Keywords: eukaryotic translation initiation, eukaryotic translation initiation factor 4E-binding protein 1 (eIF4E-BP1), liver, retinol-binding protein
Abstract
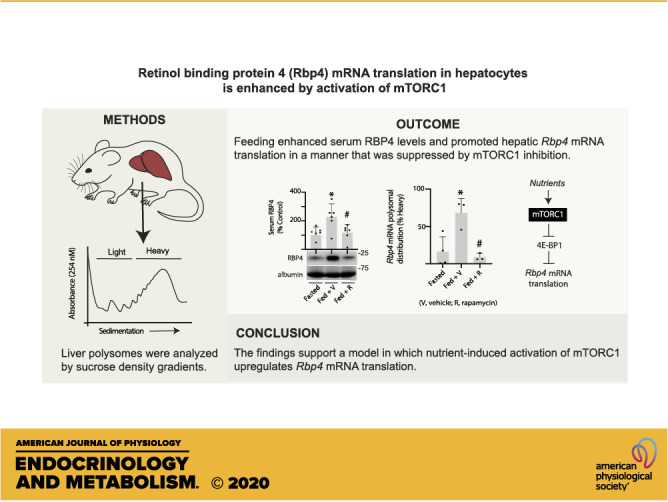
Increased expression of the peptide hormone retinol-binding protein 4 (RBP4) has been implicated in the development of insulin resistance, type 2 diabetes, and visual dysfunction. Prior investigations of the mechanisms that influence RBP4 synthesis have focused solely on changes in mRNA abundance. Yet, the production of many secreted proteins is controlled at the level of mRNA translation, as it allows for a rapid and reversible change in expression. Herein, we evaluated Rbp4 mRNA translation using sucrose density gradient centrifugation. In the liver of fasted rodents, Rbp4 mRNA translation was low. In response to refeeding, Rbp4 mRNA translation was enhanced and RBP4 levels in serum were increased. In H4IIE cells, refreshing culture medium promoted Rbp4 mRNA translation and expression of the protein. Rbp4 mRNA abundance was not increased by either experimental manipulation. Enhanced Rbp4 mRNA translation was associated with activation of the kinase mechanistic target of rapamycin in complex 1 (mTORC1) and enhanced phosphorylation of the translational repressor eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). In H4IIE cells, expression of a 4E-BP1 variant that is unable to be phosphorylated by mTORC1 or suppression of mTORC1 with rapamycin attenuated activity of a luciferase reporter encoding the Rbp4 mRNA 5′-untranslated region (UTR). Purine substitutions to disrupt a terminal oligopyrimidine (TOP)-like sequence in the Rbp4 5′-UTR prevented the suppressive effect of rapamycin on reporter activity. Rapamycin also prevented upregulation of Rbp4 mRNA translation in the liver and reduced serum levels of RBP4 in response to feeding. Overall, the findings support a model in which nutrient-induced activation of mTORC1 upregulates Rbp4 mRNA translation to promote RBP4 synthesis.
NEW & NOTEWORTHY RBP4 plays a critical role in metabolic disease, yet relatively little is known about the mechanisms that regulate its production. Herein, we provide evidence for translational control of RBP4 synthesis. We demonstrate that activation of the nutrient-sensitive kinase mTORC1 promotes hepatic Rbp4 mRNA translation. The findings support the possibility that targeting Rbp4 mRNA translation represents an alternative to current therapeutic interventions that lower serum RBP4 concentration by promoting urinary excretion of the protein.
INTRODUCTION
Retinol-binding protein 4 (RBP4) is a 21-kDa protein that transports all-trans-retinol (vitamin A) in the blood to the periphery (1). RBP4 is synthesized in hepatocytes, binds to intracellular retinol, and is secreted as a retinol-bound holo-RBP4. Previous studies demonstrate that enhanced RBP4 expression is potentially causative in the development of insulin resistance and the metabolic dysfunction that defines type 2 diabetes (2). Serum levels of RBP4 directly correlate with the magnitude of insulin resistance, glucose intolerance, and the incidence of type 2 diabetes (2, 3). Moreover, increasing serum RBP4 concentrations by transgenic overexpression or injection of purified RBP4 protein is sufficient to cause insulin resistance (2). On the other hand, RBP4-knockout mice exhibit enhanced insulin sensitivity (2).
Based on the remarkable effects of RBP4 on metabolic dysfunction, lowering serum RBP4 levels has been pursued as a potential therapy for diabetes. Specifically, the synthetic retinoid fenretinide acts to reduce serum RBP4 levels by increasing urinary RBP4 excretion (4). However, recent studies suggest that RBP4 in the serum may not be responsible for defects in glucose and energy homeostasis in mice (5). Rather, RBP4 may induce metabolic dysfunction via autocrine or paracrine signaling (6). More specifically, enhanced RBP4 production by adipocytes may act locally on adipose tissue itself to impact liver steatosis. Thus, therapeutic approaches that promote RBP4 renal clearance may be of limited benefit. One potential therapeutic alternative to promoting RBP4 excretion is to address the molecular events responsible for enhanced RBP4 expression.
Protein expression is the ultimate sum of events that include gene transcription and protein synthesis, as well as mRNA and protein degradation. Translational control plays a critical role in determining the synthesis of specific proteins in response to nutrient excess or deficiency (7). However, to date, studies investigating how RBP4 expression is controlled have focused solely on transcriptional mechanisms and have ignored translational control. A key rate-controlling step in translation is the recruitment of ribosomes to a specific mRNA, which in mammalian cells occurs through an m7GTP cap at the 5′-end of the mRNA that is recognized by the cap-binding protein eukaryotic initiation factor 4E (eIF4E). According to the traditional model of cap-dependent translation, recruitment of the ribosome onto an mRNA occurs via assembly of the eIF4F cap-binding complex, which includes eIF4E, the scaffold protein eIF4G, and the RNA helicase eIF4A (8).
Sequestration of eIF4E by the translational repressor eIF4E-binding protein 1 (4E-BP1) prevents its interaction with eIF4G and represents the best-characterized mechanism for repressing cap-dependent translation initiation. Hypophosphorylated 4E-BP1 binds tightly to eIF4E to prevent eIF4F complex assembly. In response to nutrients and growth factors, the mechanistic target of rapamycin in complex 1 (mTORC1) is activated and directly phosphorylates 4E-BP1 to promote its disassociation from eIF4E. Notably, the translation of mRNAs with unique sequence clusters of pyrimidine residues located after the m7GTP cap or a few nucleotides downstream [known as terminal oligopyrimidine (TOP) or TOP-like sequences, respectively] is particularly dependent on eIF4F complex assembly (9, 10).
Herein, we investigated translational regulation of the Rbp4 mRNA. We found that refeeding fasted rodents increased serum RBP4 levels and promoted hepatic Rbp4 mRNA translation. Stimulatory effects on Rbp4 mRNA translation and mTORC1 activation were also observed in H4IIE cell cultures upon refreshing the culture medium. Suppression of mTORC1 with rapamycin prevented the increase in Rbp4 mRNA translation in both experimental paradigms. Overall, the findings support a model wherein nutrient-induced activation of mTORC1 promotes Rbp4 mRNA translation.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (∼12 wk of age; Jackson Laboratory, Bar Harbor, ME) and male Sprague–Dawley rats (∼100–125 g body wt; Charles River Laboratories, Wilmington, MD) were maintained on a standard diet (Rodent Chow 8604; Harlan-Teklad, Indianapolis, IN). Animals were fasted overnight for 16 h with ad libitum access to water, and the following morning one-half of animals were fed. In some experiments, rats received (0.75 mg/kg) rapamycin (EMD Millipore) or a vehicle control (saline with 2% ethanol) by tail vein injection 1 h before feeding. All rodents were anesthetized with isoflurane using an E-Z Anesthesia System (Euthanex Corp.), and ∼1 g of liver was removed and immediately homogenized in low-salt sucrose homogenization buffer [50 mM HEPES, pH 7.4; 75 mM KCl; 5 mM MgCl2; 250 mM sucrose; 10% Triton X-100; 13% sodium deoxycholate; 100 μg/mL cycloheximide; 2 mM dithiothreitol; 5 μL/mL RNaseOUT Recombinant Ribonuclease Inhibitor (Invitrogen)]. The homogenate was centrifuged for 10 min at 1,000 g at 4°C, and the supernatant was collected for analysis. All procedures were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee.
Cell Culture
H4IIE cells (CRL-1548; American Type Culture Collection) were plated on CellBIND dishes (No. 0720083; Costar) at ∼70% confluency and cultured with Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum at 37°C for 48 h. Where indicated, the culture medium was replaced with fresh medium containing either DMSO or 100 nM rapamycin. Cells were washed with PBS and collected in high-salt sucrose homogenization buffer (50 mM HEPES, pH 7.4; 250 mM KCl; 5 mM MgCl2; 250 mM sucrose; 10% Triton X-100; 13% sodium deoxycholate; and 5 μL/mL RNaseOUT). For sucrose density gradient analysis, cycloheximide (A.G. Scientific) was added to the medium at a final concentration of 100 μg/mL 10 min before harvest. In addition, the wash buffer and homogenization buffers were also supplemented with 100 μg/mL cycloheximide.
Polysome Fractionation by Sucrose Density Gradient Centrifugation and RNA Isolation
Sucrose density gradient centrifugation was used to separate the subpolysomal from the polysomal ribosome fractions as described previously (11). Sucrose fractions representing the light (≤3 ribosomes) and heavy (≥4 ribosomes) portions of the gradient were collected directly into an equal volume of TRIzol reagent (Invitrogen). To improve the recovery of RNA from dense sucrose portion of the gradient, the heavy fraction was diluted with an equal volume of RNase-free water (HyClone), and the appropriate amount of TRIzol was added. RNA was extracted, precipitated with isopropanol, and resuspended in 14 μL of RNA storage solution (Ambion). An equal amount of RNA from each fraction collected was converted into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and subjected to quantitative real-time PCR using QuantiTect SYBR Green Master Mix (Qiagen). Primers for RBP4 were as follows: forward, 5′-TCTGCCTAGAGAGGCAGTACA-3′; reverse, 5′-AACTGTTTCTTGAGGGTCTGCT-3′. Actin primers were purchased from Qiagen (No. QT00193473).
Western Blot Analysis
Blood was collected into tubes containing EDTA and centrifuged at 1000 g for 5 min at 4°C. Livers were extracted, flash frozen between aluminum blocks cooled in liquid nitrogen, and later homogenized in 250 μL of extraction buffer as described previously (11). The homogenate was centrifuged at 1,000 g for 5 min at 4°C, and the supernatant was collected for analysis. A fraction of supernatant was added to SDS sample buffer, boiled for 5 min, and analyzed by Western blot as described previously (12). Antibodies used included RBP4 (Abcam Cat. No. ab109193), eIF4E (Cell Signaling Technology, Cat. No. 2067), 4E-BP1 (Cell Signaling Technology, Cat. No. 9644), phospho-T389 S6K1 (Cell Signaling Technology, Cat. No. 97596), phospho-Ser65 4E-BP1 (Cell Signaling Technology, Cat. No. 9205), albumin (Cell Signaling Technology, Cat. No. 4929), actin (Cell Signaling Technology, Cat. No. 94970), and GAPDH (Santa Cruz, Cat. No. sc-32233). Immunoprecipitations were performed by incubating cell lysates with monoclonal anti-eIF4E antibody as described previously (13).
RBP4 Luciferase Reporter Assay
The 5′-untranslated region (UTR) of human Rbp4 was generated de novo using two overlapping oligonucleotides: RBP4 HindIII Forward (5′-gcaagcttcgcctccctcgctccacgcgcgcccggactcggcggccaggcttgcgcgcgg-3′) and RBP4 NcoI Reverse (5′-agtccatggcttgcccaggaatccgcccaccgggaggggaaccgcgcgcaagcctggcc-3′). The resulting PCR product was cloned into a pGL3 firefly luciferase reporter vector (Promega) using HindIII and NcoI (New England Biolabs) and propagated in XL-10 Gold ultracompetent cells (Agilent). The final pGL3-RBP4 5′-UTR reporter plasmid was sequence verified. The start codon of the luciferase coding sequence is located in the NcoI restriction site that immediately follows the entire RBP4 5′-UTR sequence. A mutant pGL3-RBP4 5′-UTR reporter plasmid wherein the pyrimidine tract of the TOP-motif was disrupted by purine substitutions was generated using QuikChange Lightning (Agilent) and the following primers: 5′-gcgcgtggagcgacggtggcgaagcttttt-3′ and 5′-aaaaagcttcgccaccgtcgctccacgcgc-3′. H4IIE cells were cotransfected with 500 ng of pGL3-RBP4 5′-UTR and 20 ng of pRL-CMV (Promega) using jetPRIME (Polyplus) transfection reagent. After 24 h, luciferase activity was measured on a FlexStation3 (Molecular Devices) using a Dual-Luciferase Assay Kit (Promega). In some studies, cells were placed in fresh culture medium containing either rapamycin or vehicle control 4 h before being collected for luciferase assay. Alternatively, H4IIE cells were cotransfected with 500 ng of pGL3-RBP4 5′-UTR, 20 ng of pRL-CMV, and 500 ng of either hemagglutinin (HA)-tagged wild-type 4E-BP1 or the F113A 4E-BP1 variant.
Statistical Analysis
All statistical analysis was performed with GraphPad Prism (v. 8.2.0). A Student’s t test was used to compare differences between groups. Where appropriate, data were analyzed by two-way ANOVA with Dunnett’s post hoc analysis. Data are expressed as means ± SD. Significance was defined as P < 0.05 for all analyses.
RESULTS
Feeding Enhances Rbp4 mRNA Translation in Liver
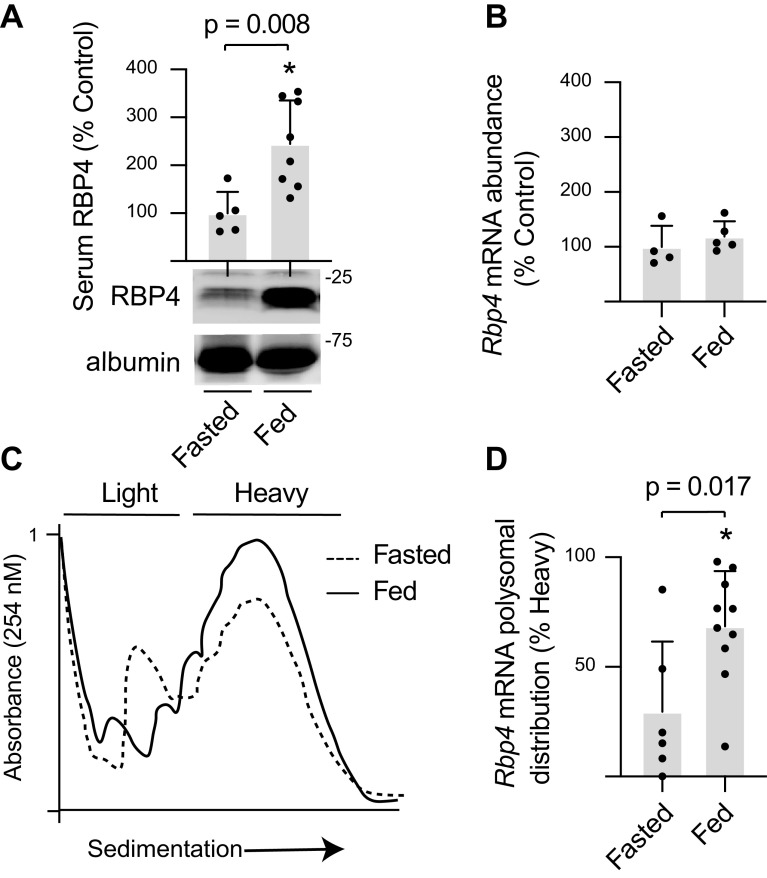
To evaluate the hepatic response to nutrients, mice were fasted overnight, and the next morning, some of the mice were refed. Serum RBP4 concentrations were increased in mice that were fed after an overnight fast (Fig. 1A). The liver is the principal source of serum RBP4 (14), yet hepatic Rbp4 mRNA abundance was similar in fasted and fed mice (Fig. 1B). To evaluate Rbp4 mRNA translation, the association of the mRNA with polysomes was assessed (Fig. 1C). Feeding promoted hepatic polysome aggregation, as indicated by an increased absorbance in the heavier sucrose density gradient fractions. In the liver of fasted mice, approximately a third of the Rbp4 mRNA was found at the top of the gradient, indicating poor ribosomal association (Fig. 1D). Upon feeding, Rbp4 mRNA abundance in the heavy polysome fraction was increased, as compared with fasted mice. The data support an increase in hepatic Rbp4 mRNA translation in response to feeding.
Figure 1.
Feeding increases serum RBP4 protein expression and promotes hepatic Rbp4 mRNA translation. A: mice were fasted overnight, and the next morning were either fed for 90 min or remained fasted. Serum RBP4 concentrations were evaluated by Western blotting. B: total Rbp4 mRNA abundance was evaluated by RT-PCR. C: hepatic ribosomes were fractionated by sucrose density gradient centrifugation. Representative tracings are shown. D: hepatic ribosomes were separated into heavy (4 or more ribosomes) or light (3 or fewer ribosomes) fractions, and RNA from each fraction was subjected to RT-PCR analysis. Values are means ± SD. *P < 0.05 versus fasted. RBP4, retinol-binding protein 4.
Refreshing the Medium on H4IIE Cell Cultures Promotes Rbp4 mRNA Translation
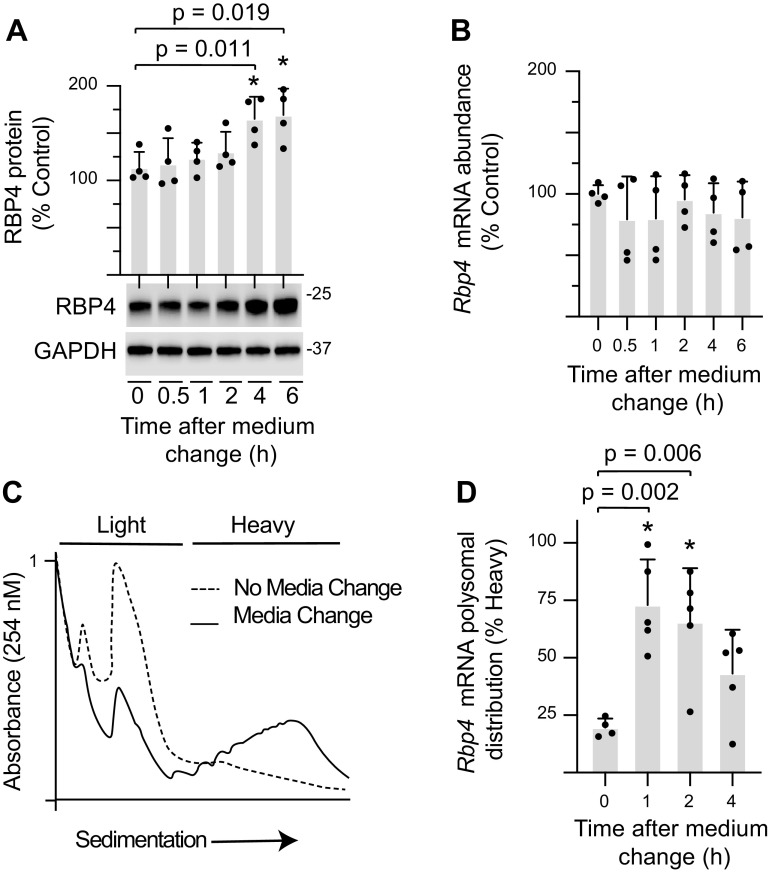
As an in vitro model of fasting/refeeding, H4IIE cells were maintained in culture for 2 days before changing the medium. Consistent with the effects of refeeding fasted mice, refreshing the culture medium enhanced RBP4 protein content (Fig. 2A). In addition, the increase in RBP4 protein was observed in the absence of an increase in Rbp4 mRNA abundance (Fig. 2B). Refreshing the culture medium promoted polysome assembly (Fig. 2C) and upregulated Rbp4 mRNA translation, as assessed by the proportion of message in the heavy sucrose density gradient fraction (Fig. 2D).
Figure 2.
Refreshing the medium on H4IIE cell cultures promotes Rbp4 mRNA translation. H4IIE cells were maintained in culture medium for 48 h before refreshing the medium. Cells were collected after exposure to the refreshed medium as indicated. A: RBP4 protein expression in whole cell lysate (WCL) was quantified by Western blot analysis. B: total Rbp4 mRNA abundance was evaluated in WCL by RT-PCR. C: cell lysates were analyzed by sucrose density gradient centrifugation. Representative tracings for no media change and 2 h after refreshing the medium are shown. D: Rbp4 translation was evaluated by comparing the abundance of Rbp4 mRNA in the heavy or light fractions by RT-PCR. Values are means ± SD. *P < 0.05 versus no medium refresh. RBP4, retinol-binding protein 4.
Activation of mTORC1 Is Necessary for Enhanced Rbp4 mRNA Translation in H4IIE Cells Exposed to Refreshed Culture Medium
Sequence analysis of the Rbp4 mRNA identified a TOP-like sequence (5′-cgcctccctcgctcc…), which has been previously associated with increased sensitivity to mTORC1 activation (9, 10). To evaluate a possible role for mTORC1 activation in the enhancement of Rbp4 mRNA translation, phosphorylation of ribosomal protein S6 kinase 1 (S6K1) on Thr389 and 4E-BP1 on Ser65 were evaluated in H4IIE cells after refreshing the culture medium. Refreshing the medium promoted rapid mTORC1 activation (Fig. 3A) and attenuated 4E-BP1 binding with eIF4E (Fig. 3B). To determine if mTORC1 activation was necessary for increased Rbp4 mRNA translation, the culture medium was refreshed with medium containing rapamycin. Rapamycin suppressed mTORC1 activation, as assessed by 4E-BP1 phosphorylation (Fig. 3C). Moreover, RBP4 protein content was reduced in cells exposed to rapamycin as compared with vehicle (Fig. 3D). The reduction in RBP4 protein content with rapamycin exposure was not associated with a change in Rbp4 mRNA abundance (Fig. 3E). The impact of rapamycin on ribosome aggregation in polysomes was modest (Fig. 3F). Nevertheless, Rbp4 mRNA translation was enhanced in cells incubated in refreshed medium supplemented with vehicle, whereas rapamycin prevented the effect (Fig. 3G). Additionally, there was a trend toward enhanced Rbp4 mRNA translation in cells exposed to refreshed medium supplemented with vehicle versus cells exposed to refreshed medium supplemented with rapamycin (P = 0.091).
Figure 3.
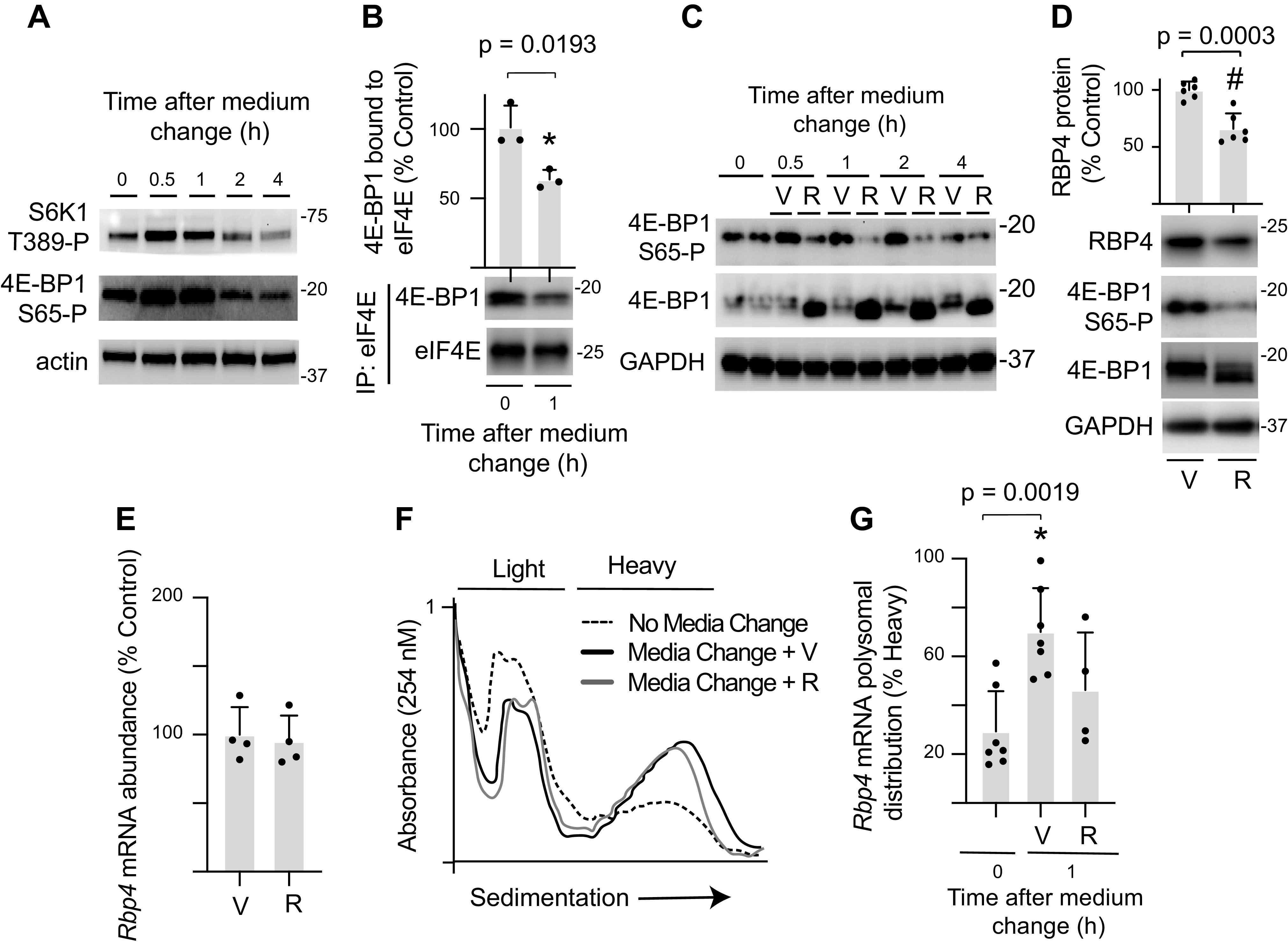
Enhanced Rbp4 mRNA translation in H4IIE cell cultures exposed to refreshed medium is mTORC1 dependent. H4IIE cells were maintained in culture medium for 48 h before refreshing the medium. Cells were collected after exposure to the refreshed medium as indicated. A: activation of mTORC1 was assessed by Western blot analysis of S6K1 phosphorylation at Thr389 and 4E-BP1 phosphorylation at Ser65 in WCL. Actin expression in WCL is also shown. B: the interaction of 4E-BP1 with eIF4E was evaluated by immunoprecipitating eIF4E from WCL and measuring the amount of 4E-BP1 in the immunoprecipitate (IP) by Western blotting. C–G: refreshed medium was supplemented with the mTORC1 inhibitor rapamycin (R) or vehicle (V). D: RBP4 protein, 4E-BP1 phosphorylation, and GAPDH expression were evaluated in WCL 4 h after medium refresh by Western blotting. E: total Rbp4 mRNA abundance 4 h after medium refresh was evaluated in WCL by RT-PCR. F: ribosomes were separated into heavy or light fractions by sucrose density gradient centrifugation. Representative tracings after no media change, 1 h after media change with V, or 1 h after media change with R are shown. G: Rbp4 mRNA translation was evaluated by comparing the abundance of Rbp4 mRNA in the heavy and light fractions. Values are means ± SD. *P < 0.05 versus no medium refresh; #P < 0.05 versus V. eIF4E, eukaryotic initiation factor 4E; mTORC1, mechanistic target of rapamycin in complex 1; RBP4, retinol-binding protein 4; WCL, whole cell lysate; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1.
Activity of the Rbp4 5′-UTR Is Repressed by 4E-BP1 Dephosphorylation
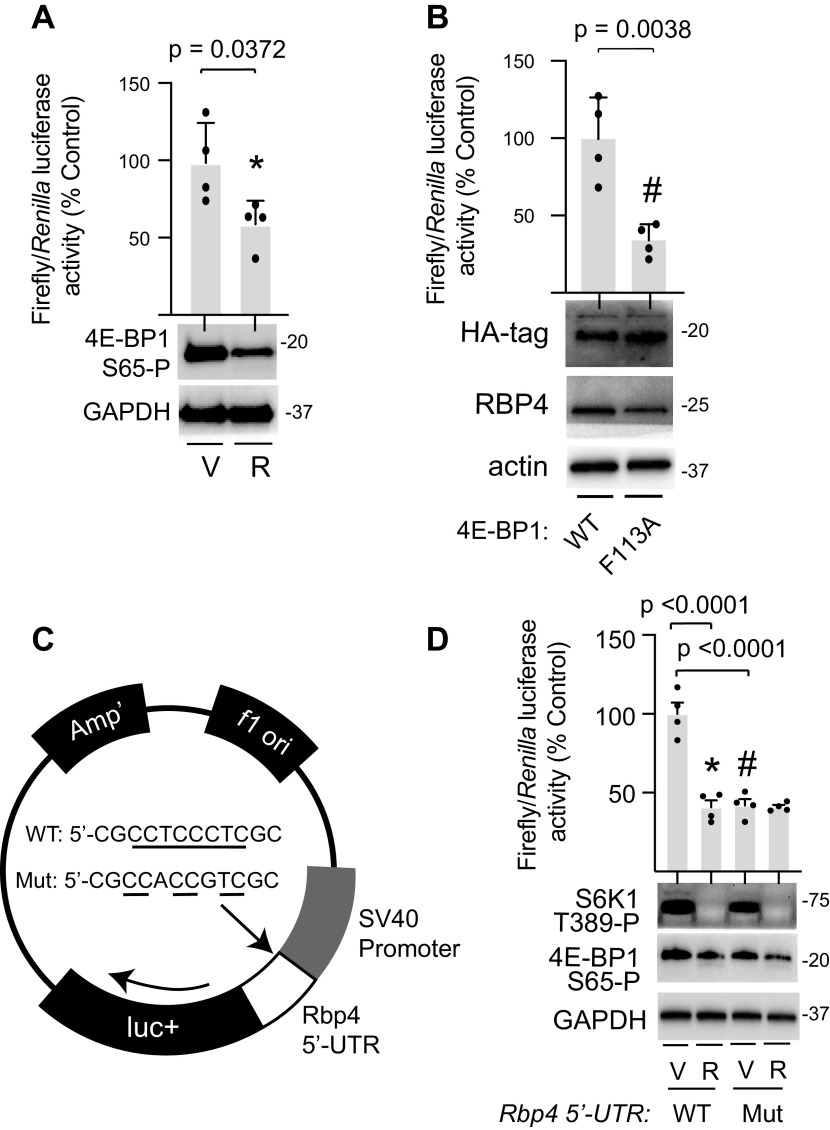
To evaluate the role of the Rbp4 5′-UTR in translational regulation, it was cloned into a luciferase reporter construct. RBP4 luciferase reporter activity was attenuated and 4E-BP1 phosphorylation was suppressed in H4IIE cells exposed to refreshed culture medium containing rapamycin, as compared with vehicle (Fig. 4A). The impact of mTOR suppression on TOP and TOP-like mRNA translation is largely mediated by 4E-BP1/2 phosphorylation (10). The 4E-BP1 F113A variant contains a disruption in the TOR signaling motif that renders it unable to be phosphorylated by mTORC1 (15). In H4IIE cells, 4E-BP1 F113A suppressed RBP4 luciferase activity as compared with wild-type 4E-BP1 (Fig. 4B). To evaluate the role of the TOP-like motif in regulation of Rbp4 mRNA translation, a mutant reporter was generated wherein the N-terminal stretch of eight pyrimidine residues was disrupted by substitution of two purine bases (Fig. 4C). As compared with the wild-type Rbp4 5′-UTR reporter, activity of the mutant reporter was attenuated in H4IIE cells cultured under conditions that activate mTORC1 (Fig. 4D). Importantly, rapamycin failed to suppress the activity of the Rbp4 5′-UTR reporter upon disruption of the TOP-like motif.
Figure 4.
Rbp4 5′-UTR activity is repressed by 4E-BP1 dephosphorylation. A firefly luciferase reporter was used to evaluate translational activity of the Rbp4 mRNA 5′-UTR. A: H4IIE cells were cotransfected with the wild-type (WT) Rbp4 5′-UTR firefly reporter and a control Renilla reporter. Cells were cultured in fresh medium containing either rapamycin (R) or a vehicle (V) for up to 4 h. B: H4IIE cells were cotransfected with the WT Rbp4 5′-UTR firefly reporter, control Renilla reporter, and either wild-type (WT) 4E-BP1 or a 4E-BP1 F113A variant that is unable to be phosphorylated by mTORC1. C: the stretch of eight pyrimidines within the TOP-like motif of the WT Rbp4 5′-UTR firefly reporter was disrupted by substitution of purine bases to generate a mutant (Mut) reporter. D: H4IIE cells were cotransfected with either WT or Mut Rbp4 5′-UTR firefly reporter and a control Renilla reporter and exposed to R as described in A. Relative luciferase expression was evaluated by a dual luciferase assay 4 h after medium refresh. Activation of mTORC1 was assessed 1 h after medium refresh by Western blot analysis of S6K1 phosphorylation at Thr389 and 4E-BP1 phosphorylation at Ser65 in whole cell lysates. GAPDH, HA-tagged 4E-BP1, RBP4, and actin expression were also evaluated in cell lysates by Western blot analysis. Values are means ± SD. *P < 0.05 versus V; #P < 0.05 versus WT. HA, hemagglutinin; mTORC1, mechanistic target of rapamycin in complex 1; RBP4, retinol-binding protein 4; TOP, terminal oligopyrimidine; UTR, untranslated region; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1.
Feeding-Induced Rbp4 mRNA Translation in the Liver Requires mTORC1 Activation
To reinforce the prior in vivo studies that were performed in mice and extend the analysis to determine the role of mTORC1 in feeding-induced Rbp4 mRNA translation in liver, rats were fasted overnight, administered either rapamycin or a vehicle control the next morning, and then refed. Phosphorylation of 4E-BP1 was assessed by gel shift, as the hypophosphorylated protein exhibits faster electrophoretic migration. As expected, 4E-BP1 phosphorylation was suppressed in the liver of rats treated with rapamycin, as compared with vehicle (Fig. 5A). Feeding increased serum RBP4 concentrations, and rapamycin prevented the effect (Fig. 5B). Hepatic Rbp4 mRNA abundance was not altered by feeding; however, a modest suppression was observed with rapamycin (Fig. 5C). Feeding promoted hepatic polysome aggregation as compared with fasted rats, and rapamycin had little effect (Fig. 5D). Rbp4 mRNA ribosome association was enhanced in the liver of fed rats, as compared with fasted rats (Fig. 5E). Rapamycin prevented the feeding effect, such that Rbp4 mRNA ribosome association in the liver of fed rats receiving rapamycin was attenuated as compared with fed rats receiving a vehicle. Overall, the findings are consistent with a model wherein nutrient-induced activation of mTORC1 promotes serum RBP4 concentrations by enhancing hepatic Rbp4 mRNA translation (Fig. 5F).
Figure 5.
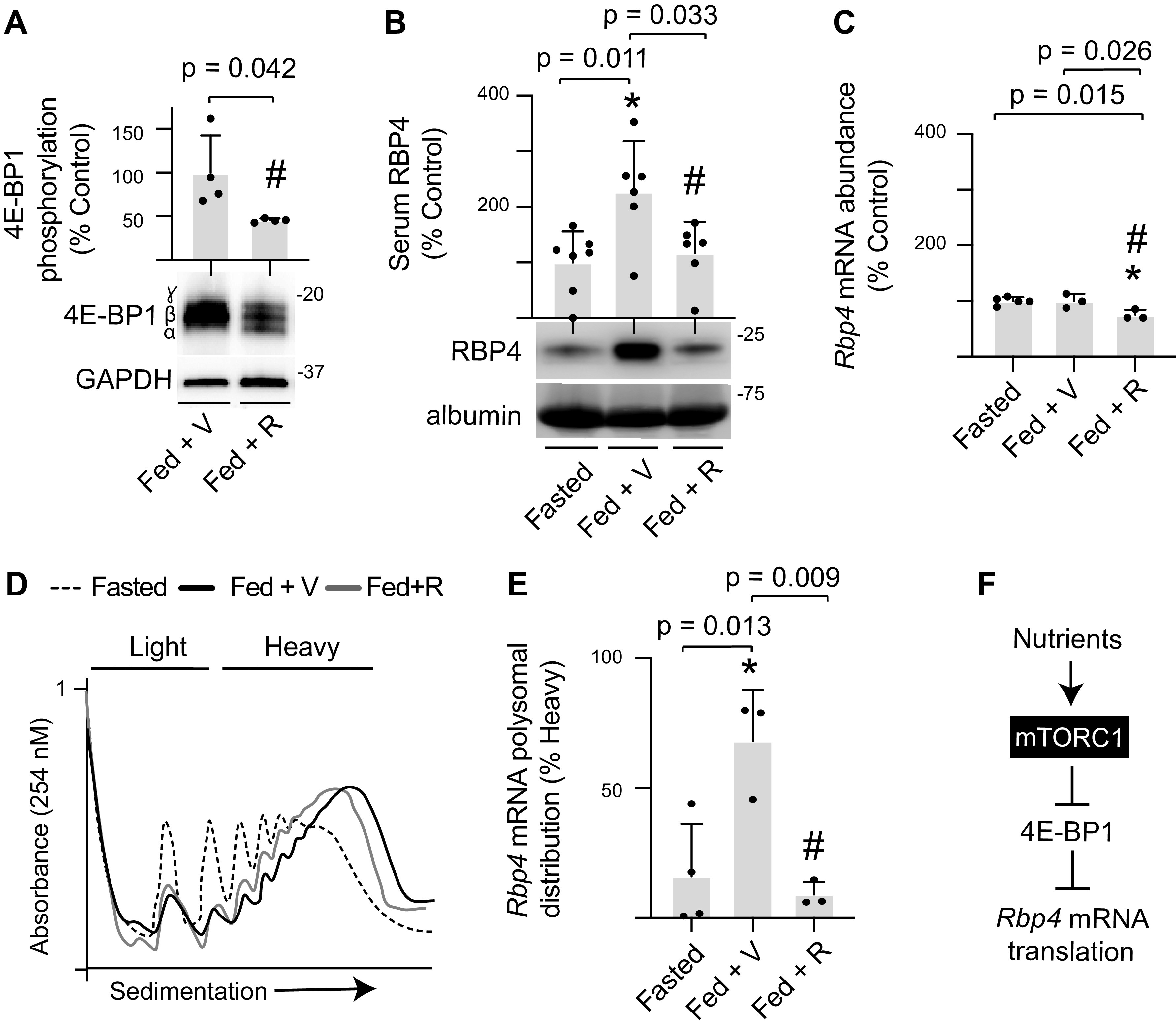
Feeding-induced serum RBP4 protein expression and hepatic Rbp4 mRNA translation are mTORC1 dependent. Rats were fasted overnight. The next morning, rapamycin (R) or a vehicle control (V) was administered by tail vein injection. One hour later, access to food was returned. All analyses were performed 6 h after the start of the feeding period. Hepatic 4E-BP1 phosphorylation (A) and serum RBP4 protein expression (B) were evaluated by Western blotting. Phosphorylation of 4E-BP1 was assessed as the proportion of the protein present in the upper γ and β-forms relative to the total amount of 4E-BP1 in all forms. C: total Rbp4 mRNA abundance in liver homogenates was evaluated by RT-PCR. D: hepatic ribosomes were separated into heavy or light fractions by sucrose density gradient centrifugation. Representative tracings are shown. E: Rbp4 mRNA translation was evaluated by comparing the abundance of Rbp4 mRNA in the heavy or light fractions. F: working model for nutrient-induced activation of Rbp4 mRNA translation. Values are means ± SD. *P < 0.05 versus fasted; #P < 0.05 versus fed + V. mTORC1, mechanistic target of rapamycin in complex 1; RBP4, retinol-binding protein 4; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1.
DISCUSSION
The synthesis of many secreted peptides is selectively regulated at the translational level in response to mitogenic and nutritional stimuli (16). Posttranscriptional regulation potentially allows for a rapid and readily reversible response to nutrients and growth factors. Since hepatocytes are the principal source of circulating RBP4 (14), we evaluated the possibility that hepatic RBP4 synthesis was influenced by translational control. We found that in the liver of fasted rodents, the Rbp4 mRNA was poorly translated, whereas feeding promoted hepatic Rbp4 mRNA translation and enhanced serum levels of the protein, independent of an increase in Rbp4 mRNA abundance. Similarly, refreshing culture medium increased RBP4 protein expression and promoted Rbp4 mRNA translation in H4IIE cell cultures. Together, the findings support translational upregulation of hepatic RBP4 production in response to nutrients.
The serine-threonine kinase mTOR is the catalytic subunit of two protein complexes known as mTORC1 and mTORC2 that are principally responsible for coordinating the cellular response to growth factors and nutrient sufficiency (17). In contrast to mTORC2, activation of mTORC1 is stimulated by nutrients. mTORC1 is also uniquely sensitive to acute inhibition by the well-known drug rapamycin, which has seen extensive clinical use (18). In the present study, refreshing culture medium enhanced 4E-BP1 phosphorylation, and rapamycin was sufficient to prevent the effect. This is consistent with the decrease in 4E-BP1 phosphorylation that was observed in the liver of rats that were administered rapamycin.
In response to feeding, global polysome aggregation was enhanced in the liver. Rapamycin had little effect on hepatic polysome aggregation, which is consistent with the prior report (19). Importantly, only a subset of mRNAs exhibit sensitivity to mTORC1 suppression with regard to their translation (10). Assembly of the eIF4F complex promotes eIF4E affinity for the 5′-cap of mRNAs and facilitates mRNA translation (20). In particular, mRNAs encoding TOP motifs are especially sensitive to changes in eIF4F assembly, a process that is controlled in part by 4E-BP1 (10). TOP motifs are defined as having a cytosine immediately after the 5′-cap, followed by an uninterrupted stretch of 4–14 pyrimidines (21). More recently, a relaxed TOP-like motif was identified based on translational sensitivity to mTOR suppression that was similar to mRNAs with sequences that strictly met the TOP definition (10). TOP-like motifs are defined as encoding a stretch of five or more pyrimidines within four nucleotides of the transcriptional start site. Sequence analysis of the Rbp4 mRNA identified a TOP-like sequence in its 5′-UTR that includes a cytosine immediately after the 5′-cap, which is proceeded by a guanine, and then eight contiguous pyrimidines. Disruption of this pyrimidine stretch by substitution of purine bases was sufficient to attenuate the activity of the Rbp4 5′-UTR in H4IIE cell cultures with active mTORC1 signaling. Rapamycin addition to suppress mTORC1 attenuated activity of the wild-type Rbp4 5′-UTR, and this effect required the TOP-like motif. Similarly, expression of the 4E-BP1 F113A variant that is unable to be phosphorylated by mTORC1 suppressed Rbp4 5′-UTR activity relative to wild-type 4E-BP1. Together, the findings support a regulatory role for the Rbp4 TOP-like sequence in RBP4 synthesis.
Despite hundreds of publications demonstrating strong correlations between serum RBP4 and metabolic disease, surprisingly few prior studies have investigated the mechanisms regulating RBP4 synthesis in hepatocytes or any other cell type. A single nucleotide polymorphism (-803 G > A) in Rbp4 promotes RBP4 production by relieving transcriptional repression of the gene by an unidentified DNA-binding protein (22). The peroxisome proliferator-activated receptor γ (PPARγ) activator pioglitazone reduces RBP4 serum levels in obese rats without altering hepatic Rbp4 mRNA abundance (23). The decline in serum RBP4 with pioglitazone is associated with transcriptional repression of Rbp4 mRNA abundance in adipose tissue; however, the contribution of adipose to serum RBP4 has more recently been questioned (14). Notably, PPARγ activation by pioglitazone also acts to suppress mTORC1 (24). Thus, an effect of PPARγ activation on hepatic Rbp4 mRNA translation may underlie the reduction in serum levels of the protein. It is important to note that in the present study rapamycin also suppressed hepatic Rbp4 mRNA abundance, but the effect was not observed in H4IIE cultures. This potential discrepancy may be explained by differences in the duration of mTORC1 suppression.
Our interest in Rbp4 mRNA translation originated from a recent investigation of transcriptome-wide variation in retinal mRNA translation in mice administered an O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) inhibitor (25). Diabetes promotes enzymatic O-GlcNAcylation of proteins in both the retina (26, 27) and liver (13, 28), and OGT catalyzes this posttranslational modification. In the prior study (25), Rbp4 was among the mRNAs most sensitive to variation translation, as it exhibited ∼16-fold change in ribosome association when O-GlcNAcylation was enhanced. To our knowledge, prior investigation of translational regulation as a mechanism for controlling RBP4 synthesis is completely absent from the literature. Yet, studies support that the cellular abundance of proteins is predominantly controlled at the level of mRNA translation (29). Together with the prior report (25), the studies herein demonstrate that RBP4 synthesis is particularly sensitive to translational control.
In addition to the metabolic consequences associated with elevated RBP4 levels, regulation of RBP4 synthesis is likely to also have important implications for understanding defects in vision. Retinol is stored in the liver, but must be delivered to the retina by RBP4 for the visual retinoid cycle leading to retinaldehyde and bisretinoid synthesis. A novel mutation in the Rbp4 gene that prevents RBP4 expression causes a common form of retinal degeneration known as retinitis pigmentosa (30). On the other hand, excessive retinol delivery is believed to be responsible for the accumulation of cytotoxic lipofuscin bisretinoid in the retinal pigment epithelium (RPE) (31). RPE lipofuscin accumulation contributes to degenerative eye diseases including macular degeneration, Stargardt’s disease, and diabetic retinopathy (32). For this reason, fenretinide and other nonretinoid antagonists of RBP4 have been pursued as therapeutic options to prevent lipofuscin bisretinoid accumulation without inhibiting the visual cycle (33, 34).
The studies herein did not investigate Rbp4 mRNA translation in the context of diabetes. However, it is tempting to speculate that overnutrition or chronic nutrient intake may lead to increased hepatic Rbp4 mRNA translation in this context. In type 2 diabetes and obesity, serum RBP4 levels are consistently increased (2, 3), and hepatocytes are believed to be the principal source of circulating RBP4 (14). Notably, a change in hepatic Rbp4 mRNA abundance does not appear to be responsible for the effect (2). However, mTORC1 signaling is enhanced in the liver of obese rats (35). Mice deficient for 4E-BP1/2 also exhibit increased sensitivity to diet-induced obesity, characterized by accelerated adipogenesis and insulin resistance (36). Thus, it will be important for future studies to evaluate Rbp4 mRNA translation in the context of metabolic disease, as targeting Rbp4 translational regulation may represent an alternative to therapeutics that promote urinary excretion of the protein.
A number of extrahepatic tissues including the eye, brain, and adipose also express RBP4. In particular, evidence supports a critical role for enhanced adipocyte RBP4 production in the development of insulin resistance (2, 3, 6, 37). In fact, RBP4 was originally described as an adipokine (2). Rbp4 mRNA in adipose tissue of obese subjects is reportedly increased relative to lean subjects (37); however, a suppressive effect of obesity on adipose Rbp4 mRNA abundance has also been reported in women (38). In rodents, adipose tissue Rbp4 mRNA abundance is enhanced in adipocyte-specific Glut4-knockout mice, whereas adipose Rbp4 mRNA levels (expressed as transcript per gram of tissue RNA) are decreased by 40%–50% in both ob/ob mice and mice fed a high-fat diet (2). Thus, there exists a potential role for mTORC1-dependent translational regulation in pathological adipose RBP4 production. Indeed, mTORC1 signaling is enhanced in adipose tissue of ob/ob mice, as well as wild-type mice fed a high-fat diet (39). This idea is conceptually consistent with a large body of work that supports a key role for overactivation of mTORC1 in the development of metabolic syndrome. An improved understanding of how diabetes and obesity influence Rbp4 mRNA translation in adipose versus hepatic tissues may provide critical new information to better understand the role of RBP4 in pathophysiological conditions.
The studies herein provide evidence for translational regulation of Rbp4 mRNA in the liver. Feeding fasted rodents promoted hepatic Rbp4 mRNA translation, and suppression of mTORC1 with rapamycin prevented the effect. Overall, the findings support a model wherein nutrient-induced activation of mTORC1 promotes Rbp4 mRNA translation via a TOP-like motif in its 5′-UTR. The findings also support that variation in Rbp4 mRNA translation should be considered in the design of therapeutic approaches to restrict RBP4 production.
GRANTS
This research was supported by the American Diabetes Association Pathway to Stop Diabetes Grant 1-14-INI-04 and National Institutes of Health Grants R01 EY029702 (to M. D. Dennis) and R01 DK13499 (to S. R. Kimball).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.W., S.S., S.R.K., and M.D.D. conceived and designed research; J.E.W., A.L.T., S.S., S.A.S., C.J.P., and S.R.K. performed experiments; J.E.W., A.L.T., S.S., S.R.K., and M.D.D. analyzed data; J.E.W., A.L.T., S.S., S.R.K., and M.D.D. interpreted results of experiments; J.E.W., S.S., S.R.K., and M.D.D. prepared figures; M.D.D. drafted manuscript; J.E.W., A.L.T., S.S., S.R.K., and M.D.D. edited and revised manuscript; J.E.W., A.L.T., S.S., S.A.S., C.J.P., S.R.K., and M.D.D. approved final version of manuscript.
REFERENCES
- 1.Blaner WS. Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev 10: 308–316, 1989. doi: 10.1210/edrv-10-3-308. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356–362, 2005. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 3.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354: 2552–2563, 2006. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 4.Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M. Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res 49: 6149–6152, 1989. [PubMed] [Google Scholar]
- 5.Fedders R, Muenzner M, Weber P, Sommerfeld M, Knauer M, Kedziora S, Kast N, Heidenreich S, Raila J, Weger S, Henze A, Schupp M. Liver-secreted RBP4 does not impair glucose homeostasis in mice. J Biol Chem 293: 15269–15276, 2018. doi: 10.1074/jbc.RA118.004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S-A, Yuen JJ, Jiang H, Kahn BB, Blaner WS. Specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 64: 1534–1546, 2016. doi: 10.1002/hep.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu XE, Swanda RV, Qian S-B. Nutrient control of mRNA translation. Annu Rev Nutr 40: 51–75, 2020. doi: 10.1146/annurev-nutr-120919-041411. [DOI] [PubMed] [Google Scholar]
- 8.Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU. Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci USA 98: 7029–7036, 2001. doi: 10.1073/pnas.111145798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, Sonenberg N. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci USA 109: 8977–8982, 2012. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485: 109–113, 2012. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welles JE, Dennis MD, Jefferson LS, Kimball SR. Glucagon-dependent suppression of mTORC1 is associated with upregulation of hepatic FGF21 mRNA translation. Am J Physiol Endocrinol Metab 319: E26–E33, 2020. doi: 10.1152/ajpendo.00555.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller WP, Ravi S, Martin TD, Kimball SR, Dennis MD. Activation of the stress response kinase JNK (c-Jun N-terminal kinase) attenuates insulin action in retina through a p70S6K1-dependent mechanism. J Biol Chem 292: 1591–1602, 2017. doi: 10.1074/jbc.M116.760868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis MD, Shenberger JS, Stanley BA, Kimball SR, Jefferson LS. Hyperglycemia mediates a shift from cap-dependent to cap-independent translation via a 4E-BP1-dependent mechanism. Diabetes 62: 2204–2214, 2013. doi: 10.2337/db12-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson SJ, Sargsyan A, Lee SA, Yuen JJ, Cai J, Smalling R, Ghyselinck N, Mark M, Blaner WS, Graham TE. Hepatocytes are the principal source of circulating RBP4 in mice. Diabetes 66: 58–63, 2017. doi: 10.2337/db16-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol 13: 797–806, 2003. doi: 10.1016/S0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 16.Karamyshev AL, Tikhonova EB, Karamysheva ZN. Translational control of secretory proteins in health and disease. Int J Mol Sci 21: 2538, 2020. doi: 10.3390/ijms21072538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol 21: 63–71, 2019. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab 19: 373–379, 2014. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol 36: 2169–2179, 2004. doi: 10.1016/j.biocel.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Ptushkina M, von der Haar T, Vasilescu S, Frank R, Birkenhager R, McCarthy JE. Cooperative modulation by eIF4G of eIF4E-binding to the mRNA 5' cap in yeast involves a site partially shared by p20. Embo J 17: 4798–4808, 1998. doi: 10.1093/emboj/17.16.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci USA 91: 4441–4445, 1994. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munkhtulga L, Nagashima S, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Zolzaya K, Lkhagvasuren T, Kagawa Y, Fujiwara H, Hosoya Y, Hyodo M, Horie H, Kojima M, Ishibashi S, Iwamoto S. Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity 18: 1006–1014, 2010. doi: 10.1038/oby.2009.358. [DOI] [PubMed] [Google Scholar]
- 23.Zhu C, Xiao Y, Liu X, Han J, Zhang J, Wei L, Jia W. Pioglitazone lowers serum retinol binding protein 4 by suppressing its expression in adipose tissue of obese rats. Cell Physiol Biochem 35: 778–788, 2015. doi: 10.1159/000369737. [DOI] [PubMed] [Google Scholar]
- 24.San YZ, Liu Y, Zhang Y, Shi PP, Zhu YL. Peroxisome proliferator-activated receptor-gamma agonist inhibits the mammalian target of rapamycin signaling pathway and has a protective effect in a rat model of status epilepticus. Mol Med Rep 12: 1877–1883, 2015. doi: 10.3892/mmr.2015.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dierschke SK, Miller WP, Favate JS, Shah P, Imamura Kawasawa Y, Salzberg AC, Kimball SR, Jefferson LS, Dennis MD. O-GlcNAcylation alters the selection of mRNAs for translation and promotes 4E-BP1-dependent mitochondrial dysfunction in the retina. J Biol Chem 294: 5508–5520, 2019. doi: 10.1074/jbc.RA119.007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai W, Dierschke SK, Toro AL, Dennis MD. Consumption of a high fat diet promotes protein O-GlcNAcylation in mouse retina via NR4A1-dependent GFAT2 expression. Biochim Biophys Acta Mol Basis Dis 1864: 3568–3576, 2018. doi: 10.1016/j.bbadis.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dierschke SK, Toro AL, Miller WP, Sunilkumar S, Dennis MD. Diabetes enhances translation of Cd40 mRNA in murine retinal Muller glia via a 4E-BP1/2-dependent mechanism. J Biol Chem 295: 10831–10841, 2020. doi: 10.1074/jbc.RA120.013711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis MD, Schrufer TL, Bronson SK, Kimball SR, Jefferson LS. Hyperglycemia-induced O-GlcNAcylation and truncation of 4E-BP1 protein in liver of a mouse model of type 1 diabetes. J Biol Chem 286: 34286–34297, 2011. doi: 10.1074/jbc.M111.259457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature 473: 337–342, 2011. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 30.Cukras C, Gaasterland T, Lee P, Gudiseva HV, Chavali VR, Pullakhandam R, Maranhao B, Edsall L, Soares S, Reddy GB, Sieving PA, Ayyagari R. Exome analysis identified a novel mutation in the RBP4 gene in a consanguineous pedigree with retinal dystrophy and developmental abnormalities. PLoS One 7: e50205, 2012. doi: 10.1371/journal.pone.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wassell J, Boulton M. A role for vitamin A in the formation of ocular lipofuscin. Br J Ophthalmol 81: 911–918, 1997. doi: 10.1136/bjo.81.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye 9: 763–771, 1995. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 33.Racz B, Varadi A, Kong J, Allikmets R, Pearson PG, Johnson G, Cioffi CL, Petrukhin K. A non-retinoid antagonist of retinol-binding protein 4 rescues phenotype in a model of Stargardt disease without inhibiting the visual cycle. J Biol Chem 293: 11574–11588, 2018. doi: 10.1074/jbc.RA118.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci 46: 4393–4401, 2005. [Erratum in Invest Ophthalmol Vis Sci 47: 3735, 2006]. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 35.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146: 1473–1481, 2005. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 36.Le Bacquer O, Petroulakis E, Paglialunga S, Poulin F, Richard D, Cianflone K, Sonenberg N. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest 117: 387–396, 2007. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 6: 79–87, 2007. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes 55: 2805–2810, 2006. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 39.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205, 2004. [Erratum in Nature 431: 485, 2004]. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]