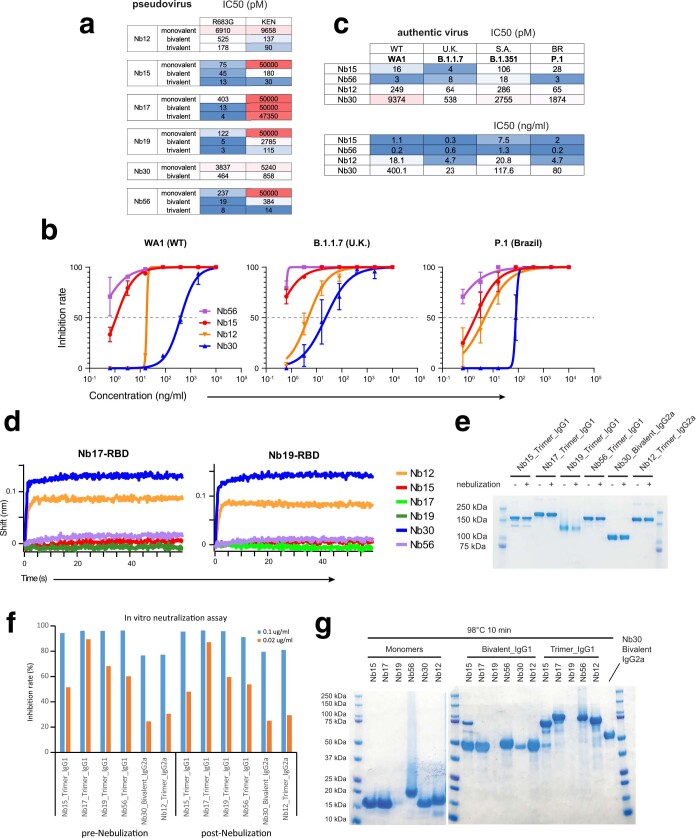
Extended Data Fig. 6. Neutralization of pseudo and authentic SARS-CoV-2 viruses.
a, Comparison of neutralization activities of leading nanobodies in monovalent, bivalent or trivalent form (results for monovalent and trivalent reproduced from Fig. 3a). b, Neutralization assays for wild-type (WA1) and SARS-CoV-2 variants B.1.1.7 and P.1 for trivalent Nb56, Nb15, Nb12 and bivalent Nb30. Data are representative of two independent experiments. Data are mean ± s.d. of triplicates. c, IC50 neutralization values for experiments shown in Fig. 3b and b. Top table values are in pM, lower table values are in ng ml−1. d, Related to Fig. 3c–f. Immunocomplexes used were Nb17–RBD (left) and Nb19–RBD (right). e, Coomassie staining showing nanobody integrity following nebulization. With the exception of Nb30 (bivalent), all nanobodies were fused to Fcs as trimers. Data are representative of two independent experiments. f, Bar graph showing in vitro neutralization (percentage) of RBD–ACE2 interactions by the different trivalent nanobody (bivalent for Nb30) before and after nebulization at two different concentrations (0.1 μg ml−1 (blue) and 0.02 μg ml−1 (orange)). g, Coomassie staining showing integrity of nanobody monomers (left) or multimers (right) following heat treatment (98 °C for 10 min). Data are representative of two independent experiments.