Abstract
Age-related blood-brain barrier (BBB) disruption and cerebromicrovascular rarefaction contribute importantly to the pathogenesis of both vascular cognitive impairment and dementia (VCID) and Alzheimer's disease (AD). Recent advances in geroscience research enable development of novel interventions to reverse age-related alterations of the cerebral microcirculation for prevention of VCID and AD. To facilitate this research, there is an urgent need for sensitive and easy-to-adapt imaging methods that enable longitudinal assessment of changes in BBB permeability and brain capillarization in aged mice and that could be used in vivo to evaluate treatment efficiency. To enable longitudinal assessment of changes in BBB permeability in aged mice equipped with a chronic cranial window, we adapted and optimized two different intravital two-photon imaging approaches. By assessing relative fluorescence changes over the baseline within a volume of brain tissue, after qualitative image subtraction of the brain microvasculature, we confirmed that, in 24-mo-old C57BL/6J mice, cumulative permeability of the microvessels to fluorescent tracers of different molecular masses (0.3 to 40 kDa) is significantly increased compared with that of 5-mo-old mice. Real-time recording of vessel cross-sections showed that apparent solute permeability of single microvessels is significantly increased in aged mice vs. young mice. Cortical capillary density, assessed both by intravital two-photon microscopy and optical coherence tomography was also decreased in aged mice vs. young mice. The presented methods have been optimized for longitudinal (over the period of 36 wk) in vivo assessment of cerebromicrovascular health in preclinical geroscience research.
NEW & NOTEWORTHY Methods are presented for longitudinal detection of age-related increase in blood-brain barrier permeability and microvascular rarefaction in the mouse cerebral cortex by intravital two-photon microscopy and optical coherence tomography.
Keywords: aging, blood-brain barrier, capillary density, intravital microscopy, microcirculation, multiphoton microscopy
INTRODUCTION
The prevalence of vascular cognitive impairment and dementia (VCID) rises exponentially with advanced age, which with its high medical and societal costs represents an important healthcare challenge in the aging societies of the Western world (1). Age-related structural and functional alterations of the cerebral microcirculation have a critical role in the pathogenesis of VCID (2). Among the age-related microvascular pathologies, endothelial dysfunction and vasomotor dysfunction, disruption of the blood-brain barrier (BBB) (3–6), and consequential neuroinflammation (7) as well as an age-related decline in brain capillary density (also known as “cerebromicrovascular rarefaction”) promoting ischemia take center stage (8–10). Importantly, both BBB disruption and capillary regression (“string vessel” formation) also contribute to the pathogenesis of Alzheimer's disease (AD) (11, 12).
A growing number of preclinical studies focus on developing interventions to delay or reverse age-related alterations of the cerebral microcirculation for prevention of VCID and AD (13–19). Yet, longitudinal in vivo assessmen of cerebromicrovascular health is usually a significant experimental challenge for laboratories in preclinical geroscience research. With the rapid expansion of NIH-funded research into the pathogenesis of AD and AD-related dementias (ADRDs), there is an urgent need for sensitive and easy-to-adapt imaging methods that enable longitudinal assessment of changes in BBB permeability and brain capillarization in aged mice, which could be used in vivo to evaluate treatment efficiency.
The objective of the present study was to adapt and optimize intravital two-photon microscopy-based imaging methods to assess BBB permeability and cerebromicrovascular density in aged mice equipped with a chronic cranial window. To enable longitudinal assessment of changes in BBB permeability in aged mice, we adapted two different two-photon imaging approaches. For monitoring cumulative permeability of the microvessels within a volume of brain tissue, we assessed relative fluorescence changes over the baseline within a three-dimensional reconstruction of volume-of-interest (VOI), after qualitative image subtraction of the brain microvasculature from a z-stack (20). Real-time recording of vessel cross-sections was used for determination of apparent solute permeability of single microvessels (21). The method was also adapted to assess changes in cortical capillary density. To enable longitudinal assessment of changes in cerebromicrovascular density in brain areas beyond the imaging depth of two-photon microscopy, we also adapted an intravital optical coherence tomography [OCT (22)]-based approach that could be used with the same chronic cranial window.
MATERIALS AND METHODS
Animals
Five- (young) and 24-mo-old (aged) male C57BL/6J mice were purchased from the colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). Animals were housed in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center, under specific pathogen-free conditions, with a controlled photoperiod (12-h:12-h light-dark), unlimited access to water, and a standard AIN-93G diet (ad libitum). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Chronic Cranial Window Surgery
Animals were anesthetized with 2–3 % isoflurane (ISOTHESIA, Henry Schein Animal Health, OH) gas via inhalation with Surgivet Classic T3 vaporizer (Smiths Medical, Minneapolis, MN) with 1- to 2-L/min flow rate before and during the surgery. Eye blink, toe, and tail pinch reflexes were monitored to determine the depth of anesthesia. The experimental animal was placed on a heating pad to maintain core body temperature during the procedures. The head of the animal was fixed in an adaptor for the stereotaxic frame by ear and nose bars. Eye ointment was applied to both eyes to prevent ocular dehydration during anesthesia. Hair removal lotion was applied to the top of the head. After hair removal, the surface of the skin was disinfected with 70% ethanol. Then, the skin was removed from the top of the skull. 2% Lidocaine (Sigma-Aldrich, St. Louis, MO) solution was dripped onto the periosteum. The periosteum was removed from the exposed area of the skull with a blade, and the area was scraped gently to establish a clean surface. To dry the surface of the skull, it was wiped with a cotton wool stick. After an area 3–4 mm in diameter had been chosen over the sensorimotor cortex, the skull was gently thinned using a pneumatic dental drill (Foredom, Blackstone Industries, Bethel, CT). During this procedure, the surface was cooled by dripping of cold, sterile PBS. Debris was blown off with compressed air. When the bone was thin enough (indicated by its slight movement when it was gently pushed on), craniotomy was performed under a drop of sterile PBS. The surface of the dura mater was carefully wiped down, and any accidental bleeding caused by the craniotomy was stopped with a Hemosponge (Goodwill Lifesciences, India) gelatin sponge immersed in sterile PBS. The cranial window was dried very carefully to remove excess fluid from the area. A glass coverslip (diameter 5 mm; Thomas Scientific, Swedesboro, NJ), which was previously soaked in 70% ethanol, was rinsed in PBS and applied to the surface of the dura mater to completely cover the cranial window. The coverslip was fixed to the skull with liquid adhesive, and after the superglue had bonded, the cranial window was secured with Jet Set-4 dental acrylic resin (Lang Dental, Wheeling, IL). The resin rim created a small pool around the cranial window. At the end of the surgery, the animals were treated with buprenorphine (1 mL/kg body wt ip; Zoopharm, WY). Enrofloxacin (5 mg/kg body wt sc, Baytril, Bayer, Germany) was administered as antibiotic prophylactic. The animals were closely monitored until the anesthesia wore off and they regained consciousness. Intravital imaging studies were conducted at least 2–3 wk after surgery.
Intravital Two-Photon Microscopy
Two-photon imaging was performed to assess microvascular density and BBB integrity.
Intravital imaging was preformed using a FluoView 1000 MPE (Olympus, Tokyo, Japan) two-photon microscope coupled with a MaiTai HP DeepSee-OL 690- to 1,040-nm (Spectra-Physics, San Jose, CA) laser and a XLPLN25XWMP ×25 water immersion objective (1.05 numeric aperture; Olympus, Tokyo, Japan). An 800-nm laser line was used for excitation. The emitted light was collected by PMT detectors. Three channels with the following filter sets were used: 420–460, 495–540, and 575–630 nm.
Mice were anesthetized with isoflurane (2-2.5% for induction and 1.5-2% for maintenance; with 1–2 L/min flow rate). The heads of the animals were fixed using a stereotaxic frame. To label the vascular glycocalyx, wheat germ agglutinin, Alexa Fluor 594 Conjugate (4 mL/kg body wt of 1 mg/mL WGA-A594; Thermo Fisher Scientific) was injected retroorbitally. Imaging of the cortex was performed through the cranial window. WGA-A594 binds to the glycocalyx in the cortical microvasculature, enabling the visualization of the microvascular network architecture and the accurate identification of the vessel wall boundaries. On the basis of the microvascular architecture, cortical areas for subsequent BBB integrity studies were identified and imaged. First, meningeal vessels were detected, and imaging depth of zero was set to them. Superior pial vessels were used as reference points for later examination. Cerebral microvessels were examined at ∼0–200 µm depth. The same laser intensity (5%) and detector sensitivity were used regardless of the depth in the tissue to maintain the reproducibility and comparability with other animals (Fig. 1). Images captured immediately after WGA-A594 injection served as a no-tracer background intensity internal control. Using two-photon microscopy, image stacks deep into the brain tissue were captured with limited photobleaching and tissue phototoxicity, as fluorescence occurs only within the plane of focus.
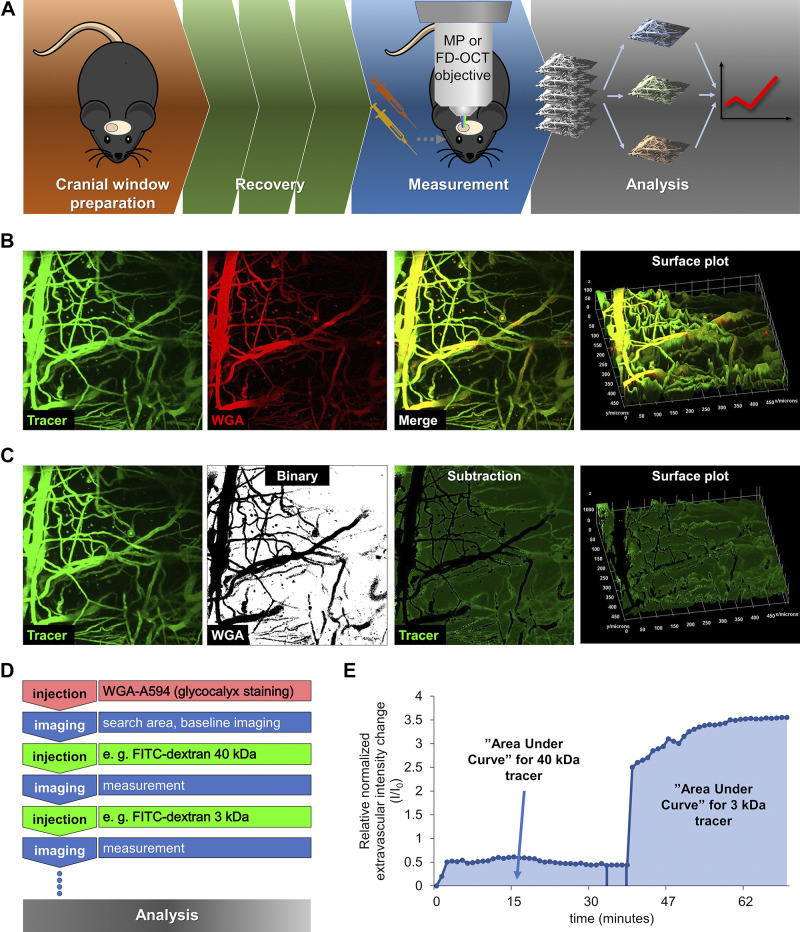
Figure 1.
Experimental setup for intravital two-photon microscopy and image analysis for measurement of blood-brain barrier permeability and cerebromicrovascular density in young and aged mice. A: experimental timeline for intravital two-photon microscopy and optical coherence tomography (OCT). Chronic cranial window preparation in the animals was followed by a recovery period (∼14 days). Then, in anesthetized and ventilated mice, tracer administration and two-photon microscopy imaging or OCT imaging were performed. B: representative z-stack images acquired by two-photon microscopy at 50–150 μm depth below the pia mater in the mouse brain. Images were processed using a custom-made macro in FIJI (ImageJ). Green fluorescence: FITC-dextran signal corresponding to the intravascular compartment (tracer channel); red fluorescence: Wheat germ agglutinin (WGA)-Alexa 594 staining of the glycocalyx of the endothelial cells. The surface plot represents the fluorescence intensity quantification of the two merged channels. C: volume image analysis from brain tissue. Tracer z-stacks were background subtracted (with background channel), while WGA z-stacks were processed to outline the brain vasculature and thresholded into binary images. Binary vasculature images were subtracted from tracer stacks to generate a time-dependent 16-bit intensity plot. Quantification is presented by surface plot of the subtracted image. Scale is visible on the surface plot. D: imaging timeline for two-photon microscopy studies. WGA-Alexa 594 was injected to stain the cerebral vasculature and find the optimal imaging area in the cerebral cortex. After recording of a baseline, fluorescence tracers were injected sequentially, in the order of highest to lowest molecular mass. Each tracer was recorded separately. E: representative time course of sequential increases in fluorescence intensity in the extravascular space upon injection of the 40-kDa FITC-dextran tracer and 3-kDa FITC-dextran tracer, indicating tracer extravasation. After quantification of tracer images, integrated pixel densities were normalized to the baseline fluorescence. Relative normalized intensity changes were expressed as a function of time. The respective areas under these curves can be used as an index for relative permeability of the microvasculature to each of the tracers injected.
To assess BBB integrity and determine the severity of BBB disruption, first a fluorescent tracer dye with higher molecular mass (4 mL/kg body wt of 2 mg/mL FITC-dextran 500 kDa; Sigma-Aldrich) was injected retroorbitally. Imaging of the preset area was performed to detect extravasation of the high-molecular-mass tracer dye, indicating severe BBB disruption. This high-molecular-mass tracer was used only for single microvessel measurement, since detectable extravasation is minimal and is hardly detectable with image subtraction analysis.
To detect mild BBB disruption, fluorescent tracer dyes with lower molecular mass (4 mL/kg body wt of 2 mg/mL FITC-dextran 40 kDa, 3 kDa, Thermo Fisher Scientific; and sodium fluorescein, Sigma-Aldrich) were injected in order of descending molecular mass, and imaging of the predetermined area was performed following the aforementioned protocol.
For image subtraction analyses, the VOIs were selected based on observable microvessels. 508 × 508 × 50–150 µm (x, y, z) VOI was imaged for z-stacks. The corresponding pixel numbers were 512 × 512 × 21; thus, the spatial resolution was ∼1 × 1 × 5 µm in x, y, z directions (objective point spread function (PSF) < 1.5 μm) (23).
In addition to large z-stacks, single capillary imaging was also performed for determination of apparent solute permeability of microvessels. To that end, the cross-sections of selected microvessels at the previously indicated depth were recorded with fast scanning mode over ∼32 s, producing 400 images as a time stack with ×25 magnification, using the high-speed, roundtrip mode of the two-photon microscope; 20 × 20-µm (x, y) ROI was imaged for time stacks, where the corresponding pixel numbers were 256 × 256.
The images were analyzed using FIJI ImageJ version 1.52p (National Institutes of Health) and Icy application version 2.0.1.0 (BioImage Analysis Unit, Institut Pasteur, Paris, France).
Image Subtraction Analysis
Image analysis of z-stack images was performed with a custom-made macro in FIJI, following the modified methods of Janiurek et al. (20). In brief, imported TIFF images were assembled, and then WGA-A594 images were processed similarly to the method described for determination of vascular density to sharpen the contours of vessels. WGA-A594 image masks were then subtracted from background-corrected tracer z-stack maximum intensity projections to remove the areas corresponding to intravascular volumes (for detailed stepwise protocol, see Fig. 2). This approach enables the selective measurement of extravasated tracer intensities at each time point separately (Fig. 1, B and C). Intensity of the intravascular volumes was also measured by inversion and subtraction of the vascularization-segmented images from the tracer channel. As a last step, intensity of images post-subtraction was measured. Area fraction and integrated density values were used for the calculations (see below). Selected recordings were median projected over time for the representative images, and original stacks were resliced z→y for further analysis, searching for focal barrier leaks.
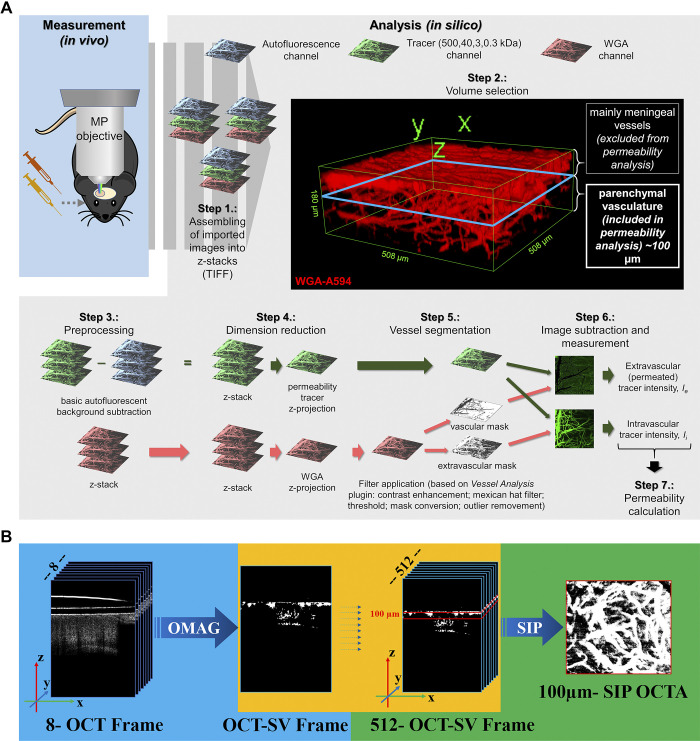
Figure 2.
Computational workflows for in silico analysis of two-photon microscopy and optical coherence tomography (OCT) images. A: computational workflow for in silico analysis of two-photon microscopy images for blood-brain barrier (BBB) permeability quantification with image subtraction analysis method. After in vivo measurement, the recorded images were exported in TIFF format and assembled into z-stacks. 3-D reconstruction of an example stack is presented in Step 2. For quantification an ∼100-μm-thick cortical volume was used in 50–150 μm depth from the “0 level” of the meningeal vessels to avoid quantification of images derived from nonparenchymal layers. After elimination of autofluorescent background and dimension reduction, filters were applied on wheat germ agglutinin (WGA)-Alexa 594 (WGA-A594) channel only to obtain vasculature as a segmented binary mask. Binary masks were then qualitatively subtracted from tracer channel images, and the resulting extravascular and intravascular intensity images were measured. Data gained from image analysis were used for permeability calculation. B: OCT image analysis. Original z-stacks were assembled and calculated with average decorrelation in OCT speckle variance frames.
Real-Time Recording of Vessel Cross-Sections for Determination of Apparent Solute Permeability of Single Microvessels
Measurement of the solute permeability of single microvessels was performed with a custom-made macro in FIJI following the modified methods of Shi et al. (24) and Kutuzov et al. (21). In brief, recorded equivalent time stacks were stacked together and shifted to align with each other with the above-mentioned Icy software applicable method. Vessel diameter was determined based on WGA-A594 glycocalyx staining, generating a local maximum of fluorescence with the following process. WGA-A594 channel images were average-intensity projected (time stack/32 s recording), and after a standard background subtraction on the projections, a Cartesian-to-polar transformation was applied based on the center of the microvessel, determined by the center of the area made with “Active Contours” level set method; x-axis summarized intensity plot profiles of the polar images were generated and measured to determine local maximum at the glycocalyx (Fig. 6, B and C) (21).
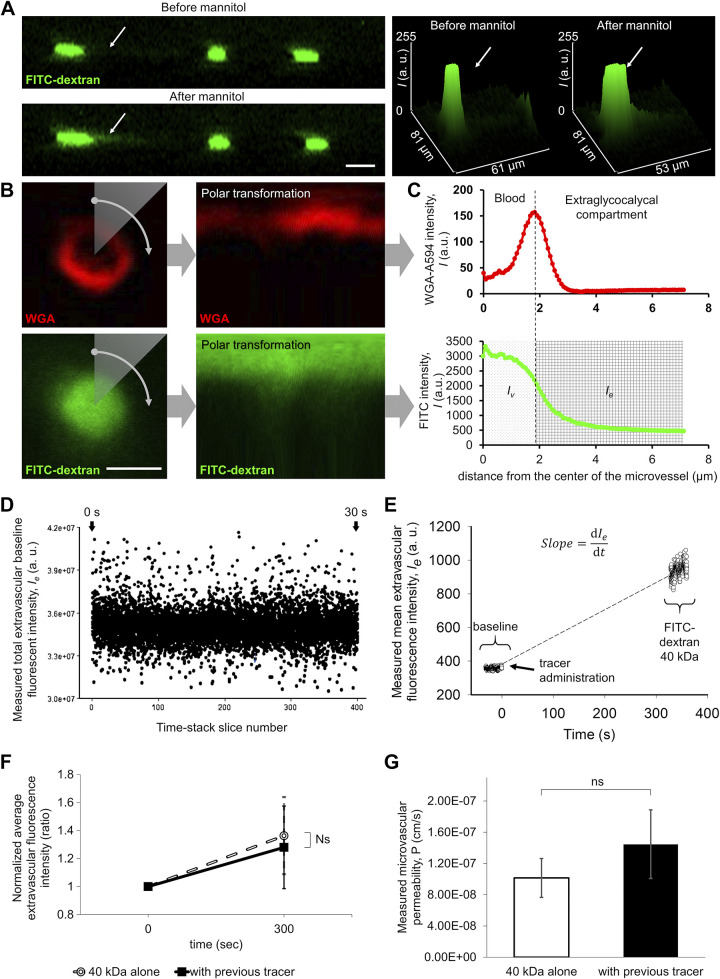
Figure 6.
Two-photon imaging-based measurement of permeability of single microvessels to fluorescent tracers. A: representative recording showing focal microvascular leakage induced by mannitol treatment. The mouse brain cortex was imaged by two-photon microscopy before (top) and after (bottom) 20% mannitol administration. Z-stacks were reassembled and resliced as a z→y transition to visualize microvascular leaks. Green fluorescence: FITC-dextran 500 kDa. Note that this large tracer is exclusively located in the intravascular compartment before mannitol administration. Arrow points to a localized increase in extravascular tracer fluorescence intensity in response to mannitol, corresponding to focal disruption of the blood-brain barrier (BBB). Right: 3-D plots showing tracer fluorescence intensity in the z dimension before and after mannitol treatment. Scale bar, 10 μm. B: cortical microvessel recording for permeability measurement. Images from microvessel cross-sections were captured at the same depth as the cortical volume imaging (50–150 μm from the brain surface); 400 images were recorded during ∼32 s with high magnification, high speed, roundtrip mode of the two-photon microscope and images were averaged (see D) as a time stack projection in each case. Red fluorescence: wheat germ agglutinin (WGA)-Alexa 594 staining of the glycocalyx of the endothelial cells. Green fluorescence: FITC-Dextran 40-kDa tracer. Right: the Cartesian-to-polar transformation of the images are shown. The image fluorescence intensities are plotted as a function of radial position for a given polar angle. Scale bar, 4 μm. C: method for quantification of extravasated tracer fluorescence intensities in the surroundings of a single microvessel. The fluorescence intensities of polar converted images are plotted as a function of distance from the center of the microvessel. Note that the signal maximum of the red fluorescence of the WGA-Alexa 594-stained glycocalyx on a radial intensity profile defines the border between the intravascular compartment and the extraluminal/extravascular (i.e., extraglycocalycal) compartment. Bottom: the radial intensity profile of the tracer (FITC-dextran 40 kDa). On the basis of the previously determined edge of the intravascular compartment (dashed line), the distribution of the intravascular and extraluminal tracer intensities can be assessed. D: representative recording showing distribution of measured total extravascular baseline intensity as a function of time. The source of this variability, in part, is flowing blood cells. To control for this variability in fluorescence intensity, average time stack projections were made from each recording. E: representative recording showing changes in extravascular tracer intensity after injection of FITC-dextran 40 kDa in an aged mouse. Extravasated tracer fluorescence intensity was calculated from every image from a time stack before and after tracer injection, and results were plotted as a function of time. The slope of the trend line is a main component of the permeability measurement formula (see Fig. 7.). F: previous injection of the 500-kDa tracer does not affect the extravascular fluorescence intensity postinjection of the 40-kDa tracer (P = 0.59, n = 6). Data are presented as means ± SE. G: calculated permeability of 40-kDa tracer with and without previously injected 500-kDa tracer, showing no significant difference (P = 0.41, n = 6). After image analysis, the permeability was calculated with the formula shown in Fig. 7.
Images of the tracer channel were also stacked together and aligned to each other, as needed. As a next step, selected images were projected with an average intensity method (time stack/32 recordings) and extra-glycocalycal (i.e., extraluminal) and intravascular fluorescent intensities were measured based on the corresponding WGA-A594 channel “Active Contours” level set segmentation method. This method generates a mask for detection of the vascular boundaries for the extraluminal fluorescence measurements (https://imagej.net/Level_Sets). Cartesian-to-polar transformation was applied based on the same center as in the case of the corresponding WGA-A594 labeling. x-axis summarized intensity plot profiles of the polar images were generated to determine intravascular and extravascular intensities (Fig. 6, B and C).
Determination of Apparent Solute Permeability of Cerebral Microvessels from Tracer Intensity Differences
Permeability calculations and formulas are based on the combination of those previously described by Kutuzov et al, Shi et al., Dreher et al., Nhan et al., and Lee et al. (21, 24–27). Both in the cortical volume analyses and in single microvascular measurements, data obtained from image analysis were used as variables in subsequent calculations.
After the image analysis described above and demonstrated on Fig. 6, B–D, the resulting numerical data were used for permeability calculations. All mean extravascular fluorescent intensity data from average projected stacks were collected. These values were compared as a function of elapsed time between the first (only WGA-A594) and second (WGA-A594 and tracer) recordings (Fig. 6E), resulting in an approximation of permeability of the microvessel based on the following Eq. 1 (28):
| (1) |
where ∼ apparent solute permeability (cm/s); ∼ initial mean extravascular intensity (arbitrary unit); ∼ slope of increasing extravascular intensity as a function of time (1/s); r ∼ measured vessel radius (cm) based on WGA-A594 staining. Previous administration of a tracer does not influence significantly the permeability measurements performed using subsequent administration of another tracer (Fig. 6, F and G).
Determination of Relative and Absolute Permeability of Cerebral Microvessels within a Volume of Brain Tissue
Measured total area integrated density values from extravascular tracer fluorescent intensity in z-stack maximum projections were normalized to the baseline intensity (WGA-A594 without the tracers) to avoid imaging error-based distortion. Intensities were represented as a function of elapsed time between the first (only WGA-A594) and further (WGA-A594 and tracer) recordings. The given function was analyzed by the calculation of the area under curve (AUC), which enabled the comparison of the extravasated tracer quantities, resulting in a relative permeability difference between subjects (Fig. 1E). These data were further analyzed and used for absolute permeability calculation with the following formula (Eq. 2):
| (2) |
where ∼ the extravascular volume; ∼ the intravascular volume, both calculated based on vascular density of the VOI; ∼ the slope of the intensity shift after tracer administration; ∼ the approximation of the surface area of the vasculature also calculated from the vascular density of the VOI; ∼ the normalized intravascular fluorescent intensity at an exact time point and ∼ the normalized extravascular or parenchymal fluorescent intensity at an exact time point, both based on the measured integrated density of the analyzed images VOI; and ∼ the hematocrit value what was given as 0.42 based on the literature (26). Permeability was measured at every single time point (∼15/tracer) with their own area fraction and normalized intensity. Then, the apparent solute permeability for different tracers was calculated from the median value of the relevant time points.
Intravital Optical Coherence Tomography
In the same mice used for two-photon imaging, the cerebral microcirculation was also imaged at least with a 1-day difference, using an OCT system to assess microvascular density.
Animals were anesthetized as described with isoflurane (2-2.5% for induction, 1.5-2% for maintenance), and their heads were fixed using a stereotaxic frame as described above.
The OCT imaging system (VEG220, Thorlabs), utilizes a wavelength-swept laser as its light source, which centered at 1,310 nm, with 100-nm bandwidth. The system had a wavelength-swept frequency of 200 KHz, with the output power of 17 mW incident onto the cronic cranial window (29, 30).
To acquire images of blood vessels in mouse cerebral cortex, the scanning protocol was optimized for OCT-based optical microangiography (OMAG) algorithm, shown in Fig. 2B. In the fast transverse scanning direction (x), 576 A-lines covering a distance of ∼2.17 mm constituted one B-scan frame. In the slow scanning axis (y), 512 sampling positions also covering a distance of ∼2.17 mm were used to capture a three-dimensional (3-D) data set.
To assess in vivo volumetric angiography, eight repeated B-frames at each location were obtained. In this scanning protocol, a complete 3-D scan data cube was composed of 832 by 576 by 4,096 (z-x-y) voxels, which took ∼31.785 s to acquire the entire data set. To be consistent with the two-photon data, images from depth range of 50–150 µm were selected for processing. To extract the blood vessels, speckle variance (SV) of the eight B-scans was estimated as described previously (31, 32). The sorted, summed intensity projection (sSIP) algorithm was then appled to the SV along each A-line and mapped to an enface blood vessels map. Finally, a Median-blur filter (with 3 × 3 kernel) was applied to denoise and smooth the obtained enface projection maps.
Longitudinal Assesment of Brain Capillarization and BBB Permeability
OCT and two-photon microscopy were used for recurrent imaging of cerebral vasculature through the chronic cranial window. With both microscopy methods, the somatosensory cortex was imaged as described above. After a first baseline measurement, animals were subjected to recurring recordings with indicated time intervals (2, 4, 34, and 36 wk). Anatomic landmarks of the vasculature were explored around the target area, which is essential to find the same area for the subsequent recording. After measurements, image subtraction analysis was used for quantification of the permeability, then all data were compared with the baseline values per tracer to reveal potential differences.
Statistics
Unless stated otherwise, data are presented as means ± SE or all measured values as a box plot with interquartile distributions and median values. Analysis was made with SigmaPlot software version 12.3 (Systat Software, Inc.). Student’s two-sample t test or two-way ANOVA with Fisher’s least significant difference post hoc test or two-way ANOVA were performed to comparing the experimental results. Differences were considered significant at P < 0.05. All data are derived from at least three independent measurements (n ≥ 3, exact “n” numbers are indicated in figure legends). Levels of significance are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
Demonstration of Age-Related Microvascular Rarefaction by Two-Photon Microscopy and OCT
In the present study, we compared microvascular densities assessed using two-photon microscopy and OCT in different tissue layers.
For determining the vascularization of the young and aged mouse brain cortex, we used a previously described computational method implemented in a FIJI (ImageJ) plugin called Vessel Analysis (33), with modifications to make it compatible with two-photon microscopy and OCT images. Briefly, z-stack two-photon microscopy images from the WGA-A594 channel and OCT recordings went through dimension reduction by maximum-intensity projection, and then different filters were applied to enhance the contrast and remove noise and outliers from the images. Finally, images were converted into binary, and pixel values were measured and visualized as colored skeletons of the vascular network (Fig. 3, A and B). Skeletonization (skeleton extraction from a digital binary picture) provides region-based shape features. The vessel skeletonization method has the potential advantage that a higher abundance of larger vessels in the imaged field can bias the analysis of the binary images, whereas vascular density parameters derived from analysis of skeletonized images of the vascular network are independent of the size distribution of vessels in the VOI.
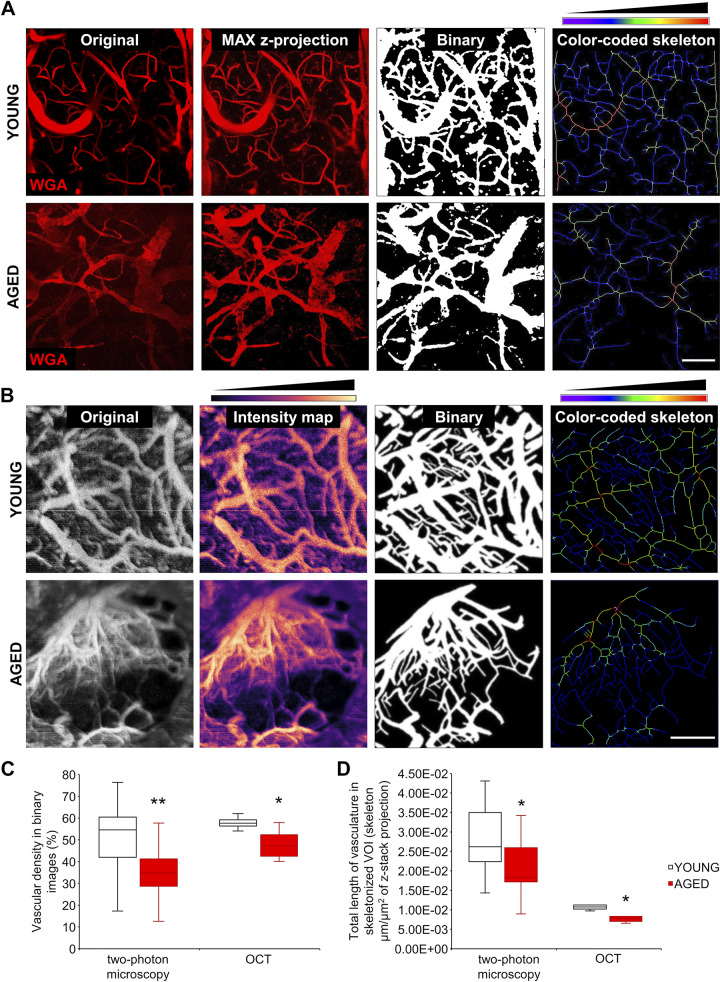
Figure 3.
Demonstration of age-related cerebromicrovascular rarefaction by intravital two-photon microscopy (MP) and optical coherence tomography (OCT). A: segmentation of blood vessels in two-photon images. Original z-stack images captured in brains of young and aged mice (red fluorescence: wheat germ agglutinin (WGA)-Alexa 594 staining of the glycocalyx of the endothelial cells) were maximally projected and processed with a modified macro from a published ImageJ plugin (see materials and methods). Thresholded images and color-coded skeleton images were used to calculate indexes corresponding to microvascular density. In the color-coded skeleton images, the pixel colors correspond to the relative vascular diameters. Scale bar, 100 μm. B: segmentation of blood vessels in brain parenchyma on OCT images. Original z-stack images acquired in brains of young and aged mice were maximally projected and processed with the same macro as the two-photon images. Thresholded binary images and color-coded skeletons were used to calculate indexes corresponding to microvascular density. Scale bar, 1 mm. C: quantification of the vascular density index, which is the ratio of the vasculature area to total imaged area (volume of interest, VOI). Aging was associated with a significant decline in the vascular density index, calculated on the basis of both the two-photon images and the OCT images (MP: P = 0.0022, n = 26/12; OCT: P = 0.0235, n = 5/5). D: quantification of the vascular length density index, which is the total length of vasculature in skeletonized images in skeleton of the z-stack projection image area (μm/μm2). Aging was associated with a significant decline in the vascular length density index, calculated either using the two-photon images or the OCT images (MP: P = 0.01, n = 25/20; OCT: P = 0.016, n = 5/5). Data are presented as interquartile distributions with median. *P < 0.05, **P < 0.01 compared with young group.
Using both two-photon microscopy and OCT, we found that vascular density was significantly decreased in the aged cortex compared with young controls (Fig. 3C). Vascular length densities (total length of vasculature in skeletonized VOI) were also found to be decreased with age, using both the two-photon and the OCT datasets (Fig. 3D). The apparent differences in the absolute results obtained with two-photon microscopy and OCT are related to the fact that two-photon microscopy has higher resolution compared with OCT.
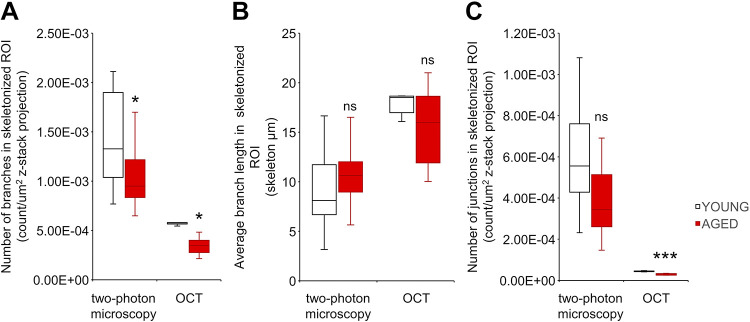
Using the vessel skeletonization method, we also assessed additional derivative parameters that could be used to characterize capillarization, including branching patterns. Figure 4, A–C, shows the count of branches, the average length of branches, and the number of junctions on the skeletonized images, respectively. Analyses of branching patterns confirmed an age-related decline in cerebral vascularization, extending the findings obtained using the methodologies presented in Fig. 3.
Figure 4.
Comparison of two-photon and optical coherence tomography (OCT) imaging-based indexes relevant for age-related cerebromicrovascular rarefaction. To evaluate the usefulness of additional imaging-based indexes relevant for age-related cerebromicrovascular rarefaction in mice, branching patterns on skeletonized images were analyzed. Comparison of indexes representing number of branches (MP: P = 0.038, n = 25/20; OCT: P = 0.008, n = 5/5) (A) and number of junctions (MP: P = 0.06, n = 25/20; OCT: P = 0.00023, n = 5/5) (C) showed significant age-related declines. In contrast, the index representing average branch lengths (MP: P = 0.34, n = 25/20; OCT: P = 0.58, n = 5/5) (B) did not differ between the two groups. Data are presented as interquartile distributions with median. *P < 0.05, **P < 0.01, ***P < 0.001 compared with young group.
Age-related microvascular rarefaction, which predominantly affects capillary-size microvessels critical for the BBB, can cause a calculation anomaly in the vascular permeability measurement. Thus, we used separate formulas for volume measurement-based permeability and single-microvessel permeability measurement.
Aging-Induced BBB Permeability Increase as Assessed with Image Subtraction Analysis
In the case of large z-stack recordings, the issues of comparability of recordings obtained at different time points and possible motion artefacts had to be solved. To that end we used an ImageJ plugin Vessel Analysis, which with minor modifications was compatible with our two-photon images. The main phases of the vessel segmentation approach are illustrated in Fig. 2A. A representative z-stack image and the ImageJ script used can be requested from the corresponding author via email (as a .zip file). With this method, we could define the vascular network in each multichannel image from WGA labeling. This solved the problem associated with minor displacements during the administration of tracers and enabled subsequent remeasurement of permeability for comparison purposes.
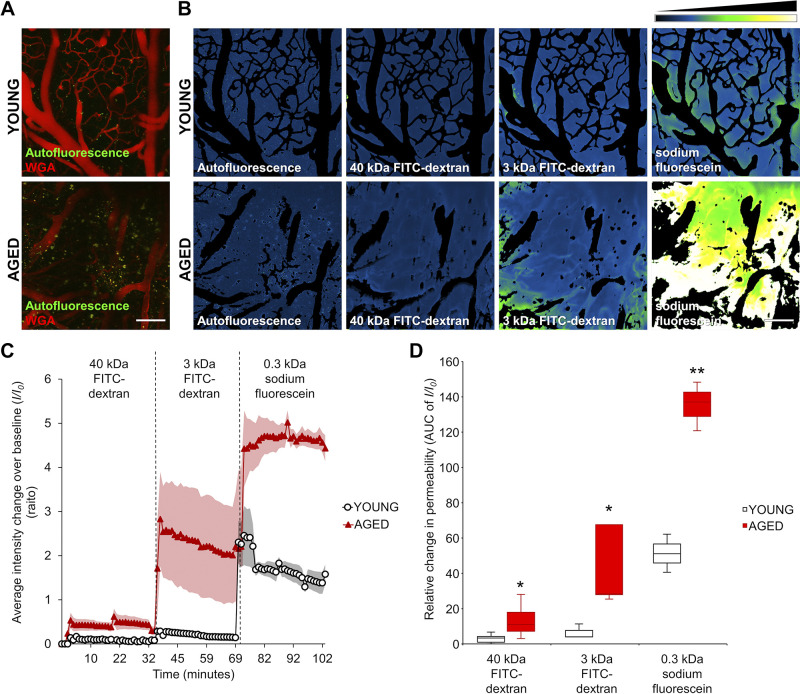
With the help of the segmentation of the vasculature, the relative permeability of the tracers could be measured. Figure 5A shows original z-stacks obtained in brains of young and aged mice before the measurement. On median summarized z-stack projections, the intensity change was visualized after administration of different size tracers. The same imaging area was used for measuring each tracer in the same animal (Fig. 5B). Figure 5C shows background-corrected fluorescent intensity changes over time in the brain parenchyma in young and aged mice after the administration of different size tracers. As a measure of relative vascular permeability, the AUC of normalized intensity changes as a function of elapsed time (32 min/tracer) was calculated in both groups. These AUC/relative permeability values for 40-kDa FITC-dextran, 3-kDa FITC-dextran, and sodium fluorescein (0.332 kDa) measured in young and aged mice are shown in Fig. 5D. We found that for each tracer the cerebral microcirculation of aged animals exhibited significantly increased permeability. The permeability differences were inversely proportional to the size of the tracers: the largest age-related permeability increase was evident for the lowest-molecular-mass tracer (sodium fluorescein). This observation is consistent with the concept that age-related disruption of the BBB is predominantly due to the increased paracellular permeability (34).
Figure 5.
Demonstration of age-related blood-brain barrier (BBB) disruption by two-photon imaging-based measurement of microvascular permeability to fluorescent tracers in a volume of brain tissue. A: z-stack projection of cerebral vasculature in young and aged mice. Red fluorescence: wheat germ agglutinin (WGA)-Alexa 594 staining of the glycocalyx of the endothelial cells. Green autofluoresent background in the tracer channel is shown. Scale bar, 100 μm. B: changes in tracer fluorescence intensity in the extravascular space and brain parenchyma of young and aged animals upon injection of the fluorescent tracers of different molecular mass. Images captured subsequent to tracer administrations were maximally projected (z-stack), and subtraction of the images of WGA-Alexa 594-stained vasculature was performed. Intensity plots derived from median projection of time stacks of the parenchymal recordings show increased extravasation of tracers of different molecular masses in brains of aged mice compared with those of young mice. Relative fluorescence intensity scale is shown at top right. Scale bar, 100 μm. C: time course of sequential increases in fluorescence intensity in the extravascular space in a volume of brain parenchyma upon injection of the fluorescent tracers of decreasing molecular mass, indicating tracer extravasation. Note the increased extravasation of fluorescent tracers in brains of aged mice compared with those in young controls. Note the more pronounced extravasation of tracers with decreasing molecular mass. Bolus injection of tracer was followed by a relatively fast increase in fluorescence intensity, which then reached a plateau phase. Data are presented as means ± SE. D: semiquantitative analysis of microvascular permeability to fluorescent tracers with different molecular masses in brains of young and aged mice. For the purpose of this analysis, the respective areas under these 32-min-long interval curves (AUC) presented in C were used as indexes for relative permeability of the microvasculature to each of the tracers injected. The AUC-based permeability indexes were calculated for both groups and are presented as a boxplot (interquartile distributions with median) (young vs. aged animals 40 kDa: P = 0.0427, young n = 5, aged n = 6; 3 kDa: P = 0.036, young n = 3, aged n = 5; SF: P = 0.0011, young n = 3, aged n = 3; note that animals with saturated staining were removed for correct quantification). Note the increased permeability for each different-sized tracers in aged animals compared with that in young mice. *P < 0.05, **P < 0.01 compared with young group.
Age-Dependent Increase in the Apparent Solute Permeability of Single Microvessel and in the Absolute Cumulative Permeability of Microvessels within a Volume of the Brain
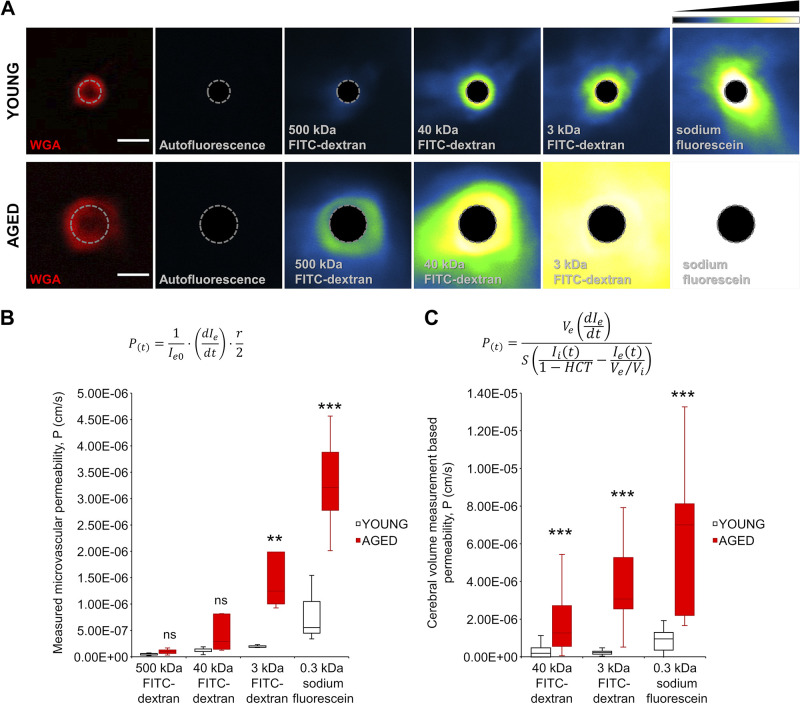
The most precise method of assessing vascular permeability is the single-vessel measurement. To demonstrate the sensitivity of our two-photon microscopy-based approach to detect transient leakage in a single microvessel, we treated the animals with 20% mannitol after FITC-dextran 500 kDa administration. Brain vasculature of the upper layers of the cortex (0–150 μm depth) were imaged (Fig. 6A) before (top) and after (bottom) the mannitol treatment. Note that mannitol caused a transient, localized leakage of the tracer. These perivascular intensity changes in single microvessel were assessed for quantification purposes. Based on the idea of Lauritzen and coworkers (21), WGA delineates the vessel lumen, enabling the differentiation of intraluminal and extraluminal spaces (Fig. 6, B and C), which makes it possible to perform absolute permeability measurements.
The measured microvascular permeabilities to the fluorescent FITC-dextran probes through the BBB in young and aged mice are shown in Fig. 7, A and B. A trend for increased microvascular permeability to 500-kDa FITC-dextran and to 40-kDa FITC-dextran was discernible in the aged mouse brains compared with young ones, although the differences did not reach statistical significance. We found significant age-related increases in microvascular permeability to 3-kDa FITC-dextran and 0.3-kDa sodium fluorescein. For quantitative comparison, permeability was also calculated based on the intensity functions (AUC values) and the area fractions for every single time point obtained during the image subtraction analysis using the formula Eq. 2 (Fig. 7C). Of note, the permeability data obtained by the two methodologies showed excellent correlation and identical trends. The numerical values of microvascular permeability obtained through single-microvessel measurement tended to be lower compared with the permeability values obtained through parenchymal volume measurement (Table 1). We attribute these differences to the presence of larger veins with higher permeability in the volume recordings. Table 1 shows that for young animals the permeability parameters calculated using our methods show an excellent agreement with data from the literature.
Figure 7.
Comparison of blood-brain barrier (BBB) permeability to different size tracers in young and aged mice. A: representative two-photon images showing measurement of the permeability of single microvessels in brains of young and aged animals. First panels show cross-sections of microvessels (red fluorescence: wheat germ agglutinin (WGA)-Alexa 594 (WGA-A594) staining of the glycocalyx of the endothelial cells; autofluorescence: baseline fluorescence in the tracer channel; dashed line: border between intravascular and extraluminal compartments based on WGA-Alexa 594 (WGA-A594) signal maximum) in brains of a young and an aged animal. Recorded images were average projected at time points corresponding to the time periods postadministration of fluorescent tracers of different molecular masses. Shown are average signal intensity projections by tracers in single microvessels in the young and aged mouse brains. Note the increased extravasation of tracers of different molecular masses in brains of aged mice compared with those of young mice. Relative fluorescence intensity scale is shown at top right. Note that in case of sodium fluorescein (SF) the intensity in aged animals was often over the dynamic range. Scale bar, 4 μm. B: boxplots are summary data\ showing increased calculated microvascular permeability in the aged mouse brain compared with young controls. After image analysis, the permeability was calculated with the formula indicated above the boxplot, where Ie0 is baseline extravascular intensity; dIe/dt is the slope of the intensity shift after tracer administration (see Fig. 6E); and r is the radius of the vessel calculated from the maximum intensity based on the radial intensity profile of WGA-A594-stained images. All data are presented as interquartile distributions with median (young vs. aged animals 500 kDa: ns, young n = 5, aged n = 4; 40 kDa: ns, young n = 6, aged n = 5; 3 kDa: P = 0.009, young n = 3, aged n = 4; SF: P = 0.00002, young n = 3, aged n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 young vs. aged. C: boxplots are summary data for cumulative microvascular permeability, calculated on the basis of two-photon imaging of extravasation of fluorescent tracers in a volume of brain tissue (see Fig. 4). After image analysis, the permeability was calculated with the formula indicated above the boxplot, where Ve is the extravascular volume; Vi is the intravascular volume, both calculated based on vascular density in the volume of interest (VOI); dIe/dt is the slope of the intensity shift after tracer administration; S is the approximation of the surface area of the vasculature also calculated from the vascular density of the VOI; Ii (t) is the normalized intravascular fluorescent intensity at an exact time point; Ie(t) is the normalized extravascular or parenchymal fluorescent intensity at an exact time point, both based on the measured integrated density of the analyzed images VOI; and HCT is the hematocrit value, what was taken as constant at 0.42 based on data from the literature. All data are presented as interquartile distributions with median [(young vs. aged animals 40 kDa: P < 0.000001 young n = 48 (from 4 animals), aged n = 90 (from 6 animals); 3 kDa: P < 0.000001, young n = 45 (from 3 animals), aged n = 58 (from 4 animals); SF: P < 0.000001, young n = 44 (from 3 animals), aged n = 45 (from 3 animals)]. Please note that measurement artifacts as outliers of the curve in given time points were removed from the analysis to evade false-positive significance). **P < 0.01, ***P < 0.001 aged vs. young.
Table 1.
Comparison of BBB permeability values obtained in brains of young and aged mice by use of intravital two-photon microscopy with data from the literature
| Molecular Mass of Tracers, kDa | Volume Imaging (Current study) |
Single Microvessel (Current study) |
Single Microvessel(Kutuzov et al. 2018) |
Single Microvessel(Shi et al. 2014) |
||
|---|---|---|---|---|---|---|
| Young | Aged | Young | Aged | Young | ||
| 500 | 0.54 ± 0.09 | 0.95 ± 0.28 | ||||
| 40 | 1.85 ± 0.52 | 12.67 ± 4.89 | 1.01 ± 0.21 | 2.91 ± 1.57 | 1.40 ± 0.15 | 1.15 ± 0.23 |
| 3 | 2.32 ± 0.41 | 30.68 ± 5.56 | 1.85 ± 0.18 | 12.91 ± 6.13 | ||
| 0.3 | 9.51 ± 0.94 | 70.01 ± 5.19* | 5.54 ± 3.71 | 32.12 ± 4.4* | 3.91 ± 0.41 | 12.92 ± 3.59 |
Values are means ± SE Measured blood-brain barrier (BBB) permeability by two-photon microscopy, P (10−7 cm/s). Calculated permeability values for fluorescent tracers with different molecular masses in brains of young and aged mice are shown. Data obtained by using both volume imaging and single-microvessel imaging are shown. data obtained in young mice from the literature are shown for comparison. Note that the absolute permeability data for 40-kDa tracer obtained both by volume imaging and by single-microvessel imaging in young mice are virtually identical to the literature data. The permeability data for the 0.3-kDa sodium fluorescein tracer obtained using volume imaging and single-microvessel imaging are comparable to the data of Shi et al. (24) (obtained by analyzing postcapillary venules, 20-40 μm in diameter) and the data of Kutuzov et al. (21) (obtained by analyzing capillaries), respectively. *Note that in case of sodium fluorescein the intensity in aged animals was often over the dynamic range; therefore, precise measurement was not always possible.
Longitudinal Tracking of Vascular Density and BBB Permeability through a Chronic Cranial Window in the Same Animal
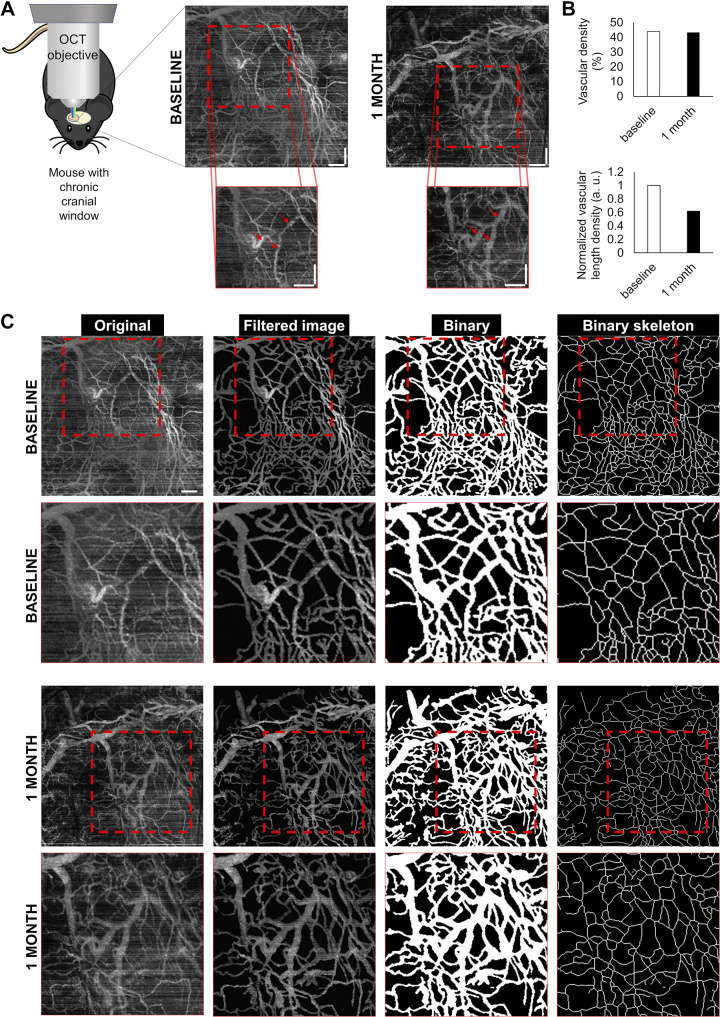
For longitudinal tracking of the vascularization and the BBB permeability, we optimized our methods and performed recurring measurements on the same animals at different time intervals. In these experiments, we used the same methodology as described above and assessed repeatability of the measurment. Mice equipped with chronic cranial windows were repeatedly imaged using OCT as described above (a representative measurement is shown in Fig. 8). For subsequent measurements, we returned to the same area of the brain (Fig. 8A), and quantified the vascular density (Fig. 8B). The main phases of the analysis are shown in Fig. 8C.
Figure 8.
Demonstration of the feasibility of longitudinal tracking of microvascular density with optical coherence tomography (OCT). A: intravital OCT imaging in mice equipped with chronic cranial window. Squares identify the same brain regions imaged at the two time points. After 1 mo, slight differences in vascular pattern are evident. Arrows in the bottom panels point to enlarged vessels. Scale bar, 250 μm. B: quantification of the vascular density index, which is the ratio of the vasculature area to total imaged area (volume of interest, VOI). Vascular density index and total length of the visible vasculature have not changed significantly. C: segmentation of blood vessels in brain parenchyma on OCT images. Original z-stack images were maximally projected and processed with the same macro as the two-photon images. Thresholded binary images and binary skeletons were used to calculate indexes corresponding to microvascular density. Bottom: magnification of the targeting area (dashed squares). Scale bar, 250 μm.
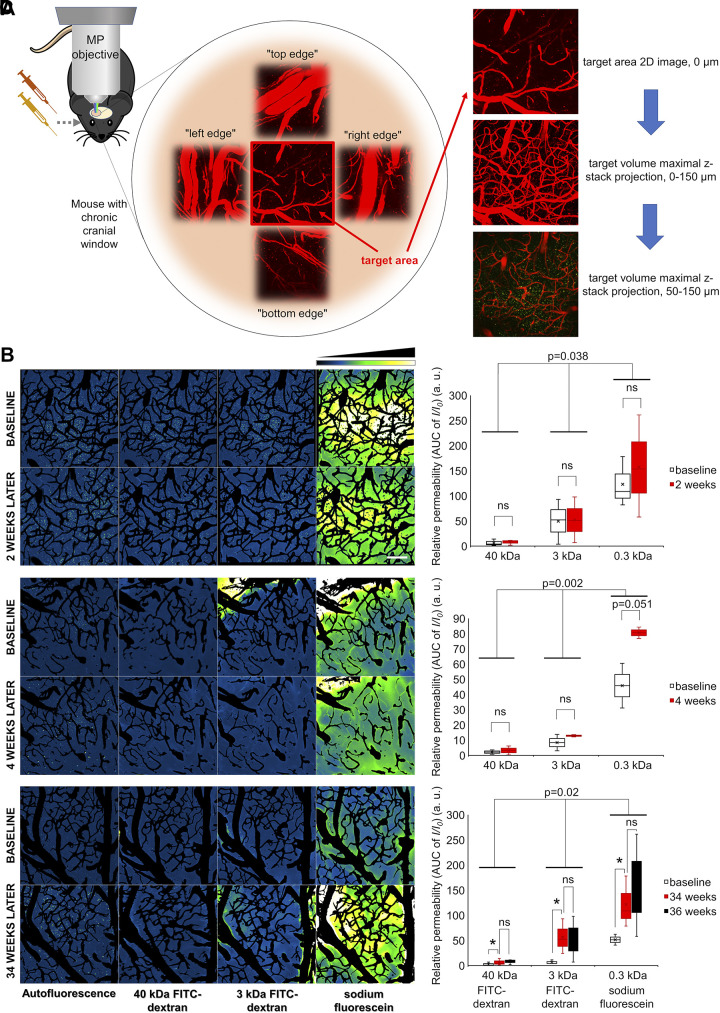
Longitudinal BBB permeability assessment was also achieved in mice equipped with chronic cranial windows (Fig. 9). Microvascular permeability was assessed in the same animals first at baseline and after 2, 4, 34, and/or 36 wk. Importantly, BBB permeability after a relatively short time period (2–4 wk) was unchanged (Fig. 9, B–D, right). Our chronic longitudinal self-controlled studies confirmed the results of the studies comparing groups of young and aged animals (Fig. 5), demonstrating that BBB permeability significantly increased over a 34-wk-long time period (Fig. 9D, right).
Figure 9.
Longitudinal tracking of blood-brain barrier (BBB) permeability. A: reconnaissance of vascular anatomy during intravital microscopy for longitudinal tracking. Target area was chosen, and surrounding vascular anatomy was recorded for determining targeting aids for recurring measurement. Target area was measured based on the image subtraction analysis method described above. B–D: longitudinal tracking of BBB permeability. Representative z-stack projections of the cerebral vasculature in the same brain area obtained at baseline and after a time interval of 2 wk (B), 4 wk (C), and 34 wk (D). Tracer fluorescence intensities in the extravascular space and brain parenchyma in the same area upon injection of the fluorescent tracers of different molecular mass are shown. Images captured subsequent to tracer administrations were maximally projected (z-stack), and subtraction of the images of wheat germ agglutinin (WGA)-Alexa 594 stained vasculature was performed. Intensity plots derived from median projection of time stacks of the parenchymal recordings are shown. Relative fluorescence intensity scale is shown at top right. Scale bar, 100 μm. Right: quantitative analysis of microvascular permeability to fluorescent tracers with different molecular masses in brains during longitudinal tracking. Relative permeability values were calculated and normalized for the first baseline measurement. Note that, after 2 (B, right) and 4 wk (C, right), there are no significant differences in tracer extravasation compared with baseline. In contrast, BBB permeability to each tracer significantly increased over a 32-wk-long time period (Fig. 9D, right; P = 0.04, n = 3). In pairwise comparison, there was significant difference between baseline and 34 mo (P = 0.04), but no significant difference between the data measured 2 wk later (P = 0.7). The results of two-way ANOVA revealing significant differences between time points and the different size tracers are indicated in the plots. All data are presented as interquartile distributions with median. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
The present study provides additional evidence that cerebral microvascular density is reduced and BBB is compromised in the aged mouse brain (34–39).
We have demonstrated that intravital two-photon microscopy-based methods can be successfully complemented by OCT-based measurement of microvascular rarefaction in the aged mouse brain. Although the spatial resolution of OCT was lower than that of two-photon microscopy, the microvascular density results obtained using the two distinct methodologies show the same tendencies. An important advantage of the OCT-based approach is that it provides structural information in larger tissue regions at greater depth than two-photon microscopy. It also does not require any special tracers, only anesthesia, which is an advantage for experiments requiring repeated measures in the same animals. It is a static measurement and the imaging part is relatively fast. However, the reconstruction and analysis of the recorded images are more complicated. We also compared our experimental results with the literature data available about vascular rarefaction in aging. Our results show an 18–35% decline in the measured vascular density (Table 2). These data correspond well to the literature. For assessment of vascular density, using OCT a higher throughput could be achieved than with two-photon microscopy.
Table 2.
Comparison of vascular density values obtained in brains of young and aged mice with data from the literature
| Age-Related Brain Vascular Rarefaction (Density Decline) | |||
|---|---|---|---|
| Species | Region | Change | Reference |
| Rat | Frontal, occipital cortex | ↓ | (40) |
| Rat | Frontal cortex | ↓ | (41) |
| Rat | Olfactory bulb | ↓ (15%) | (42) |
| Rat | Medial nucleus of the trapezoid body | ↓ (30%) | (43) |
| Rat | Whole brain | ↓ (10–30%) | (44) |
| Rat | CA1, parietal cortex | ↓ | (45,46) |
| Rat | Frontal, occipital cortex, hippocampus | ↓ | (47) |
| Mouse | Parietal cortex | ↓ (15%) | (22) |
| Mouse | Cerebral cortex | ↓ (25%) | (48) |
| Mouse | Parietal cortex | ↓ [18% (OCT), 35% (MP)] | Current study |
Age-related percentile changes in calculated murine cerebral vascular density values derived from the referenced studies and obtained in the current study are shown. MP, two-photon microscopy; OCT, optical coherence tomography.
In this study, three different measurement and calculation methods were used to determine cerebral vascular permeability. In single-vessel measurements, the radius of the microvessels based on the glycocalyx was included in the formula Eq. 1 to calculate vascular permeability. We solely compared capillary size vessels up to 8 µm in diameter. In case of relative and absolute cumulative permeability within a volume of the brain, we used different calculations. In relative permeability comparisons, vessels volume could be eliminated with the image subtraction; thus, the analysis focused on the fluorescent intensity change. In case of absolute quantification of cumulative permeability, we included the vascular area in the analysis (in the formula Eq. 2, Ve ∼ the extravascular volume, Vi ∼ the intravascular volume). These parameters were calculated on the basis of vascular density within the VOI at each time point during the measurement. This method corrects for changes in vessel size and density, eliminating calculation errors related to age-related microvascular rarefaction. In contrast, we did not include the glycocalyx partition coefficient in the calculation. To the best of our knowledge, to date only Kutuzov and colleagues have calculated exactly the glycocalyx partition coefficient and flux separately with similar method to determine the contribution of the different constituents of the BBB to the permeability (21). This aspect of the BBB analysis was beyond the scope of the present article.
On the basis of our results, we propose that both single-vessel imaging and parenchymal volume imaging are useful methods for determination of age- and/or treatment-dependent changes in BBB permeability. Both methods have their own advantages and disadvantages. Single-vessel imaging is very precise, with low variability; however, it requires the measurement of a larger number of microvessels. Parenchymal volume imaging is a very robust method, but it may yield data with somewhat higher variability. In our opinion, parenchymal volume imaging is an easier method, and in combination with the permeability calculation method used it can provide reliable data that can be used both for longitudinal comparisons (e.g., as an outcome measure for an interventional study) and for between-group comparisons. To demonstrate this potential of our method, we included a longitudinal experimental cohort in our studies. Our longitudinal studies demonstrate that by using our methods it is feasible to assess age-related changes in BBB permeability in the same animal equipped with a chronic cranial window. Of note, the image quality was excellent even after 8 mo post-implantation of the high-quality cranial windows. If the cranial windows are fixed properly with the dental acrylic, they are stable for a relatively long time; thus, the animals can be followed for a long period of time. The time window for optimal assessment of BBB using two-photon imaging was not limited by bone regrowth due to the relatively large craniotomy area and the thorough thinning of the skull. It should be noted that it is necessary to allow an adequate recovery period (over 14 days preferred) after the surgery. It is possible to perform OCT recording and assess BBB permeability in the same animal, with at least 7–10 days between the two measurements, to allow for clearance of the tracers from the brain parenchyma. Increased background fluorescence due to residual fluorescence from tracers from a previous measurement can be subtracted during image analysis, as we have demonstrated.
We propose the use of the single-vessel method for BBB assessment if the experimental design requires avoidance of possible involvement of venular permeability. Yet, it should be noted that, in case of an increased permeability of the BBB to smaller tracers, the high background can make the single-vessel measurement challenging. Note that, as a limitation of the study, in aged animals in the case of sodium fluorescein, the signal intensity was often over the dynamic range. Compensation with laser or detector power was not feasible, because the intensity difference between the fluorescence of the extravasated sodium fluorescein and the baseline was greater than the dynamic range of the method used. In similar cases, parenchymal volume imaging is the method of choice.
A potential concern is that the volume of the injected tracers may be significant compared with the blood volume of the animal. Theoretically, larger acute increases in arterial blood pressure beyond the autoregulatory capacity of cerebral blood vessels can produce changes in permeability of the BBB (49). However, previous studies have demonstrated that temporary blood pressure increases associated with the injection of the volume of fluid used in the present study are well within the autoregulatory range of cerebral blood flow (50–52). Thus, it is unlikely that BBB function was affected by injection of the fluorescent tracers per se.
Age-related BBB disruption is a universal phenomenon, as it has been demonstrated in humans (8, 9) and nonhuman primates (53) as well as in canine (54) models of aging. The BBB is a functional part of the neurovascular unit, and its main cellular components are the cerebromicrovascular endothelial cells (CMVECs), brain pericytes, and astrocytic endfeet (55, 56). The basement membrane and the endothelial glycocalyx constitute noncellular components of the BBB (57). There is increasing evidence that the BBB exhibits progressive age-related deterioration due to multifaceted structural, cellular, and molecular deficits in the neurovascular unit. Although aging may affect each component of the BBB, our current understanding is that age-related changes in CMVECs play a critical role in age-related BBB disruption (55). Tight junctions interconnecting the CMVECs (58) help to maintain the low paracellular diffusion of solutes through the endothelial layer. These junctions are composed of several special transmembrane and plaque proteins, including claudin-1, claudin-5, and claudin-12, occludin, junctional adhesion molecules (JAMs), and the membrane-associated guanylate kinases tight-junction proteins ZO1 and ZO2. Endothelial cells are also connected by adherens junctions, which are composed of cadherins, catenins, and platelet and endothelial cell adhesion molecule 1. There is evidence that in advanced aging expression of multiple junctional proteins is dysregulated (35, 37, 59, 60). Aging-induced downregulation of ATP-binding cassette transporters (ABC transporters) has also been linked to BBB disruption (61). Pericyte loss/damage in the aging brain (55, 62–65) has also been implicated in exacerbation of age-dependent BBB breakdown. Potential cellular and molecular mechanisms of aging that may alter endothelial phenotype and/or cause pericyte damage and thereby promote BBB disruption, including increased oxidative stress and mitochondrial dysfunction, heightened inflammatory status (including upregulation of components of the innate immune system), increased presence of senescent cells in the aged neurovascular unit, circulating IGF-I deficiency, decreased activity of sirtuins (37), reduction of platelet-derived growth factor receptor-β expression, and pericyte coverage and increased matrix metalloproteinase activity (34). Additionally, both hypertension (38) and increased venous congestion (66), which are also frequently present in older adults (e.g., in the presence of heart failure), can contribute to BBB disruption. The combination of these factors may often result in BBB disruption of different severity in mouse models of aging.
Through the disrupted BBB plasma constituents (including immunoglobulins, thrombin, fibrinogen) microbial agents [e.g., highly inflammatory pathogen-associated molecular patterns (PAMPs), and also internal alarmins (damage-associated molecular patterns/DAMPs)] can enter the brain and trigger inflammatory and immune responses that impair synaptic functioning, information processing, and neuronal connectivity and contribute to neurodegeneration. The severity of BBB disruption determines the size of molecules that can leak into the brain parenchyma. We used a combination of different-sized, fluorescently labeled tracers (from 500 kDa to 0.3 kDa), which is an adequate approach for qualitative and quantitative assessment of the severity of BBB disruption, predicting possible sequelae of the entry of different serum-derived factors in the brain. CMVECs do not have specific transport systems for dextran-linked tracers, and the main route of their transport is paracellular. Pinocytosis also does not play a significant role in CMVECs. Thus, our experimental approach primarily enables a detailed characterization of age-dependent changes in paracellular BBB permeability in the mouse brain. We found that in the aged mouse brain an increased permeability to the 500-kDa tracer and the 40-kDa dextran tracer was discernible. This finding has pathophysiological relevance, as leakage of higher molecular mass solutes such as plasma IgGs and fibrinogen (150–400 kDa) in the aged mouse brain has been causally linked to microglia activation and heightened state of neuroinflammation (38, 39, 67). Additionally, previous studies show that permeability to plasma IgGs is significantly exacerbated by comorbid hypertension or obesity in aged mice (38, 39). Increased permeability to larger molecules (over ∼50–60 kDa) usually indicates increased transcellular transport or compromised integrity of the vascular wall. Importantly, the molecular masses of inflammatory cytokines [e.g., IL-1β, IL-1α, IL-6, IL-18, TNFα (68)] and microbial breakdown products are between 5 and 60 kDa; thus, leakage of these proinflammatory factors through the BBB likely increases in the aging brain. We found prominent age-related paracellular BBB disruption using both the 3-kDa dextran tracer and the 0.3-kDa fluorescein tracer, consistent with the idea that in aging the BBB becomes permeable to a wide variety of small molecules, including small proteins, peptides, and toxins. We propose that because of its sensitivity the method used can be adapted for experiments assessing both exacerbated breakdown of BBB in comorbid conditions and cerebromicrovascular protective effects of antiaging interventions.
With recent developments in the field of VCID and geroscience research, there is an urgent need to evaluate the rejuvenating effects of novel antiaging treatments on multiple domains of cerebrovascular health. By use of our two-photon microscopy-based method, sensitive analysis of cortical cerebromicrovascular density can be performed in parallel on the same mice used for the BBB integrity measurements as an additional end point for cerebromicrovascular health. Using this method, we demonstrated that aging is associated with significant microvascular rarefaction in the mouse cortex, extending previous findings in laboratory rodents (22, 38, 64, 69–71). Microvascular rarefaction contributes to age-related decline in cerebral blood flow and predicts cognitive decline (2, 64, 71). The mechanisms contributing to age-related microvascular rarefaction likely include impaired angiogenic potential of aged cerebromicrovascular endothelial cells (10, 70, 72–74), increased endothelial apoptosis (75), age-related decline in angiogenic growth factors [e.g., IGF-I (76), pituitary adenylate cyclase-activating polypeptide (77)] and other circulating factors (78), reduction of cellular NAD+ levels (79) and increased endothelial senescence (80, 81). A major advantage of our methodology is that, by identification of anatomic landmarks (branching patterns of the microvascular tree), repeated measurements in the same anatomic region is feasible in longitudinal studies evaluating the effects of antiaging interventions targeting the aforementioned pathways.
In conclusion, we have quantified age-related cerebrovascular rarefaction with two different intravital imaging techniques. Additionally, we have accurately determined age-related increases in BBB permeability to four different-sized tracers in vivo. We have also demonstrated that the intravital two-photon microscopy- and OCT-based imaging methods described in our study enable longitudinal assessment of changes in BBB permeability and brain capillarization in aged mice. There is a growing understanding of the role of shared mechanisms of aging in cardiovascular and cerebrovascular aging (82), including the role of NAD+ depletion and energetic dysfunction, sirtuin 1 dysregulation (37), mitochondrial alterations (83, 84), altered mammalian target of rapamycin (mTOR) signaling (85–87), cellular senescence (88–91), nuclear regulatory factor 2 (Nrf2) dysfunction (92), impaired proteostasis (93), inflammasome activation (94), sterile inflammation (95), dysregulation of the renin-angiotensin system (96), altered Wnt signaling (97), Klotho (98), changes in autophagy (99), and amyloid and tau toxicity (100, 101). There is increasing evidence that several treatments targeting these pathways can improve endothelial and microvascular health and may thereby potentially rejuvenate the neurovascular unit and rescue cerebral blood flow regulation in murine models of aging. These include antioxidants (102) and agents conferring mitochondrial protection (103, 104), Nrf2 activators (92), and other anti-inflammatory agents (105), senolytics (106), Poly [ADP-ribose] polymerase-1 (PARP-1) inhibitors (16), drugs impacting mTOR signaling (87), metformin (107), melatonin (108), polyphenols (109, 110), IL-37 (111), agents interfering with the renin-angiotensin system (13, 112), and IGF-I (76, 113–116) as well as dietary interventions (72) and exercise regimens (117). Treatments that restore cellular NAD+ levels show great promise for endothelial (15, 118, 119) and neurovascular rejuvenation (15, 120), reducing endothelial oxidative stress (15), improving endothelial angiogenic capacity (79), rescuing mitochondrial function (15), and reversing endothelial transriptomic changes (120). Other interventions were also shown to improve endothelial function (121–124), attenuate neuroinflammation, and/or improve cognitive function in aged rodents. In contrast, there is a range of potentially preventable pathological conditions that accelerate cerebromicrovascular aging, including diabetes mellitus, obesity (39, 64, 67, 125), hypertension (38, 76, 116, 126), and accelerated DNA damage-induced senescence (106). It is also likely that chronic endotheliopathy associated with chronic viral infections [including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 (127, 128) and cytomegalovirus (129, 130)] also results in accelerated microvascular aging. Future studies should methodologically investigate the effects of the aforementioned treatments and conditions on BBB integrity and brain capillarization as well. The proposed methods can be readily incorporated in the design of future longitudinal studies for complex characterization of the effects of these interventions and other novel anti-geronic treatments on cerebromicrovascular health span and studies testing antiaging interventions in mouse models of accelerated brain aging, including models that exhibit increased cellular oxidative stress and increased neuroinflammation (131–133). There is growing evidence that cerebomicrovascular impairment, including BBB disruption play a pathogenic role in AD as well (12). The proposed methods will also be useful in experimental studies in mouse models of AD (19, 134, 135), testing the efficacy of interventions on this clinically relevant outcome measure.
GRANTS
This work was supported by Oklahoma Center for the Advancement of Science and Technology Grants (to A.C., A.Y., and Z.U.) and HR19-062 (to Q.T.), National Institutes of Health (NIH) Grants R01-AG047879, R01AG055395, R0AG068295, R01-NS100782, R01CA255840-01), and GM104938 (to A.Y.), Presbyterian Health Foundation National Institute on Aging-supported Geroscience Training Program in Oklahoma Grant T32AG052363, Oklahoma Nathan Shock Center Grant P30AG050911, Cellular and Molecular GeroScience CoBRE Grant 1P20GM125528 (sub. no. 5337), a Junior Faculty Fellowship from University of Oklahoma (to Q.T.), and Nemzeti Kutatási Fejlesztési és Innovációs Hivatal (National Research, Development and Innovation Office) Grants 132638 and 135425 projects, and a Nemzeti Szivlabor grant).
DISCLAIMERS
The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C., Z.U. and S.T. conceived and designed research; Á.N., J.D., F.Y., P.B., A.Y. and C.A. performed experiments; Á.N., A.E.F., Q.T., Z.U., S.T., J.D., F.Y., A.Y. and T.K. analyzed data; Á.N., Q.T., A.C., Z.U. S.T., F.Y. and A.Y. interpreted results of experiments; Á.N., F.Y. and A.Y. prepared figures; Á.N., A.L., Q.T., Z.U., S.T. and T.C. drafted manuscript; Á.N., A.L., A.E.F., I.W., I.A.K., Q.T., A.C., Z.U., S.T., J.D., F.Y., P.B., A.Y., C.A., T.K. and T.C. edited and revised manuscript; Á.N., A.L., A.E.F., I.W., I.A.K., Q.T., A.C., Z.U., S.T., J.D., F.Y., P.B., A.Y., C.A., T.K. and T.C. approved final version of manuscript.
REFERENCES
- 1.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P. Vascular cognitive impairment. Nat Rev Dis Primers 4: 18003, 2018. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 2.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild E, Palm WM, Hoff EI, Wardlaw JM, Jansen JFA, Verhey FR, Backes WH. White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging 85: 113–122, 2020. doi: 10.1016/j.neurobiolaging.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Noe CR, Noe-Letschnig M, Handschuh P, Noe CA, Lanzenberger R. Dysfunction of the blood-brain barrier—a key step in neurodegeneration and dementia. Front Aging Neurosci 12: 185, 2020. doi: 10.3389/fnagi.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 42: 1183–1193, 2020. doi: 10.1007/s11357-020-00211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 42: 1751–1764, 2020. doi: 10.1007/s11357-020-00282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levit A, Hachinski V, Whitehead SN. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: the shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience 42: 445–465, 2020. doi: 10.1007/s11357-020-00164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25: 270–276, 2019. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: 555–565, 2018. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riphagen JM, Ramakers I, Freeze WM, Pagen LHG, Hanseeuw BJ, Verbeek MM, Verhey FRJ, Jacobs HIL. Linking APOE-ε4, blood-brain barrier dysfunction, and inflammation to Alzheimer's pathology. Neurobiol Aging 85: 96–103, 2020. doi: 10.1016/j.neurobiolaging.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14: 133–150, 2018. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, Lecrux C, Imboden H, Hamel E. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer's disease model. Neurobiol Dis 68: 126–136, 2014. doi: 10.1016/j.nbd.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulos P, Tong XK, Imboden H, Hamel E. Losartan improves cerebrovascular function in a mouse model of Alzheimer's disease with combined overproduction of amyloid-beta and transforming growth factor-β1. J Cereb Blood Flow Metab 37: 1959–1970, 2017. doi: 10.1177/0271678X16658489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol 24: 101192, 2019. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyúl-Tóth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly (ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience 41: 533–542, 2019. doi: 10.1007/s11357-019-00101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trigiani LJ, Lacalle-Aurioles M, Bourourou M, Li L, Greenhalgh AD, Zarruk JG, David S, Fehlings MG, Hamel E. Benefits of physical exercise on cognition and glial white matter pathology in a mouse model of vascular cognitive impairment and dementia. Glia 68: 1925–1940, 2020. doi: 10.1002/glia.23815. [DOI] [PubMed] [Google Scholar]
- 18.Trigiani LJ, Royea J, Tong XK, Hamel E. Comparative benefits of simvastatin and exercise in a mouse model of vascular cognitive impairment and dementia. FASEB J 33: 13280–13293, 2019. doi: 10.1096/fj.201901002R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer's disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol 314: H693–H703, 2018. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janiurek MM, Soylu-Kucharz R, Christoffersen C, Kucharz K, Lauritzen M. Apolipoprotein M-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife 8: e49405, 2019. doi: 10.7554/eLife.49405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A 115: E9429–E9438, 2018. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YD, Choi WJ, Wei W, Song SZ, Zhang QQ, Liu JL, Wang RK. Aging-associated changes in cerebral vasculature and blood flow as determined by quantitative optical coherence tomography angiography. Neurobiol Aging 70: 148–159, 2018. doi: 10.1016/j.neurobiolaging.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negrean A, Mansvelder HD. Optimal lens design and use in laser-scanning microscopy. Biomed Opt Express 5: 1588–1609, 2014. doi: 10.1364/BOE.5.001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Zeng M, Sun Y, Fu BM. Quantification of blood-brain barrier solute permeability and brain transport by multiphoton microscopy. J Biomech Eng 136: 31005, 2014. doi: 10.1115/1.4025892. [DOI] [PubMed] [Google Scholar]
- 25.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 98: 335–344, 2006. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Kang BM, Kim JH, Min J, Kim HS, Ryu H, Park H, Bae S, Oh D, Choi M, Suh M. Real-time in vivo two-photon imaging study reveals decreased cerebro-vascular volume and increased blood-brain barrier permeability in chronically stressed mice. Sci Rep 8: 13064, 2018. doi: 10.1038/s41598-018-30875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nhan T, Burgess A, Cho EE, Stefanovic B, Lilge L, Hynynen K. Drug delivery to the brain by focused ultrasound induced blood-brain barrier disruption: quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J Control Release 172: 274–280, 2013. doi: 10.1016/j.jconrel.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu BM, Adamson RH, Curry FR. Determination of microvessel permeability and tissue diffusion coefficient of solutes by laser scanning confocal microscopy. J Biomech Eng 127: 270–278, 2005. doi: 10.1115/1.1865186. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q, Nagaya T, Liu Y, Horng H, Lin J, Sato K, Kobayashi H, Chen Y. 3D mesoscopic fluorescence tomography for imaging micro-distribution of antibody-photon absorber conjugates during near infrared photoimmunotherapy in vivo. J Control Release 279: 171–180, 2018. doi: 10.1016/j.jconrel.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Q, Wang J, Frank A, Lin J, Li Z, Chen CW, Jin L, Wu T, Greenwald BD, Mashimo H, Chen Y. Depth-resolved imaging of colon tumor using optical coherence tomography and fluorescence laminar optical tomography. Biomed Opt Express 7: 5218–5232, 2016. doi: 10.1364/BOE.7.005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baran U, Wang RK. Review of optical coherence tomography based angiography in neuroscience. Neurophotonics 3: 10902, 2016. doi: 10.1117/1.NPh.3.1.010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai MT, Zhang JW, Wei KC, Yeh CK, Liu HL. Assessment of temporary cerebral effects induced by focused ultrasound with optical coherence tomography angiography. Biomed Opt Express 9: 507–517, 2018. doi: 10.1364/BOE.9.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elfarnawany M, Pinter SZ, Lacefield JC. Improved objective selection of power Doppler wall-filter cut-off velocity for accurate vascular quantification. Ultrasound Med Biol 38: 1429–1439, 2012. doi: 10.1016/j.ultrasmedbio.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Costea L, Meszaros A, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE, Krizbai IA. The blood-brain barrier and its intercellular junctions in age-related brain disorders. Int J Mol Sci 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68: 409–427, 2010. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatovic SM, Martinez-Revollar G, Hu A, Choi J, Keep RF, Andjelkovic AV. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis 126: 105–116, 2019. doi: 10.1016/j.nbd.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci 69: 1212–1226, 2014. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein AW, Michel ME. A morphometric study of the neocortex of young adult and old maze-differentiated rats. Mech Ageing Dev 6: 441–452, 1977. doi: 10.1016/0047-6374(77)90045-8. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson JH, Hopewell JW, Reinhold HS. A quantitative study of age-related changes in the vascular architecture of the rat cerebral cortex. Neuropathol Appl Neurobiol 7: 451–462, 1981. doi: 10.1111/j.1365-2990.1981.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 42.Hinds JW, McNelly NA. Capillaries in aging rat olfactory bulb: a quantitative light and electron microscopic analysis. Neurobiol Aging 3: 197–207, 1982. doi: 10.1016/0197-4580(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 43.Casey MA, Feldman ML. Aging in the rat medial nucleus of the trapezoid body. III. Alterations in capillaries. Neurobiol Aging 6: 39–46, 1985. doi: 10.1016/0197-4580(85)90070-3. [DOI] [PubMed] [Google Scholar]
- 44.Buchweitz-Milton E, Weiss HR. Perfused capillary morphometry in the senescent brain. Neurobiol Aging 8: 271–276, 1987. doi: 10.1016/0197-4580(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 45.Jucker M, Battig K, Meier-Ruge W. Effects of aging and vincamine derivatives on pericapillary microenvironment: stereological characterization of the cerebral capillary network. Neurobiol Aging 11: 39–46, 1990. [Erratum in Neurobiol Aging 11: 675, 1990]. doi: 10.1016/0197-4580(90)90060-d. [DOI] [PubMed] [Google Scholar]
- 46.Jucker M, Meier-Ruge W. Effects of brovincamine on stereological capillary parameters in adult and old Fischer-344 rats. Microvasc Res 37: 298–307, 1989. doi: 10.1016/0026-2862(89)90048-4. [DOI] [PubMed] [Google Scholar]
- 47.Amenta F, Cavallotti D, Del VM, Mancini M, Naves FJ, Vega JA, Zeng YC. Age-related changes in brain microanatomy: sensitivity to treatment with the dihydropyridine calcium channel blocker darodipine (PY 108-068). Brain Res Bull 36: 453–460, 1995. doi: 10.1016/0361-9230(94)00210-R. [DOI] [PubMed] [Google Scholar]
- 48.Hill LK, Hoang DM, Chiriboga LA, Wisniewski T, Sadowski MJ, Wadghiri YZ. Detection of cerebrovascular loss in the normal aging C57BL/6 mouse brain using in vivo contrast-enhanced magnetic resonance angiography. Front Aging Neurosci 12: 585218, 2020. doi: 10.3389/fnagi.2020.585218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayhan WG. Regulation of blood-brain barrier permeability. Microcirculation 8: 89–104, 2001. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 50.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin 34: 465–477, 2016. doi: 10.1016/j.anclin.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grubb S, Cai C, Hald BO, Khennouf L, Murmu RP, Jensen AGK, Fordsmann J, Zambach S, Lauritzen M. Precapillary sphincters maintain perfusion in the cerebral cortex. Nat Commun 11: 395, 2020. doi: 10.1038/s41467-020-14330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res 49: 375–389, 2012. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z, Zeng W, Sun J, Chen W, Zhang R, Yang Z, Yao Z, Wang L, Song L, Chen Y, Zhang Y, Wang C, Gong L, Wu B, Wang T, Zheng J, Gao F. The quantification of blood-brain barrier disruption using dynamic contrast-enhanced magnetic resonance imaging in aging rhesus monkeys with spontaneous type 2 diabetes mellitus. Neuroimage 158: 480–487, 2017. doi: 10.1016/j.neuroimage.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Su MY, Head E, Brooks WM, Wang Z, Muggenburg BA, Adam GE, Sutherland R, Cotman CW, Nalcioglu O. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging 19: 479–485, 1998. doi: 10.1016/S01974580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 55.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev 99: 21–78, 2019. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilhelm I, Nyúl-Tóth A, Suciu M, Hermenean A, Krizbai IA. Heterogeneity of the blood-brain barrier. Tissue Barriers 4: e1143544, 2016. doi: 10.1080/21688370.2016.1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, Nirwane A, Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc Neurol 4: 78–82, 2019. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bauer HC, Krizbai IA, Bauer H, Traweger A. You shall not pass—tight junctions of the blood brain barrier. Front Neurosci 8: 392, 2014. doi: 10.3389/fnins.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osada T, Gu YH, Kanazawa M, Tsubota Y, Hawkins BT, Spatz M, Milner R, del Zoppo GJ. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β(1)-integrins. J Cereb Blood Flow Metab 31: 1972–1985, 2011. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47: 1068–1077, 2016. doi: 10.1161/STROKEAHA.115.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erdő F, Krajcsi P. Age-related functional and expressional changes in efflux pathways at the blood-brain barrier. Front Aging Neurosci 11: 196, 2019. doi: 10.3389/fnagi.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bohannon DG, Okhravi HR, Kim J, Kuroda MJ, Didier ES, Kim WK. A subtype of cerebrovascular pericytes is associated with blood-brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol Aging 96: 128–136, 2020. doi: 10.1016/j.neurobiolaging.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erdő F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 37: 4–24, 2017. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci 12: 80, 2020. doi: 10.3389/fnagi.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyúl-Tóth A, Toth P, Csiszar A, Ungvari Z. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 41: 575–589, 2019. doi: 10.1007/s11357-019-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z, Csiszar A. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci 74: 290–298, 2018. doi: 10.1093/gerona/gly127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilhelm I, Nyúl-Tóth A, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol 313: H1000–H1012, 2017. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 69.Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci 63: 12–20, 2008. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- 70.Murugesan N, Demarest TG, Madri JA, Pachter JS. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging 33: 1004, 2012. doi: 10.1016/j.neurobiolaging.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev 2: 149–168, 2003. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 72.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE, Csiszar A. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68: 877–891, 2013. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67: 821–829, 2012. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 76.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 38: 273–289, 2016. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 70: 665–674, 2015. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344: 630–634, 2014. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience 41: 619–630, 2019. doi: 10.1007/s11357-019-00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashpole NM, Warrington JP, Mitschelen MC, Yan H, Sosnowska D, Gautam T, Farley JA, Csiszar A, Ungvari Z, Sonntag WE. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells. Am J Physiol Heart Circ Physiol 307: H858–H868, 2014. doi: 10.1152/ajpheart.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A, Sonntag WE. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci 68: 1443–1457, 2013. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo S, Deng W, Xing C, Zhou Y, Ning M, Lo EH. Effects of aging, hypertension and diabetes on the mouse brain and heart vasculomes. Neurobiol Dis 126: 117–123, 2019. doi: 10.1016/j.nbd.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munro D, Baldy C, Pamenter ME, Treberg JR. The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell 18: e12916, 2019. doi: 10.1111/acel.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zampino M, Spencer RG, Fishbein KW, Simonsick EM, Ferrucci L. Cardiovascular health and mitochondrial function: testing an association. J Gerontol A Biol Sci Med Sci 76: 361–367, 2020. doi: 10.1093/gerona/glaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunt NJ, Lockwood GP, Kang SWS, Pulpitel T, Clark X, Mao H, McCourt PAG, Cooney GJ, Wali JA, Le Couteur FH, Le Couteur DG, Cogger VC. The effects of metformin on age-related changes in the liver sinusoidal endothelial cell. J Gerontol A Biol Sci Med Sci 75: 278–285, 2020. doi: 10.1093/gerona/glz153. [DOI] [PubMed] [Google Scholar]
- 86.Quarles E, Basisty N, Chiao YA, Merrihew G, Gu H, Sweetwyne MT, Fredrickson J, Nguyen NH, Razumova M, Kooiker K, Moussavi-Harami F, Regnier M, Quarles C, MacCoss M, Rabinovitch PS. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 19: e13086, 2020. doi: 10.1111/acel.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Skike CE, Lin AL, Roberts Burbank R, Halloran JJ, Hernandez SF, Cuvillier J, Soto VY, Hussong SA, Jahrling JB, Javors MA, Hart MJ, Fischer KE, Austad SN, Galvan V. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell 19: e13057, 2020. doi: 10.1111/acel.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen J, Torres C. Astrocyte senescence: evidence and significance. Aging Cell 18: e12937, 2019. doi: 10.1111/acel.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18: e12931, 2019. doi: 10.1111/acel.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18: e12945, 2019. doi: 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang D, Wei G, Long F, Nie H, Tian X, Qu L, Wang S, Li P, Qiu Y, Wang Y, Hong W, Ni T, Liu X, Zhu YZ. Histone methyltransferase Smyd3 is a new regulator for vascular senescence. Aging Cell 19: e13212, 2020. doi: 10.1111/acel.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bose C, Alves I, Singh P, Palade PT, Carvalho E, Børsheim E, Jun SR, Cheema A, Boerma M, Awasthi S, Singh SP. Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 19: e13261, 2020. doi: 10.1111/acel.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fanjul V, Jorge I, Camafeita E, Macias A, González-Gómez C, Barettino A, Dorado B, Andrés-Manzano MJ, Rivera-Torres J, Vázquez J, López-Otin O, Andrés V. Identification of common cardiometabolic alterations and deregulated pathways in mouse and pig models of aging. Aging Cell 19: e13203, 2020. doi: 10.1111/acel.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marin-Aguilar F, Lechuga-Vieco AV, Alcocer-Gómez E, Castejón-Vega B, Lucas J, Garrido C, Peralta-Garcia A, Pérez-Pulido AJ, Varela-López A, Quiles JL, Ryffel B, Flores I, Bullón P, Ruiz-Cabello J, Cordero MD. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 19: e13050, 2020. doi: 10.1111/acel.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleefeldt F, Bommel H, Broede B, Thomsen M, Pfeiffer V, Worsdorfer P, Karnati S, Wagner N, Rueckschloss U, Ergün S. Aging-related carcinoembryonic antigen-related cell adhesion molecule 1 signaling promotes vascular dysfunction. Aging Cell 18: e13025, 2019. doi: 10.1111/acel.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cosarderelioglu C, Nidadavolu LS, George CJ, Oh ES, Bennett DA, Walston JD, Abadir PM. Brain renin-angiotensin system at the intersect of physical and cognitive frailty. Front Neurosci 14: 586314, 2020. doi: 10.3389/fnins.2020.586314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown BA, Connolly GM, Mill CEJ, Williams H, Angelini GD, Johnson JL, George SJ. Aging differentially modulates the Wnt pro-survival signalling pathways in vascular smooth muscle cells. Aging Cell 18: e12844, 2019. doi: 10.1111/acel.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero A, San Hipólito-Luengo A, Villalobos LA, Vallejo S, Valencia I, Michalska P, Pajuelo-Lozano N, Sánchez-Pérez I, Leon R, Bartha JL, Sanz MJ, Erusalimsky JD, Sánchez-Ferrer CF, Romacho T, Peiró C. The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell 18: e12913, 2019. doi: 10.1111/acel.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen CY, Chao YM, Lin HF, Chen CJ, Chen CS, Yang JL, Chan JYH, Juo SH. miR-195 reduces age-related blood-brain barrier leakage caused by thrombospondin-1-mediated selective autophagy. Aging Cell 19: e13236, 2020. doi: 10.1111/acel.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michalicova A, Majerova P, Kovac A. Tau protein and its role in blood-brain barrier dysfunction. Front Mol Neurosci 13: 570045, 2020. doi: 10.3389/fnmol.2020.570045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parodi-Rullan R, Ghiso J, Cabrera E, Rostagno A, Fossati S. Alzheimer's amyloid β heterogeneous species differentially affect brain endothelial cell viability, blood-brain barrier integrity, and angiogenesis. Aging Cell 19: e13258, 2020. doi: 10.1111/acel.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie J, Hong E, Ding B, Jiang W, Zheng S, Xie Z, Tian D, Chen Y. Inhibition of NOX4/ROS suppresses neuronal and blood-brain barrier injury by attenuating oxidative stress after intracerebral hemorrhage. Front Cell Neurosci 14: 578060, 2020. doi: 10.3389/fncel.2020.578060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab 27: 1908–1918, 2007. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 104.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17: e12731, 2018. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer's disease cerebrovascular and memory deficits. J Neurosci 32: 4705–4715, 2012. [Erratum in J Neurosci 32: 7766, 2012]. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fülöp GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, Ungvari Z. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 42: 409–428, 2020. doi: 10.1007/s11357-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C, Mu N, Gu C, Liu M, Yang Z, Yin Y, Chen M, Wang Y, Han Y, Yu L, Ma H. Metformin mediates cardioprotection against aging-induced ischemic necroptosis. Aging Cell 19: e13096, 2020. doi: 10.1111/acel.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qin W, Li J, Zhu R, Gao S, Fan J, Xia M, Zhao RC, Zhang J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging (Albany NY) 11: 11391–11415, 2019. doi: 10.18632/aging.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee GH, Hoang TH, Jung ES, Jung SJ, Han SK, Chung MJ, Chae SW, Chae HJ. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 19: e13279, 2020. doi: 10.1111/acel.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Mateos A, Istas G, Boschek L, Feliciano RP, Mills CE, Boby C, Gomez-Alonso S, Milenkovic D, Heiss C. Circulating anthocyanin metabolites mediate vascular benefits of blueberries: insights from randomized controlled trials, metabolomics, and nutrigenomics. J Gerontol A Biol Sci Med Sci 74: 967–976, 2019. doi: 10.1093/gerona/glz047. [DOI] [PubMed] [Google Scholar]
- 111.Ballak DB, Brunt VE, Sapinsley ZJ, Ziemba BP, Richey JJ, Zigler MC, Johnson LC, Gioscia-Ryan RA, Culp-Hill R, Eisenmesser EZ, D'Alessandro A, Dinarello CA, Seals DR. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell 19: e13074, 2020. doi: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Royea J, Zhang L, Tong XK, Hamel E. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer's disease. J Neurosci 37: 5562–5573, 2017. doi: 10.1523/JNEUROSCI.0329-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel AM, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z, Csiszar A. IGF-1 deficiency promotes pathological remodeling of cerebral arteries: a potential mechanism contributing to the pathogenesis of intracerebral hemorrhages in aging. J Gerontol A Biol Sci Med Sci 74: 446–454, 2018. doi: 10.1093/gerona/gly144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 16: 469–479, 2017. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14: 1034–1044, 2015. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 34: 1887–1897, 2014. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roh JD, Houstis N, Yu A, Chang B, Yeri A, Li H, Hobson R, Lerchenmüller C, Vujic A, Chaudhari V, Damilano F, Platt C, Zlotoff D, Lee RT, Shah R, Jerosch-Herold M, Rosenzweig A. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell 19: e13159, 2020. doi: 10.1111/acel.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Csiszar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, Baur JA, Ungvari ZI. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol 316: H1253–H1266, 2019. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD (+)-H2S signaling network is a reversible cause of vascular aging. Cell 173: 74–89 e20, 2018. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kiss T, Nyúl-Tóth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 42: 527–546, 2020. doi: 10.1007/s11357-020-00165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307: H1754–H1763, 2014. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor {kappa}b and forkhead box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16: 17–26, 2017. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev 133: 368–371, 2012. doi: 10.1016/j.mad.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. Geroscience 40: 513–521, 2018. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation 142: 1609–1611, 2020. doi: 10.1161/CIRCULATIONAHA.120.050354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sabioni L, De LA, Lamas C, Muccillo F, Castro-Faria-Neto HC, Estato V, Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res 134: 104199, 2020. doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khoretonenko MV, Leskov IL, Jennings SR, Yurochko AD, Stokes KY. Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses. Am J Pathol 177: 2134–2144, 2010. doi: 10.2353/ajpath.2010.100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leskov IL, Whitsett J, Vasquez-Vivar J, Stokes KY. NAD (P)H oxidase and eNOS play differential roles in cytomegalovirus infection-induced microvascular dysfunction. Free Radic Biol Med 51: 2300–2308, 2011. doi: 10.1016/j.freeradbiomed.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rojo AI, Pajares M, Rada P, Nuñez A, Nevado-Holgado AJ, Killik R, Van Leuven F, Ribe E, Lovestone S, Yamamoto M, Cuadrado A. NRF2 deficiency replicates transcriptomic changes in Alzheimer's patients and worsens APP and TAU pathology. Redox Biol 13: 444–451, 2017. doi: 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Desprès S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90–94, 2011. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wyss-Coray T, Lin C, von Euw D, Masliah E, Mucke L, Lacombe P. Alzheimer's disease-like cerebrovascular pathology in transforming growth factor-beta 1 transgenic mice and functional metabolic correlates. Ann N Y Acad Sci 903: 317–323, 2000. doi: 10.1111/j.1749-6632.2000.tb06382.x. [DOI] [PubMed] [Google Scholar]
- 134.Elhaik Goldman S, Goez D, Last D, Naor S, Liraz Zaltsman S, Sharvit-Ginon I, Atrakchi-Baranes D, Shemesh C, Twitto-Greenberg R, Tsach S, Lotan R, Leikin-Frenkel A, Shish A, Mardor Y, Schnaider Beeri M, Cooper I. High-fat diet protects the blood-brain barrier in an Alzheimer's disease mouse model. Aging Cell 17: e12818, 2018. doi: 10.1111/acel.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer's disease. J Cereb Blood Flow Metab 33: 1412–1421, 2013. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]