Keywords: deuterated water, gene expression, muscle damage, protein-polyphenol
Abstract
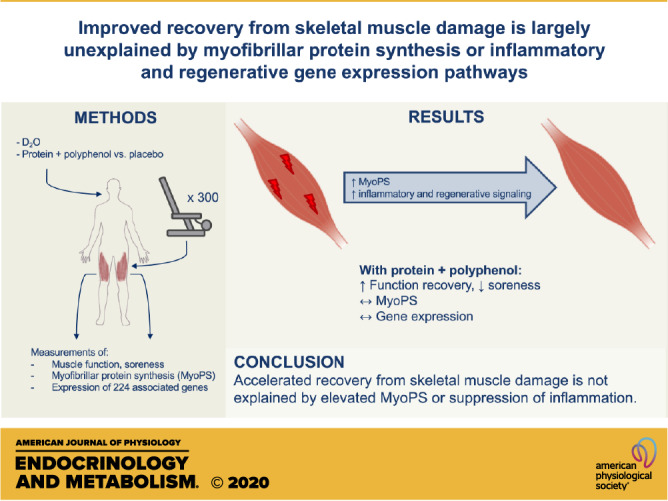
The contribution of myofibrillar protein synthesis (MyoPS) to recovery from skeletal muscle damage in humans is unknown. Recreationally active men and women consumed a daily protein-polyphenol beverage targeted at increasing amino acid availability and reducing inflammation (PPB; n = 9), both known to affect MyoPS, or an isocaloric placebo (PLA; n = 9) during 168 h of recovery from 300 maximal unilateral eccentric contractions (EE). Muscle function was assessed daily. Muscle biopsies were collected for 24, 27, 36, 72, and 168 h for MyoPS measurements using 2H2O and expression of 224 genes using RT-qPCR and pathway analysis. PPB improved recovery of muscle function, which was impaired for 5 days after EE in PLA (interaction P < 0.05). Acute postprandial MyoPS rates were unaffected by nutritional intervention (24–27 h). EE increased overnight (27–36 h) MyoPS versus the control leg (PLA: 33 ± 19%; PPB: 79 ± 25%; leg P < 0.01), and PPB tended to increase this further (interaction P = 0.06). Daily MyoPS rates were greater with PPB between 72 and 168 h after EE, albeit after function had recovered. Inflammatory and regenerative signaling pathways were dramatically upregulated and clustered after EE but were unaffected by nutritional intervention. These results suggest that accelerated recovery from EE is not explained by elevated MyoPS or suppression of inflammation.
NEW & NOTEWORTHY The present study investigated the contribution of myofibrillar protein synthesis (MyoPS) and associated gene signaling to recovery from 300 muscle-damaging, eccentric contractions. Measured with 2H2O, MyoPS rates were elevated during recovery and observed alongside expression of inflammatory and regenerative signaling pathways. A nutritional intervention accelerated recovery; however, MyoPS and gene signaling were unchanged compared with placebo. These data indicate that MyoPS and associated signaling do not explain accelerated recovery from muscle damage.
INTRODUCTION
The ability of skeletal muscle to regenerate and recover after damage is crucial for regulating muscle mass, muscle function, and disease resistance (1–3). In mice, the recovery of contractile function 48–120 h after exercise-induced muscle damage is associated with increased rates of mixed muscle protein synthesis and breakdown (4). Although a degree of inflammation appears necessary for this recovery process (4–6), aberrant inflammation suppresses rates of muscle protein synthesis (7) and may delay regeneration and recovery after muscle damage (8, 9). Indeed, polyphenols with known anti-inflammatory properties attenuate postexercise inflammatory signaling in mice (10) and accelerate functional recovery after muscle damage in humans (11). Given that muscle damage is characterized by a loss in contractile function and damage to contractile proteins (12), that satellite cells and pericytes associate with damaged myofibers, and that small heat shock proteins aggregate with myofibrils during recovery (5, 9, 13, 14), the synthesis of myofibrillar proteins (MyoPS) in particular may be critical to the recovery process. However, the time course and therefore relative importance of MyoPS during recovery, particularly in humans, is unknown because of a lack of intervention studies that utilize direct measures.
Eccentric muscle contractions safely and effectively induce transient skeletal muscle damage in humans. Maximal contractile force, considered the most reliable indirect measure of muscle damage (12), is reduced 15–30% 24 h after eccentric exercise, persisting for several days depending on the intensity of eccentric exercise (15, 16). Eccentric, rather than concentric, contractions induce greater myofibrillar disruption (17) and coincide with elevated rates of mixed muscle (18) and myofibrillar (17) protein synthesis. Protein ingestion increases rates of muscle protein synthesis and signaling through the mechanistic target of rapamycin (mTOR) pathway over and above the effect of exercise alone (19, 20). In mouse models, pharmaceutical inhibition of mTOR complex 1 delays recovery of muscle function by 20% at 7 days after eccentric contraction-induced muscle damage (21). In humans, protein ingestion has been shown to accelerate recovery of function, including measures of both peak torque and work done, after resistance exercise (22). However, to date very limited mechanistic insight exists to explain these positive findings.
Traditional stable isotope tracer methodologies allow for MyoPS rates to be quantified over the duration of an intravenous infusion, which is typically limited to several hours. Therefore, this approach is not suitable for a comprehensive measurement of MyoPS over the full duration of recovery from eccentric exercise. An alternative method involving deuterium oxide (2H2O) consumption allows for MyoPS rates to be determined in “free-living” conditions outside a laboratory setting, because of the endogenous synthesis of deuterium-labeled alanine (23–26). If the body water pool remains appropriately enriched, the 2H2O method allows for the simultaneous characterization of daily, cumulative protein synthesis rates to capture the full recovery process, as well as shorter, hourly rates to further the understanding of MyoPS across certain physiological states, such as postprandial or overnight conditions that may be influenced by immediate amino acid availability. Capturing these time periods in tandem allows for the determination of whether temporal alterations in MyoPS rates coincide with skeletal muscle recovery and are therefore consistent with being a primary mechanism dictating muscle recovery.
We hypothesized that amino acid availability and excessive inflammation would limit MyoPS after muscle damage, such that nutritionally targeting these processes with a postexercise protein and polyphenol nutritional intervention would suppress inflammation and increase MyoPS and associated signaling, resulting in improved muscle recovery. To this end, we sought to maximize rates of MyoPS with the addition of concentric exercise during the recovery period. We aimed to characterize the time course of rates of MyoPS and key gene expression pathways of inflammation, protein synthesis, proteolysis, and substrate metabolism over a week of recovery following voluntary eccentric muscle contractions in humans for the first time, while fully controlling diet and resistance exercise.
MATERIALS AND METHODS
Participants
Eighteen healthy, recreationally active, participants were recruited [11 men, 7 women; age: 22 ± 1 yr; body mass: 75.2 ± 2.7 kg; body mass index (BMI): 24.3 ± 0.8 kg·m−2], defined as participating in sporting activities > 2 h·wk−1 but not following a structured exercise training program. Exclusion criteria were 1) diagnosed metabolic or cardiovascular impairment; 2) self-reported habitual protein intake < 0.8 g·kg−1·day−1; 3) musculoskeletal injury that may impair exercise performance; and/or 4) engagement in systematic resistance training within 6 mo of participation.
All individuals provided written consent at least 24 h after verbal and written explanation of the experimental procedures, which were approved by the University of Exeter’s Sport and Health Sciences Research Ethics Committee (Ref. No. 161026/B/06). This study was registered as a clinical trial with ClinicalTrials.gov (NCT02980900).
General Study Design
Participants were randomly assigned in a double-blind, placebo-controlled, parallel-group design, counterbalanced for leg dominance, to consume either a daily postexercise protein-polyphenol beverage (PPB; n = 9) or isocaloric carbohydrate placebo (PLA; n = 9). Participant characteristics are shown in Table 1.
Table 1.
Subject characteristics and details of controlled diet
| PLA (n = 9) | PPB (n = 9) | |
|---|---|---|
| Sex (male:female) | 6:3 | 5:4 |
| Age, yr | 22 ± 0 | 22 ± 1 |
| Body mass, kg | 77.6 ± 4.1 | 72.8 ± 3.6 |
| Height, cm | 176.2 ± 1.2 | 175.5 ± 2.0 |
| BMI, kg·m−2 | 25.0 ± 1.4 | 23.6 ± 0.9 |
| Baseline function (CON leg), J | 2,824 ± 165 | 2,593 ± 140 |
| Baseline function (ECC leg), J | 2,932 ± 130 | 2,527 ± 163 |
| Energy, MJ·day−1 | 12.1 ± 0.3 | 11.9 ± 0.5 |
| Protein, g·kg−1·day−1 | 1.2 ± 0.0 | 1.5 ± 0.0*** |
| Protein, g·day−1 | 93 ± 4 | 108 ± 5* |
| Carbohydrate, g·day−1 | 394 ± 12 | 372 ± 14 |
| Fat, g·day−1 | 98.5 ± 3.5 | 94.4 ± 5.4 |
Values represent means ± SE for n subjects. BMI, body mass index; CON, concentric-only leg; ECC, eccentric + concentric leg; PLA, maltodextrin placebo condition; PPB, protein-polyphenol supplement. *P < 0.05, ***P < 0.001 significantly different from PLA.
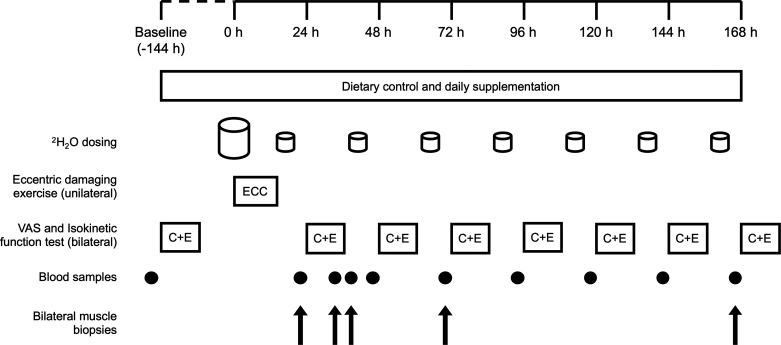
A schematic overview of the study protocol is presented in Fig. 1. After enrollment, participants visited the laboratory at least 48 h before the start of the study for anthropometric measures and familiarization with the testing procedures. Individual settings for the isokinetic dynamometer (Biodex System 3; Biodex Medical Systems, Inc., Shirley, NY) were determined on this visit and used for all subsequent visits. Full dietary control was employed from the baseline visit (−144 h) to 168 h after eccentric exercise (14 days total). The experimental beverages (see Diet and Nutritional Intervention) were consumed once daily, either immediately after exercise during laboratory visits to increase amino acid availability or at 2000 on the days before eccentric exercise, as similar durations of polyphenol dosing reportedly accelerate recovery from eccentric exercise (11). Participants were instructed to abstain from strenuous physical activity, alcohol, and anti-inflammatory or analgesic medication 48 h before and throughout the experimental protocol. Caffeine ingestion was recorded and only permitted > 6 h before a study visit.
Figure 1.
Graphical representation of the experimental protocol. Oral consumption of 70% 2H2O began at ∼0800 on the day of eccentric exercise, with 8 × 0.75 mL·kg−1 doses consumed every 1.5 h and maintained thereafter with daily doses of 0.54 mL·kg−1. Unilateral eccentric exercise performed at ∼1900 (t = 0 h) with follow-up tests of muscle function and soreness performed every 24 h thereafter. Bilateral muscle biopsies obtained at 24, 27, 36, 72, and 168 h after eccentric exercise. Blood sampling performed daily before the function test, as well as before muscle biopsies at 27 and 36 h. Full dietary control providing 1.2 g·kg−1·day−1 dietary protein was employed throughout the study, in addition to daily consumption of a protein-polyphenol beverage or an isocaloric maltodextrin placebo. C + E, control + eccentric legs; ECC, eccentric leg only; VAS, visual analog scale.
Maximal isokinetic voluntary contraction (muscle function) and soreness of the knee extensor muscles were assessed 144 h before eccentric exercise (baseline; see Muscle Soreness and Maximal Isokinetic Function). At ∼1900 on the day of eccentric exercise (t = 0 h), participants carried out 300 maximal unilateral eccentric contractions of the knee extensors designed to elicit muscle damage. Follow-up tests of muscle function and muscle soreness were made every 24 h after the eccentric protocol for 168 h. All laboratory visits, with the exception of a fasted visit at 36 h, took place 1.5 h after consumption of the final meal of the day. Bilateral muscle biopsy samples were collected from the eccentrically exercised leg (ECC) and the rested control leg (CON) before completing the functional assessment at 24, 72, and 168 h.
To investigate the effect of nutritional intervention on postprandial muscle metabolism, participants rested in a semisupine position for 3 h after the consumption of the experimental beverage at 24 h, after which time additional bilateral biopsies were taken (corresponding to 27 h after eccentric exercise). All participants were given a maltodextrin beverage before leaving the laboratory and were asked to return the following morning (36 h after eccentric exercise) for further bilateral muscle biopsies to investigate the effect of postexercise nutritional intervention on overnight recovery. Venous blood samples were collected from the antecubital vein via venipuncture technique into lithium heparin containers upon arrival at the laboratory at baseline and daily between 24 and 168 h. Additional blood samples were collected before the muscle biopsies at 27 and 36 h. Samples were immediately centrifuged at 2,850 g for 10 min at 4°C, and plasma was aliquoted, snap-frozen in liquid nitrogen, and stored at −80°C for further analysis.
Eccentric Contraction Protocol
One leg was randomly assigned to undergo the unilateral damaging exercise protocol, which consisted of 10 sets × 30 repetitions of maximal isokinetic eccentric contractions of the knee extensors. An isokinetic dynamometer was used with angular velocity of 60°·s−2. Each set was separated by 120-s rest. Knee joint range of motion was determined as 80° from full flexion, as muscle damage is more prevalent at longer muscle lengths (27). Participants received visual and verbal feedback and encouragement to ensure maximal effort throughout each repetition.
Muscle Soreness and Maximal Isokinetic Function
Upon arrival at the laboratory, participants were asked to rise from a seated position and rate general muscle soreness on a 100-mm visual analog scale (VAS) going from “no pain at all” (0 mm) to “worst possible pain” (100 mm), as described previously (28).
Bilateral assessment of maximal voluntary isokinetic muscle function was performed with one set of 30 maximal isokinetic concentric contractions of the knee extensors through 80° range of motion equidistant from full flexion and full extension. Angular velocity was 75°·s−1. A further four sets of 30 repetitions were carried out in the ECC leg after the functional assessment to provide an exercise stimulus. In the CON leg, further sets of 30 repetitions were performed at 24 h until total work was matched between legs to standardize the effects of exercise on postprandial and overnight recovery. Otherwise between 48 and 168 h, four additional sets were completed after the functional assessments. Each set of 30 repetitions was separated by 60-s rest.
Isokinetic function was calculated as the area under the torque-time graph (J), which was sampled at 100 Hz with an analog-to-digital converter (Power1401-3A; Cambridge Electronic Design Ltd., Cambridge, UK) and recorded with Spike2 software (Cambridge Electronic Design Ltd.) for off-line analysis. Data were expressed relative to the control leg (%CON) to reduce any influence of the testing procedures on contractility of the muscle.
Diet and Nutritional Intervention
Because of the hypothesis that amino acid availability would limit recovery, it was imperative that diet was strictly controlled. As such, the controlled diet was designed to maintain energy balance and provide 1.2 g·kg body mass−1·day−1 protein (Table 1), which is within American College of Sports Medicine (ACSM) guidelines to support metabolic adaptation (29) but, importantly, below current guidelines to maximize muscle protein anabolism (30). All food was weighed out, individually packaged, and provided in a container corresponding to the day of the week. Energy requirements were based on the Henry equation (31) multiplied by an activity factor of 1.6. Body mass was recorded on each laboratory visit to allow adjustments to the diet if necessary. Participants were informed of the importance of diet adherence and returned forms indicating whether each item was consumed. No other foods or energy-containing beverages were permitted throughout the study.
The nutritional intervention provided in PPB was a commercially available postexercise beverage (Beachbody Performance Recover; Beachbody LLC, Santa Monica, CA) containing 20 g of total protein (from a blend of whey, pea, and casein), 10 g of total carbohydrate, and 650 mg of pomegranate extract (211 mg polyphenols). Participants in the PLA group received an isocaloric maltodextrin placebo. Both groups were given a maltodextrin drink containing 24 g of carbohydrate to consume each night ∼30 min before sleep.
Deuterated Water Dosing Protocol
The deuterated water dosing protocol was designed to enrich the body water pool to 0.6% and consisted of one loading day followed by seven maintenance days. The protocol was adapted from previous work from our laboratory (32), as total body water (i.e., the precursor pool) is largely influenced by body mass (33) and previous loading protocols have not achieved a true steady state of precursor pool enrichment (24, 32). Thus, the present protocol was based on the assumption that the body water pool contributes 60–70% body mass in healthy lean individuals (33) and turns over at 7–10%·day−1 (34). The loading protocol commenced at ∼0800 on the day of eccentric exercise (t = −11 h) with the consumption of 6 mL·kg−1 70% 2H2O (Cambridge Isotopes Laboratories, Andover, MA), separated into eight equal doses to be consumed every 1.5 h (i.e., 8 × 0.75 mL·kg−1). Participants remained in the laboratory until four doses had been consumed to ensure that no vertigo or dizziness occurred, before leaving the laboratory with the remaining four doses. Body water enrichment was maintained thereafter with one daily dose of 0.54 mL·kg−1 consumed upon waking.
Muscle Biopsy Collection
Muscle biopsies were obtained under local anesthesia (2% lidocaine) from the midsection of the m. vastus lateralis by the Bergström needle technique modified for suction (35). All samples were rapidly dissected of visible fat and connective tissue, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until subsequent analysis.
Plasma Analyses
Plasma samples were analyzed for creatine kinase (CK) by photometric activity assay with a Cobas 8000 automated analyzer (Roche Diagnostics, Indianapolis, IN). Hydrogen isotope ratios (2H/1H) of plasma were determined in triplicate by injecting samples into a high-temperature conversion elemental analyzer (TCEA Flash 2000; ThermoFisher Scientific, Waltham, MA) coupled to an isotope ratio mass spectrometer (IRMS; Delta V; ThermoFisher Scientific). Raw isotope ratio values were normalized with in-house reference materials calibrated to Vienna Standard Mean Ocean Water (VSMOW).
Myofibrillar Bound [2H]Alanine Enrichment
Myofibrillar protein fractions were isolated from ∼50 mg wet wt of muscle tissue. Tissue was homogenized in 7.5 μL·mg−1 ice-cold homogenization buffer (in mM: 50 Tris·HCl pH 7.4, 1 EDTA, 1 EGTA, 10 β-glycerophosphate salt, 50 NaF, and 0.5 activated Na3VO4; Sigma-Aldrich Company Ltd., Poole, UK) with a complete protease inhibitor cocktail tablet (1 tablet per 50 mL of buffer; Roche, Burgess Hill, UK) with a glass pestle. Homogenates were centrifuged at 2,200 g for 10 min at 4°C, and the supernatant representing the sarcoplasmic pool was aliquoted and stored at −80°C for subsequent Western blot analysis. The remaining pellet was washed with 500 μL of homogenization buffer, followed by centrifugation at 700 g for 10 min at 4°C. Myofibrillar proteins were solubilized in 0.3 M NaOH for 30 min at 50°C and separated from the insoluble collagen fraction by centrifugation at 10,000 g for 5 min at 4°C. The remaining supernatant was aliquoted, and myofibrillar proteins were precipitated with 1 M perchloric acid and centrifuged at 700 g for 10 min at 4°C. The myofibrillar pellet was washed twice in 1 mL of 70% ethanol and hydrolyzed in 2 mL of 6 M HCl at 110°C for 24 h. The samples were subsequently dried under a vacuum (Savant SpeedVac; ThermoFisher Scientific) and reconstituted in 3 mL of 25% acetic acid. Samples were passed over cation exchange resin columns (100–200 mesh; H+ form; Dowex 50WX8; Sigma-Aldrich Company Ltd.) and eluted with 6 M NH4OH before being dried under vacuum. Samples were resuspended in 1 mL of distilled water and 1 mL of 0.1% formic acid in acetonitrile and spun at 10,000 g for 3 min at 4°C. The supernatant was aliquoted, dried under a vacuum, and stored at −20°C.
After derivatization of the purified amino acids to tert-butyl dimethylsilyl (TBDMS) esters (36), 1 μL of the sample was injected into a Delta V Advantage IRMS (ThermoFisher Scientific) fitted with a Trace 1310 gas chromatograph. The peaks were resolved on a 30 m × 0.25 mm ID × 0.25 μm film DB-5 capillary column (Agilent Technologies, Santa Clara, CA; temperature program: 110°C for 1 min; 10°C/min ramp to 180°C; 5°C/min ramp to 220°C; 20°C/min ramp to 300°C; hold for 2 min) before pyrolysis. Amino acids eluting from the gas chromatograph were combusted through use of an in-line pyrolysis reactor to thermally decompose the amino acids to their elemental components before entry into the IRMS. The enrichment of tracer was measured by monitoring ion masses 2 and 3 to determine the 2H-to-1H ratios of myofibrillar protein-bound [2H]alanine. A series of known standards were applied both to assess linearity of the mass spectrometer and to control for the loss of tracer.
Western Blot
Sarcoplasmic fractions from biopsy samples collected at 24, 27, and 36 h were prepared as described above (see Myofibrillar Bound [2H]Alanine Enrichment). Aliquots were defrosted on ice, and the protein content was determined by colorimetric assay (DC protein assay; Bio-Rad Laboratories Inc., California). After incubation at 95°C for 5 min in XT sample buffer (Bio-Rad), 20 μL of protein per lane was loaded onto 3–8% tris-acetate polyacrylamide gels (Criterion XT; Bio-Rad) and separated by electrophoresis at 150 V for 65 min in XT tricine running buffer. Proteins were transferred onto 0.2-µm nitrocellulose membranes with a Trans-blot turbo transfer system (Bio-Rad) at 2.5 A and 25 V for 10 min. Membranes were blocked in 5% BSA in Tris-buffered saline-Tween (TBST, pH 7.6) for 1 h, followed by overnight incubation at 4°C with rabbit anti-phospho-mTORSer2448 monoclonal antibody (1:1,000 in TBST; catalog no. 5536, Cell Signaling Technology Inc., Massachusetts; RRID: AB_10691552) and rabbit anti-α-tubulin (1:15,000 in TBST; catalog no. 2125, Cell Signaling Technology; RRID: AB_2619646) as a loading control. Membranes were subsequently washed three times in TBST and incubated at 23°C for 60 min in secondary horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:3,000 in TBST; catalog no. ab6721, Abcam PLC, Cambridge, UK; RRID: AB_955447). After a 3 × 10-min wash in TBST, membranes were exposed for 5 min in chemiluminescent substrate solution (Clarity; Bio-Rad), visualized with a ChemiDoc scanner, and analyzed with Image Lab software (Bio-Rad) to quantify band density.
After detection of phosphorylated mTOR (p-mTOR), membranes were incubated for 15 min in Restore stripping buffer (ThermoFisher Scientific), washed in TBST, and blocked for 60 min in 5% BSA in TBST. Membranes were reprobed overnight with anti-mTOR monoclonal antibody (1:1,000 in TBST; catalog no. 2972, Cell Signaling Technology; RRID: AB_330978) and anti-α-tubulin, and the above steps were repeated to obtain corresponding bands for mTOR. The expected migrations of p-mTOR and mTOR (both ∼289 kDa) and α-tubulin (∼52 kDa) were confirmed with an All Blue protein ladder (Bio-Rad). Samples were run in duplicate on each gel, and relative abundance was corrected against α-tubulin within each lane. Finally, the ratio of the relative abundance of p-mTORSer2448 to total mTOR (phospho/total mTOR) was calculated.
Skeletal Muscle mRNA Analyses
Total RNA was isolated from ∼20 mg of frozen muscle tissue with TRI Reagent (ThermoFisher Scientific) according to the method of Chomczynski and Sacchi (37). After spectrophotometric quantification at 260 nm (NanoDrop Lite Spectrophotometer; ThermoFisher Scientific), first-strand cDNA was synthesized from 2 μg of RNA with the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Paisley, UK). A TaqMan qPCR assay for α1 actin (ACTA1) was performed on every sample to verify the presence of cDNA. Expression levels of 224 target genes selected from PubMed literature searches and data from our laboratory for their roles in amino acid transport, apoptosis, substrate metabolism, inflammation, insulin signaling, protein synthesis, and breakdown, as well as several transcription factors, were measured by quantitative real-time PCR using 224-format OpenArray qPCR Plates (ThermoFisher Scientific; full list of analyzed genes presented in Supplemental Tables S1 and S2; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.12929111). All samples from a given participant were analyzed on the same plate alongside a pooled sample to correct for any variation in reaction efficiency. The relative expression of each transcript was calculated by the method (where CT is threshold cycle), whereby expression was normalized to the endogenous control genes β2 microglobulin (B2M), α-actin 1 (ACTA1), and β-actin (ACTB), and each subject’s control leg was used as a comparator value. No significant changes in expression of endogenous control genes were noted between leg, group, or time (Supplemental Tables S1 and S2). After log2 transformation to ensure normal distribution of variance, data were split into an acute period (i.e., gene expression at 24, 27, and 36 h after eccentric exercise) and a “temporal” period (i.e., gene expression at 24, 72, and 168 h after eccentric exercise) and analyzed for significant changes over group, time, or time × group. Because of a lack of detectable expression, 10 genes were omitted from the analysis. Lists of significantly up- or down-regulated genes were analyzed for statistical overrepresentation with the PANTHER classification system (38) against pathways defined in PANTHER and Reactome, as well as against Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with DAVID [version 6.8; Laboratory of Human Retrovirology and Immunoinformatics, Frederick, MD (39)]. The original gene list, as opposed to the whole human genome, was used as a background for the enrichment analysis to eliminate any bias from the selection of genes.
Calculations
Myofibrillar protein fractional synthesis rates (myoFSR) were calculated based on the incorporation of [2H]alanine into myofibrillar protein and mean plasma deuterium enrichment between muscle biopsy time points corrected by a factor of 3.7, based on the deuterium labeling during de novo alanine synthesis. MyoFSR was calculated with the following precursor-product equation:
| (1) |
where Em1 and Em2 are the myofibrillar protein-bound enrichments expressed as mole per cent excess (MPE), Ep represents mean precursor enrichment between given time points, and t is time between the corresponding biopsies for which myoFSR is calculated, expressed as either hours or days.
Statistical Analysis
A Student’s independent t test was used to investigate group differences in subject characteristics and plasma 2H enrichments over the postprandial and overnight periods. A two-way mixed-model analysis of variance (ANOVA) (time and group factors) was used to identify differences within and between treatments for muscle function, peak isokinetic torque, and daily plasma 2H enrichments. Plasma CK data were log10-transformed before analysis by two‐way mixed-model ANOVA (time and group factors) and are presented as back-transformed geometric means ± geometric standard deviations (40). Postprandial and overnight myoFSR was analyzed with a two-way mixed-model ANOVA (leg and group factors). Muscle mTOR phosphorylation status, muscle-bound [2H]alanine enrichment, and daily myoFSR were analyzed with three-factor mixed-model ANOVA (leg, group, and time factors). Where an interaction effect was observed with muscle-bound [2H]alanine enrichment, the change over time was assessed by a two-way mixed-model ANOVA (leg and group factors). A Tukey’s multiple comparisons test was applied to locate the individual differences when a significant main effect of time was detected. Significant interaction effects were analyzed post hoc with Sidak correction for multiple comparisons applied to locate individual differences. Relative gene expression was analyzed with a mixed error-component model with time and group factors. Separate gene lists were created from genes displaying significant time, group, or interaction effects and manually corrected for multiple comparisons with the Benjamini–Hochberg procedure (41) (false discovery rate < 5%). Hierarchical cluster analysis using Pearson’s correlation was subsequently performed on mean values of genes with significant differential expression (MeV 4.9, TM4) (42). Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA). All data are presented as means ± SE unless otherwise stated, with P < 0.05 indicating statistical significance.
RESULTS
Participants’ baseline characteristics are shown in Table 1. No differences were seen in age, body mass, height, BMI, or habitual dietary intake between groups. During the 14-day study period, body mass remained stable and did not differ between groups (PLA: 77.9 ± 4.1 to 77.3 ± 4.1 kg and PPB: 73.9 ± 3.9 to 73.8 ± 4.1 kg, baseline to 168 h, respectively), suggesting dietary compliance. No differences were observed between groups for energy, carbohydrate, or fat consumption during the control diet, but protein intake was significantly greater with PPB (1.2 ± 0.0 vs. 1.5 ± 0.0 g·kg body mass·day−1, P < 0.001; Table 1). At the end of the study, participants were asked which intervention they had been assigned to; upon unblinding, two participants in PLA and three participants in PPB had correctly identified their group. The remaining 13 participants either did not know or guessed incorrectly, suggesting effective blinding to the intervention. Skeletal muscle biopsy samples were not obtained from one participant in PLA; thus plasma deuterium and myofibrillar protein-bound [2H]alanine enrichment, myoFSR, and mTOR phosphorylation status are determined in n = 8 subjects for this group. Because of a limited quantity of remaining muscle tissue, gene expression was analyzed in n = 7 subjects for both groups.
Eccentric work completed did not differ between PLA and PPB (49,160 ± 2,542 vs. 44,647 ± 3,917 J). Peak eccentric torque during set 1 was similar between groups (254 ± 13 and 216 ± 24 n·m, PLA and PPB, respectively). This decreased over time to 182 ± 22 and 157 ± 17 n·m at set 10 for PLA and PPB, respectively (time effect P < 0.001). There was no effect of PPB on peak eccentric torque.
The matched concentric work performed at 24 h in ECC was 0.6 ± 0.2% and 0.4 ± 0.1% greater than CON for PLA and PPB, respectively (P < 0.001).
Muscle Function
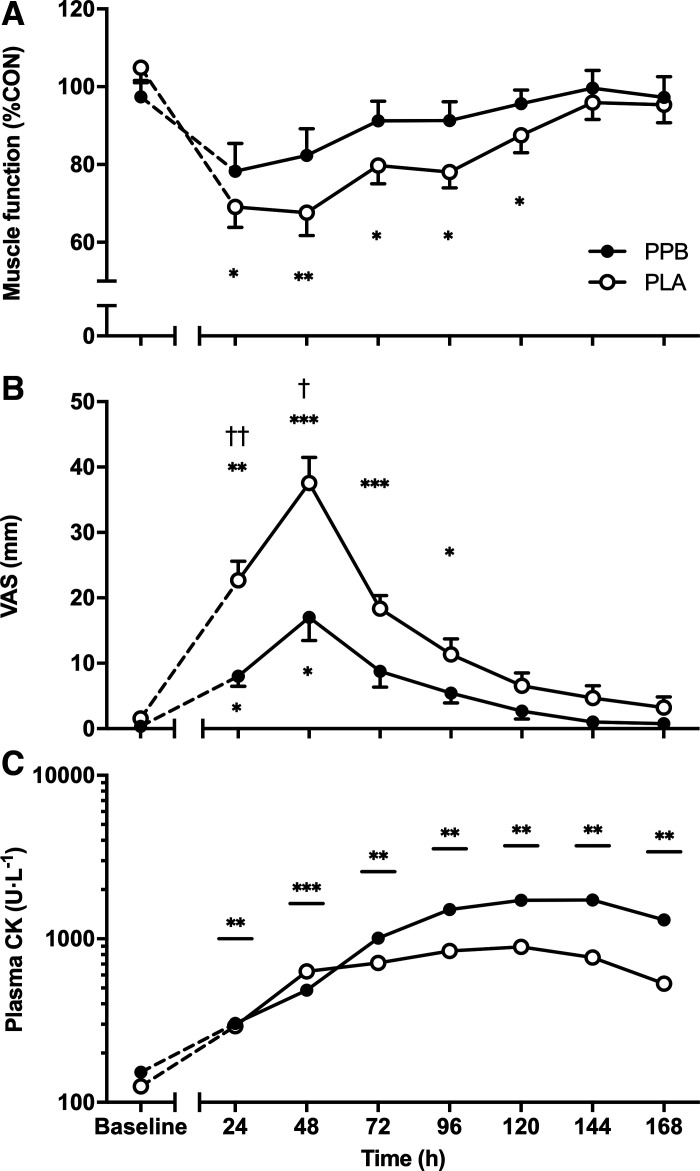
Muscle function in the ECC leg expressed relative to CON (%CON) is shown in Fig. 2A. Baseline muscle function did not differ between PLA and PPB (104.9 ± 3.3 and 97.4 ± 3.6%CON, respectively). After eccentric exercise, muscle function decreased in PLA at 24 h and remained suppressed until 120 h (69.1 ± 5.3 to 87.5 ± 4.4%CON, respectively; post hoc P < 0.05 vs. baseline). However, PPB supported the recovery of muscle function (group × time interaction P < 0.05), with post hoc tests indicating no significant differences compared to baseline, or between groups, at any time point. Muscle function recovered quicker between 24 and 72 h (8.9 ± 3.6 and 10.8 ± 4.4%·day−1, PLA and PPB respectively) than between 72 and 168 h after eccentric exercise (5.4 ± 1.5 and 1.8 ± 1.1%·day−1, PLA and PPB, respectively; time effect P < 0.05).
Figure 2.
Markers of muscle damage before (baseline) and every 24 h after performing 300 maximal, unilateral, eccentric, quadriceps contractions assessed by muscle function, defined as total work performed over 30 maximal, voluntary, isokinetic, concentric contractions of the knee extensor muscles expressed relative to the contralateral leg (%CON) (A); muscle soreness upon rising from a seated to standing position measured with 100-mm visual analog scale (VAS) going from “no pain at all” (0 mm) to “worst possible pain” (100 mm) (B); and plasma creatine kinase (CK) levels (C). A protein-polyphenol supplement (PPB; n = 9 subjects) or an isocaloric placebo (PLA; n = 9 subjects) was consumed daily, immediately after eccentric exercise or assessment of muscle function (baseline, 24–168 h). A and B: means ± SE. C: geometric mean ± geometric SD displayed on a logarithmic scale on the y-axis. Statistical analysis performed with a 2-way ANOVA. A: significant time × group interaction effect (P < 0.05). B: significant time × group interaction effect (P < 0.001). C: significant main effect of time (P < 0.001). Post hoc differences with time effect: **P < 0.01, ***P < 0.001 significantly different from baseline. Post hoc differences within time × group interaction effects: †P < 0.05, ††P < 0.01 significantly different from PLA at same time point; *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from baseline.
Eccentric exercise significantly decreased peak isokinetic torque produced during the function test (time effect P < 0.001). This decreased from 102.5 ± 3.7%CON at baseline to 67.0 ± 5.2%CON at 24 h in PLA. PPB did not influence the loss of peak torque at any time, decreasing from 96.5 ± 3.4 to 76.8 ± 8.1%CON from baseline to 24 h (post hoc P < 0.01 vs. baseline). Peak torque was 66.8 ± 5.3%CON in PLA and 82.5 ± 7.7%CON in PPB at 48 h (post hoc P < 0.01 vs. baseline) and 74.8 ± 6.4 and 88.0 ± 4.7%CON in PLA and PPB at 72 h (post hoc P < 0.05 vs. baseline). By 96 h, peak isokinetic torque had recovered to baseline (PLA: 81.8 ± 5.1%CON, PPB: 95.2 ± 5.3%CON).
Muscle Soreness
Baseline muscle soreness was 2 ± 1 and 0 ± 0 mm in PLA and PPB, respectively (Fig. 2B). Muscle soreness rose in PLA at 24 h (post hoc P < 0.01) and peaked at 48 h (post hoc P < 0.001) before returning to baseline at 120 h. In PPB, the rise in muscle soreness was significantly attenuated compared with PLA at 24 h (post hoc P < 0.01) and 48 h (post hoc P < 0.05) and had returned to baseline by 72 h after eccentric exercise (time × group interaction P < 0.001).
Plasma Creatine Kinase
Baseline plasma CK levels (Fig. 2C) were 153 ± 2 and 125 ± 2 U·L−1 for PLA and PPB, respectively. Plasma CK rose equally in both groups (time effect P < 0.001) and remained elevated at all time points after eccentric exercise (post hoc P < 0.01). PPB was not significantly different from PLA at any time point.
Plasma Precursor Enrichment
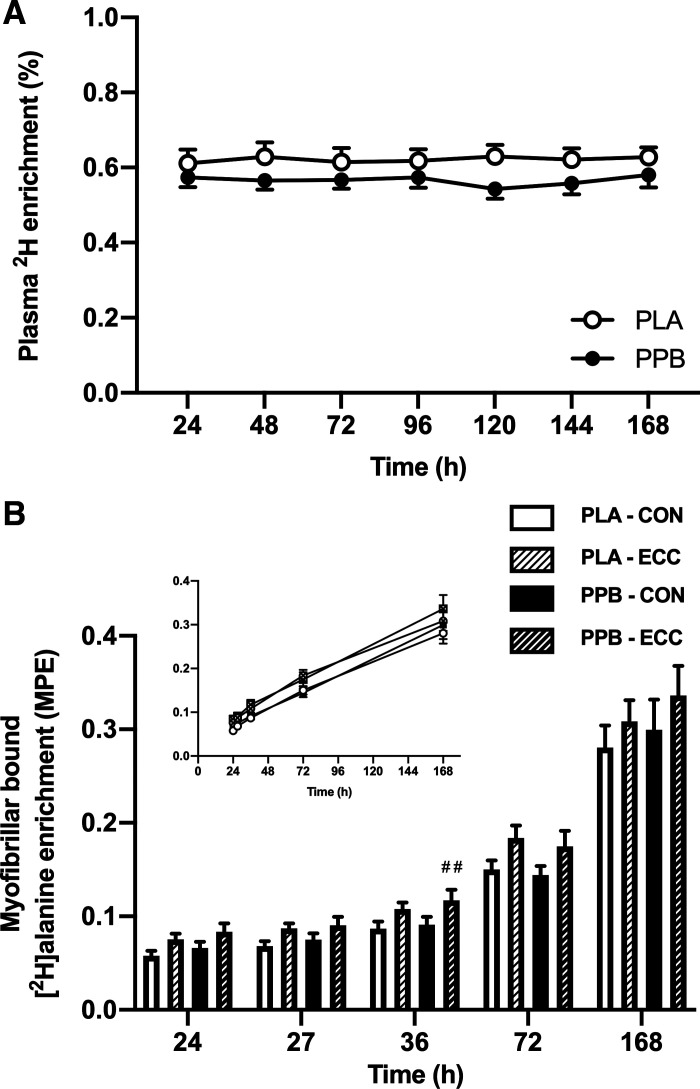
Plasma deuterium enrichment (Fig. 3A) reached 0.61 ± 0.04% at 24 h in PLA and was similar in PPB (0.57 ± 0.03%). Daily plasma enrichment remained stable and did not differ between groups (7-day average of 0.62 ± 0.01% and 0.57 ± 0.01% in PLA and PPB, respectively). Between 24 and 27 h and between 27 and 36 h after eccentric exercise, enrichment did not differ between PLA and PPB (24 and 27 h: 0.61 ± 0.03% and 0.57 ± 0.02% and 27 and 36 h: 0.63 ± 0.03% and 0.58 ± 0.02%, PLA and PPB, respectively).
Figure 3.
A: daily plasma 2H enrichment (%) after oral 2H2O ingestion, measured every 24 h after performing 300 maximal, unilateral, eccentric, quadriceps contractions. A protein-polyphenol supplement (PPB; n = 9 subjects) or an isocaloric placebo (PLA; n = 8 subjects) was consumed daily, immediately after eccentric exercise or every 24 h thereafter. B: enrichment of myofibrillar bound [2H]alanine (MPE) in skeletal muscle biopsy samples obtained 24, 27, 36, 72, and 168 h after performing 300 maximal eccentric quadriceps contractions in one leg (ECC) or the contralateral control leg (CON). PPB (n = 9 subjects) or PLA (n = 8 subjects) was consumed daily, immediately after eccentric exercise or every 24 h thereafter. Inset, data expressed relative to a linear scale on the x-axis to demonstrate increase of enrichment with respect to time. Values are means ± SE. Daily plasma 2H enrichment analyzed by 2-way ANOVA (time and group factors). Myofibrillar bound [2H]alanine enrichment analyzed by 3-way ANOVAs (time, leg, and group factors) between 24 and 27 h, 27 and 36 h, and 72 and 168 h. For myofibrillar bound [2H]alanine enrichment, significant main effect of time (P < 0.001) and leg (P < 0.001) for all comparisons. Between 27 and 36 h, significant interaction for time × leg (P < 0.01) and time × leg × group (P < 0.05). Post hoc differences within time × leg × group interaction effect: ##P < 0.01 change from previous time point significantly greater in ECC vs. CON leg in PPB.
Myofibrillar Protein Enrichment and Fractional Synthesis Rates
Myofibrillar protein-bound [2H]alanine enrichments (Fig. 3B) increased over the postprandial period 24–27 h after eccentric exercise (time effect P < 0.001) and were 32.5 ± 6.6% and 29.4 ± 4.6% higher in ECC versus CON at 24 and 27 h, respectively, in PLA (leg effect P < 0.001). A similar response was seen in PPB whereby MPE was higher in ECC versus CON by 26.5 ± 3.9% and 20.5 ± 3.6% at 24 and 27 h, respectively. Overnight, the increase in myofibrillar protein-bound [2H]alanine enrichments in PLA was similar for CON (26.9 ± 3.9%) and ECC (24.3 ± 4.3%) legs. However, with PPB, the increase was greater in ECC (29.4 ± 2.1%) than in CON (22.1 ± 2.5%; time × leg × group interaction P < 0.05). Between 72 and 168 h, myofibrillar protein-bound [2H]alanine enrichments increased by 86.4 ± 8.1% in CON and by 71.3 ± 12.5% in ECC for the PLA group (time effect P < 0.001). PPB increased by a similar extent, by 109.5 ± 21.2% and by 93.1 ± 7.0% in CON and ECC, respectively. Enrichment in the ECC leg was 23.1 ± 5.6% and 20.6 ± 6.8% greater than CON for PLA and PPB at 72 h and 14.3 ± 9.9% and 13.9 ± 5.5% greater in ECC than CON for PLA and PPB at 168 h (leg effects P < 0.001). Data were analyzed for linearity, whereby a straight-line model was preferred to explain the increase in enrichment over time (r2 = 0.794), indicating that a rise to plateau was not evident.
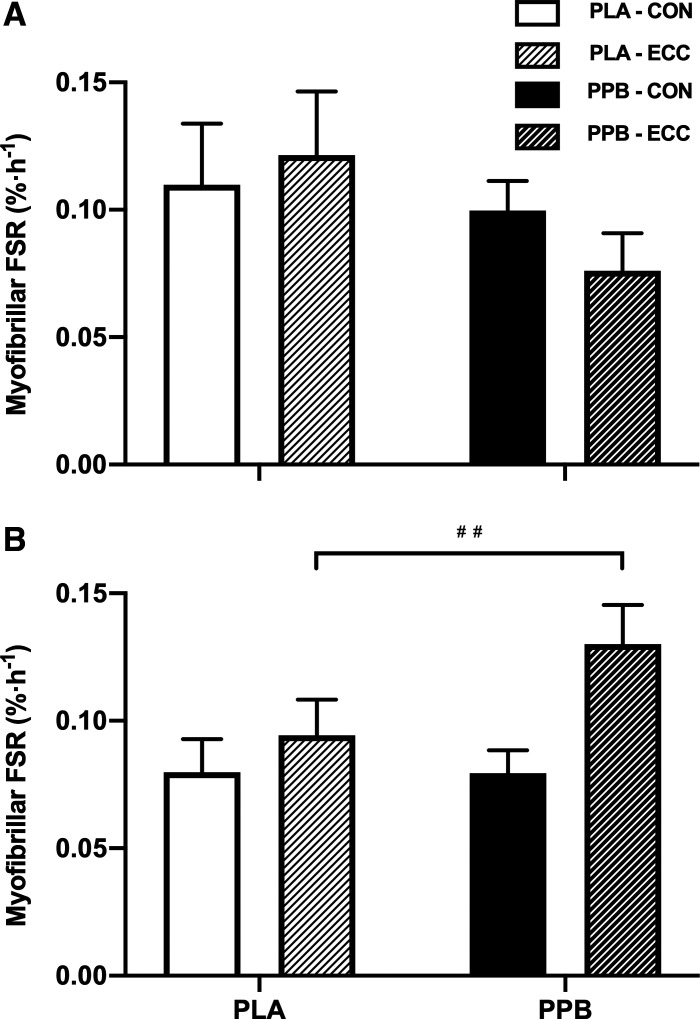
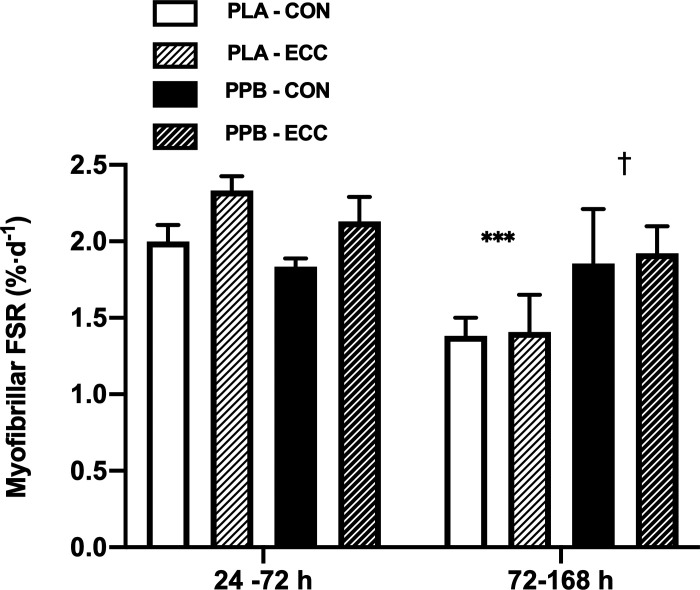
Postprandial and overnight myoFSR is displayed in Fig. 4. After exercise at 24 h, postprandial (24–27 h) myoFSR was unaffected by group or prior eccentric exercise. Overnight (27–36 h) myoFSR was greater in ECC versus CON (PLA: 0.094 ± 0.013 vs. 0.080 ± 0.012%·h−1; PPB: 0.130 ± 0.008 vs. 0.080 ± 0.008%·h−1; leg effect P < 0.01), where the difference between legs in PPB (76 ± 25%) tended to be greater than the difference in PLA (33 ± 19%; leg × group interaction P = 0.06).
Figure 4.
Myofibrillar protein fractional synthesis rate (FSR; expressed as %·h−1) over a 3-h postprandial period after maximal, bilateral, isokinetic, concentric exercise and the consumption of a postexercise protein-polyphenol supplement (PPB; n = 9 subjects) or an isocaloric placebo (PLA; n = 8 subjects) (A) and the subsequent 9-h overnight period, beginning with the consumption of a maltodextrin beverage (B). Postprandial and overnight periods correspond to between ∼24 and 27 h and ∼27 and 36 h, respectively, after performing 300 maximal eccentric quadriceps contractions in one leg (ECC). The contralateral control leg is represented as CON. Fractional synthetic rates calculated from plasma deuterium enrichment as precursor pool. Data are means ± SE. Statistical analysis performed with a 2-way ANOVA. Significant main effect of leg (i.e., CON vs. ECC): ##P < 0.01.
Daily myoFSR (Fig. 5) fell from 24–72 h to 72–168 h in PLA (CON: 2.00 ± 0.10 to 1.38 ± 0.11%·day−1 and ECC: 2.33 ± 0.09 to 1.41 ± 0.23%·day−1, respectively; post hoc P < 0.001). Conversely, daily myoFSR remained unchanged in PPB from 24–72 h to 72–168 h (CON: 1.83 ± 0.05 to 1.86 ± 0.34%·day−1 and ECC: 2.13 ± 0.15 to 1.92 ± 0.17%·day−1, respectively; group × time interaction P < 0.05) and was significantly greater than PLA between 72 and 168 h (post hoc P < 0.05). MyoFSR tended to be 16.0 ± 6.6% higher in ECC than in CON (leg effect P = 0.06).
Figure 5.
Free-living, cumulative myofibrillar protein fractional synthesis rate (FSR; expressed as %·day−1) between 24 and 72 h and between 72 and 168 h of recovery from 300 maximal, unilateral, eccentric, quadriceps contractions with daily maximal, bilateral, concentric exercise and consumption of a postexercise protein-polyphenol supplement (PPB; n = 9 subjects) or an isocaloric placebo (PLA; n = 8 subjects). Eccentric exercise was performed in one leg only (ECC). The contralateral control leg is represented as CON. Fractional synthetic rates calculated from plasma deuterium enrichment as precursor pool. Data are means ± SE. Statistical analysis performed with a 3-way ANOVA. Significant time × group interaction effect (P < 0.05). Post hoc differences within time × group interaction effect: †P < 0.05 significantly different from PLA at same time point; ***P < 0.001 significantly different from 24–72 h in PLA.
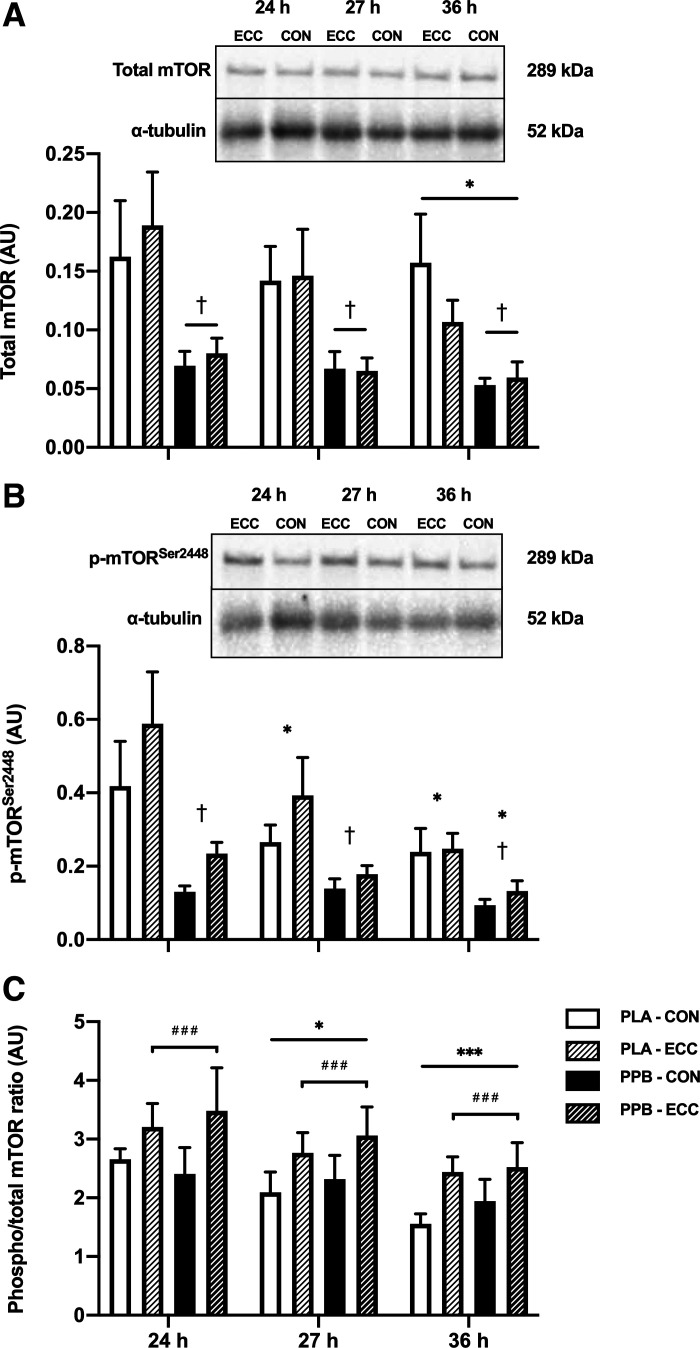
mTOR Protein Content and Phosphorylation Status
Total mTOR protein content fell between 24 and 36 h (time effect P < 0.05) and was lower in PPB than PLA (group effect P < 0.05; Fig. 6A). In ECC, p-mTORSer2448 protein content was 55 ± 17% and 100 ± 34% higher than CON in PLA and PPB, respectively (leg effect P < 0.001; Fig. 6B). In PLA this fell at 27 and 36 h from 24 h (group × time interaction P < 0.05; post hoc P < 0.05), and in PPB it was lower at 36 h than 24 and 27 h (post hoc P < 0.05).
Figure 6.
Skeletal muscle mechanistic target of rapamycin (mTOR) phosphorylation status, presented as total mTOR protein (A); mTOR phosphorylated at Ser2448 (p-mTORSer2448, B); and the ratio of phosphorylated to total protein (C) 24, 27, and 36 h after performing 300 maximal, eccentric quadriceps contractions in one leg (ECC). The contralateral control leg is represented as CON. After the biopsy at 24 h, maximal, bilateral, isokinetic, concentric exercise was performed and a postexercise protein-polyphenol supplement (PPB; n = 9 subjects) or an isocaloric placebo (PLA; n = 8 subjects) was consumed. A maltodextrin beverage was consumed immediately after the biopsy at 27 h. α-Tubulin was used as a loading control. Images obtained from a single representative participant in the placebo group. Data are means ± SE. Statistical analysis performed with a 2-way ANOVA. A: significant main effects of time (P < 0.05; post hoc differences: *P < 0.05 significantly different from baseline) and group (P < 0.05; †P < 0.05 significantly different from PLA). B: significant main effect leg (P < 0.001). Significant time × group interaction effect (P < 0.05). Post hoc differences within time × group interaction effect: †P < 0.05 significantly different from PLA at same time point; *P < 0.05 significantly different from baseline. C: significant main effect of time (P < 0.001; post hoc differences: *P < 0.05, ***P < 0.001, significantly different from baseline) and main effect of leg (P < 0.001; ###P < 0.001). AU, arbitrary units.
Skeletal muscle phospho/total mTOR fell over the 24–27 h postprandial and 27–36 h overnight periods (Fig. 6C; time effect P < 0.001). The phospho/total mTOR ratio in ECC leg was 40 ± 6% and 49 ± 13% higher than CON in PLA and PPB, respectively (leg effect P < 0.001). No group difference was evident.
Gene Expression
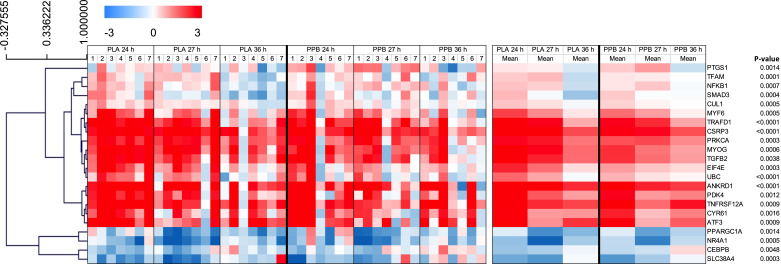
Expression of 22 genes changed between 24, 27, and 36 h after eccentric exercise (Fig. 7; Supplemental Table S1; FDR < 5%). Hierarchical cluster analysis revealed two distinct clusters: mean expression values for 18 genes were positive, indicating relatively greater expression in ECC compared with CON, and mean expression values of four genes were negative, indicating lower expression in ECC compared with CON. Analysis against the Reactome pathway database highlighted enrichment of six different pathways, with “Regulation of RUNX2 expression and activity” (UBC, CUL1, and PPARGC1A), “MAP3K8 (TPL2)-dependent MAPK1/3 activation” (UBC, CUL1, and NFKB1), “Signaling by NOTCH4” (UBC, CUL1, and SMAD3), “TNFR2 non-canonical NF-kB pathway” (UBC, CUL1, and TNFRSF12A), and “Regulation of PLK1 Activity at G2/M Transition” (UBC and CUL1) the most significant (P < 0.05). Gene functional analysis highlighted no enriched pathways against PANTHER and KEGG databases.
Figure 7.
Heatmap with hierarchical clustering of differentially expressed skeletal muscle transcripts from pathways involved in amino acid transportation, apoptosis, substrate metabolism, inflammation, insulin signaling, protein synthesis, and breakdown, as well as several transcription factors. Skeletal muscle biopsies were taken 24, ∼27, and ∼36 h after performing 300 maximal, unilateral, eccentric, quadriceps contractions, corresponding to pre, ∼3 h, and ∼12 h after maximal, bilateral, concentric exercise and consumption of a postexercise protein-polyphenol supplement (PPB; n = 7 subjects) or an isocaloric placebo (PLA; n = 7 subjects). All individual values are expressed as fold change from control leg at the same time point, with log2 transformation applied. Heatmap constructed from genes showing significant main effects for time, with corresponding P value, as assessed by a mixed error-component model with time and group factors and satisfying criterion for false discovery rate (FDR) < 5%. Hierarchical clustering performed on mean data with Pearson’s correlation.
Three genes (ACTN3, IL1RL1, FOXO3) were differentially expressed between PLA and PPB (P < 0.05), and a further seven genes (TFAM, PLIN2, CCL8, CTSL1, EIF4E, EIF4EBP1, GDF11) exhibited a leg × group interaction effect (P < 0.05), but these did not satisfy the criterion for FDR < 5% so were not analyzed for enriched pathways.
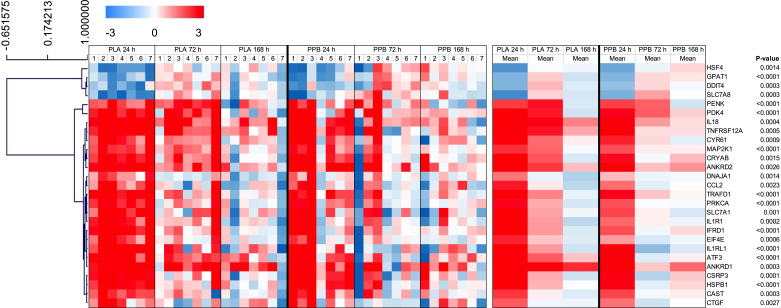
Expression of 27 genes changed between 24, 72, and 168 h after eccentric exercise (Fig. 8; Supplemental Table S2; FDR < 5%). Hierarchical cluster analysis revealed two distinct clusters: mean expression values for 23 genes were positive and decreased over time, whereas mean expression values for four genes were negative and increased over time. Gene functional analysis highlighted no enriched pathways against Reactome, PANTHER, or KEGG databases.
Figure 8.
Heatmap with hierarchical clustering of differentially expressed skeletal muscle transcripts from pathways involved in amino acid transportation, apoptosis, substrate metabolism, inflammation, insulin signaling, protein synthesis, and breakdown, as well as several transcription factors. Skeletal muscle biopsies taken in the rested state 24, 72, and 168 h after performing 300 maximal, unilateral, eccentric, quadriceps contractions with daily maximal, bilateral, concentric exercise and consumption of a postexercise protein-polyphenol supplement (PPB; n = 7 subjects) or an isocaloric placebo (PLA; n = 7 subjects). All individual values are expressed as fold change from control leg at the same time point, with log2 transformation applied. Heatmap constructed from genes showing significant main effects for time, with corresponding P value, as assessed by a mixed error-component model with time and group factors and satisfying criterion for false discovery rate (FDR) < 5%. Hierarchical clustering performed on mean data with Pearson’s correlation.
Two genes (ATF3, IL1B) were differentially expressed between PLA and PPB (P < 0.05), and six genes (CCL8, HK2, DGAT2, PIK3R1, TRAF6, and GDF11) showed a leg × group interaction (P < 0.05). However, these did not satisfy the criterion for FDR < 5% so were not analyzed for enriched pathways.
DISCUSSION
The aim of the present study was to characterize, for the first time, the time course of rates of MyoPS and key gene expression pathways of inflammation, protein synthesis, proteolysis, and substrate metabolism over a week of recovery after voluntary eccentric muscle contractions in humans. To deduce the relative importance of MyoPS, we utilized a protein and polyphenol nutritional intervention targeted at improving functional recovery while fully controlling for diet and strenuous exercise. As expected, the postexercise provision of 20 g of protein combined with 650 mg of pomegranate extract accelerated the recovery of muscle function by 120 h and suppressed soreness over 48 h after muscle damage compared with an isocaloric placebo in a healthy population. Remarkably, nutritional intervention improved recovery at 48 h, when loss of muscle function and soreness was the greatest in the placebo group (∼36% decline in muscle function). However, a major finding of the present study, and contrary to our hypothesis, was that this improvement in recovery was not underpinned by an increase in the rate of daily MyoPS compared with placebo between 24 and 72 h, when the loss of muscle function and recovery rate was the greatest, despite a trend for elevated overnight MyoPS during this time. This would suggest that amino acid availability is not limiting to MyoPS from 24 h after damaging exercise, perhaps because of increased availability from protein breakdown (18, 43). To this end, MyoPS may already be maximal during this period such that further stimulation is not possible, or elevated MyoPS may only be required for a short time period to enhance recovery. Furthermore, the upregulation in inflammatory and regenerative signaling following eccentric exercise was not affected by nutritional intervention, suggesting that accelerated recovery is not caused by the transcriptional regulation of some inflammatory and regenerative pathways.
Eccentric exercise caused a profound loss in muscle function (∼34% below baseline at 24 h) persisting for 120 h in the placebo group. Additionally, eccentric exercise caused ∼27% decrease in peak isokinetic torque produced during the muscle function assessment, in agreement with previous reports (13, 44, 45). This was observed together with ∼35% greater phospho/total mTOR, ∼41% greater rates of overnight MyoPS, and a tendency for elevated rates of daily MyoPS in the damaged versus control leg between 24–72 h and 72–168 h after eccentric exercise. Thus, by showing an accompanying, transient loss of muscle contractile ability as demonstrated in the present study, we support and extend upon existing data demonstrating that the repair and remodeling process after muscle damage is associated with elevated rates of MyoPS and mTOR phosphorylation (17, 46). Furthermore, consistent with human models of muscle damage induced by eccentric exercise (15, 16, 44, 45, 47), we show that muscle soreness peaked after 48 h and was followed by a rise in plasma CK (Fig. 2, B and C) between 96 and 168 h after eccentric exercise (48). In the present study, nutritional intervention accelerated the recovery of muscle function by 120 h, more so than has been reported with either whey protein alone (72 h) (49) or milk protein blends (48 h) (45, 50). Although the recovery of peak isokinetic torque was only qualitatively similar, function was designated our primary marker of contractile ability, as this likely reflects both peak torque and fatigability, which are known consequences of damage to contractile elements with eccentric exercise (51). This, in addition to the composition of the nutritional intervention and the strict dietary control employed to remove the influence of a variable habitual diet on amino acid availability, may explain the difference in magnitude of recovery that was observed with PPB compared with previous work. Indeed, a small but favorable effect for whey protein supplementation on recovery of function up to 96 h after resistance exercise was highlighted in a recent meta-analysis, of which only three of eight studies analyzed controlled for dietary protein intake (22). Furthermore, polyphenol-rich pomegranate extract alone improves recovery of muscle function in humans by ∼9–11% 48 h after eccentric exercise (11, 52), which may have an additive or synergistic effect when combined with protein to drastically improve the rate of muscle recovery.
To our knowledge, no study to date has determined whether an increase in MyoPS is responsible for an improvement in recovery after damaging eccentric exercise in humans. Despite significantly accelerating recovery of muscle function by 120 h, and contrary to our hypothesis, rates of postprandial MyoPS were not enhanced after nutritional intervention. Furthermore, phospho/total mTOR at 24, 27, and 36 h after eccentric exercise was similar between groups, as were rates of daily MyoPS between 24 and 72 h, when the greatest difference in recovery was observed. Taken together, these data are not consistent with MyoPS being the primary mechanism dictating muscle recovery. Although it is conceivable that nutritional intervention would not be sufficient to provide an anabolic stimulus, we are confident this was not the case, as the provision of 20 g of protein containing 2 g of leucine has been demonstrated to stimulate a robust muscle protein synthetic response over the effects of exercise alone (53, 54). Given that the rates of postprandial MyoPS in the present study (∼0.101%·h−1) are comparably larger than have previously been reported after nutrition (∼0.088%·h−1) (23) or nutrition with exercise (∼0.082%·h−1) (55) and that myofibrillar protein synthesis plateaus despite increasing amino acid availability in healthy individuals (56), we propose that exogenous amino acids are not limiting to MyoPS at this time. The proximity of the evening meal and/or the breakdown of endogenous proteins following eccentric exercise (4, 18) and injury (43) may provide sufficient substrate for MyoPS. Although we are unable to simultaneously characterize rates of muscle protein breakdown with the application of 2H2O as described here, greater rates of muscle protein breakdown would increase availability of amino acids for both synthesis and outward transport (43). As a result, this may increase amino acid delivery to both ECC and CON legs, thus accounting for the similar rates of synthesis. Moreover, should rates of muscle protein breakdown subside thereafter as is reported elsewhere (4, 18), amino acid availability may then become limiting to MyoPS. This would explain our and others’ (24) observations of a drop in daily MyoPS between 72 and 168 h after eccentric exercise in the absence of additional protein. Consistent with this proposed mechanism, the provision of exogenous protein likely afforded greater rates of MyoPS during this later period compared with placebo (Fig. 5).
At the beginning of the overnight period (27 h), phospho/total mTOR decreased by ∼13% compared with the postprandial period (24 h). Although the direct determination of mTOR activity by assessing kinase activity or investigating the phosphorylation status of additional downstream targets would have extended our mechanistic insight, these data are supported by an ∼24% decrease in rates of MyoPS overnight in the control leg. Together, it appears that the anabolic response was not maximal overnight, in accordance with previous work (57). As discussed above, we identified greater overnight MyoPS in the eccentrically exercised versus control leg, which may be due to increased amino acid availability from protein breakdown (4, 18). Accordingly, should amino acid availability dictate rates of MyoPS overnight, the protein bolus ∼3 h before and greater daily protein intake may explain why MyoPS tended to be greater still after nutritional intervention (∼18% greater with eccentric exercise alone vs. ∼64% greater with eccentric exercise and nutritional intervention). This suggests the existence of a key window within 36 h after eccentric exercise, in which basal or overnight MyoPS can be manipulated to accelerate recovery. Nonetheless, this did not manifest as a difference in daily MyoPS between 24 and 72 h, and so overnight differences may be negligible when accounting for all periods of protein synthesis 24 h after eccentric exercise. Furthermore, severe muscle-damaging protocols in mice appear to delay the rise in protein synthesis for >48 h (4), and so the existence of this window requires further investigation.
To identify changes in pathway signaling during the recovery period, we analyzed the expression of 224 genes selected for their roles in amino acid transportation, apoptosis, substrate metabolism, inflammation, insulin signaling, protein synthesis, and breakdown, as well as several transcription factors (Supplemental Table S1). Prior eccentric exercise augmented inflammatory signaling over the postprandial and overnight time frames between 24 and 36 h after eccentric exercise, in agreement with previous transcriptomic analyses (6, 58, 59). This was evidenced by enrichment of “TNFR2 non-canonical NF-kB pathway” and “MAP3K8 (TPL2)-dependent MAPK1/3 activation,” as well as clustering of PTGS1, NFKB1, TRAFD1, TNFRSF12A, and CYR61 demonstrating upregulated expression in the eccentrically exercised leg relative to the control leg. Common to all pathways enriched over this time was the expression of CUL1 and UBC, which together promote NF-κB signaling (60). Interestingly, CUL1 and UBC clustered closely with TGFB2, and to a lesser extent SMAD3 (Fig. 7), which with concurrent enrichment of “Regulation of PLK1 Activity at G2/M Transition” identifies cell cycle regulation being transcriptionally relevant during recovery (61), in support of previous work (62). Specifically, this time course approach reveals signaling related to inhibition of cell cycle progression and elevated inflammation that was initially upregulated and proceeded to fall over the postprandial and overnight phases (Fig. 7). Given that these analyses were performed on whole muscle homogenates, we cannot be sure of the definitive origin of such genes. Nonetheless, these may emanate from populations of mitotic cells that are known to proliferate in response to eccentric exercise, such as satellite cells (63). In support, we also identified the expression of two markers of myogenic differentiation within the CUL1 and UBC cluster (MYF6 and MYOG; Fig. 7). Together with clustering of PRKCA, EIF4E, and CSRP3, this signature is indicative of a regenerative milieu in the skeletal muscle after eccentric exercise, corroborating our observations of elevated overnight MyoPS and phospho/total mTOR in the eccentrically exercised leg.
To increase the temporal understanding of muscle recovery processes, and account for circadian regulation of gene expression after muscle damage (64), we performed separate bioinformatic analysis on muscle biopsy samples collected at 24, 72, and 168 h (Supplemental Table S2). Here, continued expression and clustering of genes associated with inflammatory (IL18, TNFRSF12A, CYR61, ILR1, and IL1RL1) and regenerative (EIF4E, IFRD1, PRKCA, MAP2K1, and CSRP3; Fig. 8) signaling was evident, as was identified over the acute period. Specifically, these genes support a role for NF-κB signaling beyond a 3-h period following eccentric exercise as previously identified (6). Although only the expression of the NF-κB subunit 1 (NFKB1) was identified over the acute period (Fig. 7), the expression of up- and downstream genes data together with our ontology analysis between 24 and 36 h after eccentric exercise implicate this pathway as being relevant throughout the recovery process. Interestingly, CCL2 expression was ∼4.8-fold greater at 24 h than 168 h, which is required for macrophage/monocyte infiltration and myofibrillar repair in vitro (65). Within this cluster of genes, we also show expression of HSPB1 and CRYAB heat shock proteins, which are known to colocalize with damaged myofibrillar structures between 48 and 168 h after eccentric exercise-induced muscle damage (13) and are positively associated with muscle protein accretion (66).
By capturing transcriptional events across multiple time points together with measures of muscle function, we extend on previous findings that identify transcriptional changes at single time points 3–48 h into recovery (6, 58, 59) and support the roles of upregulated inflammatory and regenerative signaling, potentially mediated by NF-κB, as being relevant to the restoration of muscle function after damage (Fig. 7). Nonetheless, despite expediting muscular recovery after eccentric exercise, we show no effect of nutritional intervention on the expression of any transcripts over either acute or temporal time frames that satisfied the criterion for FDR < 5%. Despite this, a small number of transcripts were observed to be affected by nutritional intervention with significance at the level of P < 0.05 (Supplemental Tables S1 and S2) but were discounted from the present analysis because of the necessity to control for type I errors. Of these, IL1RL1 expression appeared ∼84% lower with nutritional intervention over the acute period, consistent with attenuation of NF-κB signaling. Accordingly, IL1B and ATF3, up- and downstream of NF-κB, respectively, were ∼69% and ∼56% lower with nutritional intervention across the temporal time frame. Thus, targeted analysis into NF-κB signaling may be of interest to explore a possible relationship with accelerated recovery.
Very limited data exist investigating MyoPS or signaling pathways in cases where targeted interventions, such as protein or polyphenol supplementation, have accelerated recovery, and to our knowledge no studies that show a beneficial effect of such interventions on muscular recovery utilize comprehensive measures of MyoPS or investigate changes in signaling at the transcriptional level. Although the contribution of cytosolic protein synthesis to recovery remains unknown, we determined mTOR phosphorylation status in a cytosolic protein portion and determined rates of MyoPS to investigate the recovery of contractile elements and investigated associated gene signaling pathways. Therefore, the present data suggest that synthesis of myofibrillar proteins and gene signaling pathways may not limit muscular recovery under normal physiological conditions in humans. Indeed, eccentric exercise has been routinely demonstrated to increase intramuscular leukocyte infiltration in human (45, 47) and animal (67) models. In particular, leukocyte infiltration (47), specifically that of neutrophils (67), appears to attenuate recovery of muscle function ∼24 h after the initial injury (47, 68). Proanthocyanins have free radical scavenging ability and disrupt NADPH oxidase signaling pathway in vivo (69) and reduce proteinase release ex vivo (70). In animal models, inhibition of such pathways reduces the prevalence of damaged myofibers by ∼76% after stretch-induced injury (71). In support of this, recent work showed that acceleration of muscle function with whey protein is associated with lower circulating protein carbonylation, despite similar degrees of leukocyte infiltration in the muscle (45). Interestingly in the present study, although not satisfying the criterion for FDR < 5%, CCL8 appeared to be ∼72% lower at 24 h with nutritional intervention, suggesting possible reductions in monocyte infiltration following eccentric exercise. Thus, the combination of whey protein and pomegranate extract in the nutritional intervention employed in the present study may have influenced leukocyte numbers and/or activity, thereby accelerating muscle recovery. Although investigating this mechanism was beyond the scope of the present investigation, this warrants further attention, particularly in the first 24 h after eccentric exercise where considerable leukocyte infiltration is evident (47).
In conclusion, utilizing a specific protein-polyphenol nutritional intervention targeted increasing myofibrillar protein synthesis, and suppressing inflammation improves muscle function recovery by 120 h and suppresses soreness over 48 h after maximal eccentric exercise. We show for the first time that this acceleration in recovery occurs in the absence of elevated rates of postprandial myofibrillar protein synthesis. Overnight rates of myofibrillar protein synthesis tended to be greater with nutritional intervention, suggesting that a critical recovery window may occur within 36 h after damaging exercise. However, this was not reflected by greater daily rates between 24 and 72 h, when muscle function loss was the greatest, and as such these data are not consistent with myofibrillar protein synthesis being the primary mechanism dictating muscle recovery. Transcriptional analysis revealed initial upregulation of inflammatory and regenerative signaling following eccentric exercise, which was maintained during the recovery of muscle function. Nonetheless, nutritional intervention did not influence gene expression in these pathways, suggesting that transcriptional regulation of some inflammatory and regenerative pathways does not underpin recovery from skeletal muscle damage.
DISCLOSURES
This work was performed as part of a PhD studentship grant supported by the University of Exeter and Beachbody LLC. C. R. Mikus and N. Alamdari are employed by Beachbody LLC. F. B. Stephens has received payments as a member of the Beachbody LLC scientific advisory board.
AUTHOR CONTRIBUTIONS
G.F.P., T.S.O.J., N.A., C.R.M., B.T.W., and F.B.S. conceived and designed research; G.F.P., T.S.O.J., M.L.D., and B.P.L. performed experiments; G.F.P., T.S.O.J., B.P.L., D.R.A., A.J.M., C.P., and F.B.S., analyzed data; G.F.P., T.S.O.J., B.P.L., B.T.W., and F.B.S. interpreted results of experiments; G.F.P. prepared figures; G.F.P. and F.B.S. drafted manuscript; G.F.P., T.S.O.J., M.L.D., B.P.L., D.R.A., A.J.M., C.P., B.T.W., and F.B.S. edited and revised manuscript; G.F.P., T.S.O.J., M.L.D., B.P.L., D.R.A., A.J.M., C.P., N.A., C.R.M., B.T.W., and F.B.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Sarah R. Jackman (College of Life and Environmental Sciences, University of Exeter) for assisting with muscle biopsy collection and Prof. Lorna W. Harries (University of Exeter Medical School, University of Exeter) for providing laboratory space and equipment to run gene expression analyses. We also thank Dr. Brandon M. Invergo (College of Engineering, Mathematics and Physical Sciences, University of Exeter) for assistance with bioinformatic and statistical analysis of gene expression data and Ken Neal (Elemtex, UK) for measuring plasma deuterium enrichment.
This work was supported by a grant from Beachbody LLC.
REFERENCES
- 1.Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. Minneapolis, MN: Univ. of Minnesota Press, 1950, p. 1385. [Google Scholar]
- 2.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139: 2845–2856, 2012. doi: 10.1242/dev.069088. . [DOI] [PubMed] [Google Scholar]
- 3.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3: 260, 2012. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe DA, Warren GL, Ingalls CP, Boorstein DB, Armstrong RB. Muscle function and protein metabolism after initiation of eccentric contraction-induced injury. J Appl Physiol (1985) 79: 1260–1270, 1995. doi: 10.1152/jappl.1995.79.4.1260. [DOI] [PubMed] [Google Scholar]
- 5.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31: 384–396, 2013. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 6.Hyldahl RD, Xin L, Hubal MJ, Moeckel-Cole S, Chipkin S, Clarkson PM. Activation of nuclear factor-kappaB following muscle eccentric contractions in humans is localized primarily to skeletal muscle-residing pericytes. FASEB J 25: 2956–2966, 2011. doi: 10.1096/fj.10-177105. [DOI] [PubMed] [Google Scholar]
- 7.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 8.Raimondo TM, Mooney DJ. Functional muscle recovery with nanoparticle-directed M2 macrophage polarization in mice. Proc Natl Acad Sci USA 115: 10648–10653, 2018. doi: 10.1073/pnas.1806908115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, Aagaard P, Durigan JL, Kjaer M. Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication. FASEB J 30: 2266–2281, 2016. doi: 10.1096/fj.201500198R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol 292: R2168–R2173, 2007. doi: 10.1152/ajpregu.00858.2006. [DOI] [PubMed] [Google Scholar]
- 11.Trombold JR, Barnes JN, Critchley L, Coyle EF. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med Sci Sports Exerc 42: 493–498, 2010. doi: 10.1249/MSS.0b013e3181b64edd. [DOI] [PubMed] [Google Scholar]
- 12.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27: 43–59, 1999. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallén J, Raastad T. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol 293: R844–R853, 2007. doi: 10.1152/ajpregu.00677.2006. [DOI] [PubMed] [Google Scholar]
- 14.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2: 499, 2011. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 15.Farup J, Rahbek SK, Knudsen IS, de Paoli F, Mackey AL, Vissing K. Whey protein supplementation accelerates satellite cell proliferation during recovery from eccentric exercise. Amino Acids 46: 2503–2516, 2014. doi: 10.1007/s00726-014-1810-3. [DOI] [PubMed] [Google Scholar]
- 16.Vissing K, Overgaard K, Nedergaard A, Fredsted A, Schjerling P. Effects of concentric and repeated eccentric exercise on muscle damage and calpain-calpastatin gene expression in human skeletal muscle. Eur J Appl Physiol 103: 323–332, 2008. doi: 10.1007/s00421-008-0709-7. [DOI] [PubMed] [Google Scholar]
- 17.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288: E1153–E1159, 2005. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- 18.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 19.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab 273: E122–E129, 1997. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 20.Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 201: 365–372, 2011. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- 21.Baumann CW, Rogers RG, Otis JS, Ingalls CP. Recovery of strength is dependent on mTORC1 signaling after eccentric muscle injury. Muscle Nerve 54: 914–924, 2016. doi: 10.1002/mus.25121. [DOI] [PubMed] [Google Scholar]
- 22.Davies RW, Carson BP, Jakeman PM. The effect of whey protein supplementation on the temporal recovery of muscle function following resistance training: a systematic review and meta-analysis. Nutrients 10: 221, 2018. doi: 10.3390/nu10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson DJ, Cegielski J, Phillips BE, Boereboom C, Lund JN, Atherton PJ, Smith K. Internal comparison between deuterium oxide (D2O) and l-[ring-13C6] phenylalanine for acute measurement of muscle protein synthesis in humans. Physiol Rep 3: E12433, 2015. doi: 10.14814/phy2.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff PL, Atherton PJ, Smith K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 306: E571–E579, 2014. doi: 10.1152/ajpendo.00650.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF 3rd, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. doi: 10.1096/fj.13-246595. [DOI] [PubMed] [Google Scholar]
- 26.Dufner DA, Bederman IR, Brunengraber DZ, Rachdaoui N, Ismail-Beigi F, Siegfried BA, Kimball SR, Previs SF. Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab 288: E1277–E1283, 2005. doi: 10.1152/ajpendo.00580.2004. [DOI] [PubMed] [Google Scholar]
- 27.Newham DJ, Jones DA, Ghosh G, Aurora P. Muscle fatigue and pain after eccentric contractions at long and short length. Clin Sci (Lond) 74: 553–557, 1988. doi: 10.1042/cs0740553. [DOI] [PubMed] [Google Scholar]
- 28.Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 8: 1153–1157, 2001. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet 116: 501–528, 2016. doi: 10.1016/j.jand.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 52: 376–384, 2018. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry CJ. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8: 1133–1152, 2005. doi: 10.1079/PHN2005801. [DOI] [PubMed] [Google Scholar]
- 32.Kilroe SP, Fulford J, Holwerda AM, Jackman SR, Lee BP, Gijsen AP, van Loon LJ, Wall BT. Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates. Am J Physiol Endocrinol Metab 318: E117–E130, 2020. doi: 10.1152/ajpendo.00360.2019. [DOI] [PubMed] [Google Scholar]
- 33.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Shimamoto H, Komiya S. The turnover of body water as an indicator of health. J Physiol Anthropol Appl Human Sci 19: 207–212, 2000. doi: 10.2114/jpa.19.207. [DOI] [PubMed] [Google Scholar]
- 35.Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction-modified Bergstrom muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve 43: 716–725, 2011. doi: 10.1002/mus.21945. [DOI] [PubMed] [Google Scholar]
- 36.Molnár-Perl I, Katona ZF. GC-MS of amino acids as their trimethylsilyl/t-butyldimethylsilyl derivatives: in model solutions III. Chromatographia 51: S228–S236, 2000. doi: 10.1007/BF02492811. [DOI] [Google Scholar]
- 37.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 38.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47: D419–D426, 2019. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Karlsen A, Soendenbroe C, Malmgaard-Clausen NM, Wagener F, Moeller CE, Senhaji Z, Damberg K, Andersen JL, Schjerling P, Kjaer M, Mackey AL. Preserved capacity for satellite cell proliferation, regeneration, and hypertrophy in the skeletal muscle of healthy elderly men. FASEB J 34: 6418–6436, 2020. doi: 10.1096/fj.202000196R. [DOI] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 42.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. BioTechniques 34: 374–378, 2003. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 43.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab 87: 3378–3384, 2002. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 44.Michailidis Y, Karagounis LG, Terzis G, Jamurtas AZ, Spengos K, Tsoukas D, Chatzinikolaou A, Mandalidis D, Stefanetti RJ, Papassotiriou I, Athanasopoulos S, Hawley JA, Russell AP, Fatouros IG. Thiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exercise. Am J Clin Nutr 98: 233–245, 2013. doi: 10.3945/ajcn.112.049163. [DOI] [PubMed] [Google Scholar]
- 45.Draganidis D, Chondrogianni N, Chatzinikolaou A, Terzis G, Karagounis LG, Sovatzidis A, Avloniti A, Lefaki M, Protopapa M, Deli CK, Papanikolaou K, Jamurtas AZ, Fatouros IG. Protein ingestion preserves proteasome activity during intense aseptic inflammation and facilitates skeletal muscle recovery in humans. Br J Nutr 118: 189–200, 2017. doi: 10.1017/S0007114517001829. [DOI] [PubMed] [Google Scholar]
- 46.Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LA, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 594: 5209–5222, 2016. doi: 10.1113/JP272472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulsen G, Crameri R, Benestad HB, Fjeld JG, Mørkrid L, Hallén J, Raastad T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc 42: 75–85, 2010. doi: 10.1249/MSS.0b013e3181ac7adb. [DOI] [PubMed] [Google Scholar]
- 48.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18: 42–97, 2012. [PubMed] [Google Scholar]
- 49.Cooke MB, Rybalka E, Stathis CG, Cribb PJ, Hayes A. Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. J Int Soc Sports Nutr 7: 30, 2010. doi: 10.1186/1550-2783-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cockburn E, Hayes PR, French DN, Stevenson E, St Clair Gibson A. Acute milk-based protein-CHO supplementation attenuates exercise-induced muscle damage. Appl Physiol Nutr Metab 33: 775–783, 2008. doi: 10.1139/H08-057. [DOI] [PubMed] [Google Scholar]
- 51.Jones DA, Newham DJ, Torgan C. Mechanical influences on long-lasting human muscle fatigue and delayed-onset pain. J Physiol 412: 415–427, 1989. doi: 10.1113/jphysiol.1989.sp017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trombold JR, Reinfeld AS, Casler JR, Coyle EF. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J Strength Cond Res 25: 1782–1788, 2011. doi: 10.1519/JSC.0b013e318220d992. [DOI] [PubMed] [Google Scholar]
- 53.van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR, Burd NA. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr 106: 1401–1412, 2017. doi: 10.3945/ajcn.117.159855. [DOI] [PubMed] [Google Scholar]
- 54.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 55.Davies RW, Bass JJ, Carson BP, Norton C, Kozior M, Amigo-Benavent M, Wilkinson DJ, Brook MS, Atherton PJ, Smith K, Jakeman PM. Differential stimulation of post-exercise myofibrillar protein synthesis in humans following isonitrogenous, isocaloric pre-exercise feeding. Nutrients 11: 1657, 2019. doi: 10.3390/nu11071657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. doi: 10.3945/ajcn.112.055517. [DOI] [PubMed] [Google Scholar]
- 57.Beelen M, Tieland M, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, Saris WH, Koopman R, van Loon LJ. Coingestion of carbohydrate and protein hydrolysate stimulates muscle protein synthesis during exercise in young men, with no further increase during subsequent overnight recovery. J Nutr 138: 2198–2204, 2008. doi: 10.3945/jn.108.092924. [DOI] [PubMed] [Google Scholar]
- 58.Mahoney DJ, Safdar A, Parise G, Melov S, Fu M, MacNeil L, Kaczor J, Payne ET, Tarnopolsky MA. Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am J Physiol Regul Integr Comp Physiol 294: R1901–R1910, 2008. doi: 10.1152/ajpregu.00847.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. doi: 10.1152/physiolgenomics.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7: 758–765, 2005. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ray D, Terao Y, Nimbalkar D, Chu LH, Donzelli M, Tsutsui T, Zou X, Ghosh AK, Varga J, Draetta GF, Kiyokawa H. Transforming growth factor beta facilitates beta-TrCP-mediated degradation of Cdc25A in a Smad3-dependent manner. Mol Cell Biol 25: 3338–3347, 2005. doi: 10.1128/MCB.25.8.3338-3347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacNeil LG, Melov S, Hubbard AE, Baker SK, Tarnopolsky MA. Eccentric exercise activates novel transcriptional regulation of hypertrophic signaling pathways not affected by hormone changes. PLoS One 5: e10695, 2010. doi: 10.1371/journal.pone.0010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjær M, Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol (1985) 107: 1600–1611, 2009. doi: 10.1152/japplphysiol.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaaqoq AM, Namas RA, Abdul-Malak O, Almahmoud K, Barclay D, Yin J, Zamora R, Rosengart MR, Billiar TR, Vodovotz Y. Diurnal variation in systemic acute inflammation and clinical outcomes following severe blunt trauma. Front Immunol 10: 2699, 2019. doi: 10.3389/fimmu.2019.02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J 25: 3344–3355, 2011. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulsen G, Hanssen KE, Rønnestad BR, Kvamme NH, Ugelstad I, Kadi F, Raastad T. Strength training elevates HSP27, HSP70 and alphaB-crystallin levels in musculi vastus lateralis and trapezius. Eur J Appl Physiol 112: 1773–1782, 2012. [Erratum in Eur J Appl Physiol 113: 1101, 2013]. doi: 10.1007/s00421-011-2132-8. [DOI] [PubMed] [Google Scholar]
- 67.Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 562: 899–913, 2005. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacIntyre DL, Reid WD, Lyster DM, Szasz IJ, McKenzie DC. Presence of WBC, decreased strength, and delayed soreness in muscle after eccentric exercise. J Appl Physiol (1985) 80: 1006–1013, 1996. doi: 10.1152/jappl.1996.80.3.1006. [DOI] [PubMed] [Google Scholar]
- 69.Maraldi T. Natural compounds as modulators of NADPH oxidases. Oxid Med Cell Longev 2013: 271602, 2013. doi: 10.1155/2013/271602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michel P, Granica S, Magiera A, Rosińska K, Jurek M, Poraj Ł, Olszewska MA. Salicylate and procyanidin-rich stem extracts of Gaultheria procumbens L. inhibit pro-inflammatory enzymes and suppress pro-inflammatory and pro-oxidant functions of human neutrophils ex vivo. Int J Mol Sci 20: 1753, 2019. doi: 10.3390/ijms20071753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brickson S, Ji LL, Schell K, Olabisi R, St Pierre Schneider B, Best TM. M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J Appl Physiol (1985) 95: 969–976, 2003. doi: 10.1152/japplphysiol.00005.2003. [DOI] [PubMed] [Google Scholar]