Abstract
Blood glucose and insulin homeostasis is disrupted during the progression of type 2 diabetes. Insulin levels and action are regulated by both peripheral and central responses that involve the intestine and microbiome. The intestine and its microbiota process nutrients and generate molecules that influence blood glucose and insulin. Peripheral insulin regulation is regulated by gut-segment-dependent nutrient sensing and microbial factors such as short-chain fatty acids and bile acids that engage G-protein-coupled receptors. Innate immune sensing of gut-derived bacterial cell wall components and lipopolysaccharides also alter insulin homeostasis. These bacterial metabolites and postbiotics influence insulin secretion and insulin clearance in part by altering endocrine responses such as glucagon-like peptide-1. Gut-derived bacterial factors can promote inflammation and insulin resistance, but other postbiotics can be insulin sensitizers. In parallel, activation of small intestinal sirtuin 1 increases insulin sensitivity by reversing high fat-induced hypothalamic insulin resistance through a gut-brain neuronal axis, whereas high fat-feeding alters small intestinal microbiome and increases taurochenodeoxycholic acid in the plasma and the dorsal vagal complex to induce insulin resistance. In summary, emerging evidence indicates that intestinal molecular signaling involving nutrient sensing and the host-microbe symbiosis alters insulin homeostasis and action. Gut-derived host endocrine and paracrine factors as well as microbial metabolites act on the liver, pancreas, and the brain, and in parallel on the gut-brain neuronal axis. Understanding common nodes of peripheral and central insulin homeostasis and action may reveal new ways to target the intestinal host-microbe relationship in obesity, metabolic disease, and type 2 diabetes.
Keywords: gut, immunometabolism, inflammation, insulin, microbiota
INTRODUCTION
An influx of nutrients from the intestinal lumen into the circulation after a meal increases insulin secretion from the pancreatic β-cells. A rise in plasma insulin levels lowers blood glucose levels by increasing glucose uptake into peripheral tissues such as muscle and fat and lowers glucose production by the liver. This well-defined insulin response maintains blood glucose, but the control of insulin homeostasis and action is disrupted during the progression of type 2 diabetes (T2D). For example, an excess of calorie intake and/or obesity promotes ineffective glucose lowering by a specific level of blood insulin, which is often termed insulin resistance (1). Blood glucose control is primarily balanced by insulin levels and action, and insulin resistance is linked to hyperinsulinemia. Hyperinsulinemia can promote obesity and be both a cause and consequence of insulin resistance (2, 3). Higher insulin levels can act as a trigger or compensate for insulin resistance, and this integrated response is often able to delay or prevent the development of overt diabetes, which is characterized by increased blood glucose. Thus, several classes of therapeutics in obesity, prediabetes, and T2D aim to enhance insulin action and secretion to maintain or improve blood glucose homeostasis.
Since the discovery of insulin, a major research focus has been to characterize pancreatic insulin secretion and insulin action/signaling cascades in the liver, muscle, and fat that regulate glucose and lipid metabolism in healthy, obese, and/or diabetic conditions (4–6). Nutrient-stimulation of pancreatic beta cells increases blood insulin, which reaches the liver, muscle, and fat and these insulin-sensitive organs impact nutrient metabolism and blood glucose control. It is well established that the intestine regulates peripheral insulin levels and action and blood glucose levels. The intestine regulates glucose absorption, barrier function, and endocrine communication with the pancreas through incretins. In parallel to the investigation of peripheral insulin control and action, a seminal work described central control of insulin action. A stand-alone and landmark study published in 1979 (7) reported that intracerebroventricular administration of insulin in baboons affects energy balance by lowering food intake and body weight, and thereby preliminary extending the biological function of insulin to the regulation of energy balance by the brain. Together with the discovery of leptin that was documented in 1994, an influx of literature in the past 20 years has reported that both insulin and leptin activate their respective receptors in various regions of the brain to regulate feeding and nutrient metabolism (8–17). Although these studies are mostly conducted in mice and rats owing to feasibility issues, emerging studies conducted in humans have reported that intranasal delivery of insulin or leptin increases peripheral insulin sensitivity and regulates glucose metabolism through central-dependent mechanisms (18–21). The purpose of this perspective is to highlight the role of the intestine in peripheral and central control of insulin homeostasis and action (Fig. 1). We aim to provide historical context and potential future directions related to how the intestine can influence insulin levels and action, and the connection to blood glucose control in healthy condition as well as in progression to T2D.
Figure 1.
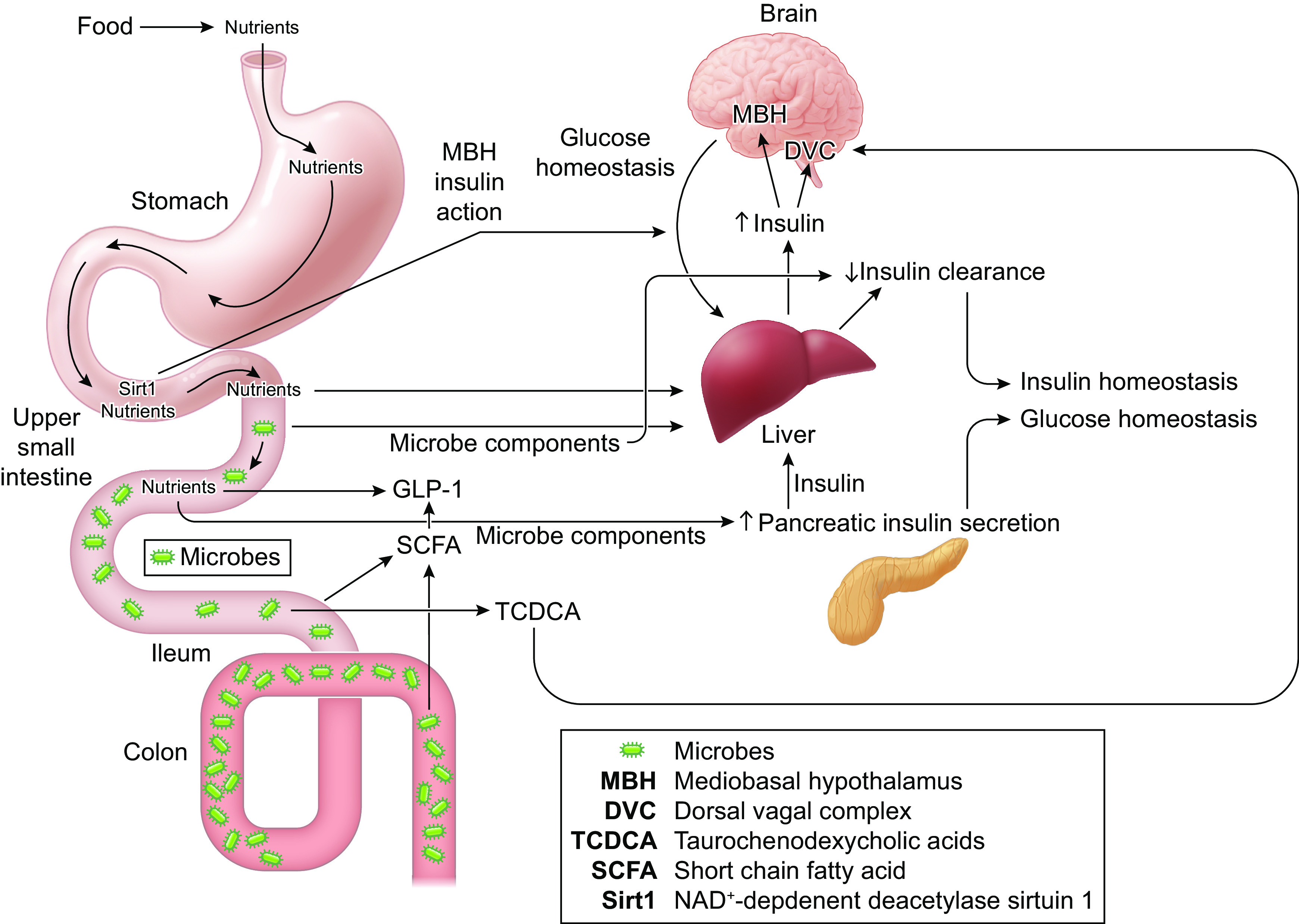
The intestine and microbiota contribute to peripheral and central insulin homeostasis and action. Mediobasal hypothalamus (MBH), dorsal vagal complex (DVC), taurochenodexycholic acids (TCDCA), NAD+-dependent deacetylase sirtuin 1 (Sirt1), and glucagon-like peptide 1 (GLP-1).
PERIPHERAL REGULATION OF INSULIN
It is well established that the intestine participates in the control of blood insulin responses. The intestine is the site of nutrient absorption. Food constituents, intestinal transit time, and intestinal transport of nutrients can all influence the magnitude and duration of postprandial blood glucose responses and consequent pancreatic insulin secretion (22). Detection of nutrients such as glucose in the gut lumen also evokes endocrine response that signals to the pancreas to promote insulin secretion. For example, sensing of glucose in the small intestine promotes enteroendocrine l-cell-mediated glucagon-like peptide-1 (GLP-1) secretion, which can augment glucose-stimulated insulin secretion from pancreatic beta cells (23). Oral xenobiotics can also influence blood glucose and insulin responses (24, 25). Historically, there has been a focus on how intestinal mediators alter insulin secretion. Blood insulin homeostasis is also controlled by insulin clearance and first-pass hepatic insulin clearance removes over half of the insulin that is secreted into the portal circulation (26). Seminal work showed that insulin has a specialized blood clearance mechanism involving carcinoembryonic antigen-related cell adhesion molecule (Ceacam-1) and insulin-degrading enzyme (IDE) (27, 28). Insulin receptor-mediated sequestration of extracellular insulin is also positioned to regulate blood insulin clearance (29). The intestine can absorb insulin and alter insulin clearance (30). For example, GLP-1 lowers insulin clearance (31). Hence an integrated response of increased insulin secretion and lower insulin clearance is relayed from gut hormones to raise blood insulin levels (Fig. 1).
The intestine harbors many bacteria and changes in these microbial communities, microbial metabolites and the host-microbe relationship are positioned to influence how the gut alters insulin homeostasis and action. Akin to the bidirectional relationship between blood glucose and gut microbes, the symbiotic relationships between intestinal microbiota and host hormones can influence insulin levels and action (32). Intestinal microbes help process dietary carbohydrates, which can influence the glycemic effect of food and consequent insulin responses (33). Gut microbes participate in an integrated response that is influenced by host genetic and environmental factors such as diet, which combine to modify blood glucose and pancreatic β-cell insulin secretion (34, 35). The exact microbial mediators and mechanisms that link features of different gut microbial communities to changes in insulin secretion are not yet known. However, there are two intriguing possibilities the link host-microbe symbiosis to insulin homeostasis. It is possible that microbes or their components penetrate the gut barrier and interact with receptors within the pancreas or insulin-responsive tissues. There is evidence for gut-derived bacterial cell wall components (i.e., postbiotics) such as muropeptides altering insulin secretion, whole-body insulin sensitivity, and cell autonomous insulin action (36–39). Diet-induced obesity can increase circulating muropeptides in mice. The type of muropeptide is critical, as those that activate Nod1 promote lipolysis and insulin resistance, where Nod2 ligands can promote insulin sensitivity (40–42). The ability of Nod1-activating muropeptides to promote inflammation in multiple metabolic tissues that control blood glucose is one way that Nod1 influences immunometabolism differently from Nod2 (37, 38). Further, Nod1 can synergize with other bacterial components such as lipopolysaccharides (LPSs) to promote profound inflammation, whereas Nod2 can tolerize immune response when combined with LPSs (37, 41). This is relevant to metabolic disease because LPSs derived from the gut microbiota can also penetrate the gut barrier during metabolic endotoxemia (43, 44). LPSs engage Toll-like receptor 4, and this innate immune response promotes inflammation in metabolic tissue that controls blood glucose. Even low doses of LPSs can compromise endocrine control of metabolism and promote hepatic and peripheral insulin resistance, thereby worsening blood glucose control (43). LPSs can also increase GLP-1 and insulin secretion (45, 46). It is possible that a feature of T2D such as hyperglycemia alters gut barrier function and promotes tissue-specific colonization of microbes or their metabolites (47, 48). Compartmentalization of microbes in different tissues and gut segments is an important consideration and site-specific changes in immune responses and microbial defenses should be considered in the regulation of insulin beyond “chronic inflammation” during obesity or T2D (49–51). Gut microbes can also modify insulin clearance. It is not yet clear how specific microbial components or tissue-specific microbial colonization influence insulin clearance. It is known that a small cluster of five related bacteria taxa explained most of the microbe influence insulin clearance during diet-induced obesity in mice (52). It was found that Enterococcaceae, Clostridiaceae, and Peptostreptococcaceae accounted for >90% of microbe-transmissible impaired insulin clearance and lowered levels of Ceacam-1 in the liver (52) (Fig. 1). Future work should focus on identifying how specific strains or molecules derived from gut bacteria alter insulin clearance, especially in the liver, which contains bacterial innate immune sensors that regulate lipid and glucose metabolism and gut dysbiosis (53).
The intestinal host-microbe relationship can influence insulin through changes in gut hormones. Again, compartmentalization of host-microbe response is important because microbes participate in the gut segment-dependent effects of incretins. Glucose is a key signal for l-cell-mediated GLP-1 release via a sodium glucose cotransporter 1-dependent pathway in the upper/small intestine and consequent changes in insulin secretion (54). Microbial metabolites such as short chain fatty acids (SCFAs) and bile acids interact with G-protein-coupled receptors and G-protein-coupled bile acid receptors and participate in upper/small intestine GLP-1 secretion and the consequent effect on insulin secretion (54). However, in the colon, these same microbial metabolites appear to regulate l-cell-mediated GLP-1 release and intestinal transit time, independent of glucose sensing (54, 55). A key future goal is to define specific mediators in each gut segment that contribute to insulin and glucose control and whether they depend on the host-microbe symbiosis. It is not yet clear if diet or pre-existing features of obesity and metabolic syndrome are the key drivers of intestinal changes that alter blood insulin. There is evidence that ingestion of specific dietary components is sufficient to coax microbes to generate endocrine responses that alter insulin homeostasis. For example, oligofructose and inulin fiber supplementation stimulates GLP-1 and peptide tyrosine tyrosine (PYY) in the ileum and colon, where the preclinical and clinical evidence has been expertly reviewed (56). Intestinal microbiota fermentation of a diet supplemented with inulin produces short-chain fatty acids, which act on the free fatty acid receptor 2 to increase the density of enteroendocrine cells that secrete PYY (57). Consistently, short-chain fatty acids activate free fatty acid receptor 2 in the ileum to regulate glucose homeostasis via a GLP-1-dependent pathway, whereas the ability of short-chain fatty acids to activate free fatty acid receptor 2 in the colon and secrete GLP-1 is also blunted by the activation of the farnesoid X receptor (58, 59). Finally, the ability of oligofructose ingestion to improve blood glucose control, increase glucose-stimulated insulin secretion, and reduce body weight gain is dependent on the action of the fermentable fiber on GLP-1 in mice as well (60).
CENTRAL REGULATION OF INSULIN ACTION
Insulin binds to the insulin receptor expressed in the hypothalamus and the dorsal vagal complex of the brain and triggers signaling cascades to regulate systemic glucose and energy homeostasis (9, 13, 61–65). Insulin resistance in both the hypothalamus and dorsal vagal complex is detected in association with excess calorie intake, obesity, and/or T2D, and are partly responsible for a disruption in metabolic homeostasis (61–63, 66–68). A direct molecular intervention in the hypothalamus and dorsal vagal complex can affect insulin action and contribute to improved blood glucose control (61, 66, 67). Alternatively, we put forward a working hypothesis that insulin resistance in the brain can be reversed by targeting the gut with small intestinal signaling molecules and/or components of the microbiota.
This working hypothesis stems from the fact that small intestinal-derived peptides such as CCK and GLP-1 act in a paracrine (via vagal afferent) and an endocrine (via the brain) fashion to trigger a gut-brain axis to regulate systemic metabolic homeostasis (69–75). Similarly, we postulate that brain insulin resistance can be reversed by targeting the small intestine via a gut-brain axis (Fig. 1). In this regard, short-term high fat (HF)-feeding induces insulin resistance in parallel to a reduction of NAD+-dependent deacetylase sirtuin 1 (Sirt1) in the small intestine of rats (76). Genetic knockdown of Sirt1 in the upper small intestinal mucosal of healthy rats for 14 days induces insulin resistance as the ability hyperinsulinemia to suppress hepatic glucose production is impaired when assessed by pancreatic-euglycemic clamps (76). Conversely, administration of insulin-sensitizer resveratrol directly into the upper small intestine of HF rats increases Sirt1 protein and activity in the small intestine mucosal, and consequently, not only rescues the ability of circulating hyperinsulinemia but also restores that ability of insulin infusion into the hypothalamus to lower hepatic glucose production during pancreatic-euglycemic clamps via a gut-brain neuronal axis (76). Importantly, the insulin-sensitizing effect of activating Sirt1 in the upper small intestine is retained in HF-induced obesity as well as in hyperglycemic diabetic rats (76). The underlying signaling mechanism within the upper small intestine of how Sirt1 signals to the hypothalamus remains unknown. Is the action of gut-derived peptides as well as any signaling cascades within the vagus necessary for small intestinal Sirt1 to increase insulin sensitivity? Is the neuronal relay between the nucleus of the solitary tract and the hypothalamus necessary for small intestinal Sirt1 to increase insulin sensitivity? If yes, which neurotransmitter(s) is involved?
Although much remains to be explored, a set of studies has demonstrated that insulin resistance in the dorsal vagal complex can also be reversed by targeting the small intestine (77). In parallel to the development of insulin resistance, short-term HF induces changes of the upper small intestinal microbiome in rats that led to an increase in small intestinal taurochenodeoxycholic acid (TCDCA) as well as in the plasma and the dorsal vagal complex (77). This is in parallel to the fact that the serum level of taurine-conjugated bile acids (i.e., TCDCA and tauroursodeoxycholic acid) is elevated and positively correlated with whole-body insulin resistance in people with T2D (78). Translation of a healthy microbiota into the upper small intestine of rats lowers TCDCA levels and also increases the ability of circulating hyperinsulinemia or insulin infusion into the dorsal vagal complex to lower hepatic glucose production in HF rats (77). Further, direct infusion of TCDCA into the dorsal vagal complex of HF rats with healthy microbiome transplant prevents the ability of the microbiome transplant to increase insulin sensitivity in HF rats (77). These findings collectively suggest that HF-induced changes in small intestinal microbiome increase TCDCA levels in the plasma and subsequently in the dorsal vagal complex to induce insulin resistance in rats (Fig. 1). It remains unknown whether changes in TCDCA levels will be found in model of obesity and diabetes, and whether the changes are small intestinal microbiome-dependent. Second, the underlying mechanism responsible for the elevation of TCDCA induced by changes in small intestinal microbiome remains elusive. Finally, the translational relevance of targeting small intestinal Sirt1 as well as changes in microbiome on insulin sensitivity in HF, obese, and/or diabetic conditions warrants further investigation.
CONCLUSIONS
The intestine relays peripheral and central signals that regulate blood insulin levels and action as well as glucose levels. Detection of nutrients in the intestine generates signals that alter blood insulin levels and action through responses in the pancreas, liver, and the brain. The intestinal microbiota participates in the paracrine and endocrine communication between the gut and other tissues involved in insulin and glucose control. Understanding how host-microbe symbiosis integrates peripheral and central control of insulin in both animal models and humans is an important future direction.
GRANTS
J.D.S is supported by a CIHR Foundation Grant (FDN-154295) and a Tier 2 Canada Research Chair in Metabolic Inflammation at McMaster University. T.K.T.L. is supported by a CIHR Foundation Grant (FDN-143204) and holds the John Kitson McIvor (1915-1942) Endowed Chair in Diabetes Research & a Tier 1 Canada Research Chair in Diabetes and Obesity at the Toronto General Hospital Research Institute and the University of Toronto. Both J.D.S and T.K.T.L. are supported by a CIHR Team Grant (MRT-168045) that focuses on investigating the metabolic impact of intestinal microbes from humans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.S. and T.K.T.L. drafted manuscript; J.D.S. and T.K.T.L. prepared figures; J.D.S. and T.K.T.L. edited and revised manuscript; J.D.S. and T.K.T.L. approved final version of manuscript.
REFERENCES
- 1.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E, the Pennington CALERIE Team. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu K-Y, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 16: 723–737, 2012. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Templeman NM, Flibotte S, Chik JHL, Sinha S, Lim GE, Foster LJ, Nislow C, Johnson JD. Reduced circulating insulin enhances insulin sensitivity in old mice and extends lifespan. Cell Rep 20: 451–463, 2017. doi: 10.1016/j.celrep.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Marette A. The fascinating physiology of insulin: celebrating a centennial hormone. Am J Physiol Endocrinol Metab. doi: 10.1152/ajpendo.00492. [DOI] [PubMed] [Google Scholar]
- 5.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 6.Vilsbøll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia 47: 357–366, 2004. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 7.Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 8.Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L. Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest 100: 3105–3110, 1997. doi: 10.1172/JCI119865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122 LP–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4: 49–60, 2006. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 3: 267–275, 2006. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz MW, Porte D. Diabetes, obesity, and the brain. Science 307: 375–379, 2005. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 16.Yue JTY, Lam TKT. Lipid sensing and insulin resistance in the brain. Cell Metab 15: 646–655, 2012. doi: 10.1016/j.cmet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [Erratum in Nature 374: 479, 1995]. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 18.Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64: 766–774, 2015. doi: 10.2337/db14-0685. [DOI] [PubMed] [Google Scholar]
- 19.Frank-Podlech S, von Schnurbein J, Veit R, Heni M, Machann J, Heinze JM, Kullmann S, Manzoor J, Mahmood S, Häring H-U, Preissl H, Wabitsch M, Fritsche A. Leptin replacement reestablishes brain insulin action in the hypothalamus in congenital leptin deficiency. Dia Care 41: 907–910, 2018. doi: 10.2337/dc17-1867. [DOI] [PubMed] [Google Scholar]
- 20.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Häring H-U, Fritsche A. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66: 1797–1806, 2017. doi: 10.2337/db16-1380. [DOI] [PubMed] [Google Scholar]
- 21.Heni M, Wagner R, Kullmann S, Veit R, Mat Husin H, Linder K, Benkendorff C, Peter A, Stefan N, Häring H-U, Preissl H, Fritsche A. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63: 4083–4088, 2014. doi: 10.2337/db14-0477. [DOI] [PubMed] [Google Scholar]
- 22.Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. Nutrients 10: 275, 2018. doi: 10.3390/nu10030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27: 740–756, 2018. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Duggan BM, Cavallari JF, Foley KP, Barra NG, Schertzer JD. RIPK2 dictates insulin responses to tyrosine kinase inhibitors in obese male mice. Endocrinology 161, 2020. doi: 10.1210/endocr/bqaa086. [DOI] [PubMed] [Google Scholar]
- 25.Kowalchuk C, Castellani LN, Chintoh A, Remington G, Giacca A, Hahn MK. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab 316: E1–E15, 2018. doi: 10.1152/ajpendo.00164.2018. [DOI] [PubMed] [Google Scholar]
- 26.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology 34: 198–215, 2019. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guénette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain. Proc Natl Acad Sci USA 100: 4162–4167, 2003. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poy MN, Yang Y, Rezaei K, Fernström MA, Lee AD, Kido Y, Erickson SK, Najjar SM. CEACAM1 regulates insulin clearance in liver. Nat Genet 30: 270–276, 2002. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 29.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97, 2000. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 30.Crane CW, Luntz GRWN. Absorption of insulin from the human small intestine. Diabetes 17: 625–627, 1968. doi: 10.2337/diab.17.10.625. [DOI] [PubMed] [Google Scholar]
- 31.Ahrén B, Thomaseth K, Pacini G. Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 48: 2140–2146, 2005. doi: 10.1007/s00125-005-1915-z. [DOI] [PubMed] [Google Scholar]
- 32.Anhê FF, Barra NG, Schertzer JD. Glucose alters the symbiotic relationships between gut microbiota and host physiology. Am J Physiol Endocrinol Metab 318: E111–E116, 2020. doi: 10.1152/ajpendo.00485.2019. [DOI] [PubMed] [Google Scholar]
- 33.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3: 289–306, 2012. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley KP, Zlitni S, Denou E, Duggan BM, Chan RW, Stearns JC, Schertzer JD. Long term but not short term exposure to obesity related microbiota promotes host insulin resistance. Nat Commun 9: 4681, 2018. doi: 10.1038/s41467-018-07146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, Vivas EI, Yandell BS, Broman AT, Hagenbuch B, Attie AD, Rey FE. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep 18: 1739–1750, 2017. doi: 10.1016/j.celrep.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denou E, Lolmède K, Garidou L, Pomie C, Chabo C, Lau TC, Fullerton MD, Nigro G, Zakaroff‐Girard A, Luche E, Garret C, Serino M, Amar J, Courtney M, Cavallari JF, Henriksbo BD, Barra NG, Foley KP, McPhee JB, Duggan BM, O'Neill HM, Lee AJ, Sansonetti P, Ashkar AA, Khan WI, Surette MG, Bouloumié A, Steinberg GR, Burcelin R, Schertzer JD. Defective NOD2 peptidoglycan sensing promotes diet‐induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med 7: 259–274, 2015. doi: 10.15252/emmm.201404169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schertzer JD, Tamrakar AK, Magalhães JG, Pereira S, Bilan PJ, Fullerton MD, Liu Z, Steinberg GR, Giacca A, Philpott DJ, Klip A. NOD1 activators link innate immunity to insulin resistance. Diabetes 60: 2206–2215, 2011. doi: 10.2337/db11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamrakar AK, Schertzer JD, Chiu TT, Foley KP, Bilan PJ, Philpott DJ, Klip A. NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology 151: 5624–5637, 2010. doi: 10.1210/en.2010-0437. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Pan Y, Zeng B, Zheng X, Wang H, Shen X, Li H, Jiang Q, Zhao J, Meng Z-X, Li P, Chen Z, Wei H, Liu Z. Intestinal lysozyme liberates Nod1 ligands from microbes to direct insulin trafficking in pancreatic beta cells. Cell Res 29: 516–532, 2019. doi: 10.1038/s41422-019-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavallari JF, Barra NG, Foley KP, Lee A, Duggan BM, Henriksbo BD, Anhê FF, Ashkar AA, Schertzer JD. Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice. Am J Physiol Endocrinol Metab 318: E579–E585, 2020. doi: 10.1152/ajpendo.00033.2020. [DOI] [PubMed] [Google Scholar]
- 41.Cavallari JF, Fullerton MD, Duggan BM, Foley KP, Denou E, Smith BK, Desjardins EM, Henriksbo BD, Kim KJ, Tuinema BR, Stearns JC, Prescott D, Rosenstiel P, Coombes BK, Steinberg GR, Schertzer JD. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab 25: 1063–1074.e3, 2017. doi: 10.1016/j.cmet.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Chi W, Dao D, Lau TC, Henriksbo BD, Cavallari JF, Foley KP, Schertzer JD. Bacterial peptidoglycan stimulates adipocyte lipolysis via NOD1. PLoS One 9: e97675, 2014. doi: 10.1371/journal.pone.0097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 44.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 45.Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros J-P, Le Guern N, Plesnik J, Thomas C, Bourgeois T, Dejong CHC, Kox M, Hundscheid IHR, Khan NA, Mandard S, Deckert V, Pickkers P, Drucker DJ, Lagrost L, Grober J. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep 21: 1160–1168, 2017. doi: 10.1016/j.celrep.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen AT, Mandard S, Dray C, Deckert V, Valet P, Besnard P, Drucker DJ, Lagrost L, Grober J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes 63: 471–482, 2014. doi: 10.2337/db13-0903. [DOI] [PubMed] [Google Scholar]
- 47.Anhê FF, Jensen BAH, Varin TV,Servant F, Van Blerk S, Richard D, Marceau S, Surette M, Biertho L, Lelouvier B, Schertzer JD, Tchernof A, Marette A. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab 2: 233–242, 2020. doi: 10.1038/s42255-020-0178-9. [DOI] [PubMed] [Google Scholar]
- 48.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383, 2018. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 49.Cavallari JF, Denou E, Foley KP, Khan WI, Schertzer JD. Different Th17 immunity in gut, liver, and adipose tissues during obesity: the role of diet, genetics, and microbes. Gut Microbes 7: 82–89, 2016. doi: 10.1080/19490976.2015.1127481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massier L, Chakaroun R, Tabei S, Crane A, Didt KD, Fallmann J, von Bergen M, Haange S-B, Heyne H, Stumvoll M, Gericke M, Dietrich A, Blüher M, Musat N, Kovacs P. Adipose tissue derived bacteria are associated with inflammation in obesity and type 2 diabetes. Gut 69: 1796–1806, 2020. doi: 10.1136/gutjnl-2019-320118. [DOI] [PubMed] [Google Scholar]
- 51.McPhee JB, Schertzer JD. Immunometabolism of obesity and diabetes: Microbiota link compartmentalized immunity in the gut to metabolic tissue inflammation. Clin Sci 129: 1083–1096, 2015. doi: 10.1042/CS20150431. [DOI] [PubMed] [Google Scholar]
- 52.Foley KP, Zlitni S, Duggan BM, Barra NG, Anhê FF, Cavallari JF, Henriksbo BD, Chen CY, Huang M, Lau TC, Plante R, Schwab M, Marette A, Schertzer JD. Gut microbiota impairs insulin clearance in obese mice. Mol Metab, 42: 101067, 2002. doi: 10.1016/j.molmet.2020.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavallari JF, Pokrajac NT, Zlitni S, Foley KP, Henriksbo BD, Schertzer JD. NOD2 in hepatocytes engages a liver-gut axis to protect against steatosis, fibrosis, and gut dysbiosis during fatty liver disease in mice. Am J Physiol Endocrinol Metab 319: E305–E314, 2020. doi: 10.1152/ajpendo.00181.2020. [DOI] [PubMed] [Google Scholar]
- 54.Greiner TU, Bäckhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab 5: 753–758, 2016. doi: 10.1016/j.molmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GÖ, Larsson T, Drucker DJ, Delzenne NM, Cani PD, Bäckhed F. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14: 582–590, 2013. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Rastelli M, Cani PD, Knauf C. The gut microbiome influences host endocrine functions. Endocr Rev 40: 1271–1284, 2019. doi: 10.1210/er.2018-00280. [DOI] [PubMed] [Google Scholar]
- 57.Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj J, Bell JD, Bell-Anderson K, Mackay CR, Ghatei MA, Bloom SR, Frost G, Bewick GA. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab 6: 48–60, 2017. doi: 10.1016/j.molmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ducastel S, Touche V, Trabelsi M-S, Boulinguiez A, Butruille L, Nawrot M, Peschard S, Chávez-Talavera O, Dorchies E, Vallez E, Annicotte J-S, Lancel S, Briand O, Bantubungi K, Caron S, Bindels LB, Delzenne NM, Tailleux A, Staels B, Lestavel S. The nuclear receptor FXR inhibits glucagon-like peptide-1 secretion in response to microbiota-derived Short. Sci Rep 10: 174, 2020. doi: 10.1038/s41598-019-56743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zadeh-Tahmasebi M, Duca FA, Rasmussen BA, Bauer P, Côté CD, Filippi BM, Lam TKT. Activation of short and long chain fatty acid sensing machinery in the ileum lowers glucose production in vivo. J Biol Chem 291: 8816–8824, 2016. doi: 10.1074/jbc.M116.718460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55: 1484–1490, 2006. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 61.Filippi BM, Abraham MA, Silva PN, Rasti M, LaPierre MP, Bauer P, Rocheleau J, Lam TKT. Dynamin-related protein 1-dependent mitochondrial fission changes in the dorsal vagal complex regulate insulin action. Cell Rep 18: 2301–2309, 2017. doi: 10.1016/j.celrep.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 62.Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JTY, Lam TKT. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes 63: 892–899, 2014. doi: 10.2337/db13-1044. [DOI] [PubMed] [Google Scholar]
- 63.Filippi BM, Yang CS, Tang C, Lam TKT. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab 16: 500–510, 2012. doi: 10.1016/j.cmet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 65.Pocai A, Lam TKT, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 66.Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 118: 2959–2968, 2008. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schriever SC, Kabra DG, Pfuhlmann K, Baumann P, Baumgart E, Nagler J, , et al. Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity. J Clin Invest 130: 6093–6108, 2020. doi: 10.1172/JCI136363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duca FA, Bauer PV, Hamr SC, Lam TKT. Glucoregulatory relevance of small intestinal nutrient sensing in physiology, bariatric surgery, and pharmacology. Cell Metab 22: 367–380, 2015. doi: 10.1016/j.cmet.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Iwasaki Y, Sendo M, Dezaki K, Hira T, Sato T, Nakata M, Goswami C, Aoki R, Arai T, Kumari P, Hayakawa M, Masuda C, Okada T, Hara H, Drucker DJ, Yamada Y, Tokuda M, Yada T. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat Commun 9: 113, 2018. doi: 10.1038/s41467-017-02488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krieger J-P, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 65: 34 LP–43, 2016. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 72.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 75.Varin EM, Mulvihill EE, Baggio LL, Koehler JA, Cao X, Seeley RJ, Drucker DJ. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep 27: 3371–3384.e3, 2019. doi: 10.1016/j.celrep.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 76.Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, Breen DM, Filippi BM, Lam TKT. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med 21: 498–505, 2015. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S-Y, Li RJW, Lim Y-M, Batchuluun B, Liu H, Waise TMZ, Lam TKT. FXR in the dorsal vagal complex is sufficient and necessary for upper small intestinal microbiome-mediated changes of TCDCA to alter insulin action in rats. Gut, in press. doi: 10.1136/gutjnl-2020-321757. [DOI] [PubMed] [Google Scholar]
- 78.Wewalka M, Patti M-E, Barbato C, Houten SM, Goldfine AB. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J Clin Endocrinol Metab 99: 1442–1451, 2014. doi: 10.1210/jc.2013-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]


