Abstract
Arsenic exposure though drinking water is widespread and well associated with adverse cardiovascular outcomes, yet the pathophysiological mechanisms by which iAS induces these effects are largely unknown. Recently, an epidemiological study in an American population with a low burden of cardiovascular risk factors found that iAS exposure was associated with altered left ventricular geometry. Considering the possibility that iAS directly induces cardiac remodeling independently of hypertension, we investigated the impact of an environmentally relevant iAS exposure on the structure and function of male and female hearts. Adult male and female C56BL/6J mice were exposed to 615 μg/L iAS for 8 wk. Males exhibited increased systolic blood pressure via tail cuff photoplethysmography, left ventricular wall thickening via transthoracic echocardiography, and increased plasma atrial natriuretic peptide via enzyme immunoassay. RT-qPCR revealed increased myocardial RNA transcripts of Acta1, Myh7, and Nppa and decreased Myh6, providing evidence of pathological hypertrophy in the male heart. Similar changes were not detected in females, and nitric oxide-dependent mechanisms of cardioprotection in the heart appeared to remain intact. Further investigation found that Rcan1 was upregulated in male hearts and that iAS activated NFAT in HEK-293 cells via luciferase assay. Interestingly, iAS induced similar hypertrophic gene expression changes in neonatal rat ventricular myocytes, which were blocked by calcineurin inhibition, suggesting that iAS may induce pathological cardiac hypertrophy in part by targeting the calcineurin-NFAT pathway. As such, these results highlight iAS exposure as an independent cardiovascular risk factor and provide biological impetus for its removal from human consumption.
NEW & NOTEWORTHY This investigation provides the first mechanistic link between an environmentally relevant dose of inorganic arsenic (iAS) and pathological hypertrophy in the heart. By demonstrating that iAS exposure may cause pathological cardiac hypertrophy not only by increasing systolic blood pressure but also by potentially activating calcineurin-nuclear factor of activated T cells and inducing fetal gene expression, these results provide novel mechanistic insight into the theat of iAS exposure to the heart, which is necessary to identify targets for medical and public health intervention.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/inorganic-arsenic-induces-cardiac-hypertrophy/.
Keywords: blood pressure, cardiac hypertrophy, environmental exposure, inorganic arsenic exposure, sex differences
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of death thoughout the world (1, 2). Traditionally, risk factors for CVD have been considered personally modifiable such that the notions of increasing physical activity, avoiding tobacco smoke, and adhering to dietary approaches to stop hypertension are recommended to the general public (3). However, growing evidence implicates environmental agents in CVD etiology and pathogenesis (4). Air pollution, for example, is now recognized as the third leading risk factor in the global burden of disease, after high blood pressure and tobacco smoke (1). Considering that the prevalence and mortality of CVD continues to escalate, part of the challenge in its prevention may result from a gap in the understanding of the interplay between chonic environmental exposures and the cardiovascular system.
Arsenic is a naturally occurring metalloid found in the Earth’s crust that can leach into drinking water, which is the primary mode of human exposure (5). The World Health Organization lists arsenic as one of 10 chemicals of major public health concern, recommending a maximum limit of 10 μg/L in drinking water. However, the levels of arsenic in drinking water, existing as inorganic arsenic (iAS), have been found to exceed 1,000 μg/L in some parts of the United States and 5,000 μg/L in other parts of the world (5, 6). Chonic iAS exposure has been linked to several disease states, including diabetes, CVD, and cancer (7, 8). Although iAS exposure is well associated with adverse cardiovascular outcomes, the mechanisms though which it induces these health consequences are largely unknown (9, 10).
Recent findings from a multistate cohort study in an American population with a low burden of CVD risk factors show that individuals exposed to iAS exhibit altered left ventricular geometry (11). Although these results highlighted stronger associations among participants with preexisting hypertension, the association was also present without hypertension, suggesting that iAS exposure may directly promote cardiac remodeling (11). Previously, our laboratory reported that a 4-wk exposure to 1,000 μg/L iAS may impart sex-disparate effects on the heart (12). We utilized an extended model of exposure in the current study to investigate the impact of long-term iAS exposure on the structure and function of male and female hearts. We report for the first time that exposure to an environmentally relevant dose of iAS induces sex-dependent pathological hypertrophy in male hearts, in part by activating the calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway. Therefore, these findings confer mechanistic significance to the detrimental impact of iAS exposure on cardiovascular health, further highlighting the need to regulate its removal from human consumption.
METHODS
Animals and Exposure Protocol
Seven-wk-old male and female C57BL/6J wild-type mice (Jackson Laboratory, Bar, Harbor, ME) were housed (5 mice per cage) under pathogen-free conditions and maintained on AIN-93G chow (Research Diets, New Brunswick, NJ) and Nestle Pure Life water (Nestlé Waters North America, Stamford, CT) for 1 wk before iAS exposure. Both the water and the chow are reported to have undetectable iAS levels, and the water was revalidated via inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500ce Octopole; Agilent Technologies, Santa Clara, CA) to have an iAS concentration less than its 0.16 μg/L limit of detection (12, 13). Following a 1-wk preexposure acclimation period, mice received water with 0 or 1,000 μg/L sodium arsenite (NaAsO2, S7400; Sigma-Aldrich, St. Louis, MO) ad libitum for 8 wk; water and chow were refreshed every 2 to 3 days to minimize oxidation, and bedding was changed weekly. Food intake, water intake, and body weight were monitored thoughout the exposure, and no significant changes were observed with iAS exposure in either sex [Supplemental Figure S1 (all supplemental materials are found at https://doi.org/10.6084/m9.figshare.12430373). Furthermore, iAS exposure was confirmed by the detection of iAS in urine samples via ICP-MS in exposed groups and the lack thereof in control groups with no sex-disparate effects. Mice were anesthetized before subsequent procedures via intraperitoneal ketamine-xylazine injection (90 mg/kg ketamine, Hofspira, Lake Forest, IL; 10 mg/kg xylazine, Sigma-Aldrich), confirmed by absent reflexes via toe pinch. All animal work in this study conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH; Publication No. 85-23, Revised 2011) and was approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
Blood Pressure Measurement
Mice were acclimated to a tail cuff pressure transduction apparatus (BP-2000 Blood Pressure Analysis System; Visitech Systems, Apex, NC) for 1 wk before the onset of exposure and data collection to minimize systolic standard deviation and ensure reproducibility. Mice were restrained by opaque, open-bottomed rodent holders (BP-MH0; Visitech Systems, Apex, NC) that facilitated breathing and limited light exposure to calm the animal and reduce stress; the platform was preheated and maintained at 38°C in the dark under a laminar flow hood, and the tails were secured to the platform with surgical tape to minimize movement. Twenty experimental measurements were taken on each mouse each session, preceded by five preliminary measurements, which allowed the animals to become accustomed to the tail cuff inflation and restraint. Briefly, the instrument reads and determines pulse though the tail, after which it starts inflating the cuff. Transmission photoplethysmography determines blood pressure by analyzing the variation in light transmitted though the tail; during systole, vessels dilate and scatter light with each pressure wave, while the reverse occurs during diastole. As such, diastolic pressure was recorded when the cuff’s pressure of occlusion caused the waveform amplitude (vessel dilation) to decrease, and systolic pressure was recorded when the occlusion pressure was sufficient to completely collapse the waveform, thus not causing any further dilation. Waveforms were continuously monitored for appropriate amplitude during each of the twenty readings, and all values were averaged for each mouse each day, excluding outliers as categorized by a systolic pressure 1.75 standard deviations beyond the mean. Measurements were taken twice a week thoughout the exposure as well as daily on week 0 (preexposure) and weeks 4 and 8. All measurements were recorded at the same time of day to minimize circadian influence.
Transthoracic Echocardiography
Transthoracic echocardiography was performed in conscious mice by an investigator blinded to experimental conditions using a preclinical ultrasound imaging system (Vevo 2100; FUJIFILM VisualSonics, Toronto, ON, Canada) with a 40-MHz linear transducer, as previously described (12). M-mode echocardiogram was acquired from the short-axis view of the left ventricle at the level of the midpapillary muscles (200 m/s sweep speed). Semiautomated continuous tracing of the left ventricular walls from this axis view measured, calculated, or extrapolated the following cardiac parameters: left ventricular anterior wall thickness at end diastole (LVAWd), left ventricular posterior wall thickness at end diastole (LVPWd), left ventricular internal diameter at end diastole (LVIDd), percent fractional shortening (FS), percent ejection fraction (EF), left ventricular mass (LV mass), stroke volume (SV), volume at end systole (Vs) and diastole (Vd), diameter at systole (Ds) and diastole (Dd), heart rate (H), and cardiac output (CO).
Inorganic Arsenic Measurement
Samples of iAS and control water were taken from bottles supplying each cage, and urine from each mouse was collected at the 6-wk time point using hydrophobic sand (Labsand, Braintree Scientific, Braintree, MA) and stored (−80°C) until analysis. Water and urine samples were blinded and analyzed independently by the laboratory of Dr. Ana Rule. Briefly, samples (50 μL) were diluted (1:50) with HNO3 (2%) and HCl (0.5%) solution. Calibration curves for arsenic were built using a standard solution (Multi-element Aqueous CRM, QC Standard 21; VHG Laboratories, Manchester, NH). Internal standard germanium [10 μg/L (vol/vol), CPI International, Santa Rosa, CA] was added to samples and calibration curves to control for potential drifts in the signal. Arsenic concentrations were then measured using ICP-MS (Agilent 7500ce Octopole inductively coupled plasma mass spectrometer; Agilent Technologies, Santa Clara, CA); the limit of detection was 0.16 μg/L for arsenic. Samples with iAS concentrations below the limit of detection were substituted by the limit of detection divided by the square root of 2. For quality control and assurance, 10% duplicates, 10% blanks, Seronorm Trace Elements Urine (Accurate, Westbury, NY), and replicate sample analysis every 12 samples were analyzed.
Langendorff Heart Perfusion
Mice were anesthetized with a mixture of ketamine (90 mg/kg; Hofspira) and xylazine (10 mg/kg; Sigma-Aldrich) via intraperitoneal injection and anticoagulated with heparin (Fresenvis Kabi, Lake Zurich, IL). Hearts were excised, cannulated onto a Langendorff apparatus, and perfused retrogradely with Krebs-Henseleit buffer (95% O2, 5% CO2; pH 7.4) under constant pressure (100 cmH2O) and temperature (37°C) as previously described (12). Buffer consisted of (in mmol/L): NaCl (120), KCl (4.7), KH2PO4 (1.2), NaHCO3 (25), MgSO4 (1.2), d-glucose (14), and CaCl2 (1.75). Hearts were subjected to either a 5-min perfusion period, after which they were sectioned into two halves and snap-frozen with liquid nitrogen, or an ischemia-reperfusion protocol as follows.
Ischemia-Reperfusion Protocol
Immediately after Langendorff cannulation, a water-filled, saran wrap balloon connected to a pressure transducer (PowerLab, AD Instruments, Dunedin, NZ) was inserted into the left ventricle via an excised left atrium to monitor and record digitized heart rate (HR) and left ventricular developed pressure (LVDP) using LabChart software (AD Instruments). Rate pressure product (RPP) was calculated as a product of these parameters and used as a metric of cardiac function. Following a period of equilibration (20 min), perfusion was stopped (20 min) to induce global normothermic ischemia in the heart and then restarted (120 min) to induce reperfusion injury. Postischemic functional recovery at 60 min into reperfusion was expressed as a percentage of the preischemic RPP to quantify functional recovery. Following ischemia-reperfusion (I/R), hearts were perfused with triphenyl tetrazolium chloride (TTC, 1%, 2 min) to stain viable cells red and leave the infarct white; hearts were then incubated (37°C, 20 min) in TTC solution and subsequently fixed in formalin solution (10%). Hearts were cut into five transverse sections, and both sides of each section were imaged under a dissecting microscope (Nikon). Infarct size was analyzed by an investigator blinded to the groups and using ImageJ software (NIH, Bethesda, MD), quantifying infarcted myocardium as a function of total myocardial area in the section plane.
Histology
Control and treatment groups for all histology experiments were collected at the same time under the same conditions. Sectioned, formalin-fixed hearts were paraffin embedded and stained with either hemotoxylin and eosin or Masson’s trichome to visualize cell morphology and collagen deposition, respectively. Whole slides of Masson’s-stained, paraffin-embedded hearts were scanned (×20, Aperio ScanScope) in a blinded fashion. Digital slides were subsequently analyzed (Aperio ImageScope) using a standard positive pixel count algorithm (Positive Pixel Count v.9.1, Aperio, Leica Biosystems) according to the following parameters, set to read blue pixels as positive and red as negative, categorizing the former into thee intensity bins: hue value = 0.62, hue width = 0.40, color saturation theshold = 0.04, intensity theshold (upper limit) of weak positive pixels = 220, intensity theshold (lower limit) of weak positive pixels = 175, intensity theshold (lower limit) of medium positive pixels = 100, intensity theshold (lower limit) of strong positive pixels = 0, intensity theshold of negative pixels = −1. Positivity, representing the number of positive pixels over the total number of positive and negative pixels, is reported as a quantification of myocardial collagen.
Plasma Enzyme Immunoassays
Blood was drawn from the inferior vena cava using a heparin-coated syringe and collected in a tube with heparin (10 μL) immediately after excision of the heart. Blood was centrifuged (15,000 g, 10 min, 4°C), supernatant was recovered as plasma, and samples were stored (−80°C). Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels were measured via enzyme immunoassay following the manufacturer’s instructions (EIA-ANP and EIA-BNP, RayBiotech, Norcross, GA).
Heart Homogenate Preparation
Hearts were homogenized with cell lysis buffer (1 mL; Cell Signaling Technology, Danvers, MA) supplemented with a protease-inhibitor cocktail (Cell Signaling Technology) in a hard tissue lysing kit (Precellys CK28 Lysing Kit, Bertin Instruments) using a bead-mill tissue homogenizer (2 × 30 s cycles, 0°C, 7,200 rpm; Precellys Evolution 24, Bertin Instruments). Supernatant was recovered as total crude homogenate, protein concentrations were determined via Bradford assay, and total homogenate aliquots were stored (−80°C).
Western Blot
Heart homogenates (30 μg protein) were separated (20 min, 75 V; 100 min, 175 V) on a gradient Bis-Tris SDS-PAGE gel (4–12%, NuPAGE; Thermo Fisher, Carlsbad, CA) and transferred (90 min, 220 mA) to a nitrocellulose membrane (Thermo Fisher). Every gel included two molecular-weight markers for separate regions of interest (High Range Color-Coded Prestained Protein Marker; Cell Signaling Technology, Danvers, MA; and Novex Prestained Protein Standard, Thermo Fisher) on opposite ends of the gel. Total protein served as the loading control; lysine residues were covalently labeled (No-Stain Protein Labeling Reagent, Thermo Fisher) following the manufacturer’s instructions and visualized by fluorescence imaging (488 nm). Membranes were blocked (1 h) with bovine serum albumin (5% wt/vol, Sigma-Aldrich) in Tris-buffered saline with Tween-20 (0.1%), and subsequently incubated (overnight, 4°C) with primary antibodies (from Cell Signaling Technology unless noted) against phosphorylated (p-)Akt S473 (1:1,000; 4060S, rabbit), total (t-)Akt (1:1,000; 4691S, rabbit), calcineurin A (1:1,000; 2614S, rabbit), p-endothelial nitic oxide synthae (eNOS) S1177 (1:500; 9570S, rabbit), p-eNOS T495 (1:500; 9574S, rabbit), t-eNOS (1:250; sc-376751, mouse, Santa Cruz Biotechnology, Dallas, TX), p-ERK1/2 T202/Y204 (1:1,000; 9101S, rabbit), t-ERK1/2 (1:1000; 9102S, rabbit), inducible (i)NOS (1:250; sc-650, rabbit, Santa Cruz Biotechnology), neuronal (n)NOS (1:250; sc-648, rabbit, Santa Cruz Biotechnology), protein kinase G (PKG-1; 1:1,000; 3248S, rabbit), p-phosphlamban (PLN) S16/T17 (1:1,000; 8496S, rabbit), t-PLN (1:1,000; PA5-26004, rabbit, Thermo Fisher Scientific), sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2; 1:1,000; sc-8094, goat, Santa Cruz Biotechnology), p-vasodilator-stimulated phosphoprotein (VASP) S239 (1:1,000; 3114S, rabbit), t-VASP (1:1000; 3132S, rabbit), p-vascular endothelial growth factor receptor 2 (VEGFR2) Y1175 (1:1,000; 2478S, rabbit), and t-VEGFR2 (1:1,000; 2479S, rabbit). Membranes were then probed (1 h) with the corresponding secondary antibody [anti-rabbit, 7074S (Cell Signaling Technology), anti-mouse (sc-2005, Santa Cruz Biotechnology), or anti-goat (R1317HP; OriGene Technologies, Rockville, MD)] and visualized using electrogenerated chemiluminescence (SuperSignal West Pico PLUS, Thermo Fisher). Membranes were stripped (Re-Blot Plus Mild Solution, EMD Millipore, Temecula, CA) and reprobed as needed, particularly to evaluate phosphorylated and total target expression on the same blot, and total protein was visualized each time. Densitometry was assessed using ImageJ and normalized to total protein. To minimize variation, comparisons were not made across multiple gels. References providing relevant examples of antibody use and validation are provided in Supplemental Table S1.
RNA Isolation, Extraction, and cDNA Conversion
Hearts were homogenized with TRIzol (1 mL, Ambion) in a hard-tissue lysing kit (Precellys CK28 Lysing Kit) using a bead-mill tissue homogenizer (2 × 30 s cycles, 0°C, 7,200 rpm; Precellys Evolution 24). Cells were harvested by adding TRIzol (1 mL) directly to the culture dish. Lysates were mixed with chloroform (200 μL, Thermo Fisher), incubated (5 min, 25°C), and centrifuged (12,000 g, 15 min, 4°C) for phase separation. RNA collected from the upper phase was precipitated by incubation (10 min, 25°C) with isopropyl alcohol (500 μL) and centrifugation (12,000 g, 8 min, 4°C). RNA pellets were washed with ethanol (500 μL, 75% EtOH), centrifuged (12,000 g, 10 min, 4°C), and air-dried under a laminar flow hood (30 min). Following RNA solubilization (100 μL, DEPC-treated water), RNA concentration and purity (A260/A280 range 1.98 to 2.06) were measured via spectrophotometry (1 mL sample, NanoDrop 100, Thermo Fisher), and RNA integrity was confirmed via 18S and 28S rRNA band visualization after agarose (1%) gel electrophoresis (1× TBE [Tris base, boric acid, and ethylenediaminetetraacetic acid (EDTA)]. RNA was subsequently converted to cDNA per manufacturer’s instructions (High-Capacity cDNA Reverse Transcription Kit, 4368814, Thermo Fisher). Briefly, the reverse transcription reaction mix was prepared on ice, RNA (2 μg) was added, and the samples were run on a thermocycler (60 min, 37°C; 5 min, 95°C; held, 4°C; Applied Biosystems); cDNA was stored (−80°C) until use.
Quantitative PCR
Expression levels of mRNA transcripts were measured using a PCR master mix (TaqMan Fast Advanced Master Mix, Applied Biosystems) and the following validated primers (TaqMan, Applied Biosystems) on a thermocycler (2 min, 50°C; 2 min, 95°C; (1 s, 95°C; 20 s, 60°C) × 40), Applied Biosystems) in a 384-well plate (Thermo Fisher): Acta1 (Mm00808218_g1), Akt1 (Mm01331626_m1), Col1a2 (Mm00483888_m1), Col3a1 (Mm00802300_m1), Gapdh (Mm99999915_g1 and Rn01775763_g1), Myh6 (Mm00440359_m1 and Rn00691721_g1), Myh7 (Mm00600555_m1 and Rn01488777_g1), Nppa (Mm01255747_g1 and Rn00664637_g1), Nppb (Mm01255770_g1 and Rn00580641_m1), Rcan1 (Mm01213406_m1 and Rn00596606_m1), Vegfa (Mm00437304_m1). Expression was determined using the ΔΔCT method and normalized to Gapdh, which did not change in cycle time with iAS treatment or sex.
Electron Paramagnetic Resonance
Briefly, heart tissue (∼20 mg) was homogenized in phosphate-buffered saline (PBS) containing 0.1 mM diethylene-24 triaminepentaacetic acid (DTPA) and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) at pH 7.4. Nonsoluble fractions were removed by centrifugation (15,000 g, 10 min, 4°C). Homogenates were kept on ice and analyzed immediately. Stock solutions of 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH; Enzo Life Sciences, Farmingdale, NY) were prepared fresh in nitrogen purged 0.9% (wt/vol) NaCl, 25 g/L Chelex 100 (Bio-Rad), and 0.1 mM DTPA and kept on ice. Samples were treated with 1 mM CMH at 37°C for 2 min, transferred to 50-μL glass capillary tubes, and analyzed on a Bruker E-Scan (Billerica, MA) electron paramagnetic resonance (EPR) spectrometer at room temperature. Instrument settings were as follows: sweep width, 100 G; microwave frequency, 9.75 GHz; modulation amplitude, 1 G; conversion time, 5.12 ms; receiver gain, 2 × 103; number of scans, 16. EPR signal intensities were normalized to homogenate protein concentrations as determined by Pierce BCA protein assay kit (Thermo Fisher).
Cell Culture Protocol
Human embryonic kidney 293 cells (HEK-293, ATCC) were maintained (5% CO2, 37°C, humidified air) in Dulbecco's modified Eagle’s medium (DMEM) with glucose (4.5 g/L) and l-glutamine (GIBCO) supplemented with fetal bovine serum (10% FBS, Sigma-Aldrich) and 100 U/mL penicillin-100 μg/mL streptomycin (1% P/S, Thermo Fisher). Neonatal rat ventricular myocytes (NRVMs) were isolated from newborn Sprague-Dawley rat pups as previously described and maintained under the conditions mentioned above (15). NRVMs seeded in six-well plates (3 × 105 cells/well) were treated (after 24 h) with sodium arsenite (0.5 and 1 μM in serum-free medium, 37°C, 48 h), and either cyclosporin A (CsA, 2 μM; 9973S, Cell Signaling Technology) or vehicle control (0.0025% DMSO) before RNA isolation and analysis.
Cell Proliferation Assay
HEK-293 cells seeded in a 96-well plate (2 × 104 cells/well) were treated (after 24 h) with sodium arsenite (0, 0.5, 1, 5, 10, 25, 50, and 100 μM in complete cell culture media, 37°C, 24 h). NRVMs similarly seeded in a 96-well plate were treated (after 24 h) with sodium arsenite (0, 0.5, 1, 5, 10, 25, 50, and 100 μM in serum-free medium, 37°C, 48 h). MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 50 μL/well; ab211091, Abcam) was then added, and the cells were incubated (37°C, 1 h). MTT solvent (150 μL/well; ab211091, Abcam) was added, and the plate was shaken (orbital shaker, dark, 40 rpm, 10 min) to solubilize the formazan crystals; absorbance (590 nm) was promptly read. Metabolic activity of MTT reduction is represented by optical density as a percentage of the average control.
NFAT Luciferase Assay
HEK-293 cells cultured in DMEM (10% FBS, 1% P/S, Thermo Fisher) to 70% confluence were transfected with plasmids expressing an NFAT-luciferase reporter (0.3 μg, Promega). Plasmids expressing Renilla-luciferase (0.001 μg, Promega) and/or TRPC6 (0.2 μg) were transfected (Xfect Transfection, Takara Bio USA, Mountain View, CA) as internal and positive controls, respectively (16, 17). Transfected cells were exposed to sodium arsenite (0 or 1 μM in complete cell culture medium, 37°C, 24 h). Cell lysate was then extracted using passive lysis buffer according to the manufacturer’s instructions (Promega), and luciferase activity was measured by Dual-Luciferase Reporter Assay (Promega).
Statistical Analysis
Sample sizes of mice and cell culture for each experiment were estimated a priori via power analysis (power = 0.80, effect size = 0.25, α = 0.05) based on data generated in previous studies from our group. Mice were also randomized during collection to minimize batch effects. Data were analyzed using GraphPad Prism (La Jolla, CA). Statistical outliers were identified by the ROUT method (Q = 1%). Statistical comparisons between groups were determined using an ordinary one-way ANOVA with Dunnett’s or Sidak’s multiple comparisons test, a two-way ANOVA with Tukey’s multiple comparisons test, or a two-tailed Mann–Whitney test as appropriate; significance was set at P < 0.05. All P values and statistical tests are reported in their respective figure legends.
RESULTS
Exposure to iAS Induces Sex-Dependent Increases in Blood Pressure and Myocardial Hypertrophy
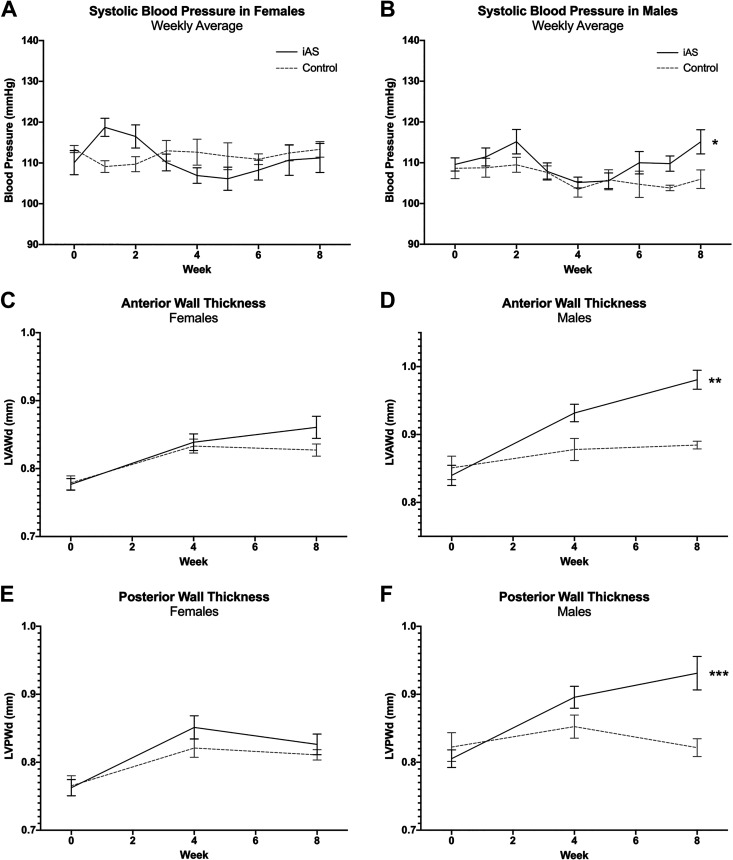
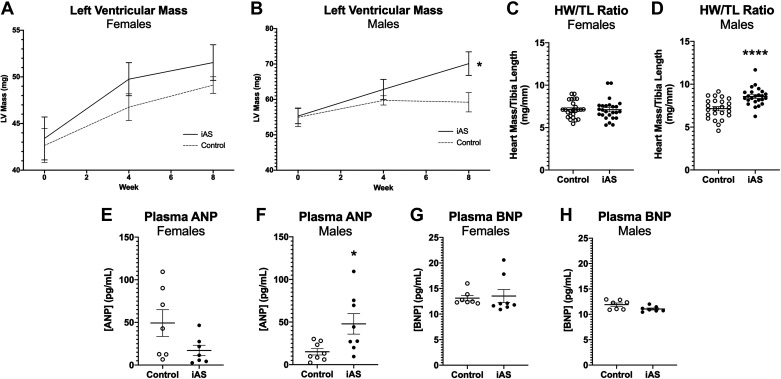
Transthoracic echocardiography was conducted at 0, 4, and 8 wk of iAS exposure (measured to be 614.6 μg/L in the current study via ICP-MS), and blood pressure was recorded thoughout. Systolic blood pressure significantly increased in iAS-exposed females during the first and second (+9.617 and +6.810 mmHg, respectively) weeks of exposure, but subsequently returned to baseline though the eighth week (Fig. 1A). Systolic blood pressure in iAS-exposed males, however, significantly increased at the seventh and eighth weeks of exposure (+5.962 and +9.162 mmHg, respectively) relative to controls (Fig. 1B). Exposure to iAS had no significant effects on LVAWd or LVPWd in females (Fig. 1, C and E). iAS-exposed males demonstrated a significant increase in LVAWd and a trending increase in LVPWd at 4 wk of exposure compared with controls (Fig. 1, D and F). Additionally, consistent with the increase in systolic blood pressure, both LVAWd and LVPWd were significantly increased in iAS-exposed males at 8 wk of exposure relative to controls (Fig. 1, D and F). Echocardiography did not reveal significant changes in other structural (LVIDd, Vd, Vs, Dd, and Ds) or functional parameters (H, EF, FS, SV, and CO) with iAS exposure in either males or females (Table 1). However, to further examine the impact of iAS exposure on cardiac function/Ca2+ handling, protein levels of p-PLN, t-PLN, and SERCA2 were probed in the heart, showing no significant differences with iAS exposure in either sex (Supplemental Fig. S2). Exposure to iAS had no effect on LV mass extrapolated from echocardiography in females at the 8-wk end point, but LV mass was significantly increased in iAS-exposed males compared with controls (Fig. 2, A and B). Similarly, there was no effect of iAS exposure on heart weight normalized to tibia length (HW/TL) in females, but HW/TL was significantly increased in iAS-exposed males relative to controls (Fig. 2, C and D). Considering the structural evidence, circulating biomarkers of a hypertrophic phenotype were probed. Plasma ANP levels were not significantly altered in iAS-exposed females but were significantly greater in iAS-exposed males compared with controls (Fig. 2, E and F). However, iAS exposure did not have a significant effect on plasma BNP levels in either sex (Fig. 2, G and H). Taken together, these data indicate that an 8-wk exposure to iAS induces sex-dependent hypertrophy of the heart.
Figure 1.
Exposure to inorganic arsenic (iAS) induces sex-dependent increases in blood pressure and left ventricular wall thickness. Weekly systolic blood pressure in females (A) and males (B) (*P = 0.0219 vs. control). Left ventricular anterior wall thickness in diastole (LVAWd) in females (C) and males (D) (**P = 0.0010 vs. control) at 0, 4, and 8 wk of exposure. Left ventricular posterior wall thickness in diastole (LVPWd) in females (E) and males (F) (***P = 0.0004 vs. control) at 0, 4, and 8 wk of exposure. Significance was determined by two-way ANOVA with Tukey’s multiple comparisons test (n = 9-10 mice/group).
Table 1.
Echocardiographic parameters for male and female hearts at 0, 4, and 8 wk of iAS exposure
| Means ± SE, mm | Male Control |
Male iAS |
Female Control |
Female iAS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 | |||
| LVAWd (mm) | 0.85 | 0.88 | 0.88 | 0.84 | 0.93 | 0.98 | ** | 0.78 | 0.83 | 0.83 | 0.78 | 0.84 | 0.86 | NS |
| SE | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | ||
| LVIDd (mm) | 2.85 | 2.89 | 2.76 | 2.95 | 2.86 | 2.86 | NS | 2.62 | 2.59 | 2.54 | 2.61 | 2.60 | 2.59 | NS |
| SE | 0.07 | 0.05 | 0.05 | 0.08 | 0.08 | 0.10 | 0.05 | 0.05 | 0.02 | 0.07 | 0.05 | 0.05 | ||
| LVPWd (mm) | 0.82 | 0.85 | 0.82 | 0.81 | 0.90 | 0.93 | *** | 0.77 | 0.82 | 0.81 | 0.76 | 0.85 | 0.83 | NS |
| SE | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | ||
| LV mass (mg) | 54.96 | 59.73 | 59.21 | 55.32 | 62.84 | 70.12 | * | 42.65 | 46.75 | 49.12 | 43.40 | 49.75 | 51.54 | NS |
| SE | 2.65 | 1.31 | 2.74 | 2.17 | 2.83 | 3.33 | 1.82 | 1.44 | 0.90 | 2.31 | 1.77 | 1.91 | ||
| CO (mL/min) | 11.97 | 13.90 | 12.94 | 12.56 | 13.54 | 13.57 | NS | 9.93 | 11.29 | 9.96 | 9.68 | 10.77 | 11.43 | NS |
| SE | 0.82 | 0.40 | 0.57 | 1.07 | 0.78 | 0.76 | 0.68 | 0.46 | 0.66 | 0.80 | 0.47 | 0.46 | ||
| FS (%) | 36.90 | 34.86 | 36.44 | 36.19 | 34.20 | 36.38 | NS | 37.26 | 35.66 | 38.27 | 37.60 | 34.64 | 38.46 | NS |
| SE | 0.80 | 0.88 | 0.62 | 0.85 | 0.69 | 1.42 | 1.00 | 0.39 | 0.69 | 1.25 | 0.65 | 0.75 | ||
| EF (%) | 68.78 | 65.76 | 68.10 | 67.78 | 64.96 | 67.60 | NS | 69.49 | 67.20 | 70.68 | 69.84 | 65.94 | 70.85 | NS |
| SE | 1.12 | 1.20 | 0.81 | 1.15 | 1.01 | 2.04 | 1.36 | 0.51 | 0.85 | 1.67 | 0.90 | 0.96 | ||
| SV (µL) | 16.73 | 19.53 | 17.84 | 17.77 | 19.22 | 19.72 | NS | 13.73 | 15.58 | 14.63 | 13.34 | 14.93 | 15.63 | NS |
| SE | 1.09 | 0.53 | 0.82 | 1.69 | 1.32 | 1.25 | 0.92 | 0.64 | 0.57 | 1.03 | 0.69 | 0.58 | ||
| HR (bpm) | 713.64 | 711.85 | 728.01 | 712.07 | 708.60 | 693.99 | NS | 725.49 | 724.52 | 680.78 | 723.52 | 723.43 | 731.22 | NS |
| SE | 8.56 | 7.42 | 8.18 | 7.32 | 11.57 | 17.02 | 7.96 | 10.34 | 34.95 | 7.47 | 7.94 | 6.51 | ||
| Vd (µL) | 24.63 | 29.91 | 26.33 | 26.54 | 29.90 | 29.79 | NS | 19.94 | 23.25 | 20.83 | 19.51 | 22.80 | 22.20 | NS |
| SE | 1.90 | 1.08 | 1.23 | 2.90 | 2.39 | 2.56 | 1.54 | 0.94 | 0.79 | 1.88 | 1.23 | 1.02 | ||
| Vs (µL) | 7.90 | 10.39 | 8.49 | 8.77 | 10.68 | 10.07 | NS | 6.21 | 7.67 | 6.20 | 6.17 | 7.87 | 6.57 | NS |
| SE | 0.85 | 0.64 | 0.47 | 1.24 | 1.10 | 1.45 | 0.70 | 0.33 | 0.30 | 0.87 | 0.57 | 0.48 | ||
| Dd (mm) | 2.58 | 2.80 | 2.66 | 2.64 | 2.79 | 2.78 | NS | 2.37 | 2.53 | 2.42 | 2.34 | 2.51 | 2.48 | NS |
| SE | 0.08 | 0.04 | 0.05 | 0.11 | 0.09 | 0.10 | 0.08 | 0.04 | 0.04 | 0.09 | 0.05 | 0.05 | ||
| Ds (mm) | 1.63 | 1.83 | 1.69 | 1.69 | 1.84 | 1.78 | NS | 1.49 | 1.63 | 1.50 | 1.47 | 1.64 | 1.53 | NS |
| SE | 0.07 | 0.05 | 0.04 | 0.08 | 0.07 | 0.10 | 0.07 | 0.03 | 0.03 | 0.09 | 0.05 | 0.04 | ||
Semiautomated continuous tracing of the left ventricular wall measured or calculated the following cardiac parameters: left ventricular anterior wall thickness at end diastole (LVAWd), left ventricle internal diameter at end diastole (LVIDd), left ventricle posterior wall thickness at end diastole (LVPWd), left ventricular mass (LV mass), cardiac output (CO), percent fractional shortening (FS), percent ejection fraction (EF), stroke volume (SV), heart rate (HR), volume at end systole (Vs) and diastole (Vd), and diameter at systole (Ds) and diastole (Dd). Significance (*P = 0.0149 vs. control, **P = 0.0010 vs. control, ***P = 0.0004 vs. control) was determined by two-way ANOVA with Tukey’s multiple comparisons test (n = 9-10 mice/group). iAS, inorganic arsenic; NS, not significant.
Figure 2.
Exposure to inorganic arsenic (iAS) induces sex-dependent hypertrophy of the heart. Left ventricular mass (LV mass) extrapolated from transthoracic echocardiography in females (A) and males (B) (*P = 0.0149 vs. control, two-way ANOVA with Tukey’s multiple comparisons) at 0, 4, and 8 wk of exposure (n = 9-10 mice/group). Heart mass normalized to tibia length (HW/TL) in females (C) and males (D) (****P < 0.0001 vs. control; n = 24-25 hearts/group). Plasma atrial natriuretic peptide (ANP) in females (E) and males (F) (*P = 0.0379 vs. control; n = 9-10 mice/group). Plasma brain natriuretic peptide (BNP) in females (G) and males (H) (n = 9-10 mice/group). Outliers were determined by the ROUT method (Q = 1%), and significance was determined by Mann–Whitney test.
Exposure to iAS Does Not Induce Myocardial Fibrosis or Angiogenesis
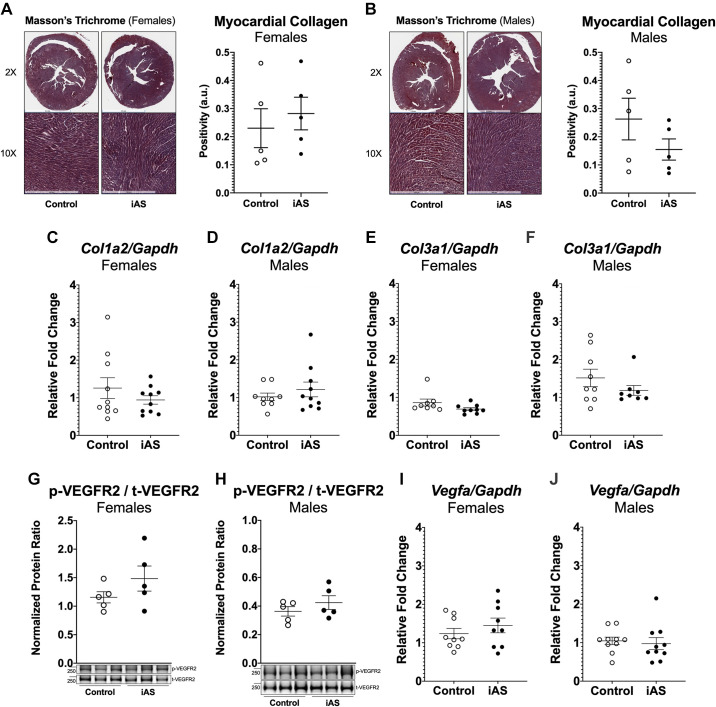
Several studies have shown that iAS activates the TGFβ/Smad pathway and induces fibrosis in mice and rats; thus, we investigated the possibility that the hypertrophic phenotype is driven by myocardial fibrosis (18). Masson’s trichome staining for collagen deposition, however, did not reveal an increase in fibrosis with iAS exposure in either sex (Fig. 3, A and B). Additionally, this was confirmed by measuring mRNA expression of profibrogenic extracellular matrix genes Col1a2 and Col3a1, for type I and type III collagen, respectively, which showed no significant changes (Fig. 3, C–F). Considering that angiogenesis in the heart may account for the lack of fibrosis and modulate the phenotype between the sexes, we evaluated the impact of iAS exposure on vascular endothelial growth factor (VEGF) by examining the mRNA levels of Vegfa in the heart. Furthermore, since VEGF can act though its tyrosine kinase receptors, we examined the protein expression of phosphorylated VEGF receptor 2 (p-VEGFR2) and t-VEGFR2. However, p-VEGFR2, t-VEGFR2, and Vegfa mRNA expression in the heart were not significantly altered with iAS exposure in either sex (Fig. 3, G–J). Since there was no evidence that an 8-wk exposure to 615 μg/L iAS stimulated either VEGF signaling or collagen transcription and deposition, angiogenesis and fibrosis of the heart are unlikely to drive the phenotype.
Figure 3.
Exposure to inorganic arsenic (iAS) does not induce angiogenesis or fibrosis in the heart. Masson’s trichome histology for collagen deposition in females (A) or males (B) (n = 9-10 hearts/group; representative images shown). Myocardial mRNA transcript levels of collagen type I α2 chain (Col1a2) in females (C) or males (D) (n = 10 hearts/group). Myocardial mRNA transcript levels of collagen type III α1 (Col3a1) in females (E) or males (F) (n = 10 hearts/group). Protein expression of phosphorylated vascular endothelial growth factor receptor 2 (p-VEGFR2) at Y1175 normalized to total VEGFR2 (t-VEGFR2) in female (G) and male (H) hearts (n = 5 hearts/group). Myocardial mRNA transcript levels of Vegfa in female (I) and male (J) hearts (n = 10 hearts/group). Transcript levels of each target were normalized to Gapdh mRNA expression, which did not change with iAS exposure in either sex. Outliers were determined by the ROUT method (Q = 1%), and significance was determined by Mann–Whitney test.
Exposure to iAS Induces Pathological Hypertrophy in the Male Heart
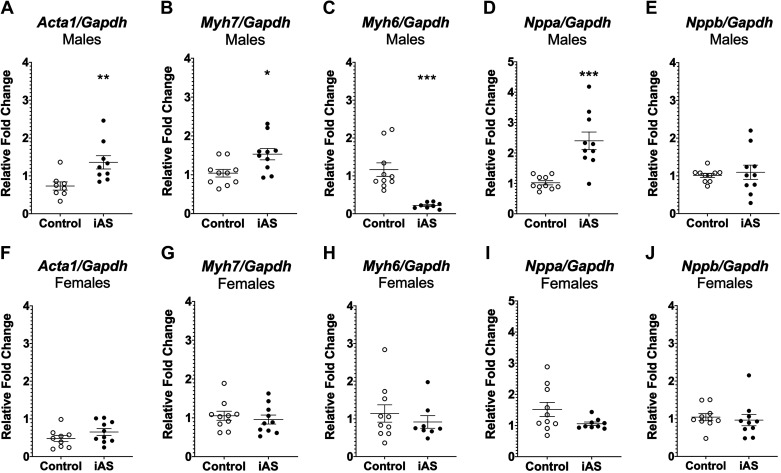
Determination of whether iAS induces maladaptive growth of the myocardium was achieved by probing for downstream transcriptional markers of pathological hypertrophy. RT-qPCR revealed that mRNA expression levels of several genes coupled to pathological hypertrophy were significantly increased with iAS exposure in males. Skeletal muscle α-actin (Acta1) and β-myosin heavy chain (Myh7) transcript levels were significantly increased in iAS-exposed males compared with controls, and alpha myosin heavy chain (Myh6) transcript levels were significantly decreased (Fig. 4, A–C). However, exposure to 615 μg/L iAS for 8 wk had no significant effects on mRNA expression levels of all such genes in females relative to controls (Fig. 4, F–H). Additionally, natriuretic peptide A (Nppa) mRNA levels were significantly increased in males but not in females, whereas no significant changes were observed in natriuretic peptide B (Nppb) transcript levels (Fig. 4, D, E, I and J), consistent with concentrations of circulating ANP and BNP, respectively (Fig. 2, E–H). Furthermore, since Akt and extracellular signal-regulated kinase (ERK) signaling can facilitate physiological hypertrophy, expression levels of p-ERK1/2, t-ERK1/2, p-Akt, and t-Akt protein, as well as Akt, RNA transcripts were probed in male and female hearts as a negative control, showing no significant effects of iAS exposure in either sex (Supplemental Fig. S3). Taken together, these data suggest that iAS exposure induces pathological hypertrophy in the male heart.
Figure 4.
Exposure to inorganic arsenic (iAS) induces markers of pathological hypertrophy in the heart. Myocardial mRNA transcript levels of skeletal muscle α-actin (Acta1; A) (**P = 0.0037 vs. control), β-myosin heavy chain (Myh7; B) (*P = 0.0138 vs. control), α-myosin heavy chain (Myh6; C) (***P = 0.0001 vs. control), natriuretic peptide A (Nppa; D) (***P = 0.0001 vs. control), and natriuretic peptide B (Nppb; E) in males (n = 10/hearts/group). Myocardial mRNA expression of Acta1 (F), Myh7 (G), Myh6 (H), Nppa (I), and Nppb (J) in female hearts (n = 10 hearts/group). Transcript levels of each target were normalized to Gapdh mRNA expression, which did not change with iAS exposure in either sex. Outliers were determined by the ROUT method (Q = 1%), and significance was determined by Mann–Whitney test.
Exposure to iAS Does Not Disrupt Nitric Oxide-Dependent Mechanisms of Cardioprotection in the Heart
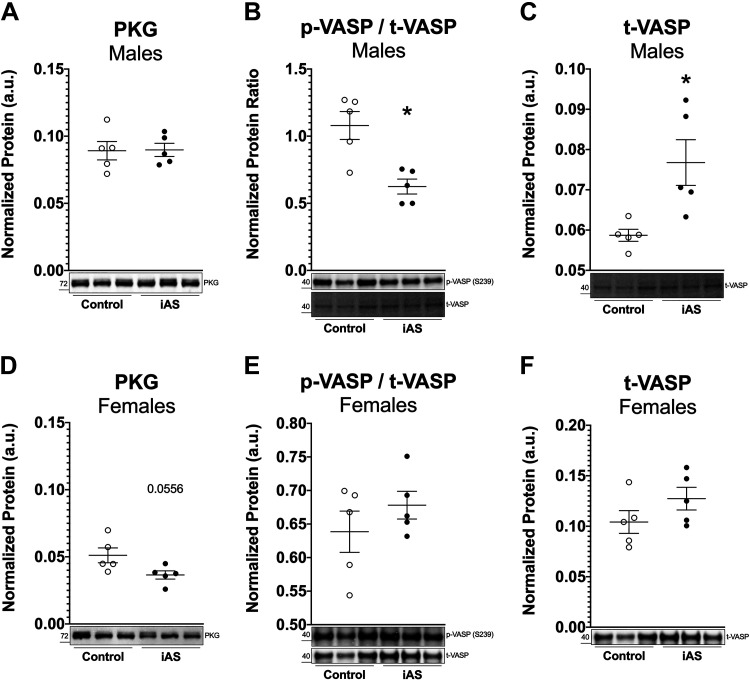
Several pathways were probed to investigate the impact of iAS exposure on cardioprotective mechanisms in the heart during this 8-wk period. Nitric oxide (NO) regulates several myocardial functions and can exert antihypertrophic effects though PKG signaling (19). Western blot for PKG, a mediator of cardioprotection, showed no difference in expression levels with iAS exposure in males but showed a decrease in iAS-exposed females trending toward significance (P = 0.0556) relative to controls (Fig. 5, A and D). Protein levels of p-VASP at S239, a downstream PKG target, and t-VASP were not altered by iAS exposure in female hearts (Fig. 5, E and F). However, protein levels of p-VASP were significantly decreased in iAS-exposed male hearts, wherease t-VASP levels were significantly increased compared with control (Fig. 5, B and C). As such, these data suggest that PKG signaling may be impaired as part of the mechanism leading to iAS-induced cardiac remodeling in males, but additional investigation along this signaling axis is necessary.
Figure 5.
Exposure to inorganic arsenic (iAS) may alter PKG signaling in the heart. Protein expression of PKG in male (A) and female (D) hearts (P = 0.0556 vs. control; n = 5 hearts/group). Protein expression of phosphorylated vasodilator-stimulated phosphoprotein (p-VASP) at S239, a PKG site, normalized to total VASP (t-VASP) in male (B) (*P = 0.0317 vs. control) and female (E) hearts (n = 5 hearts/group), and t-VASP in male (C) (*P = 0.0159 vs. control) and female (F) hearts (n = 5 hearts/group). Protein levels of each target were normalized to total transferred protein levels. Outliers were determined by the ROUT method (Q = 1%), and significance was determined by Mann–Whitney test.
Furthermore, it was hypothesized that eNOS, a significant producer of NO in the heart, might be upregulated by iAS exposure in females and either downregulated or uncoupled in males, thus modulating the sex-dependent hypertrophic response. However, Western blots for t-eNOS, p-eNOS at S1177, an activating site, and p-eNOS at T495, an inhibitory site, did not reveal significant changes with iAS exposure in either sex (Supplemental Fig. S4, A–C and F–H). Myocardial superoxide levels were measured as a marker of eNOS uncoupling in heart homogenates via EPR, but iAS exposure did not induce a change in superoxide production in either sex (Supplemental Fig. S4, D and I). Protein expression of iNOS was also probed, but iNOS bands were not detected in heart homogenates from either sex (data not shown). Although protein levels of nNOS were unchanged in iAS-exposed male hearts, these levels were significantly decreased in iAS-exposed female hearts (Supplemental Fig. S4, E and J). Since a previous study from our laboratory found that an acute 4-wk iAS exposure exacerbated myocardial I/R injury in females and reduced I/R injury in males, we examined the impact of a chonic 8-wk iAS exposure on I/R injury. However, the current treatment had no significant effects on functional recovery or infarct size in either male or female hearts, consistent with the null impact on NO signaling in the heart (Supplemental Fig. S5). Taken together, these results demonstrate that, whereas an 8-wk iAS exposure is sufficient to induce pathology in the male heart compared with a lack thereof at 4 wk, it is insufficient to (3) overcome the intrinsic mechanisms that protect the female heart and (20) alter susceptibility to ischemic heart injury.
iAS Exposure May Activate the Calcineurin-NFAT Pathway
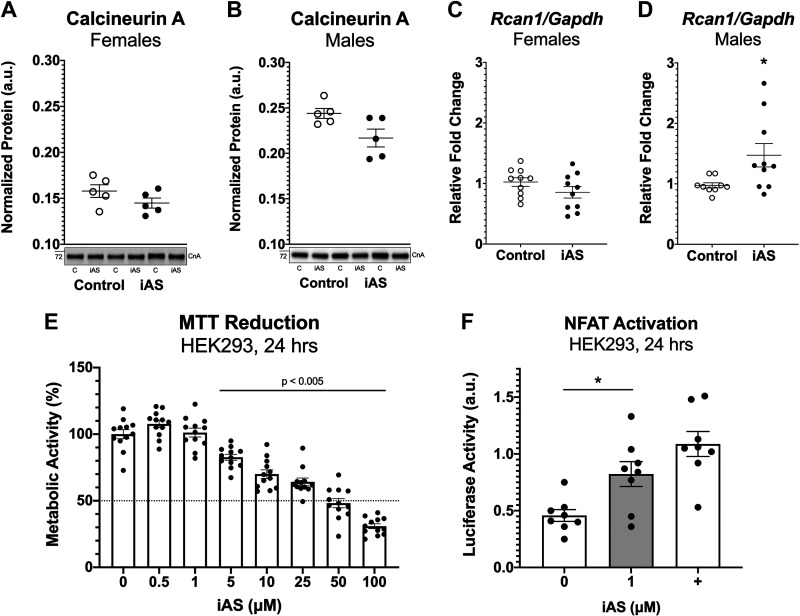
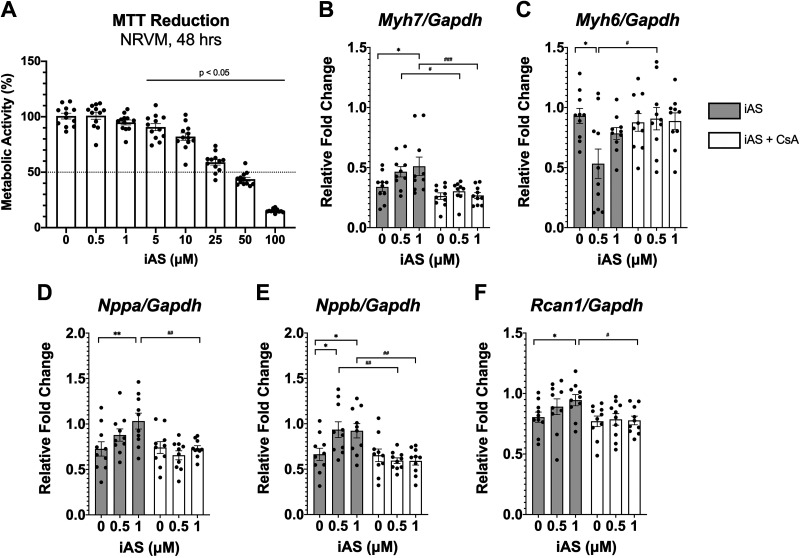
Considering that the calcineurin-NFAT signaling pathway is known to be upregulated with pathological and not physiological cardiac hypertrophy, levels of total calcineurin A protein expression in the heart were measured as a crude surrogate for calcineurin-NFAT activation, but these levels did not change with iAS exposure in either sex (Fig. 6, A and B). RT-qPCR, however, revealed that mRNA expression of regulator of calcineurin 1 (Rcan1), a specific calcineurin-NFAT target, was upregulated in iAS-exposed males relative to controls and not in females (Fig. 6, C and D), suggesting iAS may induce pathological hypertrophy of the heart in part by activation of calcineurin-NFAT. Seeing that echocardiography data characterized a significant increase in left ventricular wall thickness before the observed increase in blood pressure, an in vitro exposure model was established to determine whether iAS could activate NFAT independently of hemodynamic input. HEK-293 cells, chosen for transfection efficiency, were treated with varying concentrations of iAS for 24 h, and an MTT reduction assay was performed to assess cell viability and determine an appropriate exposure concentration; this revealed a dose-response curve indicating that 1 μM iAS does not significantly alter metabolic activity or viability relative to control (Fig. 6E). HEK-293 cells were then transfected with an NFAT-luciferase reporter, which showed that iAS exposure significantly increased NFAT-luciferase activity, indicating that iAS may induce the activation of NFAT independent of blood pressure (Fig. 6F). Furthermore, to complement these results, we examined the impact of iAS on neonatal rat ventricular myocytes (NRVMs). An MTT assay was repeated in NRVMs to confirm appropriate dosage, indicating that 0.5 and 1 μM iAS do not significantly alter cell viability relative to control (Fig. 7A). Consistent with our in vivo findings (Fig. 4), RT-qPCR revealed significantly increased mRNA expression levels of Myh7, Nppa, Nppb, and Rcan1 as well as decreased Myh6 with iAS exposure relative to control (Fig. 7, B–F). We further performed a rescue experiment using cyclosporin A, which showed that these changes in mRNA transcript levels were blocked with calcineurin inhibition (Fig. 7, B–F). Together, these data suggest that iAS has the potential to directly activate calcineurin-NFAT signaling and contribute to pathological hypertrophy in the male heart.
Figure 6.
Exposure to inorganic arsenic (iAS) may activate the calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway. Protein expression of calcineurin A in male (A) and female (B) hearts (n = 5 hearts/group). Protein levels of each target were normalized to total transferred protein levels. Myocardial mRNA transcript levels of regulator of calcineurin 1 (Rcan1) in females (C) and males (D) (*P = 0.0161 vs. control; n = 10 hearts/group). Transcript levels of each target were normalized to Gapdh mRNA expression, which did not change with iAS exposure in either sex. E: MTT reduction in HEK-293 cells treated with varying levels of iAS for 24 h, represented by optical density as a percentage of the average control, following a dose-response curve (P < 0.005 vs. control, ordinary one-way ANOVA with Dunnett’s multiple comparisons, n = 12 wells/group). F: NFAT-luciferase activity in HEK-293 cells transfected with NFAT-luciferase and Renilla-luciferase, as well as Trpc6 as a positive control (+), and treated with 1 μM iAS for 24 h (*P = 0.0214 vs. control; n = 8 wells/group). NFAT firefly luminescence was normalized to Renilla luminescence. Outliers were determined by the ROUT method (Q = 1%), and significance was determined by Mann–Whitney test.
Figure 7.
Calcineurin inhibition with cyclosporin A (CsA) blocks the impact of inorganic arsenic (iAS) exposure on neonatal rat ventricular myocytes (NRVMs). A: MTT reduction in NRVMs treated with varying levels of iAS for 48 h, represented by optical density as a percentage of the average control, following a dose response curve (P < 0.05 vs. control, ordinary one-way ANOVA with Dunnett’s multiple comparisons; n = 12 wells/group). Myocyte mRNA expression of Myh7 (B) (0.5 μM iAS vs. control, *P = 0.0280; 0.5 μM iAS + CsA vs. vehicle, #P = 0.0493; 1 μM iAS + CsA vs. vehicle, ###P = 0.0009); Myh6 (C) (0.5 μM iAS vs. control, *P = 0.0355; 0.5 μM iAS + CsA vs. vehicle, #P = 0.0433); Nppa (D) (1 μM iAS vs. control, **P = 0.0069; 1 μM iAS + CsA vs. vehicle, ##P = 0.0082); Nppb (E) (0.5 μM iAS vs. control, *P = 0.0205; 1 μM iAS vs. control, *P = 0.0290; 0.5 μM iAS + CsA vs. vehicle, ##P = 0.0022; 1 μM iAS + CsA vs. vehicle, ##P = 0.0030); and Rcan1 (F) (1 μM iAS vs. control, #P = 0.0433; 1 μM iAS + CsA vs. vehicle, *P = 0.0147) treated with 0.5 or 1 μM iAS and either 2 μM CsA or vehicle control (0.0025% DMSO) (n = 10 wells/group). Transcript levels of each target were normalized to Gapdh mRNA expression, which did not change with iAS exposure or CsA treatment. Outliers were determined by the ROUT method (Q = 1%), and significance was determined by ordinary one-way ANOVA with Sidak’s multiple comparisons test.
DISCUSSION
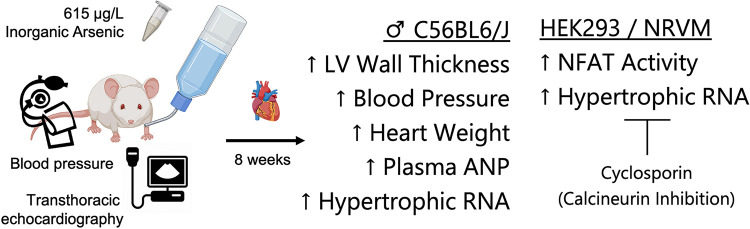
Epidemiological studies have associated chonic iAS exposure with the development of cardiovascular disease, and recent findings highlight an association with altered left ventricular geometry (9–11, 21). Herein, we find that exposure to an environmentally relevant dose of iAS though drinking water induces pathological hypertrophy of the heart in male mice but not in females (Fig. 8). Although female hearts may display a pathological phenotype in a longer model of exposure, consistent with previous findings, our data indicate that NO-dependent mechanisms of cardioprotection remain intact at this time point (22). Furthermore, we notably report, for the first time, that iAS may induce these changes, in part, by activating the calcineurin-NFAT signaling pathway.
Figure 8.
Exposure to inorganic arsenic (iAS) induces sex-dependent pathological hypertrophy in the heart, in part by activating the calcineurin-nuclear factor of activated T cells (NFAT) pathway. Graphic summary of the major findings in this study. Exposure to 615 μg/L iAS through drinking water for 8 wk was observed to increase left ventricular wall thickness, systolic blood pressure, heart mass normalized to tibia length (HW/TL), plasma atrial natriuretic peptide (ANP), and mRNA expression of the fetal gene program in male hearts. Furthermore, iAS exposure was observed to activate NFAT transcription in HEK-293 cells and stimulate fetal gene expression in neonatal rat ventricular myocytes (NRVMs), which was blocked by inhibiting calcineurin with cyclosporin treatment.
An association between iAS exposure and increased systolic blood pressure, as well as an increased prevalence ratio for hypertension, has been noted in several epidemiological studies in both biological sexes (23–29). Experimental studies corroborate this increase in systolic blood pressure in male rats exposed to 50,000 μg/L iAS for 28 wk, as well as in female mice exposed to 100 μg/L iAS for 22 wk (30, 31). However, mechanistic studies have found that the effect of chonic, low-level iAS on blood pressure often differs from that of higher levels (18). Since our previous study reported that a 4-wk exposure to an environmentally relevant dose of iAS was sufficient to induce mild hypertrophy and exacerbate I/R injury in female hearts while reducing I/R injury in male hearts, we utilized a longer, 8-wk exposure in the present study to overcome any potentially transient or compensatory phenotypes (12). We examined the impact of an 8-wk iAS exposure on myocardial I/R injury but found no significant changes in post-I/R functional recovery or infarct size in either male or female hearts (Supplemental Fig. S5). Although our current findings differ from those reported in our previous study, these differences likely resulted from time- and concentration-dependent differences in iAS exposure, providing further evidence that iAS has time- and concentration-dependent impacts on the heart (12).
Interestingly, there was evidence of cardiac remodeling in males during this 8-wk period. Systolic blood pressure was significantly increased in female mice exposed to 615 μg/L during the first and second weeks of exposure, but this increase returned to baseline though the eighth week, suggesting that the female heart remains protected at this time point (Fig. 1). Considering that a 22-wk iAS exposure has been reported to increase systolic blood pressure in female mice and that iAS is not epidemiologically reported to have a sex-disparate impact on blood pressure, systolic blood pressure between iAS-treated and control female mice is expected to diverge with an extended model of exposure (30). Systolic blood pressure in males, on the other hand, increased at the seventh and eighth weeks of exposure (Fig. 1). Additionally, LVAWd and LVPWd increased with iAS exposure in males at the eighth week, together demonstrating that iAS exposure induces cardiac remodeling in males (Fig. 1). Conversely, female hearts showed no evidence of remodeling, which differs from our previous study, in which a mild hypertrophic phenotype was observed with 4 wk of iAS exposure (12). However, these differences likely resulted from time- and concentration-dependent effects.
Further examination of biological markers substantiated an iAS-induced hypertrophic phenotype in males and not in females. Left ventricular mass extrapolated from echocardiography increased with iAS in males at the eighth week, yet there was no change with iAS in females; this was confirmed by heart mass normalized to tibia length (Fig. 2). Plasma levels of ANP, which is stimulated by mechanical stretch of the atrial wall and is upregulated in cardiac hypertrophy, were increased in iAS-exposed males and not in females (Fig. 2). However, plasma levels of BNP, which can also be upregulated with hypertrophy, did not change with iAS exposure in either sex (Fig. 2). Whereas ANP and BNP both promote overlapping antihypertrophic actions, the former is a primary regulator of cardiomyocyte size and blood pressure/volume, whereas the latter is a major regulator of cardiac fibrosis, acting to inhibit the transforming growth factor-β (TGFβ) signaling pathway (32, 33). Quantification of myocardial collagen deposition by image analysis showed no difference with iAS exposure in either sex, in accord with plasma BNP levels (Fig. 3). Additionally, measurement of Col1a2 and Col3a1 mRNA in the myocardium confirmed that iAS exposure did not upregulate TGFβ-responsive extracurricular matrix genes to induce cardiac fibrosis (Fig. 3). Although experimental literature suggests that iAS can activate the TGFβ pathway to induce the fibrosis of several organs, including the heart, liver, and lung, those studies used higher concentrations than the dose used in the current study (18). Other studies have shown that iAS exposure may stimulate VEGF as a potential mechanism of carcinogenesis, and as such, we investigated whether the current exposure had an impact on angiogenesis, but we found no evidence of altered p-VEGFR2, t-VEGFR2, or Vegfa mRNA levels in the heart (Fig. 3) (34–36). We therefore examined previously uninvestigated mechanisms though which a chonic, low-dose, and environmentally relevant iAS exposure might induce cardiac hypertrophy.
Both physiological and pathological biomarkers were probed, revealing that iAS induces pathological hypertrophy of the heart. Epidemiological evidence does not suggest that iAS confers cardiovascular benefits, but functional echocardiography parameters did not change at this 8-wk time point, consistent with the null impact on p-PLN, t-PLN, and SERCA2 protein expression (Table 1 and Supplemental Fig. S2) (12). As such, the possibility of physiological growth was examined. Akt is a downstream regulator of several critical processes involved in adaptive hypertrophy, including cell proliferation, growth, metabolism, and survival, and many studies have shown that iAS activates Akt though a variety of mechanisms in carcinogenesis (35, 37–40). Since Akt signaling is not exclusive to physiological hypertrophy, the impact of iAS exposure on ERK1/2 was also examined. ERK1/2 is another central regulator of cell survival, with a major role in promoting physiological hypertrophy, and several studies have shown that iAS exposure activates ERK1/2 in other cell types as a potential mechanism of carcinogenesis (14, 41–45). However, we found no evidence of Akt or ERK1/2 activation with iAS exposure in either sex, suggesting that iAS exposure does not induce physiological growth of the heart (Supplemental Fig. S3). Myocardial expression of a suite of genes coupled to pathological hypertrophy was therefore examined. Skeletal muscle α-actin, Acta1, decreases in the physiologically hypertrophied heart but increases with pathological hypertrophy and is associated with cardiac dysfunction. Exposure to iAS increased Acta1 mRNA expression in male and not female hearts, in accord with the hypothesis that iAS induces pathological hypertrophy (Fig. 4). Myosin heavy chain undergoes an isoform switch from its α-form Myh6 to its β-form Myh7 in the stressed, hypertrophied, and failing heart. Myocardial mRNA levels of Myh7 and Myh6 were increased and decreased, respectively, with iAS exposure in male hearts, and no changes were observed in female hearts, further demonstrating that iAS induces pathological hypertrophy (Fig. 4). Additionally, myocardial mRNA transcripts for ANP, Nppa, and BNP, Nppb, which are upregulated in cardiac hypertrophy, were increased and unchanged, respectively, in accord with protein levels found in plasma (Fig. 2 and Fig. 4). Although female hearts did not exhibit these changes, NO-dependent mechanisms of cardioprotection in the heart remained largely intact at this 8-wk timepoint (Supplemental Figs. S4 and S5). Taken together, these data highlight that iAS exposure induces pathological hypertrophy in the male heart.
Further investigation probed mechanisms of iAS-induced pathological remodeling of the myocardium. A previous study reported that a 50,000 μg/L iAS exposure for 12 wk induced systemic hypertension in male rats by increasing the expression of angiotensin II, angiotensin II type I receptor, and angiotensin-converting enzyme (46). Additionally, blocking the angiotensin II receptor ameliorated iAS-induced hypertension and TGFβ-mediated fibrosis (47). However, considering that our current study of an 8-wk, 615 μg/L iAS exposure did not find any evidence of myocardial fibrosis, the possibility that iAS directly induces hypertrophy, beyond input from elevated blood pressure, was explored. Calcineurin is a phosphatase that activates NFAT transcription factors, which have been shown to be both necessary and sufficient to induce pathological cardiac hypertrophy (48). Protein levels of calcineurin A, the catalytic subunit of the serine-theonine phosphatase, were measured in the myocardium as a crude surrogate for calcineurin-NFAT activation, but these levels were unchanged with iAS exposure in both sexes (Fig. 6). Regulator of calcineurin 1, Rcan1, is a specific NFAT target that is upregulated with calcineurin-NFAT activation in a negative feedback loop (49–51). Measuring its mRNA expression revealed that iAS exposure significantly increased Rcan1 in the male heart and not in females, in accord with the phenotype, suggesting that iAS may induce pathological cardiac hypertrophy, in part, by targeting the calcineurin-NFAT pathway (Fig. 6).
We therefore generated an in vitro model of exposure to determine whether iAS directly activates calcineurin-NFAT signaling independent of blood pressure. Considering that males presented with a significant increase in LVAWd and a trending increase in LVPWd at the fourth week of exposure, before an observed increase in systolic blood pressure, it was hypothesized that iAS might directly induce cardiac hypertrophy. Treatment with iAS significantly increased NFAT luciferase activity in HEK-293 cells, suggesting that iAS has the potential to directly activate the calcineurin-NFAT pathway (Fig. 6). Additionally, we examined the impact of iAS exposure on NRVMs, which revealed increased mRNA transcript levels of Myh7, Nppa, Nppb, and Rcan1, as well as decreased Myh6, consistent with in vivo data (Fig. 4 and Fig. 7). Finally, we found that inhibiting calcineurin with CsA blocked these changes, further demonstrating that iAS can activate the calcineurin-NFAT signaling pathway (Fig. 7). Establishing this causal mechanism clarifies observational associations of iAS exposure and cardiac remodeling in humans independent of hypertension, and it stimulates further investigations not only upstream of NFAT activation, but also in the context of other environmental exposures and CVD endpoints (11).
iAS has been shown to activate several transcription factors, including nuclear factor erythoid 2-related factor 2 (Nrf2), nuclear factor κB (NF-κB), and activating protein-1 (AP-1) (22). Whereas iAS-induced activation of Nrf2 is primarily mediated by the production of reactive oxygen species (ROS), its activation of NF-κB is mediated by both ROS and direct binding of iAS to reactive thiols on an inhibitory protein (52). Considering that NFAT is activated by an array of factors, including proteins with zinc and cysteine-rich domains, NF-κB, AP-1, and ROS, iAS exposure may act though multiple such pathways to mediate the hypertrophic response (53–56). As such, these data reasonably suggest that iAS may target blood pressure as well as the calcineurin-NFAT pathway to induce cardiac hypertrophy. Although additional studies are necessary to confirm these findings, it is understood that cardiac remodeling is an independent risk factor for adverse cardiovascular outcomes, and by demonstrating that iAS exposure may directly induce pathological hypertrophy of the heart, this investigation confers significance to upholding iAS exposure as a theat to the cardiovascular system (20, 57, 58).
Limitations
Several limitations to this study require acknowledgment. First, although the model of exposure used here doubled the duration of a model used previously by our laboratory, the divergence in systolic blood pressure during the last 2 wk in iAS-treated males, paired with the lack thereof in females, highlights not only the temporal nature of the cardiovascular effects of iAS exposure but also the limitation of not capturing the phenotypes induced by a lifetime of exposure in an 8-wk study; a longer duration is thus recommended for future investigations (12). Moreover, although echocardiography was conducted at three time points thoughout this study, additional measurements would provide further resolution. Seeing that the present study did not find epidemiologically observed alterations in the female heart, it is recommended that future studies extend this exposure beyond 8 wk such that a hypertrophic phenotype is observed in both sexes.
Conclusions
Overall, this is the first mechanistic investigation examining an environmentally relevant iAS exposure on the development of cardiac hypertrophy in male and female mice. During our 8-wk iAS exposure, males showed an increase in systolic blood pressure and altered cardiac geometry, whereas females appeared to remain protected from such effects. iAS treatment induced an array of molecular changes, observed for the first time, indicating pathological hypertrophy in the male heart, though these were not detected in female hearts. Further data support the novel finding that iAS induces pathological hypertrophy of the heart, in part, by activation of the calcineurin-NFAT pathway. Taken together, this investigation provides a mechanistic link between an environmentally relevant iAS exposure, the induction of pathological hypertrophy, and the increased the risk for adverse cardiovascular outcomes. Moreover, these findings underscore the importance of iAS removal from human consumption.
GRANTS
This work was supported by National Institutes of Health Grants R01HL136496 (to M.J.K.), R01HL136918 and R01HL063030 (to N.P.), and T32AG058527 (to G.K.) and Deutsche Forschungsgemeinschaft Grant OE 688/1-1 (to C.U.O.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.K. and M.J.K. conceived and designed research; R.K., P.S., S.M., O.V.E., N.T., C.U.O., G.K. and R.C. performed experiments; R.K., S.M., C.U.O., G.K. and R.C. analyzed data; R.K., A.M.R., M.J.K., S.M., O.V.E., C.U.O. and N.P. interpreted results of experiments; R.K. prepared figures; R.K. and M.J.K. drafted manuscript; R.K., M.J.K., and O.V.E. edited and revised manuscript; R.K., A.M.R., M.J.K., P.S., S.M., O.V.E., N.T., C.U.O., G.K., R.C. and N.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jennifer Pluznick and Jason Sanchez for scientific advice and for technical assistance.
REFERENCES
- 1.Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med 369: 448–457, 2013. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1736–1788, 2018. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC, Virani SS, Williams KA, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 74: e177–e232, 2019. [Erratum in J Am Coll Cardiol 74: 1429-1430, 2019]. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12: 627–642, 2015. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 5.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123: 305–332, 2011. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17: 517–568, 2002. doi: 10.1016/S0883-2927(02)00018-5. [DOI] [Google Scholar]
- 7.Kapaj S, Peterson H, Liber K, Bhattacharya P. Human health effects from chonic arsenic poisoning–a review. J Environ Sci Health A Tox Hazard Subst Environ Eng 41: 2399–2428, 2006. doi: 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed Abdul KS, Jayasinghe SS, Chandana EPS, Jayasumana C, MangalaDe Silva CS. Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40: 828–846, 2015. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14: 542–555, 2012. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann Intern Med 159: 649–659, 2013. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichler G, Grau-Perez M, Tellez-Plaza M, Umans J, Best L, Cole S, Goessler W, Francesconi K, Newman J, Redon J, Devereux R, Navas-Acien A. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging 12: e009018, 2019. doi: 10.1161/CIRCIMAGING.119.009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veenema RJ, Casin KM, Sinha P, Kabir R, Mackowski N, Taube N, Bedja D, Chen R, Rule A, Kohr MJ. Inorganic arsenic exposure induces sex disparate effects and exacerbates ischemia-reperfusion injury in the female heart. Am J Physiol Heart Circ Physiol 316: H1053–H1064, 2019. doi: 10.1152/ajpheart.00364.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murko M, Elek B, Styblo M, Thomas DJ, Francesconi KA. Dose and diet—sources of arsenic intake in mouse in utero exposure scenarios. Chem Res Toxicol 31: 156–164, 2018. doi: 10.1021/acs.chemrestox.7b00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Z. The molecular mechanisms of arsenic-induced cell transformation and apoptosis. Environ Health Perspect 110: 757–759, 2002. doi: 10.1289/ehp.02110s5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin BL, Matera D, Doerner JF, Zheng N, Camino D. D, Mishra S, Bian H, Zeveleva S, Zhen X, Blair NT, Chong JA, Hessler DP, Bedja D, Zhu G, Muller GK, Ranek MJ, Pantages L, McFarland M, Netherton MR, Berry A, Wong D, Rast G, Qian HS, Weldon SM, Kuo JJ, Sauer A, Sarko C, Moran MM, Kass DA, Pullen SS. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc Natl Acad Sci U S A 116: 10156–10161, 2019. doi: 10.1073/pnas.1815354116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation: novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol 48: 713–724, 2010. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarewich CA, Zhang H, Davis J, Correll RN, Trappanese DM, Hoffman NE, Troupes CD, Berretta RM, Kubo H, Madesh M, Chen X, Gao E, Molkentin JD, Houser SR. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction. Circ Res 115: 567–580, 2014. doi: 10.1161/CIRCRESAHA.115.303831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai J, Xu M, Zhang X, Niu Q, Hu Y, Li Y, Li S. Bi-directional regulation of TGF-β/Smad pathway by arsenic: a systemic review and meta-analysis of in vivo and in vitro studies. Life Sci 220: 92–105, 2019. doi: 10.1016/j.lfs.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 333: 191–201, 2010. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo PS, Polegato BF, Minicucci MF, Paiva SAR, Zornoff LAM. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol 106: 62–69, 2016. doi: 10.5935/abc.20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, van Green A, Ahsan H. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ 342: d2431–d2431, 2011. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu Rev Pharmacol Toxicol 47: 243–262, 2007. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, Bangalore S, Newman JD, Ahmed A, Islam T, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, Slavkovich V, Argos M, Bryan MS, Farzan SF, Hayes RB, Graziano JH, Ahsan H, Chen Y. Association between arsenic exposure from drinking water and longitudinal change in blood pressure among HEALS cohort participants. Environ Health Perspect 123: 806–812, 2015. doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunrath J, Gurzau E, Gurzau A, Goessler W, Gelmann ER, Thach T-T, Mccarty KM, Yeckel CW. Blood pressure hyperreactivity: an early cardiovascular risk in normotensive men exposed to low-to-moderate inorganic arsenic in drinking water. J Hypertens 31: 361–369, 2013. doi: 10.1097/HJH.0b013e32835c175f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwok RK, Mendola P, Liu ZY, Savitz DA, Heiss G, Ling HL, Xia Y, Lobdell D, Zeng D, Thorp JM Jr, Creason JP, Mumford JL. Drinking water arsenic exposure and blood pressure in healthy women of reproductive age in Inner Mongolia, China. Toxicol Appl Pharmacol 222: 337–343, 2007. doi: 10.1016/j.taap.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, Sparrow D, Vokonas P, Schwartz J. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the Normative Aging Study. Environ Health Perspect 120: 98–104, 2012. doi: 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osorio-Yáñez C, Ayllon-Vergara JC, Arreola-Mendoza L, Aguilar-Madrid G, Hernández-Castellanos E, Sánchez-Peña LC, Del Razo LM. blood pressure, left ventricular geometry, and systolic function in children exposed to inorganic arsenic. Environ Health Perspect 123: 629–635, 2015. doi: 10.1289/ehp.1307327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension 33: 74–78, 1999. doi: 10.1161/01.HYP.33.1.74. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Mao G, He S, Yang Z, Yang W, Zhang X, Qiu W, Ta N, Cao L, Yang H, Guo X. Relationship between long-term exposure to low-level arsenic in drinking water and the prevalence of abnormal blood pressure. J Hazard Materials 262: 1154–1158, 2013. doi: 10.1016/j.jhazmat.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Soria P, Broka D, Monks SL, Camenisch TD. Chronic low-level arsenite exposure though drinking water increases blood pressure and promotes concentric left ventricular hypertrophy in female mice. Toxicol Pathol 40: 504–512, 2012. doi: 10.1177/0192623311432297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang H-T, Chou H-J, Han B-C, Huang S-Y. Lifelong inorganic arsenic compounds consumption affected blood pressure in rats. Food Chem Toxicol 45: 2479–2487, 2007. doi: 10.1016/j.fct.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 32.John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267: 679–681, 1995. [Erratum in Science 267:1753, 1995]. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 33.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M, Nakao K. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A 97: 4239–4244, 2000. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao Y-H, Yu C-L, Chang LW, Yu H-S. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angiogenesis in vitro. Chem Res Toxicol 16: 460–468, 2003. doi: 10.1021/tx025652a. [DOI] [PubMed] [Google Scholar]
- 35.Liu L-Z, Jiang Y, Carpenter RL, Jing Y, Peiper SC, Jiang B-H. Role and mechanism of arsenic in regulating angiogenesis. PLoS One 6: e20858, 2011. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soucy NV, Ihnat MA, Kamat CD, Hess L, Post MJ, Klei LR, Clark C, Barchowsky A. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol Sci 76: 271–279, 2003. [Erratum in Toxicol Sci 83: 405, 2005]. doi: 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- 37.Beezhold K, Liu J, Kan H, Meighan T, Castranova V, Shi X, Chen F. miR-190-mediated downregulation of PHLPP contributes to arsenic-induced Akt activation and carcinogenesis. Toxicol Sci 123: 411–420, 2011. doi: 10.1093/toxsci/kfr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter RL, Jiang Y, Jing Y, He J, Rojanasakul Y, Liu L-Z, Jiang B-H. Arsenite induces cell transformation by reactive oxygen species, AKT, ERK1/2, and p70S6K1. Biochem Biophys Res Commun 414: 533–538, 2011. doi: 10.1016/j.bbrc.2011.09.102. [DOI] [PubMed] [Google Scholar]
- 39.Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang X-G, Shi X, Jiang B-H. Arsenite induces HIF-1α and VEGF though PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem 255: 33–45, 2004. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang W, Luo W, Zhang D, Jian J, Ma Q, Li J, Shi X, Chen J, Gao J, Huang C. PI-3K/Akt pathway-dependent cyclin D1 expression is responsible for arsenite-induced human keratinocyte transformation. Environ Health Perspect 116: 1–6, 2008. doi: 10.1289/ehp.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhury R, Chatterjee R, Giri AK, Mandal C, Chaudhuri K. Arsenic-induced cell proliferation is associated with enhanced ROS generation, Erk signaling and CyclinA expression. Toxicol Lett 198: 263–271, 2010. doi: 10.1016/j.toxlet.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Gallo S, Vitacolonna A, Bonzano A, Comoglio P, Crepaldi T. ERK: a key player in the pathophysiology of cardiac hypertrophy. IJMS 20: 2164, 2019. doi: 10.3390/ijms20092164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X-M, Ma Y-T, Yang Y-N, Liu F, Chen B-D, Han W, Zhang J-F, Gao X-M. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin Exp Pharmacol Physiol 36: 1054–1061, 2009. doi: 10.1111/j.1440-1681.2009.05243.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu T-H, Tseng T-J, Su C-C, Tang F-C, Yen C-C, Liu Y-Y, Yang C-Y, Wu C-C, Chen K-L, Hung D-Z, Chen Y-W. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis though JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol Lett 224: 130–140, 2014. doi: 10.1016/j.toxlet.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Mutlak M, Schlesinger-Laufer M, Haas T, Shofti R, Ballan N, Lewis YE, Zuler M, Zohar Y, Caspi LH, Kehat I. Extracellular signal-regulated kinase (ERK) activation preserves cardiac function in pressure overload induced hypertrophy. Int J Cardiol 270: 204–213, 2018. doi: 10.1016/j.ijcard.2018.05.068. [DOI] [PubMed] [Google Scholar]
- 46.Waghe P, Sarath TS, Gupta P, Kandasamy K, Choudhury S, Kutty HS, Mishra SK, Sarkar SN. Arsenic causes aortic dysfunction and systemic hypertension in rats: augmentation of angiotensin II signaling. Chem Biol Interact 237: 104–114, 2015. doi: 10.1016/j.cbi.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Khuman MW, Harikumar SK, Sadam A, Kesavan M, Susanth VS, Parida S, Singh KP, Sarkar SN. Candesartan ameliorates arsenic-induced hypertensive vascular remodeling by regularizing angiotensin II and TGF-beta signaling in rats. Toxicology 374: 29–41, 2016. doi: 10.1016/j.tox.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Wilkins BJ, Dai Y-S, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 49.Dirkx E, da Costa Martins PA, De Windt LJ. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta 1832: 2414–2424, 2013. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, van Rooij E, De Windt LJ, Rothenberg ME, Tschop MH, Benoit SC, Molkentin JD. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc Natl Acad Sci U S A 103: 7327–7332, 2006. doi: 10.1073/pnas.0509340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, Williams RS, Olson EN. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc Natl Acad Sci U S A 100: 669–674, 2003. doi: 10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar NV, Yang J, Pillai JK, Rawat S, Solano C, Kumar A, Grøtli M, Stemmler TL, Rosen BP, Tamás MJ. Arsenic directly binds to and activates the yeast AP-1-like transcription factor Yap8. Mol Cell Biol 36: 913–922, 2015. doi: 10.1128/MCB.00842-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian Z-Y, Huang H, Jiang H, Shen D-F, Yan L, Zhu L-H, Wang L, Cao F, Liu C, Tang Q-Z, Li H. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension 55: 257–263, 2010. doi: 10.1161/HYPERTENSIONAHA.109.135665. [DOI] [PubMed] [Google Scholar]
- 54.Erlanson DA, Chytil M, Verdine GL. The leucine zipper domain controls the orientation of AP-1 in the NFAT·AP-1·DNA complex. Chem Biol 3: 981–991, 1996. doi: 10.1016/s1074-5521(96)90165-9. [DOI] [PubMed] [Google Scholar]
- 55.Fujii T, Onohara N, Maruyama Y, Tanabe S, Kobayashi H, Fukutomi M, Nagamatsu Y, Nishihara N, Inoue R, Sumimoto H, Shibasaki F, Nagao T, Nishida M, Kurose H. Gα12/13-mediated production of reactive oxygen species is critical for angiotensin receptor-induced NFAT activation in cardiac fibroblasts. J Biol Chem 280: 23041–23047, 2005. doi: 10.1074/jbc.M409397200. [DOI] [PubMed] [Google Scholar]
- 56.Serfling E, Berberich-Siebelt F, Avots A, Chuvpilo S, Klein-Hessling S, Jha MK, Kondo E, Pagel P, Schulze-Luehmann J, Palmetshofer A. NFAT and NF-κB factors—the distant relatives. Int J Biochem Cell Biol 36: 1166–1170, 2004. doi: 10.1016/j.biocel.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat Rev Cardiol 8: 673–685, 2011. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 58.Kannel WB. Left ventricular hypertrophy as a risk factor: the Framingham experience. J Hypertens Suppl 9: S3–S8, 1991. doi: 10.1097/00004872-199112002-00002. [DOI] [PubMed] [Google Scholar]