Keywords: AgRP, food intake, GABAergic, leptin, LRP1, Stat3
Abstract
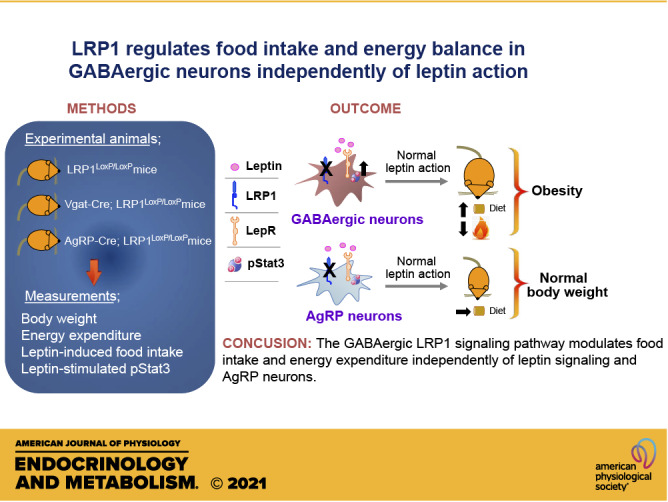
Low-density lipoprotein receptor-related protein 1 (LRP1) is a member of LDL receptor family that plays a key role in systemic glucose and lipid homeostasis. LRP1 also regulates energy balance in the hypothalamus by mediating leptin’s anorexigenic action, although the underlying neurocircuitry involved is still unclear. Because GABAergic neurons are a major mediator of hypothalamic leptin action, we studied the role of GABAergic LRP1 in energy balance and leptin action using mice lacking LRP1 in Vgat- or AgRP-expressing neurons (Vgat-Cre; LRP1loxP/loxP or AgRP-Cre; LRP1loxP/loxP). Here, we show that LRP1 deficiency in GABAergic neurons results in severe obesity in male and female mice fed a normal-chow diet. This effect is most likely due to increased food intake and decreased energy expenditure and locomotor activity. Increased adiposity in GABAergic neuron-specific LRP1-deficient mice is accompanied by hyperleptinemia and hyperinsulinemia. Insulin resistance and glucose intolerance in these mice are occurred without change in body weight. Importantly, LRP1 in GABAergic neurons is not required for leptin action, as evidenced by normal leptin’s anorexigenic action and leptin-induced hypothalamic Stat3 phosphorylation. In contrast, LRP1 deficiency in AgRP neurons has no effect on adiposity and caloric intake. In conclusion, our data identify GABAergic neurons as a key neurocircuitry that underpins LRP1-dependent regulation of systemic energy balance and body-weight homeostasis. We further find that the GABAergic LRP1 signaling pathway modulates food intake and energy expenditure independently of leptin signaling and AgRP neurons.
INTRODUCTION
Obesity is an epidemic that affects 42.4% of adults in North America (1) and a significant risk factor for a number of chronic comorbidities, including type 2 diabetes, nonalcoholic fatty liver disease, cardiovascular disease, and cancer (2). There are currently no effective therapeutic options to reduce the burden of obesity, highlighting the urgent need to understand the underlying mechanisms that drive the development of obesity.
Leptin is an adipocyte-derived hormone that plays a key role in systemic energy balance by regulating food intake, energy expenditure, and glucose and lipid homeostasis in the hypothalamus (3, 4). Obese patients and animal models are thought to develop leptin resistance, defined by the reduced ability of leptin to suppress appetite and weight gains despite elevated levels of circulating leptin (5–7). Leptin exerts its anorexigenic action through binding to and activating the long-form of leptin receptors (LepRb) that is extensively expressed in the hypothalamus (5, 8, 9). LepRb signals through the Janus kinase 2 (JAK2)-Signal Transducer and Activator of Transcription 3 (Stat3) signaling pathway, and Stat3 activation is generally used as a biochemical readout of leptin’s metabolic action (10–12). In leptin-resistant obese rodents, leptin treatment fails to stimulate Stat3 phosphorylation in the hypothalamus (5). In addition, Stat3 deletion in LepRb-expressing neurons results in hyperphagic and obese phenotype in mice (13). Although leptin resistance has been widely recognized as a key risk factor for obesity, this view has been challenged by reports that diet-induced obese mice retain hypothalamic sensitivity to the anorexigenic action of endogenous leptin (14).
Low-density lipoprotein receptor-related protein 1 (LRP1) is a large endocytic cell surface receptor that is broadly expressed throughout the body and recognizes a wide range of diverse ligands (15, 16). LRP1 has been implicated in the development of a number of diseases, including cardiovascular atherosclerosis, Alzheimer’s disease, and metabolic disorders (16–19). The central role of LRP1 in the peripheral regulation of metabolism has emerged from studies that deleted LRP1 in adipose (20), pancreatic (21), cardiovascular (22, 23), and hepatic tissue (24) and consistently resulted in metabolic dysregulation and diseases. Liu et al. (25) have studied the potential role of LRP1 in the central regulation of metabolism. They reported that disruption of the LRP1 gene in the calcium-calmodulin-dependent kinase II α (αCaMKII)-containing neurons in the central nervous system (CNS) of mice increases food intake and body weight, leading to obesity (25). Mechanistically, they found that LRP1 directly binds to leptin and the leptin receptor complex and is required for leptin-stimulated Stat3 activation (25). Based on these observations, they concluded that LRP1 is necessary for leptin’s regulation of systemic energy balance and a novel target for the treatment of obesity. However, the question of which neurocircuitry is involved, was not addressed. Identifying the neurons that mediate the LRP1 pathway will allow better understanding of LRP1 involvement in the central regulation of metabolism and leptin’s action. Specifically, the function of LRP1 in the key mediators of leptin action, GABAergic, and AgRP neurons needs to be elucidated.
In this study, we investigated the physiological role of LRP1 in energy homeostasis and leptin action in GABAergic and AgRP neurons. We show that LRP1 deletion in GABAergic neurons leads to severe obesity by increasing food intake and decreasing energy expenditure independently of leptin action and AgRP neurocircuitry.
METHODS
Animal Care
All animal care and experimental procedures were conducted in accordance with the National Institute of Health’s Guide for the Care and the Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Mice were allowed access to standard chow (Teklad F6 Rodent Diet 8664, Harlan Teklad) and water provided ad libitum and they were housed at 22°C–24°C with a 12-h light-dark cycle with the light cycle starting from 6:00 AM to 6:00 PM.
Generation of Vgat-Cre; LRP1loxP/loxP, AgRP-Cre; LRP1loxP/loxP, and Vgat-Cre, GFP; LRP1loxP/loxP Mice
Mice bearing a loxP-flanked LRP1 allele (LRP1loxP/loxP) were purchased from The Jackson Laboratory (Stock No. 012604, Bar Harbor, ME). Mice lacking LRP1 in Vgat- or AgRP-expressing neurons (Vgat-Cre; LRP1loxP/loxP or AgRP-Cre; LRP1loxP/loxP) were generated by mating LRP1loxP/loxP mice with Vgat-IRES-Cre or AgRP-IRES-Cre transgenic mice (26, 27) (gift from Dr. Brad Lowell, Beth Israel Deaconess Medical Center, Boston, MA). These mice were derived from 129 ES cells in C57BL/6 embryos. All mice we studied are mixed background with 129 and C57BL/6. Vgat-Cre, GFP mice were generated by Vgat-Cre mice mating with Cre-dependent L-GFP-reporter mice (28) (gift from Dr. Brad Lowell) and these mice were further bred with LRP1loxP/loxP mice, generating Vgat-Cre, GFP; LRP1loxP/loxP mice. GFP is only expressed in Vgat-expressing neurons. LRP1 expression was reduced in arcuate nucleus, paraventricular hypothalamic nucleus, and dorsomedial hypothalamic nucleus of Vgat-Cre; LRP1loxP/loxP mice where Vgat is expressed. In AgRP-Cre; LRP1loxP/loxP mice, LRP1 expression was only decreased in arcuate nucleus where AgRP is expressed (Supplemental Fig. S1; all Supplemental Figures are available at https://doi.org/10.6084/m9.figshare.13270598.v1) .
Body Composition and Food Intake Measurements
Mice were weighted from 4 wk of birth and weekly thereafter. Fat and lean body mass were assessed using EchoMRI (Echo Medical Systems, Houston, TX). Fat pads were harvested and weighed at the end of the experiment in male and female mice at 28 wk of age. For the measurement of daily food intake, male LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 16 wk of age were individually housed for 1 wk before the measurement of food intake. Food intake was then measured over a 7-day period. Uneaten food was collected and measured. This amount was excluded from the total amount of food intake. Fasting-induced food intake was measured for 24 h in male AgRP-Cre; LRP1loxP/loxP mice at 24 wk of age.
Blood Parameter Measurements
Blood was collected via the tail from either randomly fed mice or mice that had fasted overnight. Blood glucose was measured using a OneTouch Ultra glucose meter (LifeScan, Inc., Milpitas, CA). Serum insulin and leptin levels were measured by ELISA (Crystal Chem, Chicago, IL). Total serum cholesterol and triglyceride levels were determined by enzymatic methods (StanbioLaboratory, Boerne, TX).
Energy Expenditure and Locomotor Activity
Energy expenditure or locomotor activity was measured using a Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH). Male LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 12 wk of age were acclimated in the CLAMS chambers for 72 h before data collection. Mice had free access to food and water for the duration of the studies. During the course of the energy metabolism measurements, high variations (overlapping) in measurements emerged at individual time points between groups. To enhance the statistical power of these measurements, we combined each value from the individual time points and analyzed the data by unpaired Student’s t tests to compare the two groups.
Glucose Tolerance and Insulin Tolerance Test
For the glucose tolerance test (GTT), male or female LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice were fasted overnight, and blood glucose was measured before and at 15, 30, 60, 90, and 120 min after an intraperitoneal injection of glucose (1.0 g/kg). We used 6–7 wk of age and 20–22 wk of age of male mice and 7–8 wk of age of female mice. For the insulin tolerance test (ITT), male or female LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 20 wk of age were fasted for 4 h, and blood glucose was measured before and at 15, 30, 60, 90, and 120 min after an intraperitoneal injection of human insulin (0.75 IU/kg of body wt; Humulin R, Eli Lilly). We used 7–8 wk of age of female mice and 20–22 wk of age of male mice. Male LRP1loxP/loxP mice and AgRP-Cre; LRP1loxP/loxP mice at 8 wk of age were used for GTT and ITT. The area under the curve or above the curve for glucose was calculated using the trapezoidal rule for GTT or ITT (29).
Leptin-Stimulated Food Intake
For acute leptin-induced food intake, female LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 9–10 wk of age or male LRP1loxP/loxP mice and AgRP-Cre; LRP1loxP/loxP mice at 11 wk of age were individually housed for at least 1 wk. Mice were fasted overnight and intraperitoneally injected with leptin (3 mg/kg, National Hormone and Peptide Program, Torrance, CA). Food intake was measured at 1, 2, 4, 8, and 24 h after the injection of leptin or saline. For chronic leptin-induced food intake, male LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 30 wk of age were implanted subcutaneously with mini-osmotic pumps (ALZET Osmotic Pumps, Cupertino, CA) loaded with a physiological dose of leptin (0.5 mg/kg/day) (30, 31). Food intake was measured for 9 days after implantation of osmotic pumps.
Leptin-Induced Stat3 and S6 Phosphorylation
For the detection of pStat3 and pS6 in the hypothalamus, LRP1loxP/loxP mice and Vgat-Cre; LRP1loxP/loxP mice at 10 wk of age were fasted overnight and intraperitoneally injected with a maximal dose of leptin (3 mg/kg) (5). Forty minute after the IP leptin injection, mice were euthanized and perfused. Coronal brain sections were subjected to double immunohistochemistry (IHC) for pStat3 and pS6 as described (32). Phosphor-specific-(Tyr705) Stat3 rabbit antiserum (Cat. No. 9145, 1:1,000; Cell Signaling Technology, Danvers, MA) or phosphor-S6 rabbit antiserum (Cat. No. 4858, 1:1,000; Cell Signaling Technology) was used.
Immunoblotting Analysis
Brain lysates (20 μg protein) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes (GE Healthcare Life Sciences, Pittsburgh, PA). The membranes were incubated with polyclonal antibodies against LRP1 (Abcam, Cat. No. ab-92544) or actin (Sigma-Aldrich, Cat. No. A2066). The membranes were washed with Tris-buffered saline (TBS) containing 0.05% Tween 20 for 30 min, incubated with horseradish peroxidase secondary antibodies (GE Healthcare Life Sciences) for 1 h, and washed with TBS containing 0.05% Tween 20 for 30 min. The bands were visualized with enhanced chemiluminescence.
Quantitative Real-Time PCR
Total RNA was extracted from each tissue or cell using a TRIzol reagent (Invitrogen, CA) and subjected to quantitative real-time PCR as described previously (33). Gene-specific primer sequences are listed in Supplemental Table S1 (https://doi.org/10.6084/m9.figshare.13270601.v1).
Statistical Analysis
The assumptions for data distribution and homogeneity of variance were done by Shapiro–Wilk and Levene’s tests, respectively. Student’s t tests were used throughout this study to compare two distinct groups. Repeated-measures (RM) two-way ANOVA was performed for body weight, daily food intake, GTT, ITT, and leptin infusion/injection experiments. When intervention or interaction (intervention-by-time) was significant by RM two-way ANOVA, post hoc analyses were further performed by general linear model procedures and the Bonferroni post hoc test. A paired t test was done when comparing two dependent variables. Statistical significances were analyzed using Prizm 7.0 software (GraphPad Software, La Jolla, CA) and SPSS v 22.0 for Windows (SPSS, Inc., Chicago, IL). Differences were considered significant at P < 0.05. Data are presented as the means or individual values ± SE.
RESULTS
Selective Deletion of LRP1 in GABAergic Neurons Leads to Severe Obesity
To determine the physiological role of LRP1 in the regulation of energy balance, mice lacking LRP1 in GABAergic neurons fed a normal-chow diet were studied. Weekly body weights were measured for LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice on a normal-chow diet up to 28 wk of age. The body weight significantly increased in male Vgat-Cre; LRP1loxP/loxP mice compared with control mice starting at 6 wk of age, and this effect remained throughout the rest of the time (28 wk) that we examined the mice (Fig. 1B). At 28 wk of age, the average body weight of Vgat-Cre; LRP1loxP/loxP mice was ∼47% higher than their control littermates. This effect is due to increased fat mass in these mice (Fig. 1C), which was further confirmed by direct dissection of fat depots (Fig. 1D). Similar data are observed in female Vgat-Cre; LRP1loxP/loxP mice (Supplemental Fig. S2, A–C). Together, these data suggest that LRP1 in GABAergic neurons plays a key role in regulating adiposity and body-weight homeostasis.
Figure 1.
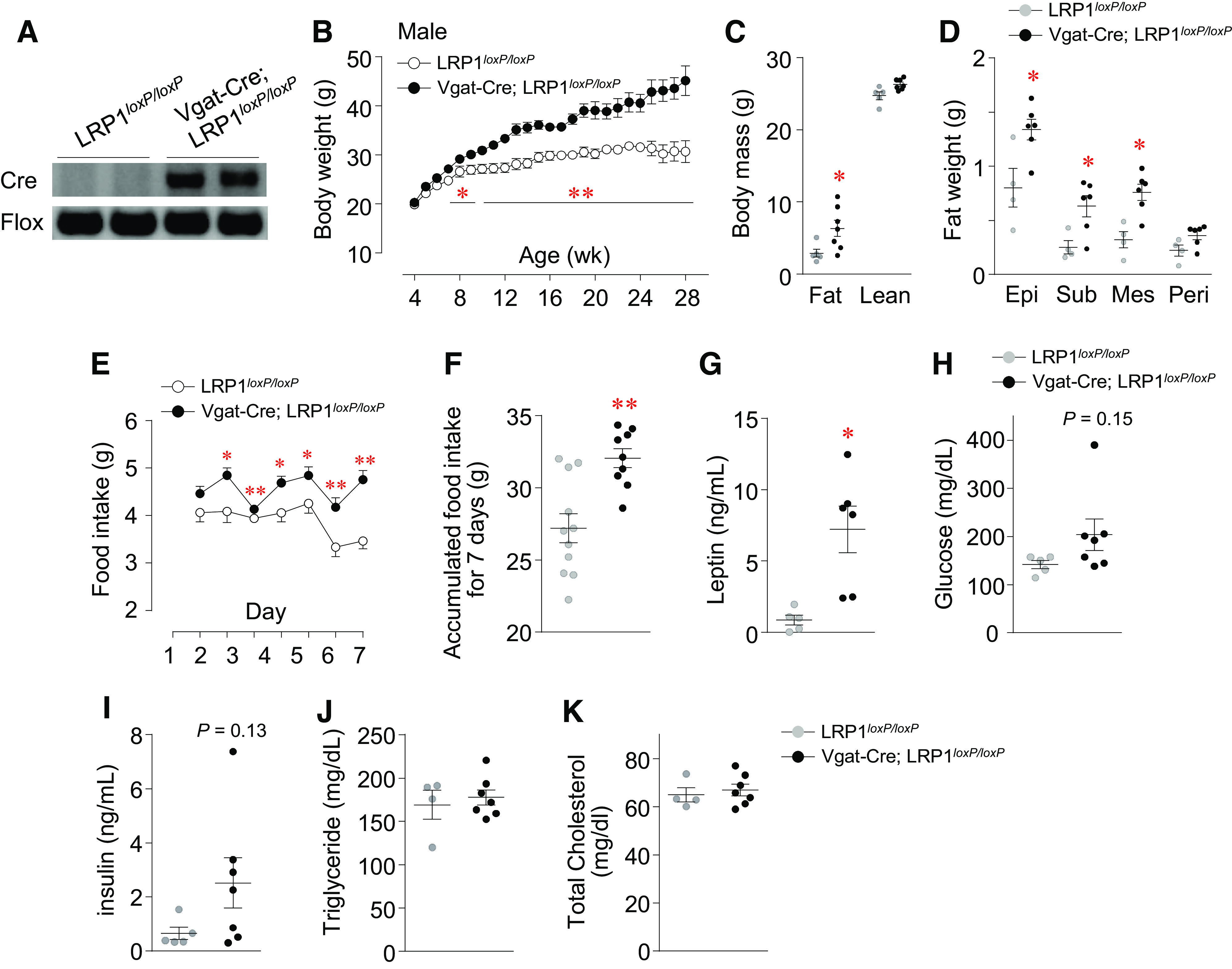
Loss of low-density lipoprotein receptor-related protein 1 (LRP1) in GABAergic neuron leads to obesity, hyperphagia, and insulin resistance. Genotyping (A), Body weight (n =5 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (B), body mass (n = 5 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (C), fat depots (n = 4 for control, n = 6 for Vgat-Cre; LRP1loxP/loxP) (D), daily food intake (n = 11 for control, n = 9 for Vgat-Cre; LRP1loxP/loxP) (E), accumulated food intake for 7 days (n = 11 for control, n = 9 for Vgat-Cre; LRP1loxP/loxP) (F), serum leptin (n = 5 for control, n = 6 for Vgat-Cre; LRP1loxP/loxP) (G), blood glucose (n = 5 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (H), serum insulin (n = 5 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (I), serum triglycerides (n = 4 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (J), and serum cholesterol (n = 4 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (K) were measured in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice. Serum parameters and fat depots were measured from overnight fasted mice at 31 wk of age. Food intake was measured at 16 wk of age for 1 wk. All graphs represent means or individual values ± SE. *P <0.05, **P <0.01 vs. LRP1loxP/loxP by two-sided Student’s t test.
Mice Lacking LRP1 in GABAergic Neurons Display Obesity-Related Metabolic Changes
We further characterized obesity-related metabolic phenotypes with male GABAergic neuron-specific LRP1-deficient mice because an obese phenotype was also seen in female Vgat-Cre; LRP1loxP/loxP mice. Daily food intake and accumulated food intake in mice lacking LRP1 in GABAergic neurons were significantly increased compared with control mice (Fig. 1, E and F). This was accompanied by increased serum leptin levels (Fig. 1G). Blood glucose and insulin levels of Vgat-Cre; LRP1loxP/loxP mice tended to be increased compared with control mice (Fig. 1, H and I), indicating a high risk for developing systemic insulin resistance. Neither serum triglyceride nor cholesterol levels were altered between the two groups (Fig. 1, J and K). Together, these data suggest that LRP1 action in GABAergic neurons is involved in the control of feeding behavior.
LRP1 Loss from GABAergic Neurons Leads to Insulin Resistance and Glucose Intolerance Independent of Body Weight
To determine whether LRP1 deletion in GABAergic neurons affects insulin sensitivity and glucose homeostasis, ITT and GTT were performed in young and old Vgat-Cre; LRP1loxP/loxP mice. Interestingly, young male and female mice lacking LRP1 in GABAergic neurons were insulin resistant, as evidenced by increased area under the curve during ITT (Fig. 2, A and C). Concurrently, we found that these mice were also glucose tolerant (Fig. 2, B and D). For this study, we used 6- to 7-wk-old male and 7- to 8-wk-old female Vgat-Cre; LRP1loxP/loxP mice, when their body weights were not different compared with control (Control vs. LRP1loxP/loxP mice, male: 23.2 ± 0.62 vs. 26.3 ± 1.6, P = 0.196; female: 22.6 ± 0.45 vs. 22.6 ± 0.52, P = 0.964). We further performed ITT and GTT with at 20- to 22-wk-old male Vgat-Cre; LRP1loxP/loxP mice because young female Vgat-Cre; LRP1loxP/loxP mice are insulin resistant and glucose tolerant. Similar ITT results were found in old male Vgat-Cre; LRP1loxP/loxP mice (Fig. 1E). Glucose tolerance was not statistically different between control and Vgat-Cre; LRP1loxP/loxP mice but there was a tendency to decrease for glucose tolerance (P = 0.097; Fig. 1F). Collectively, these data suggest that LRP1 in GABAergic neurons plays an important role in regulating insulin sensitivity and glucose metabolism independent of adiposity.
Figure 2.
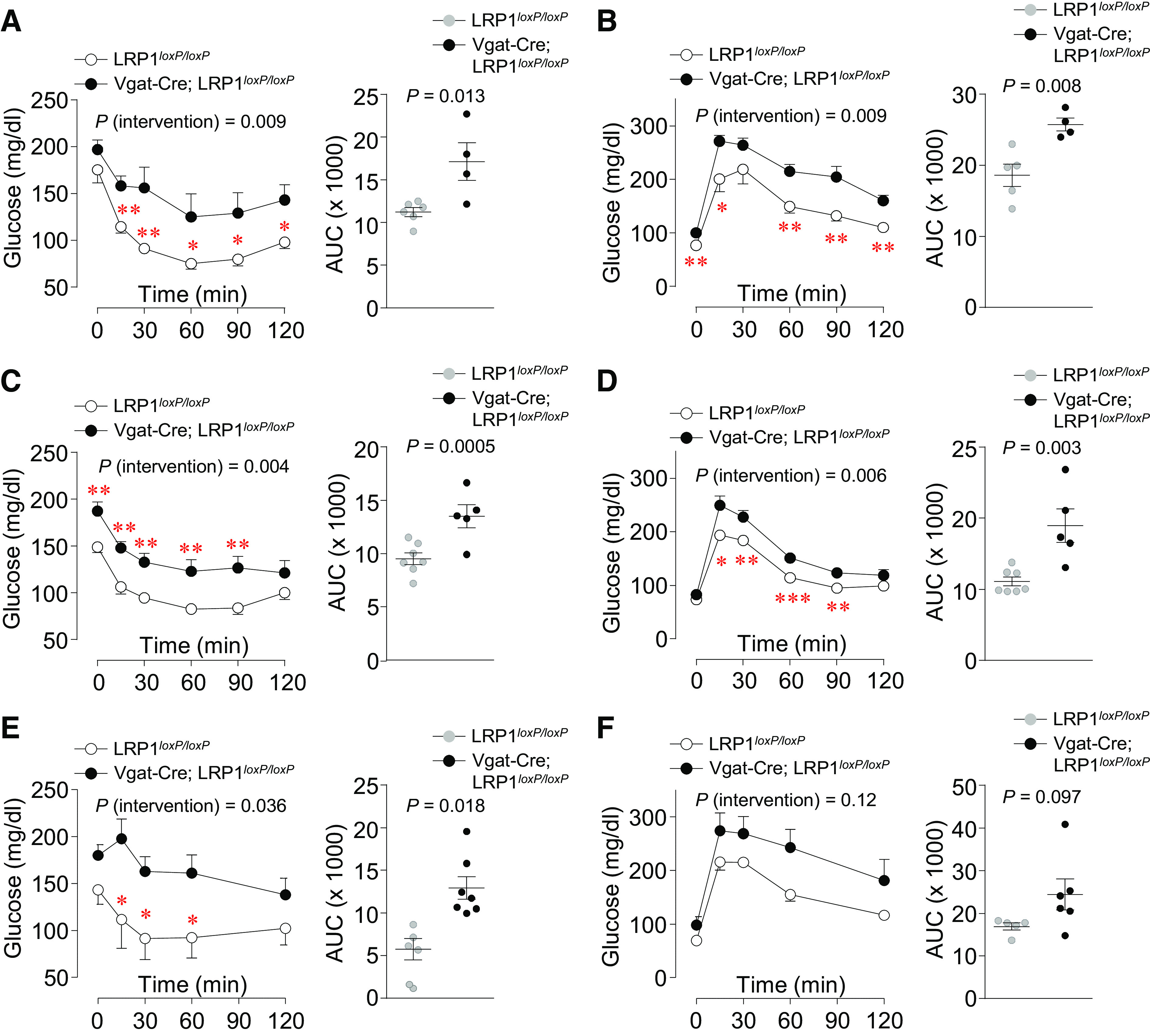
Low-density lipoprotein receptor-related protein 1 (LRP1) deletion from GABAergic neuron decreases insulin sensitivity and glucose tolerance. Insulin tolerance test (ITT) (n = 5 for control, n = 4 for Vgat-Cre; LRP1loxP/loxP) (A) and glucose tolerance test (GTT) (n = 5 for control, n = 4 for Vgat-Cre; LRP1loxP/loxP) (B) were performed in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 6–7 wk of age (n = 4–6). ITT (n = 7 for control, n = 5 for Vgat-Cre; LRP1loxP/loxP) (C) and GTT (n = 7 for control, n = 5 for Vgat-Cre; LRP1loxP/loxP) (D) were performed in female LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 7–8 wk of age (n = 4–6). ITT (n = 6 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP) (E) and GTT (n = 5 for control, n = 6 for Vgat-Cre; LRP1loxP/loxP) (F) were performed in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 20–22 wk of age (n = 5–7). Area under the curve (AUC) for ITT and GTT were calculated. All graphs represent means or individual values ± SE. P values (intervention) for ITT and GTT were evaluated by repeated-measures two-way ANOVA, and P values for AUCs were evaluated by two-sided Student’s t test. *P <0.05, **P <0.01, ***P <0.01 vs LRP1loxP/loxP mice by repeated measures two-way ANOVA.
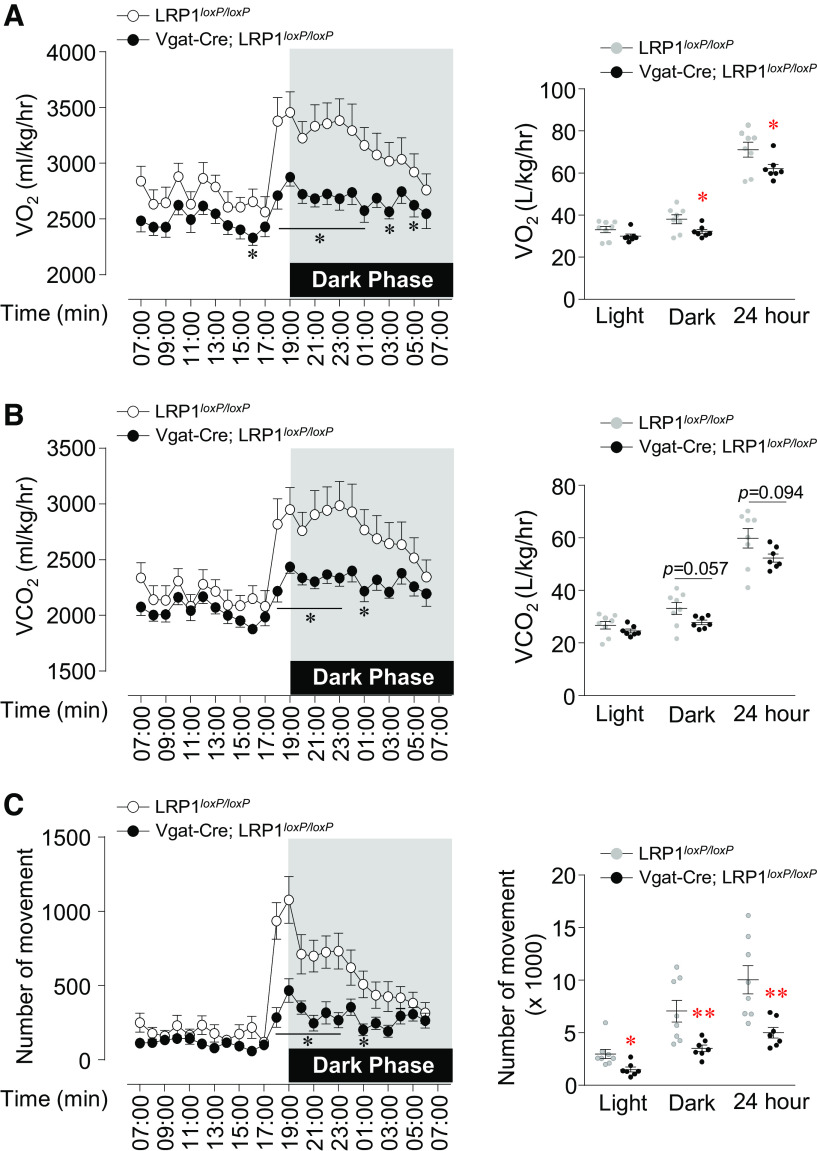
Deletion of LRP1 in GABAergic Neurons Decreases Energy Expenditure
We determined whether changes in energy expenditure contribute to the increase fat accumulation in Vgat-Cre; LRP1loxP/loxP mice. O2 consumption for 24 h in Vgat-Cre; LRP1loxP/loxP mice was significantly decreased compared with LRP1loxP/loxP mice (Fig. 3A). CO2 production tended to be decreased in Vgat-Cre; LRP1loxP/loxP mice (Fig. 3B). In addition, marked reduction in physical activity during both the light cycle and the dark cycle of Vgat-Cre; LRP1loxP/loxP mice was observed (Fig. 3C). Collectively, these data demonstrate that impaired energy expenditure and locomotor activity contribute to the increase in adiposity of Vgat-Cre; LRP1loxP/loxP mice.
Figure 3.
Deficiency of low-density lipoprotein receptor-related protein 1 (LRP1) in GABAergic neurons decreases locomotor activity. O2 consumption (A), CO2 production (B), and number of movements (C) were measured in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice (n = 8 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP). Hourly average O2 consumption or CO2 production, corresponding light and dark phase oxygen consumption (12 h average), and number of movements were assessed by Comprehensive Lab Animal Monitoring System (CLAMS) at 12 wk of age. All graphs represent means or individual values ± SE. *P < 0.05, **P <0.01 vs. LRP1loxP/loxP by two-sided Student’s t test.
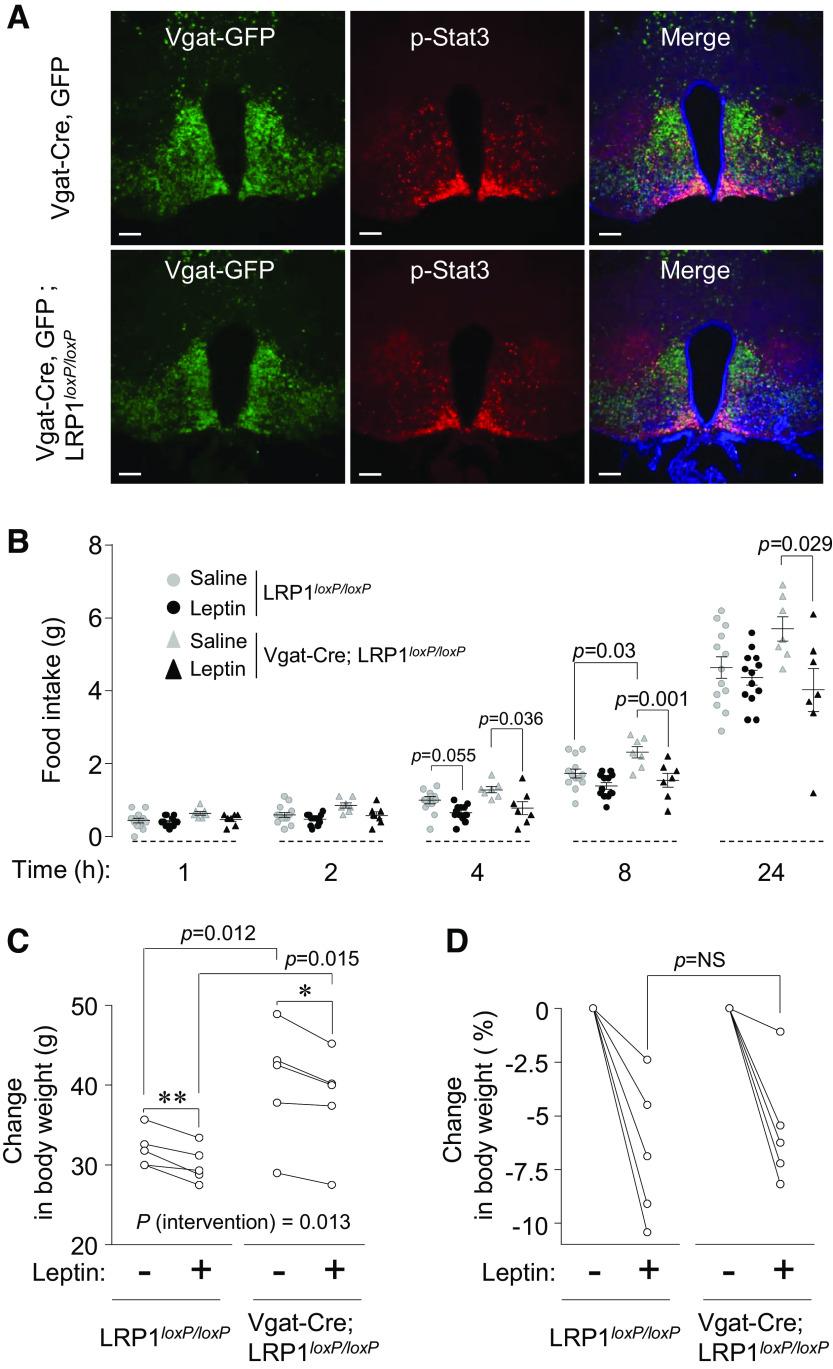
Leptin-Induced Stat3 Phosphorylation and Food Intake Are Normal in GABAergic-Neuron Specific LRP1-Deficient Mice
To determine whether LRP1 deletion in GABAergic neurons alters leptin sensitivity, leptin-stimulated hypoxthalamic Stat3 phosphorylation was measured in Vgat-Cre, GFP (control) and Vgat-Cre, GFP; LRP1loxP/loxP mice. As expected, leptin significantly increased hypothalamic Stat3 phosphorylation in control mice and this effect was also observed in Vgat-Cre, GFP; LRP1loxP/loxP mice (Fig. 4A). For Stat3 phosphorylation, leptin-responsive GABA ergic neurons were counted from Vgat-Cre, GFP and Vgat-Cre, GFP; LRP1loxP/loxP mice. Of the 338 GABAergic neurons examined in Vgat-Cre, GFP mice, leptin induced Stat3 phosphorylation in 92.6% of the neurons. Similarly, in Vgat-Cre, GFP; LRP1loxP/loxP mice, leptin induced Stat3 phosphorylation in 93.5% of the 307 GABAergic neurons. Phosphorylation of S6 induced by leptin was also not different between Vgat-Cre, GFP and Vgat-Cre, GFP; LRP1loxP/loxP mice (Supplemental Fig. S3B). These data suggest that obesity induced by LRP1 deletion in GABAergic neurons occurs despite intact leptin signaling.
Figure 4.
Leptin-induced hypothalamic Stat3 phosphorylation and food intake in Vgat-Cre; LRP1loxP/loxP mice is normal. A: immunofluorescence staining for hypothalamic Stat3 phosphorylation were shown in female Vgat-Cre, GFP and Vgat-Cre, GFP; LRP1loxP/loxP mice at 9–10 wk of age. Mice were intraperitoneally injected with saline or leptin (3 m/kg) and euthanized 40 min later. Scale bars represent 100 µm. B: leptin-induced food intake was measured in in female LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 9–10 wk of age (n = 13 for control, n = 7 for Vgat-Cre; LRP1loxP/loxP). Leptin (3 mg/kg/day) was injected in overnight-fasted mice intraperitoneally and food intake was measured as indicated time points. Changes in body weight (C) and percent changes in body weight after leptin infusion (D) were measured in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 30 wk of age (n = 5 for control and Vgat-Cre; LRP1loxP/loxP). Leptin (0.5 mg/kg/day) was infused for 9 days using mini-osmotic pump. Body weights were measured before and after leptin treatment. All graphs represent means or individual values ± SE. P values by repeated-measures two-way ANOVA are indicated. *P < 0.05, **P <0.01 vs. no leptin by Student’s paired t test.
To determine whether LRP1 deficiency in GABAergic neurons affects the metabolic action of leptin on feeding behavior in young lean animals, we measured food intake in response to leptin in young female LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 9–10 wk of age. Food intake markedly decreased at 4 h, following the administration of IP leptin in both groups. Interestingly, however, at 8- and 24-h time points, leptin-induced food intake was significantly decreased in Vgat-Cre; LRP1loxP/loxP mice but not in LRP1loxP/loxP mice (Fig. 4C). We further determined whether leptin infusion alters body weight in obese Vgat-Cre; LRP1loxP/loxP mice; mini-osmotic pumps containing leptin (0.5 mg/kg/day) in male LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice at 30 wk of age were implanted subcutaneously. Nine days after leptin infusion, body weights were significantly decreased by ∼2 g in both groups compared with preleptin treatment (Fig. 4D). Individual percentage changes of body weight between LRP1loxP/loxP and Vgat-Cre; LRP1loxP/loxP mice are similar (−6.7% ± −1.5% vs. −5.6% ± −1.2%, P = not significant) (Fig. 4E). Collectively, these data suggest that leptin action may not contribute to the increased adiposity of GABAergic neuron-specific LRP1-deficient mice and further implicate that obesity may not always be found with leptin resistance.
Hypothalamic neuropeptide gene expressions were unaltered in both groups (Supplemental Fig. S3A). Thermogenic gene mRNA levels for Cox8b and Cox7a1 were elevated in the BAT of Vgat-Cre; LRP1loxP/loxP mice, whereas the expression levels of PGC1α, UCP1, Elovl3, and Cpt1b in the brown adipose tissue (BAT) were normal (Supplemental Fig. S4B). All genes transcripts measured in the white adipose tissue (WAT) were similar for both groups (Supplemental Fig. S4C).
Increased Adiposity and Caloric Intake in LRP1 Deletion in GABAergic Neurons is Independent of LRP1 Action in AgRP Neuron
Because of the importance of AgRP neurons in energy balance regulation and the abundance of GABAergic neurons in the cluster of AgRP-expressing neurons, we determine whether LRP1 deletion in AgRP neurons leads to the obese phenotype observed in Vgat-Cre; LRP1loxP/loxP mice. In contrast to GABAergic neuron-specific LRP1-deficient mice, no significant differences in body weight or adiposity existed between LRP1loxP/loxP and AgRP-Cre; LRP1loxP/loxP mice fed a normal-chow diet (Fig. 5, B and C). Blood glucose, serum insulin levels, and fasting-induced food intake were also similar between the two groups (Fig. 5, D–F). IP injections of leptin led to a similar decrease in food intake after 8 h in both groups (Fig. 5G). Moreover, insulin sensitivity and glucose tolerance were not different between control and AgRP-Cre; LRP1loxP/loxP mice (Fig. 5, H and I). These data indicate that, unlike LRP1 action in GABAergic neurons, LRP1 in AgRP neurons may play an insignificant role in controlling energy and glucose homeostasis.
Figure 5.
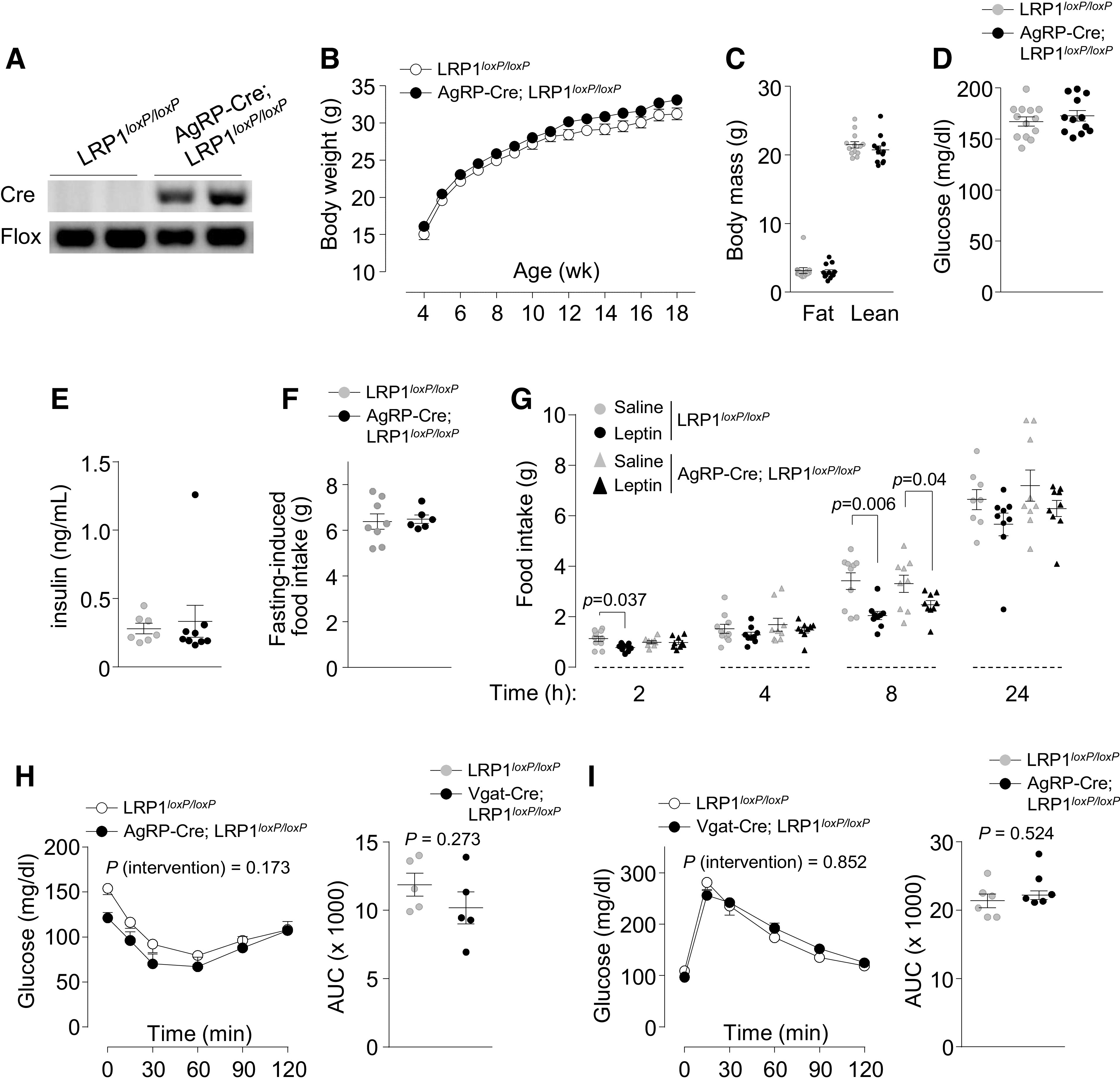
Mice lacking low-density lipoprotein receptor-related protein 1 (LRP1) in AgRP-expressing neurons are normal. Genotyping (A), body weight (n = 16 for control and n = 25 AgRP-Cre; LRP1loxP/loxP) (B), body mass (n = 13 for control and n = 12 AgRP-Cre; LRP1loxP/loxP) (C), blood glucose (n = 13 for control and n = 12 AgRP-Cre; LRP1loxP/loxP) (D), serum insulin (n = 7 for control and n = 9 AgRP-Cre; LRP1loxP/loxP) (E), fasting-induced food intake (n = 8 for control and n = 6 AgRP-Cre; LRP1loxP/loxP) (F), leptin-induced food intake (n = 8–10 for control and n = 9 AgRP-Cre; LRP1loxP/loxP) (G), results of insulin tolerance test (ITT) (n = 5 for control and AgRP-Cre; LRP1loxP/loxP) (H), and results of glucose tolerance test (GTT) (n = 6 for control and AgRP-Cre; LRP1loxP/loxP) (I) were measured in male LRP1loxP/loxP and AgRP-Cre; LRP1loxP/loxP mice. Body mass, blood glucose, serum insulin, and leptin-induced food intake were measured at 11–15 wk of age. Leptin (3 mg/kg/day) was injected in overnight fasted mice intraperitoneally, and food intake was measured as indicated time points. Fasting-induced food intake was measured at 24 wk of age. ITT and GTT were performed at 8 wk of age. All graphs represent means or individual values ± SE. P values (intervention) for ITT and GTT as well as leptin-induced food intake were evaluated by repeated-measures two-way ANOVA, and P values for area under the curves (AUCs) were evaluated by two-sided Student’s t test.
DISCUSSION
The current study uncovers the role of a GABAergic LRP1 in the regulation of body-weight homeostasis and energy balance and demonstrates that the anorexigenic signaling pathway is disconnected from leptin signaling. Deletion of LRP1 in GABAergic neurons in mice results in hyperphagia and reduced energy expenditure despite intact leptin action, leading to obesity and metabolic dysregulation.
A growing body of literature has highlighted the involvement of LRP1 in energy metabolism in periphery and brain (17, 20–25, 34). Efforts to delete LRP1 from adipose (20), pancreatic (21), cardiovascular (22, 23) and hepatic tissue (24) have all resulted in dysregulation of the metabolic homeostasis. To assess a potential role for LRP1 in the central regulation of metabolism, Liu et al. (25) have deleted LRP1 in αCaMKII-expressing neurons in the forebrain and in the hypothalamic arcuate by injection of Cre lentivirus in LRP1loxP/loxP mice. Their findings suggested that LRP1 regulates food intake and energy balance by mediating leptin’s anorexigenic action in the hypothalamus. Although our study corroborates their conclusions that hypothalamic LRP1 regulates energy balance and drives anorexigenic effect, we find that LRP1 functions independently of leptin. This discrepancy is unclear at this time, but cell type specificity could be a factor. CaMKII is expressed in a wide range of neuronal populations throughout the brain, including glutamatergic and GABAergic neurons (35, 36), whereas in the hypothalamus, αCaMKII is mainly expressed in the lateral part of arcuate nucleus as well as in the ventromedial nucleus, lateral nucleus, and dorsal-medial nucleus. Therefore, it is reasonable to assume that LRP1 action in CaMKII-expressing glutamatergic and potentially other neurons would dilute any effect resulting from a GABAergic LRP1 signaling pathway.
Notably, even though GABAergic LRP1-deficient mice develop severe obesity, metabolic disorder, and insulin resistance, we found that they retain intact hypothalamic leptin action and exhibit adequate sensitivity to leptin treatment. This, together with previous reports showing that diet-induced obese mice retain endogenous leptin action (14), challenges the view that defective hypothalamic leptin signaling is a necessary step in the development of obesity.
In this study, we demonstrate, interestingly, that systemic insulin sensitivity and glucose tolerance were significantly impaired when LRP1 was deleted in GABAergic neurons. These effects occurred independent of body weight. It is, thus, important to explore the mechanism underlying this phenomenon at peripheral levels. Investigations on hepatic glucose output and/or muscle glucose uptake will be the first.
Energy expenditure is closely linked to physical activity and hypothesized to be regulated by hypothalamic leptin signaling (37–39). Our study does not support this hypothesis given that the Vgat-Cre; LRP1loxP/loxP mice have a normal response to leptin. Importantly, recent reports have shown that modulation of GABAergic neuronal activity can affect physical activity, although it is not clear yet whether the effect is stimulatory or inhibitory (40, 41). This may explain our observations that the GABAergic LRP1 pathway is involved in the regulation of physical activity and energy expenditure.
xAgRP neurons are mostly GABAergic and play a central role in the central regulation of metabolism and in mediating leptin’s anorexigenic action (27, 42, 43). Thus, we questioned whether they also mediate LRP1 effect on systemic energy balance. Surprisingly, LRP1 deficiency in AgRP neurons had no effects on adiposity and feeding behavior, suggesting that LRP1 action in AgRP neurons is not involved in hypothalamic regulation of energy balance or in mediating GABAergic LRP1 signal. We also rule out the involvement of POMC LRP1 as POMC neurons do not express LRP1 (25). Future studies will be needed to determine if additional GABAergic neuron populations are involved in LRP1-mediated regulation of energy homeostasis, such as DMH and LH neurons.
In conclusion, our study identifies a novel GABAergic LRP1 pathway that regulates body-weight homeostasis and energy balance and drives anorexigenic action in parallel but independent of the leptin signaling pathway. Furthermore, our findings that obesity can develop in the absence of hypothalamic leptin resistance imply that conventional signature in hypothalamic leptin signal may not always be a reliable indicator for the development of obesity.
GRANTS
This work was supported by National Institutes of Health Grants R01DK111529, R01DK106076, and R01DK123002 (to Y.B.K.); grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07049123 to J.A.S.) and the Ministry of Science, ICT & Future Planning (2018R1C1B6004780). M.C.K. was a recipient of a postdoctoral fellowship award from the American Diabetes Association (1-17-PDF-146). K.C.R is a recipient of a grant by the São Paulo Research Foundation from Brazil (FAPESP 2019/19938-5). J.N.C. was supported by an American Diabetes Association Pathway to Stop Diabetes award (1-18-INI-14).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M-C.K., J.A.S., and Y-B.K. conceived and designed research; M.C.K., J.A.S., H.L., A.U., W-M.Y., K.C.R., H.J.K., W.L., and J.N.C. performed experiments; Y-B.K. analyzed data; Y.D., Y-B.K., H.J.L., W-M.Y., and H.J.K. interpreted results of experiments; M-C.K. prepared figures; H.L. drafted manuscript; Y.D. and Y-B.K. edited and revised manuscript; Y-B.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brad Lowell for providing GFP reporter Z/EG mice, Vgat-IRES-Cre, and AgRP-IRES-Cre recombinase mice; Terry Flier for helping CLAMS study; and all members of the Kim Laboratory for helpful advice and discussion. We are grateful to the Histology Core at the Beth Israel Deaconess Medical Center.
Present address of W. Li: Sanofi, 225 Second Ave, Waltham, MA 02451.
REFERENCES
- 1.Hales C, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Hyattsville, MD: National Center for Health Statistics, 2020. [Google Scholar]
- 2.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, , et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388: 776–786, 2016. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21: 263–307, 2000. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 4.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493: 63–71, 2005. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 5.El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105: 1827–1832, 2000. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1: 1311–1314, 1995. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM. A tale of two hormones. Nat Med 16: 1100–1106, 2010. doi: 10.1038/nm1010-1100. [DOI] [PubMed] [Google Scholar]
- 8.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556, 2008. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [Erratum in Nature 374: 479, 1995]. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 10.Banks AS, Davis SM, Bates SH, Myers MG Jr.. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275: 14563–14572, 2000. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108: 1113–1121, 2001. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG Jr.. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem 277: 41547–41555, 2002. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 13.Piper ML, Unger EK, Myers MG Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 22: 751–759, 2008. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D'Alessio DA, Perez-Tilve D. Diet-induced obese mice retain endogenous leptin action. Cell Metab 21: 877–882, 2015. doi: 10.1016/j.cmet.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 88: 887–918, 2008. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara M, Tachibana M, Kanekiyo T, Bu G. Role of LRP1 in the pathogenesis of Alzheimer's disease: evidence from clinical and preclinical studies. J Lipid Res 58: 1267–1281, 2017. doi: 10.1194/jlr.R075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonias SL, Campana WM. LDL receptor-related protein-1: a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am J Pathol 184: 18–27, 2014. doi: 10.1016/j.ajpath.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D, Muller C, Valet P, Liaudet-Coopman E. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One 4: e7422, 2009. doi: 10.1371/journal.pone.0007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storck SE, Pietrzik CU. Endothelial LRP1 - a potential target for the treatment of Alzheimer's disease: theme: drug discovery, development and delivery in Alzheimer's disease guest editor: avide Brambilla. Pharm Res 34: 2637–2651, 2017. doi: 10.1007/s11095-017-2267-3. [DOI] [PubMed] [Google Scholar]
- 20.Zheng XY, Yu BL, Xie YF, Zhao SP, Wu CL. Apolipoprotein A5 regulates intracellular triglyceride metabolism in adipocytes. Mol Med Rep 16: 6771–6779, 2017. doi: 10.3892/mmr.2017.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye R, Gordillo R, Shao M, Onodera T, Chen Z, Chen S, Lin X, SoRelle JA, Li X, Tang M, Keller MP, Kuliawat R, Attie AD, Gupta RK, Holland WL, Beutler B, Herz J, Scherer PE. Intracellular lipid metabolism impairs beta cell compensation during diet-induced obesity. J Clin Invest 128: 1178–1189, 2018. doi: 10.1172/JCI97702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costales P, Fuentes-Prior P, Castellano J, Revuelta-Lopez E, Corral-Rodríguez MÁ, Nasarre L, Badimon L, Llorente-Cortes V. K domain CR9 of low density lipoprotein (LDL) receptor-related protein 1 (LRP1) is critical for aggregated LDL-induced foam cell formation from human vascular smooth muscle cells. J Biol Chem 290: 14852–14865, 2015. doi: 10.1074/jbc.M115.638361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samouillan V, Revuelta-López E, Dandurand J, Nasarre L, Badimon L, Lacabanne C, Llorente-Cortés V. Cardiomyocyte intracellular cholesteryl ester accumulation promotes tropoelastin physical alteration and degradation: role of LRP1 and cathepsin S. Int J Biochem Cell Biol 55: 209–219, 2014. doi: 10.1016/j.biocel.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Ding Y, Xian X, Holland WL, Tsai S, Herz J. Low-density lipoprotein receptor-related protein-1 protects against hepatic insulin resistance and hepatic steatosis. EBioMedicine 7: 135–145, 2016. doi: 10.1016/j.ebiom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, Herz J, Muglia LJ, Bu G. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol 9: e1000575, 2011. [Erratum in PLoS Biol 17: e3000310, 2019]. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71: 142–154, 2011. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo JA, Kang MC, Yang WM, Hwang WM, Kim SS, Hong SH, Heo JI, Vijyakumar A, Pereira de Moura L, Uner A, Huang H, Lee SH, Lima IS, Park KS, Kim MS, Dagon Y, Willnow TE, Aroda V, Ciaraldi TP, Henry RR, Kim YB. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat Commun 11: 2024, 2020. [Erratum in Nat Commun 11: 2276, 2020]. doi: 10.1038/s41467-020-15963-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann A, Ebert T, Klöting N, Dokas J, Jeromin F, Jessnitzer B, Burkhardt R, Fasshauer M, Kralisch S. Leptin dose-dependently decreases atherosclerosis by attenuation of hypercholesterolemia and induction of adiponectin. Biochim Biophys Acta 1862: 113–120, 2016. doi: 10.1016/j.bbadis.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann A, Manjowk GM, Wagner IV, Klöting N, Ebert T, Jessnitzer B, Lössner U, Stukenborg JB, Blüher M, Stumvoll M, Söder O, Svechnikov K, Fasshauer M, Kralisch S. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology 157: 2461–2468, 2016. doi: 10.1210/en.2015-1966. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, Oh BC, Lee SW, Lee B, Zabolotny JM, Kim MS, Bjørbaek C, Lowell BB, Kim YB. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci 15: 1391–1398, 2012. doi: 10.1038/nn.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Lee SH, Sousa-Lima I, Kim SS, Hwang WM, Dagon Y, Yang WM, Cho S, Kang MC, Seo JA, Shibata M, Cho H, Belew GD, Bhin J, Desai BN, Ryu MJ, Shong M, Li P, Meng H, Chung BH, Hwang D, Kim MS, Park KS, Macedo MP, White M, Jones J, Kim YB. Rho-kinase/AMPK axis regulates hepatic lipogenesis during overnutrition. J Clin Invest 128: 5335–5350, 2018. doi: 10.1172/JCI63562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Au DT, Strickland DK, Muratoglu SC. The LDL receptor-related protein 1: at the crossroads of lipoprotein metabolism and insulin signaling. J Diabetes Res 2017: 8356537, 2017. doi: 10.1155/2017/8356537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Zhang C, Szábo G, Sun QQ. Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain Res 1518: 9–25, 2013. doi: 10.1016/j.brainres.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou DJ, Greer CA, Firestein S. Expression pattern of alpha CaMKII in the mouse main olfactory bulb. J Comp Neurol 443: 226–236, 2002. doi: 10.1002/cne.10125. [DOI] [PubMed] [Google Scholar]
- 37.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9: 537–547, 2009. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Brüning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab 7: 236–248, 2008. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 40.de Vrind VA, Rozeboom A, Wolterink-Donselaar IG, Luijendijk-Berg MC, Adan RA. Effects of GABA and leptin receptor-expressing neurons in the lateral hypothalamus on feeding, locomotion, and thermogenesis. Obesity (Silver Spring) 27: 1123–1132, 2019. doi: 10.1002/oby.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud HR, Morrison CD, Münzberg H. Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J Neurosci 37: 6053–6065, 2017. doi: 10.1523/JNEUROSCI.0155-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res 756: 283–286, 1997. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 43.Ovesjö ML, Gamstedt M, Collin M, Meister B. GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol 13: 505–516, 2001. doi: 10.1046/j.1365-2826.2001.00662.x. [DOI] [PubMed] [Google Scholar]