Keywords: exercise, glucose transport, GLUT4, GLUT4 translocation, muscle glucose uptake
Abstract
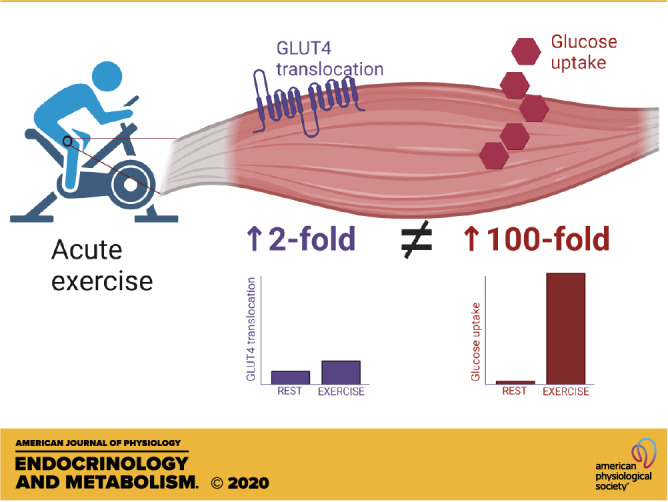
Exercise in humans increases muscle glucose uptake up to 100-fold compared with rest. The magnitude of increase depends on exercise intensity and duration. Although knockout of glucose transporter type 4 (GLUT4) convincingly has shown that GLUT4 is necessary for exercise to increase muscle glucose uptake, studies only show an approximate twofold increase in GLUT4 translocation to the muscle cell membrane when transitioning from rest to exercise. Therefore, there is a big discrepancy between the increase in glucose uptake and GLUT4 translocation. It is suggested that either the methods for measurements of GLUT4 translocation in muscle grossly underestimate the real translocation of GLUT4 or, alternatively, GLUT4 intrinsic activity increases in muscle during exercise, perhaps due to increased muscle temperature and/or mechanical effects during contraction/relaxation cycles.
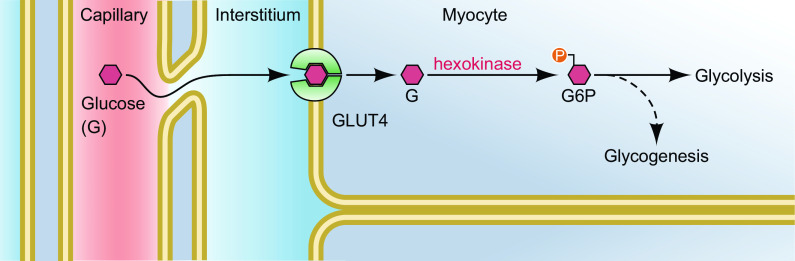
Exercise markedly increases muscle glucose uptake. Glucose entry into skeletal muscle cells occurs by facilitated diffusion provided by glucose transporter type 4 (GLUT4) and a concentration gradient of glucose from the interstitial space (the outside of the muscle cell) to the inside (cytoplasm) of the muscle cell. Studies in GLUT4 knockout mice have convincingly shown that GLUT4 is necessary for contractions as well as insulin to increase muscle glucose uptake (1–4). Nevertheless, in vivo muscle glucose uptake is dependent on four factors and three of them do not depend on GLUT4. These are as follows: 1) glucose supply (plasma glucose concentration and blood flow), 2) transcapillary transport out of the capillaries to the interstitium, 3) glucose transport across the sarcolemma and t-tubules, and 4) glucose phosphorylation and subsequent metabolism inside the muscle cell (Fig. 1).
Figure 1.
Potential sites of regulation of muscle glucose uptake during exercise. This includes plasma glucose concentration, capillary perfusion, transcapillary transport of glucose out of the capillary, transmembrane transport into the myocyte and phosphorylation, and further metabolism of glucose inside the myocyte. This figure is modified from (51). GLUT4, glucose transporter type 4; G6P, glucose 6-phosphate.
In humans, muscle glucose uptake during exercise is mostly measured across one or two legs or across the arm by measuring the arteriovenous difference of glucose (glucose extraction) multiplied by the blood flow in the leg or arm. At rest in an overnight fasted individual, leg glucose uptake is very low, reflected by the small femoral arterial minus femoral venous (A-V) glucose difference in the order of 0.05–0.1 mmol/L and the leg blood flow around 300–400 mL/min (5–12). This comes to an uptake of 15–40 µmol/min/leg. Considering that leg blood flow during hard leg exercise can reach 7–10 L/min per leg (13–15) and A-V difference may increase to 0.3–0.4 mmol/L during intense exercise (14), it follows that leg glucose uptake may increase to 2,100–4,000 µmol/min/leg. In other words, there is an approximate 100-fold increase from resting values. During more moderate exercise of 50%–60% of maximal oxygen uptake in young healthy adults, leg blood flow is around 5–6 L/min and the A-V difference is 0.2–0.3 mM (11, 14, 16), so in that case, the increase in leg glucose uptake may be more like 25- to 50-fold, still a remarkable increase.
The intriguing question is how such large increases in glucose uptake are possible? Obviously, the large increase in blood flow and maintenance of the plasma glucose concentration by increased hepatic glucose production are important to secure a continuous large supply of glucose to muscle, but the glucose would go nowhere unless the muscle membrane permeability to glucose is markedly increased as well. In fact, if leg glucose uptake can increase 100-fold, then it follows that glucose transport across the muscle membrane also increases 100-fold because if this were not the case, interstitial glucose concentrations would increase, which does not occur (17). In fact, the interstitial glucose concentration during exercise is maintained close to the plasma glucose concentration and not much different from that at rest (17). Thus, during exercise, the gradient of glucose from the interstitial space to the muscle cytoplasm is likely not much different from that at rest, since the cytoplasmic glucose concentration is close to zero both at rest and during exercise (16, 18) except during the early minutes of exercise or during very intense exercise where the cytoplasmic concentration of glucose in fact may increase (14). If the glucose gradient across the muscle cell membrane is approximately the same at rest and during exercise and the glucose flux (leg glucose uptake) increases 100-fold during hard exercise, it indicates that the permeability of the membrane for glucose is increased 100-fold to accommodate a 100-fold increase in glucose uptake. This is because of the following:
Tissue uptake = concentration difference across the membrane × membrane permeability
Rearranging the equation results in the following:
Membrane permeability = tissue uptake/concentration difference
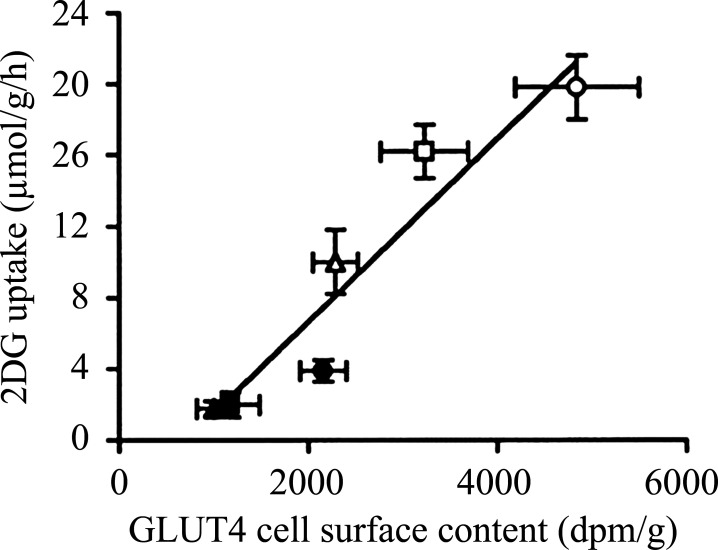
The question then arises, is this huge increase in membrane permeability during exercise due to a 100-fold increase in GLUT4 at the muscle sarcolemma and t-tubules? There have been a few attempts to measure GLUT4 translocation in muscle from rest to exercise in humans using membrane fractionation procedures or giant sarcolemmal vesicles, and the general finding is a twofold increase from rest to exercise (19–22), obviously a long way from 100-fold. In rodent skeletal muscle, subcellular fractionation (23–25) and measurements in giant vesicles (26) or immunohistochemistry (27) also typically have produced a twofold increase in surface membrane GLUT4 content. This increase in reported GLUT4 translocation is likely small due to methodological shortcomings, for example, cross-contamination of membrane fractions or other problems related to production of vesicles or obtaining a true basal level. In excised rat muscle, it was shown that subcellular fractionation underestimated insulin-induced GLUT4 translocation compared with surface photolabeling with a small hexose derivative (28). Surface labeling is likely currently the best method to measure the presence of GLUT4 at the surface membrane in the intact muscle fiber, and in incubated rat soleus muscle, maximal electrical stimulation increased GLUT4 surface labeling fourfold, which could fully account for the similar fourfold increase in muscle glucose transport (29). Furthermore, we have previously shown a tight correlation between contraction-induced muscle glucose uptake and GLUT4 surface labeling in electrically stimulated perfused rat hindlimb muscles (30) (Fig. 2).
Figure 2.
Correlation (r = 0.95, P < 0.01) between GLUT-4 cell surface content measured with exofacial labeling and 2-deoxyglucose uptake in basal (closed symbols) and contraction-stimulated (open symbols) plantaris muscles with different precontraction muscle glycogen content. High glycogen (triangles), normal glycogen (squares), and low glycogen (circles). Each point is the mean ± SE of six to eight observations. Reproduced from (30). 2DG, 2-deoxyglucose; GLUT-4, glucose transporter type 4.
Still, experiments in incubated or perfused muscle during electrical stimulation do not capture the dynamics of exercise in vivo, and correlations do not indicate causality. Experiments in mice at rest and during running do not show the large increases in muscle glucose uptake that are observed in humans, but rather 5- to 10-fold (1–4, 27, 31), perhaps due to the fact that a nonexercising mouse is not immobile but usually moves around in the cage or on the nonmoving treadmill. Furthermore, whether a maximal running effort can be obtained in a mouse is questionable.
Live imaging of GLUT4 translocation during muscle contractions in anesthetized living mice has beautifully shown the rapid kinetics of GLUT4 translocation to the sarcolemma and t-tubules, but the authors did not quantify the data (32).
So we are left with exercise-induced GLUT4 translocation data in humans that are likely not quantitatively correct, whereas in rodent models, somewhat larger fold increases in GLUT4 translocation are found but still a long way away from the 100-fold increase in muscle membrane permeability in human muscle with intense exercise.
We can then ask whether GLUT4 translocation in fact can increase 100-fold in human muscle with exercise to fully account for the 100-fold increase in membrane permeability or whether the intrinsic transporter activity of GLUT4 is increased during exercise in addition to GLUT4 translocation?
The concept of intrinsic transport activity of GLUT4, that is, the ability of each GLUT4 transporter to transport glucose, was developed because of observed mismatch between glucose transport rates and measures of GLUT4 translocation during insulin stimulation in muscle (33). Also, in adipocytes, a mismatch between insulin-stimulated glucose transport and GLUT4 translocation has been reported, as discussed previously (34). Whether changes in GLUT4 transporter activity can occur is far less studied compared with the number of studies of GLUT4 translocation. Still, there are a few studies that indicate that GLUT4 transport activity can be altered, for instance, by unmasking a GLUT4 epitope (35) or by binding of enzymes (36) or glucose analogues (37) or by vanadate (38). However, at this point, there is no clear concept of how and when GLUT4 transporter activity may change with insulin or with exercise/muscle contraction.
As regards insulin-stimulated glucose uptake in human muscle, most studies report a 10- to 20-fold increase in leg glucose uptake in insulin-sensitive young subjects at insulin concentrations that are submaximal on the upper part of the dose-response curve (39–42) or maximal (43). Recently, we performed a calculation of the increase in muscle membrane permeability to glucose in muscle at a high submaximal insulin concentration and found it increased 17-fold in rested muscle (44), whereas in muscle that displayed increased insulin sensitivity due to exercise 4 h previously, the increase in membrane permeability to glucose at the same plasma insulin concentration was 35-fold (44). These numbers are not only well beyond the known reported increase in membrane GLUT4 content with insulin stimulation but also well below the 100-fold increase with exercise.
So what is unique about exercise-induced muscle glucose transport compared with insulin-induced transport? The obvious answer is the high metabolic rate in muscle during exercise, which also increases muscle temperature. In fact, muscle temperature at rest depends on the external temperature but is typically 2–3°C below core temperature, so often 34–35°C (45, 46). During exercise, muscle temperature increases again depending on external temperature and exercise intensity but may increase to ∼40–41°C during hard exercise (45, 46). This is a potential increase in muscle temperature of up to 6–7°C. It is likely that such a temperature increase may increase the transporter activity, but detailed investigations of temperature effects on GLUT4 transporter activity have to my knowledge not been performed. Furthermore, during muscle contraction and relaxation, the mechanical deformation of the muscle tissue may cause increased GLUT4 transporter activity. In fact, passive leg movement increases muscle glucose uptake (47), and stretch of mouse muscle also increases glucose uptake (48, 49). Perhaps these two phenomena are responsible for increased glucose transporter activity during exercise, by mechanical effects on GLUT4 in a hot environment. Future studies of mechanisms changing GLUT4 transport activity may resolve this question.
In conclusion, obvious mismatches between muscle glucose uptake during exercise and available data on GLUT4 translocation in muscle exist. It is time to develop better methods for measuring GLUT4 translocation and/or study potential mechanisms for effects on GLUT4 intrinsic transporter activity during muscle contractions. The latter was also mentioned in a recent review about GLUT4 (50).
GRANTS
E. A. Richter was funded by the Novo Nordisk Foundation and the Danish Council for Independent Research/Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
E.A.R. conceived and designed research; analyzed data; interpreted results of experiments; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Amira Klip, Jørgen Wojtaszewski, Bente Kiens, and Glenn McConell for valuable comments during preparation of the perspective.
REFERENCES
- 1.Fueger PT, Li CY, Ayala JE, Shearer J, Bracy DP, Charron MJ, Rottman JN, Wasserman DH. Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J Physiol 582: 801–812, 2007. doi: 10.1113/jphysiol.2007.132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlett KF, Andrikopoulos S, Proietto J, Hargreaves M. Exercise-induced muscle glucose uptake in mice with graded, muscle-specific GLUT-4 deletion. Physiol Rep 1: e00065, 2013. doi: 10.1002/phy2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryder JW, Kawano Y, Galuska D, Fahlman R, Wallberg-Henriksson H, Charron MJ, Zierath JR. Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4-deficient mice. FASEB J 13: 2246–2256, 1999. doi: 10.1096/fasebj.13.15.2246. [DOI] [PubMed] [Google Scholar]
- 4.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 5.Capaldo B, Gastaldelli A, Antoniello S, Auletta M, Pardo F, Ciociaro D, Guida R, Ferrannini E, Saccà L. Splanchnic and leg substrate exchange after ingestion of a natural mixed meal in humans. Diabetes 48: 958–966, 1999. doi: 10.2337/diabetes.48.5.958. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68: 1468–1474, 1981. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Circ Physiol 290: H272–H278, 2006. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kjaer M, Kiens B, Hargreaves M, Richter EA. Influence of active muscle mass on glucose homeostasis during exercise in humans. J Appl Physiol 71: 552–557, 1991. doi: 10.1152/jappl.1991.71.2.552 [DOI] [PubMed] [Google Scholar]
- 9.Kristiansen S, Gade J, Wojtaszewski JFP, Kiens B, Richter EA. Glucose uptake is increased in trained vs. untrained muscle during heavy exercise. J Appl Physiol 89: 1151–1158, 2000. doi: 10.1152/jappl.2000.89.3.1151. [DOI] [PubMed] [Google Scholar]
- 10.Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JFP, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab 288: E133–E142, 2005. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- 11.Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup I-L, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab 282: E435–447, 2002. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- 12.Sjøberg KA, Frøsig C, Kjøbsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66: 1501–1510, 2017. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 13.Calbet JAL, Gonzalez-Alonso J, Helge JW, Søndergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol 103: 969–978, 2007. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- 14.Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol Endocrinol Metab 251: E65–E70, 1986. doi: 10.1152/ajpendo.1986.251.1.E65. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, González-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol 566: 273–285, 2005. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz A, Sahlin K, Broberg S. Regulation of glucose utilization in human skeletal muscle during moderate dynamic exercise. Am J Physiol Endocrinol Metab 260: E411–415, 1991. doi: 10.1152/ajpendo.1991.260.3.E411. [DOI] [PubMed] [Google Scholar]
- 17.MacLean DA, Bangsbo J, Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J Appl Physiol 87: 1483–1490, 1999. doi: 10.1152/jappl.1999.87.4.1483. [DOI] [PubMed] [Google Scholar]
- 18.Kristiansen S, Asp S, Richter EA. Decreased muscle GLUT-4 and contraction-induced glucose transport after eccentric contractions. Am J Physiol Regul Integr Comp Physiol 271: R477–R482, 1996. doi: 10.1152/ajpregu.1996.271.2.R477. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48: 1192–1197, 1999. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- 20.Kristiansen S, Hargreaves M, Richter EA. Exercise-induced increase in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am J Physiol Endocrinol Metab 270: E197–E201, 1996. doi: 10.1152/ajpendo.1996.270.1.E197. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansen S, Hargreaves M, Richter EA. Progressive increase in glucose transport and GLUT-4 in human sarcolemmal vesicles during moderate exercise. Am J Physiol Endocrinol Metab 272: E385–E389, 1997. doi: 10.1152/ajpendo.1997.272.3.E385. [DOI] [PubMed] [Google Scholar]
- 22.Richter EA, Jensen P, Kiens B, Kristiansen S. Sarcolemmal glucose transport and GLUT-4 translocation during exercise are diminished by endurance training. Am J Physiol Endocrinol Metab 274: E89–E95, 1998. doi: 10.1152/ajpendo.1998.274.1.E89. [DOI] [PubMed] [Google Scholar]
- 23.Douen A, Ramlal T, Klip A, Young D, Cartee G, Holloszy J. Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology 124: 449–454, 1989. doi: 10.1210/endo-124-1-449. [DOI] [PubMed] [Google Scholar]
- 24.Douen A, Ramlal T, Rastogi S, Bilan P, Cartee G, Vranic M, Holloszy J, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter.” J Biol Chem 265: 13427–13430, 1990. [PubMed] [Google Scholar]
- 25.Goodyear LJ, Hirshman MF, Horton ES. Exercise-induced translocation of skeletal muscle glucose transporters. Am J Physiol Endocrinol Metab 261: E795–E799, 1991. doi: 10.1152/ajpendo.1991.261.6.E795. [DOI] [PubMed] [Google Scholar]
- 26.Ploug T, Wojtaszewski J, Kristiansen S, Hespel P, Galbo H, Richter EA. Glucose transport and transporters in muscle giant vesicles: Differential effects of insulin and contractions. Am J Physiol Endocrinol Metab 264: E270–E278, 1993. doi: 10.1152/ajpendo.1993.264.2.E270. [DOI] [PubMed] [Google Scholar]
- 27.Sylow L, Nielsen IL, Kleinert M, Møller LLV, Ploug T, Schjerling P, Bilan PJ, Klip A, Jensen TE, Richter EA. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J Physiol 594: 4997–5008, 2016. doi: 10.1113/JP272039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lund S, Holman GD, Schmitz O, Pedersen O. Glut 4 content in the plasma membrane of rat skeletal muscle: Comparative studies of the subcellular fractionation method and the exofacial photolabelling technique using ATB-BMPA. Febs Lett 330: 312–318, 1993. doi: 10.1016/0014-5793(93)80895-2. [DOI] [PubMed] [Google Scholar]
- 29.Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci 92: 5817–5821, 1995. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derave W, Lund S, Holman GD, Wojtaszewski J, Pedersen O, Richter EA. Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen content. Am J Physiol Endocrinol Metab 277: E1103–E1110, 1999. doi: 10.1152/ajpendo.1999.277.6.E1103. [DOI] [PubMed] [Google Scholar]
- 31.Sylow L, Møller LLV, Kleinert M, D’Hulst G, De Groote E, Schjerling P, Steinberg GR, Jensen TE, Richter EA. Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes 66: 1548–1559, 2017. doi: 10.2337/db16-1138. [DOI] [PubMed] [Google Scholar]
- 32.Lauritzen HPMM, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes 59: 2134–2144, 2010. doi: 10.2337/db10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M. Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J Biol Chem 273: 18173–18179, 1998. doi: 10.1074/jbc.273.29.18173. [DOI] [PubMed] [Google Scholar]
- 34.Kandror KV. A long search for Glut4 activation. Sci Signal 2003: PE5, 2003. doi: 10.1126/stke.2003.169.pe5]. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Hansen PA, Marshall BA, Holloszy JO, Mueckler M. Insulin unmasks a COOH-terminal GLUT4 epitope and increases glucose transport across T-tubules in skeletal muscle. J Cell Biol 135: 415–430, 1996. doi: 10.1083/jcb.135.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaid H, Talior-Volodarsky I, Antonescu C, Liu Z, Klip A. GAPDH binds GLUT4 reciprocally to hexokinase-II and regulates glucose transport activity. Biochem J 419: 475–484, 2009. doi: 10.1042/BJ20081319. [DOI] [PubMed] [Google Scholar]
- 37.Shamni O, Cohen G, Gruzman A, Zaid H, Klip A, Cerasi E, Sasson S. Supportive data on the regulation of GLUT4 activity by 3-O-methyl-D-glucose. Data Br 14: 329–336, 2017. doi: 10.1016/j.dib.2017.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristiansen S, Youn J, Richter EA. Effect of vanadate on glucose transporter (GLUT4) intrinsic activity in skeletal muscle plasma membrane giant vesicles. Biochim Biophys Acta 1282: 71–75, 1996. doi: 10.1016/0005-2736(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 39.Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Eccentric exercise decreases maximal insulin action in humans: Muscle and systemic effects. J Physiol 494: 891–898, 1996. doi: 10.1113/jphysiol.1996.sp021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron A, Brechtel G, Wallace P, Edelman S. Rates and tissue sites of non-insulin-and insulin-mediated glucose uptake in humans. Am J Physiol Endocrinol Metab 255: E769–E774, 1988. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 41.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 42.Wojtaszewski JFP, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: Time course and effect of exercise. Diabetes 46: 1775–1781, 1997. doi: 10.2337/diabetes.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 43.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol 66: 876–885, 1989. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 44.McConell GK, Sjøberg KA, Ceutz F, Gliemann L, Nyberg M, Hellsten Y, Frøsig C, Kiens B, Wojtaszewski JFP, Richter EA. Insulin‐induced membrane permeability to glucose in human muscles at rest and following exercise. J Physiol 598: 303–315, 2020. doi: 10.1113/JP278600. [DOI] [PubMed] [Google Scholar]
- 45.González-Alonso J, Calbet JAL, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J Physiol 520: 577–589, 1999. doi: 10.1111/j.1469-7793.1999.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86: 1032–1039, 1999. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- 47.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen TE, Sylow L, Rose AJ, Madsen AB, Angin Y, Maarbjerg SJ, Richter EA. Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca2+ release. Mol Metab 3: 742–753, 2014. doi: 10.1016/j.molmet.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylow L, Møller LLV, Kleinert M, Richter EA, Jensen TE. Stretch-stimulated glucose transport in skeletal muscle is regulated by Rac1. J Physiol 593: 645–656, 2015. doi: 10.1113/jphysiol.2014.284281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klip A, McGraw TE, James DE. Thirty sweet years of GLUT4. J Biol Chem 294: 11369–11381, 2019. doi: 10.1074/jbc.REV119.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: How is it regulated?. Physiology 20: 260–270, 2005. doi: 10.1074/jbc.REV119.008351. [DOI] [PubMed] [Google Scholar]