Abstract
Uterine spiral artery remodeling (UAR) is essential for placental perfusion and fetal development. A defect in UAR underpins placental ischemia disorders, e.g., preeclampsia, that result in maternal systemic vascular endothelial dysfunction and hypertension. We have established a model of impaired UAR by prematurely elevating maternal serum estradiol levels during the first trimester of baboon pregnancy. However, it is unknown whether this experimental paradigm is associated with maternal vascular endothelial dysfunction. Therefore, in the present study baboons were administered estradiol on days 25–59 of gestation to suppress UAR and maternal vascular function determined on day 165 (term = 184 days) peripherally and in skeletal muscle, which accounts for over 40% of body mass and 25% of resting systemic vascular resistance. Maternal serum sFlt-1 levels were 2.5-fold higher (P < 0.05), and skeletal muscle arteriolar endothelial nitric oxide synthase (eNOS) protein expression and luminal area, and skeletal muscle capillary density were 30-50% lower (P < 0.05) in UAR suppressed baboons. Coinciding with these changes in eNOS expression, luminal area, and capillary density, maternal brachial artery flow-mediated dilation and volume flow were 70% and 55% lower (P < 0.05), respectively, and mean arterial blood pressure 29% higher (P < 0.01) in UAR defective baboons. In summary, maternal vascular function was disrupted in a baboon model of impaired UAR. These results highlight the translational impact of this primate model and relevance to adverse conditions of human pregnancy underpinned by improper uterine artery transformation.
NEW & NOTEWORTHY Maternal vascular dysfunction is a hallmark of abnormal human pregnancy, particularly early-onset preeclampsia, elicited by impaired UAR. The present study makes the novel discovery that maternal systemic vascular dysfunction was induced in a baboon experimental model of impaired UAR. This study highlights the translational relevance of this nonhuman primate model to adverse conditions of human pregnancy underpinned by defective UAR.
Keywords: artery, primate, remodeling, uterine, vascular
INTRODUCTION
During early human and nonhuman primate pregnancy, placental extravillous trophoblasts migrate to, invade, and replace the vascular smooth muscle and endothelial cell lining of the uterine spiral arteries (1, 2). Consequently, these arteries are remodeled into low-resistance/high flow vessels to promote placental perfusion and fetal development. A defect in uterine artery remodeling (UAR) underpins the etiology of placental ischemia disorders (3), particularly early-onset preeclampsia, fetal growth restriction, and preterm birth (4–7). As a result of impaired UAR and placental perfusion, the placenta undergoes oxidative stress and the release of potent anti-angiogenic factors, notably the soluble truncated sFlt-1 receptor that binds to and suppresses systemic tissue bioavailability of vascular endothelial growth factor (VEGF) (8–10). VEGF promotes vascular endothelial nitric oxide synthase (eNOS) expression and thus NO formation and vasodilation, as well as capillary growth (11–13). Maternal serum sFlt-1 levels are elevated in human preeclampsia (8, 9). The pathophysiological manifestations of human preeclampsia include maternal systemic vascular endothelial dysfunction and oxidative stress and hypertension, which lead to an increased risk of maternal and neonatal morbidity and mortality (14–20).
Numerous clinical and rodent studies have focused on the pathophysiological consequences of adverse pregnancy, but have not linked a defect in UAR to maternal systemic vascular dysfunction. In addition, there are fundamental differences in placental structure and development, uterine and placental vascular anatomy, UAR, and the maternal-placental-fetal endocrine axis between rodents and humans (21–24), processes that are similar in human and baboon pregnancy (21, 22, 25). We have established a model of defective UAR in the baboon by prematurely elevating maternal estradiol levels during the first trimester (26–28). This experimental paradigm caused a 75% decrease in the number of uterine spiral arteries remodeled by extravillous trophoblasts, a 30% reduction in uterine artery blood flow near term, and increase in placental expression of sFlt-1 (27–30).
However, it is unknown whether this experimental model of defective spiral artery transformation in a nonhuman primate is associated with the maternal pathophysiological hallmarks of human preeclampsia, particularly maternal vascular endothelial dysfunction. Therefore, the present study tested the hypothesis that maternal vascular eNOS expression in and vascular composition of skeletal muscle, a systemic tissue that comprises 40% of total body mass and accounts for 25% of cardiac output and resting systemic vascular resistance (31–33), as well as peripheral vascular NO-mediated endothelial function, are altered and systemic vascular oxidative stress induced in UAR suppressed baboons.
MATERIALS AND METHODS
Animals
The present in vivo animal research study was conducted in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines to enable evaluation of the rigor and reproducibility of the methods, statistical analyses, and results. Female baboons (Papio anubis) originally obtained from the Southwest National Primate Research Center (San Antonio, TX) were housed individually in large primate cages in air conditioned rooms with a 12 h/12 h light/dark cycle and received standard primate chow (Teklad Primate Diet 2050; Envigo, Frederick, MD) and fresh fruit twice daily, and water ad libitum. Females were paired with male baboons for 5 days at midcycle as estimated by menstrual cycle history and the pattern of external sex skin turgescence. Pregnancy was confirmed by ultrasound, and day 1 was designated as the day preceding perineal deturgescence. Baboons were cared for and used strictly in accordance with the United States Department of Agriculture regulations and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed.). The present experimental protocol was approved by the Institutional Animal Care and Use Committees of the University of Maryland School of Medicine and Eastern Virginia Medical School.
Pregnant baboons were randomly assigned as untreated or treated daily on days 25 to 59 of gestation (term, 184 days) with estradiol benzoate (25 µg/kg body weight/day sc), an experimental paradigm that suppresses UAR (26–28). Arterial flow-mediated dilation provides a noninvasive real-time ultrasound method of assessing primarily NO-mediated vascular endothelial function independent of renal and humoral influences (34). Thus, maternal brachial artery flow-mediated dilation and volume flow were quantified by Doppler using an Acuson Sequoia 512 ultrasound (15L8 linear transducer, Siemens, Malvern, PA) on days 100 and 165 of gestation at diastole before (basal level) and during a 5-min period (eight values obtained at 30-s intervals) after induction of hyperemia/shear-stress forearm pressure cuff inflation then deflation in baboons lightly anesthetized with iv propofol/ketamine, which does not alter blood pressure or respiration (35), and supplemented with oxygen (1 L/min) to maintain SpO2 greater than 95%. Flow-mediated dilation values were expressed as the percent increase in basal brachial artery diameter after induction of shear stress. Volume flow {time-averaged velocity measured from Doppler waveforms × cross-sectional area [π (arterial diameter ÷ 2)2]} was quantified as the percent change in basal flow after shear stress. Because vessel dilation and volume flow changes were similar on days 100 and 165, respective values were averaged to obtain a single measurement for each baboon. Maternal blood pressure and heart rate were measured throughout the flow-mediated dilation procedure via a Dinamap Pro 400 V2 (GE Medical Systems, Milwaukee, WI).
On day 165, a biopsy (7 mm3) of maternal rectus abdominis skeletal muscle was obtained at the time of cesarean section during isoflurane anesthetization and placentas and fetuses removed. Blood samples (2 mL) were obtained on day 60 from a maternal peripheral saphenous vein and on day 165 at cesarean section from saphenous and uterine veins, immediately placed on crushed ice and serum and plasma (EDTA) stored at –20°C. Rectus abdominis is an active skeletal muscle that controls posture and breathing, is perfused by the systemic vascular system, and robustly responds to the vasodilatory effects of NO (31).
Von Willebrand Factor, Lectin, and CD31 Fluorescent Immunohistochemistry
Paraffin-embedded skeletal muscle tissue sections (5 µm) were microwaved in 0.01 M sodium citrate buffer (pH 6.0) for antigen retrieval, incubated with Image-iT FX signal enhancer (Invitrogen/Thermo Fisher Scientific, Waltham, MA) and then blocked with 5% normal horse serum (Millipore Sigma, St. Louis, MO). For tissue sections incubated with von Willebrand factor (VWF) and Lectin, a streptavidin/biotin block was performed using a blocking kit (Vector Laboratories, Inc., Burlingame, CA). Tissue sections were incubated overnight at 4°C with either a primary rabbit polyclonal antibody to VWF (1:250 dilution; Cat. No. A008229-5; RRID: AB_2315602, Agilent Dako, Santa Clara, CA), mouse monoclonal antibody to CD31 (1:20 dilution; Cat. No. M082329-2; clone JC70A; RRID: AB_2114471 Agilent Dako), or biotinylated Griffonia Simplicifolia Lectin I (1:100 dilution; Cat. No. B1105; RRID: AB_2336489, Vector Labs). For VWF, tissue sections were incubated for 1 h at room temperature with a bridge biotinylated goat anti-rabbit secondary antibody (Cat. No. BA1000; RRID: AB_2313606, Vector Labs). All tissue sections were then incubated for 1 h at room temperature with either Alexa Fluor 488 donkey anti-mouse secondary antibody (Cat. No. A21202; RRID: AB_141607, Invitrogen/Thermo Fisher Scientific) or streptavidin Alexa Fluor 488 conjugate (Cat. No. S32354; RRID: AB_2315383, Invitrogen/Thermo Fisher Scientific). The sections were then washed, air-dried, and coverslipped with mounting media containing 4′,6-diamidine-2′-phenylindole (DAPI) for the detection of the nuclei. Negative controls for fluorescent immunohistochemistry included substitution of normal mouse serum or rabbit IgG isotype (Cat. No. 02–6102; RRID: AB_2532938, Invitrogen/Thermo Fisher Scientific) for primary antibody.
Proximity Ligation Assay of eNOS
PLA is a highly sensitive, PCR-based immunofluorescent method that permits cell specific in situ localization and quantification of protein at single molecule/single-cell resolution. Paraffin-embedded maternal abdominal skeletal muscle tissue sections (5 µm thick) were boiled for 40 min in 25 mM Tris-1 mM EDTA buffer (pH 8.5) for antigen retrieval, serum blocked with 5% horse serum, and incubated overnight at 4°C with primary mouse monoclonal eNOS antibody (1:1,000 dilution; Cat. No. 610297; RRID: AB_397691, NOS type III; BD Biosciences, San Jose, CA). Tissue sections were then incubated for 90 min at 37°C in a humified chamber with PLA probes consisting of two secondary anti-mouse antibodies each tagged with an oligonucleotide (diluted 1:5 in antibody diluent supplied in the Duolink In Situ PLA kit) (Cat. No. DU092001 and Cat. No. DU092004; RRID: AB_2810939 and RRID: AB_2713942, respectively, Millipore Sigma). A ligation solution (supplied in the PLA kit and diluted 1:5 in water) consisting of two oligonucleotide linkers complementary to each PLA probe and ligase (diluted 1:40 in the ligation solution) was added and tissue sections incubated for 30 min at 37°C in a humidified chamber. Tissue sections were washed and incubated for 100 min with amplification solution (supplied in PLA kit and diluted 1:5 in H2O) consisting of nucleotides and fluorescently labeled oligonucleotides and polymerase (diluted 1:80 in the amplification solution). The ligation step connects the hybridization linkers to the PLA probe oligonucleotides, forming a complete circle connecting both antibodies, and the polymerization step uses rolling circle DNA amplification to generate a concatemeric oligonucleotide product linked to the antibody complex. The fluorescently labeled oligonucleotides hybridize to the rolling circle amplification product that results in a signal that is visible as a fluorescent red dot. Tissue sections were then washed in decreasing concentrations of Tris-based buffers (PLA kit wash buffer A and B). Sections were then incubated overnight at 4°C with primary rabbit polyclonal antibody to VWF (1:250 dilution, Cat. No. A008229-5; RRID: AB_2315602, for endothelial cell-specific localization of eNOS) followed the next day by 1-h incubation with Alexa Fluor 488 donkey anti-rabbit secondary antibody (Cat. No. A21206; RRID: AB_2535792, Invitrogen/Thermo Fisher). The sections were then washed, air-dried, and coverslipped with mounting media containing DAPI for the detection of the nuclei. The eNOS protein red PLA signals were visualized by fluorescence microscopy (Nikon Eclipse E 1000) and imaged at a final magnification of ×400 using analysis software (iVision, Biovision Technologies, Inc., Exton, PA). PLA signals were quantified and expressed per VWF-stained endothelial area (µm2) within a minimum of five randomly selected arterioles and per skeletal muscle area in a minimum of 10 randomly selected areas (38,643 µm2) of the skeletal muscle using MetaMorph software (version 7.8.0.0, Molecular Devices, LLC, San Jose, CA). Arterioles (10–300 µm diameter) were differentiated from venules by their typical oval shape and thicker smooth muscle tunica media layer. Arterial luminal and VWF-stained endothelial area were quantified after circumscribing the area with a pseudo color. Correction of background fluorescence in tissue (e.g., red blood cells) unrelated to the specific signal of the proteins of interest in acquired images was performed using the background subtraction feature of the iVision software. Quantification of the proteins of interest detected by the different fluorophores was further delineated by red, green, and blue color channel separation in the Metamorph analysis software. Negative controls for PLA included substitution of normal mouse serum for the primary antibody or omission of one PLA probe.
Skeletal Muscle Capillarization
Paraffin-embedded skeletal muscle tissue sections (5 µm) were incubated in sodium citrate buffer for antigen retrieval and dual-labeled with primary rabbit antibody to VWF (1:250 dilution, Cat. No. A008229-5; RRID: AB_2315602) and mouse monoclonal antibody to Myosin FAST (1:500 dilution; Cat. No. M4276; clone MY-32; RRID: AB_477190, Millipore Sigma) for capillary and fiber identification, respectively. Tissue sections were incubated for 1 h at room temperature with a bridge biotinylated goat anti-rabbit secondary antibody (Cat. No. BA 1000; RRID: AB_2313606, Vector Labs) followed by incubation for 1 h at room temperature with streptavidin Alexa Fluor 488 conjugate and Alexa Fluor 594 donkey anti-mouse secondary antibody (Cat. No. A21203; RRID: AB_2535789, Invitrogen/Thermo Fisher Scientific). Sections were coverslipped with mounting media containing DAPI for the detection of the nuclei. Stained muscle sections were visualized by fluorescence microscopy (Nikon Eclipse E 1000) and imaged at a final magnification of ×100 using analysis software (IP Lab). Capillary density (capillaries per square mm of muscle fiber cross-sectional area) and capillary to muscle fiber ratio were quantified using MetaMorph software in six randomly selected skeletal muscle areas per section circumscribed with a pseudo-yellow color. A minimum of 120 fibers were analyzed per section.
Western Immunoblot of Oxidative Stress Markers
Maternal skeletal muscle was homogenized in buffer [phosphate-buffered saline (PBS)] with 1% deoxycholate and 0.1% sodium dodecyl sulfate (SDS) containing 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium orthovanadate, 2 mM sodium pyrophosphate, and 2× protease inhibitor cocktail (Sigma; Cat. No. P8340) and Western blotting performed essentially as described previously (36). Briefly, samples were centrifuged (13,000 rpm; 20 min) and supernatants treated with 5X Laemmli buffer and 25 µg or 50 µg of protein loaded onto 13.5% SDS-polyacrylamide gels [Oxidative Stress Defense Western Blot Cocktail—stress defense cocktail—catalase, thioredoxin (TRX), superoxide dismutase (SOD1) and smooth muscle actin], 12% SDS-polyacrylamide gels [heme oxygenase 1 (HO-1), nitrotyrosine] or 15% Tris tricine gels (malondialdehyde). Samples were electrophoresed in a Mini-Protean II electrophoresis chamber (Bio-Rad Laboratories) containing either chilled 25 mM Tris (pH 8.3), 0.192 M glycine, and 0.1% SDS buffer (stress defense panel, HO-1, nitrotyrosine) or 100 mM Tris, 100 mM tricine, 0.1% SDS (pH 8.3) cathode buffer, and 200 mM Tris (pH 8.9) anode buffer (malondialdehyde).
After transfer of proteins onto an Immobilon-P membrane (EMD Millipore, Burlington, MA), membranes were blocked for 1 h at room temperature in PBS with 0.05% Tween-20 [PBS-T] containing 5% nonfat dry milk [nfdm] (stress defense cocktail and HO-1) or blocked for 1 h with 3% nfdm in 10 mM Tris, pH 7.4, 150 mM NaCl with 0.05% Tween-20 [TBS-T] (malondialdehyde, nitrotyrosine). Membranes were then incubated overnight (4°C) with stress defense cocktail rabbit monoclonal antibodies (Cat. No. ab 179843; RRID: AB_2716714, Abcam, Cambridge, MA) or HO-1 rabbit monoclonal antibody (Cat. No. ab 52947, Abcam; RRID: AB_880536) diluted 1:250 or 1:1,000, respectively, in 5% nfdm/PBS-T or with rabbit polyclonal antibody to malondialdehyde (Cat. No. 27642; RRID: AB_776164, Abcam) diluted 1:1,000 in 3.0% nfdm/TBS-T or with nitrotyrosine (Millipore Cat. No. 06–284; RRID: AB_310089) diluted 1:1,000 in 1.5% nfdm/PBS-T. Membranes were washed in PBS-T (stress defense panel, nitrotyrosine) or TBS-T (malondialdehyde, HO-1) followed by incubation with goat anti-rabbit IgG horse radish peroxidase-conjugated (HRP) secondary antibody (Cat. No. PI-1000; RRID: AB_2336198, Vector Labs) at a 1:10,000 dilution in 5% nfdm/PBS-T (stress defense cocktail, HO-1) or 3% nfdm/TBS-T (malondialdehyde, nitrotyrosine) for 1 h (room temperature). Membranes were washed again and proteins detected using Amersham enhanced chemiluminescence (ECL, Cat. No. RPN2106, GE Healthcare, Chicago, IL) and exposure to X-ray film. The oxidative stress blots were further incubated (without stripping) with an antibody to pan-actin (loading control; Cat. No. MAB 1501; RRID: AB_2223041, EMD Millipore) at a 1:5,000 dilution in 5% nfdm/PBS-T for 75 min (room temperature), washed in PBS-T, and incubated with horse anti-mouse IgG HRP (Cat. No. PI-2000; RRID: AB_2336177, Vector Labs) at a 1:10,000 dilution in 5% nfdm/PBS-T and proteins detected with ECL as above. The specificity of the primary antibodies was determined by incubation of the samples without a primary antibody or binding to nitrated human liver homogenate standard (nitrotyrosine; Cat. No. ab 131380, Abcam).
Serum and Plasma Chemistry Analytes
Serum estradiol levels were determined using an automated chemiluminescent immunoassay system (Cat. No. LKE21; RRID: AB_2800400, Immulite; Diagnostic Products Corp., Los Angeles, CA). Serum total sFlt-1 levels were quantified by Quantikine ELISA (Cat. No. DVR100C; RRID: AB_2827807, R&D Systems, Inc., Minneapolis, MN) on samples serially diluted to 1:25, 1:50, 1:100, and/or 1:500 and 1:1,000. Serum chemistry analytes were measured by Antech Diagnostics, Lake Success, NY. For the Immulite Immunoassay of estradiol, the intra-assay and interassay CVs were 6.9% and 7.3%, respectively. For the Quantikine assay of sFlt-1, the intra-assay CV was 2.6%.
Plasma nitrate and nitrite are stable metabolites of NO, and levels have been used as an indirect measure of systemic NO secretion (37). Thus, the level of nitrate and nitrite was determined in maternal saphenous vein. Samples were centrifuged (3,800 rpm; 20 min) to remove particulates and the supernatant deproteinized by centrifugation (12,200 rpm; 45 min) through 10,000 molecular weight cutoff filters (EMD Millipore). The plasma filtrate was then diluted (1:2, 1:4, 1:8, and 1:16) and the level of nitrate plus nitrite oxidation products determined using the Griess reaction colorimetric assay (Cat. No. 880001, Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
Based on nonparametric two-sided test, it was estimated that six animals per group were required to yield at least 80% power to detect pair-wise difference between means of at least 0.5–0.8 standard deviation at α = 0.05. Serum estradiol and sFlt-1 levels on days 60 and 165 were analyzed by one-way ANOVA with post hoc comparison of the means by Newman–Keuls multiple comparison test. All other data were analyzed by Student’s t test. GraphPad software (San Diego, CA) was used for statistical analyses.
RESULTS
Serum Estradiol, sFlt-1, and Nitrate/Nitrite Levels and Weights
Maternal saphenous vein serum estradiol levels on day 60 were fourfold higher (P < 0.05, Table 1) in baboons treated with estradiol on days 25–59 of gestation, an experimental paradigm which suppresses UAR by 75% (26–28), than in untreated animals. On day 165, serum estradiol levels in untreated baboons were over 15-fold greater (P < 0.01) than on day 60, but similar in untreated and UAR suppressed animals.
Table 1.
Maternal serum estradiol, sFlt-1, and nitrate/nitrite levels and placental and fetal body weights in baboons
| Experimental | Estradiol, ng/mL | sFlt-1, ng/mL |
Nitrate/Nitrite, µmol/L | Placental | Fetal Body | ||
|---|---|---|---|---|---|---|---|
| Group | Day | Saphenous Vein | Saphenous vein | Uterine vein | Saphenous Vein | Weight, g | Weight, g |
| Untreated | 60 | 0.19 (0.06)a | 2.8 (1.7)a | — | — | — | — |
| 165 | 3.08 (0.71)b | 27.7 (23.0)b | 35.3 (34.7) | 45.1 (39.6) | 178 (16) | 802 (58) | |
| ↓ UAR | 60 | 0.85 (0.29)c | 3.2 (2.6)a | — | — | — | — |
| 165 | 3.15 (0.83)b | 72.0 (18.8)c | 97.2 (55.3)* | 32.9 (20.9) | 177 (23) | 764 (141) | |
Mean (SD) maternal saphenous vein serum estradiol, saphenous and uterine vein serum sFlt-1, and saphenous vein plasma nitrate/nitrite levels and placental and fetal body weights on days 60 and 165 of gestation in baboons untreated (n = 7) or treated daily on days 25–59 of gestation (term = 184 days) with estradiol benzoate to suppress UAR (n = 6). Values with different letter superscripts are different (P < 0.05) from one another (one-way ANOVA with post hoc comparison of the means by Neuman–Keuls multiple comparison test). *P = 0.069 vs. untreated baboons (Student’s t test).
Maternal serum sFlt-1 levels in the saphenous vein on day 60 were similar in baboons untreated and treated with estradiol (Table 1). However, serum sFlt-1 levels were ninefold higher (P < 0.05) on day 165 than on day 60 in untreated animals. Importantly, serum sFlt-1 levels on day 165 were over 2.5-fold greater in the saphenous vein (P < 0.05) and uterine vein (P = 0.06) in UAR suppressed animals than in untreated baboons (Table 1).
Plasma saphenous vein nitrate plus nitrite levels on day 165 were similar in untreated and UAR suppressed baboons (Table 1). Placental weights were similar in untreated and UAR suppressed baboons, whereas fetal body weight was 5%, but not significantly, lower in UAR defective animals (Table 1). The levels of maternal serum chemistry analytes that reflect liver, renal, and metabolic functions were within the normal range for baboon pregnancy and similar in all baboons (Table 2).
Table 2.
Maternal serum chemistry analytes in baboons
| Experimental Group |
Ast, IU/L |
Alt, IU/L |
AP, IU/L | Cholesterol, mg/dL |
BUN, mg/dL |
Glucose, mg/dL |
Creatinine, mg/dL |
|---|---|---|---|---|---|---|---|
| Untreated | 10 (3) | 5 (1) | 52 (12) | 72 (15) | 9 (4) | 49 (11) | 0.5 (0.1) |
| ↓ UAR | 15 (9) | 5 (2) | 66 (23) | 80 (10) | 10 (3) | 49 (9) | 0.7 (0.1) |
Mean (SD) maternal serum chemistry analyte levels on day 165 of gestation in untreated (n = 9) and UAR suppressed (n = 5) baboons. ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
PLA eNOS Expression within Arteriolar Endothelium and Fibers of Maternal Skeletal Muscle
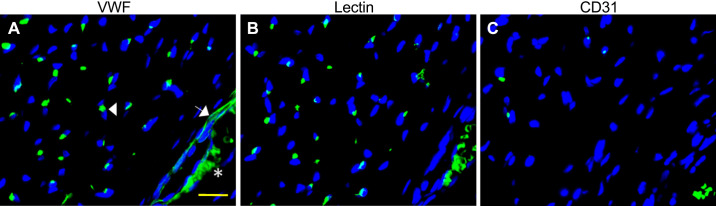
As shown in Fig. 1, skeletal muscle capillaries and the endothelial lining of arterioles were consistently labeled with VWF antibody (A). In contrast, capillaries but not vessels greater than ∼10 µm, e.g., arterioles, labeled with Lectin (B), whereas neither capillaries nor arterioles consistently labeled with CD31 antibody (C). Therefore, the VWF antibody was used throughout the study to localize maternal skeletal muscle vascular endothelial cells.
Figure 1.
Representative (from total of n = 3) photomicrographs of fluorescent immunohistochemical labeling of baboon skeletal muscle capillary and arteriole endothelial cells with VWF (A), lectin (B), and CD31 (C). White arrowhead, green immunofluorescent VWF-labeled capillary endothelial cell; white arrow, green endothelial lining of arteriole; blue, DAPI-labeled muscle fiber nuclei. *Autofluorescence of red blood cells. Scale bar = 100 µm for all panels. VWF, von Willebrand factor.
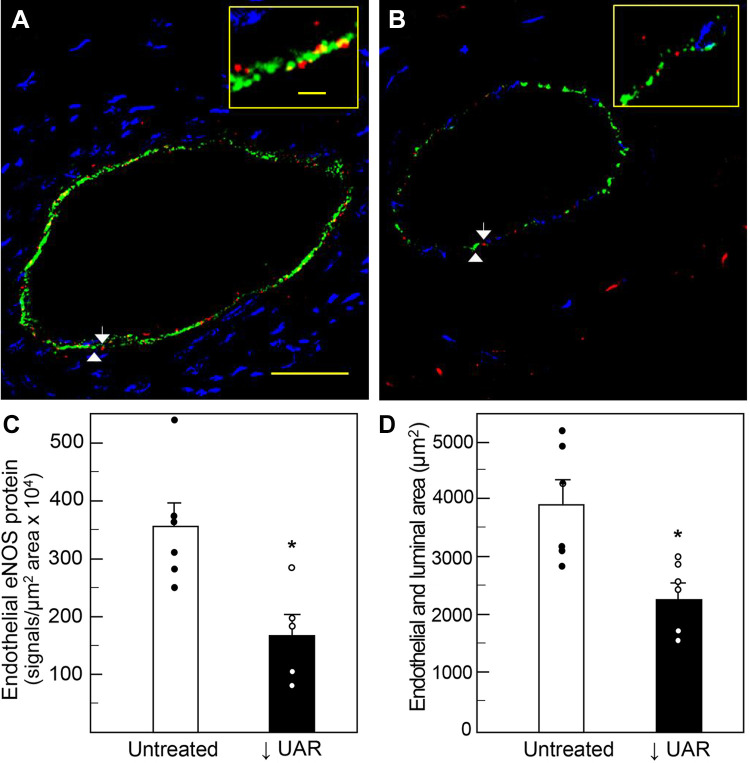
Figure 2 illustrates representative photomicrographs showing PLA localization of eNOS protein (red dots/signals) within the green VWF-labeled endothelial lining of maternal skeletal muscle arterioles on day 165 of gestation in untreated (A) and UAR impaired baboons (B). The number of eNOS PLA signals appeared less abundant in the animal with defective UAR. Thus, when quantified by image analysis, means ± SE eNOS protein levels (i.e., PLA signals/µm2 endothelial area × 104) were ∼50% lower (P = 0.009) in the endothelial lining of arterioles of UAR impaired baboons (169 ± 36) than of untreated animals (356 ± 42, Fig. 2C).
Figure 2.
Representative photomicrographs illustrating PLA localization of eNOS protein (red dots/signals, white arrows) within endothelial cell lining of maternal skeletal muscle arterioles (110 µm luminal diameter-long axis in A) on day 165 of gestation in an untreated (A) and UAR suppressed (B) baboon. Inserts in upper right corner of A and B are high magnification of endothelial cell lining of arterioles. White arrowheads, green immunofluorescent VWF-labeled endothelial cells; yellow dots, colocalization of red PLA and green VWF-labeled endothelial cells; blue, DAPI-labeled muscle fiber nuclei. Scale bar = 25 µm for A and B and 2.5 µm for inserts in A and B. C shows the means ± SE level of eNOS protein within endothelium (red signals/µm2 endothelial area × 104) on day 165 in untreated (n = 6) and UAR impaired (n = 5) baboons. *P = 0.009 vs. untreated baboons. D depicts means ± SE maternal skeletal muscle arteriolar endothelial plus luminal area (µm2) on day 165 in untreated (n = 6) and UAR suppressed (n = 6) baboons. Closed (●) and open (○) circles represent individual data points. *P = 0.008 vs. untreated baboons (Student’s t test). DAPI, 4′,6-diamidine-2′-phenylindole; eNOS, endothelial nitric oxide synthase; PLA, proximity ligation assay; UAR, uterine spiral artery remodeling; VWF, von Willebrand factor.
Corresponding with the decrease in endothelial eNOS expression, it appeared that the area encompassing the endothelium and lumen of maternal skeletal muscle blood arterioles was lower in UAR defective baboons. Thus, as quantified by image analysis, endothelial plus luminal area (µm2) was ∼40% smaller (P = 0.008) in skeletal muscle arterioles of UAR suppressed baboons (2,306 ± 245) than of untreated animals (3,933 ± 429, Fig. 2D).
Figure 3 shows the PLA expression of numerous eNOS PLA signals (red dots) within transverse sections of maternal skeletal muscle fibers (Figs. 3, A and B, and magnified in Fig. 3C). Since capillaries are superficially located in grooves within the sarcolemma (which lies immediately below the endomysium) of skeletal muscle, but do not extend below the grooves into the myofibers (38, 39), the PLA signals represent eNOS expression primarily by the myofibers. In contrast to the decrease in eNOS expression within the endothelial lining of the arterioles of UAR defective baboons (Fig. 2C), the level of eNOS protein within skeletal muscle fibers (i.e., PLA signals/µm2 skeletal muscle area × 104) was similar in untreated (43 ± 12) and UAR suppressed (57 ± 8) baboons (Fig. 3D).
Figure 3.
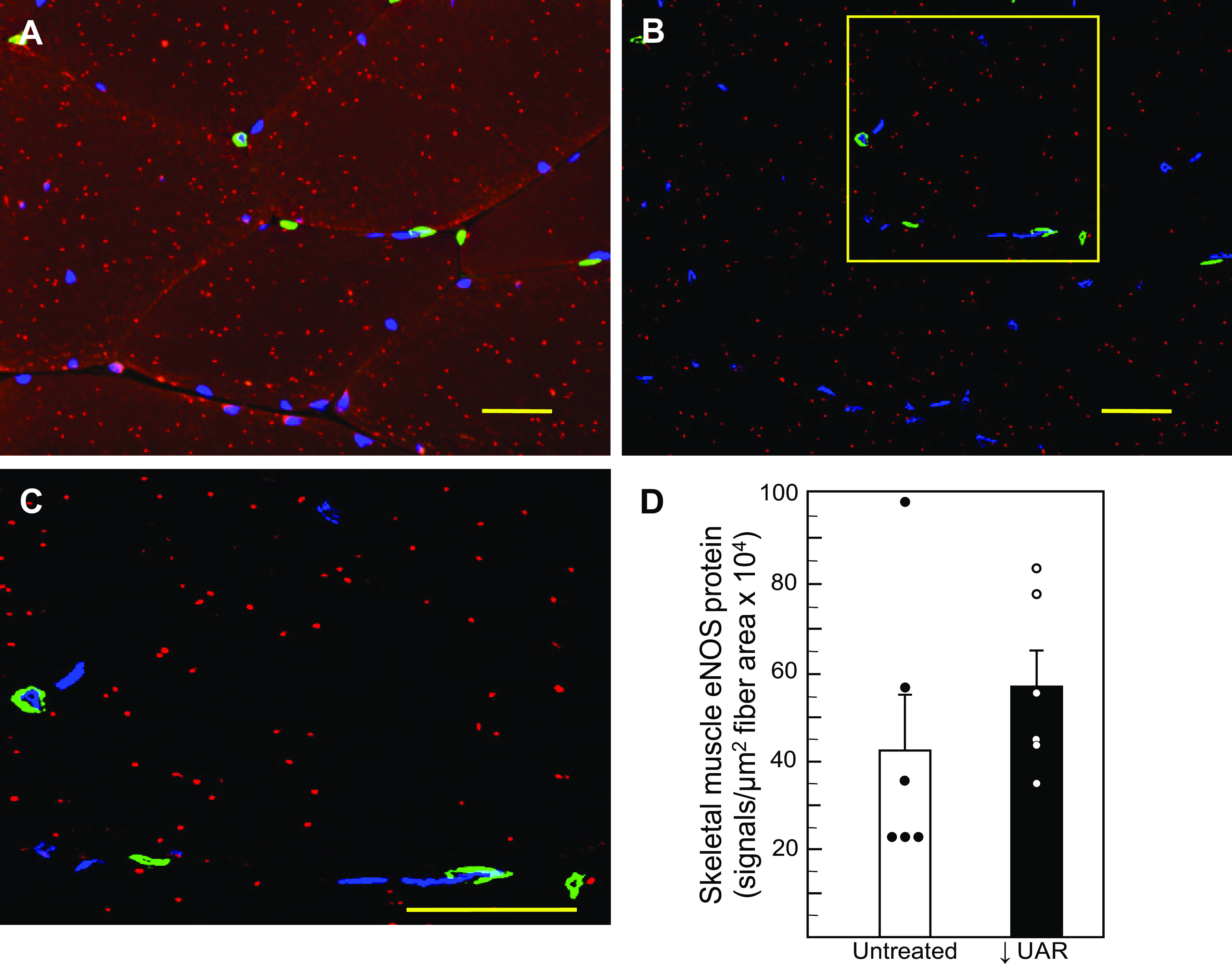
Representative photomicrographs depicting PLA localization of eNOS protein (red dots/signals) within transverse sections of maternal skeletal muscle fibers in an untreated baboon showing outline of myofiber endomysium (A) and after removal of autofluorescence background to show the presence of red PLA signals (B). Insert box in B is magnified and shown in C to more clearly illustrate red PLA eNOS signals. Green, immunofluorescent VWF-labeled endothelial cells of vessels located between myofibers. Scale bar = 25 µm (A, B, and C). D shows the means ± SE level of eNOS protein in myofibers (signals/µm2 skeletal muscle fiber area × 104) on day 165 of gestation in untreated (n = 6) and UAR suppressed (n = 6) baboons (no significant difference by Student’s t test). Closed (●) and open (○) circles represent individual data points. eNOS, endothelial nitric oxide synthase; PLA, proximity ligation assay; UAR, uterine spiral artery remodeling; VWF, von Willebrand factor.
Maternal Skeletal Muscle Capillary Density
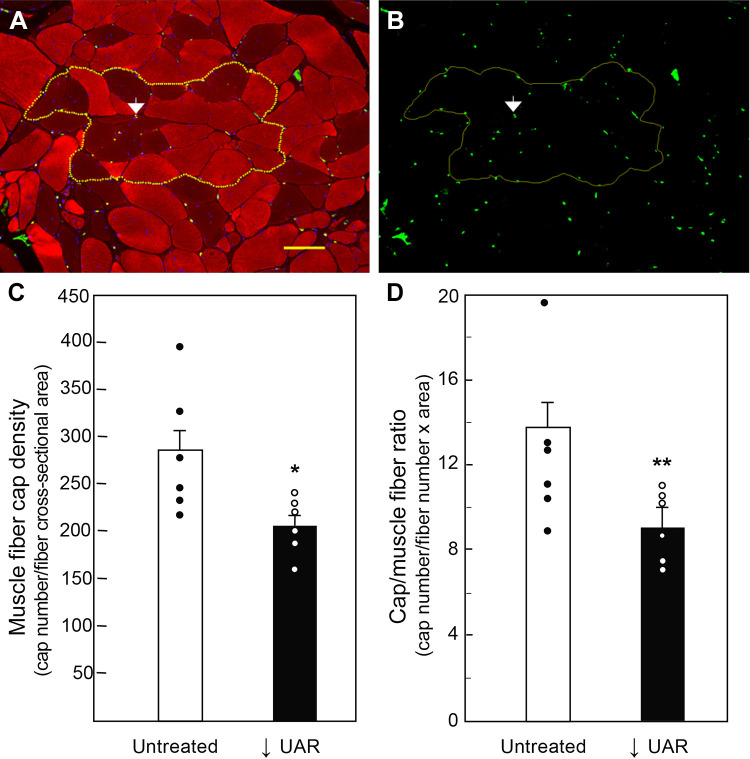
Figure 4 illustrates the localization and quantification of capillaries within maternal skeletal muscle. To quantify the density of capillaries in these myofibers, an area was circumscribed around a group of maternal skeletal muscle fibers [depicted in Fig. 4A as myosin fast (red) and slow (dark)] myofibers and in Fig. 4B as green VWF-positive capillaries). Maternal skeletal muscle capillary density (number capillaries/myofiber cross-sectional area) on day 165 was ∼30% lower (P = 0.029) in UAR suppressed (207 ± 13) than in untreated (284 ± 28) baboons (Fig. 4C). Moreover, the capillary/muscle fiber ratio was also 30% lower (P = 0.05) in UAR suppressed baboons (9.18 ± 0.68) than in untreated animals (12.85 ± 1.56, Fig. 4D).
Figure 4.
Maternal skeletal muscle [myosin fast (red) and slow (dark)] fibers of an untreated baboon shown in transverse section (A) and showing green VWF-labeled capillaries (B, white arrow). Dotted yellow line circumscribed around a group of myofibers in A and B illustrates area used to quantify by image analysis means ± SE VWF-positive capillary (cap) density (capillaries/myofiber cross-sectional area) (C) and capillary/muscle fiber ratio (capillaries/muscle fiber number × area) (D) on day 165 in untreated (n = 6) and UAR suppressed (n = 6) baboons. Scale bar = 100 µm for A and B. Closed (●) and open (○) circles represent individual data points. *P = 0.029; **P = 0.05 vs. untreated baboons (Student’s t test). UAR, uterine spiral artery remodeling; VWF, von Willebrand factor.
Maternal Brachial Artery Flow-Mediated Dilation and Volume Flow; Systemic Blood Pressure and Heart Rate
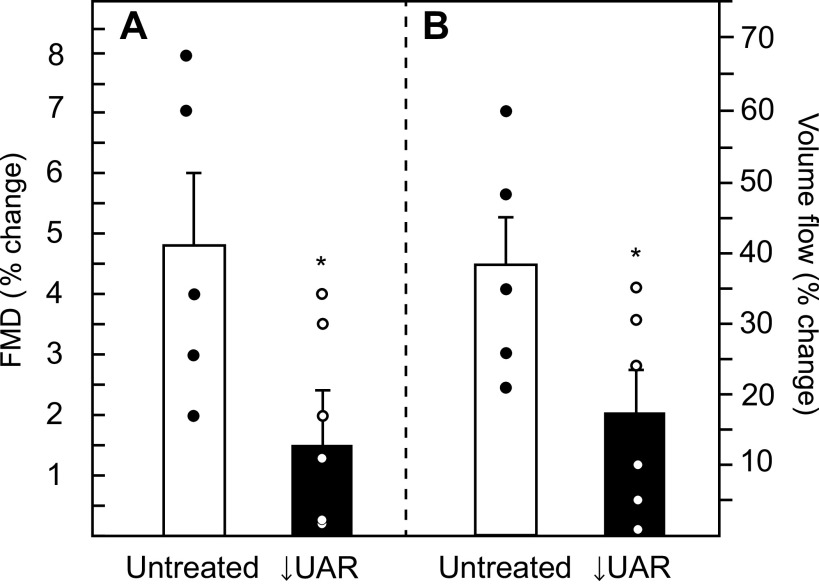
Maternal brachial artery flow-mediated dilation, quantified by ultrasound as the percent increase in artery diameter after shear stress induction, was ∼70% lower (P = 0.043) in UAR suppressed baboons (1.5% ± 0.9%, Fig. 5A) than in untreated animals (4.8% ± 1.2%). Coinciding with this reduction in shear stress induced vasodilation, the percent increase in brachial artery volume flow was 55% lower (P = 0.05) in UAR suppressed baboons (17.5% ± 6.1%, Fig. 5B) than in untreated animals (38.6% ± 7.1%).
Figure 5.
Maternal brachial artery flow-mediated dilation (FMD; A) and volume flow (B) expressed as the percent change versus basal level during the 5-min interval following induction of hyperemia/shear stress in untreated (n = 5) and UAR suppressed (n = 6) baboons. Values are the means ± SE of the average of the percent change in FMD and volume flow values obtained on days 100 and 165 of gestation. Closed (●) and open (○) circles represent individual data points. *P = 0.043 (A) and *P = 0.05 (B) vs. untreated baboons (Student’s t test). FMD, flow-mediated dilation; UAR, uterine spiral artery remodeling.
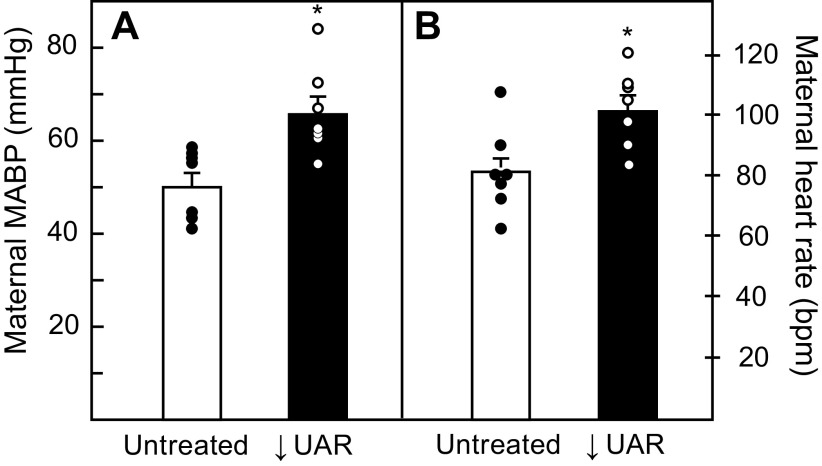
Maternal mean arterial blood pressure (mmHg) on day 165 of gestation was 29% higher (P = 0.004) in UAR impaired baboons (66 ± 3) than in untreated animals (51 ± 2, Fig. 6A). The increase in blood pressure was associated with a 26% increase (P = 0.012) in maternal heart rate in UAR defective baboons (102 ± 4 beats/min) compared with untreated animals (81 ± 4 beats/min, Fig. 6B).
Figure 6.
Maternal means ± SE arterial blood pressure (MABP, A) and heart rate (B) on day 165 in untreated (n = 6–7) and UAR defective (n = 7) baboons. Closed (●) and open (○) circles represent individual data points. *P = 0.004 (A); P = 0.012 (B) vs. untreated baboons (Student’s t test). UAR, uterine spiral artery remodeling.
Maternal Skeletal Muscle Oxidative Stress Markers
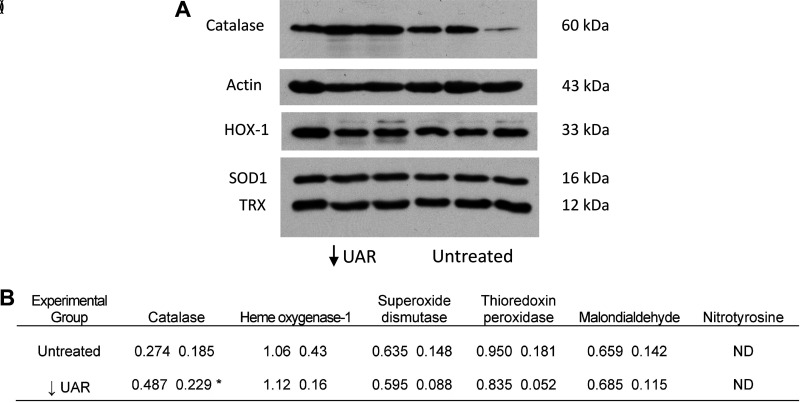
Figure 7A shows a representative Western immunoblot of catalase, pan-actin, heme oxygenase-1, superoxide dismutase, and thioredoxin peroxidase protein expression in maternal skeletal muscle of UAR suppressed and untreated baboons. Maternal skeletal muscle catalase protein levels were over 75% higher (P = 0.045) in baboons with defective UAR than in untreated animals (Fig. 7B). However, skeletal muscle heme oxygenase-1, superoxide dismutase, thioredoxin peroxidase, and malondialdehyde protein levels were similar and 3-nitrotyrosine not detectable in untreated and UAR suppressed baboons (Fig. 7B).
Figure 7.
A: representative Western immunoblot of catalase, pan-actin, heme oxygenase-1 (HO-1), superoxide dismutase (SOD1), and thioredoxin peroxidase (TRX) protein expression in maternal skeletal muscle on day 165 of UAR suppressed (n = 3) and untreated (n = 3) baboons. Catalase, actin, SOD1, and TRX electrophoresed on the same membrane and HOX-1 on a separate membrane and protein bands positioned for illustrative purposes. B: mean (SD) levels of maternal skeletal muscle oxidative stress markers quantified by Western immunoblot (expressed as a ratio of pan-actin) on day 165 of gestation in untreated (n = 7) and UAR suppressed (n = 6) baboons. *P = 0.045 vs. untreated (Student’s t test). ND, not detectable. HO-1, heme oxygenase-1; SOD1, superoxide dismutase; UAR, uterine spiral artery remodeling; TRX, thioredoxin peroxidase.
DISCUSSION
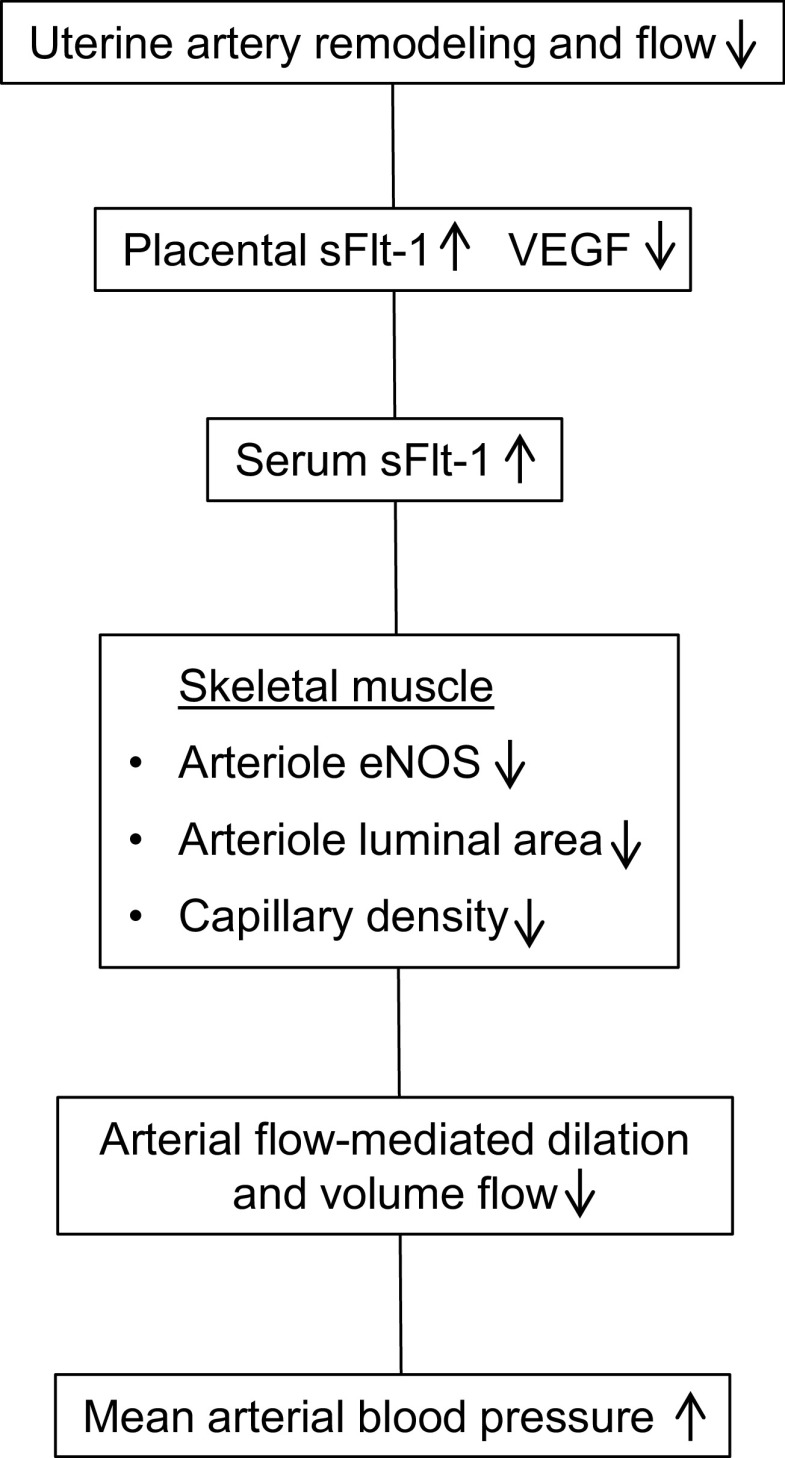
The present study shows in a nonhuman primate model of impaired UAR and elevated maternal serum sFlt-1 levels that there was striking impairment of maternal systemic vascular dynamics and function late in gestation (Fig. 8). Thus, arteriolar eNOS expression, indicative of endothelial function, and luminal area as well as capillary density were markedly reduced in maternal skeletal muscle of baboons treated early in pregnancy with estradiol, a paradigm which suppresses UAR and uteroplacental blood flow (27–29). The reduction in maternal skeletal muscle arteriolar luminal area which we suggest reflects impaired vasodilation, and capillary density would be expected to increase vascular resistance within and compromise perfusion of this systemic vascular tissue bed. Consistent with this posit, brachial artery flow-mediated dilation, which reflects primarily NO-mediated vascular endothelial function, and volume flow as indices of systemic vascular function were lower in UAR suppressed than in untreated animals.
Figure 8.
Nonhuman primate model of preeclampsia elicited by a defect in uterine spiral artery remodeling.
Along with the onset of systemic vascular dysfunction, maternal mean arterial blood pressure and heart rate were elevated in baboons in which spiral artery transformation was suppressed in early pregnancy (Fig. 8). The ability to sustain normal maternal blood pressure in pregnancy is a culmination of peripheral vasodilation and expansion of the uteroplacental vascular bed, whereas a reduction in arteriolar vasodilation peripherally and in maternal systemic tissue beds has a central role in elevating vascular resistance and thus blood pressure (reviewed in Refs. 20, 40). Presumably, the elevation in maternal blood pressure and heart rate in UAR suppressed pregnant baboons reflected the impairment in peripheral arteriolar/resistance vessel luminal area and capillary expansion, as well as the reduction in remodeling of the uterine arteries into high-capacity vessels.
Vascular function was assessed in rectus abdominis skeletal muscle because this tissue is highly vascularized, plays an active role in flexing the vertebral column and the ribcage to maintain posture and promote breathing, and can be noninvasively obtained at the time of cesarean section of baboons. In addition, the rectus abdominis muscles exhibit increased stretch in pregnancy to maintain abdominal wall stability in response to the increase in growth of the uterus and fetus. Skeletal muscle comprises 40% of body mass and accounts for 25% of the cardiac output and systemic vascular resistance in the resting state and over 80% during exercise (31–33). Thus, changes in skeletal muscle vascular resistance and blood flow greatly influence total arterial blood pressure. Moreover, human preeclampsia and other hypertensive disorders of pregnancy are associated with maternal vascular dysfunction in systemic vascular beds, including muscle, kidney, and brain (14–20, 41).
It is purported that as a consequence of impaired UAR and placental perfusion in human preeclampsia, the placenta undergoes oxidative stress and ischemia and the release of anti-angiogenic factors, particularly sFlt-1 and endoglin, that disrupt systemic vascular function (8–10, 42). The current study shows that maternal peripheral and uterine vein serum levels of sFlt-1 were 2.5-fold higher late in gestation in UAR defective baboons compared with those in untreated animals. We propose that the decrease in maternal skeletal muscle blood vessel eNOS expression, luminal area and capillary density, and systemic vascular function in UAR suppressed baboons reflected sFlt-1 suppression of the peripheral bioavailability of VEGF, a growth factor that has a pivotal role in promoting vascular eNOS expression/NO formation; vasodilation; capillary growth, i.e., angiogenesis; and vascular stability (11–13, 43).
Skeletal muscle fibers express all of the NOS isoforms (44), and myofibril NOS is upregulated by neural stimulation, contraction, hypoxia, and development (44, 45). Skeletal muscle NO appears to have a role in muscle contraction, respiration, metabolism, and oxidative stress (44, 45). However, in contrast to the reduction in arteriolar eNOS expression, eNOS protein expression by skeletal muscle fibers was unaltered in UAR defective baboons. The decline in eNOS expression in the blood vessels, but not in myofibers, indicates selectivity/specificity and points to the advantage of the proximity ligation assay to quantify cell-specific expression of protein.
Expression of catalase, an antioxidant enzyme that is elevated in a compensatory manner with the onset of oxidative stress (46), was increased in whole skeletal muscle tissue in UAR suppressed baboons. However, the levels of other antioxidants and oxidative stress markers, including heme oxygenase, an inducible enzyme that leads to the production of the antioxidant bilirubin the levels of which are correlated with sFlt-1 levels in human preeclampsia (47), were unaltered in UAR defective baboons. Thus, the decrease in systemic vascular function in UAR suppressed baboons may not have elicited a significant increase in skeletal muscle vascular oxidative stress. However, skeletal muscle fibers as well as muscle vascular cells express reactive oxygen species (48). Therefore, because we analyzed whole skeletal muscle, any potential increase in expression of antioxidants and oxidative stress markers by muscle vascular tissues in UAR suppressed baboons may have been masked by expression from the quantitatively abundant myofibers. Moreover, our studies were conducted on day 165 or approximately week 35 of human pregnancy and maternal systemic vascular inflammation and oxidative stress progressively increase with advancing human preeclampsia (10, 17, 41, 49). Thus, expression of antioxidants such as heme oxygenase and superoxide dismutase in the vascular component of skeletal muscle may be enhanced closer to term in UAR suppressed baboons as metabolic demands of the growing fetus progress. In addition, other vasodilatory factors not examined in the present study, particularly endothelium-derived hyperpolarizing factor, which is reduced in skeletal muscle of rodent models of fetal programming (50), may act via hydrogen peroxide to modulate expression of antioxidants (41).
Because of the difficulty in conducting invasive studies in pregnant women, circulating levels of factors indirectly reflecting systemic tissue dysfunction and oxidative stress, and noninvasive imaging measurements of vascular function, have been used to demonstrate maternal vascular dysfunction in human preeclampsia (14, 16, 18, 19). However, a decrease, increase, or no changes in the levels of eNOS and NO have been measured in the maternal circulation of women with preeclampsia (reviewed in Refs. 51). In the current study, plasma levels of nitrate plus nitrite were not altered in UAR suppressed baboons despite a reduction in vascular eNOS expression. This result may not be unexpected since plasma nitrate/nitrite levels were not correlated with arterial eNOS expression in male and female baboons (52). The ability to assess aberrant vascular function by direct tissue analyses along with real-time imaging as performed in pregnant baboons of the current study emphasizes the value of the experimental nonhuman primate model of defective UAR. Importantly, the observation of a decrease in capillary density in maternal skeletal muscle of baboons represents a novel finding showing a morphological, as well as functional, change in the vascular network associated with/caused by the decline in UAR.
The decrease in skeletal muscle arteriolar luminal area and capillary density on day 165 of gestation was observed 15 wk after the brief increase in serum estradiol levels induced in baboons on days 25–59, whereas serum estradiol levels progressively increased and were similar in untreated and early estradiol-treated baboons throughout the remainder of pregnancy including on day 165. Therefore, it is unlikely that the reduction in vascular function near term reflected a direct effect of the early estradiol treatment, but rather was indirectly induced subsequent to the suppression of UAR and ultimately by enhanced expression of sFlt-1/decrease in VEGF bioavailability.
The experimental paradigm of prematurely elevating estrogen in early baboon pregnancy was simply employed as a method to suppress UAR. However, high levels of estradiol are induced by superovulation during in vitro fertilization (IVF) in early human pregnancy (53) and endocrine disruptors that mimic the action of estradiol at the estrogen receptor site are present in high level in human pregnancy (54). Both IVF and elevated levels of endocrine disruptors are associated with increased incidence of human preeclampsia and fetal growth restriction (55, 56).
Despite the reduction in UAR and uterine artery blood flow, overall fetal body weight was only 5% lower in UAR defective baboons. Although 30% of infants derived from human preeclampsia pregnancies have been reported to have low birthweight, other studies have shown no change in birthweight in human preeclampsia (57, 58).
A limitation of the present study is that the direct impact of the increase in circulating sFlt-1 in UAR suppressed baboons on systemic vascular integrity and function, e.g., the addition of sFlt-1 or plasma from UAR suppressed baboons to cultures of endothelial cells, was not determined. However, culture of isolated endothelial cells may not yield definitive results since such in vitro approaches do not mimic the complex in vivo milieu within skeletal muscle and potential paracrine and intercell regulatory mechanisms that underpin the action of factors such as sFlt-1. Another limitation is that the actual levels of the reactive oxygen species were not directly measured in UAR suppressed baboons. However, the quantification of peroxides and other oxygen species is not straightforward because of their rapid metabolism and difficulty in establishing cellular origin. We suggest that these limitations do not alter the interpretation of the current findings demonstrating maternal systemic vascular dysfunction in a primate model of defective uterine spiral artery remodeling.
Maternal vascular dysfunction and hypertension are hallmarks of adverse conditions of human pregnancy, notably early onset preeclampsia, elicited by impaired UAR. Rodent models have been developed that replicate the pathophysiological consequences of preeclampsia, but the studies of these models did not focus on UAR. We suggest that the present study links defective UAR with a marked impairment of maternal systemic vascular function and capillary expansion in the baboon. This study highlights the translational significance of this nonhuman primate model and opportunity to investigate the regulatory mechanisms underpinning and potential to develop therapeutic modalities to manage and/or prevent this condition of adverse human pregnancy.
GRANTS
This work was supported by National Institutes of Health R01 HD 93070.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.D.A. and G.J.P. conceived and designed research; J.S.B., G.W.A., and M.G.B. performed experiments; E.D.A., J.S.B., G.W.A., M.G.B., and G.J.P. analyzed data; E.D.A., J.S.B., and G.J.P. prepared figures; E.D.A. and G.J.P. drafted manuscript; E.D.A. and G.J.P. edited and revised manuscript; E.D.A., J.S.B., G.W.A., M.G.B., and G.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Irene Baranyk for computer-assisted preparation of this manuscript.
REFERENCES
- 1.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat 94: 297–328, 1960. [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey EM, Houston ML, Harris JW. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am J Obstet Gynecol 124: 647–652, 1976. doi: 10.1016/0002-9378(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol 38: 131–132, 2014. doi: 10.1053/j.semperi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw 71: 222–230, 1964. doi: 10.1111/j.1471-0528.1964.tb04270.x. [DOI] [PubMed] [Google Scholar]
- 5.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049–1059, 1986. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27: 939–958, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brosens I, Puttemans P, Benagiano G. Placental bed research: I. the placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol 221: 437–456, 2019. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Gonçalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 17: 3–18, 2005. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 10.Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci 19: 1496, 2018. doi: 10.3390/ijms19051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol Heart Circ Physiol 274: H1054–H1058, 1998. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 13.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell 176: 1248–1264, 2019. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, Mclaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161: 1200–1204, 1989. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 15.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 17.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost 7: 375–384, 2009. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 18.Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation 21: 4–14, 2014. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 19.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 124: 1094–1112, 2019. [Erratum in Circ Res 126: e8, 2020]. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 20.Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 232: R27–R44, 2017. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albrecht ED, Pepe GJ. Endocrinology of pregnancy. In: Non-Human Primates in Perinatal Research, edited by Brans YW, Kuehl TJ.. New York, NY: John Wiley and Sons, 1988, p. 13–78. [Google Scholar]
- 22.Carter AM. Animal models of human placentation – a review. Placenta 28: S41–S47, 2007. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 32: 413–419, 2011. doi: 10.1016/j.placenta.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Enders AC, Blankenship TN, Fazleabas AT, Jones CJ. Structure of anchoring villi and the trophoblastic shell in the human, baboon and macaque placenta. Placenta 22: 284–303, 2001. doi: 10.1053/plac.2001.0626. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 27: 483–490, 2006. doi: 10.1016/j.placenta.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 149: 5078–5087, 2008. doi: 10.1210/en.2008-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonagura TW, Babischkin JS, Aberdeen GW, Pepe GJ, Albrecht ED. Prematurely elevating estradiol in early baboon pregnancy suppresses uterine artery remodeling and expression of extravillous placental vascular endothelial growth factor and α1β1 and α5β1 integrins. Endocrinology 153: 2897–2906, 2012. doi: 10.1210/en.2012-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. Am J Physiol Heart Circ Physiol 302: H1936–H1944, 2012. doi: 10.1152/ajpheart.00590.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babischkin JS, Aberdeen GW, Lindner JR, Bonagura TW, Pepe GJ, Albrecht ED. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology 160: 1492–1505, 2019. doi: 10.1210/en.2019-00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hably C, Vag J, Bartha J. Comparative haemodynamic studies of resting and active skeletal muscle in anaesthetized rats: role of nitric oxide. Acta Physiol Hung 88: 25–33, 2001. doi: 10.1556/APhysiol.88.2001.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Korthuis RJ. Skeletal muscle circulation. In: Integrated Systems Physiology: from Molecule to Function to Disease, edited by Granger DN, Granger J.. San Rafael, CA: Morgan & Claypool Life Sciences, 2011. doi: 10.4199/C00035ED1V01Y201106ISP023. [DOI] [PubMed] [Google Scholar]
- 33.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol 2: 321–447, 2012. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 34.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 35.Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: a randomized, controlled trial. J Trauma Acute Care Surg 73: 94–101, 2012. doi: 10.1097/TA.0b013e318250cdb8. [DOI] [PubMed] [Google Scholar]
- 36.Babischkin JS, Aberdeen GW, Pepe GJ, Albrecht ED. Estrogen suppresses interaction of melanocortin 2 receptor and its accessory protein in the primate fetal adrenal cortex. Endocrinology 157: 4588–4601, 2016. doi: 10.1210/en.2016-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang XL, Mahaney MC, Sim AS, Wang J, Wang J, Blangero J, Almasy L, Badenhop RB, Wilcken DE. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol 17: 3147–3153, 1997. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]
- 38.Kubínová L, Janáček J, Ribarič S, Čebašek V, Eržen I. Three-dimensional study of the capillary supply of skeletal muscle fibres using confocal microscopy. J Muscle Res Cell Motil 22: 217–227, 2001. doi: 10.1023/a:1012201314440. [DOI] [PubMed] [Google Scholar]
- 39.Glancy B, Hsu LY, Dao L, Bakalar M, French S, Chess DJ, Taylor JL, Picard M, Aponte A, Daniels MP, Esfahani S, Cushman S, Balaban RS. In vivo microscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers. Microcirculation 21: 131–147, 2014. doi: 10.1111/micc.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osol G, Ko NL, Mandalà M. Altered endothelial nitric oxide signaling as a paradigm for maternal vascular maladaptation in preeclampsia. Curr Hypertens Rep 19: 82, 2017. doi: 10.1007/s11906-017-0774-6. [DOI] [PubMed] [Google Scholar]
- 41.Yu W, Gao W, Rong D, Wu Z, Khalil RA. Molecular determinants of microvascular dysfunction in hypertensive pregnancy and preeclampsia. Microcirculation: e12508, 2018. doi: 10.1111/micc.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umapathy A, Chamley LW, James JL. Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis 23: 105–117, 2020. doi: 10.1007/s10456-019-09694-w. [DOI] [PubMed] [Google Scholar]
- 43.Svedas E, Islam KB, Nisell H, Kublickiene KR. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. Am J Obstet Gynecol 188: 168–176, 2003. doi: 10.1067/mob.2003.110. [DOI] [PubMed] [Google Scholar]
- 44.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 45.Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2: 21–27, 2000. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- 46.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch 459: 923–939, 2010. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 47.George EM, Warrington JP, Spradley FT, Palei AC, Granger JP. The heme oxygenases: important regulators of pregnancy and preeclampsia. Am J Physiol Regul Integr Comp Physiol 307: R769–R777, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol (1985) 102: 1664–1670, 2007. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 49.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol 16: 93–104, 1998. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 50.Stead R, Musa MG, Bryant CL, Lanham SA, Johnston DA, Reynolds R, Torrens C, Fraser PA, Clough GF. Developmental conditioning of endothelium-derived hyperpolarizing factor-mediated vasorelaxation. J Hypertens 34: 452–463, 2016. doi: 10.1097/HJH.0000000000000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanhoutte PM, Zhao Y, Xu A, Leung SWS. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 119: 375–396, 2016. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Felux D, VandeBerg J, Wang X. Discordance of endothelial nitric oxide synthase in the arterial wall and its circulating products in baboons: interactions with redox metabolism. Eur J Clin Invest 33: 288–295, 2003. doi: 10.1046/j.1365-2362.2003.01143.x. [DOI] [PubMed] [Google Scholar]
- 53.Imudia AN, Awonuga AO, Doyle JO, Kaimal AJ, Wright DL, Toth TL, Styer AK. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 97: 1374–1379, 2012. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 54.Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 110: A703–A707, 2002. doi: 10.1289/ehp.021100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol 103: 551–563, 2004. doi: 10.1097/c1.AOG.0000114989.84822.5. [DOI] [PubMed] [Google Scholar]
- 56.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol 30: 2–9, 2010. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 16: 5–15, 1998. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 58.Roberts JM, Escudero C. The placenta in preeclampsia. Pregnancy Hypertens 2: 72–83, 2012. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]