated with anxiety and depression among adults with comorbid diabetes and hypertension. J Diabetes Res 2018:4842520. 10.1155/2018/4842520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hiller H, Smith JA, de Kloet AD, Krause EG (2016) Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol Genomics 48:667–676. 10.1152/physiolgenomics.00029.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LA, Nguyen DH, Mifflin SW (2019) Corticotropin-releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increase blood pressure. J Neurophysiol 121:602–608. 10.1152/jn.00623.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Samson WK (2014) Separating thirst from hunger. In: Neurobiology of body fluid homeostasis: transduction and integration (De Luca LA Jr, Menani JV, Johnson AK, eds). Boca Raton, FL: CRC. [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Patel KP, Zucker IH, Wang W (2002) Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 282:H2039–H2045. 10.1152/ajpheart.00854.2001 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Guo SY, Gong S, Yin QZ, Hisamitsu T, Jiang XH (2005) Losartan blocks the excitatory effect of peripheral hypertonic stimulation on vasopressinergic neurons in hypothalamic paraventricular nucleus in rats: electrophysiological and immunocytochemical evidence. Neurosci Lett 380:12–16. 10.1016/j.neulet.2005.01.007 [DOI] [PubMed] [Google Scholar]


