Keywords: aging, eicosapentaenoic acid, fiber type transition, muscle strength, sarcopenia
Abstract
Age-related sarcopenia is associated with a variety of changes in skeletal muscle. These changes are interrelated with each other and associated with systemic metabolism, the details of which, however, are largely unknown. Eicosapentaenoic acid (EPA) is a promising nutrient against sarcopenia and has multifaceted effects on systemic metabolism. In this study, we hypothesized that the aging process in skeletal muscle can be intervened by the administration of EPA. Seventy-five-week-old male mice were assigned to groups fed an EPA-deprived diet (EPA−) or an EPA-enriched diet with 1 wt% EPA (EPA+) for 12 wk. Twenty-four-week-old male mice fed with normal chow were also analyzed. At baseline, the grip strength of the aging mice was lower than that of the young mice. After 12 wk, EPA+ showed similar muscle mass but increased grip strength compared with EPA−. EPA+ displayed higher insulin sensitivity than EPA−. Immunohistochemistry and gene expression analysis of myosin heavy chains (MyHCs) revealed fast-to-slow fiber type transition in aging muscle, which was partially inhibited by EPA. RNA sequencing (RNA-Seq) analysis suggested that EPA supplementation exerts pathway-specific effects in skeletal muscle including the signatures of slow-to-fast fiber type transition. In conclusion, we revealed that aging skeletal muscle in male mice shows lower grip strength and fiber type changes, both of which can be inhibited by EPA supplementation irrespective of muscle mass alteration.
NEW & NOTEWORTHY This study demonstrated that the early phenotype of skeletal muscle in aging male mice is characterized by muscle weakness with fast-to-slow fiber type transition, which could be ameliorated by feeding with EPA-enriched diet. EPA induced metabolic changes such as an increase in systemic insulin sensitivity and altered muscle transcriptome in the aging mice. These changes may be related to the fiber type transition and influence muscle quality.
INTRODUCTION
Sarcopenia, defined by muscle weakness with low muscle quantity or quality (1), is a critical health problem in the geriatric population. Sarcopenia is associated with an increased risk of falls, fractures, frailty, and metabolic diseases, and it correlates with a range of adverse outcomes (2, 3). In general, a decrease in muscle strength reflects a decrease in muscle mass. However, aging is considered to be a complicated process, and reduction in muscle strength and subsequent impairment of physical performance could precede the evident loss of muscle mass (4). In this line, the updated recommendation of the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) focuses on low muscle strength as a key characteristic of sarcopenia (1).
Age-related decrease in muscle mass and strength is mechanistically based on the various changes that occur during the aging process. These include anatomical changes such as fast-to-slow type transition of muscle fibers, intramuscular and intermuscular fat infiltration, alterations of neuromuscular junction (NMJ), microvascular changes, and biochemical changes such as mitochondrial dysfunction, imbalances in protein metabolism, inflammation, oxidative stress, apoptosis, and satellite cell dysfunction (5). These age-related changes are not only interrelated with each other but also associated with other organs and/or biological processes (6, 7). Understanding the age-related changes in skeletal muscle, therefore, is essential for the development of early intervention strategies against sarcopenia.
Eicosapentaenoic acid (EPA) is one of the omega-3 (n-3) polyunsaturated fatty acids (PUFAs), which is abundant in fish oil. EPA is used in clinical settings to lower serum triglyceride levels (8, 9) and is also expected to reduce the risk of cardiovascular events (10). Several human studies have suggested that EPA supplementation protects against sarcopenia (11–14), although other reports have not reported any significant changes (15–17). Considering multifaceted effects of EPA on metabolism (18), we hypothesized that EPA might influence various age-related biological processes, thereby combating development of sarcopenia.
In this study, to elucidate the effect of EPA on the muscle aging, we administered EPA to male mice at an early stage of sarcopenia. We demonstrated that EPA augments grip strength in aging mice without an increase in muscle mass. Moreover, aging mice fed with an EPA-enriched diet showed various metabolic changes, and muscle histopathological and transcriptomic analyses revealed that a fast-to-slow fiber type transition occurs in aging muscle, which can be partially inhibited by EPA.
MATERIALS AND METHODS
Animals
Seventy-one-week-old C57BL/6J male mice were obtained from Charles River Laboratories, Inc. (CRL) and housed individually under a 12:12-h light/dark cycle in a specific pathogen-free (SPF) environment at Laboratory Animal Research Center, the Institute of Medical Science, the University of Tokyo. The mice were subjected to a 4-wk acclimatization period before the initiation of experiments. In the latter half of this acclimatization period, mice were fed standard powder chow. At the start of the study, 75-wk-old mice (Aging, n = 12) were randomly assigned to groups fed with control (EPA−, n = 6) or EPA-enriched diet (EPA+, n = 6). EPA− diet was based on CE-2 but deprived of fish powder (65.3% kcal carbohydrate, 23.4% kcal protein, and 11.3% kcal fat; CLEA Japan, Inc.) and contained nitrogen-free extracts (55.3% by weight), crude protein (19.9%), crude fat (4.3%), crude fiber (5.3%), crude ash (6.2%), and moisture (9.0%). For EPA-enriched diet, purified EPA ethyl ester (EPA-E) (purity > 96%, Nippon Suisan Kaisha, Ltd.) was added to the control diet with a final EPA concentration of 1 wt% (Mochida Pharmaceutical Co., Ltd.). A comparison of diet compositions and calorie counts of EPA− and EPA+ diet is presented in Table 1. We assessed food consumption by measuring the weight of diet and calculated the calorie content of the food based on diet composition. Both powder diets were stored at −20°C in a vacuum pack with oxygen scavenger. Throughout the study, mice were provided free access to food and water. Body weight (BW) was measured once a week. After 12 wk of diet supplementation, mice were euthanized following 4-h fasting. Twenty-four-week-old mice (Young) fed with standard chow were also euthanized. Mice were anesthetized with an intraperitoneal injection with a dose of 75 mg/kg body wt pentobarbital sodium salt (Kyoritsu Seiyaku Co.). Blood was collected from the inferior vena cava using a heparinized syringe and centrifuged at 10,000 rpm at 4°C for 5 min. Plasma and organ samples were immediately frozen in liquid nitrogen. Frozen tissues were crushed using a Cryo-Press (Microtec Co., Ltd.) and a mini compressor (Kiso Power Tool Co.). The crushed tissues were dispensed into a 1.5-mL tube in liquid nitrogen and stored at −80°C. All animal experiments were performed under the approval of the Animal Ethics Committee of the Institute of Medical Science, the University of Tokyo (Permission number: PA13-64, PH14-34, PH15-16, PH15-24, PH16-11, PH16-13, PH16-26, PA18-26).
Table 1.
Diet composition
| Content | EPA− |
EPA+ |
||
|---|---|---|---|---|
| g/100 g | kcal/100 g | g/100 g | kcal/100 g | |
| Nitrogen-free extracts | 55.3 | 221 | 54.8 | 219 |
| Crude protein | 19.9 | 80 | 19.7 | 79 |
| Crude fat | 4.3 | 39 | 4.2 | 38 |
| Crude fiber | 5.3 | 0 | 5.2 | 0 |
| Crude ash | 6.2 | 0 | 6.2 | 0 |
| Moisture | 9.0 | 0 | 8.9 | 0 |
| EPA-E | 0 | 0 | 1.0 | 0 |
| Total | 340 | 336 | ||
EPA-E, eicosapentaenoic acid ethyl ester.
Measurement of Grip Strength
At baseline and 4 days before euthanasia, grip strength was measured using Grip Strength Meter for Mice Model (MK-380M; Muromachi Kikai Co., Ltd.). After placing forelimbs and hind limbs on a mesh, each mouse was gently pulled back until it lost its grip from the mesh. The maximal force generated at the point where the animal lost its grip was measured in grams of resistance by a strain gauge. The median score of five trials was recorded.
Treadmill Exhaustion Test
Treadmill exhaustion test was performed using MK-680AT/02M (Muromachi Kikai Co., Ltd.). Uphill inclination (slopes of 10°) was used for this test. The initial speed was 10 m/min, and the speed was increased by 1 m/min subsequently after every consecutive minute to obtain a maximum speed of 25 m/min. The mice ran for 30 min totally or until exhaustion. Exhaustion was defined as the inability to run on the treadmill despite repeated electric prodding to the mice. The running time was measured, and the running distance was calculated.
Measurement of Triglyceride Levels
Plasma triglyceride level and triglyceride contents of liver and skeletal muscle were measured with a Triglyceride Quantification kit (K622-100, BioVision) by colorimetric analysis according to the manufacturer’s instructions.
Measurement of Plasma Glucose, Insulin, and Corticosterone Levels
Plasma glucose levels were measured with a Glucose CII Test Wako kit (439-90901, Wako) by using a DeNovix Spectrophotometer (VBI Core Labs). Plasma levels of insulin and corticosterone were measured with an Ultra Sensitive Mouse Insulin ELISA kit (MS303, Morinaga Institute of Biological Science, Inc.) and a Corticosterone EIA kit (YK240, Yanaihara Institute Inc.) by using an iMark Microplate Absorbance Reader (Bio-Rad), respectively. Measurements were carried out according to the manufacturer’s instructions. Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = plasma insulin (µU/mL) × plasma glucose (mg/dL)/405.
Histological Analysis
Considering its role in grip force production, plantar flexor complex except for soleus muscle, that is, the medial and lateral gastrocnemius and plantaris muscle (Gas + Pl) was used in this study. Gas + Pl samples were stood upright on a piece of cork using tragacanth gum and frozen in liquid nitrogen-cooled isopentane. They were cut into 8-µm sections with a cryostat (Leica, CM1950) at −25°C and either processed for immunostaining or hematoxylin & eosin (HE) staining.
For fluorescence immunostaining of myosin heavy chains (MyHCs), we employed the method described in a previous report (19) with minor modifications. Briefly, serial sections were prepared, and the first section was stained by primary antibodies against MYH7, MYH2, and MYH4, whereas the second section was stained by a primary antibody against MYH1. Cryosections were first fixed in cold acetone (Nacalai Tesque Inc.) for 15 min and dried for 1 h. After blocking with 5% goat serum containing 1% bovine serum albumin (BSA) in PBS for 15 min, sections were incubated overnight at 4°C with primary antibodies diluted by 1% BSA in PBS. Sections were washed with PBS, incubated with secondary antibodies diluted in 1% BSA in PBS at room temperature for 1 h, washed with PBS, and then mounted in Mount-Quick aqueous mounting medium (Daido Sangyo Co., Ltd.). The first section was incubated with a cocktail of three primary antibodies or a cocktail of three secondary antibodies. The primary antibodies used in this staining were as follows: mouse IgG2b MyHC type 1 antibody (1:50, BA-D5), mouse IgG1 MyHC type 2A antibody (1:400, SC-71), mouse IgM MyHC type 2B antibody (1:50, BF-F3), and mouse IgM MyHC type 2X antibody (1:100, 6H1). All MyHC antibodies were obtained from Developmental Studies Hybridoma Bank (DSHB). The secondary antibodies used in this staining were as follows: goat anti-mouse IgG2b labeled with Alexa Fluor 350 (1:200, A-21140), goat anti-mouse IgG1 labeled with Alexa Fluor 568 (1:1,000, A-21124), and goat anti-mouse IgM labeled with Alexa Fluor 488 (1:1,000, A-21142). All secondary antibodies were obtained from Thermo Fisher Scientific. Images of the immunofluorescence staining were obtained under a BZ-X700 fluorescence microscope (KEYENCE) and analyzed using Image J software (National Institutes of Health). Double-positive fibers were defined as hybrid fibers, MYH7 and MYH2 double-positive fibers were type 1/2A, MYH2 and MYH1 double-positive fibers were type 2AX, and MYH1 and MYH4 double-positive fibers were type 2XB. Single-positive fibers stained by MYH7, MYH2, MYH1, and MYH4 were defined as fiber types 1, 2A, 2X, and 2B, respectively. The number and percentage of types 1, 2A, 2X, 2B, 1/2A, 2AX, and 2XB fibers were evaluated. The number of fibers was counted using HE-stained sections as reference. For HE staining (ScyTek Laboratories, Inc.), sections were fixed in 10% formaldehyde neutral buffer solution (Nacalai Tesque Inc.) for 10 min. After washing in running tap water for 10 min, sections were immersed in Mayer’s hematoxylin solution (Lillie’s Modification) for 3 min, washed in running tap water for 10 min, and immersed in Eosin Y solution (modified alcoholic) for 10 min. Subsequently, sections were washed and dehydrated in 99.5% ethanol and rendered transparent by xylene. Finally, sections were mounted with a malinol mounting agent (Muto Pure Chemicals Co., Ltd.) and observed under an optical microscope (BZ-X700 system).
Quantitative Reverse-Transcription Polymerase Chain Reaction
Total RNA from Gas + Pl was extracted using Sepasol-RNA I Super G (Nacalai Tesque Inc.) and subjected to reverse-transcription with oligo-dT primer (Invitrogen) using Super Script III First-Strand Synthesis System for RT-PCR (Invitrogen). PCR was performed with THUNDERBIRD probe qPCR mix (TOYOBO Co., Ltd.), Universal probe library sets (Roche), and CFX96 real-time PCR detection system (Bio-Rad). Expression levels of mRNA were calculated based on standard curves generated for each primer pair, and 36B4 mRNA was used as an internal control. The sequences of the primer used in the assays are listed in Table 2.
Table 2.
Primers for quantitative RT-PCR
| Target Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Gene Number |
|---|---|---|---|
| 36B4 | actggtctaggacccgagaag | ctcccaccttgtctccagtc | NM_007475 |
| GR | tgacgtgtggaagctgtaaagt | catttcttccagcacaaaggt | NM_008173 |
| FKBP5 | aaacgaaggagcaacggtaa | tcaaatgtccttccaccaca | NM_010220 |
| KLF15 | acaggcgagaagcccttt | catctgagcgggaaaacct | NM_023184 |
| FoxO1 | cttcaaggataagggcgaca | gacagattgtggcgaattga | NM_019739 |
| FoxO3a | gctaagcaggcctcatctca | ttccgtcagtttgagggtct | NM_019740 |
| FoxO4 | aaggacaagggtgacagcaa | ctgtgcaaggacaggttgtg | NM_018789 |
| MuRF1 | cctgcagagtgaccaagga | ggcgtagagggtgtcaaact | NM_001039048 |
| Atrogin-1 | agtgaggaccggctactgtg | gatcaaacgcttgcgaatct | NM_026346 |
| LC3 | catgagcgagttggtcaaga | ccatgctgtgctggttga | NM_025735 |
| BNIP3 | cctgtcgcagttgggttc | gaagtgcagttctacccaggag | NM_009760 |
| Myh7 | cgcatcaaggagctcacc | ctgcagccgcagtaggtt | NM_080728 |
| Myh2 | aactccaggcaaaagtgaaatc | cttggatagatttgtgttggattg | NM_001039545 |
| Myh1 | aatcaaaggtcaaggcctacaa | gaatttggccaggttgacat | NM_030679 |
| Myh4 | aatcaaaggtcaaggcctacaa | gaatttggccaggttgacat | NM_030679 |
RNA Sequencing
RNA samples of Gas + Pl derived from Young (n = 3), EPA− (n = 6), and EPA+ (n = 6) were pooled. The quality and quantity of total RNA were verified using DeNovix and Agilent 2100 Bioanalyzer. Library construction and sequencing were performed on DNBSEQ-G400, and 100-bp paired-end reads were generated (Beijing Genomics Institute, China). After data filtering, reads were aligned to the GRCm38 reference genome using Hisat2 (v2.1.0) and counted using Stringtie (v1.3.4d). Gene annotation was performed using an Ensembl gene annotation file downloaded from University of California, Santa Cruz (UCSC) genome browser. Ensembl gene IDs were converted to gene names using biomaRt (v2.38.0) in R (v3.5.0). Counts per million (CPM) were used for comparison of gene expression between samples. Differentially expressed genes (DEGs) were determined using NOISeq package in R (20). The NOISeq is a data-adaptive and nonparametric method for analyzing RNA-sequencing (RNA-Seq) data, which can also handle samples without replicates by adopting some simulations. Genes having no counts (CPM = 0) in both samples to compare were denoted as NA (not assessed), but otherwise, all expression data were used. DEGs were explored using the default setting (pnr = 0.2, nss = 5, v = 0.02, lc = 0), and a threshold of q = 0.5. Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using DAVID 6.8 (https://david.ncifcrf.gov). The super-computing resource was provided by the Human Genome Center, the Institute of Medical Science, the University of Tokyo (http://sc.hgc.jp/shirokane.html). The data were deposited in the Gene Expression Omnibus (GEO) under accession number GSE149307.
Statistical Analysis
Results are presented as the means ± SE. Comparisons between two groups were analyzed using unpaired Student’s t test, and comparisons among three groups were analyzed using one-way ANOVA followed by Bonferroni’s post hoc test. Paired Student’s t test was used to compare pre- and poststudy results. Results were considered statistically significant at P < 0.05.
RESULTS
Aging Male Mice Fed with EPA Showed an Increase in Grip Strength without a Change in Muscle Mass
We observed that 75-wk-old male mice (Aging) showed significantly higher body weight and lower grip strength than 24-wk-old male mice (Young) (Fig. 1A). Because muscle weakness during aging could reflect the initial phenotype of sarcopenia, we focused on the effects of EPA on skeletal muscle in these mice. Aging mice were fed with EPA-deprived diet (EPA−) or EPA-enriched diet (EPA+) for 12 wk. There were no differences in body weight between the two groups at any time point (Fig. 1B). Although food intake of EPA+ was slightly higher than that of EPA−, the calorie intake calculated from the composition of each diet was comparable (Fig. 1C). EPA+, however, showed significantly greater grip strength than EPA− (Fig. 1D). Upon normalizing the grip strength values with measurements such as body weight, tissue weight of Gas + Pl, and tibial length, significantly higher values were observed in EPA+ than in EPA− (Fig. 1E). Body weight-normalized grip strength significantly increased from baseline to the end of the study in EPA+ (Fig. 1E, left panel). In contrast, there was no significant difference between EPA− and EPA+ in either distance or duration of the treadmill test (Fig. 1F). Taken together, EPA supplementation in aging male mice with muscle weakness improved the ability of the mice to generate muscle force rather than endurance.
Figure 1.
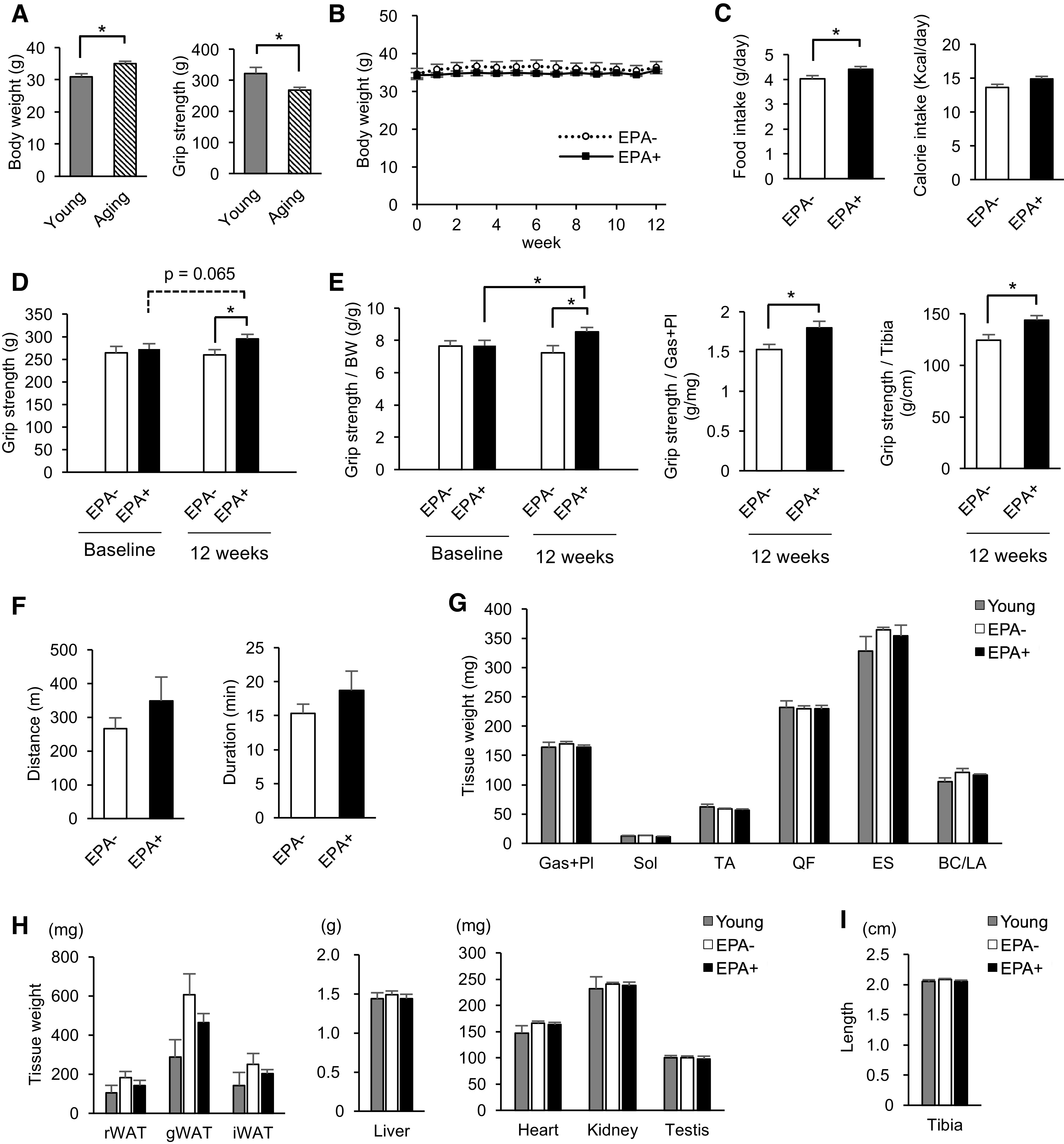
Aging male mice fed with EPA-enriched diet show increased grip strength but no change in endurance capacity or muscle mass. A: basal body weight and grip strength of 24-wk-old male mice (Young, n = 8) and 75-wk-old male mice (Aging, n = 12). B: aging were fed with control diet (EPA−, n = 6) or EPA-enriched diet (EPA+, n = 6) for 12 wk, and body weight was measured once a week. C: daily average food intake and calorie intake in EPA− and EPA+ for the observational period (n = 6). D: grip strength of EPA− and EPA+ at baseline and at the end of the study (n = 6). E: grip strength normalized by body weight, tissue weight of Gas + Pl, and tibial length (n = 6). F: running distance and duration of treadmill exercise of EPA− and EPA+ at the end of the study (n = 6). G: muscle weight of Young (n = 4), EPA− (n = 6), and EPA+ (n = 6) fed the 12-wk diet. H: tissue weight of Young (n = 4), EPA− (n = 6), and EPA+ (n = 6) fed the 12-wk diet. I: tibial length of Young (n = 4), EPA− (n = 6), and EPA+ (n = 6) fed the 12-wk diet. Data are expressed as means ± SE, *P < 0.05, assessed by Student’s t test (A–F), paired Student’s t test between baseline and the end of the study (D and E), or one-way ANOVA followed by Bonferroni’s post hoc test (G–I). EPA, eicosapentaenoic acid; Gas, gastrocnemius; Pl, plantaris; Sol, soleus; TA, tibialis anterior; QF, quadriceps femoris; ES, erector spinae; BC/LA, bulbocavernosus and levator ani; rWAT, retroperitoneal white adipose tissue; gWAT, gonadal white adipose tissue; iWAT, inguinal white adipose tissue.
Because muscle strength generally reflects muscle mass, we next evaluated the weight of various skeletal muscles in these mice. However, there was no difference among Young, EPA−, and EPA+ in the weight of any muscles including medial and lateral gastrocnemius and plantaris (Gas + Pl), soleus (Sol), tibialis anterior (TA), quadriceps femoris (QF), erector spinae (ES), and bulbocavernosus and levator ani (BC/LA) (Fig. 1G). These results indicated that muscle mass at the early stage of aging was not reduced compared with that in young mice, irrespective of EPA supplementation. In addition, EPA augmented muscle strength without increase in muscle mass, suggesting that EPA affects muscle quality.
Muscle quality is influenced by changes in body composition such as systemic fat accumulation (21). Indeed, EPA− and EPA+ tended to exhibit increased retroperitoneal, gonadal, and inguinal adipose tissue weight compared with Young, which tended to be inhibited by EPA supplementation (Fig. 1H). No difference was observed in weights of the liver, heart, kidney, and testis among Young, EPA−, and EPA+ (Fig. 1H). Tibial length was found to be similar in the three groups (Fig. 1I). These changes in adipose tissue weight may reflect that the effect of EPA on the aging process is accompanied by systemic metabolic alterations.
Aging Male Mice Fed EPA-Enriched Diet Exhibited Improved Glucose Tolerance
Considering the multifaceted metabolic effects of EPA (18), we further examined the metabolic alterations in response to EPA supplementation in aging male mice. Plasma triglyceride and liver triglyceride levels showed a tendency to decrease in EPA+ (Fig. 2A). Muscle triglyceride content showed no difference between EPA− and EPA+ (Fig. 2A). EPA+ showed lower plasma glucose levels and a tendency toward lowered plasma insulin levels in a 4-h fasted state, resulting in lower HOMA-IR (Fig. 2B).
Figure 2.
EPA improves glucose tolerance in aging male mice. A: plasma, liver, and muscle triglyceride levels in EPA− and EPA+ (n = 6). B: plasma glucose and insulin concentrations, and calculated HOMA-IR of EPA− and EPA+ (n = 6). Data are expressed as means ± SE, *P < 0.05, assessed by Student’s t test. EPA, eicosapentaenoic acid; HOMA-IR, homeostasis model assessment-insulin resistance.
Not only fat accumulation but also redistribution of fat depots to ectopic sites such as liver and muscle is closely related to age-related sarcopenia and insulin resistance (21). Our results, therefore, indicate that EPA supplementation to aging male mice induces the improvement in whole-body insulin resistance, probably via metabolic alterations including inhibition of fat accumulation in adipose tissue and liver. These metabolic aspects might have contributed to increased muscle strength in EPA+.
EPA Supplementation Partially Modifies the Changes in Catabolic Gene Expression in Aging Muscle
Because EPA− and EPA+ exhibited comparable muscle mass (Fig. 1G), the catabolic process in skeletal muscle was presumed to be primarily unaffected by EPA supplementation. One of the critical pathways controlling protein catabolism is glucocorticoid receptor (GR) signaling (22). GR is activated by glucocorticoids, and it is reported that elevated cortisol levels might increase the risk of sarcopenia (23). We, therefore, studied the effects of EPA on GR signaling in aging skeletal muscle. The level of plasma corticosterone increased slightly but not significantly with aging, and no difference was seen between EPA− and EPA+ (Fig. 3A). Both groups exhibited lower GR mRNA levels than Young, and no difference was observed between them. We also evaluated expression levels of GR direct target genes (FKBP5, KLF15, FoxOs, and MuRF1) (24), and further downstream genes regulated by KLF15 or FoxOs (Atrogin-1, LC3, and BNIP3) (24). mRNA levels of the GR direct target genes were significantly lower (FKBP5, FoxO3a, and FoxO4) or tended to be lower (KLF15, FoxO1, and MuRF1) in EPA− than in Young. EPA supplementation did not significantly alter the levels of these genes, which corroborated with GR expression levels. Expression levels of MuRF1, Atrogin-1, and LC3 did not significantly differ between the three groups, but EPA+ showed a tendency toward increased expression of these genes compared with EPA−. BNIP3 level was significantly lower in EPA− than in Young, but higher in EPA+ than in EPA− (Fig. 3B).
Figure 3.
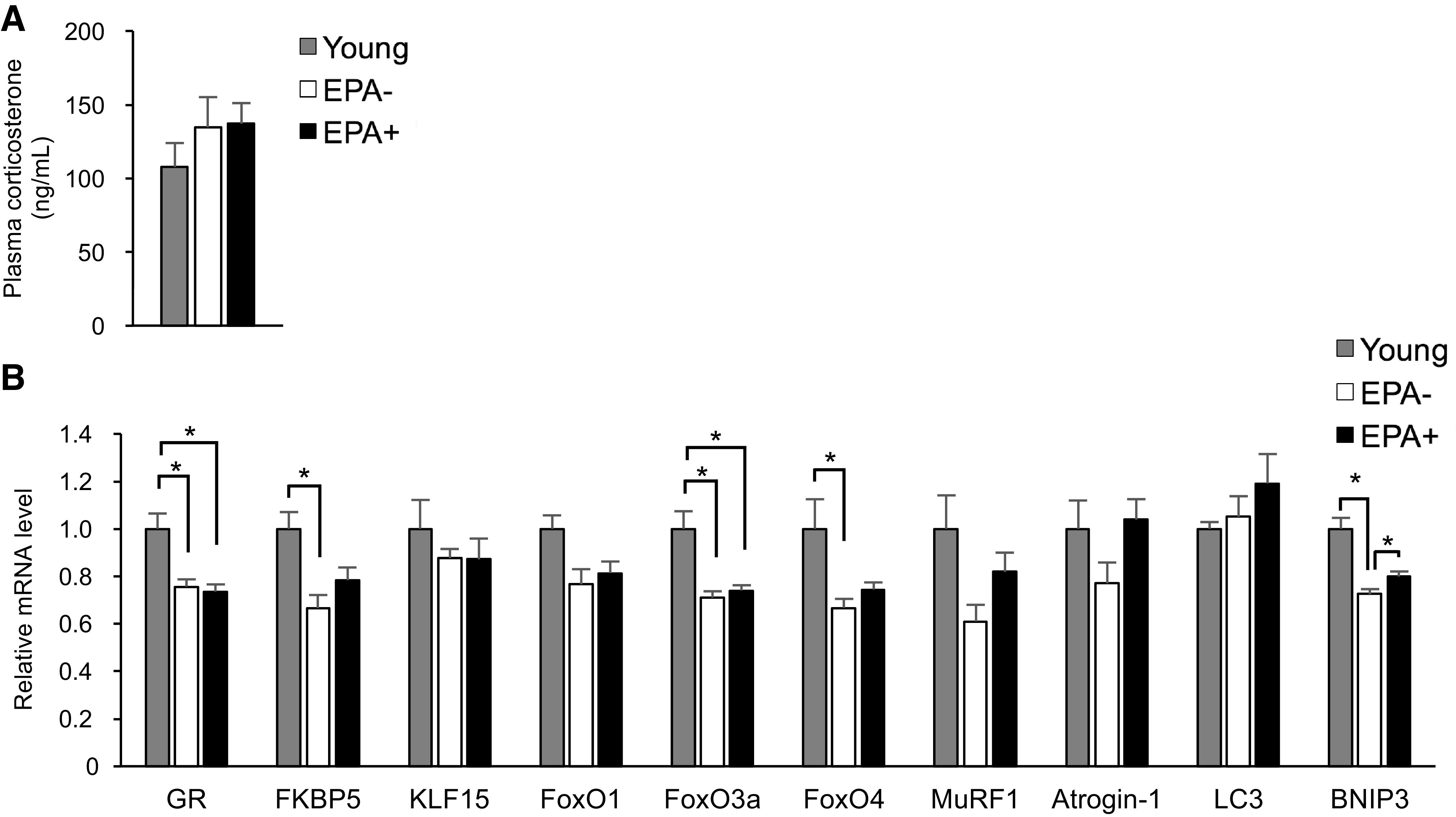
Age-related changes in the expression levels of catabolic genes are partially inhibited by EPA supplementation. A: concentration of plasma corticosterone in Young (n = 4), EPA− (n = 6), and EPA+ (n = 6). B: mRNA expression levels in Gas + Pl muscle from Young (n = 4), EPA− (n = 6), and EPA+ (n = 6) were evaluated by qRT-PCR. Data are normalized by 36B4 mRNA levels and presented as fold change relative to that in Young. Data are expressed as means ± SE, *P < 0.05, assessed by one-way ANOVA followed by Bonferroni’s post hoc test. EPA, eicosapentaenoic acid; Gas, gastrocnemius; Pl, plantaris; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
These results suggest that glucocorticoid signaling is downregulated in aging muscle and that EPA supplementation does not exert robust effects on GR signaling. Nonetheless, the ubiquitin proteasome pathway (MuRF1, Atrogin-1), as well as the autophagy pathway (LC3, BNIP3), might be modified by EPA supplementation independently of glucocorticoid signaling. We thought that these changes in the muscle transcriptome are possibly related to EPA-induced metabolic alterations in skeletal muscle.
Aging Male Mice Demonstrated Fast-to-Slow Fiber Type Transition in Skeletal Muscle, Which Is Inhibited by EPA Supplementation
To further gain mechanistic insights into the increase in grip strength in aging male mice fed EPA-enriched diet, we performed histological analysis of skeletal muscle focusing on muscle fiber type. Muscle fibers in mice are composed of types 1, 2A, 2X, 2B, and hybrid fibers (19). Because fast-twitch (type 2) fibers can generate more force than slow-twitch (type 1) fibers (25), we performed immunostaining of MyHCs (MYH7, MYH2, MYH4, and MYH1) to evaluate the proportion of fiber types in Gas + Pl, as described in materials and methods (Fig. 4A). We assessed the proportion of fiber types in the lateral part of gastrocnemius as well as whole plantaris. In gastrocnemius, the number and percentage of types 1 and 2A fibers were significantly higher, whereas those of type 2B fibers were significantly lower in EPA− than in Young (Fig. 4, B and C). This was consistent with a previously reported fast-to-slow fiber type transition during aging (26). However, EPA+ exhibited a significantly lower proportion of type 2A fibers and a significantly higher proportion of type 2B fibers than EPA− (Fig. 4C). In plantaris, as previously reported (19), no type 1 fibers were detected. In plantaris, the number and percentage of type 2A fibers were significantly higher, whereas those of type 2B fibers were significantly lower in EPA− than in Young (Fig. 4D and E). EPA+ tended to exhibit a lower proportion of type 2A fibers and a higher proportion of type 2B fibers than EPA− (Fig. 4E). In accordance with the changes in the proportion of fiber types, mRNA levels of Myh7 tended to increase in EPA− and EPA+ compared with those in Young, and mRNA levels of Myh4 decreased in EPA− and EPA+ compared with those in Young (Fig. 4F). Additionally, mRNA levels of Myh1 increased in EPA− and EPA+ compared with those in Young (Fig. 4F). These findings suggest that the number of fibers expressing MYH4 decreases during aging and they transition to the fibers expressing other types of MyHCs. It is important to note that the decrease in mRNA levels of Myh4 was partially, albeit significantly, restored in EPA+ (Fig. 4F). The mRNA levels of Myh2 showed no significant differences among the three groups.
Figure 4.
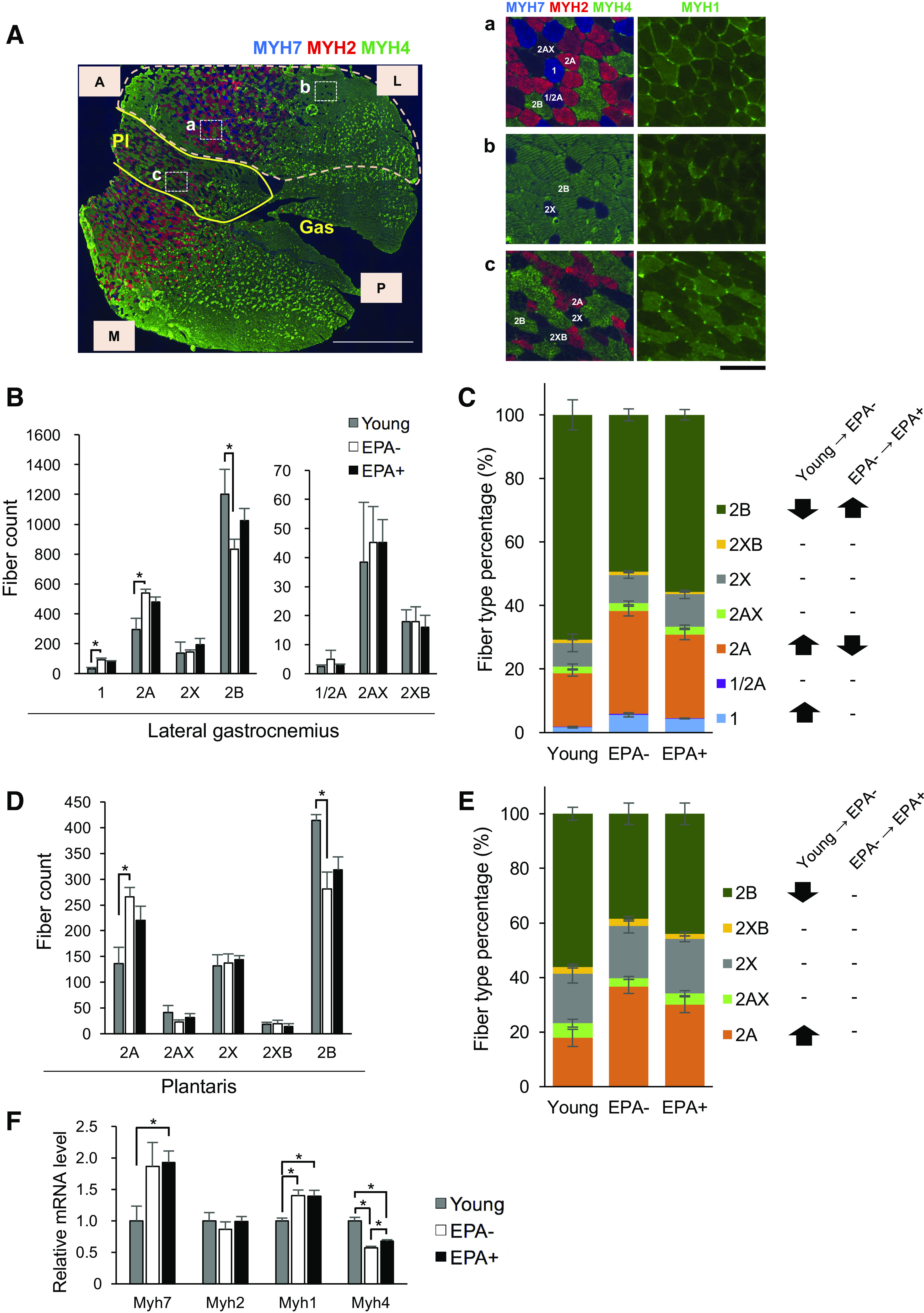
Aging muscle shows fast-to-slow fiber type transition, which is inhibited by EPA supplementation. A: representative images of transverse sections of gastrocnemius–plantaris complex (Gas + Pl) for fiber type determination. Co-immunostaining of myosin heavy chains (MyHCs) was performed; blue, red, and green represent positive areas for MYH7 (type 1), MYH2 (type 2A), and MYH4 (type 2B), respectively. An entire image of gastrocnemius–plantaris complex (left) and magnified images of specific regions (a, b, and c), with images obtained by immunostaining for MYH1 (type 2X) (right) shown. Types 1, 2A, 2X, and 2B fibers as well as hybrid fibers (1/2A, 2AX, and 2XB) were identified. In the following analysis, the lateral portion of gastrocnemius (area surrounded by the dashed line) and plantaris were used for quantification. Gas, gastrocnemius; Pl, plantaris; A, anterior; P, posterior; L, lateral; M, medial. Scale bars represent 1 mm in the left and 100 μm in the right. The number (B) and the proportion (C) of fiber type in the gastrocnemius were assessed by immunostaining. Comparisons between two groups were performed: Young versus EPA− and EPA− versus EPA+. Significant changes are depicted by asterisks (B) or arrows (C). Nonsignificant changes are denoted as “−” (C). Young (n = 3), EPA− (n = 6), and EPA+ (n = 6). Means ± SE; *P < 0.05 assessed by Student’s t test. The number (D) and the proportion (E) of fiber type in the plantaris were assessed by immunostaining. Comparisons between two groups were performed: Young versus EPA− and EPA− versus EPA+. Significant changes are depicted by asterisks (D) or arrows (E). Nonsignificant changes are denoted as “−” (E). Young (n = 3), EPA− (n = 6), and EPA+ (n = 6). Means ± SE; *P < 0.05 assessed by Student’s t test. F: expression levels of the indicated mRNA of Gas + Pl muscle from Young (n = 3), EPA− (n = 6), and EPA+ (n = 6) were evaluated by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Data are normalized by 36B4 mRNA levels and presented as fold change relative to Young. Means ± SE, *P < 0.05, assessed by one-way ANOVA followed by Bonferroni’s post hoc test. EPA, eicosapentaenoic acid.
Collectively, aging male mice presented a fast-to-slow fiber type transition, and EPA supplementation partially mitigated such changes occurring during the aging process.
Muscle Transcriptome Analysis Validated Fast-to-Slow Fiber Type Transition in Aging Muscle and Its Inhibition by EPA
Because age-related or EPA-induced transcriptional changes were observed in skeletal muscle (Figs. 3B and 4F), we finally investigated the transcriptomic program induced by aging or supplementation of EPA in skeletal muscle. RNA-Seq analyses were performed using Gas + Pl samples from Young, EPA−, and EPA+. Upon comparison of Young and EPA−, NOISeq analysis revealed 256 upregulated and 257 downregulated genes in EPA−. GO enrichment analysis and KEGG pathway analyses revealed that the enriched GO terms (Biological Process) in EPA− contained “transition between fast and slow fiber” (Fig. 5A). Transcriptome analysis, thus, supported the fiber type transition during aging. Upon comparison of EPA− and EPA+, 165 upregulated and 177 downregulated genes were identified in EPA+. The most enriched GO term (Biological Process) in EPA+ was “ubiquitin-dependent protein catabolic process” (Fig. 5B). It is compatible with the results of quantitative reverse-transcription polymerase chain reaction (qRT-PCR), where the levels of MuRF1 and Atrogin-1 tended to increase in EPA+ compared to EPA−. Moreover, other GO terms related to metabolism such as “positive regulation of TOR signaling” and “cellular response to fructose stimulus” were enriched in EPA+ (Fig. 5B). These data support the idea that the administration of EPA alters muscle metabolism.
Figure 5.
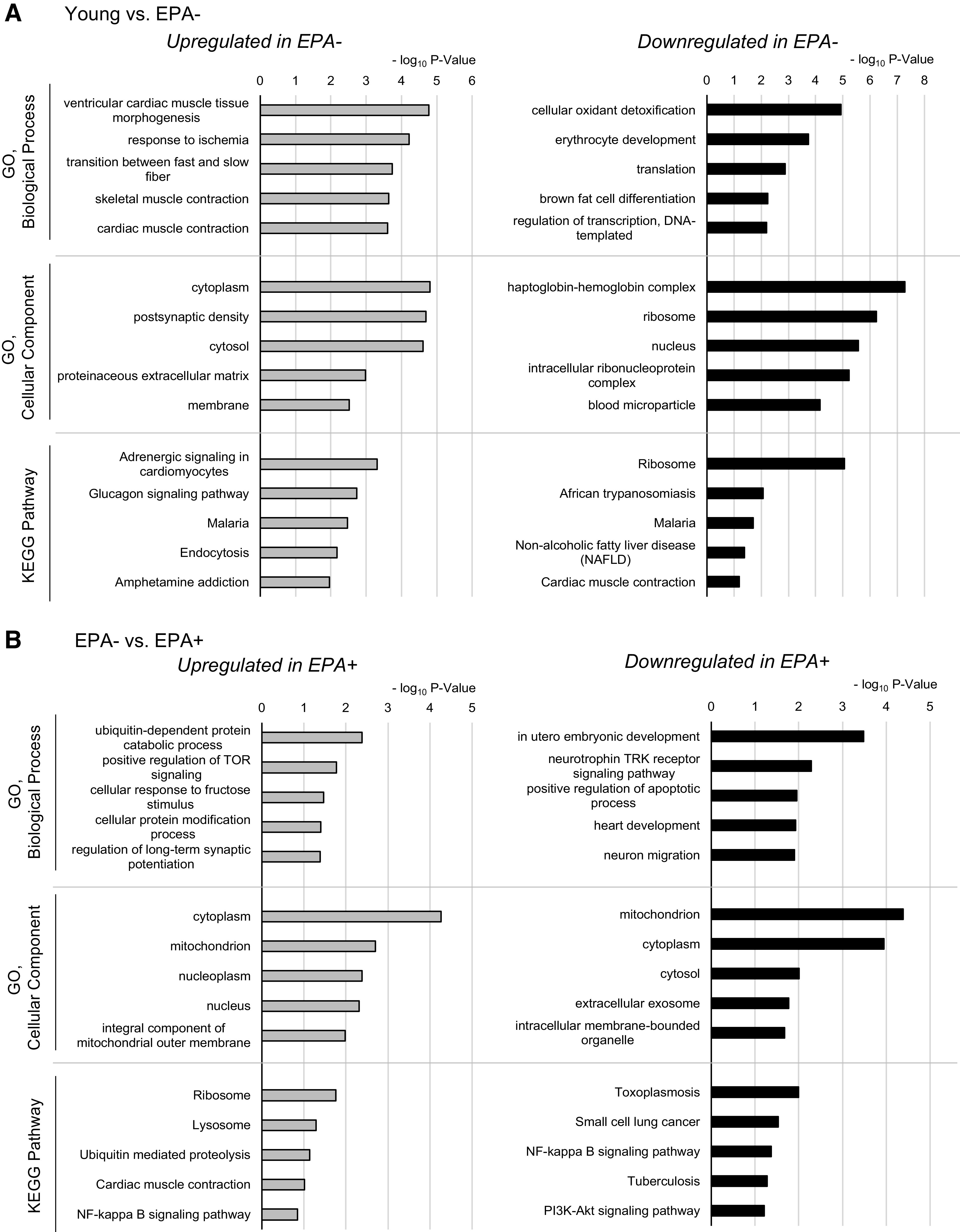
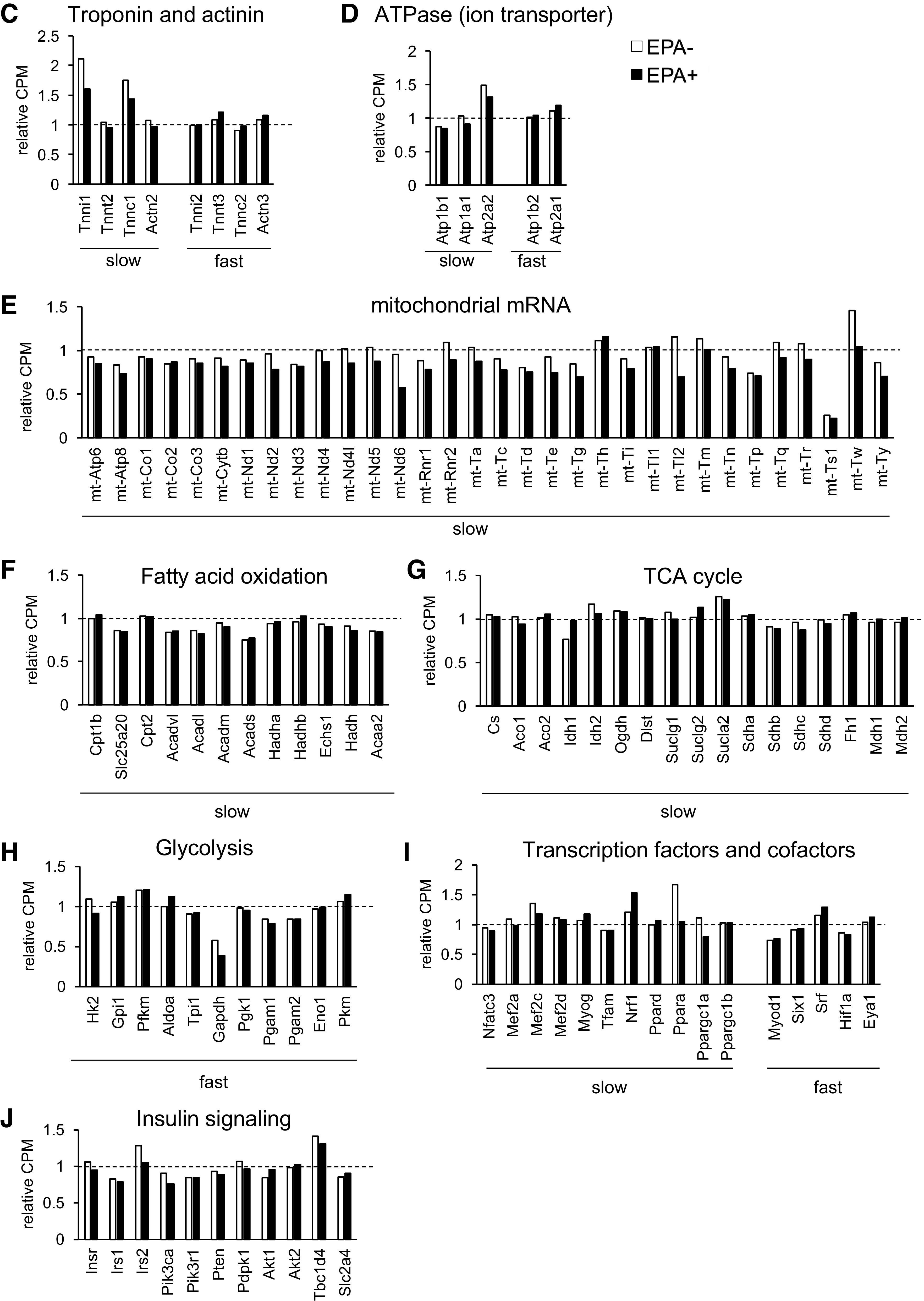
Transcriptomic analysis of skeletal muscle validates fast-to-slow fiber type transition in aging muscle and its inhibition by EPA. RNA samples were extracted from Gas + Pl muscle from Young, EPA−, and EPA+, and pooled samples comprising equal amounts of each sample (Young, n = 3; EPA−, n = 6; EPA+, n = 6) were used for RNA sequencing. A and B: comparisons were performed in Young versus EPA− (A) and EPA− versus EPA+ (B). Upregulated or downregulated gene sets were determined using NOISeq, followed by GO enrichment or KEGG pathway analysis. The top five terms enriched in each category are represented with the bar graphs of negative log10-transformed P values. Expressions of genes related to fiber types or signaling pathways are shown; troponin and actinin (C), ATPase (ion transporter) (D), mitochondrial mRNA (E), fatty acid oxidation (F), TCA cycle (G), glycolysis (H), transcription factors and cofactors (I), and insulin signaling (J). Data indicate the counts per million (CPM) presented as fold change relative to that in Young. EPA, eicosapentaenoic acid; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
To further explore the transcriptomic changes that occur during aging and upon EPA supplementation, we compared the gene expression levels using RNA-Seq data focusing on some gene sets based on existing knowledge. Each fiber type in skeletal muscle comprises contractile components and has functional properties (27). Given this, we evaluated the genes characteristic of, but not restricted to, types 1 or 2 fibers (Fig. 5, C–I), to validate the fast-to-slow transition in aging muscle and its inhibition by EPA. First, we evaluated the expression of troponin and actinin (Fig. 5C), as well as ATPase (ion transporter) (Fig. 5D) enriched in either fiber type. The prominent changes in EPA− compared to Young was an increase in type 1 fiber marker genes including Tnni1, Tnnc1, and Atp2a2, all of which were downregulated in EPA+ compared with in EPA− (Fig. 5, C and D). Supplementation with EPA showed tendencies toward a decrease in other type 1 fiber markers (Tnnt2, Actn2, Atp1b1, and Atp1a1) and an increase in type 2 fiber markers (Tnnt3, Tnnc2, Actn3, Atp1b2, and Atp2a1). Next, we evaluated the expression of other gene sets considering mitochondria-rich oxidative type 1 fibers and glycolytic type 2 fibers. Expression of some of the mitochondrial mRNAs and genes related to fatty acid oxidation, tricarboxylic acid (TCA) cycle, and glycolysis seemed to change with aging (Fig. 5, E–H). EPA supplementation led to the global downregulation of mitochondrial mRNA levels with a few exceptions (Fig. 5E), whereas it had no global effects on gene sets involved in fatty acid oxidation, TCA cycle, and glycolysis (Fig. 5, F–H). Some transcription factors or cofactors contributing to fiber type identities were also compatible with slow-to-fast fiber transition: a decrease in the expression levels of Nfatc3, Mef2a, Mef2c, Ppara, and Ppargc1a (Pgc1a) and an increase in Myod1, Srf, and Eya1 in EPA+ (difference > 5%). The expression levels of other factors were comparable (Mef2d, Tfam, Pgc1b, Six1, and Hif1a) or showed inverse patterns (Myog, Nrf1, and Ppard) (Fig. 5I). As far as we studied, we could not explain higher insulin sensitivity in EPA+ from the gene expression levels of some components of insulin signaling involved in glucose uptake (Fig. 5J).
These data indicate that age-related fast-to-slow fiber type transition is not necessarily associated with a global increase in such gene markers characteristic of the metabolic properties of slow-type fibers. Nevertheless, expression of several sarcomeric or ATPase genes showed changes with aging, leading to fast- to slow-type signatures. Furthermore, EPA induced transcriptomic signatures of slow-to-fast fiber type transition in skeletal muscle. EPA supplementation altered the expression of specific gene sets, including genes of sarcomeric proteins, ATPase, or mitochondrial mRNA, possibly via alteration in the metabolic profile of skeletal muscle.
DISCUSSION
In this study, we investigated age-related changes in skeletal muscle of 75-wk-old mice presenting with muscle weakness. EPA supplementation for 12 wk augmented grip strength without increasing muscle mass. Muscle strength is based on the function of motor units and the interrelation with the environment of muscle fibers, and all of them are affected by aging (28). Moreover, aging is a systemic process that induces diverse changes in various organs (6), and skeletal muscle is incorporated in these organ communications (29, 30). Therefore, EPA may influence muscle quality and systemic metabolism and improve insulin sensitivity. Of course, many direct effects of EPA on skeletal muscle (31–33) might contribute to the changes in muscle properties. Additionally, EPA has a neuroprotective effect (34), suggesting that the improvement of integrity in neurons or NMJs might be the case. In any case, EPA could influence systemic aging process and cause qualitative changes in motor units in skeletal muscle, resulting in an increase in muscle strength.
Age-related systemic changes in this study included a tendency toward increased levels of plasma corticosterone as previously reported (35), whereas mRNA levels of GR target genes in skeletal muscle were globally downregulated. Of note, EPA appeared to upregulate the levels of genes related to ubiquitin proteasome system or autophagy. Because the expression of these genes is regulated by many signaling cascades such as insulin/IGF-1 signaling, transforming growth factor β (TGFβ) signaling, inflammation, and glucocorticoid signaling (36), it is possible that EPA modifies the expression of genes related to some specific pathways in the aging process and controls muscle protein quality.
Contractile properties of skeletal muscle are coordinated by the regulation of distinct myosin isoforms and troponins in each fiber type (37), as well as other components such as actinin (38). In this study, histological and gene expression analyses supported that aging muscle shows altered expression of these sarcomeric proteins, indicating a fast-to-slow fiber type transition. In a previous report (39), the transcriptome of skeletal muscle from 24-mo-old mice exhibited upregulation of type 1 markers compared with that from 3-mo-old mice. We not only supported the fast-to-slow fiber type transition during aging but also clarified that this transition precedes an apparent decline in muscle mass. Furthermore, we showed that EPA supplementation ameliorates the aging-related fiber type transition. EPA might directly intervene in the determination of fiber type composition irrespective of aging, as the administration of n-3 PUFA is reported to reduce the proportion of type 1 and 2A fibers and increase that of type 2B fibers in young rats (40). Type 2 fibers have more force-producing capacity than type 1 fibers (25), and a change of fiber type proportions affects muscle strength (41). EPA-induced slow-to-fast fiber type transition, therefore, may inhibit age-related muscle weakness.
Our transcriptomic analysis revealed complicated gene expression signatures related to characteristics of muscle fiber types in EPA+. Regarding transcriptional regulators, EPA seemed to affect mRNA levels of Pgc1a. Because PGC-1α accelerates fast-to-slow fiber type switching (42), the effects of EPA might occur partially via the downregulation of Pgc1a expression. EPA did not globally affect the expression of genes related to fatty acid oxidation and TCA cycle, whereas EPA changed gene expression levels of sarcomeric protein, ATPase, and mitochondrial mRNA in a slow-to-fast fiber type transition. Some or all of these changes may be involved in fiber type transition in various ways. Together, although further studies are needed, our results indicate that EPA may modify the integrity of the force production machinery and signaling cascade related to fiber type transition in the aging process.
There are several limitations to this study. First, all experiments were conducted in male but not in female mice. There might be sex differences in the effectiveness of intervention against sarcopenia, as shown in studies on fish-oil supplementation (43) or others (44). Future studies should clarify the crosstalk between nutritionally driven metabolic alterations and sexually determined metabolic pathways. Second, to extend our findings to the clinical settings, we should consider the potential differences between mice and humans, not only with regard to the aging process but also the effects of EPA. Third, our transcriptomic analysis of genes such as Pgc1a may not necessarily correlate with the protein abundancy or functional properties influenced by various posttranslational regulations. Indeed, it is reported that EPA improves the mitochondrial function in aged mice, possibly through posttranslational modifications (45).
In conclusion, the early phenotype of skeletal muscle in aging male mice was characterized by muscle weakness with fast-to-slow fiber type transition, which could be ameliorated by EPA supplementation. We speculate that EPA-driven transcriptomic changes in skeletal muscle may be related to this transition and influence muscle quality. It should be emphasized that, in humans, oral administration of certain nutrients can prevent muscle damage in clinical settings (46). Further studies will not only clarify the precise mechanism underlying the aging process but also identify novel nutritional or pharmacological approaches against critical pathways involved in the development of sarcopenia.
GRANTS
This work was supported by JSPS KAKENHI—Grant Numbers JP16H05330 and JP18KT0017 (to H.T.), JP16K09230 (to N.Y.), JP17K16158, and JP20K17528 (to H.Y.)—and by AMED Grant “Project for Whole Implementation to Support and Ensure the Female Life” 18gk0210019h0001 (to H.T).
DISCLOSURES
This work was financially supported by Mochida Pharmaceutical Co., Ltd.
AUTHOR CONTRIBUTIONS
H.Y. and H.T. conceived and designed research; H.Y., M.N., M.U., A.K-S., and M.Y. performed experiments; H.Y. and M.N. analyzed data; H.Y., M.Y., N.Y., K-I.M., and H.T. interpreted results of experiments; H.Y. prepared figures; H.Y. drafted manuscript; H.Y., M.Y., N.Y., K-I.M., and H.T. edited and revised manuscript; H.Y., M.N., M.U., A.K-S., M.Y., N.Y., K-I.M., and H.T. approved final version of manuscript.
REFERENCES
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48: 16–31, 2019. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, Iliodromiti S, Sillars A, Graham N, Mackay DF, Pell JP, Gill JM, Sattar N, Gray SR. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361: k1651, 2018. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayer AA, Kirkwood TBL. Grip strength and mortality: a biomarker of ageing? Lancet 386: 226–227, 2015. doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 393: 2636–2646, 2019. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 6.Schaum N, Lehallier B, Hahn O, Pálovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, Fehlmann T, Webber JT, McGeever A, Calcuttawala K, Zhang H, Berdnik D, Mathur V, Tan W, Zee A, Tan M, Pisco AO, Karkanias J, Neff NF, Keller A, Darmanis S, Quake SR, Wyss-Coray T; The Tabula Muris Consortium. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583: 596–602, 2020. doi: 10.1038/s41586-020-2499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shavlakadze T, Morris M, Fang J, Wang SX, Zhu J, Zhou W, Tse HW, Mondragon-Gonzalez R, Roma G, Glass DJ. Age-related gene expression signature in rats demonstrate early, late, and linear transcriptional changes from multiple tissues. Cell Rep 28: 3263–3273.e3, 2019. doi: 10.1016/j.celrep.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 110: 984–992, 2012. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 108: 682–690, 2011. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380: 11–22, 2019. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 11.Logan SL, Spriet LL. Omega-3 fatty acid supplementation for 12 weeks increases resting and exercise metabolic rate in healthy community-dwelling older females. PLoS One 10: e0144828, 2015. doi: 10.1371/journal.pone.0144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA; Hertfordshire Cohort Study Group. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc 56: 84–90, 2008. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 95: 428–436, 2012. doi: 10.3945/ajcn.111.021915. [DOI] [PubMed] [Google Scholar]
- 14.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr 102: 115–122, 2015. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krzymińska-Siemaszko R, Czepulis N, Lewandowicz M, Zasadzka E, Suwalska A, Witowski J, Wieczorowska-Tobis K. The effect of a 12-week omega-3 supplementation on body composition, muscle strength and physical performance in elderly individuals with decreased muscle mass. Int J Environ Res Public Health 12: 10558–10574, 2015. doi: 10.3390/ijerph120910558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolland Y, Barreto PS, Maltais M, Guyonnet S, Cantet C, Andrieu S, Vellas B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain lifestyle intervention on muscle strength in older adults: secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT). Nutrients 11: 1931, 2019. doi: 10.3390/nu11081931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, Winzell MS, Wahle KW, Ahren B, Williams LM. Effect of a conjugated linoleic acid and omega-3 fatty acid mixture on body composition and adiponectin. Obesity (Silver Spring) 16: 1019–1024, 2008. doi: 10.1038/oby.2008.41. [DOI] [PubMed] [Google Scholar]
- 18.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr 142: 592S–599S, 2012. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 19.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarazona S, Garcia-Alcalde F, Dopazo J, Ferrer A, Conesa A. Differential expression in RNA-seq: a matter of depth. Genome Res 21: 2213–2223, 2011. doi: 10.1101/gr.124321.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buch A, Carmeli E, Boker LK, Marcus Y, Shefer G, Kis O, Berner Y, Stern N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—an overview. Exp Gerontol 76: 25–32, 2016. doi: 10.1016/j.exger.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Shimizu N, Yoshikawa N. Role of skeletal muscle glucocorticoid receptor in systemic energy homeostasis. Exp Cell Res 360: 24–26, 2017. doi: 10.1016/j.yexcr.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Yanagita I, Fujihara Y, Kitajima Y, Tajima M, Honda M, Kawajiri T, Eda T, Yonemura K, Yamaguchi N, Asakawa H, Nei Y, Kayashima Y, Yoshimoto M, Harada M, Araki Y, Yoshimoto S, Aida E, Yanase T, Nawata H, Muta K. A high serum cortisol/DHEA-S ratio is a risk factor for sarcopenia in elderly diabetic patients. J Endocr Soc 3: 801–813, 2019. doi: 10.1210/js.2018-00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, Nakae J, Tagata Y, Nishitani S, Takehana K, Sano M, Fukuda K, Suematsu M, Morimoto C, Tanaka H. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab 13: 170–182, 2011. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 9: 87–95, 1999. doi: 10.1016/S1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 26.Ohlendieck K. Proteomic profiling of fast-to-slow muscle transitions during aging. Front Physiol 2: 105, 2011. doi: 10.3389/fphys.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 28.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 9: 213–228, 2008. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu N, Maruyama T, Yoshikawa N, Matsumiya R, Ma Y, Ito N, Tasaka Y, Kuribara-Souta A, Miyata K, Oike Y, Berger S, Schutz G, Takeda S, Tanaka H. A muscle-liver-fat signalling axis is essential for central control of adaptive adipose remodelling. Nat Commun 6: 6693, 2015. doi: 10.1038/ncomms7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara M, Yamazaki H, Yoshikawa N, Kuribara-Souta A, Tanaka H. Correlation among body composition and metabolic regulation in a male mouse model of Cushing's syndrome. Endocr J 67: 21–30, 2020. doi: 10.1507/endocrj.EJ19-0205. [DOI] [PubMed] [Google Scholar]
- 31.Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun 432: 593–598, 2013. doi: 10.1016/j.bbrc.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 32.Magee P, Pearson S, Whittingham-Dowd J, Allen J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J Nutr Biochem 23: 1440–1448, 2012. doi: 10.1016/j.jnutbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Saini A, Sharples AP, Al-Shanti N, Stewart CE. Omega-3 fatty acid EPA improves regenerative capacity of mouse skeletal muscle cells exposed to saturated fat and inflammation. Biogerontology 18: 109–129, 2017. doi: 10.1007/s10522-016-9667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalo-Gobernado R, Ayuso MI, Sansone L, Bernal-Jimenez JJ, Ramos-Herrero VD, Sanchez-Garcia E, Ramos TL, Abia R, Muriana FJG, Bermudez B, Montaner J. Neuroprotective effects of diets containing olive oil and DHA/EPA in a mouse model of cerebral ischemia. Nutrients 11: 1109, 2019. doi: 10.3390/nu11051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strijbos PJ, Horan MA, Carey F, Rothwell NJ. Impaired febrile responses of aging mice are mediated by endogenous lipocortin-1 (annexin-1). Am J Physiol Endocrinol Metab 265: E289–E297, 1993. doi: 10.1152/ajpendo.1993.265.2.E289. [DOI] [PubMed] [Google Scholar]
- 36.Vainshtein A, Sandri M. Signaling pathways that control muscle mass. Int J Mol Sci 21: 4759, 2020. doi: 10.3390/ijms21134759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin J-P. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol 290: C567–C576, 2006. doi: 10.1152/ajpcell.00422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet 21: 353–354, 1999. doi: 10.1038/7675. [DOI] [PubMed] [Google Scholar]
- 39.Lin I-H, Chang J-L, Hua K, Huang W-C, Hsu M-T, Chen Y-F. Skeletal muscle in aged mice reveals extensive transformation of muscle gene expression. BMC Genet 19: 55, 2018. doi: 10.1186/s12863-018-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fappi A, Neves JDC, Kawasaki KA, Bacelar L, Sanches LN, P. da Silva F, Larina-Neto R, Chadi G, Zanoteli E. Omega-3 multiple effects increasing glucocorticoid-induced muscle atrophy: autophagic, AMPK and UPS mechanisms. Physiol Rep 7: e13966, 2019. doi: 10.14814/phy2.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K, Kakigi R, Okada T, Sakurai T, Kawamori R, Watada H. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep 5: e13250, 2017. doi: 10.14814/phy2.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 43.Da Boit M, Sibson R, Sivasubramaniam S, Meakin JR, Greig CA, Aspden RM, Thies F, Jeromson S, Hamilton DL, Speakman JR, Hambly C, Mangoni AA, Preston T, Gray SR. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am J Clin Nutr 105: 151–158, 2017. doi: 10.3945/ajcn.116.140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Spiegeleer A, Beckwee D, Bautmans I, Petrovic M; Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs Aging 35: 719–734, 2018. doi: 10.1007/s40266-018-0566-y. [DOI] [PubMed] [Google Scholar]
- 45.Johnson ML, Lalia AZ, Dasari S, Pallauf M, Fitch M, Hellerstein MK, Lanza IR. Eicosapentaenoic acid but not docosahexaenoic acid restores skeletal muscle mitochondrial oxidative capacity in old mice. Aging cell 14: 734–743, 2015. doi: 10.1111/acel.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshikawa N, Shimizu N, Uehara M, Oda A, Matsumiya R, Matsubara E, Kobayashi H, Hosono O, Kuribara-Souta A, Baba H, Nagamura F, Kiryu S, Tanaka H. The effects of bolus supplementation of branched-chain amino acids on skeletal muscle mass, strength, and function in patients with rheumatic disorders during glucocorticoid treatment. Mod Rheumatol 27: 508–517, 2017. doi: 10.1080/14397595.2016.1213480. [DOI] [PubMed] [Google Scholar]