Keywords: β-cell function, insulin secretion, obesity, Roux-en-Y gastric bypass, type 2 diabetes
Abstract
Reductions in β-cell number and function contribute to the onset type 2 diabetes (T2D). Roux-en-Y gastric bypass (RYGB) surgery can resolve T2D within days of operation, indicating a weight-independent mechanism of glycemic control. We hypothesized that RYGB normalizes glucose homeostasis by restoring β-cell structure and function. Male Zucker Diabetic Fatty (fa/fa; ZDF) rats were randomized to sham surgery (n = 16), RYGB surgery (n = 16), or pair feeding (n = 16). Age-matched lean (fa/+) rats (n = 8) were included as a secondary control. Postprandial metabolism was assessed by oral glucose tolerance testing before and 27 days after surgery. Fasting and postprandial plasma GLP-1 was determined by mixed meal tolerance testing. Fasting plasma glucagon was also measured. β-cell function was determined in isolated islets by a glucose-stimulated insulin secretion assay. Insulin and glucagon positive areas were evaluated in pancreatic sections by immunohistochemistry. RYGB reduced body weight (P < 0.05) and improved glucose tolerance (P < 0.05) compared with sham surgery. RYGB reduced fasting glucose compared with both sham (P < 0.01) and pair-fed controls (P < 0.01). Postprandial GLP-1 (P < 0.05) was elevated after RYGB compared with sham surgery. RYGB islets stimulated with 20 mM glucose had higher insulin secretion than both sham and pair-fed controls (P < 0.01) and did not differ from lean controls. Insulin content was greater after RYGB compared with the sham (P < 0.05) and pair-fed (P < 0.05) controls. RYGB improves insulin secretion and pancreatic islet function, which may contribute to the remission of type 2 diabetes following bariatric surgery.
NEW & NOTEWORTHY The onset and progression of type 2 diabetes (T2D) results from failure to secrete sufficient amounts of insulin to overcome peripheral insulin resistance. Here, we demonstrate that Roux-en-Y gastric bypass (RYGB) restores islet function and morphology compared to sham and pair-fed controls in ZDF rats. The improvements in islet function were largely attributable to enhanced insulin content and secretory function in response to glucose stimulation.
INTRODUCTION
The onset and progression of type 2 diabetes (T2D) results from failure to secrete sufficient amounts of insulin to overcome peripheral insulin resistance. Current lifestyle, behavioral, and pharmacological therapeutic strategies can improve glycemic control but require lifelong adherence and typically lack durability. There is now substantial level 1 evidence that metabolic surgeries, including Roux-en-Y gastric bypass (RYGB), improve glycemic control and result in a greater number of patients in diabetes remission (HbA1c ≤ 6.0%, off all diabetes medications) up to 5 years after surgery compared to conventional lifestyle and medical therapies (1, 2). Calorie restriction and durable weight loss contribute immensely to the normalization of glycemia following RYGB (3). However, fasting glucose can normalize within days of surgery where no weight loss has been observed (4), supporting a surgical-derived mechanism of remission. Several hypotheses have been put forth to explain the surgical contribution to glycemic control, including improved insulin sensitivity, altered gut hormone signaling, and elevated insulin secretion (5–9). Improved peripheral insulin sensitivity is an attractive idea, but skeletal muscle glucose uptake does not improve in the early postoperative period (10). We and others have shown that RYGB can alter gut hormone signaling and enhance insulin secretion (11, 12). However, it remains unclear if remodeling of pancreatic islet function and structure contributes to enhanced secretory function. Therefore, the aim of this study was to determine the structural and functional changes in pancreatic islets following RYGB.
METHODS
Animal Care and Surgery
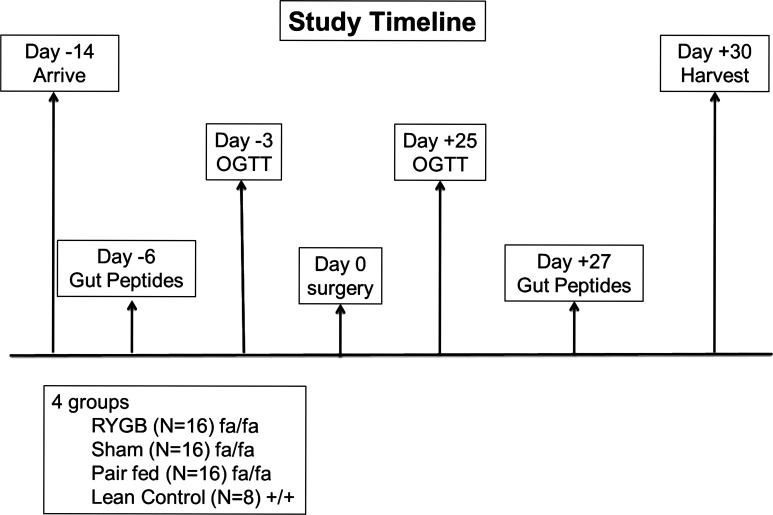
The protocol was approved and performed in compliance with the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC). Adult male Zucker Diabetic Fatty (fa/fa, n = 48) and lean (fa/+, n = 8) rats were purchased from Charles River Laboratories. The animals were received at 12 wk of age and housed in individual cages. Rats were kept at a constant temperature and ambient humidity in a 12-h light/dark cycle. Preoperatively, the rats had free access to tap water and were fed an ad libitum chow diet (Purina 5008; 56.5% carbohydrate, 26.5% protein, and 17% fat). After a 2-wk acclimatization period the animals were randomly assigned to sham surgery (n = 16), RYGB surgery (n = 16), or pair feeding (pair fed, n = 16). Age-matched lean (fa/+) controls (n = 8) were included for comparison (Fig. 1).
Figure 1.
Schematic illustration of study design and timeline. RYGB, Roux-en-Y gastric bypass; OGTT, oral glucose tolerance test.
Animals undergoing surgery were fasted overnight (∼12 h). Ceftriaxone (75 mg/kg) was administered intramuscularly for prophylaxis; an isoflurane gas chamber was used for anesthesia induction, which was switched to nose cone flow for maintenance of anesthesia during the procedure. We used the gastric bypass model and bowel limb lengths as previously described (13) to achieve durable weight loss. The rats were maintained on an ad libitum liquid diet with Boost (Nestle, Buffalo Grove, IL) for up to 7 days after surgery. Pain was controlled with subcutaneous buprenorphine for the first 2 days after surgery. Animals were monitored daily by a licensed veterinarian for signs of pain or distress. RYGB- and sham-treated rats were fed ad libitum postoperatively, whereas pair-fed rats were calorie restricted to match the consumption rates of the RYGB rats. The lean control group was fed a normal chow diet ad libitum. All rats were euthanized on postoperative day 30.
Oral Glucose Tolerance Test
A fasting glucose tolerance test (GTT) was performed preoperatively and 21 days postoperatively as previously described (12). Briefly, after an oral gavage of a 3.0 mg/kg glucose solution, glucose levels were measured via tail vain puncture at baseline and at 10, 30, 60, and 120 min after gavage. Data are presented as glucose concentrations (mg/dL) over time and area under the curve (AUC). Insulin sensitivity was estimated by the homeostatic model assessment of insulin resistance (HOMA-IR) using the following equation: fasting glucose (mg/dL) × fasting insulin (µU/mL)/405.
Mixed Meal Tolerance Test and Gut Peptide Analysis
A fasting mixed meal tolerance test (MMTT) was performed preoperatively and repeated on postoperative day 25. A baseline blood sample was collected from the femoral vein, which was exposed using a cut-down technique after rats were anesthetized using isoflurane gas. After the animal emerged from anesthesia, a 2.4 mL/kg liquid meal, containing 45 g of carbohydrate, 14 g of fat, and 14 g of protein (Boost - 360 kcal, 237 mL), was given by oral gavage. Blood samples were centrifuged at 4°C for 10 min, and the plasma was stored at −80°C for subsequent analysis.
Plasma Hormone Analysis
Blood samples obtained during the MMTT were analyzed for GLP-1 using a commercially available rat gut hormone multiplex panel (Bio-Rad Laboratories, Hercules, CA). Plasma insulin was determined using a commercially available enzyme-linked immunosorbent assay kit for rat plasma (Linco Research, St. Charles, MO). Plasma glucagon was assessed using a commercially available enzyme-linked immunosorbent assay kit for rat plasma (Mercodia AB, Uppsala, Sweden).
Islet Isolation
Pancreatic islet isolation was performed on postoperative day 30. Islets were isolated by collagenase digestion as previously described (6). Briefly, the common bile duct was cannulated proximally with a 26-gauge needle and 20 mL of 0.2% collagenase (Sigma-Aldridge, St. Louis, MO) was infused. With retrograde flow into the pancreatic duct, the pancreas was inflated and then excised. The inflated pancreas was then incubated at 37°C for 30–35 min and shaken vigorously. Islets were separated from acinar tissue after a series of washes with 10% fetal bovine serum in Roswell Park Memorial Institute medium (Gibco, Carlsbad, CA). The islets were then manually picked under a dissecting microscope.
Glucose-Stimulated Insulin Secretion
Following isolation, islets were cultured overnight in Roswell Park Memorial Institute medium (Gibco) containing fetal bovine serum, Pen-Strep, and 11 mM glucose. The islets were preincubated in oxygenated Krebs–Ringer bicarbonate buffer [1 mM nicotinamide, 1.2 mM KH2PO4, 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, and 0.25% radioimmunoassay-grade bovine serum albumin (BSA)] supplemented with 2 mM glucose for 1 h at 37°C. Islets of similar size were handpicked into groups of 10 islets in triplicate and incubated with 1 mL of Krebs–Ringer bicarbonate buffer containing either 2 mM glucose or 20 mM glucose for 1 h at 37°C. The insulin concentration from the medium was subsequently determined using a rat insulin enzyme-linked immunosorbent assay kit (ALPCO, Salem, NH).
Immunohistochemistry
Immunohistochemical analysis of β- and α-positive cells was performed in paraffin-embedded pancreatic sections using insulin (Cat. No. ab7842, Abcam) and glucagon antibodies (Cat. No. ab10988, Abcam) counterstained with 4′,6-diamidino-2-phenylindole (Cat. No. ab228549, Abcam). Insulin and glucagon contents were determined by relative florescent intensity using the average of three sections per animal expressed as percent area of the total image field using Image J software (NIH).
Statistical Analysis
Main effects of treatment were assessed by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons. Data are reported as means ± standard error of the mean (SEM) unless otherwise denoted in the figure legend. Prism 8 software (GraphPad, San Diego) was used for statistical testing. Animals were randomized 1:1:1 by an independent biostatistician and in a blinded fashion. Normality of the models was assessed by Kolmogorov–Smirnov and D’Agostino–Pearson test where appropriate. Significance was accepted as P < 0.05.
RESULTS
RYGB Restores Insulin Sensitivity and Resolves Hyperglycemia
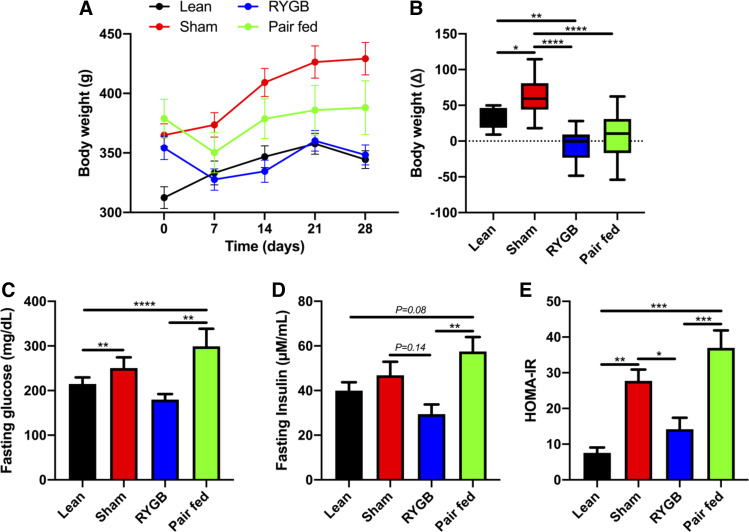
Post surgery, the RYGB group weighed significantly less than the sham group (P < 0.001) starting at day 14 and continuing through the duration of the study (Fig. 2A). The sham group also weighed significantly more than the lean control group (P < 0.05) starting at day 14 and continuing through the duration of the study (Fig. 2A). Both RYGB and pair-fed rats were equally protected from weight gain throughout the study period (Fig. 2B). Sham and pair-fed rats displayed increased fasting glucose and insulin compared with both lean and RYGB groups (Fig. 2, C and D). Based on HOMA-IR, RYGB improved insulin sensitivity compared with both sham and pair-fed controls and reached a level that was comparable to lean rats (Fig. 2E).
Figure 2.
RYGB surgery reduces bodyweight and improves insulin sensitivity in obese rats. Body weight over time (A) and change from pre-post treatment in lean, pair-fed (PF), sham-, and RYGB-treated rats (B). Fasting glucose (C), insulin (D), and insulin sensitivity assessed by HOMA-IR (E). Lean n = 8 rats, Sham n = 16 rats, RYGB n = 16 rats, Pair-fed n = 13 rats. Data are shown as the means ± SE with exception to B which is displayed as a box (means ± 5-95% CI) and whiskers (minimum to maximum). B, C, D, and E were assessed by one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.01, ****P < 0.001. RYGB, Roux-en-Y gastric bypass; HOMA-IR, homeostatic model assessment of insulin resistance.
RYGB Improves Glucose Tolerance
Preoperatively, there were no differences between sham, pair-fed, and RYGB rats, and all of these groups displayed reduced glucose tolerance compared with lean control rats (Fig. 3, A–C). Postoperatively, RYGB improved glucose tolerance, unlike both sham and pair feeding, which continued to worsen (Fig. 3, B–E). Sham, pair-fed, and RYGB rats all remained glucose intolerant compared with lean controls (Fig. 3D).
Figure 3.
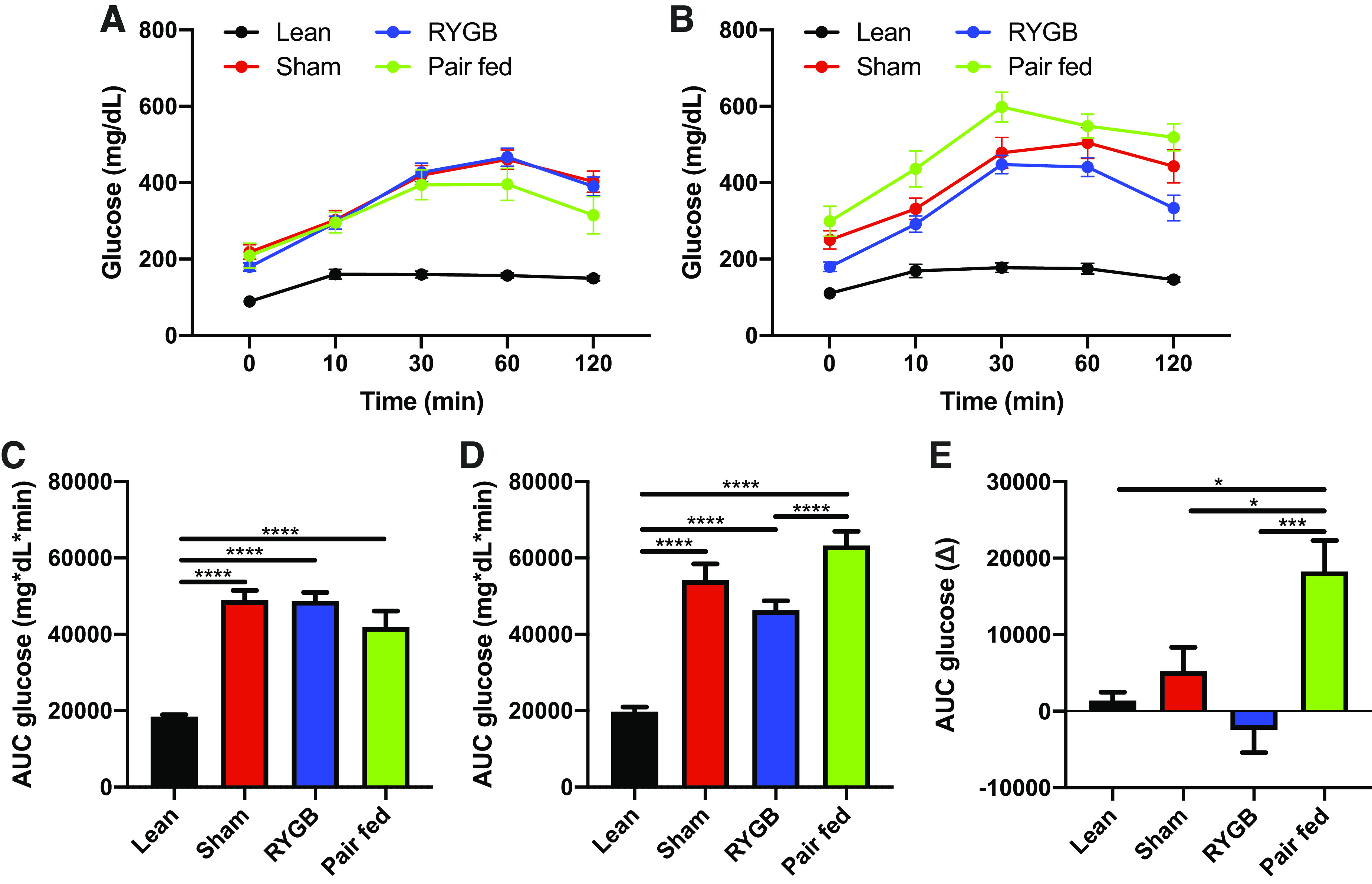
RYGB improves oral glucose tolerance. Responses to a 3.0 mg/kg gavage of glucose on day 0 (A) and day 25 (B) in lean, pair- fed (PF), sham-, and RYGB-treated rats. AUC glucose on day 0 (C), day 25 (D), and the change from day 0–25 (E). Lean n = 8 rats, Sham n = 16 rats, RYGB n = 16 rats, Pair-fed n = 13 rats. Data are shown as the means ± SE. C, D, and E were assessed by one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, ***P < 0.01, ****P < 0.001. AUC, area under the curve; RYGB, Roux-en-Y gastric bypass.
Pancreatic Islet Structure and Function
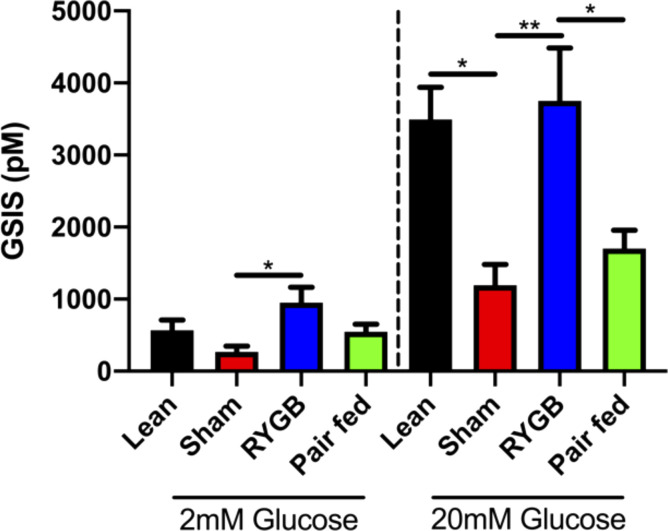
Insulin positive area was significantly greater in RYGB rats compared with both sham and pair-fed groups (Fig. 4, A and B). The increase in insulin content appeared to be the result of a larger and more integrated collection of β-cells within the islet (Fig. 4A). The glucagon positive area did not differ among treatments (Fig. 4C). Using a static-well glucose-stimulated insulin secretion assay we then assessed insulin secretory capacity of isolated islets. Under low glucose conditions, the RYGB islets secreted more insulin compared with the sham group (P < 0.01) (Fig. 5). When using the 20 mM glucose solution we found that the RYGB group secreted significantly more insulin than the sham (P < 0.05) and the pair-fed (P < 0.01) groups, and the response started to resemble the insulin secretion response of the lean group (Fig. 5).
Figure 4.
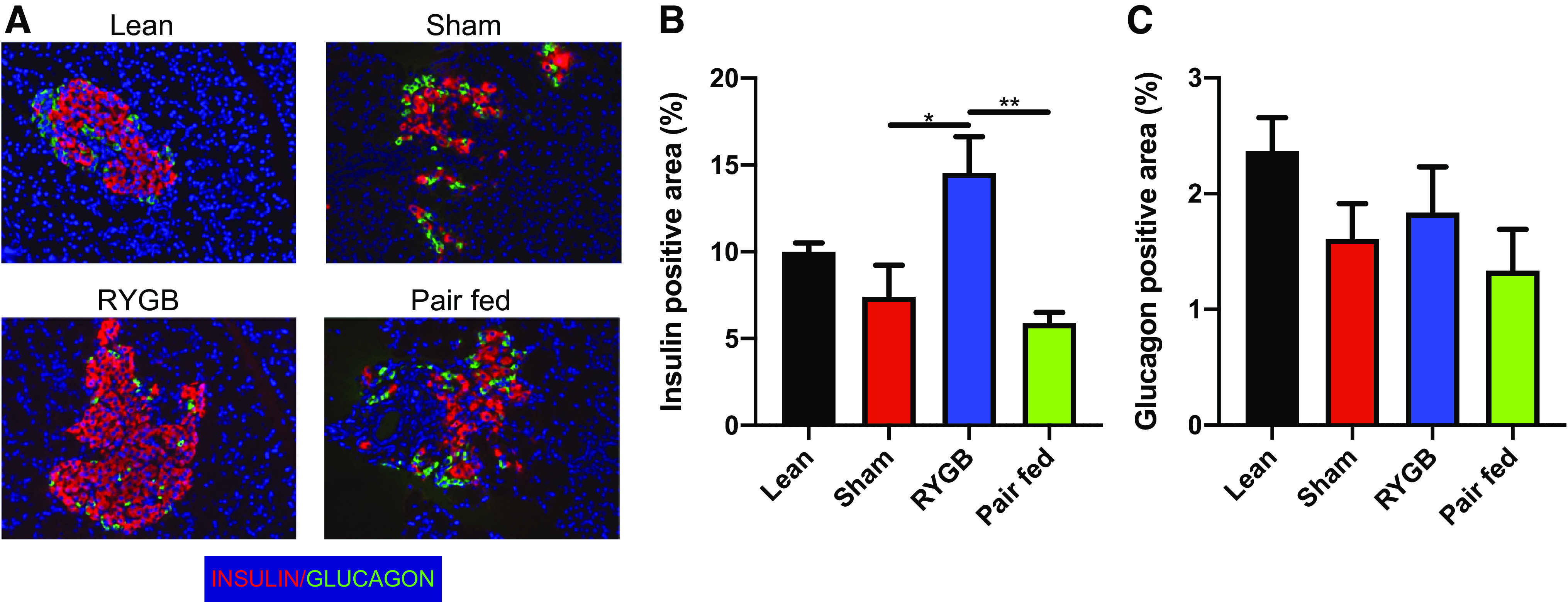
RYGB increases insulin content and improves islet structure. Immunohistochemical analysis of insulin and glucagon content and morphology in lean, pair-fed (PF), sham-, and RYGB-treated rats. A: representative immunohistochemical sections of islets stained with insulin (red), glucagon (green), and DAPI (blue). Quantification of insulin (B) and glucagon (C) positive area. Lean n = 8 rats, Sham n = 5 rats, RYGB n = 9 rats, pair-fed n = 7 rats. Data are shown as the means ± SE. B was assessed by one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P < 0.01. DAPI, 4′,6-diamidino-2-phenylindole; RYGB, Roux-en-Y gastric bypass.
Figure 5.
RYGB surgery restores glucose-stimulated insulin secretion in pancreatic islets. Cell medium insulin concentration after incubation of isolated islets in either 2 mM or 20 mM glucose solutions. Lean n = 4 rats, Sham n = 7 rats, RYGB n = 7 rats, pair-fed n = 9 rats. Data are shown as the means ± SE Data were assessed by one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P < 0.01. RYGB, Roux-en-Y gastric bypass.
Mixed Meal Tolerance Test and Incretin Secretion
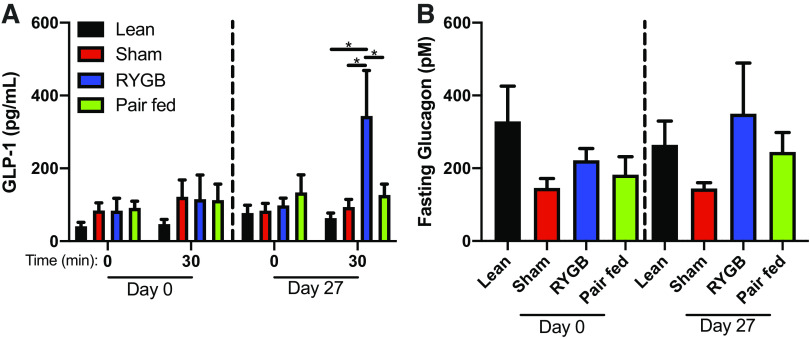
Fasting and the GLP-1 response to the MMTT were similar between all groups preoperatively (Fig. 6A). The postoperative fasting GLP-1 was also similar between groups, but the response to the oral MMTT revealed a significantly greater increase in GLP-1 in the RYGB group when compared to all other groups (P < 0.05) (Fig. 6A). Fasting plasma glucagon was unchanged after RYGB compared with both the sham and pair-fed animals (Fig. 6B) and was similar to lean controls.
Figure 6.
RYGB increases meal-stimulated GLP-1 secretion. A Preoperative (day 0) and postoperative (day 17) fasting and meal-stimulated GLP-1 (A) and fasting glucagon concentrations (B) in lean, sham, RYGB, and pair-fed rats. n = 6 rats/group. Data are shown as the means ± SE. A and B were assessed by one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05. RYGB, Roux-en-Y gastric bypass.
DISCUSSION
It has been previously demonstrated that RYGB improves glycemic control in patients with T2D before significant weight loss (14). Several hypotheses have emerged but the mechanisms driving this response remain largely unknown. A recent study found that glucose absorption and utilization in the gut is increased, leading to an overall decrease in the rate of appearance of glucose in the blood (15). Other studies suggest that a more rapid delivery of glucose to the duodenum and pancreas causes a spike in gut hormone secretion and an increase in insulin secretion (8, 16). Finally, some studies have suggested that rerouting the intestine generates a rapid change in gut hormone signaling, which leads to an increase in insulin secretion (17). Based upon the findings that RYGB may independently alter insulin secretion, we investigated the role of pancreatic β-cell structure and function in the remission of diabetes. The main findings are that RYGB surgery: 1) improved insulin sensitivity, 2) improved glucose tolerance, and 3) increased insulin expression and insulin secretion for pancreatic islets in rats with obesity and type 2 diabetes.
Several studies have reported improved β-cell function in human subjects following RYGB surgery (18, 19). These studies describe increased postprandial insulin secretion in conjunction with elevated circulating incretins including GLP-1. Although these observations provide useful insight into the whole body physiological mechanisms that may be responsible for the improved glucose control, it is unclear if improved insulin secretion occurs due to the indirect effects stemming from improved metabolic function, or if surgery has some direct effect on β-cell structure and/or function. For these reasons, we focused on the overall functionality and morphological structure of the β-cells. We observed that RYGB surgery increased nutrient-stimulated insulin secretion and restored secretory capacity to that of the control animals. Pancreatic islets serve as the body’s sensor for fluctuations in blood glucose and regulate homeostasis by secreting insulin to counteract hyperglycemia. Our data suggest that RYGB increases islet sensitivity to glucose. Although 20 mM glucose represents a supraphysiologic condition, it does provide a stimulus that allowed us to evaluate insulin secretory capacity. Data herein suggest that RYGB restored the ability to respond to severe hyperglycemic stimulation to the level that is comparable to that of healthy control animals.
We also examined whether the enhanced insulin function that was observed in the RYGB islets was contemporaneous with changes in islet architecture. We found that RYGB surgery appears to restore islet integrity to a degree that is comparable to islets from nondiabetic lean controls and clearly shifts the structural appearance of the islets away from the dispersed and fragmented features that are seen in the sham animals. These data raise the exciting possibility that RYGB surgery induces pleiotropic effects that include restoration of pancreatic islet integrity through a regenerative program that transforms the islet structure and reestablishes islet function. The cellular and molecular adaptations that underlie this process warrant further investigation.
Our observations on islet structure are consistent with data showing that hyperglycemia (20, 21) is associated with islet disruption and this is readily reversed when normoglycemia is restored (20). There is also evidence to suggest that prolonged periods of hyperglycemia lead to endoplasmic reticulum (ER) stress in β-cells, and chronic ER stress does cause cellular dysfunction and cell death. The presence of ER stress in β-cells is well documented in both diabetic animals (22–24) and humans (25, 26). We have also previously reported that RYGB surgery reduces reactive oxygen species (ROS) in liver (27). This allows us to speculate that reduced energetic demands following surgery may have attenuated ROS production and dampened ER stress, thus facilitating or contributing to the restoration of islet structure. Another factor to consider in the context of islet architecture is the effects of RYGB surgery on GLP-1. Following surgery there is typically a rise in the postprandial GLP-1 response. Studies have shown that GLP-1 can decrease cellular stress in several tissue types including β-cells (28, 29). Herein, we show an elevated postprandial GLP-1 response in the RYGB group when compared with the sham animals. This raises the possibility that GLP-1 may play a role in restoration and perhaps the preservation of pancreatic islet structure in the RYGB animals.
In conclusion, this study provides evidence to support the hypothesis that the remission of type 2 diabetes following RYGB surgery may be related to cellular adaptations that include enhanced β-cell sensitivity to the prevailing glucose milieu. Intriguingly, this metabolic adaptation is accompanied by a remarkable remodeling and restoration of islet architecture.
Limitations
RYGB can alter glucose delivery and nutrient absorption. As such, differences in glucose tolerance may not be attributable to improved insulin sensitivity. Pair-fed animals did not undergo a sham surgery and as such did not incur surgery-related stress and recovery like RYGB animals. Immunofluorescence was used to measure islet and glucagon expression and as such, islet size was not discernable. Rat metabolism varies greatly from humans and as such, may limit clinical translation of the observations.
GRANTS
This research was supported in part by a Grant from the American Society of Metabolic and Bariatric Surgery, internal funding from the Cleveland Clinic Research Program Committee (Grant No. 2010-1009), and NIH Grants DK108089 (to J. P. Kirwan) and U54GM104940 (to J. P. Kirwan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.M., S.A.B., P.R.S., and J.P.K. conceived and designed research; J.D.M., S.A.B., A.A., E.B., H.R.T., C.D., A.M., and A.S. performed experiments; J.D.M., C.L.A., and A.M. analyzed data; J.D.M. and C.L.A. interpreted results of experiments; J.D.M. and C.L.A. prepared figures; J.D.M. and C.L.A. drafted manuscript; J.D.M., A.A., C.L.A., E.B., H.R.T., C.D., A.M., P.R.S., and J.P.K. edited and revised manuscript; J.D.M., S.A.B., J.P.K., A.A., C.L.A., E.B., H.R.T., C.D., A.M., A.S., and P.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study staff for considerable time and effort on this project.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Courcoulas AP, Gallagher JW, Neiberg RH, Eagleton EB, DeLany JP, Lang W, Punchai S, Gourash W, Jakicic JM. Bariatric surgery vs. lifestyle intervention for diabetes treatment: five year outcomes from a randomized trial. J Clin Endocrinol Metab 105: 866–876, 2020. doi: 10.1210/clinem/dgaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR, Investigators S. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 376: 641–651, 2017. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshino M, Kayser BD, Yoshino J, Stein RI, Reeds D, Eagon JC, Eckhouse SR, Watrous JD, Jain M, Knight R, Schechtman K, Patterson BW, Klein S. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med 383: 721–732, 2020. doi: 10.1056/NEJMoa2003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240: 236–242, 2004. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikman BT, Zheng D, Pories WJ, Chapman W, Pender JR, Bowden RC, Reed MA, Cortright RN, Tapscott EB, Houmard JA, Tanner CJ, Lee J, Dohm GL. Mechanism for improved insulin sensitivity after gastric bypass surgery. J Clin Endocrinol Metab 93: 4656–4663, 2008. doi: 10.1210/jc.2008-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatmaitan P, Huang H, Talarico J, Moustarah F, Kashyap S, Kirwan JP, Schauer PR, Brethauer SA. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis 6: 532–537, 2010. doi: 10.1016/j.soard.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 33: 1438–1442, 2010. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu XJ, Apovian C, Hess D, Carmine B, Saha A, Ruderman N. Improved insulin sensitivity 3 months after RYGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes 64: 3155–3159, 2015. doi: 10.2337/db14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, Chaim EA, Geloneze B. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 95: 3871–3875, 2010. doi: 10.1210/jc.2010-0085. [DOI] [PubMed] [Google Scholar]
- 11.Douros JD, Tong J, D’Alessio DA. The effects of bariatric surgery on islet function, insulin secretion, and glucose control. Endocr Rev 40: 1394–1423, 2019. doi: 10.1210/er.2018-00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu H, Eldar S, Heneghan HM, Schauer PR, Kirwan JP, Brethauer SA. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg Obes Relat Dis 10: 29–35, 2014. doi: 10.1016/j.soard.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Gatmaitan P, Huang H, Talarico J, Moustarah F, Kashyap S, Kirwan JP, Schauer PR, Brethauer SA. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis 6: 532–537, 2010. doi: 10.1016/j.soard.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248–256.e5, 2009. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 142: 74–85, 2007. doi: 10.1016/j.surg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244: 741–749, 2006. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindqvist A, Spegel P, Ekelund M, Garcia Vaz E, Pierzynowski S, Gomez MF, Mulder H, Hedenbro J, Groop L, Wierup N. Gastric bypass improves beta-cell function and increases beta-cell mass in a porcine model. Diabetes 63: 1665–1671, 2014. doi: 10.2337/db13-0969. [DOI] [PubMed] [Google Scholar]
- 20.Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK, Ramracheya R, Gribble FM, Reimann F, Clark A, Rorsman P, Ashcroft FM. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 5: 4639, 2014. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53: 624–632, 2004. doi: 10.2337/diabetes.53.3.624. [DOI] [PubMed] [Google Scholar]
- 22.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 23.Omikorede O, Qi C, Gorman T, Chapman P, Yu A, Smith DM, Herbert TP. ER stress in rodent islets of Langerhans is concomitant with obesity and beta-cell compensation but not with beta-cell dysfunction and diabetes. Nutr Diabetes 3: e93, 2013. doi: 10.1038/nutd.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirakawa J, Togashi Y, Sakamoto E, Kaji M, Tajima K, Orime K, Inoue H, Kubota N, Kadowaki T, Terauchi Y. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-cells. Diabetes 62: 3448–3458, 2013. doi: 10.2337/db13-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 121: 2308–2318, 2008. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50: 2486–2494, 2007. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 27.Sacks J, Mulya A, Fealy CE, Huang H, Mosinski JD, Pagadala MR, Shimizu H, Batayyah E, Schauer PR, Brethauer SA, Kirwan JP. Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol Rep 6: e13600, 2018. doi: 10.14814/phy2.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunha DA, Ladriere L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes 58: 2851–2862, 2009. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4: 391–406, 2006. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]