Abstract
Interoceptive signals from gut and adipose tissue and sensory cues from the environment are integrated by hubs in the brain to regulate feeding behavior and maintain homeostatic control of body weight. In vivo neural recordings have revealed that these signals control the activity of multiple layers of hunger neurons and eating is not only the result of feedback correction to a set point, but can also be under the influence of anticipatory regulations. A series of recent technical developments have revealed how peripheral and sensory signals, in particular, from the gut are conveyed to the brain to integrate neural circuits. Here, we describe the mechanisms involved in gastrointestinal stimulation by nutrients and how these signals act on the hindbrain to generate motivated behaviors. We also consider the organization of multidirectional intra- and extrahypothalamic circuits and how this has created a framework for understanding neural control of feeding.
Keywords: feeding, gut-to-brain, hypothalamus
INTRODUCTION
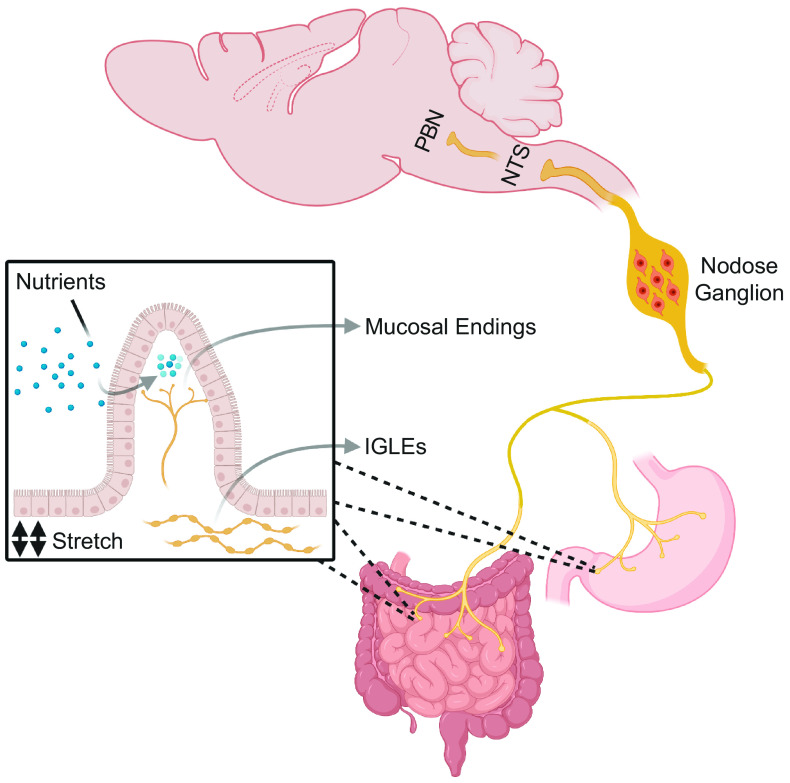
Gut vagal terminals act as polymodal sensors of gastrointestinal (GI) content responding to stimuli, such as stretching, osmolarity, pH, and nutrients, and connecting with the brain in order to elicit energy homeostatic responses (1, 2). Vagal afferent nerve terminals are anatomically distributed in different layers of the GI tract, as shown in Fig. 1. Intraganglionic laminar endings (IGLEs) act as mechanoreceptors, sensing GI stretching, whereas vagal mucosal endings can sense chemical stimuli. A large heterogeneous group of nerve terminals also express receptors for enteroendocrine hormones and their activation, in part by mechanosensing, generates signals that can regulate food intake (3). These diverse afferent signals are processed by the nodose ganglia (NG) that contains the cell bodies of ∼2,300 neurons (4) comprising the vagal afferent system and conveys the chemical and mechanical information from the GI tract (and other organs) to the nucleus of the solitary tract (NTS) and area postrema (AP) in the hindbrain. NTS neurons integrate the information and in turn excite other hindbrain regions, such as the parabrachial nucleus (PBN), which in turn project broadly to higher centers in the brain.
Figure 1.
Vagal afferent neurons. The gastrointestinal tract is densely innervated by the vagus nerve and its mucosal endings acts as chemosensory terminals detecting nutrients and hormones, whereas the IGLEs are anatomically concentrated in muscle layers and detect gastrointestinal stretch. The cell bodies of the afferent fibers are located in the nodose ganglia and the signals from their terminals are relayed to the NTS. The PBN, in turn, receives ascending inputs from the NTS and coordinates meal termination. IGLEs, intraganglionic laminar endings; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus.
Recent studies have also reshaped our understanding about the roles of hypothalamic neurons, revealing that they function primary as interoceptive sensors of hormone levels that reflect the milieu interne that can be further modulated by sensory cues that modulate their firing (5). The identification of these novel intra- and extrahypothalamic populations has elucidated how the central nervous system adjusts food consumption and energy expenditure to maintain energy homeostasis.
In this mini-review, we describe recent advances obtained from mouse studies in the characterization of GI-brain connections involved in the regulation of appetite and also the neuronal networks integrating hypothalamic and extrahypothalamic signals.
THE ASCENDING PATHWAY FOR FEEDING CONTROL
The enteroendocrine cells in the gut are equipped with an array of nutrient, chemical, and mechanical sensors and influence food digestion and appetite by releasing a plethora of hormones (6). A new identified class of epithelial cells in the colon and small intestine, termed neuropod cells, release glutamate in response to a sugar stimulus and synapse with vagal neurons, suggesting a new mechanism by which a luminal stimulus is rapidly conveyed to the brain (7).
Recent genetic mapping, anatomical tracing, and optogenetic activation of different nodes of afferent vagal neurons have defined their neurochemical phenotypes and effects on feeding behavior. The role of Gpr65- and Glp1r-expressing vagal neurons was extensively explored after the identification of G protein-coupled receptors (GPCRs) in distinct vagal afferents. The Gpr65-expressing vagal neurons were found in mucosal-ending terminals, mainly in duodenal villi, and Glp1r-expressing afferents were identified in IGLEs in the stomach muscle. Interestingly, in vivo calcium image (GCaMP3) in nodose ganglia of the respective Cre-knockin mice showed that GPR65 neurons are responsive to nutrients in the intestinal lumen whereas GLP1R neurons in nodose respond to gastrointestinal distension but not to GLP1 (8).
A recent study evaluated the putative appetite-suppressant role of afferent vagal neurons. The genetic characterization of vagal sensory neurons as revealed by single-cell RNA sequence (sc-RNAseq) of GI-targeted afferent neurons identified 12 clusters; eight of them expressing unique markers: Oxtr+, Olfr78+, Npas1a+, Sst+, Calca+, Vip+/Utsb2b+, Prom1+, and Edn3+. However, considering that the vagus nerve innervates most organs in the thoracic and abdominal cavities, it is worth mentioning that none of these unique genetic markers have been confirmed to exclusively innervate the gut. Following a GI tract-nodose ganglia neuronal retrograde tracing, the authors identified Vip+- and Gpr65+-expressing neurons in mucosal-endings and Oxtr+- and Glp1r+-expressing neurons in IGLEs. Surprisingly, only IGLE-targeted neurons (Oxtr+and Glp1r+) inhibited food intake after optogenetic (ChR2) and chemogenetic (hM3D) activation; however, these findings do not rule out the role of chemosensing on feeding control. Mechanosensing signaling triggered by Oxtr+-expressing vagal neurons activated tyrosine hydroxylase (Th)-expressing neurons in the NTS, calcitonin (Calca)-expressing neurons localized in the external lateral parabrachial nucleus (PBNel), and another neuronal population in the dorsal lateral parabrachial nucleus (PBNdl) (9). In addition, signaling by mechanosensing vagal afferents to the hindbrain through the nodose ganglion also led to the identification of neurons in the PBN that express prodynorphin (PBNPdyn) that are responsive to liquid and solid food consumption (10). Two-photon calcium imaging demonstrated rapid and reversible activation of PBNPdyn neurons upon gastric distension with an appetite-suppressing effect after chemogenetic activation of PBNPdyn neurons, suggesting that these neurons might be components of a rapid anorexigenic feedback response to avoid overconsumption. Interestingly, the synaptic inputs shown by tracing experiments demonstrated connections between NTS regions that receive oral and oropharyngeal sensory information and, consistently, the PBNPdyn neurons were rapidly activated by tongue and esophagus sensation from a gavage needle (10). The cocaine- and amphetamine-regulated transcript (CART), which is co-released with pro-opiomelanocortin (POMC) in neurons in the arcuate nucleus (ARC) of the hypothalamus, also plays a role in the gut-brain axis (11). The NG, in particular the right NG, expresses CART peptide and its release into the NTS is necessary to inhibit food intake (11).
Gut-innervating sensory vagal afferents have also been implicated as having roles in the transmission of reward signals to the brain. Han et al. (12) have shown that the selective activation of the right NG, but not the left NG, produced reward-like behaviors. Because the right NG neurons do not project directly to the substantia nigra (SNc), which in turn release dopamine onto dorsal striatum (DS) neurons producing behavioral reinforcement, the authors found that the increased dopamine release in the DS after optogenetic activation of the right NG is mediated by the circuit NTS-PBNdl-SNc (12). Interestingly, another work has recently shown that vagal sensory neurons are responsive to intestinal sugar and are implicated in the development of preference for sugar. The activation of vagal sensory neurons by sugar is dependent on the sodium-glucose-linked transporter-1 (SGTL1) expressed in enterocytes and endocrine cells in the gut, as its pharmacological inhibition abrogated the vagal activation (13).
As a gateway for ascending information from the GI tract, the NTS is at the intersection of the central nervous system and digestive system and its activity is controlled by a number of different neuropeptides and neuromodulators. Feeding results in rapid activation of cholecystokinin (Cck)-expressing neurons in NTS (NTSCCK) (14). NTSCCK circuit mapping showed that calcitonin gene-related peptide (CGRP)-expressing neurons in the lateral parabrachial nucleus (LPBNCGRP) (14) and melanocortin-4 receptor (MC4R)-expressing neurons in the paraventricular hypothalamus (PVH) (14) are potential downstream mediators of the anorexigenic effects of NTSCCK activation. It was recently shown that calcitonin receptor-expressing neurons in NTS (NTSCALCR) (15), which do not overlap with NTSCCK, mediates nonaversive suppression of food intake, as mice consumed more of the flavor paired with the activation of NTSCALCR in a two-flavor preference essay. The NTSCALCR suppress food intake via projections to a PBN node yet to be identified but do not project to LPBNCGRP, which mediates feeding aversion in response to GI malaise (15). The visceral malaise is also associated with increased levels of growth differentiation factor 15 (GDF15), a potent anorectic factor implicated in the cancer-associated cachexia (16, 17). Recent studies have reported that the anorectic effects of GFD15 are mediated through GDNF-family receptor-α-like (GFRAL), which is expressed exclusively in the AP and NTS (18–20). Further neurochemical characterization of GFRAL expression has demonstrated that the majority of GFRAL neurons are CCK-positive and the deletion of CCK in the AP and NTS significantly reduces the anorectic effects of GFD15 (21). Interestingly, the administration of recombinant GDF15 results in an aversive response pattern to flavored food (22).
Because many of the identified NTS neuronal types comprise of key circuits for satiation, some antiobesity drugs may influence feeding through this node. In concert, it was recently shown that the antiobesity effects of lorcaserin depends, at least partially, on a subset of pro-opiomelanocortin (POMC) neurons in the NTS that also express the 5-hydroxytryptamine 2 C receptor (5-HT2CR) (11). The GLP1R is also a target for obesity treatment and GLP1R agonists, such as the liraglutide, reduce appetite. In the NTS, a portion of GLP1R-expressing neurons also express γ-aminobutyric acid (GABA), and the chemogenetic silencing of GABAergic neurons in the NTS reduces the appetite-suppressant effect of liraglutide (23). To analyze the endogenous effects of GLP-1 in the NTS, Cheng et al. (24) ablated the preproglucagon (Ppg), whose selective cleavage gives rise to GLP-1, in leptin receptor (LepR)- and Ppg-expressing neurons in the NTS. Although the Ppg deletion in both populations did not alter body weight and food intake, Cheng et al. (24) found that the chemogenetic activation of LepR- and Ppg-expressing neurons reduced the food intake.
In contrast to the most NTS neurons described so far that convey satiety, tyrosine hydroxylase (Th)- and epinephrine-expressing NTS populations (NTSTH and NTSE, respectively) with appetite-stimulant properties were recently identified (25, 26). The NTSTH neurons densely project to the ARC and drives agouti-related peptide (AgRP) neural activation through direct norepinephrine (NE) signaling; the NTSE, in turn, coexpress the orexigenic neuropeptite Y (NPY) and its chemogenetic activation stimulates feeding (25, 26).
AgRP-expressing neurons in the hypothalamus potently induce feeding when stimulated and are key neurons in the regulation of energy balance (27, 28). For many years, AgRP neurons were exclusively considered to function as long-term homeostatic neurons. However, measurements of AgRP neuron dynamics in awake, behaving mice demonstrated that sensory cues, such as sight and smell of food, can rapidly inhibit these neurons (29, 30). Caged food presentation induces rapid and transient AgRP neuron inhibition in fasted mice, but if the food is subsequently consumed the inhibition is sustained, pointing to a key role for signals from the GI tract in the rapid control of AgRP neurons. Consistent with this, it was shown that intragastric infusion of calorie-containing nutrients promote persistent AgRP inhibition (31, 32). Moreover, intragastric infusion of water or consumption of a calorie-free gel resulted in only a small reduction in AgRP neuron activity (31, 32). Consistent with the known role of IGLEs, mechanoreceptors sensing GI distention, chemogenetic activation of Oxtr+-expressing neurons in IGLEs, and Glp1r+ with a lesser magnitude also inhibited AgRP neurons (9). Also consistent with this, the Oxtr+ IGLE is specifically expressed in the intestine and a calorie-free volumetric load in the intestine, but not in the stomach, sustained AgRP neuron inhibition (9).
Gut microbiota landscape is yet another factor affecting the gut-brain axis (33). Studies with germ-free rodents have that shown elevated levels of PYY and enteroglucagon (34) and the metabolites generated by enzymatic processing of nutrients, such as the short-chain fatty acids produced by the microbiota, can stimulate GLP1 release from L cells, suggesting that the gut bacteria participate in endocrine physiology (35). In addition to controlling metabolites, the microbiota is also able to produce signaling molecules with putative functions on feeding control. The Escherichia coli, for instance, produces a caseinolytic peptidase B protein homologue (ClpB), an αMSH-like peptide, whose plasma levels are associated with increased POMC neuronal activation (36).
NOVEL INTRA- AND EXTRAHYPOTHALAMIC CIRCUIT NODES IN FEEDING CONTROL
The sustained feeding behavior seen after activating AgRP neurons is quenched by sensory cues, raising the possibility that sustained hunger is mediated by another long-lasting neuropeptide. The AgRP neurons also release NPY and GABA, and the contribution of each of these neuromodulators to sustained hunger signal was recently assessed. AgRP neurons were optogenetically activated in animals in which GABA or NPY signaling was ablated by a cell-specific knockout for 15 min and food intake was subsequently measured (37). Mice lacking NPY presented a time-locked feeding and less drive for food-seeking upon stimulation, establishing NPY as the neuromodulator responsible for the sustained hunger and motivated behaviors produced by AgRP neuronal activation (37). In addition, the activation of AgRP neurons induces peripheral insulin resistance and this effect is also NPY dependent (38).
AgRP- and POMC-expressing neurons project to the PVH, leading to increased and decreased food intake, respectively. Optogenetic activation of AgRP terminals in the PVH rapidly stimulate food intake through the inhibitory and fast-acting transmitters GABA and NPY (39). Conversely, the slow-acting, PVHMC4R agonist, αMSH (cleaved from POMC) decreases food intake but only after hours (40). Thus, it was further hypothesized that there is another unknown fast-acting satiety neuron in the ARC. A group of glutamatergic neurons (ARCVGLUT2) was recently identified as the source of excitatory input onto PVH neurons, and they were shown to rapidly induce satiety (41). Moreover, there is plasticity of glutamatergic transmission to PVHMC4R and this is potentiated by αMSH. ChR2-assisted circuit mapping (CRACM) demonstrated that ARCVGLUT2 neurons receive light-evoked inhibitory postsynaptic currents (IPSCs) from ARCAgRP neurons. Thus, ARCVGLUT2 neurons are inhibited by GABAergic projections from ARCAgRP neurons under fasting conditions (41).
The PVHMC4R is an important downstream effector site for ARC neurons and a critical node for satiety signaling. However, PVHMC4R does not account for all satiety-related signaling of PVH neurons, as demonstrated by the comparison between the chemogenetic activation of single-minded-1-expressing neurons in PVH (PVHSIM1), which is expressed by most PVH neurons, and the chemogenetic activation of PVHMC4R neurons on food intake. It was recently shown that the chemogenetic activation of glucagon-like peptide 1-expressing neurons in PVH (PVHGLP1R) acutely suppress food intake and their silencing induced body weight gain and hyperphagia (42). Nevertheless, the significant overlap between PVHMC4R and PVHGLP1R neurons rules out the possibility that the putative PVHSIM1-positive/PVHMC4R-negative neurons are this satiety-inducing population. The investigation of a prodynorphin-expressing neuron in PVH (PVHPDYN), which does not overlap with PVHMC4R, led to the identification of this putative PVHSIM1-positive appetite-suppressing population, as their silencing also triggered obesity and hyperphagia (43). Anterograde viral tracing demonstrated that PVHPDYN project to the central compartment of the lateral parabrachial nucleus (cLPBN) and prelocus coeruleus (pLC), but PVHPDYN make glutamatergic synapses onto neurons in the pLC but not the cLPBN. Finally, the authors found that PVHPDYN neurons receive GABAergic input from ARCAgRP neurons, as demonstrated by light-evoked IPSCs (43).
In contrast to the short-term and gut-derived signals to the brain, leptin secretion by adipose tissue acts as long-term afferent signal to modulate food intake and body weight by controlling the activity of ARC, and other, neurons (44). Previous reports have indicated that leptin receptor-expressing (LepR) ARCPOMC neurons are important for feeding and body weight regulation. However, LepR deletion in ARCPOMC of adult mice does not affect body weight and food intake (45). Rather, leptin signaling in ARCPOMC is required for the regulation of glucose homeostasis independent of its effect on energy balance (45). A recent work demonstrated that CRISPR-mediated deletion of LepR in ARCAgRP induced severe obesity, diabetes, and food intake, suggesting that leptin largely suppresses appetite by targeting ARCAgRP and not ARCPOMC neurons (46). This work has now been challenged by a recent finding showing that the antiobesity effects of leptin are mediated by GABA-positive neurons in the ARC and its chronic activation induces massive obesity (47). The authors also observed that leptin administration in ARCAgRP -ablated ob/ob mice is sufficient to normalize the body weight. Interestingly, the chronic chemogenetic activation of ARCAgRP neurons increases feeding initially and induces significant weight gain, however the food intake and body weight return to baseline after 7 and 60 days, respectively (48). Taken together, the aforementioned studies highlight the complexity of hypothalamic circuits involved in the energy homeostasis.
Whereas increased leptin levels inhibit the food intake, a fall in leptin levels disinhibits ARC neurons and stimulate appetite. This hormonal programming to conserve fuel stores was recently extended by a recent work demonstrating that food-restricted mice display increased serum concentration of growth-hormone (GH) and the activation of its receptor (GHR) in ARCAgRP induces metabolic responses consistent with energy conservation, such as reduction in energy expenditure and increased food intake (49).
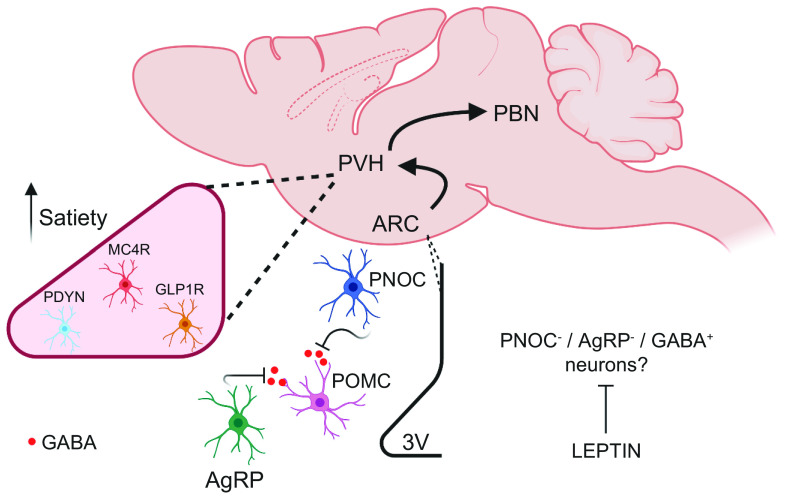
Another novel GABAergic neuronal population regulating food intake was recently identified in the ARC. Prepronociceptin-expressing neurons (ARCPNOC) are distinct from ARCAgRP and ARCPOMC and are glucose-excited (50). ARCPNOC project locally to the ARC and further assessment of its innervations identified an inhibitory connectivity onto ARCPOMC neurons. The optogenetic activation of ARCPNOC neurons promotes feeding but does not trigger acute effects on glucose homeostasis or insulin sensitivity. Interestingly, the Pnoc gene was one of the most enriched transcripts in the hypothalamus in mice fed an acute high-fat diet (HFD), indicating that PNOC-expressing neurons may have a role in the overconsumption of mice fed a HFD (Fig. 2) (50). A recent report demonstrated that ARCAgRP neurons receive input from a separate population of nociceptin-expressing neurons in the anterior bed nuclei of the stria terminalis (aBNST) (51). Moreover, the ablation of nociceptin-expressing neurons in aBNST increased body weight and food intake, suggesting a putative role of these neurons in energy homeostasis by controlling ARCAgRP neurons activity (51). PNOC-expressing neurons are also distributed in other extrahypothalamic areas, such as lateral septum (LS) and central amygdala (CeA). The latter is recognized as an important integrative brain region and it receives excitatory glutamatergic inputs from PBNCGRP neurons, and it is also activated by CCK. PNOC-expressing neurons in CeA (CeAPNOC) were recently identified as a novel population (52). Consumption of HFD acutely activated CeAPNOC neurons, and mice with prior chemogenetic inhibition of CeAPNOC neurons reduced their HFD consumption on first exposure. The optogenetic activation of CeAPNOC terminals in the ventral BNST, PBN, and NTS induced a reward-like behavior (52).
Figure 2.
Novel ARC and PVH neurons. PNOC-expressing neurons are activated by short-term HFD feeding and, in concert with AgRP neurons, inhibit POMC neurons through GABAergic projections. Recent studies suggest a distinct GABAergic neuronal population as the primary effector of leptin signaling in the ARC. The ARC neurons project to and target PVH neurons to control feeding. GLP1R, MC4R, and PDYN are expressed in different neurons in PVH and their activation induce satiety through different efferent circuitry in PBN. AgRP, agouti-related peptide; ARC, arcuate nucleus; GABA, gamma-aminobutyric acid; GLP1R, glucagon-like peptide-1 receptor; MC4R, melanocortin-4 receptor; PBN, parabrachial nucleus; PDYN, prodynorphin; PNOC, prepronociceptin; POMC, pro-opiomelanocortin; PVH, paraventricular nucleus; 3V, third ventricle.
The development of tissue-clearing techniques (e.g., iDISCO-based methods) combined with Fos staining in the whole brain have provided further progress in the field by allowing the comparison of neuronal activation in different contexts and the identification of unappreciated brain regions involved in controlling energy homeostasis. In this regard, a study identified two molecularly and anatomically distinct neuronal populations in the dorsal raphe nucleus (DRN): a vesicular GABA transporter (DRNVGAT) and a vesicular glutamate transporter type 3 (DRNVGLUT3) (53). It was shown that fasting increased Fos-positive activation of DRNVGAT population. Optogenetic activation of DRNVGAT population increased food intake and inhibition decreased food intake. The DRNVGLUT3 were activated by refeeding and they inhibited food intake when activated whereas photoinhibition of DRNVGLUT3 increased food intake. Chronic chemogenetic inhibition of DRNVGAT neurons in leptin-deficient ob/ob mice led to a significant reduction in body weight (53). Further investigation of DRNVGAT neurons in controlling energy homeostasis also identified a key regulatory role in thermogenesis, as their activation suppresses energy expenditure through reduction of interescapular brown adipose tissue (iBAT) temperature (54). Using iBAT retrograde viral tracing, the authors found that DRNVGAT neurons send descending projections to raphe pallidus (RPa), which in turn innervates iBAT (54).
CONCLUDING REMARKS
Interoceptive neurons process internal-state information to control appetite. Although most of the experimental approaches in neuroscience have been useful to probe neural mechanisms and circuits, the extent to which artificial activation/inhibition of neurons recapitulate their function under physiological circumstances is still unclear. The integration of sensory cues and caloric value of food is a permanent task for neurons and how these pathways are disturbed by obesity-predisposing factors, such as the consumption of HFD, is an ongoing debate (55–57). There is also a complex CNS network that controls iBAT thermogenesis and white adipose tissue (WAT) metabolism by controlling autonomic outflow. Similarly, peripheral insulin sensitivity and glucose metabolism are also potently, but not exclusively, governed by CNS. Finally, there is an emergent need to comprehend how the brain deciphers palatable food and drive reinforcing effects, intermingling hedonic and homeostatic feeding (58).
GRANTS
This study was supported by the São Paulo Research Foundation (FAPESP) 2019/12969-2 (to A.M-A.), the JPB Foundation (to J.M.F), and the São Paulo Research Foundation (FAPESP) 2013/07607-8 (to L.A.V.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M-A. prepared figures; A.M-A. drafted manuscript; A.M-A., J.M.F., and L.A.V. edited and revised manuscript; A.M-A., J.M.F., and L.A.V. approved final version of manuscript.
REFERENCES
- 1.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16: 28–33, 2004. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR, Neuhuber WL. Vagal mechanisms as neuromodulatory targets for the treatment of metabolic disease. Ann NY Acad Sci 1454: 42–55, 2019. doi: 10.1111/nyas.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waise TMZ, Dranse HJ, Lam TKT. The metabolic role of vagal afferent innervation. Nat Rev Gastroenterol Hepatol 15: 625–636, 2018. doi: 10.1038/s41575-018-0062-1. [DOI] [PubMed] [Google Scholar]
- 4.Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci 21: 8602–8615, 2001. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. Bioessays 38: 316–324, 2016. doi: 10.1002/bies.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 15: 226–237, 2019. doi: 10.1038/s41574-019-0168-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohorquez DV. A gut-brain neural circuit for nutrient sensory transduction. Science 361: eaat5236, 2018. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209–221, 2016. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, Madisen L, Zeng H, Krasnow MA, Knight ZA. Genetic identification of vagal sensory neurons that control feeding. Cell 179: 1129–1143.e23, 2019. doi: 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, Jung S, An M, Ahn BH, Park JH, Park HE, Lee M, Lee JW, Schwartz GJ, Kim SY. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580: 376–380, 2020. doi: 10.1038/s41586-020-2167-2. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino G, Lyons D, Cristiano C, Lettieri M, Olarte-Sanchez C, Burke LK, Greenwald-Yarnell M, Cansell C, Doslikova B, Georgescu T, Martinez de Morentin PB, Myers MG Jr, Rochford JJ, Heisler LK. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab 28: 619–630.e15, 2018. doi: 10.1016/j.cmet.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohorquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE. A neural circuit for gut-induced reward. Cell 175: 665–678.e23, 2018. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, Zuker CS. The gut-brain axis mediates sugar preference. Nature 580: 511–516, 2020. doi: 10.1038/s41586-020-2199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Agostino G, Lyons DJ, Cristiano C, Burke LK, Madara JC, Campbell JN, Garcia AP, Land BB, Lowell BB, Dileone RJ, Heisler LK. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. Elife 5: e12225, 2016. doi: 10.7554/eLife.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng W, Gonzalez I, Pan W, Tsang AH, Adams J, Ndoka E, Gordian D, Khoury B, Roelofs K, Evers SS, MacKinnon A, Wu S, Frikke-Schmidt H, Flak JN, Trevaskis JL, Rhodes CJ, Fukada SI, Seeley RJ, Sandoval DA, Olson DP, Blouet C, Myers MG Jr.. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab 31: 301–312.e5, 2020. doi: 10.1016/j.cmet.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med 13: 1333–1340, 2007. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 17.Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, Li D, Starck SR, Chen HH, McEntee M, Katewa SD, Phung V, Wang M, Kekatpure A, Lakshminarasimhan D, White A, Olland A, Haldankar R, Solloway MJ, Hsu JY, Wang Y, Tang J, Lindhout DA, Allan BB. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med 26: 1264–1270, 2020. doi: 10.1038/s41591-020-0945-x. [DOI] [PubMed] [Google Scholar]
- 18.Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, Wu X. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23: 1215–1219, 2017. doi: 10.1038/nm.4393. [DOI] [PubMed] [Google Scholar]
- 19.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, Rangwala SM. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23: 1150–1157, 2017. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Chang C-C, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Nørgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, Jørgensen SB. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23: 1158–1166, 2017. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 21.Worth AA, Shoop R, Tye K, Feetham CH, D'Agostino G, Dodd GT, Reimann F, Gribble FM, Beebe EC, Dunbar JD, Alexander-Chacko JT, Sindelar DK, Coskun T, Emmerson PJ, Luckman SM. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. Elife 9: e55164, 2020. doi: 10.7554/eLife.55164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borner T, Wald HS, Ghidewon MY, Zhang B, Wu Z, De Jonghe BC, Breen D, Grill HJ. GDF15 induces an aversive visceral malaise state that drives anorexia and weight loss. Cell Rep 31: 107543, 2020. doi: 10.1016/j.celrep.2020.107543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin SM, Lipsky RK, Lhamo R, Chen J, Kim E, Borner T, Schmidt HD, Hayes MR. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci Transl Med 12: eaay8071, 2020. doi: 10.1126/scitranslmed.aay8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng W, Ndoka E, Hutch C, Roelofs K, MacKinnon A, Khoury B, Magrisso J, Kim KS, Rhodes CJ, Olson DP, Seeley RJ, Sandoval D, Myers MG Jr.. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight 5: e134359, 2020. doi: 10.1172/jci.insight.134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aklan I, Sayar Atasoy N, Yavuz Y, Ates T, Coban I, Koksalar F, Filiz G, Topcu IC, Oncul M, Dilsiz P, Cebecioglu U, Alp MI, Yilmaz B, Davis DR, Hajdukiewicz K, Saito K, Konopka W, Cui H, Atasoy D. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab 31: 313–326.e5, 2020. doi: 10.1016/j.cmet.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, Wang F, Herzog H, Song S, Zhan C. A vagal-NTS neural pathway that stimulates feeding. Curr Biol 30: 3986–3998.e5, 2020. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 27.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, Tannous BA, Krashes MJ, Lowell BBA. Neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18: 863–871, 2015. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521: 180–185, 2015. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160: 829–841, 2015. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, Knight ZA. Dynamics of gut-brain communication underlying hunger. Neuron 96: 461–475.e5, 2017. doi: 10.1016/j.neuron.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep 21: 2724–2736, 2017. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, , et al. The microbiota-gut-brain axis. Physiol Rev 99: 1877–2013, 2019. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 34.Ghatei MA, Ratcliffe B, Bloom SR, Goodlad RA. Fermentable dietary fibre, intestinal microflora and plasma hormones in the rat. Clin Sci (Lond) 93: 109–112, 1997. doi: 10.1042/cs0930109. [DOI] [PubMed] [Google Scholar]
- 35.Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc 74: 328–336, 2015. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 36.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J, Goichon A, Guerin C, Peltier J, Pestel-Caron M, Chan P, Vaudry D, do Rego JC, Lienard F, Penicaud L, Fioramonti X, Ebenezer IS, Hokfelt T, Dechelotte P, Fetissov SO. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab 23: 324–334, 2016. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Essner RA, Kosar S, Miller OH, Lin YC, Mesgarzadeh S, Knight ZA. Sustained NPY signaling enables AgRP neurons to drive feeding. Elife 8: e46348, 2019. doi: 10.7554/eLife.46348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engstrom Ruud L, Pereira MMA, de Solis AJ, Fenselau H, Bruning JC. NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons. Nat Commun 11: 442, 2020. doi: 10.1038/s41467-020-14291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 18: 588–595, 2013. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci 19: 206–219, 2016. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, Lowell BB. A rapidly acting glutamatergic ARC–>PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci 20: 42–51, 2017. [Erratum in Nat Neurosci 20: 1189, 2017]. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Navarrete J, Liang-Guallpa J, Lu C, Funderburk SC, Chang RB, Liberles SD, Olson DP, Krashes MJ. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab 29: 681–694.e5, 2019. doi: 10.1016/j.cmet.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li MM, Madara JC, Steger JS, Krashes MJ, Balthasar N, Campbell JN, Resch JM, Conley NJ, Garfield AS, Lowell BB. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102: 653–667.e6, 2019. doi: 10.1016/j.neuron.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab 1: 754–764, 2019. doi: 10.1038/s42255-019-0095-y. [DOI] [PubMed] [Google Scholar]
- 45.Caron A, Dungan Lemko HM, Castorena CM, Fujikawa T, Lee S, Lord CC, Ahmed N, Lee CE, Holland WL, Liu C, Elmquist JK. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife 7: e33710, 2018. doi: 10.7554/eLife.33710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, Kong D. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556: 505–509, 2018. doi: 10.1038/s41586-018-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu C, Jiang Z, Xu Y, Cai ZL, Jiang Q, Xu Y, Xue M, Arenkiel BR, Wu Q, Shu G, Tong Q. Profound and redundant functions of arcuate neurons in obesity development. Nat Metab 2: 763–774, 2020. doi: 10.1038/s42255-020-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewbank SN, Campos CA, Chen JY, Bowen AJ, Padilla SL, Dempsey JL, Cui JY, Palmiter RD. Chronic Gq signaling in AgRP neurons does not cause obesity. Proc Natl Acad Sci USA 117: 20874–20880, 2020. doi: 10.1073/pnas.2004941117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perello M, Frazao R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr.. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun 10: 662, 2019. [Erratum in Nat Commun 10(1):980, 2019]. doi: 10.1038/s41467-019-08607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jais A, Paeger L, Sotelo-Hitschfeld T, Bremser S, Prinzensteiner M, Klemm P, Mykytiuk V, Widdershooven PJM, Vesting AJ, Grzelka K, Minere M, Cremer AL, Xu J, Korotkova T, Lowell BB, Zeilhofer HU, Backes H, Fenselau H, Wunderlich FT, Kloppenburg P, Bruning JC. PNOC(ARC) neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106: 1009–1025.e10, 2020. doi: 10.1016/j.neuron.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith MA, Choudhury AI, Glegola JA, Viskaitis P, Irvine EE, de Campos Silva PCC, Khadayate S, Zeilhofer HU, Withers DJ. Extrahypothalamic GABAergic nociceptin-expressing neurons regulate AgRP neuron activity to control feeding behavior. J Clin Invest 130: 126–142, 2019. doi: 10.1172/JCI130340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL. Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102: 1037–1052.e37, 2019. doi: 10.1016/j.neuron.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nectow AR, Schneeberger M, Zhang H, Field BC, Renier N, Azevedo E, Patel B, Liang Y, Mitra S, Tessier-Lavigne M, Han MH, Friedman JM. Identification of a brainstem circuit controlling feeding. Cell 170: 429–442.e11, 2017. doi: 10.1016/j.cell.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 54.Schneeberger M, Parolari L, Das Banerjee T, Bhave V, Wang P, Patel B, Topilko T, Wu Z, Choi CHJ, Yu X, Pellegrino K, Engel EA, Cohen P, Renier N, Friedman JM, Nectow AR. Regulation of energy expenditure by brainstem GABA neurons. Cell 178: 672–685.e12, 2019. doi: 10.1016/j.cell.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai D, Khor S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab 30: 19–35, 2019. doi: 10.1016/j.cmet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Cavadas C, Aveleira CA, Souza GF, Velloso LA. The pathophysiology of defective proteostasis in the hypothalamus - from obesity to ageing. Nat Rev Endocrinol 12: 723–733, 2016. doi: 10.1038/nrendo.2016.107. [DOI] [PubMed] [Google Scholar]
- 57.Razolli DS, Moura-Assis A, Bombassaro B, Velloso LA. Hypothalamic neuronal cellular and subcellular abnormalities in experimental obesity. Int J Obes 43: 2361–2369, 2019. doi: 10.1038/s41366-019-0451-8. [DOI] [PubMed] [Google Scholar]
- 58.Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27: 42–56, 2018. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]