Abstract
The goal of this work was to investigate the role of t-tubule (TT) remodeling in abnormal Ca2+ cycling in ventricular myocytes of failing dog hearts. Heart failure (HF) was induced using rapid right ventricular pacing. Extensive changes in echocardiographic parameters, including left and right ventricular dilation and systolic dysfunction, diastolic dysfunction, elevated left ventricular filling pressures, and abnormal cardiac mechanics, indicated that severe HF developed. TT loss was extensive when measured as the density of total cell volume, derived from three-dimensional confocal image analysis, and significantly increased the distances in the cell interior to closest cell membrane. Changes in Ca2+ transients indicated increases in heterogeneity of Ca2+ release along the cell length. When critical properties of Ca2+ release variability were plotted as a function of TT organization, there was a complex, nonlinear relationship between impaired calcium release and decreasing TT organization below a certain threshold of TT organization leading to increased sensitivity in Ca2+ release below a TT density threshold of 1.5%. The loss of TTs was also associated with a greater incidence of triggered Ca2+ waves during rapid pacing. Finally, virtually all of these observations were replicated by acute detubulation by formamide treatment, indicating an important role of TT remodeling in impaired Ca2+ cycling. We conclude that TT remodeling itself is a major contributor to abnormal Ca2+ cycling in HF, reducing myocardial performance. The loss of TTs is also responsible for a greater incidence of triggered Ca2+ waves that may play a role in ventricular arrhythmias arising in HF.
NEW & NOTEWORTHY Three-dimensional analysis of t-tubule density showed t-tubule disruption throughout the whole myocyte in failing dog ventricle. A double-linear relationship between Ca2+ release and t-tubule density displays a steeper slope at t-tubule densities below a threshold value (∼1.5%) above which there is little effect on Ca2+ release (T-tubule reserve). T-tubule loss increases incidence of triggered Ca2+ waves. Chemically induced t-tubule disruption suggests that t-tubule loss alone is a critical component of abnormal Ca2+ cycling in heart failure.
Keywords: calcium, calcium waves, heart failure, transverse tubule, ventricle
INTRODUCTION
T-tubule (TT) remodeling has been identified in left ventricular myocytes in every animal model of heart failure (HF) examined so far including mice, rats, goats, pigs, and sheep, as well as in left ventricular myocardium of patients with HF (1–10). In general, it is widely accepted that TT remodeling may be a consequence of the processes underlying HF development. However, there are now numerous studies strongly suggesting that TT loss is likely to be responsible for diminished ventricular performance at both the myocyte and whole heart level (3, 11), that pharmacological prevention of HF development improves cardiac function and prevents TT remodeling (12), and that the reversal of HF in animal models is associated with the recovery of TTs (13). These studies support the notion proposed by several investigators that TT loss may not be a consequence of HF, but rather the cause (1, 9, 10, 14–16), although this idea is far from certain based on our current understanding of this relationship.
It is known that the loss of TTs has profound effects on Ca2+ release properties, which in turn affect contractile behavior at the cellular level (1, 9, 10, 15, 17, 18). TT remodeling causes dyssynchrony of Ca2+ release along the cell length because the distance between ryanodine receptors (RyRs) within the Ca2+ release units (CRUs) and the nearest source of trigger Ca2+ entry increases as TT loss reduces the number of L-type Ca2+ channels (LTCCs). The loss of an extensive TT network results in many regions of the cell interior no longer having a ready source of Ca2+ influx that initiates Ca2+-induced Ca2+ release. Many RyRs are removed from the normal CRU arrangement, and these orphaned RyRs must await activation by Ca2+ that diffuses from distant sites, causing a delay and dyssynchrony in Ca2+ release in cellular regions that no longer have TTs (1, 19–21). One consequence of TT loss and the resulting gap between LTCCs and orphaned RyRs is that some cell regions will release early following the action potential if it is both located near remaining TTs and maintains intact junctional CRUs. Ca2+ release where orphaned RyRs predominate was delayed (1). It is likely that this fractionation in Ca2+ release along the cell causes intact regions to contract while TT-depleted areas are passively stretched until Ca2+ diffusion arrives to induce contraction. We have reported that the development of HF is likely to be the result of the cumulative effects of inefficient Ca2+ release and resulting contraction as progressively more cells are affected during the development of HF in rats (7). Thus the fractionation of Ca2+ release in failing myocytes is likely to contribute directly to reduced myocardial performance.
These results support the idea that TT remodeling plays a critical role in the decline in cardiac function found in clinical HF. Our goal in this study was to investigate the role of TT loss in both Ca2+ release properties and abnormal Ca2+ cycling that contributes to both impaired cellular and cardiac performance and potential contribution to the dangerous ventricular arrhythmias found in many HF patients. We wished to explore both the true changes in cardiac Ca2+ cycling as well as the mechanisms underlying these changes in cell function. We also wanted to investigate the role of TT remodeling, using an acute detubulation procedure, to determine what role the removal of the TT itself plays in abnormal Ca2+ cycling that not only alters contraction but could also contribute to arrhythmogenesis.
METHODS
Ventricular myocytes were isolated from either unpaced control dog hearts or dogs with pacemakers implanted with leads in the right ventricle. Hounds from 25 to 35 kg of either sex were used in this study. HF was induced by pacing at ∼240 beats/min for ∼4 wk. The end point of pacing was severity of impaired myocardial performance and not duration of rapid RV pacing, with an ejection fraction of <25% determined to indicate extreme HF. Myocytes were isolated from a left ventricular (LV) wedge perfused with liberase at 36°C for ∼40 min for control hearts and 50 min for HF dogs. Physiology and ultrastructure experiments were performed in live cells within 24 h after isolation. All animal experiments were performed in accordance with guidelines of the National Institutes of Health and following approval of all experimental protocols by the Northwestern University Institutional Animal Care and Use Committee.
Echocardiography
Comprehensive two-dimensional (2-D), Doppler, tissue Doppler, and speckle-tracking echocardiography were performed using a GE Vivid 7 ultrasound system (GE Healthcare, General Electric, Corp, Waukesha, WI) with a M3S transducers for M-mode, 2-D, and Doppler acquisition using a standardized acquisition protocol by a single experienced sonographer. All 2-D, Doppler, and M-mode echocardiographic measurements were performed using GE EchoPAC software (version 201, GE Healthcare, General Electric, Corp., Waukesha, WI) by a single experienced research sonographer blinded to all other data. Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (22, 23). Speckle-tracking analysis was also performed using GE EchoPAC software as described previously (24). Left ventricular longitudinal systolic strain and early diastolic strain rate and left atrial conduit, booster, and reservoir strains were quantified. The P wave was used as the reference point to calculate all atrial strain values. For ease of reporting and interpretation, all strain values were reported as absolute values (with lower absolute strain values corresponding to worse cardiac mechanics).
Line Scan Imaging for Intracellular Ca2+ Measurements
Myocytes were loaded with rhod-4AM (15 µM for 25 min) and imaged using a Zeiss LSM510 or LSM510 META confocal microscope with a ×25 objective (NA 1.2) as described previously (22). Note that this dye was used instead of a more typical bright dye like Cal520-AM because this dye localized to the cell organelles and did not respond to changes in cytoplasmic Ca2+ in the usual manner. Cells were superfused with modified Tyrode’s solution maintained at 36°C in an experimental chamber with temperature control and pacing capability (Cell MicroControls, Stony Brook, NY) on the stage of the microscope. Intracellular Ca2+ was measured by placing the scan line along the long axis of an individual cell, and high-resolution X-t line scan images of intracellular calcium transients were obtained (1.92 ms/scan, 512 pixels/line). Excitation was performed at 543 nm with emission collected at >560 nm. Pacing protocols were delivered at basic cycle lengths (BCL) of 1,000, 500, 400, 300, and 200 ms for ∼10 s at each rate. Data were analyzed using customized MatLab programs developed in-house (22). To analyze the synchrony of Ca2+ release along the cell length, each linescan image was partitioned into 2-µm-wide lengths to approximate Ca2+ release along each TT-RyR dyadic junction. The dyssynchrony of Ca2+ release at all 2-µm intervals was then measured, and the standard deviation at this resolution was reported as the heterogeneity index (HI).
Measurements of Nearest Neighbor and Three-Dimensional TT Density
Myocytes were loaded with di-8-aminonaphthylethenylpyridinium (di-8-ANEPPS; 4 µM for 20 min) and allowed to settle to the floor of the experimental chamber before imaging was initiated. Images were obtained as z-stacks using a ×63 NA 1.2 objective (excitation at 488 nm; emission ≥520 nm). Distance from each point to the nearest TT or sarcolemmal membrane in the cell was calculated using customized MatLab software as previously described (23).
TT density was calculated from z-stacks acquired at 0.2- to 0.3-µm intervals throughout each ventricular myocyte, including anywhere from ∼70 to over 100 slices. Each slice was analyzed using AutoTT software (24, 25) to obtain an estimate of the total cellular TT density, which was obtained as the percentage of bright pixels compared with total pixels in each slice, and then all slices were added to obtain total cellular TT density. Because it was not possible to measure TT density in the total cell volume in all myocytes due to geometrical limitations of cell architecture, the actual cell volumes used for three-dimensional (3-D) density and density calculations ranged from 85 to 100% of the total cell volume.
Acute Detubulation of Dog Ventricular Myocytes Using Formamide
Experimental detubulation has been studied in rodent ventricular myocytes (VMs) for nearly 20 yr using an experimental model developed by Orchard and colleagues (26, 27). Formamide was added to Tyrode’s solution containing freshly dissociated dog VMs at a concentration of 1.5 M for 9–10 min after which the cells are washed twice in fresh solution to allow osmotic shock-induced cell swelling. Cells were allowed to rest for 20 min before use. Formamide-treated (FORM) VMs were used for Ca2+ and ultrastructural imaging for ∼1.5 h after which a fresh treatment was prepared.
Use of Rat Ventricular Myocytes
Rat ventricular myocytes were isolated using techniques we have described previously. Myocytes were stained with di-8-ANEPPS and imaged using the same 3-D z-stack approach as described for dog myocytes.
Statistics
Echocardiographic parameters at baseline (healthy control) and after ∼4 wk of rapid RV pacing (HF) were compared using paired t tests. Results from three or more comparisons were performed using an ANOVA with Tukey’s or Dunnett’s post hoc tests for significance between means. Comparisons between two groups was performed using an unpaired t test. Comparisons were performed using each cell as an individual observation as recommended by our statistical consultant. Nonparametric comparisons were performed using a chi-square test. Differences between sample means were considered significant when P < 0.05.
RESULTS
Effects of Rapid RV Pacing on Cardiac Structure/Function
Tables 1 and 2 display the results of comprehensive echocardiographic analysis of dogs at baseline (healthy control dogs) and after ∼4 wk of rapid RV pacing (HF dogs). As shown in Table 1, there were profound changes in cardiac structure and function in the HF dogs: biventricular enlargement and systolic dysfunction, LV diastolic dysfunction, and elevated E/e’ ratio (indicating increased LV filling pressures). Marked left atrial enlargement and dysfunction were also present in the HF dogs. As shown in Table 2, there were also profound changes in cardiac mechanics with reduced LV longitudinal strain, LV early diastolic strain rate, and LA conduit, booster, and reservoir strain.
Table 1.
Changes in cardiac structure and function on echocardiography in the rapid RV rapid pacing model of heart failure
| Echocardiographic Parameter | n | Normal | Heart Failure | P Value* |
|---|---|---|---|---|
| LV structure | ||||
| Septal wall thickness, cm | 12 | 0.93 ± 0.09 | 0.88 ± 0.10 | 0.33 |
| Posterior wall thickness, cm | 12 | 0.89 ± 0.10 | 0.86 ± 0.12 | 0.38 |
| LV end-diastolic dimension, cm | 12 | 3.8 ± 0.3 | 4.6 ± 0.3 | <0.0001 |
| LV end-systolic dimension, cm | 12 | 2.8 ± 0.2 | 4.2 ± 0.4 | <0.0001 |
| LV mass, g | 12 | 102.8 ± 19.5 | 131.4 ± 29.2 | 0.0008 |
| LV end-diastolic volume, ml | 12 | 52.3 ± 19.8 | 87.1 ± 18.0 | <0.0001 |
| LV end-systolic volume, ml | 12 | 24.3 ± 10.1 | 70.0 ± 19.0 | <0.0001 |
| LV systolic and diastolic function | ||||
| LV ejection fraction, % | 12 | 53.9 ± 7.4 | 20.7 ± 11.0 | <0.0001 |
| Transmitral E velocity, cm/s | 12 | 61.4 ± 10.8 | 83.1 ± 23.6 | 0.003 |
| Transmitral A velocity, cm/s | 11 | 34.9 ± 11.5 | 48.1 ± 21.4 | 0.015 |
| E deceleration time, ms | 12 | 166.6 ± 22.5 | 101.6 ± 31.7 | 0.0003 |
| E/A ratio | 11 | 1.9 ± 0.4 | 1.9 ± 0.8 | 0.72 |
| Early diastolic (e’) velocity | 12 | 10.5 ± 1.9 | 7.4 ± 3.5 | 0.012 |
| E/e’ ratio | 12 | 6.0 ± 1.4 | 12.6 ± 4.9 | 0.0005 |
| LA structure/function | ||||
| LA maximal volume, ml | 12 | 21.6 ± 5.1 | 46.8 ± 14.0 | <0.0001 |
| LA minimal volume, ml | 12 | 12.0 ± 3.3 | 37.9 ± 12.2 | <0.0001 |
| LA emptying fraction, % | 12 | 44.2 ± 11.0 | 19.4 ± 8.2 | 0.0004 |
| RV structure/function | ||||
| RV end-diastolic area, cm2 | 12 | 9.2 ± 1.3 | 12.7 ± 3.8 | 0.012 |
| RV end-systolic area, cm2 | 12 | 6.1 ± 1.1 | 10.8 ± 3.3 | 0.002 |
| RV fractional area change, % | 12 | 33.4 ± 6.5 | 14.8 ± 4.4 | <0.0001 |
| TAPSE, cm | 12 | 1.15 ± 0.27 | 0.97 ± 0.25 | 0.034 |
Values represent means ± SD. LV, left ventricular; E, early diastolic; A, late (atrial) diastolic; LA, left atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion. *Paired t test.
Table 2.
Changes in cardiac mechanics on speckle-tracking echocardiography in the rapid RV rapid pacing model of heart failure
| Speckle-Tracking Echocardiographic Parameter* | n | Normal | Heart Failure | P Value† |
|---|---|---|---|---|
| LV longitudinal systolic strain, % | 12 | 13.6 ± 2.2 | 6.3 ± 2.2 | <0.0001 |
| LV early diastolic strain rate, 1/s | 12 | 1.50 ± 0.49 | 0.83 ± 0.26 | 0.0006 |
| LA conduit strain, % | 12 | 10.1 ± 3.7 | 4.4 ± 2.7 | 0.001 |
| LA booster strain, % | 12 | 7.4 ± 3.4 | 4.2 ± 2.2 | 0.020 |
| LA reservoir strain, % | 12 | 17.5 ± 2.8 | 8.7 ± 3.2 | 0.0001 |
Values represent means ± SD. RV, right ventricular; LV, left ventricular; LA, left atrial. *All strain values are presented as absolute values. †Paired t test.
Consequences of Detubulation of Dog VMs
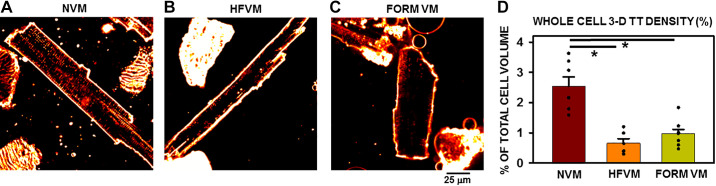
One of the fundamental changes induced by HF in VMs is the loss of TTs. While TT remodeling has been reported in dog VMs, this study investigated the extent of TT loss throughout the entire cell depth rather than at a single plane through the middle of the cell. Examples of normal ventricular myocytes (NVMs) and HF ventricular myocytes (HFVMs) are shown in Fig. 1, A and B. TT density was clearly decreased in the HF myocyte compared with the normal cell. It has been demonstrated before that TT density is decreased in virtually all models of HF; however, previous studies have relied exclusively on 2-D measurements of TT density to quantify TT remodeling. To obtain a more complete and meaningful estimate of the extent of TT remodeling throughout the entire cell volume, total TT density in the entire cell volume was measured using z-stacks obtained from each myocyte to study total 3-D TT density remodeling. Normal TT density was 1.94 ± 0.17% of total cell volume (n = 8 myocytes/5 dogs). Note that rat VMs show significantly greater TT density in total cell volume (9.84 ± 0.27, n = 15 myocytes/6 rats, P < 0.001, compared with dog VMs). Overall, we observed a significant decrease in TT density in HFVMs across the entire cell of nearly 50% (Fig. 1D), similar to previously reported values. When compared with FORM-treated, acutely detubulated myocytes (Fig. 1, C and D), there was no significantly different TT loss observed in HF-induced myocytes over a 4-wk period of rapid pacing. This important observation offers the possibility of studying the effects of pure detubulation in isolation from any of the multitude of other changes in myocyte ultrastructure, function and protein expression that are known to accompany HF in this and other experimental models of HF.
Figure 1.
T-tubule (TT) remodeling in heart failure (HF) and after acute formamide (FORM) treatment. A–C: 2-dimensional confocal images of normal ventricular myocyte (NVM), HF ventricular myocyte (HFVM), and FORM ventricular myocyte (VM). D: summary of whole cell [3-dimensional (3-D)] TT density in each experimental group. *P < 0.05; n = 7 myocytes from 6 dogs (NVM), 6 myocytes from 5 failing dogs (HFVM), and 10 myocytes treated with FORM (FORM VM) from 3 dogs for the 3 groups. Data were compared using a single-tailed ANOVA with a Dunn’s test for secondary comparisons.
To get a more complete picture of TT density and organization in normal and failing dog ventricular myocytes, we also performed several 3-D reconstructions of TTs from the high-density imaging performed on both NVMs and HFVMs. Supplemental Video S1 (see https://doi.org/10.6084/m9.figshare.14115734) shows an example of a 3-D rendering of a NVM in which the middle 20 slices (0.2-mm thickness) so that it is clear how TTs are organized within the cell. Unlike rat ventricular myocytes, there are very few TTs in the central cylindrical core of a NVM and nearly all TTs are located around this mostly empty core. In the movie of the HFVM showing the 20 middle slices around the cell center (Supplemental Video S2; see https://doi.org/10.6084/m9.figshare.14115725), much of the TTs are absent in a very much hypertrophied myocyte. Now the TTs are located in the cell periphery and are present in a much lower density than the NVM in Supplemental Video S1. It is clear from the videos that not only is there less TT density overall but that the result is that the cell center has large regions that are very distant from the nearest sarcolemma and TT membrane.
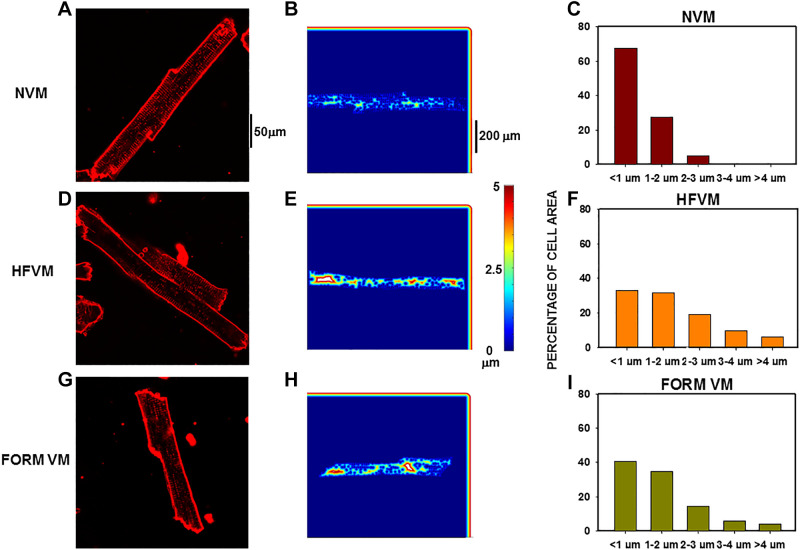
In HF, increased distances are observed between the cell membrane at the outer sarcolemma and the TT network to all regions of the myocyte interior compared with normal myocytes. Nearest neighbor analysis gave an estimate of the shortest distance between each pixel in the cell interior to the closest pixel at the cell membrane where LTCCs are located. Cells with diminished TT networks have greater distances from each pixel to nearest sarcolemma. Color-coded spatial maps showed the distances from each pixel to its nearest region of cell membrane where LTCCs were located. Figure 2A shows an example of a NVM with extensive TT network resulting in mostly short distances within the cell interior (Fig. 2B). When we plotted the distribution of distances (Fig. 2C), most pixels (∼70%) in the cell interior fell within 1 µm of the membrane as the distances between TTs at z-lines is ∼2 µm. It follows that most pixels are located within half that distance. There was a relatively small proportion of distances in this cell that ranged between 1 and 3 µm. The HFVM in Fig. 2D shows fewer TTs, resulting in greater distances to the sarcolemma (Fig. 2E). 34% of measured distances in this cell (Fig. 2F) were <1 µm and a larger fraction of pixels ranged in distances from 1 to 4 µm. Nearly identical results were observed in a FORM VM (Fig. 2, G–I) demonstrating both greater populations of pixels with increased distances and greater maximal distances as well.
Figure 2.
Distance mapping in normal, heart failure (HF), and formamide (FORM) detubulated dog ventricular myocytes (VMs). Nearest neighbor analysis for distance to closest sarcolemmal or t-tubule membrane from each pixel in the cell interior. A and B: confocal image of a normal VM (NVM) (A) with the distance plot for that cell (B). C: distribution of al pixel distances for this myocyte. D–F: similar presentations for a HFVM. G–I: similar presentations for a FORM myocyte.
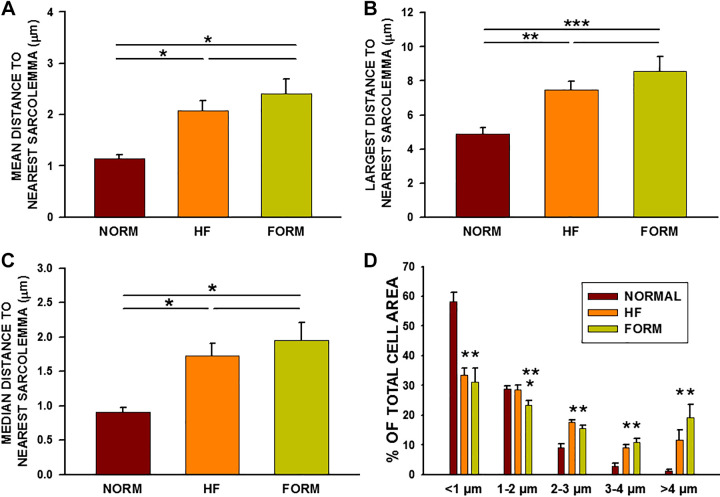
We found that the result of acute detubulation of NVMs significantly increases the distances between the cell interior and the cell membrane (Fig. 3). In addition, the extent of these increased distances is nearly the same in HF and FORM myocytes compared with normal VMs. Thus mean distances (Fig. 3A), maximal distances (Fig. 3B), and median distances (Fig. 3C) showed the same pattern of nearly identical increases in HF and FORM compared with NVMs. Finally, the percentage of cell interior area decreased at the shortest distances (<1 µm) and increased at 2–3 µm and above, demonstrating a significant increase in the distribution of pixels with greater distances between sarcolemma and cell interior. Note that there is crossover at the 1- to 2-µm distances for NVMs and HFVMs, which is accompanied by a decline in shorter distances and an increase in longer distances.
Figure 3.
Summary of distance mapping in normal, heart failure (HF), and formamide (FORM) detubulated dog ventricular myocytes (VMs). A: average distance to nearest sarcolemma for all myocytes from each experimental group. B: greatest distance measured for each group. C: median distance to nearest sarcolemma. D: percentage of total cell area for each distance. A–C: *P < 0.05. **P < 0.01; ***P < 0.001. D: P < 0.05, compared with normal VMs (NVMs); **P < 0.01, compared with HFVMs. Data were compared using a single-tailed ANOVA with a Dunn’s or Holm-Sidak tests for secondary comparisons; n = 14 myocytes in 8 dogs (NVM), 13/5 (HFVM), and 10/3 (FORM VM).
Effects of TT Remodeling on Ca2+ Release
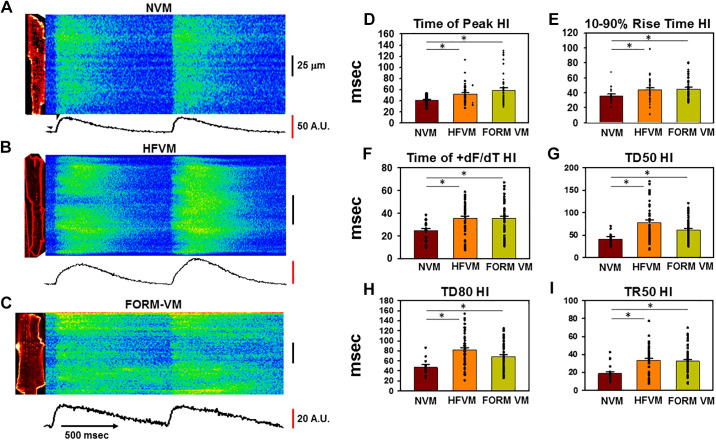
Profound changes in TT organization would be expected to be accompanied by equally dramatic effects on Ca2+ release during the Ca2+ transient. These changes have been reported in several animal models of HF and indicate a primary interference of Ca2+ release along the cell length because of the variability in TT loss within the cell. To investigate how detubulation affects Ca2+ release, we investigated the heterogeneity in Ca2+ release along the cell length under our experimental conditions. Simultaneous measurements of Ca2+ and TTs were obtained in each myocyte for these experiments. Figure 4A shows Ca2+ transients during pacing at BCL = 300 ms in NVM. Ca2+ release is uniform in response to each stimulus with some variability in the duration of the transient at different cell regions as indicated by the differences in decline in Ca2+ during recovery. In contrast, there is an enormous variability in Ca2+ release along the HFVM shown in Fig. 4B, with some regions failing entirely while other regions are delayed compared with early release sites along the cell. Similar results were observed in FORM VMs (Fig. 4C) which also showed great heterogeneity in Ca2+ release.
Figure 4.
Defects in intracellular Ca2+ release variability [heterogeneity index (HI)] following TT remodeling in heart failure (HF) and formamide (FORM)-treated dog ventricular myocytes (VMs). A–C: confocal linescan images of Ca2+ transients during pacing at 2 Hz in a normal VM (NVM; A), HFVM (B), and FORM-treated myocyte (C). A.U., arbitrary units. The corresponding 2-dimensional image is shown to the left of each linescan. Average fluorescence along the length of the line is shown below each image. D–I: summary data showing HI for variability in time to peak, rise time, time of maximum dF/dt, duration at 50% of peak (TD50), transient duration at 80% of recovery (TD80), and time to 50% of release (TR50). *P < 0.05, as measured using a single-tailed ANOVA with a Dunn’s test for secondary comparisons; n = 19-20 myocytes in 4 dogs (NVM), 42 in 4 dogs (HFVM), and 49 in 6 dogs (FORM VM).
Summary data from numerous experiment (Fig. 4, D–I) showed that there were significant increases in the heterogeneity index (HI; defined as the standard deviation along the cell length) (28) in HF and FORM for time to peak, rise time, time of maximal rate of release (+dF/dt), transient duration at 50% of recovery (TD50), transient duration at 80% of recovery (TD80), and transient rise time to 50% of peak (TR50). Furthermore, the increases in variability were the same for HF and FORM compared with normal VMs, again suggesting that it is the detubulation itself that is at least partly responsible for the changes in Ca2+ release observed in HF.
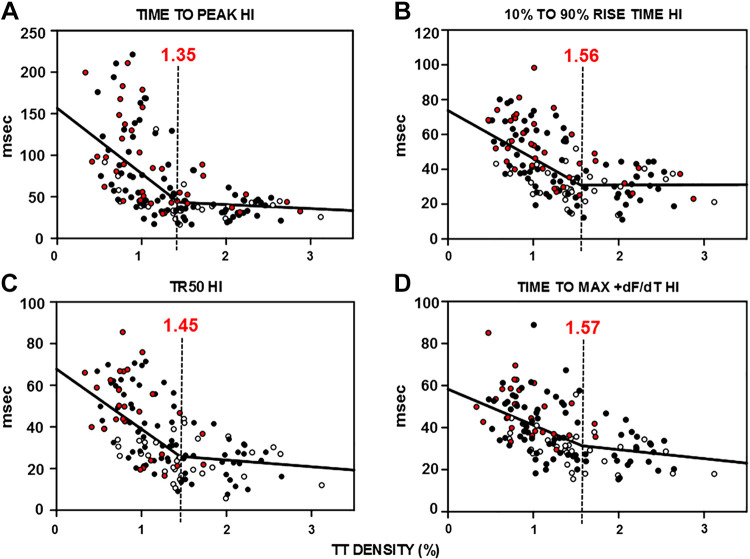
Relationship between TT Remodeling and Ca2+ Release and the TT Reserve
To investigate the relationship between the extent of TT loss and changes in intracellular Ca2+ release more precisely, we plotted the variability in the timing of several critical variables of Ca2+ release as a function of measured TT density for a large population of myocytes from each experimental group. We found that the HI for time to peak Ca2+ release, rise time, TR50, and time to maximum release rate showed a significant reliance on TT density such that variability increased as TT density decreased (Fig. 5, A–D). When we fitted these relationships between each variable and TT density to a double linear fit, we found that there was a much steeper slope for each graph at values of TT density below 1.35–1.57 such that there was an increase in variability in Ca2+ release with decreasing TT density. These results indicate that not only is there an important reliance of Ca2+ release on TT density but that this relationship accelerates as TT remodeling increases. The result is that at high TT densities there is very little change in Ca2+ release over a wide range of declining TT density. This range represents a TT reserve where loss of TTs causes little change in Ca2+ release. However, below a certain extent of TT loss, there is an increasing heterogeneity in Ca2+ release indicating an increase in sensitivity of Ca2+ release to TT remodeling. One interpretation of these results is that there is a “threshold” of ∼1.5% density below which there is an acceleration in variability in Ca2+ release properties. Moreover, there appears to be no difference between HF and FORM in this relationship between Ca2+ release and TT density, indicating that it is the TT density itself that is responsible for the increase in variability Ca2+ release along the cell length. These data show that there is a close relationship between TT density and critical Ca2+ release characteristics such that TT loss causes a slowing and delay in Ca2+ release along the cell length in HF that is a result of the TT remodeling itself. In addition, this relationship is characterized by a relative insensitivity of Ca2+ release to TT loss over a wide range of TT remodeling (a TT reserve), but when some critical threshold value of remodeling is achieved, the resulting impairments in Ca2+ release are greatly accelerated with decreasing TT density.
Figure 5.
Relationships between t-tubule (TT) density and heterogeneity index (HI) of Ca2+ release properties and the concept of TT reserve. Summary graphs for all myocytes showing the relationship between TT density and HI for time to peak (A), rise time (B), time to 50% of release (TR50; C), and time to maximum dF/dt (D). Each graph was fitted to a double linear curve with the inflection point indicated by the dashed vertical line with the TT density indicated in red above each line; n = 32–33 in 5 dogs [normal ventricular myocyte (VM), open circles], 26–28 in 3 dogs (heart failure VM, red filled circles), and 75–77 in 7 dogs (formamide VM, black filled circles).
TT Remodeling and Triggered Ca2+ Waves in Normal, HF, and Detubulated VMs
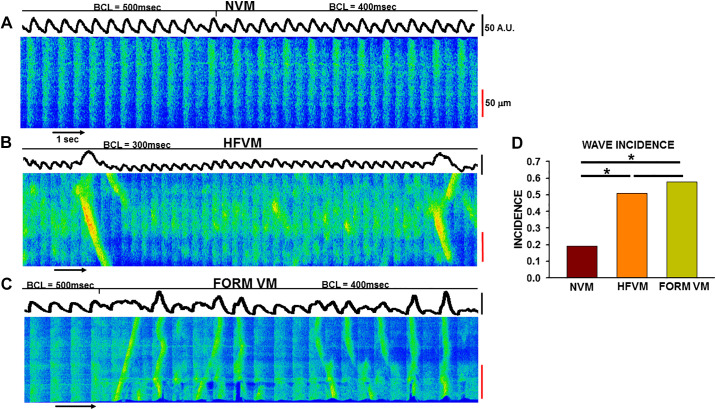
We have previously reported that a unique form of abnormal Ca2+ release occurs during rapid pacing in atrial myocytes in contrast to spontaneous Ca2+ waves that occur after pacing is terminated (22, 29). Triggered Ca2+ waves arise in the atrium specifically because of the low density of TTs in this tissue. These triggered Ca2+ waves have important effects not only on Ca2+ release but are highly effective in producing depolarization and prolongation of action potential (AP) duration because they occur during the AP (29). Because of the likelihood that the changes in cellular electrophysiology could establish the conditions for repolarization gradients and reentry, we have proposed that triggered Ca2+ waves might contribute to the initiation and/or maintenance of atrial arrhythmias. We also investigated whether or not triggered Ca2+ waves occur in VMs and if their presence was related to TT remodeling.
The linescan image in Fig. 6A shows that no triggered waves arose in this NVM during pacing at BCL= 400 and 500 ms. This result was typical of NVMs, and we found that <20% showed triggered waves during rapid pacing. It is also worth noting that, in this example as well as in some others, Ca2+ alternans developed during pacing that could also contribute to the generation of ventricular arrhythmias. In contrast, prominent and frequent triggered waves were initiated during rapid pacing in both HFVMs and in FORM VMs (Fig. 6, B and C). Overall, we found that over 50% of HF and FORM VMs demonstrated triggered waves compared with <20% on NVMs (Fig. 6D). These results demonstrate that triggered waves do occur at a low incidence even in normal VMs but that the likelihood of initiating triggered waves is much greater in both HFVMs and FORM VMs. Furthermore, the fact that the incidence is the same in both forms of detubulated myocytes strongly suggests it is the detubulation itself (and possibly the resulting RyR reorganization) and not some other epiphenomenon of HF that is responsible for establishing the conditions for triggered wave initiation. This is consistent with the fact that nearly 80% of normal atrial myocytes and 90% of HF atrial myocytes demonstrated this behavior during rapid pacing (22). It is possible that, as we have reported in the atrium (22), this form of abnormal Ca2+ cycling could contribute to the establishment of repolarization gradients and reentry in the ventricle, thus establishing the substrate for reentrant arrhythmias especially in the setting of HF where detubulation is significant.
Figure 6.
Incidence of triggered Ca2+ waves in heart failure (HF) and formamide (FORM) detubulated dog ventricular myocytes (VMs). A: linescan image during rapid pacing [basic cycle lengths indicated above the image (BCL)] in a normal VM (NVM; A), a HFVM (B), and a FORM myocyte (C). D: summary of incidence of triggered Ca2+ waves in each experimental group compared using a chi-square test (*P < 0.05).
Supplemental data are available at: https://figshare.com/articles/media/NORMAL_DVM_-_slice_original_file_size_gif/13296560; https://figshare.com/articles/media/HF_DVM/13296584.
DISCUSSION
It has been known for over 15 yr that TT remodeling is a hallmark of virtually every model of HF that has been studied in both animals and humans. In addition, numerous studies have reported that detubulation produces profound effects on intracellular Ca2+ release, which affect cellular contraction and are likely to also contribute to impairment of overall myocardial performance. More recently, it has been shown that TT loss itself contributes to the decline in cardiac function during the development of HF, suggesting that detubulation may not be a mere outcome of HF but may in fact be a direct contributor to its development, making this critical feature of HF more than just an accompaniment to the underlying causes of the disease. It is important to note that this view is still unproven but it raises the possibility of a new understanding of the molecular mechanisms underlying HF development with possible new approaches to treatment.
TT Remodeling, Excitation-Contraction Coupling, and Cardiac Performance
Our results demonstrate that TT loss produces major deficiencies in the uniformity of intracellular Ca2+ release that is critical to a normal, efficient Ca2+ transient essential to maintaining cellular contraction and cardiac function. The loss of TTs causes an increase in the distances between internal RyRs and their source of trigger Ca2+, which results in poor coupling between trigger and release mechanisms at the CRU. This increase in distance means that orphaned RyRs must wait for Ca2+-induced Ca2+ release at distant sites, nearer to the membrane where LTCCs reside, to diffuse into the cell interior to induce Ca2+ release at nonjunctional release sites that is both slower (because of slow delivery) and delayed. The result is that some cellular regions will release Ca2+ and contract earlier than other regions, causing a highly heterogeneous contraction along the cell length. It is even possible that those cell regions that are most delayed may undergo stretch before contraction is initiated, making for an extremely inefficient contraction at the cellular level. Overall, we found significant differences in Ca2+ release that result from TT remodeling and reorganization due to HF and acutely induced detubulation. Ca2+ release in NVMs is characterized by fast release and reuptake, resulting in a sharp rise in Ca2+ in response to electrical stimulation. Interestingly, different patterns of calcium release were observed in HFVMs, which were characterized by a rounding of the Ca2+ release peak as has been reported previously in several species (1, 30). The rise to peak is bimodal, showing fast Ca2+ release before slowing down, producing a rounded shape. This is likely due to the fact that not all TTs are remodeled in HF and there is still a population of TT that operate optimally (17). We found similar Ca2+ release behavior in FORM VMs insofar as Ca2+ release demonstrates bimodality, similarly characterized by an initial fast release of Ca2+ and immediately followed by slowed phase of Ca2+ rise, rounding out the Ca2+ release peak.
We and others have previously shown that the development of HF is associated with an increase in the number of cells that demonstrate poor TT organization with a commensurate decline in the number of myocytes with normal, high TT organization, suggesting that TT remodeling itself may be responsible for the progression in poor cardiac function with HF development (1, 9, 10, 15, 16). The current results expand on this work by showing why and in what specific ways Ca2+ release is impaired during detubulation. The result is that contraction in cells with a normal TT network will contract early and uniformly along the cell length while neighboring myocytes with poor TT organization will be both delayed and fragmented. Delayed release and fragmented TT networks likely contribute to both impairments in contraction and relaxation at the organ level as the variability in the ratio of normal to abnormal myocytes decreases with disease progression. It remains to be seen how the shift in balance of normal to abnormal TT organization affects the two phases of the cardiac cycle since it is possible that the shift in balance may be responsible for either contraction or relaxation separately, which could have major implications for the development of HF with preserved ejection fraction as opposed to reduced ejection fraction. It may be that a higher ratio is able to maintain normal contraction despite early changes in diastolic function but that ejection fraction falls as the balance shifts to more poorly performing myocytes, which then have a greater influence on systolic performance and diastolic performance.
Relationship between TT Remodeling and Ca2+ Release
We have previously reported that TT remodeling in failing rat VMs is characterized by a wide range across which Ca2+ cycling is unaffected until organization is severely compromised, at which point Ca2+ cycling shows an accelerating sensitivity to further disruption (31). We suggested that this concept of a TT reserve was a fundamental property of cardiac cellular function since it ensured that modest TT loss had little effect on Ca2+ release. However, this protective effect failed when TT loss exceeded some threshold beyond which dyssynchrony on Ca2+ release follows an accelerating path as TT organization and density continue to fall. We found similar results in the current study in dog VM; our data show progressive and nonlinear sensitivity to TT disruption as TT density falls below ∼1.5%, but above which minimal disruption to Ca2+ cycling is observed. Thus the TT reserve and a heightened sensitivity to TT loss once some critical value is achieved are also relevant in large animal hearts. This is indicated by the abrupt increase in the slope of this relationship as TT density falls below this threshold value. Our previous results suggested a nonlinear, exponential relationship between TT loss and Ca2+ release in the rat, but our current results are best fit by a double linear relationship. This difference may be related in part to the fact that rodents start with approximately five times the TT density compared with that observed in dog heart although is it also possible that a double-linear fit might be equally appropriate for the rat. We had expected that there would be a narrow TT reserve in the dog heart where less TT density was found but this was not the case; about half of TT density could be lost before Ca2+ release showed appreciable indications of heterogeneity that might influence myocardial mechanics. Furthermore, this relationship is observed in both the HF and detubulation models, suggesting that critical sensitivity to TT loss is a result of detubulation and not of other cellular changes in function or protein expression that might affect cell performance.
Inverse Square Rule and Ca2+-Induced Ca2+ Release in Detubulated Myocytes
In our previous publication, we also used computer simulations to demonstrate that nonlinear effects of RyR remodeling on Ca2+ release are most likely explained by the rule of square (31). Those results were obtained in rat ventricular myocytes using a computational approach, but we would expect that similar rules would apply in dog even though we did not use modeling to address this possibility in the current study in dog ventricle. As TT loss develops, the activation of core RyRs is achieved through the spread of Ca2+ from distant triggering sites and the density of Ca2+ over distance that is determined by this rule. Thus the fidelity of coupling and release is determined by the distance between LTCCs and RyRs and between activated RyRs and neighboring RyRs downstream that must wait for local Ca2+ density to reach threshold, inducing release from the closest release sites. Since the density of Ca2+ declines as the square of the distance from the trigger source, it is the growing distance due to detubulation that is responsible for this critical nonlinearity of Ca2+ release as the LTCC-RyR distance increases. It is very likely that these changes in cell architecture following TT loss are responsible for dyssynchronous Ca2+ release and ultimately cause both diastolic and systolic dysfunction during HF development.
Role of Detubulation Alone on Impaired Cellular Performance in HF
One of the most important observations in our current work is that the effects of TT loss in HF and following acute detubulation are nearly the same in almost all critical features of intracellular Ca2+ release. We found that the extent of TT loss, the resulting increase in distances from internal release sites to nearest membrane (and resident LTCCs), and the effects on intracellular Ca2+ release of the increased distances are virtually identical in HF and FORM VMs. This is an important observation since it indicates that TT remodeling itself is critical to the changes in excitation-contraction (E-C) coupling responsible for diminished cardiac performance during HF development. Despite the fact that our results support the notion that TT loss itself is likely to play a critical role in impaired Ca2+ release in HF, it is important to acknowledge that it is unlikely that TT loss alone is the only effect of HF that is responsible for abnormal Ca2+ cycling since it is well known that many other changes in contractile proteins, energetics, mitochondrial function, and other sequelae of HF probably also contribute to the decline in contractile function at both the cellular and organ levels. Finally, the observation that TT loss contributes to an increase in the incidence of arrhythmogenic Ca2+ waves during the AP is consistent with the relative abundance of these abnormal Ca2+ events in atrium, but, more significantly, it explains why the incidence of triggered waves is increased in HF. Low incidence in NVMs likely results from low baseline TT density in dog VMs compared with rodents where we and others have found no evidence of triggered waves during rapid pacing. Thus, when TT loss is exaggerated in HF and FORM, the conditions favoring triggered waves are all the more prominent and both HF and FORM VMs produce nearly identical likelihood of triggered waves, underscoring the central role of detubulation in this potentially highly arrhythmogenic setting. It should be noted, however, that other factors besides TT density and organization in HF might affect triggered wave vulnerability in HF including Ca2+ release properties (RyR refractoriness and cluster reorganization in HF) as well as sarcoplasmic reticulum Ca2+ uptake and load both locally and globally that determine Ca2+ release, an essential determinant of triggered wave development.
Conclusions
The unique observations presented in this study, namely new measurements of whole cell TT density rather than conclusions based on 2-D TT density, distance mapping in a large animal model of HF, and the results of osmotic-shock-induced TT loss as a surprisingly accurate mimic of E-C coupling changes in HF, all provide novel support for the critical role of TT disruption in altered Ca2+ release as well as in impaired myocardial performance. Our results provide new evidence that supports the idea that TT remodeling itself is a major contributor to abnormal Ca2+ cycling in HF and that this underlies the reduction in myocardial performance leading to HF. In addition, the fact that we found a nonlinear relationship between TT loss and altered Ca2+ cycling in a large animal model of HF provides confirmation of previous observations in rodents and that there is a TT reserve such that a substantial loss of TTs is tolerated in the dog heart but below a certain level TT loss causes progressively more severe impairment in the ability of the myocyte to maintain normal Ca2+ cycling. Finally, our results also suggest that the loss of TTs itself, whether through chronic TT remodeling during HF development or in response to an acute osmotic disruption of TT organization, is also responsible for an increased incidence of triggered Ca2+ waves that may play a role in ventricular arrhythmias arising in HF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants R01-HL-119095 (to Y.S. and J.A.W.) and R01-HL-125881 and R01-HL-140061 (to R.A.). S. J. Shah is supported by NHLBI Grants R01-HL-107577, R01-HL-127028, R01-HL-140731, and R01- HL-149423) and American Heart Association Grant 16SFRN28780016.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.Y., M.F., W.E.L., J.R., S.J.S., and J.A.W. conceived and designed research; S.Y., D.W., A.B., J.R., L.N., J.Z., W.M., and J.A.W. performed experiments; S.Y., D.W., M.D., H.P., B.G., R.M., M.M., C.O., A.B., J.R., L.N., M.C., and S.J.S. analyzed data; S.Y., D.W., H.P., B.G., R.M., M.M., C.O., W.E.L., A.B., J.R., L.N., M.C., S.J.S., G.A., W.M., and J.A.W. interpreted results of experiments; S.Y., D.W., M.D., H.P., B.G., R.M., C.O., S.J.S., and J.A.W. prepared figures; S.Y., D.W., and S.J.S. drafted manuscript; S.Y., D.W., M.D., B.G., M.F., W.E.L., R.A., Y.S., S.J.S., G.A., J.Z., and J.A.W. edited and revised manuscript; S.Y., D.W., M.D., H.P., B.G., R.M., M.M., C.O., M.F., W.E.L., R.A., Y.S., A.B., J.R., L.N., M.C., S.J.S., G.A., J.Z., W.M., and J.A.W. approved final version of manuscript.
REFERENCES
- 1.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A 103: 4305–4310, 2006. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73, 2004. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Aistrup GL, Gupta DK, O'Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol 306: H88–H103, 2014. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res 59: 67–77, 2003. doi: 10.1016/S0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 5.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A 106: 6854–6859, 2009. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei S, Guo A, Chen B, Kutschke WJ, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res 107: 520–531, 2010. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aistrup GL, Gupta DK, Kelly JE, O'Toole MJ, Nahhas A, Chirayil N, Misener S, Beussink L, Singh N, Ng J, Reddy M, Mongkolrattanothai T, El-Bizri N, Rajamani S, Shryock JC, Belardinelli L, Shah SJ, Wasserstrom JA. Inhibition of the late sodium current slows t-tubule disruption during the progression of hypertensive heart disease in the rat. Am J Physiol Heart Circ Physiol 305: H1068–H1079, 2013. doi: 10.1152/ajpheart.00401.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc Res 49: 298–307, 2001. doi: 10.1016/S0008-6363(00)00256-X. [DOI] [PubMed] [Google Scholar]
- 9.Manfra O, Frisk M, Louch WE. Regulation of cardiomyocyte t-tubular structure: opportunities for therapy. Curr Heart Fail Rep 14: 167–178, 2017. doi: 10.1007/s11897-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roe AT, Frisk M, Louch WE. Targeting cardiomyocyte Ca2+ homeostasis in heart failure. Curr Pharm Des 21: 431–448, 2015. doi: 10.2174/138161282104141204124129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisk M, Ruud M, Espe EKS, Aronsen JM, Røe ÅT, Zhang L, Norseng PA, Sejersted OM, Christensen GA, Sjaastad I, Louch WE. Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis. Cardiovasc Res 112: 443–451, 2016. doi: 10.1093/cvr/cvw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CK, Chen BY, Guo A, Chen R, Zhu YQ, Kutschke W, Hong J, Song LS. Sildenafil ameliorates left ventricular T-tubule remodeling in a pressure overload-induced murine heart failure model. Acta Pharmacol Sin 37: 473–482, 2016. doi: 10.1038/aps.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim M, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, Gorelik J, Yacoub MH, Terracciano CM. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+)release in a rodent model of heart failure. Eur J Heart Fail 14: 571–580, 2012. doi: 10.1093/eurjhf/hfs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balijepalli RC, Kamp TJ. Cardiomyocyte transverse tubule loss leads the way to heart failure. Future Cardiol 7: 39–42, 2011. doi: 10.2217/fca.10.113. [DOI] [PubMed] [Google Scholar]
- 15.Louch WE, Stokke MK, Sjaastad I, Christensen G, Sejersted OM. No rest for the weary: diastolic calcium homeostasis in the normal and failing myocardium. Physiology (Bethesda) 27: 308–323, 2012. doi: 10.1152/physiol.00021.2012. [DOI] [PubMed] [Google Scholar]
- 16.Shah SJ, Aistrup GL, Gupta DK, O'Toole MJ, Nahhas AF, Schuster D, Chirayil N, Bassi N, Ramakrishna S, Beussink L, Misener S, Kane B, Wang D, Randolph B, Ito A, Wu M, Akintilo L, Mongkolrattanothai T, Reddy M, Kumar M, Arora R, Ng J, Wasserstrom JA. Ultrastructural and cellular basis for the development of abnormal myocardial mechanics during the transition from hypertension to heart failure. Am J Physiol Heart Circ Physiol 306: H88–H100, 2014. doi: 10.1152/ajpheart.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsett DB, Frisk M, Aronsen JM, Norden ES, Buonarati OR, Cataliotti A, Hell JW, Sjaastad I, Christensen G, Louch WE. Cardiomyocyte substructure reverts to an immature phenotype during heart failure. J Physiol 597: 1833–1853, 2019. doi: 10.1113/JP277273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolstad TR, van den Brink J, MacQuaide N, Lunde PK, Frisk M, Aronsen JM, Norden ES, Cataliotti A, Sjaastad I, Sejersted OM, Edwards AG, Lines GT, Louch WE. Ryanodine receptor dispersion disrupts Ca(2+) release in failing cardiac myocytes. Elife 7: e39427, 2018. doi: 10.7554/eLife.39427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca(2+) sparks in myocytes from infarcted hearts. Circ Res 87: 1040–1047, 2000. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 20.Louch WE, Hake J, Mork HK, Hougen K, Skrbic B, Ursu D, Tonnessen T, Sjaastad I, Sejersted OM. Slow Ca(2)(+) sparks de-synchronize Ca(2)(+) release in failing cardiomyocytes: evidence for altered configuration of Ca(2)(+) release units? J Mol Cell Cardiol 58: 41–52, 2013. doi: 10.1016/j.yjmcc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Oyehaug L, Loose KO, Jolle GF, Roe AT, Sjaastad I, Christensen G, Sejersted OM, Louch WE. Synchrony of cardiomyocyte Ca(2+) release is controlled by T-tubule organization, SR Ca(2+) content, and ryanodine receptor Ca(2+) sensitivity. Biophys J 104: 1685–1697, 2013. doi: 10.1016/j.bpj.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aistrup GL, Arora R, Grubb S, Yoo S, Toren B, Kumar M, Kunamalla A, Marszalec W, Motiwala T, Tai S, Yamakawa S, Yerrabolu S, Alvarado FJ, Valdivia HH, Cordeiro JM, Shiferaw Y, Wasserstrom JA. Triggered intracellular calcium waves in dog and human left atrial myocytes from normal and failing hearts. Cardiovasc Res 113: 1688–1699, 2017. doi: 10.1093/cvr/cvx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frisk M, Koivumaki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol 307: H609–20, 2014. doi: 10.1152/ajpheart.00295.2014. [DOI] [PubMed] [Google Scholar]
- 24.Arora R, Aistrup GL, Supple S, Frank C, Singh J, Tai S, Zhao A, Chicos L, Marszalec W, Guo A, Song LS, Wasserstrom JA. Regional distribution of T-tubule density in left and right atria in dogs. Heart Rhythm 14: 273–281, 2017. doi: 10.1016/j.hrthm.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo A, Song LS. AutoTT: automated detection and analysis of T-tubule architecture in cardiomyocytes. Biophys J 106: 2729–2736, 2014. doi: 10.1016/j.bpj.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol Heart Circ Physiol 283: H1720–H1728, 2002. doi: 10.1152/ajpheart.00347.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kawai M, Hussain M, Orchard CH. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol Heart Circ Physiol 277: H603–H609, 1999. doi: 10.1152/ajpheart.1999.277.2.H603. [DOI] [PubMed] [Google Scholar]
- 28.Wasserstrom JA, Kapur S, Jones S, Faruque T, Sharma R, Kelly JE, Pappas A, Ho W, Kadish AH, Aistrup GL. Characteristics of intracellular Ca2+ cycling in intact rat heart: a comparison of sex differences. Am J Physiol Heart Circ Physiol 295: H1895–H1904, 2008. doi: 10.1152/ajpheart.00469.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gussak G, Marszalec W, Yoo S, Modi R, O'Callaghan C, Aistrup GL, Cordeiro JM, Goodrow R, Kanaporis G, Blatter LA, Shiferaw Y, Arora R, Zhou J, Burrell AR, Wasserstrom JA. Triggered Ca(2+) waves induce depolarization of maximum diastolic potential and action potential prolongation in dog atrial myocytes. Circ Arrhythm Electrophysiol 13: e008179, 2020. doi: 10.1161/circep.119.008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Rourke B, Kass DA, Tomaselli GF, KääB S, Tunin R, Marbán E, Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84: 562–570, 1999. doi: 10.1161/01.RES.84.5.562. [DOI] [PubMed] [Google Scholar]
- 31.Singh JK, Barsegyan V, Bassi N, Marszalec W, Tai S, Mothkur S, Mulla M, Nico E, Shiferaw Y, Aistrup GL, Wasserstrom JA. T-tubule remodeling and increased heterogeneity of calcium release during the progression to heart failure in intact rat ventricle. Physiol Rep 5: e13540, 2017. doi: 10.14814/phy2.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]